ABSTRACT
Transforming Growth Factor (TGF)-β inhibitors have been in development for decades with the outmost results of being promising candidates. From the latest clinical results at the 2016 ASCO meeting converging evidences suggest that we have moved from promising to effective drug nominees.
KEYWORDS: Belagenpumatucel-L, clinical trials, fresolimumab, galunisertib, gemogenovatucel-T, trabedersen, XOMA089
Abbreviations
- AFP
alpha fetoprotein
- ELISPOT
enzyme-linked immunospot
- GM-CSF
granulocyte macrophage colony-stimulating factor
- HCC
hepatocellular carcinoma
- HR
hazard ratio
- IFNγ
interferon gamma
- ITT
intention-to-treat
- MHC
major histocompatibility complex
- NK
natural killer
- NSCLC
non-small cell lung cancer
- OS
overall survival
- PBMC
peripheral blood mononuclear cells
- PFS
progression-free survival
- RFS
relapse-free survival
- RNAi
ribonucleic acid interference
- shRNAi
small hairpin ribonucleic acid interference
- TGF-β
transforming growth factor-β
- Th1
type 1 helper T cell
- Th2
type 2 helper T cell
TGF-β pathway stands as one of the most universal and conserved pathways in the animal kingdom and one of the most potent immunomodulators albeit remaining a challenge to tackle for oncological applications. Considering the whole TGF-β family, more than 30 family members have been shown involved in various physiological and pathological processes. In oncology, TGF-β has been involved in cell proliferation, angiogenesis, epithelial-to-mesenchymal transition, immune infiltration, metastases dissemination, and drug resistance.1 Regarding immunomodulation, TGF-β has been shown to switch Th1/Th2 (type 1 and type 2 helper T cells) balance toward Th2 via IL-10 and direct inhibition of the Th1 response, to inhibit M1-type while promoting M2-type macrophages, and to suppress CD8+ T, NK, and dendritic cell functions while increasing CD4+CD25+ T-reg cell functions.1 Crosstalk between the canonical TGF-β signaling (SMADs) and several non-canonical pathways including MAPK, PI3K, WNT, HH, and NOTCH has been described. Furthermore, the seemingly paradox that most cancer cells display altered or non-functional TGF-β signaling further added complexity for recognizing the value of TGF-β and TGF-β receptors as targets for drug development in oncology. Nonetheless, continuous endeavor to understand the role of TGF-β has started highlighting the crucial importance of the tumor microenvironment, unraveling how TGF-β was every so often behaving as an oncogene at late stage or as a tumor suppressor gene at early stage of tumor development.2 In advanced tumors, TGF-β inhibition may result in anti-proliferative effects at low concentrations and pro-proliferative effects at higher doses, making uneasy the selection of appropriate concentration in clinical trials. Furthermore, TGF-β may have limited direct effects on tumor cells, and thereby, TGF-β inhibitors may be expected to exert only limited effects on cancer cell proliferation. Interestingly, TGF-β inhibitors primarily exert their antitumor activity by affecting TGF-β responsive cells that are fibroblastic and endothelial cells as well as T-cells in the tumor microenvironment. As a result of modulating tumor microenvironment with limited direct effects on cancer cells, TGF-β inhibitors are expected to yield cytostatic effects and lead to tumor stasis translating in few tumor responses but delayed tumor progression in clinical trials. The immune-modulatory cytokine effects of TGF-β inhibition has also recently gained much interest in the bursting onco-immunology field, which is now rushing to evaluate TGF-β pathway inhibition together with checkpoint inhibitors.
Development of small molecules TGF-β pathway inhibitors has long been impeded by on-target toxicities, especially cardiac injuries. However, significant work on drug pharmacokinetic and pathway inhibition threshold using pharmacodynamic biomarkers can considerably reduce their toxicities.3 Other strategies using RNAi or monoclonal antibodies are usually displaying manageable toxicities.
Small molecules inhibiting TGF-β
Several TGF-β pathway inhibitors have now reached the clinic (Fig. 1, Table 1), and some evidences, especially using small molecule inhibitors have started to emerge. Small-molecule inhibitors are primarily aimed at inhibiting TGF-β receptors and are based on a dihydropyrrolopyrazole scaffold (LY550410 and LY580276 from Eli Lilly Research Laboratories) or on imidazole scaffolds (SB-505124 from GlaxoSmithKline). Other inhibitors of interest are based on pyrazolopyridine, pyrazole, imidazopyridine, triazole, pyridopyrimidine, and isothiazole scaffolds. Among small molecule inhibitors galunisertib (LY2157299 Monohydrate), a drug developed by Eli Lilly, is one of the most advanced and have shown interesting results in two phase II trials. An interesting figure is that galunisertib displays a very safe toxicity profile across various clinical trials with no dose-limiting event, including no cardiac toxicity in human that was of primary concern with first-generation TGF-β inhibitors tested in the clinic.
Figure 1.
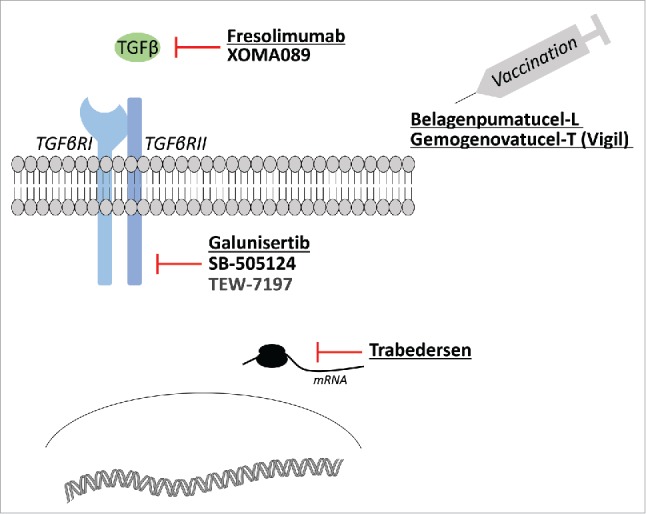
TGF-β inhibition strategies. Examples of TGF-β pathway inhibitors and their use in anticancer strategies. Underlined molecules have reached phase II clinical development.
Table 1.
Summary of the main clinical interventions and results.
| Clinical development | Main results | |
|---|---|---|
| Small molecule TGF-β inhibitors | ||
| Galunisertib (LY2157299) | PhII (NCT01373164): galunisertib + gemcitabine vs. gemcitabine in PAC | Trend to OS benefit |
| PhII (NCT01246986): monotherapy in HCC | OS benefit in patients with >20% decrease in TGF-β1, AFP, and CDH1 levels from baseline | |
| PhII (NCT01246986): combination with sorafenib in HCC | Ongoing | |
| PhIb (NCT02734160): combination with durvalumab in PAC | Ongoing | |
| PhIb (NCT02423343): combination with nivolumab in glioblastoma, NSCLC and HCC | Ongoing | |
| Monoclonal antibodies against TGF-β | ||
| Fresolimumab (GC1008) | PhIb (NCT00356460): monotherapy in melanoma and RCC | Preliminary evidence of antitumor activity |
| PhII (NCT01401062): combination with radiotherapy in mBC | Ongoing | |
| PhI/II (NCT02581787): combination with radiotherapy in early stage NSCLC | Ongoing | |
| Vaccinations strategies | ||
| Lucanix (Belagenpumatucel-L) | PhIII (NCT00676507): monotherapy as maintenance therapy in NSCLC | OS benefit in patients with prior radiotherapy and/or randomized within 12 weeks of chemotherapy completion |
| Vigil (Gemogenovatucel-T) | PhII/III (NCT02346747): monotherapy as maintenance in high-risk ovarian cancer | Preliminary evidence of improved relapse-free survival |
| PhIIb (NCT02511132): monotherapy vs. gemcitabine + docetaxel in Ewing's sarcoma | Ongoing | |
| PhIII (NCT02639234): combination with nivolumab in NSCLC after platinum-based therapy | Ongoing | |
| Antisense oligonucleotides | ||
| Trabedersen | PhIIb (NCT00761280): monotherapy vs. SOC in refractory GBM or anaplastic astrocytoma | Non significative OS benefit |
| PhI/II (NCT00844064): monotherapy in melanoma | Preliminary evidence of improved overall survival | |
GBM: glioblastoma multiforme; HCC: hepatocellular carcinoma; mBC: metastatic breast cancer; NSCLC: non-small cell lung cancer; PAC: pancreatic adenocarcinoma; PhII: phase II trial; RCC: renal cell carcinoma.
From the 2016 American Society of Clinical Oncology (ASCO), data were presented comparing galunisertib in combination with gemcitabine (GG) versus gemcitabine plus placebo (GP) in a 2:1 comparative phase II trial that randomized 156 patients with pancreatic cancer,4 a disease in which SMAD4 abnormalities are reported in near 60% of cases. Using advanced statistical analyses, the study was considered positive on its primary endpoint with a median overall survival of 8.9 mo versus 7.1 mo in the galunisertib plus gemcitabine and gemcitabine plus placebo arms, respectively (Hazard ratio: 0.8 (95% Confidence Interval 0.6–1.09), the probability for the hazard ratio to be <1 being 92%. Secondary endpoints consistently showed an increase in the disease control rate of 49% in patients receiving galunisertib versus 20% receiving placebo. Interestingly, analyses of plasma biomarkers showed that patients with low baseline level of TGF-β1 <4,224 pg/mL might benefit more from galunisertib.4 A phase Ib trial is currently exploring the combination of the anti-PD-L1 durvalumab (MEDI4736) with galunisertib in advanced pancreatic adenocarcinoma failing standard treatments (NCT02734160).
Another large phase II trial performed in hepatocellular carcinoma (HCC), involving several cohorts, was also presented at ASCO. The initial cohort A included 109 patients with elevated AFP ≥ 1.5 UNL treated with galunisertib as single agent in second-line after sorafenib failure, yielding a median overall survival of 8.3 mo.5 Interestingly, patients under exposure to galunisertib who decreased the expression levels of any or all the prespecified blood biomarkers, AFP, TGF-β1, and CDH1 from baseline, had improved outcomes. For instance, patients with high baseline AFP > 200 ng/mL and experiencing >20% reduction in AFP level at anytime during treatment with galunisertib presented an outstanding median OS of 21.4 mo, suggesting that a subgroup of this poor prognostic population derived a pronounced effect of galunisertib. Cohort B, including 40 patients with normal AFP and treated with galunisertib monotherapy in second line, reported a median OS of 16.8 mo; patients showing >20% decrease in blood TGF-β level (∼30%) reaching a median OS of 21.8 mo.5 Expectedly, galunisertib has now moved to first line setting in a randomized phase II trial evaluating the benefit of adding galunisertib to sorafenib (NCT02178358). As well expected, and justified by recent clinical data,6 a phase I/II trial has been initiated, evaluating addition of the anti-PD-1 nivolumab to galunisertib in advanced recurrent or refractory HCC, NSCLC, pancreatic adenocarcinoma, and glioblastoma patients (NCT02423343).
Monoclonal antibodies inhibiting the TGF-β pathway
Other major companies may also be interested for clinical development of monoclonal antibodies in oncology. For instance, fresolimumab (GC1008), a human monoclonal antibody initially co-developed by Cambridge Antibody Technology and Gemzyme then acquired by Sanofi-Aventis for the treatment of idiopathic pulmonary fibrosis and focal segmental glomerulosclerosis, may be further investigated in renal cell carcinoma and melanoma. Novartis and Xoma also joined for co-development of XOMA089, a fully human, high-affinity, monoclonal antibody designed to neutralize TGF-β1 and TGF-β2 while sparing TGF-β3. Preclinical data have shown that XOMA089 displays activity against tumor growth in preclinical models of squamous cell head and neck carcinoma and breast cancer (BC). Animal data also suggested that XOMA089 might be synergistic with PD-1 inhibition.
Vaccinations strategies
Belagenpumatucel-L is the most advanced vaccine that uses four TGF-β2-antisense gene-modified, irradiated, allogeneic non-small cell lung cancer (NSCLC) cell lines to stimulate immune reactions. After promising phase II data, the double blind, randomized phase III trial in patients with stage III/IV NSCLC after frontline platinum-based induction chemotherapy with or without irradiation, did not meet its pre-specified primary efficacy endpoint and was terminated at the second interim analysis for futility.7 Median OS in the intention-to-treat (ITT) population was 20.3 mo for belagenpumatucel-L compared to 17.8 mo for placebo (hazard ratio (HR) = 0.94, p = 0.594). However, in pre-specified analyses, patients who were randomized within 12 weeks after induction chemotherapy, who received prior radiation therapy, or both, seemed to benefit most of the treatment; in these three patient groups, median OS in the vaccine arm were 20.7, 24.8, and 24.8 months, respectively, compared to 13.4, 16.0, and 10.3 mo for the placebo arm (HR = 0.77, p = 0.092; HR = 0.61, p = 0.032; HR = 0.47, p = 0.013, respectively). Owing to the fact that both chemotherapy and radiotherapy can have beneficial effects on antitumor immunity (decreased Treg cell population, improvement of the Treg/CD4+ and CD8+ T cells ratios, upregulation of MHC class I), the authors suggested that delaying vaccine treatment allow time for the immune system to recover and for the tumor to rebuild an immunosuppressive microenvironment. Following this hypothesis, a randomized phase IIb has been initiated to evaluate the combination of gemogenovatucel-T (see below) with nivolumab in advanced or metastatic NSCLC after one prior platinum-based systemic therapy (NCT02639234).
Based on the same vaccination principle, the personalized approach of the gemogenovatucel-T (Vigil, formerly FANG) vaccine consists in ex vivo transfection of autologous tumor cells with the GM-CSF transgene together with a bi-functional short hairpin RNAi (bi-shRNAi) targeting the furin convertase to revert immune tolerance by inhibiting endogenous TGF-β1 and TGF-β2 proteins maturation and function. In a phase Ib trial of 12 patients with recurrent advanced/metastatic Ewing's sarcoma, expression of TGF-β1 and TGF-β2 was dramatically downregulated and treatment was associated with 100% conversion of immune response to unmodified autologous tumor by IFNγ ELISPOT assay.8 Given a 1-y survival rate of 75% in this small patient population, a randomized phase IIb comparing gemogenovatucel-T against a combination of gemcitabine and docetaxel was initiated in advanced refractory Ewing's sarcoma (NCT02511132). Gemogenovatucel-T is also being pursued in a phase II/III trial as maintenance treatment in high-risk stage III/IV ovarian cancer following clinical complete response after primary surgery and adjuvant chemotherapy (NCT02346747). First data on 21 patients in this setting showed a median relapse-free survival (RFS) of 13.3 mo compared to 3.1 mo in the patients that did not received maintenance treatment, urging the investigators to move directly to the phase III part of the trial.
Antisense oligonucleotides to suppress TGF-β expression
Trabedersen, a synthetic TGF-β2 antisens oligodeoxynucleotide, was compared to standard chemotherapy (temozolomide or procarbazine/lomustine/vincristine) in a phase IIb trial that recruited 145 patients with recurrent or refractory glioblastoma multiforme or anaplastic astrocytoma.9 The trial did not meet its primary efficacy endpoint (tumor growth control at 6 mo) but delayed responses were observed even after treatment discontinuation. Median OS for 10 µM trabedersen was 39.1 mo compared to 21.7 mo for chemotherapy although the difference was not statistically significant. In a phase I/II trial in stage III/IV melanoma patients no longer amenable to established therapy, trabedersen (OT-101) was tested as decloaking agent facilitating immune response.10 Authors claimed outstanding OS not related to PFS associated with therapeutic benefit primarily in patients who were subsequently treated by chemotherapies.
Biomarkers
Owing to the complex nature of the TGF-β pathway, its role in cell fate and differential activity in tumor cells and their microenvironment, predictive biomarkers may be challenging to identify. Moreover, biomarkers that would tailored TGF-β inhibitors to specific patient population as single agent may not be relevant in combination therapies. Nonetheless, TGF-β signatures have been highlighted in several tumor types such as colon, breast, liver, or pancreatic cancer and may be worth investigating in clinical trials. Given its role in immunomodulation, predictive expression, or mutation in TGF-β receptors (TGFBR1/2) may also be investigated when TGF-β inhibitors are combined with immunotherapy.
In contrast to the still prospective nature of predictive biomarkers, utility of few pharmacodynamic biomarkers to monitor drug effectiveness have been highlighted. Modulation of SMAD phosphorylation is the most often used biomarker of TGF-β pathway activity in preclinical investigations. In the phase I of Galunisertib, inhibition of pSMAD2 in PBMC has been observed in 64% of the patients.11 However, the inherent difficulty of evaluating phosphorylated biomarkers will likely limit their use in the clinic. In contrast, circulating blood biomarkers are easier to asses and decrease in >20% of TGF-β1, AFP, or CDH1 levels from baseline has been shown to be associated with strong improvement of disease control in the phase II trial of galunisertib in HCC.5,12 However, the delay between treatment initiation and decrease in biomarker level seemed highly variable and remain unpredictable. Several other biomarkers have been tested in clinical trials, but they either lacked of significant association with disease control or were tested on a very limited number of patients to be reliably robust, warranting further investigations.
Discussion
These results highlight that inhibiting TGF-β pathways can yield activity in patients with cancer, slowing down tumor progression and translating into prolonged disease control. Albeit active as single agents, TGF-β inhibitors may be poor inhibitors of proliferation or apoptosis inducers; it is thus likely that those agents will express their best potential in combination with other drugs. Favorable safety profile of novel generation of TGF-β inhibitors make them suitable in combinations with cytotoxic agents as well as combinations with drugs aiming at restoring a functional microenvironment such as antiangiogenic drugs and/or checkpoint inhibitors for which combination trials are ongoing. Importantly, identification of reliable predictive biomarkers to identify patients who are most likely to benefit from these treatments is also a foreseeable issue. Expression levels of biomarkers related to the TGF-β pathway, or its downstream consequences on cell fate, may be difficult to evaluate as predictive biomarkers owing to the influence of the tumor and its microenvironment on TGF-β functions. Nonetheless, their use as monitoring biomarkers can be a valuable tool, as may be the evaluation of the antitumor immune response.
The future of TGF-β inhibitors most immediately relies on a combination strategy that will produce truly identifiable antitumor benefit. The past 10 y have highlighted discrete steps yielding TGF-β inhibitors to be extremely close to a definitive success in clinical research. The future will tell, but for certain, TGF-β inhibitors are ready for prime time.
Disclosure of potential conflicts of interest
SF and ER have received consultancy compensation from Eli Lilly.
References
- 1.Neuzillet C, Tijeras-Raballand A, Cohen R, Cros J, Faivre S, Raymond E, de Gramont A. Targeting the TGFβ pathway for cancer therapy. Pharmacol Ther 2015; 147:22-31; PMID:25444759; http://dx.doi.org/ 10.1016/j.pharmthera.2014.11.001 [DOI] [PubMed] [Google Scholar]
- 2.Massagué J. TGFβ signalling in context. Nat Rev Mol Cell Biol 2012; 13:616-30; PMID:22992590; http://dx.doi.org/ 10.1038/nrm3434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gueorguieva I, Cleverly AL, Stauber A, Sada Pillay N, Rodon JA, Miles CP, Yingling JM, Lahn MM. Defining a therapeutic window for the novel TGF-β inhibitor LY2157299 monohydrate based on a pharmacokinetic/pharmacodynamic model. Br J Clin Pharmacol 2014; 77:796-807; PMID:24843434; http://dx.doi.org/ 10.1111/bcp.12256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Melisi D, Garcia-Carbonero R, Macarulla T, Pezet D, Deplanque G, Fuchs M, Trojan J, Oettle H, Kozloff M, Cleverly A et al.. A phase II, double-blind study of galunisertib+gemcitabine (GG) vs gemcitabine+placebo (GP) in patients (pts) with unresectable pancreatic cancer (PC). J Clin Oncol 2016; 34, (suppl; abstr 4019). [Google Scholar]
- 5.Faivre S, Santoro A, Kelley RK, Merle P, Gane E, Douillard J-Y, Waldschmidt D, Mulcahy MF, Costentin C, Minguez B et al.. A phase 2 study of a novel transforming growth factor-beta (TGF-β1) receptor I kinase inhibitor, LY2157299 monohydrate (LY), in patients with advanced hepatocellular carcinoma (HCC). J Clin Oncol 2014; 32, (suppl 3; abstr LBA173). [Google Scholar]
- 6.El-Khoueiry AB, Melero I, Crocenzi TS, Welling TH, Cheung Yau T, Yeo W, Chopra A, Grosso J, Lang L, Anderson J et al.. Phase I/II safety and antitumor activity of nivolumab in patients with advanced hepatocellular carcinoma (HCC): CA209-040. J Clin Oncol 2015; 33, (suppl; abstr LBA101). [Google Scholar]
- 7.Giaccone G, Bazhenova LA, Nemunaitis J, Tan M, Juhász E, Ramlau R, van den Heuvel MM, Lal R, Kloecker GH, Eaton KD et al.. A phase III study of belagenpumatucel-L, an allogeneic tumour cell vaccine, as maintenance therapy for non-small cell lung cancer. Eur J Cancer Oxf Engl 1990 2015; 51:2321-9. [DOI] [PubMed] [Google Scholar]
- 8.Ghisoli M, Barve M, Schneider R, Mennel R, Lenarsky C, Wallraven G, Pappen BO, LaNoue J, Kumar P, Nemunaitis D et al.. Pilot trial of FANG immunotherapy in Ewing's sarcoma. Mol Ther J Am Soc Gene Ther 2015; 23:1103-9; PMID:25917459; http://dx.doi.org/ 10.1038/mt.2015.43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bogdahn U, Hau P, Stockhammer G, Venkataramana NK, Mahapatra AK, Suri A, Balasubramaniam A, Nair S, Oliushine V, Parfenov V et al.. Targeted therapy for high-grade glioma with the TGF-β2 inhibitor trabedersen: results of a randomized and controlled phase IIb study. Neuro-Oncol 2011; 13:132-42; PMID:20980335; http://dx.doi.org/ 10.1093/neuonc/noq142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hwang L, Ng K, Wang W, Trieu VN. OT-101: An anti-TGF-beta-2 antisense- primed tumors to subsequent chemotherapies. J Clin Oncol 2016; 34, (suppl; abstr e15727). [Google Scholar]
- 11.Rodón J, Carducci M, Sepulveda-Sánchez JM, Azaro A, Calvo E, Seoane J, Braña I, Sicart E, Gueorguieva I, Cleverly A et al.. Pharmacokinetic, pharmacodynamic and biomarker evaluation of transforming growth factor-β receptor I kinase inhibitor, galunisertib, in phase 1 study in patients with advanced cancer. Invest New Drugs 2015; 33:357-70; PMID:25529192; http://dx.doi.org/ 10.1007/s10637-014-0192-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Faivre S, Santoro A, Gane E, Kelley RK, Ollivier-Hourmand I, Gueorguieva I, Cleverly A, Desaiah D, Lahn MM, Raymond E et al.. A phase 2 study of galunisertib, a novel transforming growth factor-beta (TGF-β) receptor I kinase inhibitor, in patients with advanced hepatocellular carcinoma (HCC) and low serum alpha fetoprotein (AFP). J Clin Oncol 2016; 34, (suppl; abstr 4070) [Google Scholar]


