ABSTRACT
Immunological strategies to treat pancreatic cancer offer new therapeutic approaches to improve patient outcomes. Understanding alterations in the immune systems of pancreatic cancer patients will likely lead to advances in immunotherapy for the disease. We profiled peripheral blood leukocytes from pancreatic cancer patients (n = 22) and age-matched controls (n = 20) using flow cytometry. Immune profiling of pancreatic cancer patients identified phenotypic changes in various immune cell populations, including a population of immunosuppressive monocytes (CD14+HLA-DRlo/neg), which were shown to be increased in these patients. There was a correlation between the levels of CD14+ monocytes and the levels of CD14+HLA-DRlo/neg monocytes in peripheral blood from pancreatic cancer patients. HLA-DR downregulation of monocytes was shown to occur through pancreatic cancer-derived exosome interactions with monocytes. In an in vitro model, exosomes from patient-derived xenograft cell lines and patient plasma decreased HLA-DR expression on CD14+ monocytes. Additionally, tumor-derived exosomes caused immune suppression in monocytes through altered STAT3 signaling, induction of arginase expression, and reactive oxygen species. These findings provide novel insights into the mechanisms that govern immunosuppression in pancreatic cancer. Understanding monocyte–exosome interactions could lead to novel immunotherapies for this disease.
KEYWORDS: Exosomes, HLA-DR, immune profiling, immunosuppression, monocytes, pancreatic cancer
Abbreviations
- cDNA
complement DNA
- CM
conditioned media
- DC
dendritic cell
- EV
extracellular vesicle
- HLA
human leukocyte antigen
- MHC
major histocompatibility complex
- MFI
mean fluorescence intensity
- MV-free
microvesicle-free
- MDSC
myeloid-derived suppressor cell
- NAC
N-acetyl cysteine
- NK cell
natural killer cell
- NO
nitric oxide
- PC
pancreatic cancer
- NOS2
nitric oxide synthase
- PC-Exo
pancreatic cancer-derived exosomes
- PBMC
peripheral blood mononuclear cell
- PBS
phosphate-buffered saline solution
- ROS
reactive oxygen species
- RT-PCR
real-time polymerase chain reaction
- Tregs
regulatory T cells
Introduction
Pancreatic cancer (PC) is the fourth leading cause of cancer-related death in the United States and is projected to become the second leading cause as early as 2030.1 PC has a dismal prognosis (∼5% 5-y survival) mainly because the majority of patients are diagnosed when the cancer is already at an advanced stage. Current therapies such as gemcitabine and FOLFIRINOX (5-flouroacil, leucovorin, oxaliplatin, and irinotecan) have provided only minimal survival benefits to advanced PC patients.2 Recent evidence suggests that immunotherapies for PC offer a promising new approach for improving patient survival2, however understanding optimal immunotherapeutic regimens will require comprehending the immunological deficits of PC patients.
The immune system's role in promoting PC has been mostly described by the interactions of immune cells and cancer cells in the tumor microenvironment. For example, the tumor microenvironment has been shown to be responsible for creating an environment favorable to immune suppression. This can occur through changes in the function of immune cells, such as T-helper cells acquiring Th2 phenotypes which prevent the destruction of tumor cells and macrophages that switch to a M2 anti-inflammatory tumor-promoting stage.3 In addition, T-regulatory cells and myeloid-derived suppressor cells (MDSCs) will block effector immune cell responses.3
The tumor microenvironment is rich in innate and adaptive immune and stromal cells. Communication between these cells can occur through direct cell-to-cell contact or the secretion of cytokines and chemokines acting in either an autocrine or a paracrine manner.4 However, soluble factors generally act in a localized manner and therefore do not explain the systemic effects that are witnessed in the peripheral blood and tissues of cancer patients. Recently, extracellular vesicles (EVs) have emerged as a new delivery method for tumor-derived factors that can contribute to immune suppression.
EVs have garnered a lot of attention in recent years due to their relevance in a variety of biological systems. EVs were found to be important mediators of cell-to-cell communication because they harbor a variety of biologically active cargo of which can be transferred to target cells. There are three classes of EVs: microvesicles (500–2,000 nm in size), apoptotic blebs (100–1,000 nm), and nanoparticle-sized exosomes (30–150 nm).5 Interest in exosomes and immunity began in the 1990s when it was observed that B lymphocytes secrete major histocompatibility complex (MHC) class II enriched exosomes.6 The B lymphocyte exosomes had the ability to present MHC–peptide complexes to T cells, suggesting that they play a role in mediating adaptive immune responses. Dendritic cells (DCs) also secrete exosomes containing MHC class I–peptide complexes, which showed T-cell-mediated antitumor effects.7 These initial observations led to the hypothesis that exosomes could impact immunity. Our group has previously shown that pancreatic tumors shed more exosomes than any other EV, and that these tumor-derived exosomes can mediate β-cell dysfunction and induce lipolysis in both in vitro and in vivo models of new-onset diabetes for PC.8,9 This work suggests that tumor-derived exosomes are mediators of paraneoplastic syndromes in PC. Therefore, we sought to examine the role of PC-derived exosomes (PC-Exo) in immunity for PC.
In this study, we investigated the compositional changes in peripheral blood leukocytes from PC patients to identify the immune phenotypes that are associated with immunosuppression and/or disease progression in these patients. As a result of this comprehensive approach, we identified a population of immunosuppressive monocytes (CD14+HLA-DRlo/neg) that are elevated in PC patients. The mechanism for the alterations in this population of monocytes remains to be clarified. Therefore, we investigated the role of PC-Exo in the “priming” of these CD14+HLA-DRlo/neg monocytes. Understanding the immunological mechanisms that govern immunosuppression in PC could potentially lead to novel therapeutic opportunities for inhibiting tumor immune escape.
Results
Immunophenotypic changes in PC patients
We sought to determine the extent of the phenotypic changes seen in peripheral blood leukocytes after the development of PC. We assessed immunophenotypes by flow cytometry using unmanipulated whole-blood samples. The flow cytometry protocols were designed to encompass all major leukocytes and additional subphenotypes from T cells, B cells, and myeloid cells.10 The data comparing the 96 phenotypes of the control subjects and PC patients are listed in Table S1. Of the 96 phenotypes that included T cells, B cells, natural killer (NK) cells, regulatory T cells (Tregs), granulocytes, and monocytes, we saw significant differences in 11 of these phenotypes. However, due to many of the lymphocyte-related phenotypes not being significantly different between the groups (Fig. S1), we focused on the myeloid phenotypes that showed the most variation. As shown in Fig. 1 we looked at granulocyte and monocyte populations in cells/µL, including Lin-DR+ monocytes, circulating DCs, and two types of MDSCs (immature and granulocytic MDSCs). One of the most striking differences we saw in the myeloid phenotypes was the increase in a population of immunosuppressive monocytes termed CD14+HLA-DRlo/neg or monocytic MDSCs. The gating strategy for these monocytes is shown in Fig. S2. We chose to focus on these cells as this particular group was specifically elevated in PC, whereas the other MDSC populations were not.
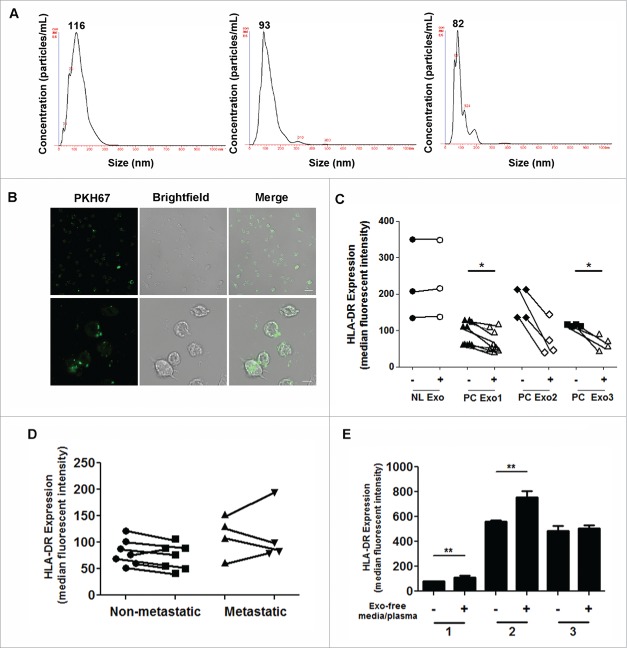
Figure 1.
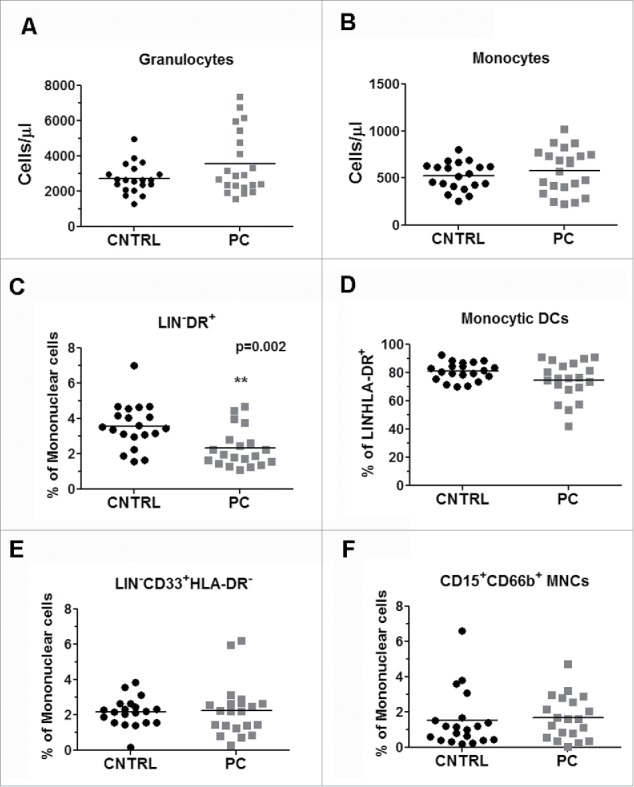
Comparison of immune phenotypes observed in PC patients. Whole blood from PC patients and healthy volunteers was assessed for several immune phenotypes. (A) Granulocytes and (B) monocytes were reported as cells/µL. (C) Lin−DR+ cells, Lin−CD33+HLA-DR+ (E), and CD15+CD66b+ mononuclear cells (F) were all reported as percentage of mononuclear cells. (D) Monocytic dendritic cells were reported as percentage of Lin−HLA-DR+.
CD14+HLA-DRlo/neg monocytes are elevated in PC patients
CD14+HLA-DRlo/neg monocytes have been implicated in tumor-mediated immunosuppression in various cancers. HLA-DR loss on CD14+ monocytes has been shown to be a major event in tumor-induced immunosuppression.11-18 We have shown in our results that this population of immunosuppressive monocytes is elevated in PC in terms of the percentage of total monocytes and in cell count (data not shown) (Fig. 2A, left panel). Further subdividing the PC patients into locally advanced (nonresectable) and metastatic disease shows increasing CD14+HLA-DRlo/neg monocytes with advanced disease progression (Fig. 2A, right panel). CD14+ monocytes were further stratified into three classes: classical (CD14+CD16−), intermediate (CD14+CD16+), and nonclassical (CD14loCD16+). In all three subsets of monocytes, HLA-DR expression was significantly decreased (Fig. 2B, left panel). The intermediate monocyte population had the most significant decrease in HLA-DR expression; therefore, we further subdivided the values based on the disease staging (locally advanced/nonresectable vs. metastatic). The severity of HLA-DR loss corresponded to advanced disease progression (Fig. 2B, right panel). Lastly, we investigated the correlation between the number of monocytes (cells/µL) and the percentage of those that were CD14+HLA-DRlo/neg cells. The results showed a positive correlation in PC patients, indicating an overall higher monocyte (cells/µL) count and therefore a higher percentage of CD14+HLA-DRlo/neg compared to healthy volunteers (Fig. 2C). These data indicate that monocytes are very responsive to tumor-mediated signals through the loss of HLA-DR and the expansion of cells in circulation.
Figure 2.
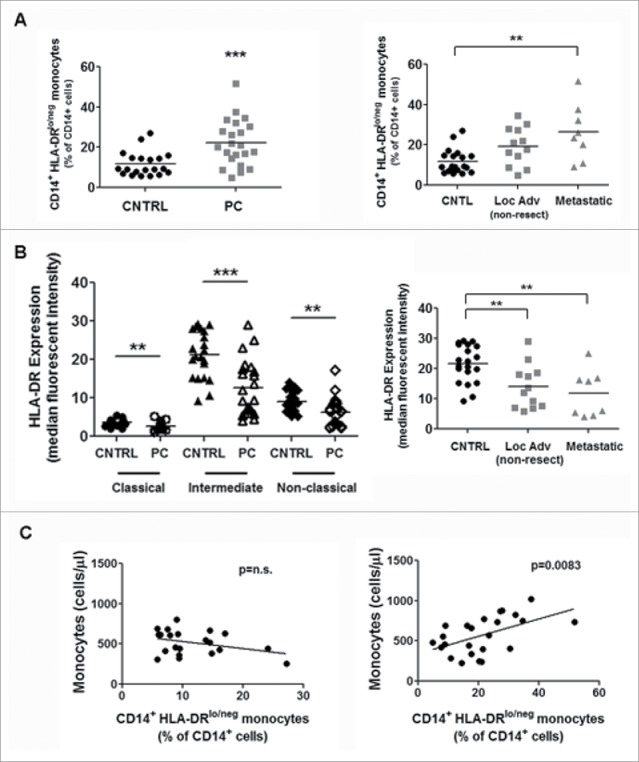
CD14+HLA-DRlo/neg monocytes are expanded in PC compared to healthy volunteers. (A) CD14+HLA-DRlo/neg monocytes were quantified from whole blood in PC and healthy volunteers (CNTRL) (left panel). PC patients were further subdivided into locally advanced (non-metastatic) and metastatic patients (right panel). (B) Monocyte subpopulations were assessed in control and PC patients. Classical (CD14+CD16−), intermediate (CD14+CD16+), and non-classical (CD14loCD16+) monocytes were reported as HLA-DR mean fluorescence intensity (MFI; geometric mean) (left panel). The intermediate monocyte population was further subdivided into monocytes from locally advanced (non-metastatic) and metastatic PC patients (right panel). (C) We correlated the amount of monocytes (cells/µL) and the amount of CD14+HLA-DRlo/neg as a percentage of CD14+ cells. The left panel refers to the healthy volunteers and the right panel is the PC patients. **p < 0.001; ***p < 0.0001.
Exosomes are abundantly secreted in PC and readily enter monocytes
Although several reports have shown this immunosuppressive population of monocytes to be elevated in a variety of cancers, the possible mechanisms for HLA-DR loss have yet to be addressed. Exosomes have long been implicated in immunity due to their importance in the communication between immune cells and cancer cells.19 Additionally, exosomes have previously been shown to alter the tumor microenvironment by transferring important cellular cargo, such as cytokines and growth factors.19 We therefore hypothesized that PC-Exo downregulate HLA-DR expression on CD14+ monocytes.
EVs (microvesicles and exosomes) were isolated from the peripheral blood of PC patients by differential centrifugation (see section Patients, Materials, and Methods). The size of the vesicles was ∼80 to 100 nm. This range indicates that the majority of EVs isolated from patient plasma were exosomes (Fig. 3A). To determine if monocytes could internalize exosomes, CD14+ monocytes were isolated from the PBMCs of healthy volunteers and cultured with PKH67-dyed PANC-1 exosomes. Monocytes internalized the PKH67-dyed exosomes within hours of co-incubation (Fig. 3B).
Figure 3.
PC-Exo downregulates HLA-DR expression on CD14+ monocytes. (A) NanoTracker analysis of the particle size distribution of microparticles that were isolated from three different PC patient plasma samples from the 22 patient cohort. The graph indicates the concentration (particles/mL) vs. size (nm) with the mode for each sample indicated at the top of the peak. (B) Confocal microscopy was conducted using CD14+ healthy donor monocytes incubated with 10 µL of PKH67-dyed PANC-1 exosomes. Images were taken after 24 h. Scale bar, 20 µm. The bottom three panels indicate zoomed images (4.5×). Scale bar, 5 µm. (C) Exosomes were isolated from PANC-1 (PC Exo1) and two PC patient xenograft cell lines (PC Exo 2 and 3) with normal CD14+ monocytes. HLA-DR expression (MFI) was assessed on the monocytes using flow cytometry after 4 d of incubation. Exosomes isolated from pooled healthy volunteer plasma were used as a control (NL Exo). (D) Exosomes isolated from selected patients from the 22 patient cohort were co-incubated with CD14+ monocytes, and HLA-DR expression was measured in non-metastatic and metastatic patients. (E) Fifty microgram of exosome-free media from PANC-1 supernatants (1), PC patient-derived xenograft cell line (2), and exosome-free plasma from a PC patient (3) were cultured with CD14+ monocytes for 4 d. The MFI of HLA-DR expression was assessed on the monocytes. *p < 0.05; **p < 0.001.
PC-Exo can decrease HLA-DR expression on CD14+ monocytes
Having shown that monocytes and exosomes interact, we sought to determine whether the CM isolated from PANC-1 cells could affect HLA-DR levels on CD14+ monocytes. CD14+ monocytes were cultured with or without PANC-1 CM (50% by volume) in either the presence or absence of growth factor GM-CSF for 96 h. The inclusion of GM-CSF in culture was used as a survival factor for monocytes by pushing monocytes to an immature DC phenotype. The results showed a decrease in HLA-DR levels on CD14+ monocytes in the presence of PANC-1 CM supplemented with GM-CSF (Fig. S3). This is in contrast with the results from CD14+ monocytes cultured with only PANC-1 CM, which showed no significant changes in HLA-DR levels (Fig. S3). These results suggest that in the presence of GM-CSF, the addition of PANC-1 CM was able to decrease HLA-DR expression on monocytes.
We sought to test if exosomes secreted in the CM of PANC-1 and two PC patient-derived cell lines could downregulate HLA-DR expression on normal CD14+ monocytes. Co-incubation of 50 µg of exosomes isolated from all PC cell lines decreased DR expression on monocytes compared to monocytes cultured without PC-Exo (Fig. 3C). Additionally, exosomes isolated from pooled normal-human plasma were co-incubated with CD14+ monocytes (Fig. 3C). Interestingly, the results showed no change in HLA-DR expression on monocytes, suggesting that tumor-derived exosomes contain unique contents that can promote an immunosuppressive environment. We then sought to determine if exosomes isolated from the plasma of PC patients in our cohort could decrease the HLA-DR levels on CD14+ monocytes. Data were obtained from 11 PC patients (7 nonmetastatic vs. 4 metastatic). The results showed that PC patient exosomes had the ability to downregulate HLA-DR expression levels. Some patient-derived monocytes showed either an increase in DR expression or no change (Fig. 3D). These differences could be attributed to variability in the exosomes from different patients because of the staging or grade of their tumors, to other health issues of the patient, or to the heterogeneous population (normal and tumor-derived) of exosomes in patient-derived blood samples. Additional testing using more patient samples is needed to confirm this observation. Lastly, to ensure that the exosomes were solely responsible for downregulating HLA-DR levels on monocytes, the exosome-free media (medium devoid of exosomes) from PANC-1, a PC patient-derived cell line, and the exosome-free plasma from a PC patient were cultured with CD14+ monocytes isolated from healthy donors. The results showed no effect on the suppression of HLA-DR on the monocytes (Fig. 3E). Although we found that the CM from PANC-1 supplemented with GM-CSF could decrease HLA-DR levels on normal CD14+ monocytes, we have shown that the immunosuppressive effects are due to the exosomes and not any other secreted factors in the media.
PC-Exo contribute to converting monocytes into immunosuppressive monocytes
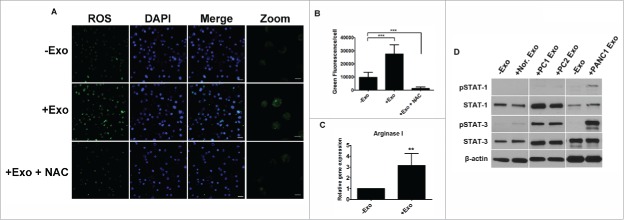
The mechanisms for HLA-DR loss on monocytes have been investigated for quite some time.20 Arginase metabolism has been shown to play a key role in controlling T-cell-mediated responses. It has been reported that myeloid cells suppress T-cell function by either depleting L-arginine in the presence of arginase or by inducing nitric oxide synthase (NOS2) that produces NO.21 To assess the immunosuppressive effects of PC-Exo on monocytes, we checked reactive oxygen species (ROS) production using the CellROX® detection assay system. Fifty micrograms of PC-Exo were incubated with isolated CD14+ from a normal, healthy volunteer. Co-incubation of PC-Exo showed an increase in ROS production indicated by an increase in green fluorescence per cell compared to untreated cells (−Exo) (Fig. 4A). To block the effect of PC-Exo on increasing oxidative stress, N-acetyl-cysteine (NAC) was added to the culture with 50 µg PC-Exo. The results showed a significant decrease in ROS production, which has been quantified in Fig. 4B. We next looked at total arginase I expression measured through qRT-PCR analysis of CD14+ monocytes cultured with 50 µg PANC-1 exosomes. The results showed an increase in arginase I expression in the presence of PC-Exo compared to the untreated cells (Fig. 4C). STAT signaling has also been implicated in the generation and proliferation of myeloid cells.22 We therefore tested whether PC-Exo could activate this pathway by checking the phosphorylation status of STAT1 and STAT3. Through western blotting, we showed that both pSTAT1 and pSTAT3 levels were increased in the presence of both PANC-1 exosomes and exosomes isolated from PC-patient plasma. Conversely, exosomes isolated from pooled healthy-volunteer plasma did not activate pSTAT-1 and only moderately activated pSTAT-3 (Fig. 4D).
Figure 4.
Mechanism of HLA-DR downregulation on monocytes mediated by PC-Exo. (A) CD14+ monocytes were co-incubated with 50 µg of PANC-1 exosomes or exosomes that had been treated with NAC for 4 d. Reactive oxygen species (ROS, shown as green) were detected using the Cell ROX® detection assay. Scale bar, 20 µm. The right panel shows zoomed images (4×). Scale bar, 5 µm. (B) Quantification of ROS (green) in control and treatment groups. (C) mRNA expression of arginase I in CD14+ monocytes after treatment with 50 µg PANC-1 exosomes (+Exo) compared to untreated cells (−Exo). (D) Exosomes isolated from two PC patient plasma samples (PC1 and PC2 Exo) and PANC-1 Exo were co-incubated with monocytes. pSTAT1, pSTAT3, total STAT3, and total STAT1 protein expression levels were assessed compared to untreated monocytes (−Exo). **p < 0.001; ***p < 0.0001.
Discussion
We have previously demonstrated that PC-Exo from PC cell lines and patient samples contain powerful mediators of cell transformation.8,9 These important cellular cargos can aid in metabolic alterations that can help facilitate tumor growth. Exosomes have been strongly implicated in tumor immunity as mediators of both immune activation and suppression.23 Due to the complex etiology of PC, the immunological mechanisms that lead to this disease are not fully understood. In this study, we measured the immunological cellular components of peripheral blood to assess the breadth and depth of systemic alterations in PC patients. From this approach, we identified a population of immunosuppressive monocytes (CD14+HLA-DRlo/neg) that is expanded in PC. As a consequence of tumor-derived exosome-mediated immunesuppression, we provided evidence of increased ROS production, arginase metabolism, and STAT3 signaling. Taken together, this model of immune suppression may serve as a potential biomarker for the early detection of PC.
The failure of an adequate immune response to cancer cells is a topic of significant interest. The tumor must escape proper immune recognition through downregulation of MHC class I and II molecules24 or through inhibition of the ability for the MHC to complex with antigenic peptides.25 In addition, inadequate immune responses may also depend on altered immune cells mediated by the tumor. The myeloid cell compartment in cancer patients is negatively affected due to the influence of the tumor and surrounding microenvironment.11,26 As a consequence of interacting with tumor cells, monocytes become highly immunosuppressive and contribute to T-cell inhibition, reduce antigen-presentation, induced arginase and iNOS production, and cause improper maturation of DCs.11,12,16,17 The functional abnormalities described are thought to be a result of direct tumor–immune cell interactions or the release of soluble factors.26,27 However, the localized effects from secreted factors cannot solely describe what is occurring systemically in the peripheral blood and tissues. We therefore hypothesized a novel mechanism through which PC-Exo downregulate HLA-DR on monocytes. This work provides evidence that direct contact of CD14+ monocytes with PC-Exo has the ability to transform seemingly normal monocytes into highly immunosuppressive CD14+HLA-DRlo/neg. The work of Gustafson et al. has shown that direct monocyte–tumor cell interactions can induce GM-CSF production in tumor cells and is known to aid in the survival of monocytes.11 Additionally, it was shown that RCC cells downregulated HLA-DR expression on monocytes and increased certain proangiogenic factors such as FGF2 and IL-1β.11 This work suggests that tumor-infiltrating monocytes and tumor cells work synergistically to promote tumor progression, and the consequences of these interactions significantly affect patient survival. Whether tumor-derived exosomes play a key mechanistic role in supplying monocytes with the necessary soluble factors to enhance tumorigenesis is a topic that is being investigated further by our group.
We have shown that PC-Exo has the ability to downregulate HLA-DR expression on monocytes. However, what is specifically contained in PC-Exo that mediates this alteration is still unknown. In one report, cytokines were shown to contribute to MDSC production in a murine model of PC.28 Similarly, several “proMDSC” cytokines, including IL-1β, IL-4, IL-8, G-CSF, and VEGF, were elevated in the plasma of PC patients.29 Future studies will delve into which factors are contained within PC-Exo that can mediate the immunosuppressive effect seen on monocytes. Due to the highly dynamic nature of exosomes, it is possible that multiple factors may be playing functional roles in the downregulation of HLA-DR on monocytes. This notion will be further explored in future studies.
We found a correlation between the amount of CD14+HLA-DRlo/neg monocytes and total circulating monocytes. Based on the data presented here, we provide evidence of tumor-mediated immune suppression in which PC-Exo promotes CD14+HLA-DRlo/neg monocyte survival through several potential mechanisms which include STAT3 phosphorylation in addition to downregulating HLA-DR expression. STAT3 is a known activator of several anti-apoptotic and cell cycle progressive genes.22 In animal models, STAT3 is a critical factor for promoting myeloid-mediated tumorigenesis.30 Others have shown that STAT3 signaling in monocytes correlated to progressive disease and poor prognosis in patients with liver cancer.31 Cytokines that activate STAT3, such as IL-10, and IL-1β, have been implicated in the conversion of normal monocytes to immunosuppressive monocytes.11,32,33 Interestingly, we conducted a 10-plex cytokine analysis on exosomes isolated from the plasma of healthy volunteers and normal pancreas cell lines compared to PC patient exosomes and cell lines. Preliminary results showed an increase in IL-1β in PC-Exo compared to the controls (data not shown). Our data suggests several potential mechanisms that govern monocyte reprogramming which include STAT3 signaling. Future studies will further explore both STAT3-dependent and STAT3-independent signaling pathways that contribute to CD14+HLA-DRlo/neg monocyte survival and the specific mechanisms that govern this process.
Immune modulation strategies are generating increasing interest as new options are becoming available. However, to optimize the use of these therapies, the relationship between the responses to different types of immunotherapies and the extent of immune suppression in cancer patients will need to be further investigated. Here, in addition to characterizing the immunological alterations in PC patients, we identified an immunosuppressive population of monocytes that are influenced by direct interactions with tumor-derived exosomes. CD14+HLA-DRlo/neg monocytes have been shown to negatively impact responses to DC vaccines.20,34,35 In combination with tumor-derived exosomes, this cell type may serve as prognostic biomarkers and potential targets for novel therapeutic approaches for PC.
To our knowledge, this is the most comprehensive immune phenotypic analysis of PC patients to date. We identified a population of immunosuppressive monocytes elevated in PC (CD14+HLA-DRlo/neg). We showed that exosomes potentially reprogram normal monocytes into immunosuppressive monocytes through downregulation of HLA-DR, altered STAT3 signaling, and induction of arginase expression and ROS. Taken together, these results provide novel mechanistic insight into a highly immunosuppressive environment. This knowledge, in turn, can aid in the development of future immunotherapeutic approaches for PC.
Patients, materials, and methods
Study subjects
The acquisition of patient samples was conducted by the staff in the Clinical Research and Patient Registry Core of the Mayo Clinic Pancreatic Cancer SPORE. The protocol for obtaining data and biospecimens was approved by the Mayo Clinic Institutional Review Board. Peripheral blood was collected in K2EDTA tubes. Samples from 22 PC patients and 20 age-matched healthy volunteers were identified for this project. Patient characteristics are listed in Table 1.
Table 1.
Pancreatic cancer patient demographics.
| Age at pancreas cancer diagnosis | Total (N = 22) |
|---|---|
| Mean (SD) | 66.7 (10.6) |
| Median (range) | 65.0 (51.0–87.0) |
| Gender | |
| Female | 10 (45.5%) |
| Male | 12 (54.5%) |
| Stage of disease | |
| Local (surgically resectable) | 2 (8.3%) |
| Locally advanced (unresectable) | 12 (50.0%) |
| Metastatic | 8 (33.3%) |
| Diabetes status | |
| No diabetes | 15 (68.2%) |
| Diabetes | 7 (31.8%) |
Flow cytometry of peripheral blood
Flow cytometric analysis of immunophenotypes was performed on whole blood from the participants. The methods for processing samples including protocols, antibodies, gating strategies, and instrument settings were previously outlined by Gustafson et al.10,11 Minor adjustments were made, such as CD69 PC7 and CD3 KrO were used instead of CD3PC7 and CD8+ KrO in the T-cell-1 protocol.
CD14+ monocyte isolation
Peripheral blood mononuclear cells (PBMCs) were isolated by density-gradient centrifugation using Histopaque-1077 (Sigma Aldrich, no. 10771). Per the manufacturer's protocol, monocytes were isolated from PBMCs by incubation with anti-CD14 immunomagnetic beads (Miltenyi Biotec, no. 130-050-201) and selection with AutoMACS (Miltenyi Biotec, no. 130-090-273).12
In vitro culture of CD14+ monocytes
CD14+ monocytes were cultured in six-well plates using DMEM media supplemented with 1% human AB serum (Corning, no. 35-060-Cl) and penicillin–streptomycin (Thermo Fisher Scientific, no. 15140122). For experiments using PANC-1-conditioned media (CM), 50% CM by volume was added to the wells in either the presence or absence of GM-CSF (2,800 U/mL) (CellGenix, no. 1012–050). In all additional experiments, microvesicle-free (MV-free) media was made consisting of human AB serum centrifuged at 100,000g for 1 h which was added to the media. GM-CSF (2,800 U/mL) and IL-4 (1,000 U/mL) (R&D Systems, no. 204-IL) were added to the cultured CD14+ monocytes as survival factors.
Exosome isolation from pancreatic cancer cell lines and peripheral blood
Exosomes were isolated by differential ultracentrifugation of conditioned media from PANC-1, primary patient-derived xenograft cell lines, or peripheral blood taken from PC patients. The isolation procedure was adapted from Javeed et al.8
Exosome internalization into CD14+ monocytes
Exosomes were isolated from PANC-1 CM 72 h post-incubation with MV-free media as described above. The resulting exosomes were dyed with the green fluorescent linker PKH67 (Sigma-Aldrich, no. PKH67GL-1KT) according to the manufacturer's protocol. PKH67-dyed PANC-1 exosomes (10 µL) were added to isolated CD14+ monocytes that were obtained from a healthy donor. Confocal microscopy images were taken at 4, 8, and 21 h post-incubation with PC-Exo using a Zeiss LSM 780 confocal microscope.
NanoTracker analysis of exosomes
Exosomes isolated from the blood plasma of three representative PC patients were analyzed on a NanoSight NS300. Exosomal fractions were diluted to meet the appropriate particle/frame concentration, and three 30 s videos were taken for each sample. The videos were merged and analyzed using NanoSight® software program. The resulting graphs show the particle size distribution vs. concentration of microparticles (particles/mL).
Real-time polymerase chain reaction
Isolated CD14+ monocytes were plated in six-well plates (8 × 106 cells/well) and treated with or without 50 µg of PANC-1 exosomes for 96 h. Total RNA was isolated using the RNeasy kit (Qiagen, no. 74106). Complement DNA (cDNA) was reverse transcribed using 300–500 ng of RNA using an iScript cDNA Synthesis kit (Bio-Rad, no. 1708891). Real-time polymerase chain reaction (RT-PCR) analysis was performed using the ABI 7500 RT-PCR system. We used the following primers for arginase I: Forward: 5′-TGGAAACTTGCATGGACA-3′; Reverse: 5′-CCTGGCACATCGGGAATCTTT-3′.
Reactive oxygen species detection in monocytes
CD14+ monocytes were cultured in four-well chamber slides (Thermo Fisher Scientific, no. 12-565-7), and 30 µg of PANC-1 exosomes were incubated with the monocytes for 96 h. The monocytes were treated with the reactive oxygen inhibitor NAC (10 mM) (Sigma-Aldrich, no. A7250) for 30 min in wells treated with PANC-1 exosomes or in untreated wells (control). The media in each well was removed and replaced with media containing CellROX® Reagent (Thermo Fisher Scientific, no. C10444) at a final concentration of 5 µM for 30 min at 37°C. The media was removed and washed with 1X phosphate-buffered saline solution (PBS), and the cells were fixed with 4% paraformaldehyde for 10 min, washed three times with 1X PBS, and mounted with DAPI-containing mounting media (Vector Labs, no. H-1200). We obtained images using a Zeiss LSM 780 confocal microscope. The green fluorescence per cell was quantified using Zeiss KS400 Image Analysis software. To analyze each image, the nuclei were counted, and total green fluorescence for each image was divided by the nuclei count to obtain green fluorescence per cell.
Western blot analysis
CD14+ monocytes were isolated from healthy donors (see procedure above) and plated in six-well plates (6–8 × 106 cells/well) with the cytokines previously mentioned. PANC-1 exosomes (50 µg) were added to the cultures with wells without exosomes serving as a control. After incubation with the PANC-1 exosomes for 96 h, cell lysates were isolated using Tergitol-type NP-40 (Boston BioProducts, no. BP-119). Lysates were quantified using a bicinchoninic acid assay (Pierce, no. 23225), and 20 µg of each sample was analyzed by western blotting according to standard protocols. Samples were probed with phospho-STAT1, phospho-STAT3, total STAT1, and total STAT3 (Cell Signaling Technology, no. 9167S, 9145P, 9172P, 4904P).
Statistical analyses
Categorical variables (e.g., sex, stage of disease, and diabetes status) were summarized and presented using frequency and percentage. Continuous variables (e.g., age at diagnosis and 96 immune cell phenotypes) were summarized using means and standard deviations, and comparisons between PC patients and healthy volunteers were drawn using the Wilcoxon Rank Sum test. Scatterplots were used to explore the relationship between measures of interest (i.e., immune profiling of phenotypes), and Pearson correlation coefficients were used to quantify the nature of those relationships. qRT-PCR statistical analysis was conducted using a paired t-test. All values were normalized where 1.0 was equivalent to 100%. All statistical analyses were conducted using GraphPad software (San Diego, CA), and p values less than 0.05 were considered statistically significant.
Supplementary Material
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors would like to thank the Mayo Clinic SPORE in Pancreatic Cancer; Peggy Bulur for her assistance in monocyte isolation; Petra Hirsova, PhD, for her assistance in using the NanoSight® NS300; and Sandhya Davarajan, MBBS, for help in assembling the immune phenotypes table.
Funding
This work was partly supported by the Florida Department of Health Cancer Research Grants CA78383 and CA150190 to DM; P50 CA102701 to GMP and STC; T32 CA148073 and Mayo Graduate School to NJ.
References
- 1.Matrisian LM, Aizenberg R, Rosenzweig A. The alarming rise of pancreatic cancer deaths in the United States: Why we need to stem the tide. Pancreatic Cancer Action Network 2013. [Google Scholar]
- 2.Gunturu KS, Rossi GR, Saif MW. Immunotherapy updates in pancreatic cancer: are we there yet? Ther Adv Med Oncol 2013; 5:81-9; PMID:23323149; http://dx.doi.org/ 10.1177/1758834012462463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sideras K, Braat H, Kwekkeboom J, van Eijck CH, Peppelenbosch MP, Sleijfer S, Bruno M. Role of the immune system in pancreatic cancer progression and immune modulating treatment strategies. Cancer Treat Rev 2014; 40:513-22; PMID:24315741; http://dx.doi.org/ 10.1016/j.ctrv.2013.11.005 [DOI] [PubMed] [Google Scholar]
- 4.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell 2010; 140:883-99; PMID:20303878; http://dx.doi.org/ 10.1016/j.cell.2010.01.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mathivanan S, Ji H, Simpson RJ. Exosomes: extracellular organelles important in intercellular communication. J Proteomics 2010; 73:1907-20; PMID:20601276; http://dx.doi.org/ 10.1016/j.jprot.2010.06.006 [DOI] [PubMed] [Google Scholar]
- 6.Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ, Geuze HJ. B lymphocytes secrete antigen-presenting vesicles. J Exp Med 1996; 183:1161-72; PMID:8642258; http://dx.doi.org/ 10.1084/jem.183.3.1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zitvogel L, Regnault A, Lozier A, Wolfers J, Flament C, Tenza D, Ricciardi-Castagnoli P, Raposo G, Amigorena S. Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nat Med 1998; 4:594-600; PMID:9585234; http://dx.doi.org/ 10.1038/nm0598-594 [DOI] [PubMed] [Google Scholar]
- 8.Javeed N, Sagar G, Dutta SK, Smyrk TC, Lau JS, Bhattacharya S, Truty M, Petersen GM, Kaufman RJ, Chari ST et al.. Pancreatic cancer-derived exosomes cause paraneoplastic beta-cell dysfunction. Clin Cancer Res 2015; 21:1722-33; PMID:25355928; http://dx.doi.org/ 10.1158/1078-0432.CCR-14-2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sagar G, Sah RP, Javeed N, Dutta SK, Smyrk TC, Lau JS, Giorgadze N, Tchkonia T, Kirkland JL, Chari ST et al.. Pathogenesis of pancreatic cancer exosome-induced lipolysis in adipose tissue. Gut 2015; 65:1165-74; PMID:26061593; http://dx.doi.org/ 10.1136/gutjnl-2014-308350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gustafson MP, Lin Y, Maas ML, Van Keulen VP, Johnston PB, Peikert T, Gastineau DA, Dietz AB. A method for identification and analysis of non-overlapping myeloid immunophenotypes in humans. PLoS One 2015; 10:e0121546; PMID:25799053; http://dx.doi.org/ 10.1371/journal.pone.0121546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gustafson MP, Lin Y, Bleeker JS, Warad D, Tollefson MK, Crispen PL, Bulur PA, Harrington SM, Laborde RR, Gastineau DA et al.. Intratumoral CD14+ cells and circulating CD14+HLA-DRlo/neg monocytes correlate with decreased survival in patients with clear cell renal cell carcinoma. Clin Cancer Res 2015; 21:4224-33; PMID:25999436; http://dx.doi.org/ 10.1158/1078-0432.CCR-15-0260 [DOI] [PubMed] [Google Scholar]
- 12.Cornec D, Varache S, Morvan J, Devauchelle-Pensec V, Berthelot JM, Le Henaff-Bourhis C, Hoang S, Martin A, Chales G, Jousse-Joulin S et al.. Comparison of ACR 1987 and ACR/EULAR 2010 criteria for predicting a 10-year diagnosis of rheumatoid arthritis. Joint Bone Spine 2012; 79:581-5; PMID:22405855; http://dx.doi.org/ 10.1016/j.jbspin.2012.01.015 [DOI] [PubMed] [Google Scholar]
- 13.Gustafson MP, Lin Y, New KC, Bulur PA, O'Neill BP, Gastineau DA, Dietz AB. Systemic immune suppression in glioblastoma: the interplay between CD14+HLA-DRlo/neg monocytes, tumor factors, and dexamethasone. Neuro Oncol 2010; 12:631-44; PMID:20179016; http://dx.doi.org/ 10.1093/neuonc/noq001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vuk-Pavlovic S, Bulur PA, Lin Y, Qin R, Szumlanski CL, Zhao X, Dietz AB. Immunosuppressive CD14+HLA-DRlow/- monocytes in prostate cancer. Prostate 2010; 70:443-55; PMID:19902470; http://dx.doi.org/ 10.1002/pros.21078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Filipazzi P, Valenti R, Huber V, Pilla L, Canese P, Iero M, Castelli C, Mariani L, Parmiani G, Rivoltini L. Identification of a new subset of myeloid suppressor cells in peripheral blood of melanoma patients with modulation by a granulocyte-macrophage colony-stimulation factor-based antitumor vaccine. J Clin Oncol 2007; 25:2546-53; PMID:17577033; http://dx.doi.org/ 10.1200/JCO.2006.08.5829 [DOI] [PubMed] [Google Scholar]
- 16.Hoechst B, Ormandy LA, Ballmaier M, Lehner F, Kruger C, Manns MP, Greten TF, Korangy F. A new population of myeloid-derived suppressor cells in hepatocellular carcinoma patients induces CD4(+)CD25(+)Foxp3(+) T cells. Gastroenterology 2008; 135:234-43; PMID:18485901; http://dx.doi.org/ 10.1053/j.gastro.2008.03.020 [DOI] [PubMed] [Google Scholar]
- 17.Jitschin R, Braun M, Buttner M, Dettmer-Wilde K, Bricks J, Berger J, Eckart MJ, Krause SW, Oefner PJ, Le Blanc K et al.. CLL-cells induce IDOhi CD14+HLA-DRlo myeloid-derived suppressor cells that inhibit T-cell responses and promote TRegs. Blood 2014; 124:750-60; PMID:24850760; http://dx.doi.org/ 10.1182/blood-2013-12-546416 [DOI] [PubMed] [Google Scholar]
- 18.Poschke I, Mougiakakos D, Hansson J, Masucci GV, Kiessling R. Immature immunosuppressive CD14+HLA-DR-/low cells in melanoma patients are Stat3hi and overexpress CD80, CD83, and DC-sign. Cancer Res 2010; 70:4335-45; PMID:20484028; http://dx.doi.org/ 10.1158/0008-5472.CAN-09-3767 [DOI] [PubMed] [Google Scholar]
- 19.Akers JC, Gonda D, Kim R, Carter BS, Chen CC. Biogenesis of extracellular vesicles (EV): exosomes, microvesicles, retrovirus-like vesicles, and apoptotic bodies. J Neurooncol 2013; 113:1-11; PMID:23456661; http://dx.doi.org/ 10.1007/s11060-013-1084-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laborde RR, Lin Y, Gustafson MP, Bulur PA, Dietz AB. Cancer vaccines in the world of immune suppressive monocytes (CD14(+)HLA-DR(lo/neg) Cells): the gateway to improved responses. Front Immunol 2014; 5:147; PMID:24772111; http://dx.doi.org/ 10.3389/fimmu.2014.00147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bronte V, Serafini P, Mazzoni A, Segal DM, Zanovello P. L-arginine metabolism in myeloid cells controls T-lymphocyte functions. Trends Immunol 2003; 24:302-6; PMID:12810105; http://dx.doi.org/ 10.1016/S1471-4906(03)00132-7 [DOI] [PubMed] [Google Scholar]
- 22.Siveen KS, Sikka S, Surana R, Dai X, Zhang J, Kumar AP, Tan BK, Sethi G, Bishayee A. Targeting the STAT3 signaling pathway in cancer: role of synthetic and natural inhibitors. Biochim Biophys Acta 2014; 1845:136-54; PMID:24388873; http://dx.doi.org/ 10.1016/j.bbcan.2013.12.005 [DOI] [PubMed] [Google Scholar]
- 23.Clayton A, Mason MD. Exosomes in tumour immunity. Curr Oncol 2009; 16:46-9; PMID:19526085; http://dx.doi.org/ 10.3747/co.v16i3.367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ryschich E, Notzel T, Hinz U, Autschbach F, Ferguson J, Simon I, Weitz J, Frohlich B, Klar E, Buchler MW et al.. Control of T-cell-mediated immune response by HLA class I in human pancreatic carcinoma. Clin Cancer Res 2005; 11:498-504; PMID:15701833 [PubMed] [Google Scholar]
- 25.Lu T, Ramakrishnan R, Altiok S, Youn JI, Cheng P, Celis E, Pisarev V, Sherman S, Sporn MB, Gabrilovich D. Tumor-infiltrating myeloid cells induce tumor cell resistance to cytotoxic T cells in mice. J Clin Invest 2011; 121:4015-29; PMID:21911941; http://dx.doi.org/ 10.1172/JCI45862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iero M, Valenti R, Huber V, Filipazzi P, Parmiani G, Fais S, Rivoltini L. Tumour-released exosomes and their implications in cancer immunity. Cell Death Differ 2008; 15:80-8; PMID:17932500; http://dx.doi.org/ 10.1038/sj.cdd.4402237 [DOI] [PubMed] [Google Scholar]
- 27.Almand B, Resser JR, Lindman B, Nadaf S, Clark JI, Kwon ED, Carbone DP, Gabrilovich DI. Clinical significance of defective dendritic cell differentiation in cancer. Clin Cancer Res 2000; 6:1755-66; PMID:10815894 [PubMed] [Google Scholar]
- 28.Bayne LJ, Beatty GL, Jhala N, Clark CE, Rhim AD, Stanger BZ, Vonderheide RH. Tumor-derived granulocyte-macrophage colony-stimulating factor regulates myeloid inflammation and T cell immunity in pancreatic cancer. Cancer Cell 2012; 21:822-35; PMID:22698406; http://dx.doi.org/ 10.1016/j.ccr.2012.04.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Markowitz J, Brooks TR, Duggan MC, Paul BK, Pan X, Wei L, Abrams Z, Luedke E, Lesinski GB, Mundy-Bosse B et al.. Patients with pancreatic adenocarcinoma exhibit elevated levels of myeloid-derived suppressor cells upon progression of disease. Cancer Immunol Immunother 2015; 64:149-59; PMID:25305035; http://dx.doi.org/ 10.1007/s00262-014-1618-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kujawski M, Kortylewski M, Lee H, Herrmann A, Kay H, Yu H. Stat3 mediates myeloid cell-dependent tumor angiogenesis in mice. J Clin Invest 2008; 118:3367-77; PMID:18776941; http://dx.doi.org/ 10.1172/JCI35213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu WY, Li J, Wu ZS, Zhang CL, Meng XL. STAT3 activation in monocytes accelerates liver cancer progression. BMC Cancer 2011; 11:506; PMID:22136659; http://dx.doi.org/ 10.1186/1471-2407-11-506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Loercher AE, Nash MA, Kavanagh JJ, Platsoucas CD, Freedman RS. Identification of an IL-10-producing HLA-DR-negative monocyte subset in the malignant ascites of patients with ovarian carcinoma that inhibits cytokine protein expression and proliferation of autologous T cells. J Immunol 1999; 163:6251-60; PMID:10570318 [PubMed] [Google Scholar]
- 33.Xiu B, Lin Y, Grote DM, Ziesmer SC, Gustafson MP, Maas ML, Zhang Z, Dietz AB, Porrata LF, Novak AJ et al.. IL-10 induces the development of immunosuppressive CD14(+)HLA-DR(low/-) monocytes in B-cell non-Hodgkin lymphoma. Blood Cancer J 2015; 5:e328; PMID:26230952; http://dx.doi.org/ 10.1038/bcj.2015.56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walter S, Weinschenk T, Stenzl A, Zdrojowy R, Pluzanska A, Szczylik C, Staehler M, Brugger W, Dietrich PY, Mendrzyk R et al.. Multipeptide immune response to cancer vaccine IMA901 after single-dose cyclophosphamide associates with longer patient survival. Nat Med 2012; 18:1254-61; PMID:22842478; http://dx.doi.org/ 10.1038/nm.2883 [DOI] [PubMed] [Google Scholar]
- 35.Olin MR, Low W, McKenna DH, Haines SJ, Dahlheimer T, Nascene D, Gustafson MP, Dietz AB, Clark HB, Chen W et al.. Vaccination with dendritic cells loaded with allogeneic brain tumor cells for recurrent malignant brain tumors induces a CD4(+)IL17(+) response. J Immunother Cancer 2014; 2:4; PMID:24829761; http://dx.doi.org/ 10.1186/2051-1426-2-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.