ABSTRACT
Gene microarray and bioinformatic analysis showed that S100A8 was more abundant in the stroma surrounding tumor buddings (TBs) than in the stroma surrounding primary tumor cells in colorectal carcinomas. Here, S100A8+ cells in 419 colorectal carcinoma samples were stained by immunohistochemistry and counted using Image-pro plus 6.0. TBs were also counted and biomarkers associated with the epithelial–mesenchymal transition and apoptosis were assessed by immunohistochemistry. We evaluated the association between S100A8+ cells and clinico-pathological variables as well as survival. Migration and invasion as well as biomarkers of the epithelial–mesenchymal transition and apoptosis were tested in CRC cells, treated with graded concentrations of recombinant human S100A8 protein.
We found that the density of S100A8+ cells in the tumor invasive front (S100A8+TIF) clearly distinguished patients with 5-y survival from those who did not survive (p = 0.01). The S100A8+-associated tumor budding (SATB) index determined by the S100A8+TIF and TB was an independent predictor of overall survival (p = 0.001) other than the S100A8+TIF or TB alone. Migration and invasion properties of CRC cells were inhibited by recombinant human S100A8 treatment. The particular S100A8+ cells in the stroma were associated with important biomarkers of the epithelial–mesenchymal transition (E-cadherin and SNAIL) and apoptosis (BCL2).
In conclusion, S100A8+ cells in the stroma predict a good prognosis in colorectal carcinoma. An index combining S100A8+ cells and TB independently predicts survival. Recombinant human S100A8 inhibited CRC cell migration and invasion, which was involved in epithelial–mesenchymal transition (E-cadherin and SNAIL) and apoptosis (BCL2).
KEYWORDS: EMT, immune microenvironment, invasion, migration, prognosis, S100A8, tumor budding
Abbreviations
- BCL2
B-cell lymphoma-2
- CRC
colorectal carcinoma
- EMT
epithelial–mesenchymal transition
- E-CAD
E-cadherin
- HE
hematoxylin–eosin
- LCM
laser capture microdissection
- N-CAD
N-cadherin
- qRT-PCR
quantitative real-time polymerase chain reaction
- RAGE
receptor for advanced glycation end products
- rhS100A8
recombinant human S100A8 protein
- S100A8+TIF
S100A8+ in the invasive front
- S100A8+TC
S100A8+ in the tumor center
- SATB
S100A8+-associated tumor budding
- TIF
tumor-invasive front
- TC
tumor center
- TNM
tumor-node-metastasis.
Introduction
Colorectal carcinoma (CRC) is one of the top three diseases leading to cancer-related death in the Western world.1 Tumor tissues are heterogeneous, including tumor cells and tumor stroma cells.2,3 In order to better understand the tumor microenvironment, it is preferred to segregate different stroma cells around tumor cells. To discriminate stroma cells from tumor cells, we utilized laser capture microdissection (LCM) to separate stroma cells in the tumor-invasive front (TIF) and stroma cells in the tumor center (TC) in CRC models. Several differentially expressed genes were identified through gene expression microarray and bioinformatic analysis. Among these, the S100A8 gene aroused our interest because S100A8+ cells have paradoxical effects on tumor growth and metastasis.4-6 S100A8 is mainly produced by the myeloid lineage including granulocytes and monocytes, and even in stimulated endothelial cells, epithelial cells, and myofibroblasts.7-9 A recent study has demonstrated that treating CRC cells with a low concentration of recombinant S100A8 protein enhances their invasive and proliferative properties by activating the Akt1-Smad5-Id3 axis.10 S100A8 or A9 promotes tumor cell migration and invasion by upregulating the expression of matrix metalloproteinases.11,12 Mac 1+-myeloid cells and lung endothelial cells in the pre-metastatic niche secrete S100A8 and S100A9 to prepare for a suitable environment, increasing malignant cell adhesion and invasion.13 On the contrary, a high S100A9+ stroma cell count predicts a good prognosis in gastric cancers, whereas the S100A8+ stroma cell count has no association with survival.14
Metastasis is the main biological behavior of malignantly transformed cells and leads to an unfavorable outcome. During the last few decades, the epithelial–mesenchymal transition (EMT) has been regarded as the main cause of the transformation of tumor cells that metastasize to lymph nodes or distant organs.15 The EMT process is accompanied by the loss of epithelial markers like E-cadherin (E-CAD), and by the presence of mesenchymal markers like N-cadherin (N-CAD), along with activated transcription factors like SNAIL.16 Cells that acquire an EMT phenotype are resistant to apoptosis, which facilitates tumor cell survival from physical, chemical, or biological insults.17 Tumor budding (TB), which is defined as single cell or cell clusters composed of at most five de-differentiated cells at the invasive front,18 is associated with other clinico-pathological features and has been regarded as an independent but poor prognostic factor in many studies.19-21 Prevailing opinion acknowledges that TB is at least a morphological characteristic of the EMT.22 However, the environmental regulation of the EMT is complex, and the host response to the EMT has still not been elucidated. So, TB offers an appropriate model to study the EMT and its specific environment in CRC patients.
Although S100A8+ stroma cells are distributed unevenly in the center and margin of colorectal tumor lesions,23 there still lacks definitive data about the functions of S100A8+ stroma cells at the TIF on tumor metastasis and patient survival or even the effect of S100A8+ stroma cells on the EMT and apoptosis. It has been reported that S100A8/9-positive granulocytes have a perivascular distribution in the adventitia in giant-cell arteritis.24 However, the significance of the perivascular distribution of S100A8+ cells in CRC has not been elucidated.
We therefore counted S100A8+ stroma cells in the TIF and evaluated the S100A8+TIF cell counts in tumor metastasis and overall survival in 419 CRC cases. The transformation of migration and invasion capacities in CRC cells were observed, treated by graded concentrations of recombinant human S100A8. The correlations between the S100A8+TIF cell counts and the important biomarkers associated with the EMT and apoptosis, E-CAD, N-CAD, SNAIL, and BCL2 (B-cell lymphoma-2) were also investigated.
Results
S100A8+ cells in the tumor invasion front and determination of cut-off values
By gene expression microarray and bioinformatic analysis, the expression of S100A8 in stroma cells surrounding normal epithelial cells (p = 0.042) and tumor cells (p = 0.0048) was lower than in those surrounding TB cells (Fig. 1 A). S100A8 expression in the stroma surrounding parenchymal cells gradually increased: normal epithelia < tumor cells < TB cells. S100A8 expression did not differ among normal epithelia, tumor cells, and TB cells (p > 0.05; Fig. 1 B).
Figure 1.
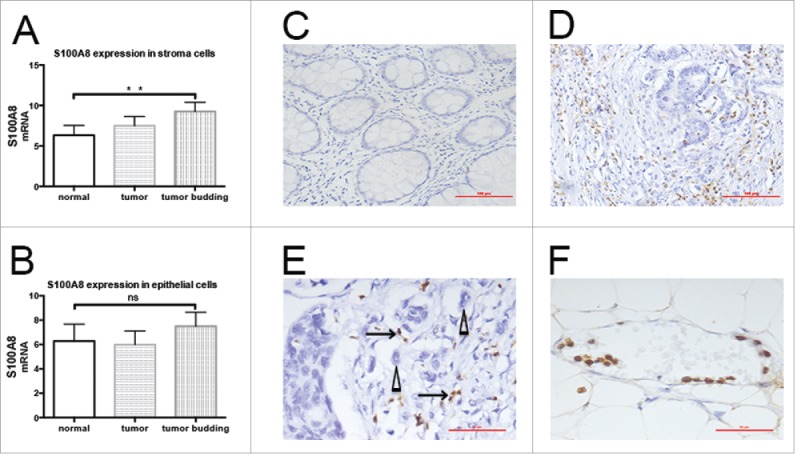
Distribution of S100A8+ cells in CRC tissues. A, S100A8 expression in stroma cells surrounding normal epithelial cells (p = 0.042) and tumor cells (p = 0.0048) was lower than in those surrounding tumor budding cells. B, S100A8 expression did not differ among normal epithelia, tumor cells, and tumor budding cells. C, S100A8+ cells in normal epithelial stroma (20×), scale bar: 100μm. D, S100A8+ cells (brown) in the tumor invasive front (20×), scale bar: 100μm; E, S100A8+ cells (arrow) distributed around tumor budding (triangle) (40×), scale bar: 50μm; tumor buddings are defined as comprising five tumor cells or less, appearing at the invasive front of a subset of colorectal adenocarcinomas of tiny cords of neoplastic epithelium that extend from the neoplastic glands into the adjacent stroma, and small aggregates of neoplastic epithelium that appear to have detached and migrated a short distance into a usually quite desmoplastic stroma; Patients parameters: TNM III, lymphonde metastasis, metastasis and vessel infiltration positive. F, S100A8+ cell-marginated vessels (40×), scale bar: 50μm; Patients parameters: TNM II, lymphonde metastasis, metastasis and vessel infiltration negative.
Immunohistochemistry showed S100A8+ cells were mainly monocytes and polymorphonuclear neutrophils (Fig. 1C–F). S100A8+TIF cells were distributed close to TBs (Fig. 1E). The S100A8+TIF counts were introduced in the 5-y survival-specific ROC curve analysis to discriminate surviving from non-surviving patients. These patients were manifestly distinguished by the ROC curve according to the S100A8+TIF counts [p = 0.025, AUC (95%) = 0.595 (0.514–0.676)]. The median value of S100A8+TIF cell number was 39. According to the Youden index, the sensitivity and specificity of the cut-off value 39 were 0.643 and 0.457, respectively (Fig. 2B).
Figure 2.
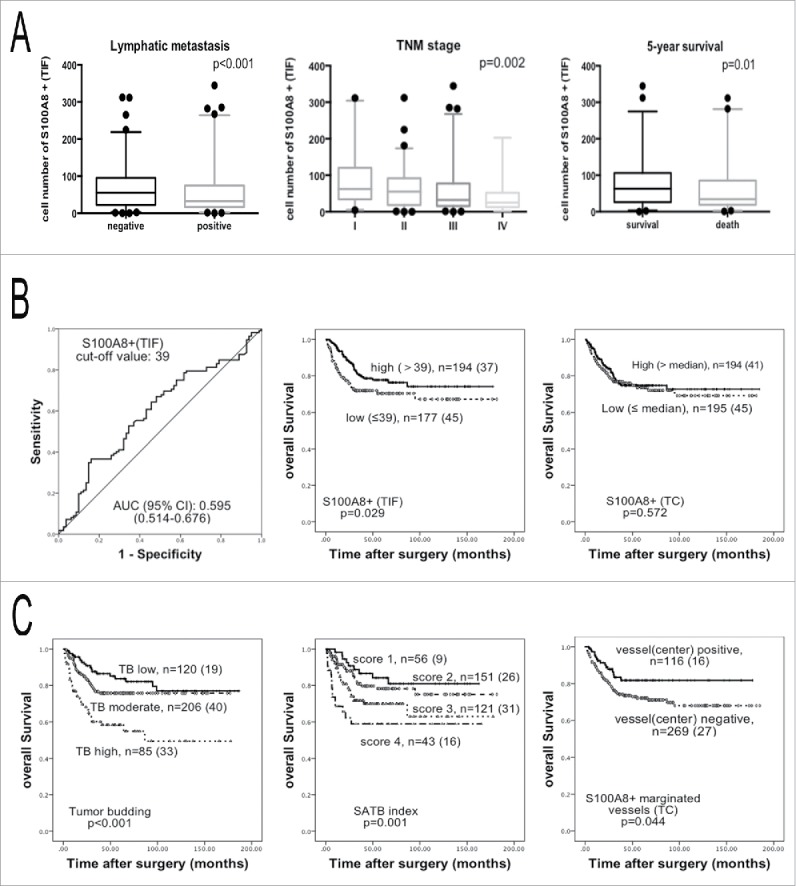
Analysis of the S100A8+TIF, S100A8+TC, TB, and SATB index. (A) The S100A8+TIF cell counts were less in the lymph node metastasis groups than that in the non-lymph node metastasis groups (left); the S100A8+TIF cell counts were less in the higher TNM stage (middle) groups; the S100A8+TIF cell counts were more in 5-y alive groups than that in death groups (right); p value was calculated by unpaired t test. (B) ROC curve analysis of the S100A8+TIF (left), and the Kaplan–Meier survival analysis (log-rank test) of the S100A8+TIF (middle) and the S100A8+TC (right). (C) TB grade (left) and SATB index defined by the S100A8+TIF and TB grade (middle) predicted overall survival by the Kaplan–Meier survival analysis (log-rank test); the density of S100A8+ cell-marginated vessels in the TC was favorable for overall survival (right); n = total cases (death cases).
Based on the cut-off value of 39 for S100A8+TIF, two groups were obtained: low S100A8+TIF and high S100A8+TIF. Low S100A8+TIF was associated with increased lymph node metastasis (p < 0.001), a higher tumor-node-metastasis (TNM) stage (p = 0.002), and a higher grade (p < 0.001; Fig. 2A and Table 1). In low S100A8+TIF group, 20.7% of patients had vessel infiltration than 10.6% of that in high S100A8+TIF group (p = 0.007; Table 1 and Fig. S3). The S100A8+TIF counts discriminated surviving from non-surviving patients 5-y after surgery (p = 0.01; Fig. 2A and Table 1). From the Kaplan–Meier survival analysis, higher S100A8+TIF was associated with a better overall survival (p = 0.029; Fig. 2B). The same procedure was performed on S100A8+ in the TC. Low and high S100A8+TC groups were determined by the median value. The S100A8+TC groups had no correlation with overall survival in these CRC patients according to the Kaplan–Meier survival analysis (p = 0.572; Fig. 2B).
Table 1.
Association of the S100A8+TIF and TB with clinico-pathological parameters.
| S100A8+TIF |
TB |
|||||||
|---|---|---|---|---|---|---|---|---|
| Parameters | Low ≤ 39 | High >39 | p | Low | Moderate | High | p | |
| Age (y) | <60 | 61(34.1) | 75(37.9) | 0.443 | 40(33.3) | 74(35.4) | 42(47.7) | 0.074 |
| ≥ 60 | 118(65.9) | 123(62.1) | 80(66.7) | 135(64.6) | 46(52.3) | |||
| Gender | Male | 93(52.0) | 109(55.1) | 0.547 | 73(60.8) | 112(53.6) | 41(46.6) | 0.122 |
| Female | 86(48.0) | 89(44.9) | 47(39.2) | 97(46.4) | 47(53.4) | |||
| Lymph node metastasis | Negative | 76(42.5) | 120(60.6) | <0.001 | 89(74.2) | 107(51.2) | 26(29.5) | <0.001 |
| Positive | 103(57.5) | 78(39.4) | 31(25.8) | 102(48.8) | 62(70.5) | |||
| Distant metastasis | Negative | 157(87.7) | 183(92.4) | 0.124 | 112(93.3) | 192(91.9) | 73(83.0) | 0.026 |
| Positive | 22(12.3) | 15(7.6) | 8(6.7) | 17(8.1) | 15(17.0) | |||
| TNM | I | 14(7.8) | 31(15.7) | 0.002 | 34(28.3) | 19(9.1) | 3(3.4) | <0.001 |
| II | 55(30.7) | 83(41.9) | 50(41.7) | 80(38.3) | 22(25.0) | |||
| III | 88(49.2) | 69(34.8) | 28(23.3) | 93(44.5) | 48(54.5) | |||
| IV | 22(12.3) | 15(7.6) | 8(6.7) | 17(8.1) | 15(17.0) | |||
| Histological grade | Low | 62(34.6) | 35(82.3) | <0.001 | 38(31.7) | 36(17.2) | 44(50.0) | <0.001 |
| High | 117(65.4) | 163(17.7) | 82(68.3) | 173(82.8) | 44(50.0) | |||
| Vessel infiltration | Negative | 142(79.3) | 177(89.4) | 0.007 | 110(91.7) | 189(90.4) | 56(63.6) | <0.001 |
| Positive | 37(20.7) | 21(10.6) | 10(8.3) | 20(9.6) | 32(36.4) | |||
| 5-y survival status | Alive | 40(47.6) | 72(66.1) | 0.01 | 58(78.4) | 54(57.4) | 18(35.3) | <0.001 |
| Dead | 44(52.4) | 37(33.9) | 16(21.6) | 40(42.6) | 33(64.7) | |||
The TB grade was determined as low TB (0–4 TBs), moderate TB (5–14 TBs), and high TB (more than 15 TBs). The correlations between the TB grades and clinico-pathological parameters were evaluated and were found to be significantly associated (p < 0.05; Table 1). Higher TB was also associated with an unfavorable overall survival (p < 0.001) according to the Kaplan–Meier survival analysis (Fig. 2C).
The SATB index based on the S100A8+TIF and the TB was an independent prognostic predictor of overall survival
Above results indicated that S100A8+ cells played an antitumor role, whereas TBs had a pro-tumor role in metastasis and prognosis. A new scoring system termed the S100A8+-associated tumor budding (SATB) index was used by integrating the S100A8+TIF cell and the TB. First, the high S100A8+TIF group was defined as a score of “1” (better survival) and the low S100A8+TIF group was defined as a score of “2” (worse survival). Low, moderate, and high TB groups were given scores of “0,” “1” and “2” (good, moderate, and poor survival), respectively. Second, the S100A8+ and TB scores were added together in each case. Finally, each case was given a new score ranging from “1” to “4.” A higher score on the SATB index was positively associated with lymph node metastasis (p < 0.001), distant metastasis (p = 0.041), histological grade (p < 0.001), vessel invasion (p < 0.001), TNM stage (p < 0.001), and 5-y mortality (p < 0.001; Table 2).
Table 2.
Association between the SATB index and clinico-pathological parameters.
| SATB index |
||||||
|---|---|---|---|---|---|---|
| Parameters | Score: 1 | Score: 2 | Score: 3 | Score:4 | p | |
| Age (y) | <60 | 19(33.9) | 54(35.3) | 43(34.7) | 20(45.5) | 0.586 |
| ≥ 60 | 37(66.1) | 99(64.7) | 81(65.3) | 24(54.5) | ||
| Gender | Male | 34(60.7) | 84(54.9) | 64(51.6) | 20(45.5) | 0.455 |
| Female | 22(39.3) | 69(45.1) | 60(48.4) | 24(54.5) | ||
| Lymph node metastasis | Negative | 45(80.4) | 90(58.8) | 52(41.9) | 9(20.5) | <0.001 |
| Positive | 11(19.6) | 63(41.2) | 72(58.1) | 35(79.5) | ||
| Distant metastasis | Negative | 52(92.9) | 143(93.5) | 110(88.7) | 35(79.5) | 0.041 |
| Positive | 4(7.1) | 10(6.5) | 14(11.3) | 9(20.5) | ||
| TNM | I | 17(30.4) | 20(13.1) | 7(5.6) | 1(2.3) | <0.001 |
| II | 24(42.9) | 68(44.4) | 38(30.6) | 8(18.2) | ||
| III | 11(19.6) | 55(35.9) | 65(52.4) | 26(59.1) | ||
| IV | 4(7.1) | 10(6.5) | 14(11.3) | 9(20.5) | ||
| Histological grade | Low | 12(21.4) | 30(19.6) | 30(24.2) | 25(56.8) | <0.001 |
| High | 44(78.6) | 123(80.4) | 94(75.8) | 19(43.2) | ||
| Vessel infiltration | Negative | 52(92.9) | 140(91.5) | 103(83.1) | 24(54.5) | <0.001 |
| Positive | 4(7.1) | 13(8.5) | 21(16.9) | 20(45.5) | ||
| 5-y survival status | Alive | 31(79.5) | 50(66.7) | 22(40.7) | 9(36.0) | <0.001 |
| Dead | 8(20.5) | 25(33.3) | 32(59.3) | 16(64.0) | ||
According to the Kaplan–Meier survival curve analysis, the SATB index predicted overall survival (p = 0.001; Fig. 2C), as did the S100A8+TIF and the TB grade alone. In order to clarify whether the S100A8+TIF, TB grade, and SATB index were independently associated with overall survival, univariate and multivariate Cox proportional hazards models were applied to the 419 CRC cases. The S100A8+TIF, TB grade, and SATB index, along with lymph node metastasis, distant metastasis, and TNM stage were associated with overall survival (p < 0.05; Table S3) according to this analysis, but not histological grade (p = 0.054) and vessel invasion (p = 0.420). Next, the S100A8+TIF, TB grade, and SATB index, as well as TNM stage, which were significant predictors from the univariate analysis, were integrated into the multivariate Cox proportional hazards model. Only the SATB index and TNM stage were preserved in the final model (Table 3). The SATB index was an independent predictor of overall survival in CRC patients rather than the S100A8+TIF or the TB alone.
Table 3.
Results of the multivariate Cox proportional hazards model (n = 419).
| Parameters | RR (95% CI) | p |
|---|---|---|
| SATB index | 0.054 | |
| Score 2 | 1.173 (0.544–2.529) | 0.684 |
| Score 3 | 1.594 (0.740–2.728) | 0.234 |
| Score 4 | 2.728 (1.168–6.371) | 0.020 |
| TNM stage | <0.001 | |
| II | 1.575 (0.532–4.662) | 0.412 |
| III | 2.411 (0.838–6.941) | 0.103 |
| IV | 8.589 (2.884–25.580) | <0.001 |
| Tumor budding | – | 0.797 |
| Moderate | – | 0.511 |
| High | – | 0.697 |
| S100A8+TIF (low vs. high) | – | 0.939 |
All parameters were modified by gender and age.
S100A8+TC cells in vessels
S100A8+ cells were also observed in vessels, so we counted the number of vessels that were marginated by S100A8+ cells. There was a positive correlation between the number of S100A8+TIF cells and the number of S100A8+-marginated vessels in the TC (r = 0.138, p = 0.008). This implied that S100A8+ cells in the stroma of primary tumors might be derived from these typical vessels (Fig. 1F). Intriguingly, increased densities of vessels marginated with S100A8+ cells in the TC were correlated with an increased overall survival (p = 0.029; Table S4). We also found that the high TB grade was accompanied by the decreased S100A8+-marginated vessels in the TC (p = 0.019; Table S4). By the Kaplan–Meier survival analysis, more S100A8+ cell-marginated vessels in the TC had a better prognosis for overall survival in CRC patients (p = 0.044; Fig. 2C).
Recombinant S100A8 protein inhibited migration and invasion of CRC cells
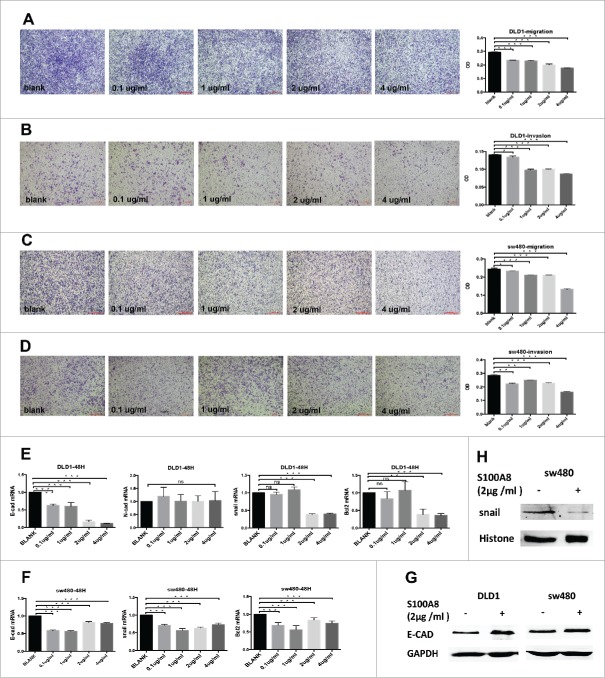
The survival analysis above indicated that S100A8 in stroma cells benefited survival (Fig. 2B and C). In vitro, migration and invasion of CRC cells were conducted by sequential concentrations of recombinant S100A8 stimulation at 0, 0.1, 1, 2, and 4 µg/mL for 48 h. At high concentration (4 µg/mL), the migration and invasion capacities of DLD1 and sw480 cells were significantly inhibited (Fig. 3A–D).
Figure 3.
Migration and invasion of DLD1 and sw480 cells within graded concentrations of recombinant S100A8 treatment (A-D, 4×), scale bar: 500μm. mRNA expression of E-CAD, N-CAD, SNAIL and BCL2 in DLD1 and sw480 cells after rhS100A8 treatment (E and F). Protein levels of E-CAD, SNAIL in DLD1 and sw480 cells after rhS100A8 treatment (G and H).
S100A8 was associated with important biomarkers of the EMT and apoptosis
Finally, we set out to determine whether the effects of S100A8 on patient survival and cell migration and invasion were due to critical proteins like EMT markers (E-CAD and N-CAD), EMT-related transcription factors (SNAIL) and an apoptosis marker (BCL2). So, these proteins were also assessed by immunohistochemistry and observed in tumor cells (Table S5). The S100A8+TIF was negatively associated with SNAIL (p = 0.010). The density of S100A8+ cell-marginated vessels in the TC was negatively associated with N-CAD (p = 0.009). E-CAD expression had no correlation with the S100A8+TIF (p = 0.689; Table S5). Higher S100A8+TIF was associated with lower BCL2 expression (p = 0.027).
Further, DLD1 and sw480 were treated with rhS100A8 at 0, 0.1, 1, 2, and 4 µg/mL for 48 h. Then, the transcriptional levels of E-CAD, N-CAD, SNAIL, and BCL2 were evaluated by qRT-PCR. In accordance with the immunohistochemistry results, the transcriptional levels of SNAIL and BCL2 were significantly downregulated after 2 or 4 µg/mL rhS100A8 in both DLD1 and sw480 cells (p <0.05), whereas their expression was not influenced by low rhS100A8 concentrations (Fig. 3E/F). N-CAD did not change in DLD1 cells (p >0.05; Fig. 3E), whereas it was not expressed in sw480 cells. However, the expression of E-CAD was also downregulated (Fig. 3E/F). To further verification, the protein levels of E-CAD expression and SNAIL were detected. E-CAD was upregulated in DLD1 and sw480 cells (Fig. 3G) and nuclear SNAIL (Fig. 3H) was downregulated in sw480 cells, whereas no expression was detected in DLD1 cells when treated by 2 µg/mL rhS100A8. To some extent, S100A8+ cells in the stroma impeded the EMT and accelerated apoptosis.
Discussion
The observation of immune cells within tumors by Rudolf Virchow in the 19th century implied a possible link between the immune microenvironment and tumors.25 Different types, locations, and subclasses of immune cells have different functions and predictive value in CRC.26 In general, natural killer cells, CD8+ T cells, and M1 tumor-infiltrating macrophages are associated with a better outcome, whereas regulatory T cells and M2 tumor-infiltrating macrophages are involved in a poor outcome.7,8,26 Most of these studies are based on the immunophenotype of one or several immune cell subtypes. The S100A8 is mainly expressed in the myeloid lineage including granulocytes and monocytes, but not lymphocytes.7-9 Our observation of S100A8+ cells in the tumor stroma could reflect an immune state in CRC. We showed S100A8+TIF was a protective factor for survival, which was based on the immune associated protein expression in cells, rather than the conventional immunophenotyping.
Tumor microenvironment plays critical roles in tumor cells malignance and patients survival.27,28 Given the existing significance of tumor microenvironment, we sought to create a new scoring system, the SATB index, to better predict tumor progression and survival. In addition to the TB grade, which is a well-documented predictor of survival,19-21 the Kaplan–Meier analysis indicated that the S100A8+TIF was a protective factor for survival. The SATB index based on the S100A8+TIF and TB was a better independent predictor of overall survival than the S100A8+TIF or TB alone because the SATB index was retained in the multivariate Cox proportional hazards model. For the first time, our research focused on counting S100A8+TIF cells and their effect on the prognosis in CRC patients. The new SATB index is better for evaluating the prognosis in CRC patients, compared with S100A8+TIF and TB grade alone.
We chose S100A8 because its expression differed between the stroma cells of TBs and those of primary tumors as measured by the gene expression array analysis. S100A8 and often the co-expressed S100A8/A9 complex, known as a damage-associated molecular pattern, have been emphasized not only as inflammation markers,29 but also as regulators of the immune response and tumor progression.7 However, S100A8 overexpression in diseases associated with inflammation implied a possible role in tumorigenesis. Many studies have confirmed the tumorigenesis-inducing effect of S100A8 in gastric, colon, pancreatic, bladder, ovarian, breast, and skin cancers, both in tumor cells and stroma cells.30 We concluded that the S100A8+TIF was negatively correlated with lymph node metastasis, vessel invasion, and TNM stage (p < 0.05). We also found that a high S100A8+TIF predicted favorable survival both by the Kaplan–Meier method and univariate Cox proportional hazards model analysis (p < 0.05). Our results suggested an antitumor role of S100A8+ cells.
S100A8 expression in tumors plays dual roles in both inflammation-induced cancer and cancer-induced inflammation.7,27 One possible antitumor mechanism is that extracellular S100A8 plays a role in chemotaxis, the induction of leukocyte recruitment, and promotion of the immune response,7,31 which is in agreement with our finding that increasing S100A8+TIF cells were associated with increasing CD8+ CTLs (cytotoxic T lymphocytes, p = 0.032, r = 0.122), CD4+ T cells (p = 0.015, r = 0.137), and CD68+ macrophages (p <0.001, r = 0.235) (data not shown).
Another possible antitumor mechanism is that extracellular S100A8 induces apoptosis by zinc exclusion from target cells or the suppression of intracellular zinc and mediates Bcl2 family members through the mitochondrial death pathway.4 BCL2 is not only an oncogene, but also has gained most attention with regard to apoptosis.32 Studies in vitro have strongly demonstrated apoptotic induction by S100A8 in many tumor cell lines33-35; however, its antitumor action has not been established in vivo, and needs further validation. In this study, we showed that increasing S100A8+TIF cells in the stroma were accompanied by downregulation of BCL2 in tumor cells (p = 0.027). But S100A8 still possesses a pro-tumor property. The antitumor or pro-tumor response may be concentration-dependent, because at high concentrations (80–100 mg/mL), S100A8 has apoptotic effects on tumor cells, whereas at low concentrations (< 25 mg/mL) it promotes tumor cell growth, migration, and invasion.27,36 At low concentrations, extracellular S100A8 binds to RAGE (receptor for advanced glycation end products) on tumor cells, and then activates the MAPK and NF-κB signaling pathways, which are associated with tumor growth and metastasis.5,37
Another phenomenon is that S100A8+-marginated vessels appeared in CRC patients and this was associated with an increased overall survival. We also found a positive correlation between the number of S100A8+ cell-marginated vessels in the TC and S100A8+TIF cells (p = 0.007). S100A8 plays an important role in tubulin-dependent cytoskeletal rearrangements during the trans-endothelial migration of phagocytes.38 The S100A8/A9 complex helps the CD11b–CD18 molecules of leukocytes bind to ICAM-1 on endothelium and also impairs endothelial integrity.39,40 When S100A8 is blocked, neutrophil migration in response to lipopolysaccharide is also suppressed.41 It is indicated that S100A8+ cells move along with the blood-flow in vessels when they receive signals from lesions; they marginate, penetrate vessels, and directionally migrate toward tumor lesions, ultimately performing their antitumor function against tumor cells in the primary tumor.
Our results showed that stimulation with recombinant S100A8 protein at 4 µg/mL suppressed DLD1 and sw480 cells migration and invasion capacities. This was largely due to that S100A8+ cells in the stroma influence the EMT and apoptosis. Research on S100A8 and the EMT is rare. One study indicated that recombinant S100A8 and S100A9 proteins increase expression of the EMT marker β-catenin and its target genes c-myc and MMP7, with enhanced viability and migration in HCT116 and SW480 cells.42 Most research has focused on stimulating cells with recombinant S100A8 or S100A8/A9 protein,43 whereas our study emphasized the density of S100A8+ cells in the stroma using immunohistochemistry. Our results showed opposite effects of S100A8 on the EMT. A higher density of S100A8+TIF cells was associated with downregulation of nuclear SNAIL expression, and a higher density of S100A8+ cell-marginated vessels in the TC was associated with downregulation of N-CAD. Therefore, S100A8+ cells seem to inhibit the EMT. Based on studies on the serum levels of S100A8, which in healthy controls range from 1058 to 6175 ng/mL,44,45 we used rhS100A8 at four concentrations (0.1, 1, 2, and 4 µg/mL) compared with untreated controls to simulate the extracellular actions of S100A8 on tumor cells. Our in vitro results revealed that the transcriptional levels of SNAIL and BCL2 were significantly downregulated after high concentrations (2 or 4 µg/mL) of rhS100A8. Protein levels revealed that high concentration of rhS100A8 increased E-CAD expression. Cell experiments also demonstrated that rhS100A8 could affect apoptosis because BCL2 expression was also inhibited after rhS100A8 treatment.
Immune surveillance spares no effort in fighting against the EMT. The invasive front of CRC could be regarded as a battlefield between pro-tumor and antitumor factors. TB promotes tumor progression and metastasis, but the host's innate and adaptive immune responses attempt to block pro-tumor factors by recruiting phagocytes and CTLs.46 It has also been hypothesized that the specific immune response acts against the EMT with strong lymphocyte infiltration by targeting and then destroying TBs.47 We considered that the infiltration of S100A8+ phagocytes, especially in the TIF, is an innate immune response to restrain the EMT. Our results showed that the S100A8+TIF, S100A8+ cell-marginated vessels were negatively correlated with EMT phenotype. The changes of EMT markers were also induced by rhS100A8 treatment. We speculated that S100A8+TIF monocytes and polymorphonuclear neutrophils could directly or indirectly recognize TBs with an EMT phenotype and act against them, perhaps by inducing tumor cell apoptosis since we found a downregulation of BCL2.
In conclusion, a higher density of S100A8+ cells in the TIF and vessels with marginating S100A8+ cells in the TC are favorable predictors of overall survival. The SATB index, based on the S100A8+TIF and TB, is an independent prognostic factor for overall survival. S100A8+ cells in the stroma or vessels potentially impede the EMT and induce apoptosis.
Materials and methods
Laser capture microdissection and gene microarray analysis
Fifty CRC samples from operation without chemotherapy were collected. Hematoxylin–eosin (HE) staining was performed and three samples with >10 TB cells were selected after microscopic examination (10×). Then, each selected sample was stained with an LCM frozen section staining kit (Life Technologies) to identify TB cells. Later, laser capture microdissection (Life Technologies) was used according to the manufacturer's instructions. Normal epithelial cells, tumor cells, TB cells, and their corresponding stroma cells were separately dissected from three CRC samples. To guarantee the quality of RNA amplification, >100 cells in each section were collected. RNA amplification (Picopure RNA isolation kit) and the Affymetrix U133 Plus 2.0 array were used. The original data from the Affymetrix U133 Plus 2.0 array was transformed by a log2 algorithm and standardized by quantile normalization. After t-testing between samples, differential genes were selected when the p-value was <0.05 and the fold-change filtering value was >2 or <0.5.
Cases and tissue microarrays
All 419 CRC biopsies were collected from the First Peoples Hospital of Xiaoshan, China, during 1990–2006, with follow-up ranging from 1 mo to 186.58 mo. The median follow-up was 33 mo, 90 (21.5%) patients died during 5-y follow-up, and 97 (23.2%) died during the entire follow-up. Patients' ages ranged from 24 to 91 y (mean 62.5, median 64 y). A total of 227 patients (54.2%) were males and 192 (45.8%) were females. A total of 56 patients were classified as in tumor-node-metastasis (TNM) stage I, 152 in stage II, 170 in stage III, and 41 in stage IV. A total of 299 patients had a low histological grade (well differentiated) and 120 had a high histological grade (poorly differentiated). Tissue microarrays were also constructed with samples from 419 CRC patients treated at the First Peoples Hospital of Xiaoshan. Tissues were obtained from the TC and TIF, as initially verified on the corresponding H&E slides.
This study was approved by the Ethics Committee of the School of Medicine, Zhejiang University, Hangzhou, China.
Immunohistochemistry
The expression of S100A8 was assessed by immunohistochemistry in the paraffin sections from the 419 CRC cases. Primary S100A8 antibody (goat S100A8 polyclonal antibody) was obtained from Santa Cruz, USA (dilution 1:100). The detailed procedure was performed as described.48 The antibody information for E-CAD (n = 108), N-CAD (n = 104), SNAIL (n = 419) and BCL2 (n = 131) is listed in Table S1.
Staining score determination
S100A8 sections were scanned by NanoZoomer slide scanners (NanoZoomer-XR C12000, Hamamatsu) and viewed with NDP.view software (NDP.view2 U12388-01, Hamamatsu). Then, the sections were divided into those with the TIF and those with the TC. The TIF area was determined as a 20× field (545 × 577 μm2) viewed by NDP.view within the most distal tumor cells located in the center. The TC area was determined as at least a distance of 20× fields from the border of normal mucosa. The numbers of S100A8+ cells were counted in four hotspots (20×) using a computer-automated method (Image-pro plus 6.0, Media Cybernetics Inc.), and then mean densities were calculated. The cut-off value of the S100A8+ cell counts was determined by the 5-y survival-specific ROC curve analysis according to the Youden index. Then, a low S100A8+ cell was not more than the cut-off value and a high S100A8+ cell was greater than the cut-off value. Vessels marginated with S100A8+ cells were defined as vessels with at least five marginating S100A8+ cells. The number of vessels marginated with S100A8+ cells was counted in the two hotspots (10×) of TIF and TC, and then summed values were calculated.
The numbers of TBs were counted in the four most intense fields (500 μm × 250 μm)49 in the TIF and mean densities were calculated. The TB grade was determined as low with 0–4 TBs, moderate with 5–14 TBs, and high with >15 TBs.
Sections stained for E-CAD, N-CAD, SNAIL, and BCL2 were viewed under a light microscope. They were evaluated by multiplying two parameters: percentage of positive cells and positive intensity. The evaluation criteria and ultimate scores of each marker are listed in Table S1.
Cell cultures
CRC cells, DLD1, and sw480 were introduced from American Type Culture Collection (ATCC, Manassas, VA, 2011) and cultured as described.50 Cells were treated with recombinant human S100A8 protein (rhS100A8, Proteintech, China) at 0, 0.1, 1, 2, and 4 µg/mL for 48 h.
Quantitative real-time PCR
RNA extraction, reverse transcription, and quantitative real-time polymerase chain reaction (qRT-PCR) were performed as described.51 The primer sequences were listed in Table S2.
Cell migration and invasion assays
The transwell chambers (8-µm pore size; Corning Costar, New York, USA) were placed in a 24-well culture plate with 10% serum medium, containing recombinant protein S100A8 at 0, 0.1, 1, 2, and 4 µg/mL in each lower chamber. 1.0 × 105 DLD1 and sw480 cells were seeded into the upper chambers. After 48 h, cell motility and invasiveness were measured as described.50
Western blot analysis
Treated cells were lysed in RIPA Lysis Buffer (Beyotime, China) and nucleoprotein was extracted using nuclear and cytoplasmic protein extraction kit (KeyGEN, China). The protein expression levels were assessed by western blot, with antibodies against E-CAD (1:1000, CST) and SNAIL (1:500, CST). Blots were then incubated with IRdye secondary antibodies, visualized by Odyssey® Imager (LI-COR, USA).
Statistical analysis
The 5-y survival-specific ROC curve analysis was performed to evaluate the prognostic significance of S100A8+ cells. A comparison between the clinico-pathological variables and the S100A8+ or S100A8+-marginated vessels was made using the χ2 test or Fisher's exact test using SPSS 20.0 statistical software (SPSS, Inc.). Univariate survival analysis was performed and survival curves were constructed using the Kaplan–Meier method. The differences between curves were tested by the log-rank test. Multivariate analyses were done using the Cox proportional hazard model and a forward stepwise method was used to bring variables into the model. Student's t-test was used in the cell migration and invasion analysis. A significant difference was identified at p <0.05.
Supplementary Material
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
The research is supported by the grants of National Natural Science Foundation of China under Grant 81090420/81090421, Key Project for Science and Technology of Zhejiang Province under Grant 2012C1301-3, The National High Technology Research and Development Program (863) of China under Grant 2012AA02A601 and Program of Introducing Talents of Discipline to Universities under Grant B13026.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA 2011; 61:69-90; PMID:21296855; http://dx.doi.org/ 10.3322/caac.20107 [DOI] [PubMed] [Google Scholar]
- 2.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011; 144:646-74; PMID:21376230; http://dx.doi.org/ 10.1016/j.cell.2011.02.013 [DOI] [PubMed] [Google Scholar]
- 3.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 2000; 100:57-70; PMID:10647931; http://dx.doi.org/ 10.1016/S0092-8674(00)81683-9 [DOI] [PubMed] [Google Scholar]
- 4.Ghavami S, Chitayat S, Hashemi M, Eshraghi M, Chazin WJ, Halayko AJ, Kerkhoff C. S100A8/A9: a Janus-faced molecule in cancer therapy and tumorgenesis. European J Pharmacol 2009; 625:73-83; PMID:19835859; http://dx.doi.org/ 10.1016/j.ejphar.2009.08.044 [DOI] [PubMed] [Google Scholar]
- 5.Ghavami S, Rashedi I, Dattilo BM, Eshraghi M, Chazin WJ, Hashemi M, Wesselborg S, Kerkhoff C, Los M. S100A8/A9 at low concentration promotes tumor cell growth via RAGE ligation and MAP kinase-dependent pathway. J Leukocyte Biol 2008; 83:1484-92; PMID:18339893; http://dx.doi.org/ 10.1189/jlb.0607397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drews-Elger K, Iorns E, Dias A, Miller P, Ward TM, Dean S, Clarke J, Campion-Flora A, Rodrigues DN, Reis-Filho JS et al.. Infiltrating S100A8+myeloid cells promote metastatic spread of human breast cancer and predict poor clinical outcome. Breast Cancer Res Tr 2014; 148:41-59; PMID:25270120; http://dx.doi.org/20213444 10.1007/s10549-014-3122-4 [DOI] [PubMed] [Google Scholar]
- 7.Goyette J, Geczy CL. Inflammation-associated S100 proteins: new mechanisms that regulate function. Amino Acids 2011; 41:821-42; PMID:20213444; http://dx.doi.org/ 10.1007/s00726-010-0528-0 [DOI] [PubMed] [Google Scholar]
- 8.Lood C, Stenstrom M, Tyden H, Gullstrand B, Kallberg E, Leanderson T, Truedsson L, Sturfelt G, Ivars F, Bengtsson AA. Protein synthesis of the pro-inflammatory S100A8/A9 complex in plasmacytoid dendritic cells and cell surface S100A8/A9 on leukocyte subpopulations in systemic lupus erythematosus. Arthritis Res Therapy 2011; 13:R60; PMID:21492422; http://dx.doi.org/ 10.1186/ar3314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roth J, Vogl T, Sorg C, Sunderkotter C. Phagocyte-specific S100 proteins: a novel group of proinflammatory molecules. Trends Immunol 2003; 24:155-8; PMID:12697438; http://dx.doi.org/ 10.1016/S1471-4906(03)00062-0 [DOI] [PubMed] [Google Scholar]
- 10.Zhang X, Ai F, Li X, She X, Li N, Tang A, Qin Z, Ye Q, Tian L, Li G et al.. Inflammation-induced S100A8 activates Id3 and promotes colorectal tumorigenesis. Int J Cancer 2015; 137:2803-14; PMID:26135667; http://dx.doi.org/25236491 10.1002/ijc.29671 [DOI] [PubMed] [Google Scholar]
- 11.Silva EJ, Argyris PP, Zou X, Ross KF, Herzberg MC. S100A8/A9 regulates MMP-2 expression and invasion and migration by carcinoma cells. Int J Biochem Cell Biol 2014; 55:279-87; PMID:25236491; http://dx.doi.org/ 10.1016/j.biocel.2014.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lim SY, Yuzhalin AE, Gordon-Weeks AN, Muschel RJ. Tumor-infiltrating monocytes/macrophages promote tumor invasion and migration by upregulating S100A8 and S100A9 expression in cancer cells. Oncogene 2016; 35:5735-45; PMID:27086923; http://dx.doi.org/17128264 10.1038/onc.2016.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hiratsuka S, Watanabe A, Aburatani H, Maru Y. Tumour-mediated upregulation of chemoattractants and recruitment of myeloid cells predetermines lung metastasis. Nat Cell Biol 2006; 8:1369-75; PMID:17128264; http://dx.doi.org/ 10.1038/ncb1507 [DOI] [PubMed] [Google Scholar]
- 14.Fan B, Zhang LH, Jia YN, Zhong XY, Liu YQ, Cheng XJ, Wang XH, Xing XF, Hu Y, Li YA et al.. Presence of S100A9-positive inflammatory cells in cancer tissues correlates with an early stage cancer and a better prognosis in patients with gastric cancer. BMC Cancer 2012; 12:316; PMID:22838504; http://dx.doi.org/ 10.1186/1471-2407-12-316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest 2009; 119:1420-8; PMID:19487818; http://dx.doi.org/ 10.1172/JCI39104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zeisberg M, Neilson EG. Biomarkers for epithelial-mesenchymal transitions. J Clin Invest 2009; 119:1429-37; PMID:19487819; http://dx.doi.org/ 10.1172/JCI36183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robson EJD, Khaled WT, Abell K, Watson CJ. Epithelial-to-mesenchymal transition confers resistance to apoptosis in three murine mammary epithelial cell lines. Differentiation 2006; 74:254-64; PMID:16759291; http://dx.doi.org/ 10.1111/j.1432-0436.2006.00075.x [DOI] [PubMed] [Google Scholar]
- 18.Mitrovic B, Schaeffer DF, Riddell RH, Kirsch R. Tumor budding in colorectal carcinoma: time to take notice. Modern Pathol 2012; 25:1315-25; PMID:22790014; http://dx.doi.org/ 10.1038/modpathol.2012.94 [DOI] [PubMed] [Google Scholar]
- 19.Ueno H, Murphy J, Jass JR, Mochizuki H, Talbot IC. Tumour ‘budding’ as an index to estimate the potential of aggressiveness in rectal cancer. Histopathology 2002; 40:127-32; PMID:11952856; http://dx.doi.org/ 10.1046/j.1365-2559.2002.01324.x [DOI] [PubMed] [Google Scholar]
- 20.Prall F, Nizze H, Barten M. Tumour budding as prognostic factor in stage I/II colorectal carcinoma. Histopathology 2005; 47:17-24; PMID:15982319; http://dx.doi.org/ 10.1111/j.1365-2559.2005.02161.x [DOI] [PubMed] [Google Scholar]
- 21.Hase K, Shatney C, Johnson D, Trollope M, Vierra M. Prognostic value of tumor “budding” in patients with colorectal cancer. Dis Colon Rectum 1993; 36:627-35; PMID:8348847; http://dx.doi.org/ 10.1007/BF02238588 [DOI] [PubMed] [Google Scholar]
- 22.Zlobec I, Lugli A. Epithelial mesenchymal transition and tumor budding in aggressive colorectal cancer: tumor budding as oncotarget. Oncotarget 2010; 1:651-61; PMID:21317460; http://dx.doi.org/ 10.18632/oncotarget.199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stulik J, Osterreicher J, Koupilova K, Knizek , Macela A, Bures J, Jandík P, Langr F, Dedic K, Jungblut PR. The analysis of S100A9 and S100A8 expression in matched sets of macroscopically normal colon mucosa and colorectal carcinoma: the S100A9 and S100A8 positive cells underlie and invade tumor mass. Electrophoresis 1999; 20:1047-54; PMID:10344284; http://dx.doi.org/ 10.1002/(SICI)1522-2683(19990101)20:4/5%3c1047::AID-ELPS1047%3e3.0.CO;2-E [DOI] [PubMed] [Google Scholar]
- 24.Foell D, Hernandez-Rodriguez J, Sanchez M, Vogl T, Cid MC, Roth J. Early recruitment of phagocytes contributes to the vascular inflammation of giant cell arteritis. J Pathol 2004; 204:311-6; PMID:15476267; http://dx.doi.org/ 10.1002/path.1660 [DOI] [PubMed] [Google Scholar]
- 25.Fridman WH, Pages F, Sautes-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer 2012; 12:298-306; PMID:22419253; http://dx.doi.org/ 10.1038/nrc3245 [DOI] [PubMed] [Google Scholar]
- 26.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P et al.. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 2006; 313:1960-4; PMID:17008531; http://dx.doi.org/ 10.1126/science.1129139 [DOI] [PubMed] [Google Scholar]
- 27.Srikrishna G. S100A8 and S100A9: new insights into their roles in malignancy. J Innate Immunity 2012; 4:31-40; PMID:21912088; http://dx.doi.org/ 10.1159/000330095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Funk S, Mark R, Bayo P, Flechtenmacher C, Grabe N, Angel P, Plinkert PK, Hess J. High S100A8 and S100A12 protein expression is a favorable prognostic factor for survival of oropharyngeal squamous cell carcinoma. Int J Cancer 2015; 136:2037-46; PMID:25302747; http://dx.doi.org/ 10.1002/ijc.29262 [DOI] [PubMed] [Google Scholar]
- 29.Kang JH, Hwang SM, Chung IY. S100A8, S100A9 and S100A12 activate airway epithelial cells to produce MUC5AC via extracellular signal-regulated kinase and nuclear factor-kappa B pathways. Immunology 2015; 144:79-90; PMID:24975020; http://dx.doi.org/ 10.1111/imm.12352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gebhardt C, Nemeth J, Angel P, Hess J. S100A8 and S100A9 in inflammation and cancer. Biochem Pharmacol 2006; 72:1622-31; PMID:16846592; http://dx.doi.org/ 10.1016/j.bcp.2006.05.017 [DOI] [PubMed] [Google Scholar]
- 31.De Filippo K, Neill DR, Mathies M, Bangert M, McNeill E, Kadioglu A, Hogg N. A new protective role for S100A9 in regulation of neutrophil recruitment during invasive pneumococcal pneumonia. FASEB J 2014; 28:3600-8; PMID:24776746; http://dx.doi.org/ 10.1096/fj.13-247460 [DOI] [PubMed] [Google Scholar]
- 32.Cory S, Adams JM. The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer 2002; 2:647-56; PMID:12209154; http://dx.doi.org/ 10.1038/nrc883 [DOI] [PubMed] [Google Scholar]
- 33.Yui S, Nakatani Y, Mikami M. Calprotectin (S100A8/S100A9), an inflammatory protein complex from neutrophils with a broad apoptosis-inducing activity. Biol Pharmaceutical Bulletin 2003; 26:753-60; PMID:12808281; http://dx.doi.org/ 10.1248/bpb.26.753 [DOI] [PubMed] [Google Scholar]
- 34.Ghavami S, Kerkhoff C, Los M, Hashemi M, Sorg C, Karami-Tehrani F. Mechanism of apoptosis induced by S100A8/A9 in colon cancer cell lines: the role of ROS and the effect of metal ions. J Leukocyte Biol 2004; 76:169-75; PMID:15075348; http://dx.doi.org/ 10.1189/jlb.0903435 [DOI] [PubMed] [Google Scholar]
- 35.Zali H, Rezaei-Tavirani M, Kariminia A, Yousefi R, Shokrgozar MA. Evaluation of growth inhibitory and apoptosis inducing activity of human calprotectin on the human gastric cell line (AGS). Iranian Biomed J 2008; 12:7-14; PMID:18392090 [PubMed] [Google Scholar]
- 36.Ichikawa M, Williams R, Wang L, Vogl T, Srikrishna G. S100A8/A9 activate key genes and pathways in colon tumor progression. Mol Cancer Res 2011; 9:133-48; PMID:21228116; http://dx.doi.org/ 10.1158/1541-7786.MCR-10-0394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Turovskaya O, Foell D, Sinha P, Vogl T, Newlin R, Nayak J, Nguyen M, Olsson A, Nawroth PP, Bierhaus A et al.. RAGE, carboxylated glycans and S100A8/A9 play essential roles in colitis-associated carcinogenesis. Carcinogenesis 2008; 29:2035-43; PMID:18689872; http://dx.doi.org/ 10.1093/carcin/bgn188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vogl T, Tenbrock K, Ludwig S, Leukert N, Ehrhardt C, van Zoelen MA, Nacken W, Foell D, van der Poll T, Sorg C et al.. Mrp8 and Mrp14 are endogenous activators of Toll-like receptor 4, promoting lethal, endotoxin-induced shock. Nat Med 2007; 13:1042-9; PMID:17767165; http://dx.doi.org/ 10.1038/nm1638 [DOI] [PubMed] [Google Scholar]
- 39.Newton RA, Hogg N. The human S100 protein MRP-14 is a novel activator of the beta 2 integrin Mac-1 on neutrophils. J Immunol 1998; 160:1427-35; PMID:957056315598812 [PubMed] [Google Scholar]
- 40.Viemann D, Strey A, Janning A, Jurk K, Klimmek K, Vogl T, Hirono K, Ichida F, Foell D, Kehrel B et al.. Myeloid-related proteins 8 and 14 induce a specific inflammatory response in human microvascular endothelial cells. Blood 2005; 105:2955-62; PMID:15598812; http://dx.doi.org/ 10.1182/blood-2004-07-2520 [DOI] [PubMed] [Google Scholar]
- 41.Vandal K, Rouleau P, Boivin A, Ryckman C, Talbot M, Tessier PA. Blockade of S100A8 and S100A9 suppresses neutrophil migration in response to lipopolysaccharide. J Immunol 2003; 171:2602-9; PMID:12928412; http://dx.doi.org/23637971 10.4049/jimmunol.171.5.2602 [DOI] [PubMed] [Google Scholar]
- 42.Duan L, Wu R, Ye L, Wang H, Yang X, Zhang Y, Weng Y, Luo J, Tang M, Shi Q et al.. S100A8 and S100A9 are associated with colorectal carcinoma progression and contribute to colorectal carcinoma cell survival and migration via Wnt/beta-catenin pathway. PLoS One 2013; 8:e62092; PMID:23637971; http://dx.doi.org/ 10.1371/journal.pone.0062092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yin C, Li H, Zhang B, Liu Y, Lu G, Lu S, Sun L, Qi Y, Li X, Chen W. RAGE-binding S100A8/A9 promotes the migration and invasion of human breast cancer cells through actin polymerization and epithelial-mesenchymal transition. Breast Cancer Res Treat 2013; 142:297-309; PMID:24177755; http://dx.doi.org/ 10.1007/s10549-013-2737-1 [DOI] [PubMed] [Google Scholar]
- 44.Andres Cerezo L, Mann H, Pecha O, Plestilova L, Pavelka K, Vencovsky J, Senolt L. Decreases in serum levels of S100A8/9 (calprotectin) correlate with improvements in total swollen joint count in patients with recent-onset rheumatoid arthritis. Arthritis Res Ther 2011; 13:R122; PMID:21791097; http://dx.doi.org/ 10.1186/ar3426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pepper RJ, Hamour S, Chavele KM, Todd SK, Rasmussen N, Flint S, Lyons PA, Smith KG, Pusey CD, Cook HT et al.. Leukocyte and serum S100A8/S100A9 expression reflects disease activity in ANCA-associated vasculitis and glomerulonephritis. Kidney Int 2013; 83:1150-8; PMID:23423260; http://dx.doi.org/ 10.1038/ki.2013.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zlobec I, Lugli A. Invasive front of colorectal cancer: dynamic interface of pro-/anti-tumor factors. World J Gastroenterol 2009; 15:5898-906; PMID:20014453; http://dx.doi.org/17516584 10.3748/wjg.15.5898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zlobec I, Lugli A, Baker K, Roth S, Minoo P, Hayashi S, Terracciano L, Jass JR. Role of APAF-1, E-cadherin and peritumoral lymphocytic infiltration in tumour budding in colorectal cancer. J Pathol 2007; 212:260-8; PMID:17516584; http://dx.doi.org/ 10.1002/path.2164 [DOI] [PubMed] [Google Scholar]
- 48.Wang L, Lin D, Fu Y, Lai M. Nuclear aldehyde dehydrogenase 1A1 (ALDH1A1) expression is a favorable prognostic indicator in colorectal carcinoma. Pathol Res Practice 2016; 212:791-9; PMID:27461829; http://dx.doi.org/27443606 10.1016/j.prp.2016.06.009 [DOI] [PubMed] [Google Scholar]
- 49.Morodomi T, Isomoto H, Shirouzu K, Kakegawa K, Irie K, Morimatsu M. An index for estimating the probability of lymph node metastasis in rectal cancers. Lymph node metastasis and the histopathology of actively invasive regions of cancer. Cancer 1989; 63:539-43 [DOI] [PubMed] [Google Scholar]
- 50.Kong J, Sun W, Li C, Wan L, Wang S, Wu Y, Xu E, Zhang H, Lai M. Long non-coding RNA LINC01133 inhibits epithelial-mesenchymal transition and metastasis in colorectal cancer by interacting with SRSF6. Cancer Lett 2016; 380:476-84; PMID:27443606; http://dx.doi.org/ 10.1016/j.canlet.2016.07.015 [DOI] [PubMed] [Google Scholar]
- 51.Ruan W, Zhu S, Wang H, Xu F, Deng H, Ma Y, Lai M. IGFBP-rP1, a potential molecule associated with colon cancer differentiation. Mol Cancer 2010; 9:281; PMID:20977730; http://dx.doi.org/ 10.1186/1476-4598-9-281 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.