ABSTRACT
The clinical relevance of tumor-infiltrating lymphocytes (TIL) in breast cancer (BC) has been clearly established by their demonstrated correlation with long-term positive outcomes. Nevertheless, the relationship between protective immunity, observed in some patients, and critical features of the infiltrate remains unresolved. This study examined TIL density, composition and organization together with PD-1 and PD-L1 expression in freshly collected and paraffin-embedded tissues from 125 patients with invasive primary BC. Tumor and normal breast tissues were analyzed using both flow cytometry and immunohistochemistry. TIL density distribution is a continuum with 25% of tumors identified as TIL-negative at a TIL density equivalent to normal breast tissues. TIL-positive tumors (75%) were equally divided into TIL-intermediate and TIL-high. Tumors had higher mean frequencies of CD4+ T cells and CD19+ B cells and a lower mean frequency of CD8+ T cells compare with normal tissues, increasing the CD4+/CD8+ T-cell ratio. Tertiary lymphoid structures (TLS), principally located in the peri-tumoral stroma, were detected in 60% of tumors and correlated with higher TIL infiltration. PD-1 and PD-L1 expression were also associated with higher TIL densities and TLS. TIL density, TLS and PD-L1 expression were correlated with more aggressive tumor characteristics, including higher proliferation and hormone receptor negativity. Our findings reveal an important relationship between PD-1/PD-L1 expression, increased CD4+ T and B-cell infiltration, TIL density and TLS, suggesting that evaluating not only the extent but also the nature and location of the immune infiltrate should be considered when evaluating antitumor immunity and the potential for benefit from immunotherapies.
KEYWORDS: Breast cancer, PD-1, PD-L1, tertiary lymphoid structures, tumor-infiltrating lymphocytes
Abbreviations
- Ab
antibody
- BC
breast cancer
- CI
confidence interval
- CTLA-4
cytotoxic T lymphocyte-associated protein 4
- ER
estrogen receptor
- FFPE
formalin-fixed paraffin-embedded
- HD
healthy donor
- H&E
hematoxylin and eosin
- HER2
human epidermal receptor 2
- HR
hormone receptor
- IC
immune cells
- ICC
intra-class correlation coefficient
- IHC
immunohistochemistry
- NANT
non-adjacent non-tumor
- PB
peripheral blood
- PD-1
programmed death 1
- PD-L1
programmed death 1 ligand
- PR
progesterone receptor
- TC
tumor cells
- Tfh
T follicular helper
- Ti-BALT
tumor-induced bronchus-associated lymphoid tissue
- TIL
tumor-infiltrating lymphocytes
- TLS
tertiary lymphoid structure
- TNBC
triple negative breast cancer.
Introduction
Tumor-infiltrating immune cells have been associated with favorable clinical outcomes, including disease-free survival, and overall survival in various solid tumors, including melanoma,1 colorectal,2 lung3 and breast cancer (BC).4 Large BC clinical trials have validated the prognostic and predictive significance of tumor infiltrating lymphocytes (TIL), particularly in TNBC and HER2+ BC.5 These studies principally scored TIL on hematoxylin and eosin-stained (H&E) sections, with occasional use of immunohistochemistry (IHC) and immune gene signatures. Measuring the overall extent of TIL infiltration does not distinguish between the relative contributions made by the diversity of innate and adaptive immune cells potentially present; however, higher magnitudes do appear to reflect antitumor immune responses that are clinically relevant. Recent evidence suggests that the balance between immune cells whose functions actively control or restrain neoplastic progression and those that promote tumor activities may be a critical determinant for clinical outcome.4,6 Some immune cells are direct effectors of antitumor immune responses, exemplified by CD8+ T cells, whereas others such as CD4+ regulatory T cells and M2-macrophages can negatively regulate immune responses and have been associated with worse clinical outcomes.7-10
The immune infiltrate in breast tumors is heterogeneous, ranging from a few detectable lymphocytes to extensive infiltration.4,6 Immune cells, when present, are scattered throughout the tumor bed and/or aggregated in the peri-tumoral stroma. These immune cell clusters are frequently organized in tertiary lymphoid structures (TLS),6,11 structurally similar to secondary lymphoid organs. TLS are thought to be a key contributing factor to the chronic inflammation associated with autoimmune diseases and persistent infections (reviewed in ref.12). TLS were first associated with better clinical outcomes in colorectal and lung cancer.13,14 Recent studies have more closely investigated the nature of the immune infiltrate in BC,15,16 including our work identifying TLS and their specific association with specialized CD4+ T follicular helper (Tfh) cells.6 We found that their presence, as detected by an eight gene signature dominated by CXCL13, is positively associated with survival and response to pre-operative chemotherapy.
The emergence of immunotherapy as an effective cancer treatment highlights the need to fully understand variation in immune responses between different tumor microenvironments. Checkpoint receptors play an important role in regulating conventional lymphocyte responses by limiting antigen activation through binding to their specific ligands. These ligands are upregulated on immune cells in response to chronic stimulation and inflammatory cytokines as well as other cell types, including tumor cells.17-19 Clinical use of immune checkpoint inhibitors, initially with antibodies blocking CTLA-4 and PD-1 or PDL-1,20 have shown remarkable promise via their induction of long-lasting responses and survival benefits in various tumor types, including melanoma, lung and more recently renal21 and breast cancer patients.22 An increased TIL presence at the tumor site has been linked with the efficacy of immune checkpoint blockade.23 PD-L1 upregulation on tumor cells and/or immune cells has been detected on many human tumors including BC, with triple-negative (TN) and HER2+ BC most frequently characterized by higher TIL and PD-L1 upregulation.24,25 PD-L1 upregulation has been correlated with a better response to PD-1 blockade;26,27 however, recent studies suggest that the appropriate immune microenvironment is key to positive responses.28-30 The goal of the present study was to examine TIL density, composition and organization in breast tumor compare with normal tissues, establish a threshold identifying TIL-positive (TILpos) and TIL-negative (TILneg) BC, evaluate PD-1 and/or PD-L1 expressing cells in the tumor microenvironment and analyze the relationship between specific TIL characteristics and standard clinicopathological parameters.
Results
TIL density and a lymphocyte-infiltration threshold for human BC
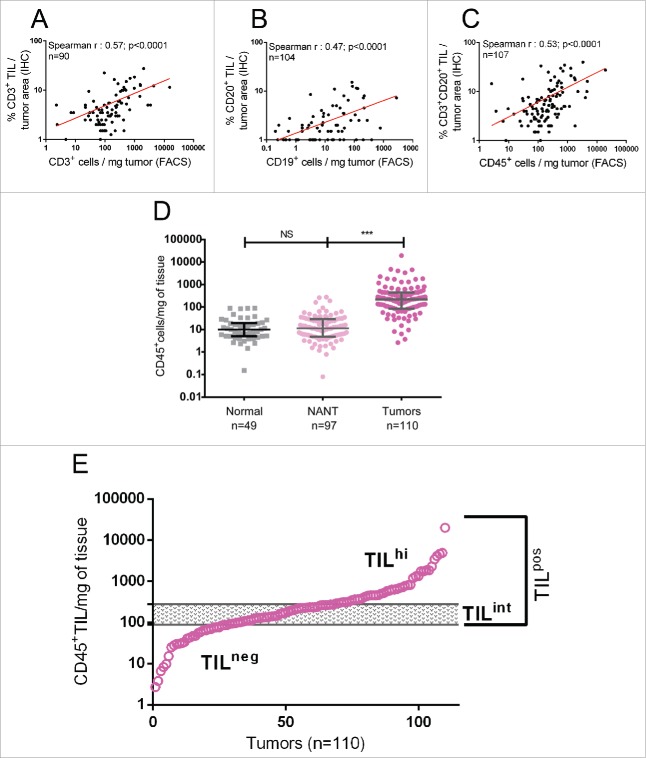
In this study, TIL density determined by flow cytometry was compared with TIL assessment on IHC-stained full-face tissue sections from formalin-fixed, paraffin-embedded (FFPE) of the same BC surgical specimens (Figs. 1A–C; gating strategies shown in Fig. S1). We found a significant positive correlation between IHC- and flow cytometry-based scoring of T-cell and B-cell TIL. Consistent with the observation that a sizeable majority of TIL are lymphocytes (Fig. 2A), a combined IHC score for CD3+ T cells and CD20+ B cells was positively correlated with total CD45+ TIL density quantified by flow cytometry (Fig. 1C). Overall, our data demonstrate that quantification of TIL density by flow cytometry accurately reflects lymphocyte-infiltration levels scored by experienced pathologists.
Figure 1.
The density (cells/mg of tissue) of (A) CD3+ TIL (T cells) and (B) CD19+ TIL (B cells) quantified by flow cytometry (x-axis) was correlated with the percentage of tumor surface area occupied by CD3+ TIL or CD19+ TIL evaluated by trained pathologists on IHC-stained full-face tissue sections (y-axis), respectively. (C) The density of total CD45+ TIL quantified by flow cytometry was correlated with the percentage of tumor surface area occupied by CD3+ plus CD19+ TIL evaluated on IHC-stained tissue sections. (D) CD45+ cell density was compared between normal breast tissues, NANT and breast tumor tissues. Median and interquartile ranges are represented for each group. The level of significance is shown as a p value (*p < 0.05, **p < 0.01, ***p < 0.001). (E) The distribution of CD45+ cell density determined by flow cytometry for 110 breast tumors is shown graphically. The 99th percentile of CD45+ cell density in normal breast tissue is identified by the lower line, whereas the upper line delimits that for NANT. These thresholds are used to define TIL-negative (TILneg; below the lower threshold) and TIL-high tumors (TILhi; above the upper threshold), respectively, with TIL-intermediate tumors (TILint) falling between the two thresholds.
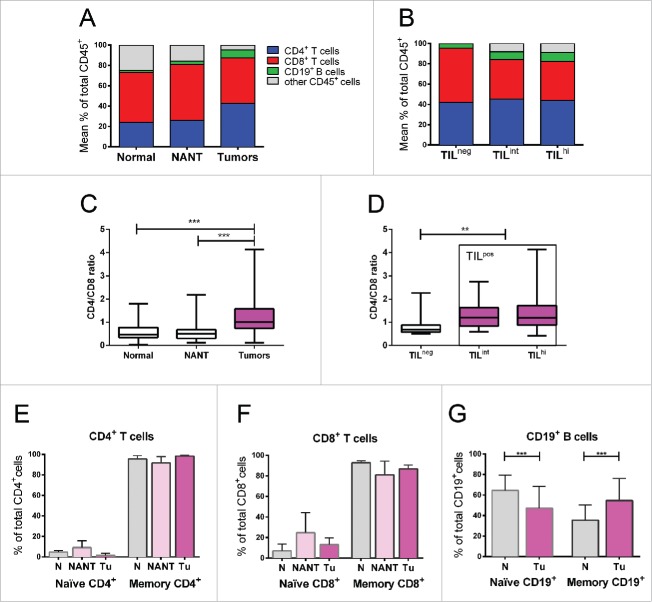
Figure 2.
(A) Flow cytometry data represented as the mean percentage of CD4+ T cells, CD8+ T cells and B cells among total CD45+ cells in breast tumors compared with normal breast tissues or NANT. (B) The same lymphocyte subsets are shown for breast tumors divided into TILneg, TILint and TILhi density groups. (C) CD4+/CD8+ ratio in breast tumors compared with normal breast tissues or NANT. (D) CD4+/CD8+ ratio in breast tumors divided into TILneg, TILint and TILhi density groups. (E-G) CD4+ T cell, CD8+ T cell and CD19+ B-cell TIL in normal, NANT or tumor tissues separated into naïve vs. memory cell CD45RA/RO (CD45RA+/− for T cells and CD27+/− for B cells). The level of significance is shown as a p value (*p < 0.05, **p < 0.01, ***p < 0.001).
We next compared CD45+ immune cell infiltration in breast tissues from patients undergoing mammary reduction surgery as well as matched NANT (non-adjacent non-tumor) from the primary tumors. Our analysis revealed remarkably similar CD45+ cell densities in NANT and mammary reduction tissues (median: 5.4 and 3.7 infiltrating CD45+ cells/mg of tissue, respectively (Fig. 1D)). No correlation was found between CD45+ cell density in a tumor specimen and its matched NANT nor based on the distance between the two samples (Fig. S3). Finally, our data importantly establish that CD45+ TIL distribution in breast tumors forms a continuum with a median at 218 CD45+ TIL/mg of tumor tissue (Fig. 1E; interquartile range: 85–445 CD45+ TIL/mg).
With the goal of setting a threshold that discriminates between normal immune trafficking in breast tissue and tumor-associated inflammation, CD45+ cell densities in normal tissue and NANT were used to identify relevant cutoffs. TILneg tumors are defined as those with a TIL density <99th percentile of CD45+ cell density in normal tissue (≤89 TIL/mg; Fig. 1E). TILpos tumors (>89 TIL/mg) are subdivided into TIL-high (TILhi; ≥275 TIL/mg), which is equivalent to a TIL density >99th percentile of CD45+ cell density in NANT and TIL-intermediate (TILint; >89 and <275 TIL/mg). Applying these thresholds identified approximately 25% of our BC cohort as TILneg with CD45+ cell infiltration levels similar to normal breast tissue. The TILpos tumors (75%) are evenly divided into TILint tumors (36% of BC; median: 163, interquartile range: 121–229 CD45+ TIL/mg) and TILhi tumors (39% of BC; median: 598, interquartile range: 379–1,407 CD45+ TIL/mg).
We next investigated potential correlations between the frequency of TIL, both as continuous and categorical (three TIL density groups) variables, and clinicopathological parameters. Higher TIL densities were significantly associated with a younger age, higher tumor cell proliferation (Ki67), histological grade and hormone receptor (HR) negativity (Table 1 and Fig. S4, Table S4) consistent with previous studies.4
Table 1.
Clinicopathological characteristics of the 125 patients with invasive breast cancer included in this study.1
| TIL density |
TLS presence |
PD-L1 status |
PD-1 status |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients | TILneg | TILint | TILhi | TLSneg | TLSpos | PD-L1neg | PD-L1pos | PD-1neg | PD-1pos | |||||||||
| N = (%) | 125 (100) | 28 (25.5) | 40 (36.4) | 42 (38.2) | χ2 p-value | 49 (40.2) | 73 (59.8) | χ2 p-value | 84 (76.4) | 26 (23.6) | χ2 p-value | 89 (84.5) | 17 (15.5) | χ2 p-value | ||||
| Age(years) | 0.025 | 0.538 | 0.02 | 1 | ||||||||||||||
| <50 | 32 (26) | 2 (7.1) | 11 (39.3) | 15 (53.3) | 11 (35.5) | 20 (64.5) | 17 (58.6) | 12 (41.4) | 25 (86.2) | 4 (13.8) | ||||||||
| ≥50 | 95 (74) | 26 (31.7) | 29 (35.4) | 27 (32.9) | 38 (41.8) | 53 (58.2) | 64 (80) | 16 (20) | 68 (84) | 13 (16) | ||||||||
| Histology | 0.617 | 0.282 | 0.107 | 0.101 | ||||||||||||||
| Ductal | 100 (80) | 23 (25.6) | 32 (35.6) | 35 (38.9) | 42 (42.9) | 56 (57.1) | 62 (71.3) | 25 (29.1) | 73 (83.9) | 14 (16.1) | ||||||||
| Lobular | 20 (16) | 4 (26.7) | 6 (40) | 5 (33.3) | 6 (31.6) | 13 (68.4) | 18 (100) | 0(0) | 17 (94.4) | 1 (5.6) | ||||||||
| Mixed | 3 (2.4) | 0 (0) | 2 (66.6) | 1 (33.3) | 0 (0) | 3 (100) | 2 (66.7) | 1 (33.3) | 2 (66.7) | 1 (33.1) | ||||||||
| others | 2 (1.6) | 1 (50) | 0 (0) | 1 (50) | 1 (50) | 1 (50) | 2 (100) | 0 (0) | 1 (50) | 1 (50) | ||||||||
| Size | 0.924 | 0.966 | 0.073 | 1 | ||||||||||||||
| <20 mm | 73 (58) | 16 (24.2) | 24 (36.4) | 26 (39.4) | 28 (40) | 42 (60) | 44 (69.8) | 19 (30.2) | 53 (84.1) | 10 (15.9) | ||||||||
| ≥ 20 mm | 52 (42) | 12 (27.3) | 16 (36.4) | 16 (36.4) | 21 (40.4) | 31 (59.6) | 40 (85.1) | 7 (14.9) | 40 (85.1) | 7 (14.9) | ||||||||
| Node status | 0.399 | 0.959 | 0.811 | 0.574 | ||||||||||||||
| Negative | 42 (33.6) | 21 (28.8) | 27 (37) | 25 (34.2) | 32 (40) | 48 (60) | 58 (77.3) | 17 (22.7) | 62 (82.7) | 13 (17.3) | ||||||||
| Positive | 83 (66.4) | 7 (18.9) | 13 (35.1) | 17 (45.9) | 17 (40.5) | 25 (59.5) | 26 (76.5) | 9 (23.5) | 31 (88.6) | 4 (11.4) | ||||||||
| Stage(AJCC) | 0.623 | 0.762 | 0.227 | 0.52 | ||||||||||||||
| I | 63 (50.4) | 14 (25) | 22 (39.3) | 20 (35.7) | 25 (41.7) | 35 (58.3) | 40 (71.4) | 16 (28.6) | 46 (82.1) | 10 (17.9) | ||||||||
| II | 49 (39.2) | 13 (29.5) | 22 (29.5) | 18 (40.9) | 20 (40.8) | 29 (59.2) | 35 (85.4) | 6 (14.6) | 35 (85.4) | 6 (14.6) | ||||||||
| III | 13 (10.4) | 1 (10) | 4 (50) | 4 (40) | 4 (30.8) | 9 (69.2) | 9 (69.2) | 4 (30.8) | 12 (92.3) | 1 (7.7) | ||||||||
| Histological grade | 0.016 | 0.032 | 0.002 | 0.294 | ||||||||||||||
| 1 | 20 (16) | 5 (23.8) | 9 (42.9) | 7 (33.3) | 9 (45) | 11 (55) | 16 (88.9) | 3 (16.7) | 15 (83.3) | 3 (16.7) | ||||||||
| 2 | 53 (42.4) | 16 (35.6) | 19 (42.2) | 10 (22.2) | 26 (52) | 24 (48) | 39 (88.6) | 5 (11.4) | 40 (90.9) | 4 (9.1) | ||||||||
| 3 | 52 (41.6) | 7 (15.9) | 12 (27.3) | 25 (56.8) | 14 (26.9) | 38 (73.1) | 29 (60.4) | 19 (39.6) | 38 (79.2) | 10 (20.8) | ||||||||
| Ki67(IHC) | 0.002 | 0.007 | <0.0001 | 0.033 | ||||||||||||||
| < 20% | 71 (56.8) | 21 (33.9) | 26 (41.9) | 15 (24.2) | 35 (50.7) | 34 (49.3) | 54 (90) | 6 (10) | 55 (91.7) | 5 (8.3) | ||||||||
| ≥20% | 54 (43.2) | 7 (14.6) | 14 (29.2) | 27 (56.3) | 14 (26.4) | 39 (73.6) | 30 (60) | 20 (40) | 38 (76) | 12 (24) | ||||||||
| Lymphovascular Embolism | 0.056 | 0.19 | 0.069 | 0.186 | ||||||||||||||
| absent | 76 (60.8) | 22 (32.8) | 24 (35.8) | 21 (31.3) | 33 (45.2) | 40 (54.8) | 56 (82.4) | 12 (17.6) | 60 (88.2) | 8 (11.8) | ||||||||
| present | 49 (39.2) | 6 (14) | 16 (37.2) | 21 (48.8) | 16 (32.7) | 31 (67.3) | 27 (66.7) | 14 (33.3) | 33 (78.6) | 9 (21.4) | ||||||||
| HR(IHC) | ||||||||||||||||||
| ERneg | 22 (17.6) | 2 (10.5) | 4 (21.1) | 13 (68.4) | 0.011 | 5 (22.7) | 17 (77.3) | 0.065 | 11 (55) | 9 (45) | 0.02 | 13 (65) | 7 (35) | 0.014 | ||||
| ERpos | 103 (82.4) | 26 (28.6) | 36 (39.6) | 29 (31.9) | 44 (44) | 56 (56) | 73 (81.1) | 17 (18.9) | 80 (88.9) | 10 (11.1) | ||||||||
| PRneg | 34 (27.2) | 3 (11.1) | 8 (29.6) | 16 (59.3) | 0.023 | 8 (23.5) | 26 (76.5) | 0.02 | 17 (54.8) | 14 (45.2) | 0.002 | 22 (71) | 9 (29) | 0.02 | ||||
| PRpos | 91 (72.8) | 25 (30.1) | 32 (51.7) | 26 (31.3) | 41 (46.6) | 47 (53.4) | 67 (84.8) | 12 (15.2) | 71 (89.9) | 8 (10.1) | ||||||||
| HER2(FISH) | 0.356 | 0.606 | 0.544 | 0.464 | ||||||||||||||
| HER2neg | 104 (83.2) | 24 (26.4) | 35 (38.5) | 32(35.2) | 42 (41.2) | 60 (58.8) | 72 (77.4) | 21 (22.6) | 77 (82.8) | 16 (17.2) | ||||||||
| HER2pos | 21 (16.8) | 4 (21.1) | 5 (26.3) | 10 (52.6) | 7 (35) | 13 (65) | 12 (70.6) | 5 (29.4) | 16 (94.1) | 1 (5.9) | ||||||||
| TIL density | <0.0001 | 0.003 | <0.0001 | |||||||||||||||
| TILneg | 28 (25.5) | 19 (67.9) | 9 (32.1) | 23 (92) | 2 (8) | 25 (100) | 0 (0) | |||||||||||
| TILint | 40 (36.4) | 18 (47.4) | 20 (52.6) | 30 (85.7) | 5 (14.3) | 32 (91.4) | 3 (8.6) | |||||||||||
| TILhi | 42 (38.2) | 6 (14.6) | 35 (85.4) | 21 (58.3) | 15 (41.7) | 23 (63.9) | 13 (36.1) | |||||||||||
| TLS presence | <0.0001 | 0.007 | ||||||||||||||||
| TLSneg | 49 (40.2) | 43 (93.5) | 3 (6.5) | 44 (95.7) | 2 (4.3) | |||||||||||||
| TLSpos | 73 (59.8) | 41 (64.1) | 23 (35.9) | 49 (76.6) | 15 (23.4) | |||||||||||||
| PD-L1 status | 0.001 | |||||||||||||||||
| PD-L1neg | 81 (74.3) | 77 (91.7) | 7 (8.3) | |||||||||||||||
| PD-L1pos | 28 (25.7) | 16 (61.5) | 10 (38.5) | |||||||||||||||
The association between TIL density groups, TLS presence, PD-1 postivity, PD-L1 positivity and clinciopathological characteristics were evaluated using the Pearson's chi-square test
The composition and phenotype of BC TIL
Flow cytometry was used to quantify the major lymphocyte lineages in the TIL including CD4+ and CD8+ T cells and CD19+ B cells. Tumors were determined to have higher mean frequencies of CD4+ T cells (42% of TIL) and CD19+ B cells (8% of TIL) and a lower mean frequency of CD8+ T cells (44% of TIL) compare with control tissues (Fig. 2A, Fig. S5 and Table S2), which signifies an increased CD4+/CD8+ ratio in tumors (Fig. 2C). We also observed differences in lymphocyte subpopulations between the TIL density groups with an increased CD4+/CD8+ ratio in TILpos compare with TILneg BC (Fig. 2D). TILhi tumors were distinguished by a significantly lower frequency of CD8+ T cells and a higher density of CD4+ T cells and B cells (Fig. S6 and Table S3). Interestingly, B cells were identified (≥1% of total CD45+ TIL identified by flow cytometry) in more than 80% of tumors and matching NANT and 66% of normal breast tissues.
The majority of T cell TIL in BC were previously shown to have gene expression patterns characteristic of CD45RO+ antigen-experienced memory cells with an effector/memory phenotype.6 In this study, flow cytometric analysis confirmed that CD4+ and CD8+ TIL are principally composed of CD45RO+ memory T cells (Figs. 2E–G and Table S2). Similar frequencies of memory T cells were detected in both normal (reduction and NANT) and malignant breast tissues. In contrast, tumors were enriched in CD19+CD27+ B cells making the proportion of tumor-resident memory B cells significantly higher than normal breast tissues (Fig. 2G and Table S2). No correlation was observed between standard clinicopathological parameters or TIL density and the distribution of naïve or memory T- and B-cell TIL subpopulations. These observations appear to be specific for the TIL because differences in the percentage of CD4+, CD8+ and CD19+ cells and their maturation state were not detected in the peripheral blood (PB) of patients and healthy donors (HD) or between patients with TILpos and TILneg tumors (Fig. S7).
BC TIL organization in TLS
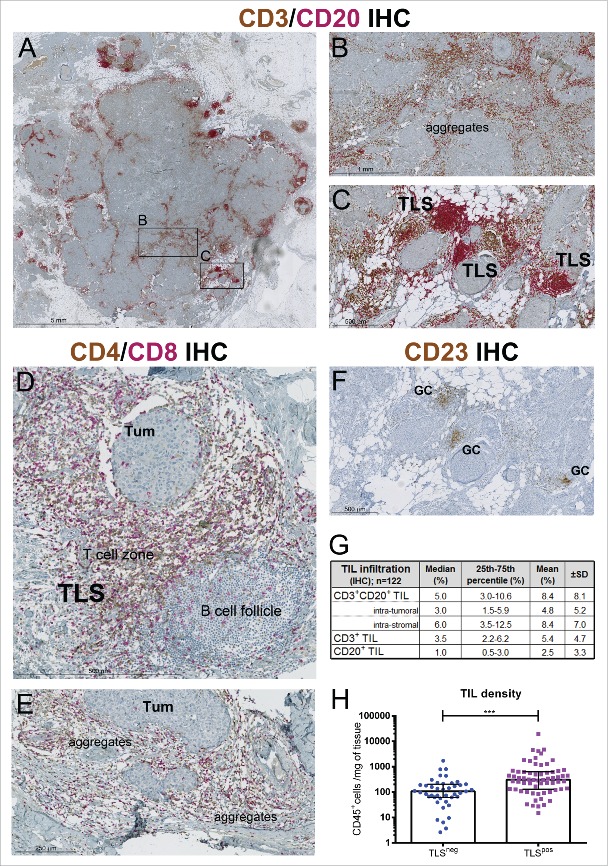
TIL spatial distribution and organization cannot be determined by flow cytometry; therefore, dual IHC-stained sections (CD3 plus CD20) from the same tumors were evaluated to link these parameters with TIL density and composition. The pattern of lymphocyte distribution in TILpos BC reveals that T- and B-cell TIL are scattered in the tumor core with more dense aggregates located in the peri-tumoral stroma where a substantial number are organized TLS (Fig. 3). Intra-tumoral and stromal TIL densities are highly correlated (Spearman r: 0.89, p < 0.0001), with the median percentage for intra-tumoral TIL at 3%, which is significantly lower than stromal TIL at 6% (p < 0.0001) (Fig. 3G). Consistent with the flow cytometry data, the IHC analyses show that T cells dominate the immune infiltrate with a median infiltration of 3.5% in the tumor. B cells were detected in 72% of the IHC-stained samples, most frequently located in TLS, with a median infiltration of 1% in the invasive tumor area.
Figure 3.
(A) A representative TILhi breast tumor IHC-stained for CD3+ T cells (brown) and CD20+ B cells (red). Enlargement of areas showing random aggregates of CD3+ and CD20+ TIL (B) or highly organized infiltrates in TLS (C) with the latter characterized a B-cell follicle adjacent to T-cell zone. (D, E) A different section from the same TILhi tumor IHC-stained for CD4+ (brown) and CD8+ T cells showing their location in TLS (D) and TIL aggregates (E). (F) CD23+ IHC staining of follicular dendritic cells (FDC) and germinal center B cells in the same TILhi tumor. (G) Table showing mean and median values for the percent TIL in different regions of the tumor evaluated on CD3 plus CD20 IHC-stained slides. (H) CD45+ TIL density determined by flow cytometry in TLSneg and TLSpos tumors, scored within the tumor area on CD3 plus CD20 IHC-stained slides.
TLS were detected in 60% of all tumors (70% of TILpos BC) with a median of 2.5 TLS per cm2 of tissue (interquartile range: 1.0–5.2) among the TLS-positive (TLSpos) tumors. A TLS presence is correlated with higher TIL infiltration (Fig. 3H) making the proportion of TLSpos tumors significantly larger in the TILhi group (Table 1). Flow cytometry experiments confirmed that TLSpos BC also contain a higher proportion of B cells (CD19+) associated with a parallel decrease in CD8+ T cells (Fig. S8) as previously shown for melanoma31 and lung cancer.14 The positioning of the major lymphocyte subpopulations was further analyzed using a double IHC stain for CD4+ plus CD8+ (Figs. 3D and E) and a single stain for CD23 (Fig. 3F; follicular dendritic cells). TLS are principally composed of CD4+ T cells and B cells with fewer CD8+ T cells (Fig. 3D). A network of CD23+ follicular dendritic cells is present in the TLS with particularly dense staining in the germinal center (GC) due to the presence of GC B cells, which are also CD23+ (Fig. 3F). Both CD4+ and CD8+ T cells are scattered throughout the B-cell follicles of TLS (Fig. 3D). In addition to their correlation with TIL density, TLS were also associated with higher histological grades, increased proliferation and HR-negativity (Table 1, Fig. S4), suggesting that TLS more readily form in extensively-infiltrated, high proliferative tumors.
PD-1 and PD-L1 expression in BC
The prevalence of PD-1 and PD-L1 expression on cells in the BC microenvironment and their potential correlation with TIL, TLS and clinicopathological parameters were evaluated using a double IHC stain on FFPE tissue sections from the same primary tumors (Fig. 4). These analyses revealed heterogeneity of expression with PD-1 expressed only on immune cells (Figs. 4C and D) and PD-L1 principally detected on immune cells (Figs. 4B–D) with only a small percentage of positive tumor (Fig. 4A) or stromal cells. Morphological evaluation of PD-L1 together with CD68 and CD3+CD20 IHC staining of serial sections reveals that positive cells are principally from the macrophage lineage with some positive lymphoid lineage immune cells (Fig. S9).
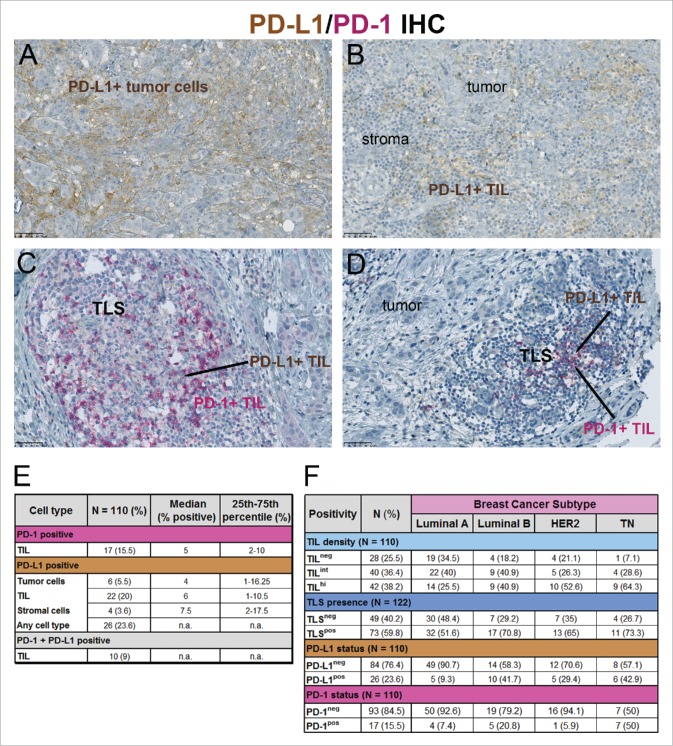
Figure 4.
(A–D) Representative tumors were IHC-stained for PD-1 (red) plus PD-L1 (brown) tumor cells (TC) and/or immune cells (IC), showing PD-L1+ tumor cells (A), PD-L1+ IC at the tumor-stroma interface (B) and PD-1+ plus PD-L1+ IC in TLS (C, D). (E) PD-1 and PD-L1 expression on different cell types is represented numerically for PD-L1+ tumors (>1% PD-L1+ among all cell types). (F) The four BC molecular subtypes (Luminal A (HR+, Ki67 <20%), Luminal B (HR+, Ki67 ≥20%), HER2+ (HR+ or HR−) and TN (HR− HER2−)) are numerically divided based on different TIL densities, TLS, PD-1 and PD-L1. “n.a” for not applicable.
Using a threshold of ≥1% positive cells, 15.5% of our tumors contained PD-1+ immune cells, 23.6% PD-L1+ cells (all cell types) and 9% positive for both (Fig. 4E). PD-1+ TIL and PD-L1+ tumor cells (just over the limit of 1% positivity) were detected in two TN tumors; however, they were not bordering as PD-1+ TIL were primarily restricted to TLS in the peri-tumoral stroma. Only three tumors (2.8%) contained both PD-L1+ immune and tumor cells and all were TILhi. PD-L1 positivity was correlated with standard clinicopathological and immune parameters revealing a significant association with younger age, ductal histology, higher tumor grade, HR negativity, higher Ki67 expression, higher TIL density, a TLS presence and a higher proportion of PD-1+ cells (Table 1 and Fig. S4; the distribution between the four BC molecular subtypes is shown in Fig. 4F). No relationship was observed with tumor size or nodal involvement suggesting that PD-L1 expression is not linked to BC stage.
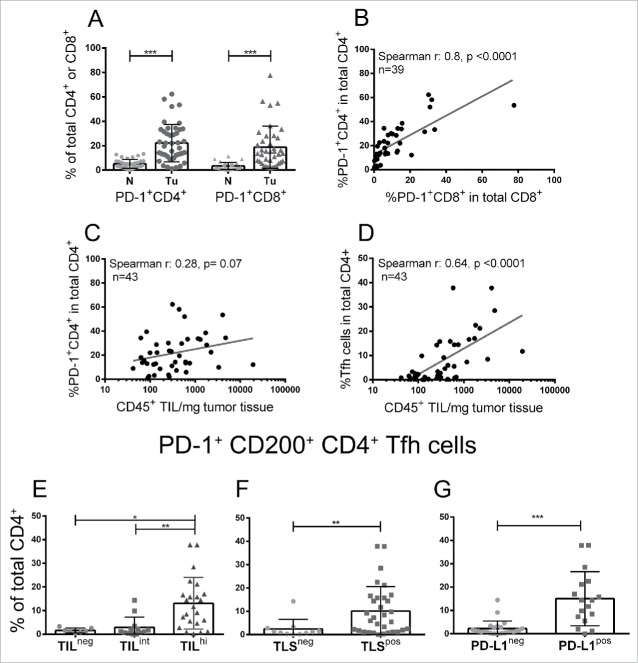
We also evaluated PD-1 expression on T cells in tumors compare with normal breast tissue using flow cytometry, finding higher mean frequencies of expression on both CD4+ TIL (22% vs. 5% in normal) and CD8+ TIL (19% vs. 3% in normal) (Fig. 5A and Table S2). PD-1+CD4+ and PD-1+CD8+ TIL frequencies were positively correlated with one another (Fig. 5B) but not with TIL density (total CD45+ TIL) (Fig. 5C). PD-1+CD200+CD4+ Tfh cells constitute 40% of the PD-1+CD4+ TIL (Table S3) and are significantly correlated with TIL density (Figs. 5D and E) and specifically associated with TLS (Fig. 5F) and PD-L1 positivity (Fig. 5G). Contrary to the PD-1+CD4+ TIL, the proportion of Tfh cells was not correlated with PD1+CD8+ TIL. No other significant differences in immune subpopulation composition were detected between the positive or negative tumors for either marker.
Figure 5.
(A) Graphic representation of the proportion of PD-1+ T cells determined by flow cytometry for tumors (Tu) compare with normal breast tissue (N). (B) The correlation between PD-1+CD4+ T cells (y-axis) and PD-1+CD8+ T cells (x-axis) in tumor tissue. (C, D) The correlation between PD-1+CD4+ (C) and PD-1+CD200+CD4+ Tfh cells (D) and CD45+ TIL density all determined by flow cytometry. (E–G) Graphic representation of the proportion of PD-1+CD200+ (Tfh cells) in total CD4+ T cells relative to TIL density (E), TLS presence (F) and PD-L1 status (G).
Discussion
The data presented in this study show that beyond the extent of infiltration, the balance and organization of individual TIL subpopulations are important parameters of the BC immune microenvironment. The correlation between TIL and clinical outcome was shown to be linear in large BC trials.32-34 Here, we used normal breast tissues to establish thresholds that define three density groups: TILneg tumors (25%), infiltrated at levels in the range determined for normal breast tissue; TILhi tumors (38%), above the NANT threshold; and TILint falling in between the normal and NANT thresholds (36%). This approach of setting TIL density thresholds based on infiltration in non-malignant breast tissues and linking the analysis of fresh tissues by flow cytometry with standard histo-pathological analysis of FFPE tumor sections is novel. Although we were unable to analyze small tumors due to a lack of residual material, we did analyze a sizeable cohort (n = 125) of untreated, primary BC tumors and matching NANT, which included tumors from clinical stage IA to IIIC, the four BC molecular subtypes and the three histological grades.
Our data show that the BC immune infiltrate forms a continuum (Fig. 1E) where the TIL density for a given tumor is only incrementally different from those proximate but distinct from those at a distance on the curve. These data provide a different view of TIL in BC and suggest that stratification of tumors using our TIL density thresholds for TILneg, TILint or TILhi could have clinical relevance for evaluating treatment options, particularly for cancer immunotherapies. TILneg tumors appear to be seen by the immune response as “normal tissue” with this threshold possibly identifying patients tolerant to their tumors. These patients are unlikely to respond to immunotherapies designed to augment or release existing immune responses but might benefit from treatments that increase immune recognition and infiltration. Patients with TILhi tumors may benefit most from therapies that amplify their existing immune responses, particularly treatments that induce immunogenic cell death.35 This notion is supported by studies showing TIL predicts the response to neo-adjuvant chemotherapy in BC.36,37 TILint tumors have moderate degrees of immune activity that might be actively suppressed. These tumors likely need treatments that relieve immune suppression and/or expand immune responses. Immune checkpoint inhibitors in combination with conventional treatments administered in the neo-adjuvant setting could potentially reactivate antitumor immunity to exploit the tumor as a source of antigen, with clinical trials in TNBC currently ongoing (NCT02622074 and NCT 02620280). The use of flow cytometry to analyze fresh tissue homogenates provides information that is difficult to obtain from archival tissues. These data were used to determine the frequency of a given lymphocyte subpopulation within the total leukocyte population (i.e., % of x in total CD45+ cells) and calculate the relative density of individual subpopulations per mg of tissue, to fully reflect the nature of a tissue infiltrate (detailed in M&M). In some tumors, the distribution of TIL subpopulations, reflected by their frequency (%) within the total CD45+ population, was similar to normal tissues; however, examination of their density revealed an overall significant increase. In TILpos tumors, this increase was repeatedly associated with higher frequencies of CD4+ and CD19+ TIL (and consequently lower CD8+ TIL) compare with normal tissues and TILneg tumors. This observation shows that the quality as well as quantity of TIL is an important consideration with an increased ratio of CD4+ helper T cells and B cells potentially reflecting a role for humoral immunity in tumors with higher TIL infiltration. Specific CD4+ T cell and B-cell subpopulations have been correlated with antitumor (Th1,38 Tfh,6 follicular B cells39) as well as pro-tumor (Treg40 and Breg41) activities, reinforcing the notion that their balance is an important consideration.
The precise cellular and molecular mechanisms that drive antitumor immune responses in some tumors and not others remain unknown, although mutation load and neoantigen presentation is thought to be a contributing factor.42 Recent data from human tumors suggests that critical responses may originate in tumor-associated TLS.43 One study using a genetically engineered murine model, where lung adenocarcinomas can develop from untransformed cells in a “natural” microenvironment, demonstrated that TLS recruit T cells to the tumor and facilitate their interactions with antigen presenting cells to produce potent antitumor immune responses.44 TLS were initially detected in colorectal and lung carcinomas in close proximity to tumor nests with their presence linked to a positive prognosis.13,14 We identified TLS in BC, a tumor type previously thought to be modestly immunogenic and linked their presence with higher TIL infiltration and a favorable prognosis.6 TLS were detected in the stroma of 60% of our BC cohort and correlated with the presence of CD4+ Tfh cells and higher TIL density and histological grade. These data linking a TLS presence with greater TIL infiltration agrees with recent retrospective studies of primary BC; however, it differs in the frequency detected. Figenschau et al.45 found TLS in 40% of tumors but they used H&E slides previously scored for immune cell infiltration to pre-select tumors for subsequent IHC staining and had a higher proportion of low grade tumors, both potential factors in their lower TLS frequency. Two other retrospective studies detected at least one TLS in 92.7% of TN46 and 89.9% of HER2-positive47 tumors by scoring H&E sections and including the adjacent tissue and in situ component. The evaluation of TLS on H&E sections does not distinguish between organized TLS and lymphoid aggregates, potentially a factor in the higher percentage they detected. Our recent methodological study found that IHC is a more accurate than H&E for scoring TLS in BC (Buisseret et al., submitted to Mod. Pathol.). The growing scientific literature reporting that TLS are a significant prognostic or predictive factor in a variety of solid tumors suggests that their accurate identification and in-depth functional analysis are important future directions.12,43,48 Sequestration of immune cells in TLS could deliver a measure of protection from tumor-mediated immune suppression while providing an appropriate niche for the generation of protective immune responses.
Why some tumors elicit an immune reaction and others do not remains unknown but an important link has emerged between pre-existing antitumor immunity and clinical responses to immune checkpoint blockade for a variety of solid tumor types.49 Treatments blocking these immune regulatory pathways have produced durable clinical outcomes in some patients.50 In BC, PD-L1 expression has been associated with poor-prognostic features, including larger tumor size, higher tumor grade, higher proliferation index (Ki-67), HR-negativity and HER2-positivity.51 Alternatively, some studies reported a good prognostic and predictive value for PD-L1, particularly in ER-negative BC.25,52-54 We show here that in BC, PD-1 and PD-L1 positivity is most frequently detected on immune cells (T cells and macrophage lineage cells, respectively) and correlated with higher levels of TIL and TLS, supporting previous findings that increased PD-L1 expression is associated with more extensive infiltration.54,55
A recent evaluation of PD-L1 expression in 3,916 BC TMA cores from the SEARCH and NEAT studies found a low frequency of expression (6% of TIL and 1.7% of tumor cells).25 We detected a higher prevalence of PD-L1 positivity (23.6% of BC on any cell type) that was dominated by PD-L1+ immune cells (20%) potentially because our assessment of full-face sections included the peri-tumoral stroma. Variation in PD-L1 positivity between BC studies could be linked with the PD-L1 antibody used and/or differences between scoring on TMA cores, biopsies and full-face sections. It is important to standardize these analyses, particularly since recent clinical data suggest that PD-1 and PD-L1 expression on immune cells is a critical parameter linked with response to treatments targeting these molecules.56
TIL in breast tumors are a key element currently motivating the testing of immunotherapies for the treatment of specific BC subtypes. The success of immunotherapy in some but not all BC patients highlights our need to understand differences in tumor-associated immune responses and identify immune biomarkers of response. The classification of BC based on the extent of TIL, their organization in TLS, altered CD4+/CD8+ ratios, the balance between infiltrating T and B cells and their association with PD-1/PD-L1 expression shown here is only a beginning. We need to understand why some primary, untreated BC are naturally receptive to significant immune infiltration, which subsequently establishes a functional organization. Paradoxically, highly infiltrated tumors are frequently associated with more aggressive pathological features, including higher proliferation and HR negativity, but it is expected that these BC patients will have more positive clinical outcomes due to the presence of TIL and TLS.
Materials and methods
Human specimens
Tumor and NANT breast tissues from surgical specimens were prospectively collected from untreated primary BC patients diagnosed and treated in the adjuvant setting at the Institut Jules Bordet between September 2012 and December 2013. Inclusion criteria: all untreated invasive primary carcinomas, stage I to III at diagnosis, from female patients who accepted to be included and signed an informed consent (clinicopathological parameters in Table 1). Normal breast tissues were collected from 56 patients undergoing mammary reduction surgery as a control. PB samples from 59/125 BC patients and 23 HD were also analyzed. All specimens were acquired after signed informed consent using procedures approved by the Institut Jules Bordet's Medical Ethics Committee (Accepted project CE 1895).
Flow cytometry
Blood samples were freshly collected (prior to surgery for BC patients) and processed. PB mononuclear cells were isolated by Ficoll Paque density gradient centrifugation prior to antibody (Ab) labeling for multi-color flow cytometric analysis. Fresh breast tissue specimens from mammary reductions (n = 56), NANT (n = 114) and tumors (n = 125) were collected immediately following surgery and dissociated (without enzymatic digestion) using the GentleMacs Dissociator (Miltenyi Biotec) prior to Ab labeling and analysis.57 With the goal of setting a threshold that discriminates between normal lymphocyte traffic in breast epithelium and tumor-driven infiltration, we tested a variety of approaches for measuring TIL density. We have previously shown that mechanical (non-enzymatic) tissue dissociation coupled with flow cytometry is rapid, reproducible and sufficiently quantitative for comparing TIL densities in fresh tissue samples prepared directly from the operating room.57 TIL density based on the weight of the tissue fragment together with the number of viable CD45+ cells in the lymphocyte gate (Fig. S1) was determined for 110 tumor, 97 NANT samples and 49 normal breast samples as controls. Fluorescently-labeled cells were acquired on a GALLIOS 10/3 cytometer and analyzed using Kaluza® 1.3 Flow Analysis Software (Beckman Coulter). The antibodies used for flow cytometry are listed in Table S1.
Immunohistochemistry (IHC)
FFPE full-face tissue sections from available tumors were dual IHC stained as detailed in supplementary text. All antibodies were initially tested on positive and negative control tissues with the Abs chosen for IHC detailed in the supplementary text file. Dual IHC stains were employed to examine the proximity of different lymphocyte subpopulations using the following markers: CD3 for total T cells, CD4+ and CD8+ for T-cell subsets, CD20 for total B cells (chosen for IHC instead of CD19 (used in flow cytometry experiments) because it has been shown to be more sensitive and specific for B cells in FFPE tissues58), CD23 for follicular dendritic cells and GC B cells, PD-1 and PD-L1. IHC staining for PD-L1 (B7-H1) was compared between several different Abs (polyclonal Abs Ab58810 (Abcam) and GTX104763 (GeneTex) and monoclonal Ab E1L3N (Cell Signaling Technology)) and a non-commercial reference Ab kindly provided by Dr Lieping Chen's lab (murine IgG1, clone 5H159) with the best results achieved using E1L3N (control tissue staining shown in Fig. S1). Tumors with PD-L1 positivity on infiltrating immune cells were stained with CD68 (macrophage lineage marker).
Pathological assessment
Scoring TIL infiltration, lymphocyte subpopulation markers and TLS on IHC-stained tissues was independently performed by two trained pathologists (RdW, GVdE) who were blinded to the clinical and experimental data. TIL infiltration was assessed as a continuous variable based on the percentage of tissue area occupied by CD3+ T cells plus CD20+ B cells. Lymphocytes in direct contact with tumor cells were identified as intra-tumoral TIL and those in the stroma (within the borders of the invasive carcinoma or its immediate surroundings) as stromal TIL following the proposed guidelines for BC.4 TLS were defined as dense aggregates of B cells with an adjacent T-cell zone. GC in TLS were identified by the presence of CD23+ cells. The threshold for PD-L1 positivity was set at ≥1% surface positive cells, including tumor, TIL and/or stromal cells. Regions of in situ carcinoma, normal glandular epithelium and necrosis were excluded from the tissue evaluated.
A high degree of reliability (measured by the intra-class correlation coefficient (ICC)) was detected between the two pathologists and the mean values are presented. The ICC's were: 0.909 with a 95% confidence interval (CI) from 0.866 to 0.938, p < 0.001 for global infiltration; 0.805 with a 95% CI from 0.717 to 0.864, p < 0.001 for CD3 positivity; and 0.856 with a 95% CI from 0.721 to 0.917, p < 0.001 for CD20 positivity. Discordant results for TLS and PD-L1 scoring were jointly re-evaluated by the pathologists.
Statistical analysis
All statistical analyses were performed using SPSS software (IBM SPSS Statistics for Macintosh ver.20.0). The two-tailed Spearman rank test was used between continuous variables. The Mann–Whitney and Kruskal–Wallis non-parametric tests were used for correlations between binary or categorical and continuous variables. The Chi-square test was used between categorical variables. Binary logistic regression analysis was used to identify predictors for high TIL infiltration, TLS presence and PD-1/PD-L1 positivity. Two-tailed tests were considered significant if the overall p-value was ≤0.05.
Supplementary Material
Disclosure of potential conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Dr Sébastien Duquenne for help reading some of the IHC slides; Dr Jalal Valaki for help constructing a patient database; Dr Christophe Lelubre for initial insights into the statistical analysis, Dr Matthew Silver at Cell Signaling for providing a pre-commercial sample of the anti-PD-L1 antibody E1L3N and Dr Lieping Chen for providing the B7-H1 antibody. This work was supported by grants from the Belgian Fund for Scientific Research (FNRS), Les Amis de l'Institut Bordet, FNRS-Opération Télévie, Plan Cancer of Belgium, MEDIC Foundation and Fonds Lambeau-Marteaux. LB is a fellow and CS is a research director of the FRS-FNRS.
References
- 1.Weiss SA, Hanniford D, Hernando E, Osman I. Revisiting determinants of prognosis in cutaneous melanoma. Cancer 2015; 121:4108-23; PMID:26308244; http://dx.doi.org/ 10.1002/cncr.29634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reissfelder C, Stamova S, Gossmann C, Braun M, Bonertz A, Walliczek U, Grimm M, Rahbari NN, Koch M, Saadati M et al.. Tumor-specific cytotoxic T lymphocyte activity determines colorectal cancer patient prognosis. J Clin Invest 2015; 125:739-51; PMID:25562322; http://dx.doi.org/ 10.1172/JCI74894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brambilla E, Le Teuff G, Marguet S, Lantuejoul S, Dunant A, Graziano S, Pirker R, Douillard JY, Le Chevalier T, Filipits M et al.. Prognostic effect of tumor lymphocytic infiltration in Resectable Non-small-cell lung cancer. J Clin Oncol 2016; 34:1223-30; PMID:26834066; http://dx.doi.org/ 10.1200/JCO.2015.63.0970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salgado R, Denkert C, Demaria S, Sirtaine N, Klauschen F, Pruneri G, Wienert S, Van den Eynden G, Baehner FL, Penault-Llorca F et al.. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol 2015; 26:259-71; PMID:25214542; http://dx.doi.org/ 10.1093/annonc/mdu450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Savas P, Salgado R, Denkert C, Sotiriou C, Darcy PK, Smyth MJ, Loi S. Clinical relevance of host immunity in breast cancer: from TILs to the clinic. Nat Rev Clin Oncol 2016; 13:228-41; PMID:26667975; http://dx.doi.org/ 10.1038/nrclinonc.2015.215 [DOI] [PubMed] [Google Scholar]
- 6.Gu-Trantien C, Loi S, Garaud S, Equeter C, Libin M, de Wind A, Ravoet M, Le Buanec H, Sibille C, Manfouo-Foutsop G et al.. CD4(+) follicular helper T cell infiltration predicts breast cancer survival. J Clin Invest 2013; 123:2873-92; PMID:23778140; http://dx.doi.org/ 10.1172/JCI67428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Palucka AK, Coussens LM. The basis of oncoimmunology. Cell 2016; 164:1233-47; PMID:26967289; http://dx.doi.org/ 10.1016/j.cell.2016.01.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ali HR, Provenzano E, Dawson SJ, Blows FM, Liu B, Shah M, Earl HM, Poole CJ, Hiller L, Dunn JA et al.. Association between CD8+ T-cell infiltration and breast cancer survival in 12,439 patients. Ann Oncol 2014; 25:1536-43; PMID:24915873; http://dx.doi.org/ 10.1093/annonc/mdu191 [DOI] [PubMed] [Google Scholar]
- 9.Bates GJ, Fox SB, Han C, Leek RD, Garcia JF, Harris AL, Banham AH. Quantification of regulatory T cells enables the identification of high-risk breast cancer patients and those at risk of late relapse. J Clin Oncol 2006; 24:5373-80; PMID:17135638; http://dx.doi.org/ 10.1200/JCO.2006.05.9584 [DOI] [PubMed] [Google Scholar]
- 10.Lindau D, Gielen P, Kroesen M, Wesseling P, Adema GJ. The immunosuppressive tumour network: myeloid-derived suppressor cells, regulatory T cells and natural killer T cells. Immunology 2013; 138:105-15; PMID:23216602; http://dx.doi.org/ 10.1111/imm.12036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dieu-Nosjean MC, Giraldo NA, Kaplon H, Germain C, Fridman WH, Sautes-Fridman C. Tertiary lymphoid structures, drivers of the anti-tumor responses in human cancers. Immunol Rev 2016; 271:260-75; PMID:27088920; http://dx.doi.org/ 10.1111/imr.12405 [DOI] [PubMed] [Google Scholar]
- 12.Jones GW, Jones SA. Ectopic lymphoid follicles: inducible centres for generating antigen-specific immune responses within tissues. Immunology 2016; 147:141-51; PMID:26551738; http://dx.doi.org/ 10.1111/imm.12554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P et al.. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 2006; 313:1960-4; PMID:17008531; http://dx.doi.org/ 10.1126/science.1129139 [DOI] [PubMed] [Google Scholar]
- 14.Dieu-Nosjean MC, Antoine M, Danel C, Heudes D, Wislez M, Poulot V, Rabbe N, Laurans L, Tartour E, de Chaisemartin L et al.. Long-term survival for patients with non-small-cell lung cancer with intratumoral lymphoid structures. J Clin Oncol 2008; 26:4410-7; PMID:18802153; http://dx.doi.org/ 10.1200/JCO.2007.15.0284 [DOI] [PubMed] [Google Scholar]
- 15.Ruffell B, Au A, Rugo HS, Esserman LJ, Hwang ES, Coussens LM. Leukocyte composition of human breast cancer. Proc Natl Acad Sci U S A 2012; 109:2796-801; PMID:21825174; http://dx.doi.org/ 10.1073/pnas.1104303108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leong PP, Mohammad R, Ibrahim N, Ithnin H, Abdullah M, Davis WC, Seow HF. Phenotyping of lymphocytes expressing regulatory and effector markers in infiltrating ductal carcinoma of the breast. Immunol Letters 2006; 102:229-36; PMID:16246429; http://dx.doi.org/ 10.1016/j.imlet.2005.09.006 [DOI] [PubMed] [Google Scholar]
- 17.Pauken KE, Wherry EJ. SnapShot: T cell exhaustion. Cell 2015; 163:1038-e1; PMID:26544946; http://dx.doi.org/22461641 10.1016/j.cell.2015.10.054 [DOI] [PubMed] [Google Scholar]
- 18.Taube JM, Anders RA, Young GD, Xu H, Sharma R, McMiller TL, Chen S, Klein AP, Pardoll DM, Topalian SL et al.. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med 2012; 4:127ra37; PMID:22461641; http://dx.doi.org/ 10.1126/scitranslmed.3003689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Teng MW, Ngiow SF, Ribas A, Smyth MJ. Classifying cancers based on T-cell infiltration and PD-L1. Cancer Res 2015; 75:2139-45; PMID:25977340; http://dx.doi.org/ 10.1158/0008-5472.CAN-15-0255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sharma P, Allison JP. The future of immune checkpoint therapy. Science 2015; 348:56-61; PMID:25838373; http://dx.doi.org/ 10.1126/science.aaa8172 [DOI] [PubMed] [Google Scholar]
- 21.Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S et al.. Nivolumab versus Everolimus in advanced Renal-Cell Carcinoma. N Engl J Med 2015; 373:1803-13; PMID:26406148; http://dx.doi.org/ 10.1056/NEJMoa1510665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nanda R, Chow LQ, Dees EC, Berger R, Gupta S, Geva R, Pusztai L, Pathiraja K, Aktan G, Cheng JD et al.. Pembrolizumab in patients with advanced triple-negative breast cancer: Phase 1b KEYNOTE-012 study. J CLin Oncol 2016; 34(21):2460-7, PMID:27138582; http://dx.doi.org/ 10.1200/JCO.2015.64.8931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu V et al.. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014; 515:568-71; PMID:25428505; http://dx.doi.org/ 10.1038/nature13954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cimino-Mathews A, Thompson E, Taube JM, Ye X, Lu Y, Meeker A, Xu H, Sharma R, Lecksell K, Cornish TC et al.. PD-L1 (B7-H1) expression and the immune tumor microenvironment in primary and metastatic breast carcinomas. Hum Pathol 2016; 47:52-63; PMID:26527522; http://dx.doi.org/ 10.1016/j.humpath.2015.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ali HR, Glont SE, Blows FM, Provenzano E, Dawson SJ, Liu B, Hiller L, Dunn J, Poole CJ, Bowden S et al.. PD-L1 protein expression in breast cancer is rare, enriched in basal-like tumours and associated with infiltrating lymphocytes. Ann Oncol 2015; 26:1488-93; PMID:25897014; http://dx.doi.org/ 10.1093/annonc/mdv518.22 [DOI] [PubMed] [Google Scholar]
- 26.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB et al.. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012; 366:2443-54; PMID:22658127; http://dx.doi.org/ 10.1056/NEJMoa1200690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger SN et al.. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 2014; 515:563-7; PMID:25428504; http://dx.doi.org/ 10.1038/nature14011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bhatia S, Margolin K. Disparate clinical activity of PD-1 blockade in melanoma subtypes: Know thy enemy!. Cancer 2016; 122:3263-6; PMID:27533794; http://dx.doi.org/ 10.1002/cncr.30260 [DOI] [PubMed] [Google Scholar]
- 29.Algazi AP, Tsai KK, Shoushtari AN, Munhoz RR, Eroglu Z, Piulats JM, Ott PA, Johnson DB, Hwang J, Daud AI et al.. Clinical outcomes in metastatic uveal melanoma treated with PD-1 and PD-L1 antibodies. Cancer 2016; 122:3344-53; PMID:27533448; http://dx.doi.org/ 10.1002/cncr.30258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shoushtari AN, Munhoz RR, Kuk D, Ott PA, Johnson DB, Tsai KK, Rapisuwon S, Eroglu Z, Sullivan RJ, Luke JJ et al.. The efficacy of anti-PD-1 agents in acral and mucosal melanoma. Cancer 2016; 122:3354-62; PMID:27533633; http://dx.doi.org/ 10.1002/cncr.30259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cipponi A, Mercier M, Seremet T, Baurain JF, Theate I, van den Oord J, Stas M, Boon T, Coulie PG, van Baren N. Neogenesis of lymphoid structures and antibody responses occur in human melanoma metastases. Cancer Res 2012; 72:3997-4007; PMID:22850419; http://dx.doi.org/ 10.1158/0008-5472.CAN-12-1377 [DOI] [PubMed] [Google Scholar]
- 32.Denkert C, von Minckwitz G, Brase JC, Sinn BV, Gade S, Kronenwett R, Pfitzner BM, Salat C, Loi S, Schmitt WD et al.. Tumor-infiltrating lymphocytes and response to neoadjuvant chemotherapy with or without carboplatin in human epidermal growth factor receptor 2-positive and triple-negative primary breast cancers. J Clin Oncol 2015; 33:983-91; PMID:25534375; http://dx.doi.org/ 10.1200/JCO.2014.58.1967 [DOI] [PubMed] [Google Scholar]
- 33.Loi S, Sirtaine N, Piette F, Salgado R, Viale G, Van Eenoo F, Rouas G, Francis P, Crown JP, Hitre E et al.. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J Clin Oncol 2013; 31:860-7; PMID:23341518; http://dx.doi.org/ 10.1200/JCO.2011.41.0902 [DOI] [PubMed] [Google Scholar]
- 34.Adams S, Gray RJ, Demaria S, Goldstein L, Perez EA, Shulman LN, Martino S, Wang M, Jones VE, Saphner TJ et al.. Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J Clin Oncol 2014; 32(27):2959-66; PMID:25071121; http://dx.doi.org/27748397 10.1200/JCO.2013.55.0491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Galluzzi L, Buque A, Kepp O, Zitvogel L, Kroemer G. Immunogenic cell death in cancer and infectious disease. Nat Rev Immunol 2016; PMID:27748397; http://dx.doi.org/ 10.1038/nri.2016.107 [DOI] [PubMed] [Google Scholar]
- 36.Denkert C, Loibl S, Noske A, Roller M, Muller BM, Komor M, Budczies J, Darb-Esfahani S, Kronenwett R, Hanusch C et al.. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol 2010; 28:105-13; PMID:19917869; http://dx.doi.org/ 10.1200/JCO.2009.23.7370 [DOI] [PubMed] [Google Scholar]
- 37.Ignatiadis M, Singhal SK, Desmedt C, Haibe-Kains B, Criscitiello C, Andre F, Loi S, Piccart M, Michiels S, Sotiriou C. Gene modules and response to neoadjuvant chemotherapy in breast cancer subtypes: a pooled analysis. J Clin Oncol 2012; 30:1996-2004; PMID:22508827; http://dx.doi.org/ 10.1200/JCO.2011.39.5624 [DOI] [PubMed] [Google Scholar]
- 38.Datta J, Fracol M, McMillan MT, Berk E, Xu S, Goodman N, Lewis DA, DeMichele A, Czerniecki BJ. Association of depressed Anti-HER2 T-Helper Type 1 response with recurrence in patients with completely treated her2-positive breast cancer: Role for immune monitoring. JAMA Oncol 2016; 2:242-6; PMID:26719971; http://dx.doi.org/ 10.1001/jamaoncol.2015.5482 [DOI] [PubMed] [Google Scholar]
- 39.Germain C, Gnjatic S, Tamzalit F, Knockaert S, Remark R, Goc J, Lepelley A, Becht E, Katsahian S, Bizouard G et al.. Presence of B cells in tertiary lymphoid structures is associated with a protective immunity in patients with lung cancer. Am J Respir Crit Care Med 2014; 189:832-44; PMID:24484236; http://dx.doi.org/ 10.1164/rccm.201309-1611OC [DOI] [PubMed] [Google Scholar]
- 40.Liu S, Foulkes WD, Leung S, Gao D, Lau S, Kos Z, Nielsen TO. Prognostic significance of FOXP3+ tumor-infiltrating lymphocytes in breast cancer depends on estrogen receptor and human epidermal growth factor receptor-2 expression status and concurrent cytotoxic T-cell infiltration. Breast Cancer Res 2014; 16:432; PMID:25193543; http://dx.doi.org/ 10.1186/s13058-014-0432-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bodogai M, Lee Chang C, Wejksza K, Lai J, Merino M, Wersto RP, Gress RE, Chan AC, Hesdorffer C, Biragyn A. Anti-CD20 antibody promotes cancer escape via enrichment of tumor-evoked regulatory B cells expressing low levels of CD20 and CD137L. Cancer Res 2013; 73:2127-38; PMID:23365136; http://dx.doi.org/ 10.1158/0008-5472.CAN-12-4184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science 2015; 348:69-74; PMID:25838375; http://dx.doi.org/ 10.1126/science.aaa4971 [DOI] [PubMed] [Google Scholar]
- 43.Sautes-Fridman C, Lawand M, Giraldo NA, Kaplon H, Germain C, Fridman WH, Dieu-Nosjean MC. Tertiary lymphoid structures in cancers: Prognostic value, regulation, and manipulation for therapeutic intervention. Front Immunol 2016; 7:407; PMID:27752258; http://dx.doi.org/ 10.3389/fimmu.2016.00407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Joshi Nikhil S, Akama-Garren Elliot H, Lu Y, Lee DY, Chang Gregory P, Li A, DuPage M, Tammela T, Kerper NR, Farago AF et al.. Regulatory T cells in tumor-associated tertiary lymphoid structures suppress anti-tumor T cell responses. Immunity 2015; 43:579-90; PMID:26341400; http://dx.doi.org/ 10.1016/j.immuni.2015.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Figenschau SL, Fismen S, Fenton KA, Fenton C, Mortensen ES. Tertiary lymphoid structures are associated with higher tumor grade in primary operable breast cancer patients. BMC Cancer 2015; 15:101; PMID:25884667; http://dx.doi.org/ 10.1186/s12885-015-1116-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee HJ, Park IA, Song IH, Shin S-J, Kim JY, Yu JH, Gong G. Tertiary lymphoid structures: prognostic significance and relationship with tumour-infiltrating lymphocytes in triple-negative breast cancer. J Clin Pathol 2016; 69(5):422-30; PMID:26475777; http://dx.doi.org/ 10.1136/jclinpath-2015-203089 [DOI] [PubMed] [Google Scholar]
- 47.Lee HJ, Kim JY, Park IA, Song IH, Yu JH, Ahn J-H et al.. Prognostic significance of tumor-infiltrating lymphocytes and the tertiary lymphoid structures in HER2-positive breast cancer treated with Adjuvant Trastuzumab. Am J Clin Pathol 2015; 144:278-88; PMID:26185313; http://dx.doi.org/ 10.1309/AJCPIXUYDVZ0RZ3G [DOI] [PubMed] [Google Scholar]
- 48.Silina K, Rulle U, Kalnina Z, Line A. Manipulation of tumour-infiltrating B cells and tertiary lymphoid structures: a novel anti-cancer treatment avenue? Cancer Immunol Immunother 2014; 63:643-62; PMID:24695950; http://dx.doi.org/ 10.1007/s00262-014-1544-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smyth MJ, Ngiow SF, Ribas A, Teng MW. Combination cancer immunotherapies tailored to the tumour microenvironment. Nat Rev Clin Oncol 2016; 13:143-58; PMID:26598942; http://dx.doi.org/ 10.1038/nrclinonc.2015.209 [DOI] [PubMed] [Google Scholar]
- 50.Callahan MK, Postow MA, Wolchok JD. Targeting T cell co-receptors for cancer therapy. Immunity 2016; 44:1069-78; PMID:27192570; http://dx.doi.org/ 10.1016/j.immuni.2016.04.023 [DOI] [PubMed] [Google Scholar]
- 51.Muenst S, Schaerli AR, Gao F, Daster S, Trella E, Droeser RA, Muraro MG, Zajac P, Zanetti R, Gillanders WE et al.. Expression of programmed death ligand 1 (PD-L1) is associated with poor prognosis in human breast cancer. Breast Cancer Res Treat 2014; 146:15-24; PMID:24842267; http://dx.doi.org/ 10.1007/s10549-014-2988-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Baptista MZ, Sarian LO, Derchain SF, Pinto GA, Vassallo J. Prognostic significance of PD-L1 and PD-L2 in breast cancer. Hum Pathol 2016; 47:78-84; PMID:26541326; http://dx.doi.org/ 10.1016/j.humpath.2015.09.006 [DOI] [PubMed] [Google Scholar]
- 53.Wimberly H, Brown JR, Schalper K, Haack H, Silver MR, Nixon C, Bossuyt V, Pusztai L, Lannin DR, Rimm DL. PD-L1 expression correlates with Tumor-Infiltrating Lymphocytes and response to neoadjuvant chemotherapy in breast cancer. Cancer Immunol Res 2015; 3:326-32; PMID:25527356; http://dx.doi.org/ 10.1158/2326-6066.CIR-14-0133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schalper KA, Velcheti V, Carvajal D, Wimberly H, Brown J, Pusztai L, Rimm DL. In situ tumor PD-L1 mRNA expression is associated with increased TILs and better outcome in breast carcinomas. Clin Cancer Res 2014; 20:2773-82; PMID:24647569; http://dx.doi.org/ 10.1158/1078-0432.CCR-13-2702 [DOI] [PubMed] [Google Scholar]
- 55.Webb JR, Milne K, Kroeger DR, Nelson BH. PD-L1 expression is associated with tumor-infiltrating T cells and favorable prognosis in high-grade serous ovarian cancer. Gynecol Oncol 2016; 141:293-302; PMID:26972336; http://dx.doi.org/ 10.1016/j.ygyno.2016.03.008 [DOI] [PubMed] [Google Scholar]
- 56.Zou W, Wolchok JD, Chen L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: Mechanisms, response biomarkers, and combinations. Sci Transl Med 2016; 8:328rv4; PMID:26936508; http://dx.doi.org/ 10.1126/scitranslmed.aad7118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Garaud S, Gu-Trantien C, Lodewyckx JN, Boisson A, De Silva P, Buisseret L, Migliori E, Libin M, Naveaux C, Duvillier H et al.. A simple and rapid protocol to non-enzymatically dissociate fresh human tissues for the analysis of infiltrating lymphocytes. J Vis Exp 2014; PMID:25548995; http://dx.doi.org/ 10.3791/52392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Adams H, Liebisch P, Schmid P, Dirnhofer S, Tzankov A. Diagnostic utility of the B-cell lineage markers CD20, CD79a, PAX5, and CD19 in paraffin-embedded tissues from lymphoid neoplasms. Appl Immunohistochem Mol Morphol 2009; 17:96-101; PMID:18838917; http://dx.doi.org/ 10.1097/PAI.0b013e3181845ef4 [DOI] [PubMed] [Google Scholar]
- 59.Bigelow E, Bever KM, Xu H, Yager A, Wu A, Taube J, Chen L, Jaffee EM, Anders RA, Zheng L. Immunohistochemical staining of B7-H1 (PD-L1) on paraffin-embedded slides of pancreatic adenocarcinoma tissue. J Vis Exp 2013; PMID:23328703; http://dx.doi.org/ 10.3791/4059 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.