ABSTRACT
The intestinal microbiota plays a key role in the pathogenesis of acute graft-versus-host disease (aGVHD). High-dose conditioning regimens given prior to allogeneic hematopoietic stem cell transplantation (aHSCT) modulate the composition of gut microbiota and damage the gut epithelial barrier, resulting in increased systemic inflammation. We assessed whether gut decontamination with antibiotics (ATB) prior to aHSCT influenced the frequency of aGVHD and mortality in 500 patients from two Canadian centers between 2005 and 2012. The rate of grade II–IV aGVHD was higher in the ATB arm compared with the arm without ATB (42% vs 28%; p < 0.001). This difference was mainly driven by a 2-fold higher rate of grade II–IV gastrointestinal aGVHD (GI-GVHD) in the ATB arm compared with the arm without ATB (20.7% vs 10.8%; p = 0.003). Multivariate analyses adjusted for known aGVHD risk factors revealed that more patients in the ATB group developed clinically significant GI-GVHD and liver aGVHD; adjusted odds ratio (aOR) = 1.83; p = 0.023 and aOR = 3.56; p = 0.047, respectively. Importantly, median overall survival (OS) was significantly lower in the group receiving ATB and the OS at 10 y remained decreased in the ATB group; adjusted hazard ratio (aHR) = 1.61 (p < 0.001).
Without undermining the role of ATB prophylaxis to prevent infection in aHSCT, we have shown that the use of ATB that targets intestinal bacteria is associated with a more severe aGVHD that involves the GI organs and impacts OS. Prospective studies that evaluate the contribution of bacterial decontamination to aGVHD are warranted.
KEYWORDS: Acute graft-versus-host disease, allogeneic hematopoietic stem cell transplantation, antibiotics, gut decontamination, microbiota
Abbreviations
- aGVHD
acute graft-versus-host disease
- aHR
adjusted hazard ratio
- aHSCT
allogeneic hematopoietic stem cell transplantation
- aOR
adjusted odds ratio
- ATB
antibiotics
- DC
dendritic cells
- GI-GVHD
gastrointestinal GVHD
- IEC
intestinal epithelial cells
- LPS
lipopolysaccharide
- MAMPs
microbe-associated molecular patterns
- OS
overall survival
- PAMPs
pathogen-associated molecular patterns
- PRR
pathogen recognition receptors
- ST2
suppression of tumorigenicity 2
- SCTAs
short-chain fatty acids
Introduction
Microbes present in the human gastrointestinal (GI) tract are 1.3 times more than thee human cells in the body.1 The complex interactions between theses microbes, known as the intestinal microbiota, and the host contribute to immune system homeostasis; this relationship is the focus of a growing number of cancer therapy research initiatives.1-3 In allogeneic hematopoietic stem cell transplantation (aHSCT), acute graft-versus-host disease (aGVHD) remains the principal hurdle for favorable patient outcome. In early the stages of aGVHD, the disruption of the GI barrier caused by the conditioning regimen results in the leakage of bacterial lipopolysaccharide (LPS) and other microbe/pathogen-associated molecular patterns (MAMPS and PAMPs) into the systemic circulation.4 This translocation triggers the secretion of proinflammatory cytokines such as tumor necrosis factor (TNF) and interleukins 1 and 6 (IL1 and IL6). The donor T cells recruited into host organs by these cytokines are responsible for the aGVD complications.5,6
This intertwined host–microbiota relationship has pushed the scientific community to identify strategies to control the influence of bacteria on aGVHD. Pioneering experiments in which germ-free mice housed in sterile conditions or mice treated with antibiotics developed less severe aGVHD following aHSCT identified the troll of the microbiota as an independent contributor to the pathogenesis of aGVHD.7 Similarly, the incidence of aGVHD was reduced in monkeys that had undergone bacterial decontamination prior to aHSCT.8 However, the concept that gut decontamination prevents aGVHD following aHSCT in human is controversial given that clinical trials have failed to demonstrate consistent benefits.9,10
The gut microbiota may also contribute to the therapeutic response to chemotherapy by mechanisms, which involve bacterial translocation, inflammation, and adaptive immunity.11,12 Therapy with cyclophosphamide disrupts the gut epithelial barrier to allow bacterial translocation into the blood of gram-positive bacteria such as Enteroccocus hirae. This promotes the upregulation of pathogenic T-helper 17 cells and modulates antitumoral activity.11,13 Furthermore, cyclophosphamide induces gut dysbiosis and inverses the Firmicutes to Bacteroides ratio, thus, promoting a new microbiota signature which might influence the immune response.14
In addition, changes of the microbiota may influence the outcome of aHSCT by other mechanisms. Recent studies found that low bacterial diversity had significant impact on transplant-related mortality. This increased mortality appeared to be related to an increased risk of infections and of more severe aGVHD.15,16 Furthermore, in aHSCT, delayed lymphopenia is considered to be one of the key factors which predict the incidence of aGVHD.17,18 However, it remains unknown if lymphocyte recovery is influenced by the changes in the composition of the microbiota that are caused by prophylactic ATB treatment.
Given the fragile balance between the composition of the microbiota and the health of the host, the question of whether it is beneficial to manipulate the gut microbiota with prophylactic ATB is a conundrum. Although some experts from transplant centers continue to reduce bacterial colonization prior to aHSCT, others have stopped doing so. In order to gain insight into this issue, we conducted a retrospective study of patients who had undergone aHSCT in two transplant centers in the province of Quebec (Canada); these patients differed with respect to whether they had received ATB to decontaminate the gut before undergoing aHSCT. We assessed the effect of ATB prophylaxis prior to allogeneic stem cell infusion on the frequency and severity of aGVHD and its impact on overall survival (OS).
Results
A total of 602 patient charts were reviewed. A total of 58 patients were excluded as a result of missing data and 44 met at least one of the exclusion criteria. In total, 500 patients were included in the study (HMR = 376 and CHUQ = 124), with n = 239 in the ATB and n = 261 in the no ATB group (Table 1).
Table 1.
Demographic characteristics of the allogeneic hematopoietic stem cell transplant recipients.
| Antibiotic therapy |
|||
|---|---|---|---|
| No, n (%) | Yes, n (%) | p-value | |
| Cohort/transplant center | |||
| HMR | 195 (74.7) | 181 (75.7) | 0.795 |
| CHUQ | 66 (25.3) | 58 (24.3) | |
| Gender | |||
| Female | 105 (40.2) | 94 (39.3) | 0.839 |
| Male | 156 (59.8) | 145 (60.7) | |
| Age group | |||
| <50 | 119 (45.6) | 121 (50.6) | 0.263 |
| >50 | 142 (54.4) | 118 (49.4) | |
| Stem cell source | |||
| Blood | 230 (88.1) | 187 (78.2) | 0.015* |
| Bone marrow | 26 (10.0) | 41 (17.2) | |
| Cord | 5 (1.9) | 11 (4.6) | |
| Donor | |||
| Related | 186 (71.3) | 149 (62.3) | 0.003** |
| Unrelated | 75 (28.7) | 90 (37.7) | |
| Intensity | |||
| Non-myeloablative | 160 (61.3) | 78 (32.6) | <0.001*** |
| Myeloablative | 101 (38.7) | 161 (67.4) | |
| Regimen | |||
| Fludarabine–cyclophosphamide | 104 (40.0) | 37 (15.5) | <0.001*** |
| Fludarabine–melphalan | 10 (3.8) | 13 (5.4) | |
| Fludarabine–busulfan(2) | 22 (8.5) | 26 (10.9) | |
| Fludarabine–busulfan(3) | 8 (3.1) | 0 (0.0) | |
| Fludarabine–busulfan(4) | 8 (3.1) | 7 (2.9) | |
| Busulfan–cyclophosphamide | 60 (23.1) | 77 (32.2) | |
| Cyclophosphamide–total body irradiation | 31 (11.9) | 49 (20.5) | |
| Cyclophosphamide–cytarabine–topotecan–busulfan | 6 (2.3) | 11 (4.6) | |
| Others | 11 (4.2) | 19 (8.0) | |
| Antibiotics | |||
| Ciprofloxacin | x | 157 (65.7) | |
| Trimethoprim–sulfamethoxazole | x | 29 (12.1) | |
| Levofloxacin | x | 18 (7.5) | |
| Moxifloxacin | x | 17 (7.1) | |
| Carbapenem | x | 16 (6.7) | |
| Vancomycin | x | 2 (0.8) | |
p < 0.05.
p < 0.01.
p < 0.001.
Both groups were equally distributed for age and sex. Median age was 51.6 y (IQR: 18–69) in the ATB arm and 49.9 y (IQR 16–70) in the no ATB arm (p = 0.263). Stem cell source differed in both populations, with the ATB group receiving more bone marrow and cord cells than the no ATB group who most frequently collected from donor peripheral blood (88.1%; p = 0.015). Conditioning regimens intensity was also different between the two groups; the ATB treated patients received significantly more myeloablative transplant compared with the group without ATB (67.4% vs 38.7%, respectively; p < 0.001). With respect to donor selection, a lower proportion of patients in the ATB group had a HLA-matched related donor (62.3% vs 71.3%; p = 0.04). Accordingly to antibioprophylaxis guidelines, the ATB group 72.8% received either ciprofloxacin or moxifloxacin. The remaining patients on ATB were prescribed trimethoprim–sulfamethoxazole, carbapenem, levofloxacin, and vancomycin (12.1%, 6.7%, 7.5%, and 0.8%, respectively).
Contribution of prophylactic antibiotics on the frequency of aGVHD
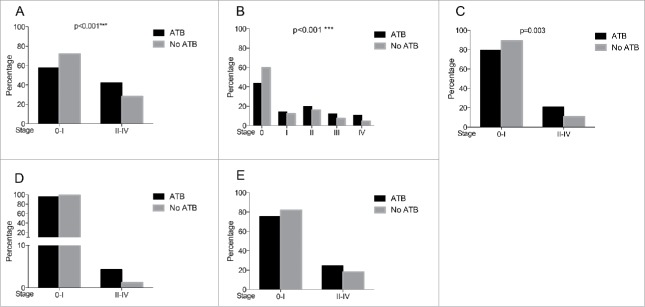
Proportion of patients who developed clinically significant aGVHD (stage II–IV) in ATB group was higher compared with the no ATB group (42.3% vs 28%; p < 0.001; Fig. 1). Using an ordinal representation for aGVHD stages, the rate of more severe aGVHD from stage O to stage IV was significantly higher in the ATB group. The odds of developing any aGVHD stage increased (OR = 1.96 [1.41;2.74]; p < 0.001) in patients receiving ATB compared with the no ATB group. This effect of higher frequency of aGVHD in the ATB group remained significant after accounting for clinical parameters (aOR = 1.56 [1.09;2.25]; p = 0.015; Table S1).
Figure 1.
Incidence of aGVHD and sub-types in patients in the ATB and no ATB groups. (A) Percentage of patients that developed Stage 0–I vs II–IV aGVHD in the ATB and no ATB groups. (B) Percentage of patients that developped each stage of aGVHD in both groups. (C–E) Percentage of aGVHD sub-types in patients receiving or no ATB for gastrointestinal-aGVHD, liver-aGVHD, and skin-aGVHD, respectively. Raw p values are shown based on comparaison of aGVHD incidence using a ordinal regression based on the aGVHD stage. aGVHD: acute graft-versus-host disease, ATB: antibiotics; *p < 0.05, **p < 0.01,***p < 0.001, ns = not significant.
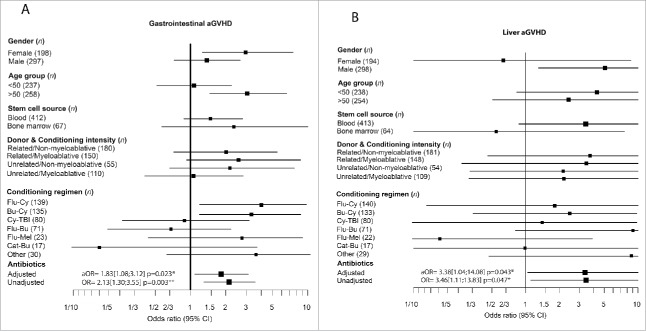
In both GI organs (gastrointestinal tract and liver) more patients in the ATB group experienced more grade II–IV aGVHD. In the ATB arm, incidence of GI-GVHD was twice as high as it was in the no ATB group (20.7% vs 10.8%; p = 0.003). Severe hepatic aGVHD was documented in 4.3% in the ATB group vs 1.2% in the counterpart and was represented by a strong trend (p = 0.055; Fig. 1). The odds of observing GI-GVHD of grade II or more were found to be almost two times (2.13 [1.30;3.55]; p = 0.003; aOR = 1.83 [1.08;3.12]; p = 0.023) in the ATB-treated group (Fig. 2A and Table S2). Similarly, a positive association between ATB and liver GVHD was observed (OR = 3.46 [1.11;13.83]; p = 0.047; aOR = 3.38 [1.04;14.04]; p = 0.043; Fig. 2B and Table S3). For skin aGVHD, the frequency was similar in both groups (data not shown). In the ATB group there was no aGVHD difference when looking at each classification of ATB or ATB prescribed either as prophylaxis or for treatment. These findings indicate that patients receiving ATB developed more severe grade II–IV aGVHD mostly involving GI organs than those without ATB.
Figure 2.
Forest plots demontrating the impact of antibiotics in aGVHD after multivariable analysis. (A) Association of incidence of GI-GVHD with demographic and clinical parameters. Measurement of the odds ratio of patients developing GI-aGVHD comparing patients that received ATB or not ATB. After adjusting for the clinical parameters, patients on ATB develop more severe GI-aGVHD compared with the no ATB group. (B) Forest plot for liver-aGVHD, aOR was higher in the ATB group and they experienced more liver-aGVHD. Odds ratios are presented adjusting for all relevant clinical parameters, than the one tested. Confidence intervals is censored at 1/10 and 10. Raw p values are provided. aGVHD: acute graft-versus-host disease, GI: gastrointestinal, ATB: antibiotics, CI: confidence interbal, OR:odds ratio, aOR: adjusted odds ratio, Flu-CY: fludarabine–cyclophosphamide, Bu-Cy: busulfan–cyclophosphamide, Cy-TBI: cyclophosphamide–total body irradiation, Flu-Bu: fludarabine–busulfan, Flu-Mel: fludarabine– melphalan, Cat-Bu: cyclophosphamide–cytarabine–topotecan–busulfan; *p < 0.05, **p < 0.01,***p < 0.001, ns = not significant.
Influence of prophylactic antibiotics on transplant survival outcome
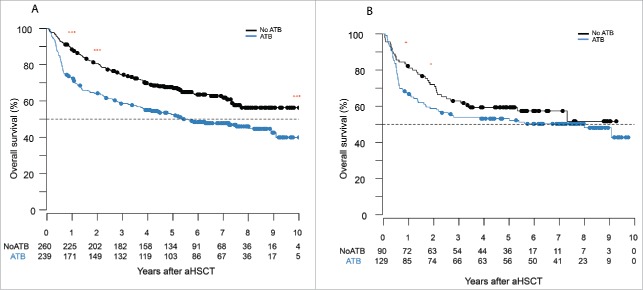
Median survival was estimated to be 5.4 y [3.8;9.1] from the date of stem cell infusion in the cohort that received ATB and was not reached in the no ATB arm. Furthermore, the survival rate at 1 y and 2 y were estimated to be 72.4% [66.9;78.3] and 64.2% [58.4;70.6] in the ATB prophylaxis group and 88.1% [84.2;92.1] and 80.6% [75.9;85.6] for its counterpart (p < 0.001; Figs. 3 and S4) Patients that received ATB prophylaxis presented with a reduced survival compared with the no ATB group (HR = 1.61 [1.23;2.10]; p< 0.001) regardless of the clinical characteristics (aHR = 1.35 [1.02;1.79]; p = 0.034; Fig. 4).
Figure 3.
Antibiotics given to patients pre-aHSCT worsen overall survival. (A) Survival analysis by Kaplan–Meier curves of all aHSCT patients either in the ATB or no ATB group. Day 0 was the day of stem cell infusion. The OS was reduced in patient receiving ATB before their transplant at 1 y, 2 y, and 10 y. (B) Survival analysis by Kaplan–Meier cruves of patients that undergone a myeloablative aHSCT and either received or not ATB before the transplant. OS was reduced in the ATB group at 1 y and 2 y. Log-rank (Mantel–Cox); *p < 0.05, **p < 0.01,***p < 0.001, ns = not significant.
Figure 4.
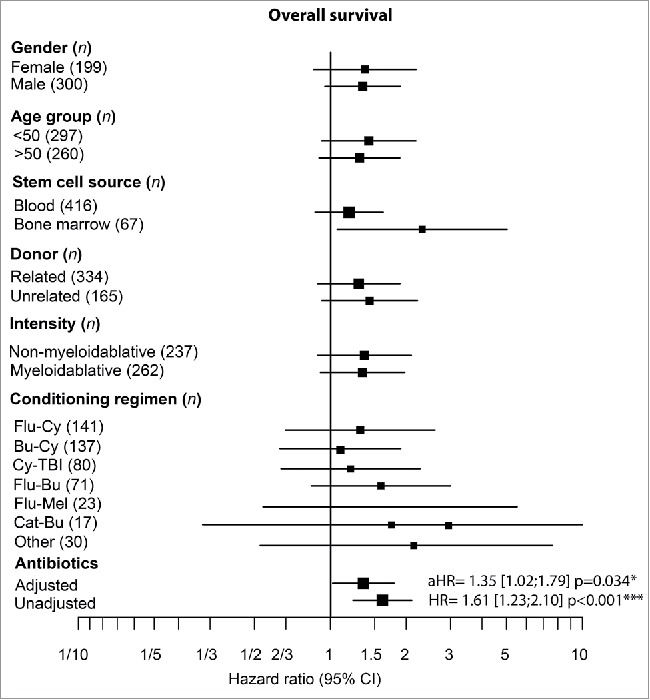
Decrease overall survival in patients on ATB after multvariable analysis. (A) Forest plot analysis on overall survival assessing the role of each clinical parameters and the influence of ATB on HR and aHR. ATB pre-aHSCT lead to a higher aHR compared with the no ATB group. For each comparison, HR were adjusted for all other clinical parameters, confidence intervals were censored at 1/3 and 5. aGVHD: acute graft-versus-host disease, ATB: antibiotics, CI: confidence interbal, HR: hazard ratio, aHR: adjusted hazard ratio, Flu-CY: fludarabine–cyclophosphamide, Bu-Cy: busulfan–cyclophosphamide, Cy-TBI: cyclophosphamide–total body irradiation, Flu-Bu: fludarabine–busulfan, Flu-Mel: fludarabine–melphalan, Cat-Bu: cyclophosphamide–cytarabine–topotecan–busulfan; *p < 0.05, **p < 0.01,***p < 0.001, ns = not significant.
Influence of myeloablative conditioning regimens on survival outcome
Given the relationship between conditioning intensity and GVHD risk, we determined the impact of ATB on the rate of aGVHD and OS in the subgroup of patients receiving only myeloablative regimens. A total of 210 patients received myeloablative-conditioning regimens, with the following treatments: cyclophosphamide + total body irradiation in ATB arm n = 31 vs no ATB n = 49, busulfan + cyclophosphamide ATB n = 77 and no ATB n = 60, fludarabine + busulfan ATB n = 7 and no ATB n = 8. There was no difference in rate of aGVHD in both groups but the 1-y and 2- y OS rates were lower in patients that received ATB (82% vs 69%; p = 0.019 and 76% vs 59%; p = 0.046; Fig. 3). However, when taking the entire follow-up period in the myeloablative subgroup into account, patients in the ATB arm had a numerically higher overall mortality than those in the no ATB group (aHR = 1.49 [0.96;2.29]; p = 0.074) after adjusting for confounding factors (Fig. S1).
Leukocytes recovery rate at day +14 and correlation with antibiotics
Taking into consideration the entire cohort there was a significant faster lymphocyte rate recovery at day +14 in the no ATB group compared with the ATB group ( [0–7.3] and 0.2 [0–5.4], respectively; p = 0.015). A similar difference was observed when taking into account the neutrophil count at day +14. The mean absolute number was 0.6 [0–9.0] in no ATB and 0.3 [0–11.9] the ATB group (p < 0.001; Fig. S2).
Nevertheless, when adjusting for important clinical parameters that strongly influence the engraftment such as conditioning intensity and source of the stem cells, there was no evidence of difference in the no ATB and ATB groups in both neutrophils and lymphocytes at day +14.
Discussion
aGVHD is an immune disorder affecting organs colonized with microorganisms and its related mortality remains the most challenging issue to the wider application of aHSCT.
In this retrospective study, we report that the addition of prophylactic ATB in a large cohort of patients receiving aHSCT after both myeloabaltive and non-myeloablative conditioning regimens significantly increases the rate of clinically significant aGVHD. Our data reveal that severe GI-GVHD is significantly increased in patients receiving ATB compared with those who did not. A similar trend is also observed for liver-GVHD but not for skin GVHD. Furthermore, multivariate analyses in myeloablative and non-myeloablative regimens demonstrated that the positive association between prophylactic ATB and aGVHD represents an independent risk factor after adjusting for the known predictors of aGVHD. Similar analysis but comparing benign aGVHD (grade 0–1) vs deleterious one (grade II–IV) also demonstrated that ATB independently increase the rate of GI-GVHD and liver-GVHD. We also examined the influence of ATB on survival outcome. We determined that the 1-y and 2-y OS for patients who received prophylactic ATB was inferior to those not receiving ATB including in the subgoup of patients receiving myeloablative regimens. Lastly, the OS was lower in the patients receiving ATB.
This study has several limitations. First, this is a retrospective study examining aGVHD in two distinct centers. Although these centers follow national and international guidelines for transplant indications and both are Foundation for the Accreditation of Cellular Therapy (FACT) accredited, we encountered significant differences in patient characteristics. To overcome this limitation, we conducted multivariate analyses and forest plots to adjust for key components involved in aGVHD pathogenesis. As residual-confounding factors may still persist, a randomized clinical trial comparing ATB and no ATB in patients receiving aHSCT would be invaluable. Second, the analysis did not take into account antibiotics and/or antifungal medications to treat febrile neutropenia and infection following stem cell infusion or immunosuppressive agents given for GVHD prophylaxis and treatment.
Prophylactic ATB administered with conditioning regimens have a broad-spectrum activity in order to reduce the risk of gram-negative bacteremia. By targeting multiple bacterial species to prevent infections antibiotics also decrease microbiota diversity and biomass, which take several weeks to recover.19-21
Over the last decade, the role of microbiota on aGVHD development has gained more recognition owing to the development of culture-independent DNA sequencing techniques, which have emerged to play a key role in the accurate identification of multiple bacterial species.22 Alteration of microbiota composition and epithelial barrier dysfunction promote switch in MAMPs recognition by pathogen recognition receptors (PRR) on intestinal epithelial cells (IEC) and dendritic cells (DC).23 Such imbalance between microbes and the host can lead to the production of proinflammatory cytokines by macrophages and activated T cells contributing to the immune damage observed in GI-GVHD. In addition to the epithelial damage owing to conditioning regimens, the microbiota composition and its diversity is altered by (i) antibiotics prescribed for infection prophylaxis and during episodes of febrile neutropenia, (ii) fasting due to painful mucositis, and (iii) parenteral nutrition.
Taur et al. have shown in a prospective clinical trial enrolling 80 patients that low bacterial diversity driven by the use of antibiotics translates into higher probability of transplants related death.15 Subsequently, the same group demonstrated the impact of recipient intestinal flora with high diversity and the abundance of the genus of Blautia as two independent protecting factors for aGVHD.16
More recently, Soho et al. reported in a single-center retrospective study, an increased GVHD related mortality associated to broad spectrum ATB used following aHSCT in adults. Feces composition of patients treated post-aHSCT with piperacillin–tazobactam was associated with a greater loss of diversity and most notably loss of Bacteroidetes and Lactobacillus. These results were further confirmed in a rodent model treated with different ATB.24
When analyzing the entire cohort, we showed a faster lymphocytes and neutrophils recovery at day +14 in the no ATB group. However, this correlation was not maintained after correction for other clinical factors that are known to influence engraftment. As a result, we cannot conclude that the difference in aGVHD rate in both groups is due to a difference in leukocyte count at day +14 as a direct association with ATB prescription.
We speculate that the findings of this study favor a model of depletion of bacteria through the prophylactic use of ATB prior to stem cell infusion, for the modification of MAMP.25
Furthermore, as each bacterium produces important metabolites such as butyrate and kynurenine, a bacterial shift caused by antibiotics modifies gut metabolomic profile. Enrofloxacin, another fluroquinolone, dramatically alters the metabolomic profile of short-chain fatty acids (SCFAs) in mice and represents an important modulator of the immune system.26,27
Numerous studies have identified several risk factors for aGVHD such as source of the stem cell graft, HLA mismatch, and intensity of the conditioning regimen.28-30
According to recent evidence, the single best biomarker to predict GVHD is soluble suppression of tumorigenicity 2 (ST2) secreted by apoptotic or inflamed epithelial cells as a consequence of chemotherapy directly leading to gut toxicity.31 The “alarmin” IL-33/ST2 axis has recently been delineated to play a key role in the loss of epithelial integrity caused by microbial translocation of the mucosal barrier in mice and humans.32-35 Despite this relevant axis, an impact of the microbiota remains a less well established and controversial risk factor for aGVHD pathogenesis.36,37
In conclusion, this study highlights the potentially detrimental role of ATB in aGVHD severity and in OS. Future prospective studies should be conducted to fully address the issue of antibioprophylaxis for gut decontamination. To better understand the influence of ATB on aGVHD, these studies should analyze patient feces using shotgun sequencing metagenomic approaches. The hematopoietic cell transplantation-comorbidity index, which assesses 17 categories of organ dysfunction, remains the best predictive score stratifying transplant risk. Based on the results of our study, it appears that the microbiota dysbiosis induced by ATB is another risk factor that influences transplant outcome. We recommend, therefore, that the use of antibioprophylaxis at the time of conditioning should also be included as a contributing factor in the hematopoietic cell transplantation-comorbidity index.
Methods
Patients
We retrospectively identified 602 adult patients who underwent a single HLA compatible myeloablative or non-myeloabaltive aHSCT for hematological malignancy between January 2005 and December 2012 in two academic centers in the province of Quebec (Canada): (1) Hôpital Maisonneuve Rosemont (HMR), Montreal, Canada; and (2) Centre Universitaire de Québec (CHUQ), Québec, Canada. Syngeneic and T-cell depleted haploidentical aHSCT were excluded from this study to prevent further confounding effect of these factors on the incidence of aGVHD. Data were extracted from hospital patient records and pharmacy databases. Each university hospital had implemented its own pretransplant antibioprophylaxis guideline. At HMR, ciprofloxacin 500 mg twice daily or moxifloxacine 400 mg once daily were started at initiation of myeloablative and reduced-intensity conditioning regimen for gut decontamination, and were omitted in patients with fluoroquinolone or penicillin allergy and during outbreak nosocomial infection outbreaks such as Clostridium difficile and at time of non-myeloablative transplant. At CHUQ, gut decontamination was not incorporated in the conditioning regimens regardless of the intensity. However, patients who initiated ATB at any time from the beginning of the conditioning regimen up to 24 h before stem cell infusion for suspected infections were included in the ATB arm, as well as patients on trimethoprim–sulfamethoxazole prophylaxis. Calcineurin based inhibitor for GVHD prophylaxis was used in all patients starting at day −1 and targeting therapeutic level (cyclosporine (CsA) 250–500 ng/mL and tacrolimus 10–15 ng/mL). Patients undergoing myeloablative aHSCT received a standard combination of CsA 2.25 mg/kg q 12 h starting at day 1 and methotrexate 15 mg/m2 IV day +1, and 10 mg/m2 at days +3, +6, and +11 in related transplant and of tacrolimus (0.0225 mg/kg IV daily starting day −1) and methotrexate at the same dose in unrelated donor transplants.
Patients undergoing non-myeloablative received a combination of CsA or tacrolimus with mofetyl mycophenolate 15 mg/kg q 12 h in related and q 8 h in unrelated donor aHSCT. There was no in vivo or ex vivo T-cell depletion.
Patient supportive care in both centers was protectively isolated in reverse isolation rooms equipped with high efficiency particulate air filtration systems.
Data collection and outcomes
Data extraction included: demographics, indication of aHCT, type of aHCT, source of graft, conditioning regimen, and last follow up visit or date of death for all patients. The primary endpoint of this study was the development of a severe clinical aGVHD grade >II and the organ involvement recorded by the treating physician. aGVHD was diagnosed clinically and confirmed pathologically whenever possible and was classified based on the Glucksberg classification. Intent to treat analysis was used and antibiotics prescribed after the day of stem cell infusion (day 0) were not included in the ATB group. Secondary end point was mortality at 1 y, OS and time to the last follow-up that were recorded on hospital chart. The data for the day +14 leukocyte count in absolute number were also collected from the medical record.
The research and ethical boards of both institutions approved this study.
Statistical analyses
Patient characteristics were summarized using median (IQR) and contingency (percentage) tables for continuous and categorical variables respectively. Inferential analyses were conducted with Mann–Whitney U tests, Chi square, and Fisher exact when appropriate.
Incidences in the GVHD grades were compared by ordinal (aGVHD) or logistic regression for outcomes that did not meet proportional assumptions.
Sensitivity analysis was performed on clinical parameters (age, sex, graft origin: bone marrow vs peripheral stem cells, donor type/match, and conditioning regimens) and no further model of reduction was attempted. Of note, to decrease patient characteristics variables reduced intensity and non-myeloabaltive transplantation were pooled together.
Forest plots for GI-GVHD, liver-GVHD, and OS were depicted using odds ratio or hazard ratio, which were calculated adjusting for all other clinical parameters than the one tested and confidence intervals were censored at 1/10 and 10 and 1/3 and 5, respectively.
Survival time was defined as being from the time of graft infusion to either the date of the last follow up or the date of death. Survival curves of both ATB and no ATB arms were estimated by the Kaplan–Meier product-limit method and their distributions were compared using Cox regression with stratification by clinical centers (HMR/CHQ).
All statistical analyses were performed using the statistical environment R 65.
Supplementary Material
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We are grateful to the transplant program clinicians, nurses, and pharmacists at HMR and CHU de Québec. We would like to thank Dr. Geneviève Gallagher from CHU de Québec for providing access to the CHU de Québec transplant data and Dr. Marco Spazianno from McGill University for his constructive input regarding the analyses.
Funding
BR is supported by Gustave Roussy Philanthropia foundation and a McGill University Townsend hematology research fellowship award.
Author contributions
BR and SL designed the research study, analyzed the data and wrote and edited the manuscript. BR, CL, MCP, and KG collected the data. P-L W and DG edited the paper. DE and VM performed the statistical analyses and edited the manuscript. NCS and KG provided the pharmacology data.
References
- 1.Sender R, Fuchs S, Milo R. Are we really vastly outnumbered? Revisiting the ratio of bacterial to host cells in humans. Cell 2016; 164:337-40; PMID:26824647; http://dx.doi.org/ 10.1016/j.cell.2016.01.013 [DOI] [PubMed] [Google Scholar]
- 2.Garrett WS. Cancer and the microbiota. Science 2015; 348:80-6; PMID:25838377; http://dx.doi.org/ 10.1126/science.aaa4972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zitvogel L, Ayyoub M, Routy B, Kroemer G. Microbiome and anticancer immunosurveillance. Cell 2016; 165:276-87; PMID:27058662; http://dx.doi.org/11413166 10.1016/j.cell.2016.03.001 [DOI] [PubMed] [Google Scholar]
- 4.Cooke KR, Gerbitz A, Crawford JM, Teshima T, Hill GR, Tesolin A, Rossignol DP, Ferrara JL. LPS antagonism reduces graft-versus-host disease and preserves graft-versus-leukemia activity after experimental bone marrow transplantation. J Clin Invest 2001; 107:1581-9 PMID:11413166; http://dx.doi.org/ 10.1172/JCI12156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zeiser R, Socie G, Blazar BR. Pathogenesis of acute graft-versus-host disease: from intestinal microbiota alterations to donor T cell activation. Br J Haematol 2016; 175:191-207; PMID:27619472; http://dx.doi.org/ 10.1111/bjh.14295 [DOI] [PubMed] [Google Scholar]
- 6.Chen Y, Zhao Y, Cheng Q, Wu D, Liu H. The role of intestinal microbiota in acute graft-versus-host disease. J Immunol Res 2015; 2015:145859; PMID:26090477; http://dx.doi.org/ 10.1155/2015/145859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Bekkum DW, Knaan S. Role of bacterial microflora in development of intestinal lesions from graft-versus-host reaction. J Natl Cancer Inst 1977; 58:787-90; PMID:14265 [DOI] [PubMed] [Google Scholar]
- 8.Wagemaker G, Heidt PJ, Merchav S, van Bekkum DW. Abrogation of histocompatibility barriers to bone marrow transplantation in rhesus monkeys In: Baum SD, Ledney GD, Thierfelder S, eds. Experimental Hematology Today. Basel, Switzerland: Karger, 1982 [Google Scholar]
- 9.Whangbo J, Ritz J, Bhatt A. Antibiotic-mediated modification of the intestinal microbiome in allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant 2016; PMID:27526283; http://dx.doi.org/ 10.1038/bmt.2016.206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kimura S, Akahoshi Y, Nakano H, Ugai T, Wada H, Yamasaki R, Ishihara Y, Kawamura K, Sakamoto K, Ashizawa M et al.. Antibiotic prophylaxis in hematopoietic stem cell transplantation. A meta-analysis of randomized controlled trials. J Infect 2014; 69:13-25; PMID:24583063; http://dx.doi.org/24264990 10.1016/j.jinf.2014.02.013 [DOI] [PubMed] [Google Scholar]
- 11.Viaud S, Saccheri F, Mignot G, Yamazaki T, Daillere R, Hannani D, Enot DP, Pfirschke C, Engblom C, Pittet MJ et al.. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science 2013; 342:971-6; PMID:24264990; http://dx.doi.org/ 10.1126/science.1240537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iida N, Dzutsev A, Stewart CA, Smith L, Bouladoux N, Weingarten RA, Molina DA, Salcedo R, Back T, Cramer S et al.. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science 2013; 342:967-70; PMID:24264989; http://dx.doi.org/ 10.1126/science.1240527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zitvogel L, Galluzzi L, Viaud S, Vetizou M, Daillere R, Merad M, Kroemer G. Cancer and the gut microbiota: an unexpected link. Sci Transl Med 2015; 7:271; PMID:25609166; http://dx.doi.org/24939656 10.1126/scitranslmed.3010473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu X, Zhang X. Effects of cyclophosphamide on immune system and gut microbiota in mice. Microbiol Res 2015; 171:97-106; PMID:25553830; http://dx.doi.org/24939656 10.1016/j.micres.2014.11.002 [DOI] [PubMed] [Google Scholar]
- 15.Taur Y, Jenq RR, Perales MA, Littmann ER, Morjaria S, Ling L, No D, Gobourne A, Viale A, Dahi PB et al.. The effects of intestinal tract bacterial diversity on mortality following allogeneic hematopoietic stem cell transplantation. Blood 2014; 124:1174-82; PMID:24939656; http://dx.doi.org/ 10.1182/blood-2014-02-554725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jenq RR, Taur Y, Devlin SM, Ponce DM, Goldberg JD, Ahr KF, Littmann ER, Ling L, Gobourne AC, Miller LC et al.. Intestinal blautia is associated with reduced death from graft-versus-host disease. Biol Blood Marrow Transplant 2015; 21:1373-83; PMID:25977230; http://dx.doi.org/ 10.1016/j.bbmt.2015.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsirigotis PD, Resnick IB, Or R, Elad S, Zilberman I, Yoffe L, Levovic A, Miron S, Gesundheit B, Slavin S et al.. Post-hematopoietic stem cell transplantion immune-mediated cytopenias. Immunotherapy 2009; 1:39-47; PMID:20635972; http://dx.doi.org/18043614 10.2217/1750743X.1.1.39 [DOI] [PubMed] [Google Scholar]
- 18.Han DK, Baek HJ, Kim SY, Hwang TJ, Kook H. Implication of early lymphocyte recovery after allogeneic hematopoietic stem cell transplantation in children with leukemia. Yonsei Med J 2013; 54:62-70; PMID:23225800; http://dx.doi.org/18043614 10.3349/ymj.2013.54.1.62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jernberg C, Lofmark S, Edlund C, Jansson JK. Long-term ecological impacts of antibiotic administration on the human intestinal microbiota. ISME J 2007; 1:56-66; PMID:18043614; http://dx.doi.org/ 10.1038/ismej.2007.3 [DOI] [PubMed] [Google Scholar]
- 20.Willing BP, Russell SL, Finlay BB. Shifting the balance: antibiotic effects on host-microbiota mutualism. Nat Rev Microbiol 2011; 9:233-43; PMID:21358670; http://dx.doi.org/ 10.1038/nrmicro2536 [DOI] [PubMed] [Google Scholar]
- 21.Blaser MJ. Antibiotic use and its consequences for the normal microbiome. Science 2016; 352:544-5; PMID:27126037; http://dx.doi.org/ 10.1126/science.aad9358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taguer M, Maurice CF. The complex interplay of diet, xenobiotics, and microbial metabolism in the gut: Implications for clinical outcomes. Clin Pharmacol Ther 2016; 99:588-99; PMID:26950037; http://dx.doi.org/ 10.1002/cpt.366 [DOI] [PubMed] [Google Scholar]
- 23.Heidegger S, van den Brink MR, Haas T, Poeck H. The role of pattern-recognition receptors in graft-versus-host disease and graft-versus-leukemia after allogeneic stem cell transplantation. Front Immunol 2014; 5:337; PMID:25101080; http://dx.doi.org/ 10.3389/fimmu.2014.00337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shono Y, Docampo MD, Peled JU, Perobelli SM, Velardi E, Tsai JJ, Slingerland AE, Smith OM, Young LF, Gupta J et al.. Increased GVHD-related mortality with broad-spectrum antibiotic use after allogeneic hematopoietic stem cell transplantation in human patients and mice. Sci Transl Med 2016; 8:339ra71; PMID:27194729; http://dx.doi.org/ 10.1126/scitranslmed.aaf2311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Becattini S, Taur Y, Pamer EG. Antibiotic-induced changes in the intestinal microbiota and disease. Trends Mol Med 2016; 22:458-78; PMID:27178527; http://dx.doi.org/19768747 10.1016/j.molmed.2016.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Romick-Rosendale LE, Goodpaster AM, Hanwright PJ, Patel NB, Wheeler ET, Chona DL, Kennedy MA. NMR-based metabonomics analysis of mouse urine and fecal extracts following oral treatment with the broad-spectrum antibiotic enrofloxacin (Baytril). Magn Reson Chem 2009; 47 (Suppl 1):S36-46; PMID:19768747; http://dx.doi.org/ 10.1002/mrc.2511 [DOI] [PubMed] [Google Scholar]
- 27.Woodmansey EJ, McMurdo ME, Macfarlane GT, Macfarlane S. Comparison of compositions and metabolic activities of fecal microbiotas in young adults and in antibiotic-treated and non-antibiotic-treated elderly subjects. Appl Environ Microbiol 2004; 70:6113-22; http://dx.doi.org/ 10.1128/AEM.70.10.6113-6122.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee SJ, Klein J, Haagenson M, Baxter-Lowe LA, Confer DL, Eapen M, Fernandez-Vina M, Flomenberg N, Horowitz M, Hurley CK et al.. High-resolution donor-recipient HLA matching contributes to the success of unrelated donor marrow transplantation. Blood 2007; 110:4576-83; PMID:17785583; http://dx.doi.org/ 10.1182/blood-2007-06-097386 [DOI] [PubMed] [Google Scholar]
- 29.Jagasia M, Arora M, Flowers ME, Chao NJ, McCarthy PL, Cutler CS, Urbano-Ispizua A, Pavletic SZ, Haagenson MD, Zhang MJ et al.. Risk factors for acute GVHD and survival after hematopoietic cell transplantation. Blood 2012; 119:296-307; PMID:22010102; http://dx.doi.org/ 10.1182/blood-2011-06-364265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anasetti C, Logan BR, Lee SJ, Waller EK, Weisdorf DJ, Wingard JR, Cutler CS, Westervelt P, Woolfrey A, Couban S et al.. Peripheral-blood stem cells versus bone marrow from unrelated donors. N Engl J Med 2012; 367:1487-96; PMID:23075175; http://dx.doi.org/ 10.1056/NEJMoa1203517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vander Lugt MT, Braun TM, Hanash S, Ritz J, Ho VT, Antin JH, Zhang Q, Wong CH, Wang H, Chin A et al.. ST2 as a marker for risk of therapy-resistant graft-versus-host disease and death. N Engl J Med 2013; 369:529-39; PMID:23924003; http://dx.doi.org/ 10.1056/NEJMoa1213299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boga S, Alkim H, Koksal AR, Ozagari AA, Bayram M, Tekin Neijmann S, Sen I, Alkim C. Serum ST2 in inflammatory bowel disease: a potential biomarker for disease activity. J Invest Med 2016; 64:1016-24; PMID:27001944; http://dx.doi.org/ 10.1136/jim-2016-000062 [DOI] [PubMed] [Google Scholar]
- 33.Schiering C, Krausgruber T, Chomka A, Frohlich A, Adelmann K, Wohlfert EA, Pott J, Griseri T, Bollrath J, Hegazy AN et al.. The alarmin IL-33 promotes regulatory T-cell function in the intestine. Nature 2014; 513:564-8; PMID:25043027; http://dx.doi.org/ 10.1038/nature13577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mehraj V, Jenabian MA, Ponte R, Lebouche B, Costiniuk C, Thomas R, Baril JG, LeBlanc R, Cox J, Tremblay C et al.. The plasma levels of soluble ST2 as a marker of gut mucosal damage in early HIV infection. AIDS 2016; 30:1617-27; PMID:27045377; http://dx.doi.org/ 10.1097/QAD.0000000000001105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mehraj V, Ponte R, Routy JP. The dynamic role of the IL-33/ST2 axis in chronic viral-infections: alarming and adjuvanting the immune response. EBioMedicine 2016; 9:37-44; PMID:27397514; http://dx.doi.org/ 10.1016/j.ebiom.2016.06.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Molofsky AB, Savage AK, Locksley RM. Interleukin-33 in tissue homeostasis, injury, and inflammation. Immunity 2015; 42:1005-19; PMID:26084021; http://dx.doi.org/ 10.1016/j.immuni.2015.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Molofsky AB, Van Gool F, Liang HE, Van Dyken SJ, Nussbaum JC, Lee J, Bluestone JA, Locksley RM. Interleukin-33 and interferon-gamma counter-regulate group 2 innate lymphoid cell activation during immune perturbation. Immunity 2015; 43:161-74; PMID:26092469; http://dx.doi.org/ 10.1016/j.immuni.2015.05.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.