ABSTRACT
PD-L1 expression and regulation by mesenchymal tumor cells remain largely undefined. Here, we report that among different EMT-activated MCF7 human breast cancer cell clones, PD-L1 was differentially upregulated in MCF7 sh-WISP2, MCF7–1001/2101, and MDA-MB-231 cells but not in MCF7 SNAI1 and MCF7 SNAI1–6SA cells. Mechanistic investigations revealed that siRNA silencing of ZEB-1, but not SNAI1, TWIST, or SLUG and overexpression of miR200 family members in MCF7 sh-WISP2 cells strongly decreased PD-L1 expression. Thus, we propose that PD-L1 expression in EMT-activated breast cancer cells depends on the EMT-TF involved in EMT activation. Interestingly, siRNA-mediated targeting of PD-L1 or antibody-mediated PD-L1 block restored the susceptibility of highly resistant MCF7 sh-WISP2 and MCF7–2101 cells to CTL-mediated killing. Additionally, these results provide a novel preclinical rationale to explore EMT inhibitors as adjuvants to boost immunotherapeutic responses in subgroups of patients in whom malignant progression is driven by different EMT-TFs.
KEYWORDS: Breast cancer, epithelial-to-mesenchymal transition, miR-200 and immunotherapy, PD-L1, SLUG (SNAI2), SNAI1, ZEB-1
Introduction
Epithelial cancer cells can convert into a more mobile and invasive mesenchymal phenotype through “Epithelial-to-mesenchymal transition” (EMT). Orchestrated by a group of pleiotropic EMT-promoting transcription factors (EMT-TFs) and a subset microRNA's (miRs), EMT promotes stemness and enhances drug resistance. While the reverse process mesenchymal epithelial transition (MET) is required for the growth of micrometastatic tumors.1,2 A revolution in immune checkpoint immunotherapies has begun and new combination strategies with potent curative potential are emerging.3 Programmed death 1 (PD-1) or Programmed death ligand 1 (PD-L1) blockade has proven to be successful in many cancers.4,5 We and others6 have reported that Hypoxia-inducing factors (HIF-1α7 and HIF-2α8) regulate PD-L1 under hypoxia. PD-L1 expression is also regulated by signaling pathways, transcription factors, and miRs.9 However, the expression and regulation of PD-L1 in metastatic mesenchymal tumors versus primary epithelial tumors remain unclear. Furthermore, the potential contribution of various EMT-TFs on PD-L1 expression is still largely unknown.
In the present study, using multiple EMT-activated human breast cancer cell lines, we compared the expression and regulation of PD-L1 and we showed that not all EMT-activated cells upregulated PD-L1. Furthermore, upregulated PD-L1 rendered EMT-activated cells resistant to CTL-mediated lysis.
Materials and methods
Culture of tumor cells and CTLs
The tumor-infiltrating cytotoxic T lymphocyte (TIL CTL) clone Heu 33 and the human breast cancer cell lines were maintained in culture as described.10,11
RNA isolation and SYBR-GREEN qRT-PCR (real time-quantitative polymerase chain reaction) and Western blot
RNA isolation and SYBR-GREEN qRT-PCR were performed as described.12 Expression level of 18S was used as endogenous control. Western blotting was performed as previously.7
Flow cytometry analysis
Flow cytometry was performed using FACS LSR-II. Data were further analyzed by FACS DIVA 7.0 or Flow Jo 7.6.5 software.7
Gene silencing by RNA interference
Pre-designed siRNAs against PD-L1 and scrambled control were obtained from Life Technologies and transfected by electroporation as described earlier.7
Confocal microscopy
Confocal microscopy was performed as described.11
Cytotoxicity assay
The cytotoxic activity of the TIL CTL clone (Heu33) was measured by a conventional 4-h Cr51 release assay.13,14
Statistical analysis
Data were analyzed with GraphPad Prism. Student's t-test was used for single comparisons. Statistically significant differences (indicated by asterisks) are shown (* = p < 0.05, ** = p < 0.005, and *** = p < 0.0005). Error bars indicate SD.
Results and discussion
Differential upregulation of PD-L1 in MCF7 sh-WISP2 and MCF7–1001/2101 cells vs. MCF7, MCF7 SNAI1, and MCF7 SNAI1–6SA cells
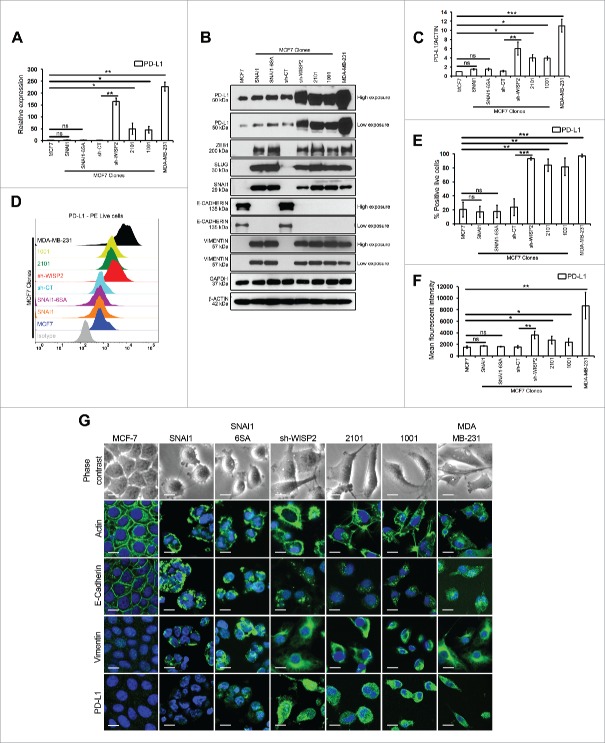
We first compared the expression of PD-L1 in MCF7 and different EMT-activated MCF7 clones (MCF7 SNAI1, MCF7 SNAI1–6SA, MCF7 sh-WISP2, and MCF7 1001/2101 cells),10,11 as well as in mesenchymal MDA-MB-231 cells. We showed that among the different EMT-activated MCF7 clones, PD-L1 was differentially upregulated only in MCF7 sh-WISP2 (more than 150-fold) and MCF7–1001/2101 cells (more than 50-fold) vs. MCF7, MCF7 SNAI1, and MCF7 SNAI1–6SA cells both at mRNA (Fig. 1A) and protein (Figs. 1B and C) levels. Similarly, as depicted in Figs. 1D–F and G, surface expression of PD-L1 was significantly upregulated in MCF7 sh-WISP2, MCF7–1001/2101, and MDA-MB-231 cells as compare with MCF7, MCF7 SNAI1, and MCF7 SNAI1–6SA cells. Moreover, IFNγ strongly upregulated PD-L1 at both mRNA (Fig. S1A) and protein (Figs. S1B–D) levels in all cell lines. Surprisingly, although all of the EMT-activated MCF7 clones and MDA-MB-231 cells expressed EMT markers (Fig. 1B), we did not observe any difference in PD-L1 expression in MCF7 SNAI1 and MCF7 SNAI1–6SA cells vs. MCF7 cells. Recently, a new molecular link between EMT-upregulated PD-L1 expression and CD8+ TIL immunosuppression was established in human lung cancer.15 Subsequently, in a patient-derived mesenchymal tumor, a pan-cancer EMT signature was identified that showed high expression of multiple immune checkpoints including PD-L1.16
Figure 1.
MCF7 sh-WISP2 and MCF7 1001/2101 cells selectively upregulate PD-L1 as compare with MCF7, MCF7 SNAI1, and MCF7 SNAI1–6SA cells. (A) SYBR-GREEN RT-qPCR was used to monitor PD-L1 mRNA expression. The experiment was performed in triplicate and repeated five times with the same results. (B) Western blot was performed to show PD-L1, ZEB1, SLUG, SNAI1, E-CADHERIN, and VIMENTIN protein levels. (C) Densitometry was performed to compare PD-L1 protein levels. The experiment was repeated five times with the same results. (D–F) Surface expression of PD-L1 on live cells was evaluated by flow cytometry as compare with isotype control (gray-shaded histogram). The experiment was repeated five times with the same results. (G) Confocal microscopy analysis of PD-L1, VIMENTIN, E-CADHERIN, and ACTIN expression in indicated cells (magnification 40×, bar: 10 μm).
Our data strongly demonstrate that among the different EMT-activated MCF7 clones, PD-L1 is differentially upregulated in MCF7 sh-WISP2 and MCF7–1001/2101 cells, but not in MCF7 SNAI1 and MCF7 SNAI1–6SA cells. Additionally, we provide new evidence here that not all EMT-activated breast cancer cells upregulate PD-L1 expression.
TGFβ-1 and TNFα have no effect on PD-L1 expression in MCF-7 and MCF7–2101 cells, and the selective upregulation of PD-L1 in MCF7 sh-WISP2 does not involve TGFβ-1
We next investigated the mechanisms involved in the upregulation of PD-L1 in EMT-activated MCF7 sh-WISP2, MCF7–1001/2101, and MDA-MB-231 cells. Both TGFβ1 and TNFα have been reported to control PD-L1.17,18 By treating MCF7 and MCF7 2101 cells with TGFβ-1 (Figs. S2A–C) and TNFα (Figs. S2D–F), we showed that none affected PD-L1 expression at either the mRNA or protein level in MCF7 and MCF7 2101 cells.
We have previously shown that the loss of WISP2 in MCF7 cells resulted in increased TGF-β signaling, thereby promoting EMT.11,19 To examine directly whether the TGF-β signaling pathway modulated PD-L1 expression, MCF7 sh-WISP2 and MDA-MB-231 cells were treated with two different inhibitors of TGFβ signaling. However, as illustrated in Fig. 2B, although both SB 431542 and A83–01 strongly inhibited SMAD-2 activation, they did not modulate PD-L1 expression at either the mRNA (Fig. 2A) or protein (Fig. 2B) level. Similarly, surface expression of PD-L1 remained highly upregulated in both MCF7 sh-WISP2 (Figs. 2C–E) and MDA-MB-231 (Figs. 2F–H) when treated with SB 431542 and A83–01. These data clearly indicate that upregulated PD-L1 in EMT-activated MCF7 clones (MCF7 sh-WISP2, MCF7–2101, and MDA-MB-231 cells) is not regulated by TGF-β and TNF-α signaling.
Figure 2.
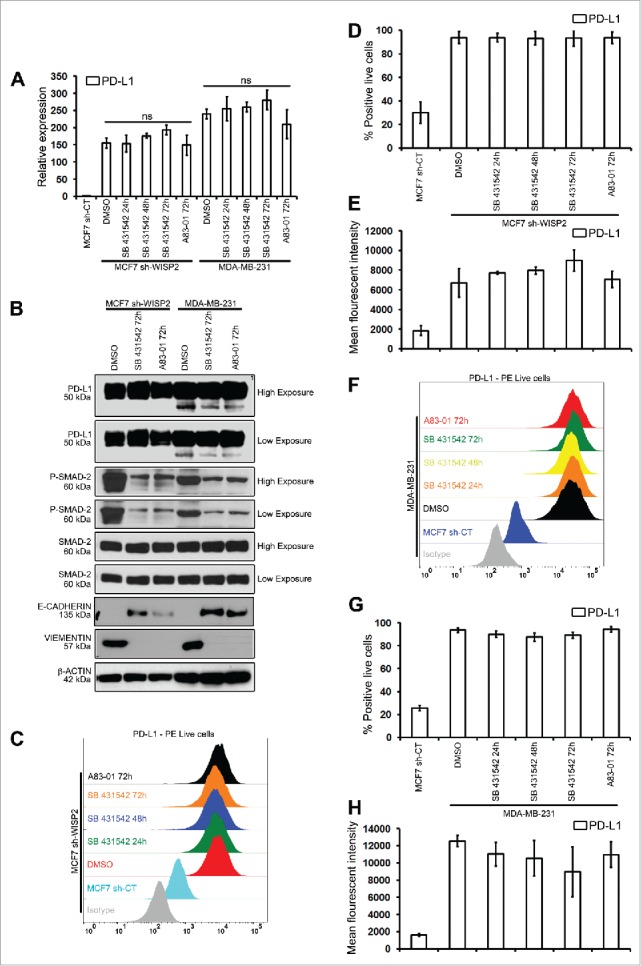
The inhibition of TGFβ-1 has no effect on PD-L1 expression in MCF-7 shWISP2 and MDA-MB-231 cells. MCF7 sh-WISP2 and MDA-MB-231 cells were treated with TGFβ-1 (5 ng/mL) in the absence (DMSO) or presence of SB 431542 (25 μM) or A83–01 (10 μM) at indicated times. (A) SYBR-GREEN RT-qPCR was used to monitor PD-L1 mRNA expressions levels. The experiment was performed in triplicate and repeated three times with the same results. (B) Western blot was performed to show protein levels. The experiment was repeated three times with the same results. (C–E) Surface expression of PD-L1 on live MCF7 sh-WISP2 cells was evaluated by flow cytometry as compare with isotype control (gray-shaded histogram). The experiment was repeated five times with the same results. (F–H) Surface expression of PD-L1 on live MDA-MB-231cells was evaluated by flow cytometry as compare with isotype control (gray-shaded histogram). The experiment was repeated five times with the same results.
Recently, MAPK, IFNγ, and PI3K/Akt9 signaling pathways have been reported to regulate PD-L1. Whether these signaling pathways are activated in our different EMT-activated MCF7 clones and whether they are involved in the upregulation of PD-L1 remains to be explored.
The selective upregulation of PD-L1 is dependent on ZEB-1/miR-200 axis in MCF7 sh-WISP2 cells
Stress-induced activation of EMT-TFs (TWIST, SNAIL, and ZEB families) results in EMT and cancer metastasis.1 In order to assess a putative role for these different EMT-TFs in the regulation of PD-L1 expression, we silenced SNAI1, TWIST, SLUG, or ZEB-1 in MCF7 sh-WISP2 cells. Interestingly, siRNA silencing of ZEB-1, but not SNAI1, TWIST, or SLUG in MCF7 sh-WISP2 strongly decreased PD-L1 at mRNA (Fig. 3A) and protein (Fig. 3B) levels. Similarly, as represented in Fig. 3C, surface expression of PD-L1 significantly decreased in MCF7 sh-WISP2 only after ZEB-1 silencing.
Figure 3.
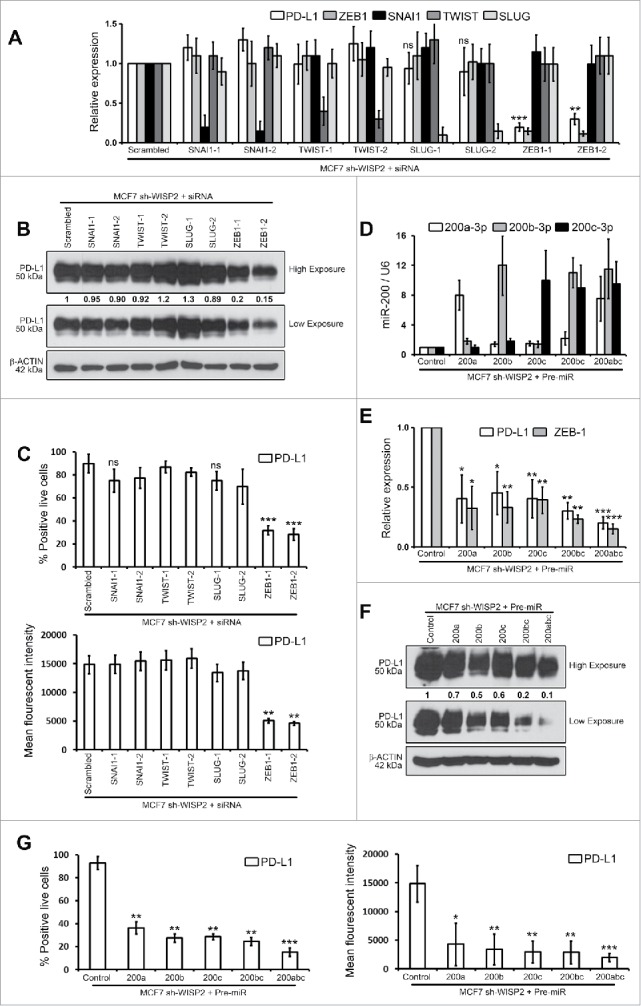
ZEB-1 and miR-200 selectively controls upregulated PD-L1 in MCF7 sh-WISP2 cells. (A–C) MCF7 sh-WISP2 cells were transfected with different siRNA targeting SNAI1, TWIST, SLUG, ZEB-1, or scrambled control. (A) SYBR-GREEN RT-qPCR was used to monitor PD-L1, ZEB-1, SNAI1, TWIST, and SLUG mRNA expressions levels. The experiment was performed in triplicate and repeated three times with the same results. (B) Western blot was performed to show PD-L1 protein levels. The experiment was repeated three times with the same results. (C) Surface expression of PD-L1 on live cells was evaluated by flow cytometry. The experiment was repeated three times with the same results. (D–G) MCF7 sh-WISP2 cells were transfected with different Pre-microRNA: (Pre-miR) Pre-miR200a, Pre-miR200b, Pre-miR200c, Pre-miR200bc, Pre-miR200abc, or Pre-miR control. (D) Taqman RT-qPCR was used to evaluate expression of different microRNA's at indicated conditions. Expression levels of U6 were used as endogenous control. The experiment was performed in triplicate and repeated three times with the same results. (E) SYBR-GREEN RT-qPCR was used to monitor PD-L1 and ZEB-1 mRNA expressions levels. The experiment was performed in triplicate and repeated three times with the same results. (F) Western blot was performed to show PD-L1 protein levels. The experiment was repeated three times with the same results. (G) Surface expression of PD-L1 on live cells was evaluated by flow cytometry. The experiment was repeated three times with the same results.
It is noteworthy that in MCF7–2101 and MDA-MB-231 cells, upregulated of PD-L1 was found to be dependent on ZEB-1 and SNAI1 but not SLUG at both mRNA (Figs. S3A and D) and protein (Figs. S3B–C and S3E–F) levels. Both SLUG and SNAIL occupy the ZEB1 promoter, but ZEB-1 expression is controlled by SNAIL but not SLUG in MDAMB-231 cells.20 In light of our observations (multiple EMT-TF comparison study), we propose that PD-L1 expression in EMT-activated breast cancer cells depends on the EMT activation by EMT-TF. Future experiments will attempt to study whether other EMT-TFs, such as SLUG or TWIST-driven EMT-activated cells upregulate PD-L1 expression.
miR-200 and ZEB-1 are well known to form a double negative feedback loop to regulate EMT in various cancers.1 We therefore asked whether members of miR200 family (miR200a, miR200b, and miR200c) can regulate PD-L1 expression in MCF7 sh-WISP2 cells (Fig. 3D). PD-L1 expression in MCF7 sh-WISP2 cells strongly decreased after transfection with Pre-miR200a, Pre-miR200b, Pre-miR200c, Pre-miR200bc, or Pre-miR200abc as compare with Pre-miR control at both mRNA (Fig. 3E) and protein (Figs. 3F and G) levels. This is in complete agreement with a recent finding, which showed that ZEB-1 activates EMT by repressing miR-200, increasing PD-L1 on lung cancer cells and hence promoting intratumoral CD8+ T cells immunosuppression and metastasis.15 Taken together, our data strongly points to the regulation of PD-L1 in EMT-activated breast cancer cells through the ZEB-1/miR-200 axis (MCF7 sh-WISP2 cells) and ZEB-1 and SNAI1 but not SLUG (MCF7–2101 and MDA-MB-231 cells).
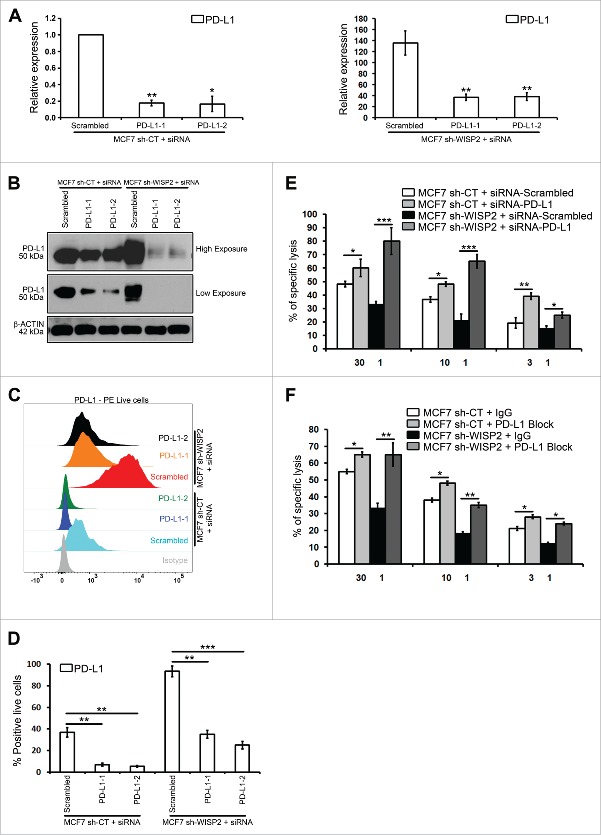
Targeting PD-L1 and PD-L1 block restores the susceptibility of MCF7 sh-WISP2 and MCF7–2101 cells to CTL-mediated lysis
To investigate directly the functional consequences of upregulated PD-L1 in EMT-activated cells, MCF7 sh-WISP2 (Figs. 4A–D) and MCF7–2101 (Figs. 4SA–D) cells were transfected with different siRNAs against PD-L1, and Cr51 cytotoxicity assays were performed using the allogeneic HLA-A2-restricted H33 CTL clone. We have previously shown that both MCF7 sh-WISP211 and MCF7–210110 cells were resistant to CTL-mediated lysis. Importantly, targeting PD-L1 significantly increased the CTL-mediated killing of both MCF7 sh-WISP2 (Fig. 4E) and MCF7–2101 (Fig. S4E) cells. Similarly, PD-L1 block also significantly restored the susceptibility of MCF7 sh-WISP2 (Fig. 4F) and MCF7–2101 (Fig. S4F) cells to CTL-mediated lysis. In conclusion, EMT activation-mediated PD-L1 upregulation in EMT-activated cells is associated with resistance to CTL-mediated lysis. Whether there is a bidirectional crosstalk between PD-L1 expression and EMT activation remains uninvestigated. Future experiments will attempt to dissect whether there is a relationship between resistance to CTL-mediated lysis, autophagy, and PD-L1 expression in EMT+ cells.
Figure 4.
siRNA-mediated PD-L1 silencing and PD-L1 block increase the susceptibility of MCF7 sh-WISP2 cells to CTL-mediated killing. (A–D) MCF7 sh-CT and MCF7 sh-WISP2 cells were transfected with different siRNA targeting PD-L1 or scrambled control. (A) SYBR-GREEN RT-qPCR was used to monitor PD-L1 mRNA expressions levels. The experiment was performed in triplicate and repeated three times with the same results. (B) Western blot was performed to show PD-L1 protein levels. The experiment was repeated three times with the same results. (C and D) Surface expression of PD-L1 on live cells was evaluated by flow cytometry as compare with isotype control (gray-shaded histogram). The experiment was repeated three times with the same results. (E) Conventional 4 h Cr51 cytotoxicity assays were performed on MCF7 sh-CT and MCF7 sh-WISP2 cells transfected with either siRNA targeting PD-L1 or scrambled control as targets at different effector to target (E:T) ratios. The experiment was repeated two times with the same results. (F) MCF7 sh-CT and MCF7 sh-WISP2 cells were pretreated for 30 min on ice with 5 ug/mL control antibody (IgG) or antibody against PD-L1 (PD-L1 Block). Conventional 4 h Cr51 cytotoxicity assay was performed at different E:T ratios. The experiment was repeated two times with the same results.
It would be of major interest to study whether metastatic mesenchymal tumors with increased PD-L1 expression16 as compare with primary tumors will respond better to anti-PD-1/anti-PDL1 immunotherapy. It would also be interesting to compare CD8+ T cell immunosuppression mediated by increased PD-L1 in various EMT-activated tumors driven by differentially expressed multiple EMT-TFs (ZEB, SNAIL, and TWIST families).
Taken together, PD-L1 was highly upregulated in EMT-activated breast cancer cells driven by various EMT-TFs (ZEB-1/miR200 or SNAI1) and PD-L1 renders EMT-activated cells resistant to CTL-mediated lysis. Therefore, the use of EMT inhibitors as adjuvants with new emerging immunotherapeutic strategies may be beneficial for boosting the immune system in cancer patients with mesenchymal metastatic tumors.
Supplementary Material
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Annette K. LARSEN and Michèle SABAH (INSERM U938, Center de Recherche Saint-Antoine, Paris, France) for different EMT-activated MCF7 clones (MCF7 SNAI1, MCF7 SNAI1–6SA, and MCF7 sh-WISP2).
Funding
This work was supported by a grant GEFLUC (R15079LL), “Equipe labellisée par la Ligue Contre le Cancer” (EL2015.LNCC/SaC), FNRS-Televie (7.4664.15), LIH (2013 11 05), Luxembourg and the INCA for an INCA grant PLBIO15–266.
References
- 1.Puisieux A, Brabletz T, Caramel J. Oncogenic roles of EMT-inducing transcription factors. Nat Cell Biol 2014; 16:488-94; PMID:24875735; http://dx.doi.org/ 10.1038/ncb2976 [DOI] [PubMed] [Google Scholar]
- 2.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell 2009; 139:871-90; PMID:19945376; http://dx.doi.org/ 10.1016/j.cell.2009.11.007 [DOI] [PubMed] [Google Scholar]
- 3.Sharma P, Allison JP. The future of immune checkpoint therapy. Science 2015; 348:56-61; PMID:25838373; http://dx.doi.org/ 10.1126/science.aaa8172 [DOI] [PubMed] [Google Scholar]
- 4.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K et al.. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. New Engl J Med 2012; 366:2455-65; PMID:22658128; http://dx.doi.org/ 10.1056/NEJMoa1200694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB et al.. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. New Engl J Med 2012; 366:2443-54; PMID:22658127; http://dx.doi.org/ 10.1056/NEJMoa1200690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barsoum IB, Smallwood CA, Siemens DR, Graham CH. A mechanism of hypoxia-mediated escape from adaptive immunity in cancer cells. Cancer Res 2014; 74:665-74; PMID:24336068; http://dx.doi.org/ 10.1158/0008-5472.CAN-13-0992 [DOI] [PubMed] [Google Scholar]
- 7.Noman MZ, Desantis G, Janji B, Hasmim M, Karray S, Dessen P, Bronte V, Chouaib S. PD-L1 is a novel direct target of HIF-1alpha, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J Exp Med 2014; 211:781-90; PMID:24778419; http://dx.doi.org/ 10.1084/jem.20131916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Messai Y, Gad S, Noman MZ, Le Teuff G, Couve S, Janji B, Kammerer SF, Rioux-Leclerc N, Hasmim M, Ferlicot S et al.. Renal cell carcinoma programmed Death-ligand 1, a new direct target of Hypoxia-inducible Factor-2 Alpha, is regulated by von Hippel-Lindau gene mutation status. Eur Urol 2015; PMID:26707870; http://dx.doi.org/ 10.1016/j.eururo.2015.11.029 [DOI] [PubMed] [Google Scholar]
- 9.Chen J, Jiang CC, Jin L, Zhang XD. Regulation of PD-L1: a novel role of pro-survival signalling in cancer. Ann Oncol 2016; 27:409-16; PMID:26681673; http://dx.doi.org/ 10.1093/annonc/mdv615 [DOI] [PubMed] [Google Scholar]
- 10.Akalay I, Janji B, Hasmim M, Noman MZ, Andre F, De Cremoux P, Bertheau P, Badoual C, Vielh P, Larsen AK et al.. Epithelial-to-mesenchymal transition and autophagy induction in breast carcinoma promote escape from T-cell-mediated lysis. Cancer Res 2013; 73:2418-27; PMID:23436798; http://dx.doi.org/ 10.1158/0008-5472.CAN-12-2432 [DOI] [PubMed] [Google Scholar]
- 11.Akalay I, Tan TZ, Kumar P, Janji B, Mami-Chouaib F, Charpy C, Vielh P, Larsen AK, Thiery JP, Sabbah M et al.. Targeting WNT1-inducible signaling pathway protein 2 alters human breast cancer cell susceptibility to specific lysis through regulation of KLF-4 and miR-7 expression. Oncogene 2015; 34:2261-71; PMID:24931170; http://dx.doi.org/ 10.1038/onc.2014.151 [DOI] [PubMed] [Google Scholar]
- 12.Noman MZ, Janji B, Hu S, Wu JC, Martelli F, Bronte V, Chouaib S. Tumor-promoting effects of myeloid-derived suppressor cells are potentiated by hypoxia-induced expression of miR-210. Cancer Res 2015; 75:3771-87; PMID:26206559; http://dx.doi.org/ 10.1158/0008-5472.CAN-15-0405 [DOI] [PubMed] [Google Scholar]
- 13.Noman MZ, Janji B, Kaminska B, Van Moer K, Pierson S, Przanowski P, Buart S, Berchem G, Romero P, Mami-Chouaib F et al.. Blocking hypoxia-induced autophagy in tumors restores cytotoxic T-cell activity and promotes regression. Cancer Res 2011; 71:5976-86; PMID:21810913; http://dx.doi.org/ 10.1158/0008-5472.CAN-11-1094 [DOI] [PubMed] [Google Scholar]
- 14.Noman MZ, Buart S, Romero P, Ketari S, Janji B, Mari B, Mami-Chouaib F, Chouaib S. Hypoxia-inducible miR-210 regulates the susceptibility of tumor cells to lysis by cytotoxic T cells. Cancer Res 2012; 72:4629-41; PMID:22962263; http://dx.doi.org/ 10.1158/0008-5472.CAN-12-1383 [DOI] [PubMed] [Google Scholar]
- 15.Chen L, Gibbons DL, Goswami S, Cortez MA, Ahn YH, Byers LA, Zhang X, Yi X, Dwyer D, Lin W et al.. Metastasis is regulated via microRNA-200/ZEB1 axis control of tumour cell PD-L1 expression and intratumoral immunosuppression. Nat Commun 2014; 5:5241; PMID:25348003; http://dx.doi.org/ 10.1038/ncomms6241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mak MP, Tong P, Diao L, Cardnell RJ, Gibbons DL, William WN, Skoulidis F, Parra ER, Rodriguez-Canales J, Wistuba II et al.. A patient-derived, pan-cancer EMT signature identifies global molecular alterations and immune target enrichment following epithelial-to-mesenchymal transition. Clin Cancer Res 2016; 22:609-20; PMID:26420858; http://dx.doi.org/ 10.1158/1078-0432.CCR-15-0876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ou JN, Wiedeman AE, Stevens AM. TNF-alpha and TGF-beta counter-regulate PD-L1 expression on monocytes in systemic lupus erythematosus. Sci Rep 2012; 2:295; PMID:22389764; http://dx.doi.org/ 10.1038/srep00295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Starke A, Wuthrich RP, Waeckerle-Men Y. TGF-beta treatment modulates PD-L1 and CD40 expression in proximal renal tubular epithelial cells and enhances CD8 cytotoxic T-cell responses. Nephron Exp Nephrol 2007; 107:e22-9; PMID:17671397; http://dx.doi.org/ 10.1159/000106506 [DOI] [PubMed] [Google Scholar]
- 19.Ferrand N, Gnanapragasam A, Dorothee G, Redeuilh G, Larsen AK, Sabbah M. Loss of WISP2/CCN5 in estrogen-dependent MCF7 human breast cancer cells promotes a stem-like cell phenotype. PloS one 2014; 9:e87878; PMID:24498388; http://dx.doi.org/ 10.1371/journal.pone.0087878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ye X, Tam WL, Shibue T, Kaygusuz Y, Reinhardt F, Ng Eaton E, Weinberg RA. Distinct EMT programs control normal mammary stem cells and tumour-initiating cells. Nature 2015; 525:256-60; PMID:26331542; http://dx.doi.org/ 10.1038/nature14897 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.