ABSTRACT
Tumor-associated or -infiltrating lymphocytes (TALs or TILs) co-express multiple immune inhibitory receptors that contribute to immune suppression in the ovarian tumor microenvironment (TME). Dual blockade of PD-1 along with LAG-3 or CTLA-4 has been shown to synergistically enhance T-cell effector function, resulting in a delay in murine ovarian tumor growth. However, the mechanisms underlying this synergy and the relative contribution of other inhibitory receptors to immune suppression in the ovarian TME are unknown. Here, we report that multiple immune checkpoints are expressed in TALs and TILs isolated from ovarian tumor-bearing mice. Importantly, blockade of PD-1, LAG-3, or CTLA-4 alone using genetic ablation or blocking antibodies conferred a compensatory upregulation of the other checkpoint pathways, potentiating their capacity for local T-cell suppression that, in turn, could be overcome through combinatorial blockade strategies. Whereas single-agent blockade led to tumor outgrowth in all animals, dual antibody blockade against PD-1/CTLA-4 or triple blockade against PD-1/LAG-3/CTLA-4 resulted in tumor-free survival in 20% of treated mice. In contrast, dual blockade of LAG-3 and CTLA-4 pathways using PD-1 knockout mice led to tumor-free survival in 40% of treated mice, suggesting a hierarchical ordering of checkpoint function. Durable antitumor immunity was most strongly associated with increased numbers of CD8+ T cells, the frequency of cytokine-producing effector T cells, reduced frequency of Tregs and arginine-expressing monocytic myeloid-derived suppressor cells in the peritoneal TME. These data provide a basis for combinatorial checkpoint blockade in clinical intervention for ovarian cancer.
KEYWORDS: Antibody blockade, CTLA-4, immune inhibitory checkpoint, LAG-3, PD-1, ovarian cancer
Introduction
In the tumor microenvironment (TME), the presence of CD8+ T cells correlates with ovarian cancer patient survival.1 However, T cells in the TME often upregulate markers consistent with T-cell exhaustion, providing tumors with the potential to escape immune control.2 These multiple inhibitory receptors (including PD-1, CTLA-4, LAG-3, and TIM-3) and their cognate ligands, expressed on tumors and suppressive or tolerogenic antigen-presenting cells, are listed in Table 1.3-6 Paradoxically, these checkpoint receptors are also markers of T-cell activation, highlighting the complex array of signals that govern T-cell activation and suppression. Specifically, although it is known that these receptors can interact with identified cognate ligands to regulate T-cell signaling, precisely how and under what context these receptors act to enhance, control, or limit T-cell function is not yet clear. Whether these receptors can work in concert or alter the expression of additional checkpoint receptors also remains unclear. Blockade of CTLA-4 or PD-1 with specific antibodies has shown significant promise in overcoming immune suppression and mediating tumor regression.7-9 Based on results from preclinical models and clinical trials,7-11 the Food and Drug Administration (FDA) has approved anti-CTLA-4 antibody for treating melanoma and anti-PD-1 antibody for treating melanoma and several cancers including non-small cell lung, kidney, and head and neck cancer. However, approximately one half of treated patients remain unresponsive to these therapies12 and certain cancers, including ovarian cancer, respond poorly (11–25% overall) to single-agent checkpoint blocking strategies,13-15 suggesting combinatorial strategies may be required. Several pre-clinical studies by our group and others have demonstrated synergistic effects of combinatorial blockade of at least two immune checkpoints in inhibiting murine tumor growth.16-19 Simultaneous inhibition of PD-1 and CTLA-420 or TIM-321 in phase I studies have also shown enhanced efficacy in advanced melanoma patients. However, these combinatorial treatments are often associated with immune-related adverse events,12 adding additional complexity to developing combinatorial therapies. In addition, the mechanism of this synergy is not completely clear. One possibility is that these checkpoints function through non-overlapping cellular mechanisms. For example, although PD-1 acts directly via PD-L1/PD-L2 interaction, CTLA-4 is able to outcompete CD28 for interaction with CD80/86 to limit co-stimulation and leads to suppression.22,23 Others suggest that a redundancy or hierarchy order may exist among the immune checkpoints.3 Alternatively, the synergy could involve targeting multiple checkpoint receptors on one cell, or different receptors on different cells. Thus, understanding the underlying mechanisms of how checkpoint receptors are expressed, their relative kinetics, and targetability, would be of tremendous value in designing effective combinatorial treatments and avoiding autoimmune pathology at the same time.
Table 1.
The immune inhibitory receptors and their cognate ligands.
| Receptors | Full name | Ligands |
|---|---|---|
| PD-1 | Programmed cell death-1 | PD-L1, PD-L2 |
| LAG-3 | Lymphocyte activation gene-3 | HLA-class-II |
| CTLA-4 | Cytotoxic T lymphocyte antigen 4 | CD80 (B7-1), CD86 (B7-2) |
| TIM-3 | T cell immunoglobulin and mucin protein 3 | Galectin-9 |
| BTLA | B- and T-lymphocyte attenuator | B7-H4 and herpes virus entry mediator (HVEM, or NFR-SF14) |
| KLRG1 | Killer-cell lectin like receptor G1 | E-cadherin |
| 2B4 | Signaling lymphocyte activation | CD48, B-lymphocyte activation molecule marker (BLAST-1) |
| CD160 | GPI-anchored lymphocyte surface receptor | Herpes virus entry mediator (HVEM), or MHC class I molecules |
| TIGIT | T cell immunoglobulin and immunoreceptor tyrosine-based inhibitory motif [ITIM] domain | CD155 (poliovirus receptor) and CD112 |
We have previously demonstrated that a subset of tumor antigen-specific CD8+ T cells in human ovarian cancer that co-express PD-1 and LAG-3 are impaired in IFNγ and TNFα production compared with PD-1 or LAG-3 single positive cells.24 Simultaneous blockade of PD-1 or LAG-3 ex vivo restored effector function of human ovarian tumor antigen-specific T cells to a level that is above the additive effects of single blockade of PD-1 or LAG-3 alone.24 We have further shown in mice that dual blockade with LAG-3 synergizes with PD-1 blockade to enhance CD8+ tumor-infiltrating lymphocyte (TIL) functions and promoted better control of transplanted IE9mp1 ovarian tumors, whereas single-agent blockade had little or no effect. Combinatorial blockade with anti-LAG-3 and anti-PD-1 antibodies significantly increased the number of T cells in the TME, enhanced CD8+ T-cell function, and reduced CD4+CD25+Foxp3+ Treg cells. The synergistic effect of blocking both LAG-3 and PD-1 pathways in enhancing antitumor immunity was also demonstrated using LAG-3 and PD-1 knockout mice. Based on the current promise of checkpoint inhibitors and the early success of combinatorial blockade in melanoma,20 it is likely that combinatorial blockade strategies will be implemented as immunotherapy for additional cancers as new data emerges. Therefore, it is critical to identify the optimal blockade combinations, administration methods, and treatment schedules that will achieve the greatest benefit for cancer patients.
In investigating the potential mechanisms of synergy between PD-1 and LAG-3 blockade, we previously showed that PD-1 and LAG-3 may collaborate in recruiting SHP1 or SHP2 to the TCR complex, thereby, negatively co-regulating T-cell signaling and function.19 However, the molecular interaction of PD-1 and LAG-3 appeared weak and transient, suggesting that other mechanisms may be involved in the PD-1-LAG-3 functional synergy. In the current study, we tested the hypothesis that a compensatory cellular mechanism exists whereby blockade of a single inhibitory receptor leads to upregulation of additional checkpoint receptors. Using PD-1 and LAG-3 genetic knockout mice and single antibody blockade of each individual pathway in wild-type mice, we found that blocking one of the checkpoint pathways results in pronounced elevation of the others. These results have implications both for understanding the mechanisms of resistance to checkpoint inhibitors and rational design of combinatorial immune checkpoint blockade.
Results
Multiple immune inhibitory receptors are expressed in a murine model of metastatic ovarian cancer
Previous reports have shown that multiple immune inhibitory receptors are expressed by antigen-specific T cells during chronic viral infection25 and in cancers,4 which may promote tumor escape from immune surveillance. To understand which pathways may drive immune suppression and limit T-cell activity beyond PD-1 and LAG-3, we examined the expression profile of multiple immune inhibitory receptors in tumor-associated lymphocytes (TALs) isolated from the ascites of our IE9mp1 murine ovarian cancer model.19 In this model, implanted IE9mp1 tumor implants develop primarily in the omentum and ovary following injection, and metastasize to peritoneal surfaces and organs such as liver, diaphragm, and serosal surface of the intestines, with progressive development of ascites fluid, resembling disease progression of human ovarian cancer. The expression of the receptors in spleen and TALs from tumor-bearing mice was first analyzed at days 25–30 after tumor implantation (Fig. 1A), corresponding to ascites onset. Compared with the CD8+ and CD4+ T cells isolated from spleen, the levels of PD-1, LAG-3, CTLA-4, and CD160 were significantly increased in CD8+ and CD4+ TALs (Fig. 1A and B). In particular, PD-1 and CTLA-4 were most abundant among all checkpoints examined. Some of these TALs co-expressed at least two checkpoint receptors (Fig. 1C and D). Interestingly, the percentage of cells co-expressing PD-1 and LAG-3 was clearly highest in CD8+ TALs (Fig. 1C), whereas PD-1 and CTLA-4 were highest in CD4+ TALs (Fig. 1D). To determine whether these checkpoints are similarly upregulated on TALs derived from the human ovarian cancer microenvironment, we examined TALs from a cohort of ovarian cancer patients for PD-1, LAG-3, TIM-3, and CTLA-4. We confirmed the upregulation of PD-1 and LAG-3 in both CD8+- and CD4+-derived TALs, and of CTLA-4 and TIM-3 in a subset of the cells (Fig. 2A). Similar to the observations made in murine OVC, many of the interrogated cells expressed dual checkpoints (Fig. 2B and C). These data suggest and support the idea that many immune checkpoint receptors are upregulated in TALs and TILs in both murine and human ovarian cancer and may serve as candidate immunotherapeutic targets.
Figure 1.
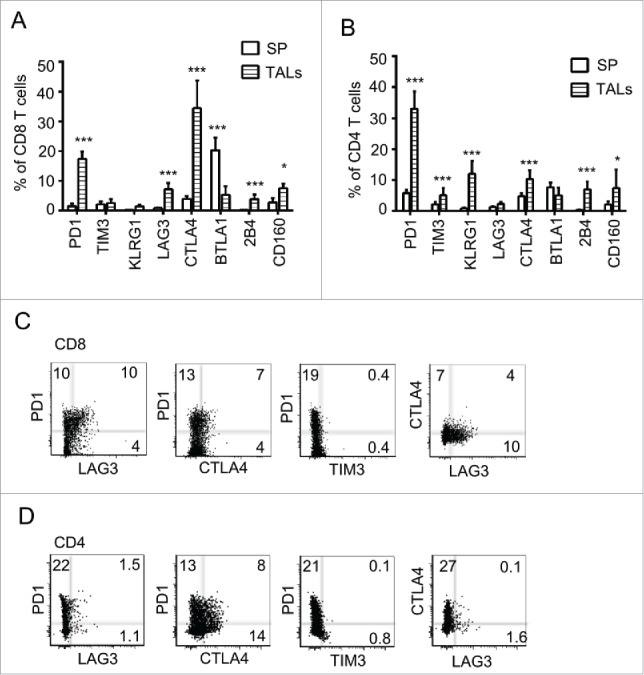
Multiple immune checkpoints are expressed on TALs of murine ovarian cancer microenvironment. Pooled data of eight inhibitory receptor expression on CD8+ (A) and CD4+ (B) TALs from tumor-bearing mice. IE9mp1 tumor cells (1×107) were injected intraperitoneally. TALs were isolated at day 25 (Materials and Methods) and stained for surface expression of the checkpoints and analyzed with flow cytometry. Expression of eight checkpoints in T cells from splenocytes is shown for comparison. Data were obtained from 10 animals and are representative of two independent experiments. Data were analyzed using GraphPad Prism 6. Error bars represent SD. Statistical significance was determined by Student's t-test. *p < 0.05; **p < 0.01; ***p < 0.001. Examples of CD8+ (C) and CD4+ (D) TALs coexpressing two checkpoint molecules are shown. Representative flow cytometry analysis of TALs stained with Live/Dead dye, mAbs to CD4+, CD8+, PD-1, LAG-3, TIM-3, and CTLA-4. Dot plot analyses were gated on live cells, then on CD8+ or CD4+ and showed percentages of single and double stained PD-1, LAG-3, CTLA-4, and TIM-3-positive cells.
Figure 2.
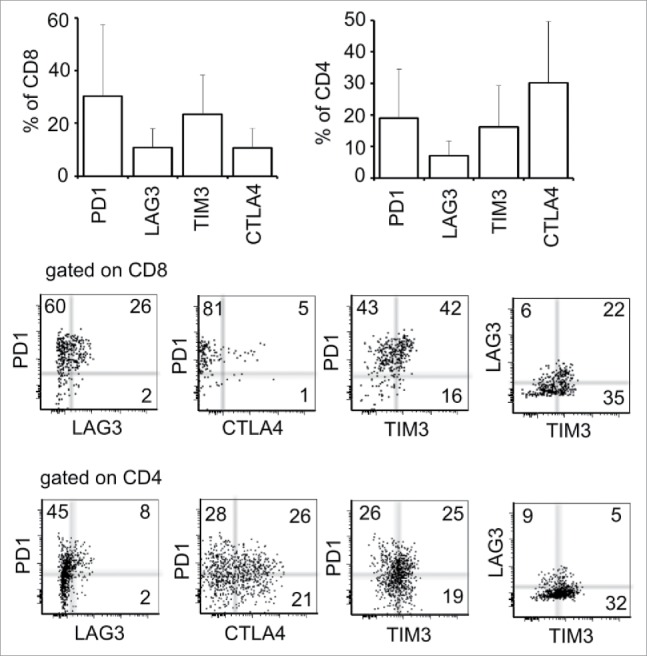
Expression of four immune checkpoints on TALs from the human ovarian cancer microenvironment. (A) Pooled data of the PD-1, LAG-3, TIM-3, and CTLA-4 expression on CD8+ (left panel) and CD4+ (right panel) TALs from 10 human ovarian cancer patients. (B, C) Examples of flow cytometry analysis displaying co-expression of dual immune checkpoints. TALs were isolated as described in Materials and Methods, stained with antibodies against CD8+, CD4+, and the above mentioned checkpoints and analyzed by flow cytometry. Numbers represent the percentage of each population.
Compensatory upregulation of co-inhibitory checkpoints results from blockade of a single checkpoint receptor
We have previously shown that dual blockade of PD-1 and LAG-3 synergistically delayed IE9mp1 tumor growth as compared with single-agent treatment.19 In that study, we found that PD-1 and LAG-3 may interact in both the cytoplasm and cell membrane compartments to exert their synergistic effect. However, the interaction was weak, suggesting the involvement of additional mechanisms in mediating suppression. For example, PD-1/LAG-3 may act in concert with other binding partners or additional pathways. Furthermore, it has been reported that blockade of PD-1 induces the upregulation of CTLA-4 expression in a murine melanoma model and vice versa.16 Based on these data, we hypothesized that compensatory and/or overlapping functionality for these inhibitory receptors may exist that could limit the efficacy of single-agent checkpoint blockade. To begin investigating this, we analyzed the level of other inhibitory receptors when PD-1 or LAG-3 was blocked. Taking advantage of our existing PD-1KO and LAG-3KO mouse strains, we found that the expression of LAG-3, TIM-3, or CTLA-4 genes was increased in the TALs from PD-1KO mice bearing IE9mp1 tumors. Similarly, the level of PD-1, TIM-3, and 2B4 genes was elevated in the tumor-bearing LAG-3KO mice (Fig. 3A and B). Next, we examined whether antibody blockade of these pathways in wild-type mice would result in a similar pattern of compensatory receptor upregulation as was seen in the tumor-bearing knockout mice. As shown in Fig. 3C and D, the level of LAG-3 and CTLA-4 is increased by treatment with anti-PD-1 antibody, whereas PD-1 is increased after treatment with anti-LAG-3 antibody. Interestingly, CTLA-4 level was not elevated after blockade of LAG-3 pathway either by genetic ablation or inhibition by antibody. In addition, PD-1 and LAG-3 were also elevated by the treatment with anti-CTLA-4 antibody. Thus, our data indicate that blockade of one immune inhibitory pathway leads to compensatory upregulation of other checkpoint receptors in T cells infiltrating the ovarian TME.
Figure 3.
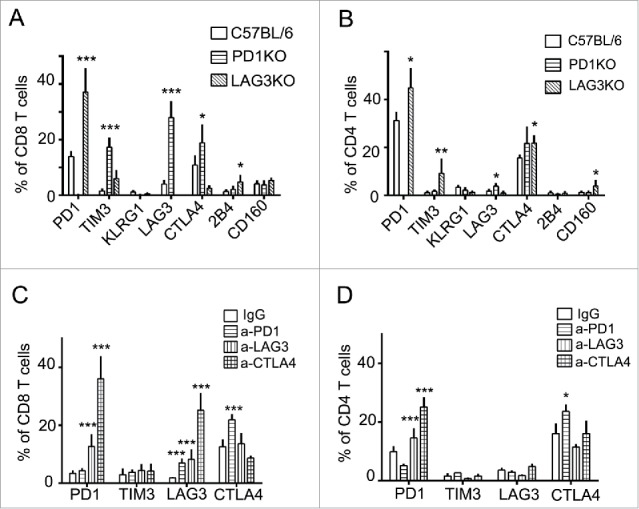
Compensatory upregulation of immune checkpoints in murine OVC after blockade of single checkpoint pathway. (A, B) Elevated expression of other checkpoints in tumor-bearing PD-1KO or LAG-3KO mice. The levels of LAG-3, CTLA-4, and TIM-3 are elevated in the TALs from the tumor-bearing PD-1KO mice, whereas PD-1, TIM-3, and 2B4 are increased in tumor-bearing LAG-3KO mice. Tumor implantation was performed as described in Fig. 1. TALs and TILs (data not shown) were collected from tumor-bearing C57BL6 (wild-type), PD-1KO, and LAG-3KO mice (five animals per group) at early time points and stained for the antibody against the indicated checkpoints. Data are representative of two independent experiments. (C, D) Individual blockade with immune checkpoint antibody anti-PD-1, or anti-LAG-3, or anti-CTLA-4 in the wild-type mice results in elevated expression of the other immune inhibitory checkpoints. Ten days after tumor implantation, mice were treated intraperitoneally with antibody (200 μg per mouse) every other day for four times. T cells were analyzed 7 d after the end of the treatment. Data were analyzed using GraphPad Prism 6. Error bars represent SD. Statistical significance was determined by Student's t-test. *p < 0.05; **p < 0.01; ***p < 0.001. Data were obtained from five animals and are representative of three independent experiments.
Combinatorial blockade of PD-1/CTLA-4/LAG-3 leads to differential tumor control in a murine model of metastatic ovarian cancer
The data described above provided a basis for testing rational combination treatments of antibodies targeting additional checkpoints in ovarian cancer beyond the previously studied PD-1/LAG-3 combinations.19 Although genetic ablation of PD-1 or LAG-3 upregulated TIM-3 expression antibody blockade of either pathway did not have the same effect; therefore, we examined the effect combining any two or all three blocking antibodies against PD-1, LAG-3, or CTLA-4 pathway on tumor growth control in the IE9mp1 model. Similar to previous reports,16-19 single-agent blockade of PD-1, LAG-3, or CTLA-4 did not have a significant effect on delaying ovarian cancer growth in our model (Fig. 4A and B). Combinations of either PD-1/LAG-3 or LAG-3/CTLA-4 showed modest improvement over single-agent blockade. In contrast, PD-1/CTLA-4 dual blockade exhibited superior effects compared to the other two combinations (Fig. 4C, p=0.002). Surprisingly, additional blockade of LAG-3 pathway did not further enhance the synergistic effect of blocking PD-1/CTLA-4 on antitumor immunity in the ovarian tumor-bearing mice (Fig. 4C, p = 0.2). Approximately 20% of the treated mice in the triple blockade group showed declined health and splenic inflammation upon necropsy possibly due to immune-related cytotoxicity. Taken together, these data suggest that combinational checkpoint blockades against PD-1, CTLA-4, and LAG-3 pathways exhibit potential in controlling ovarian tumor growth. However, appropriate dosing and scheduling may be required to achieve optimal benefits of the treatment.
Figure 4.
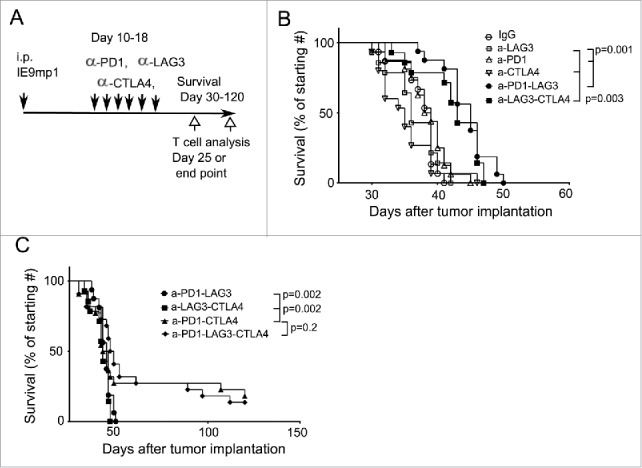
Combinatorial and triple blockade of PD-1, CTLA-4, and LAG-3 pathways exerts differential antitumor immunity. (A) Experimental scheme and antibody blockade treatment. C57BL/6 mice were implanted with IE9mp1 tumors as described in Fig. 3, randomized and treated with control IgG, single antibody, and combination of two or three antibodies were administered every other day for six times (Materials and Methods). Tumor progression was monitored by measuring the abdominal circumference due to accumulation of ascites. Survival was determined when the abdominal circumference reached 12 cm or moribund. (B) Combinatorial blockade of PD-1/LAG-3 or CTLA-4/LAG-3 pathways mildly improve antitumor immunity. Antibody treatment was as described above. Survival data were analyzed with the Mantel–Cox log-rank test. Data were obtained from 14 to 15 mice per group and were representative of two independent experiments. (C) Triple blockade of three checkpoints and dual antibody blockade of PD-1 and CTLA-4 significantly enhanced the survival of ovarian tumor-bearing wild-type mice. Antibody treatment and data analysis were performed at the same time as that described in (B).
Inhibition of different immune checkpoint receptors differentially affects the functionality of CD8+ TALs and TILs
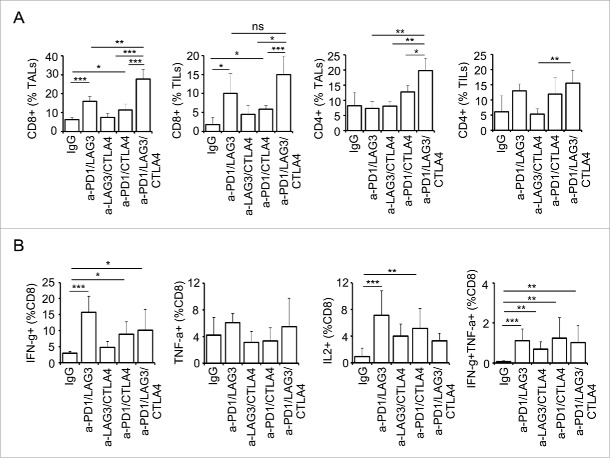
We have previously shown that dual blockade with PD-1 and LAG-3 enhances antitumor activity by increasing the number of functional CD8+ TILs/TALs as well as reducing the number of Treg cells.19 Based on these data, we next compared the effect of the various dual checkpoint blocking interventions with the triple blockade on TALs and TILs at an early time point (day 25, Fig. 5) and experimental endpoint when the abdominal circumference reached 12 cm or the mouse became moribund (Fig. S1). Both PD-1/LAG-3 and PD-1/CTLA-4 treatments significantly increased the CD8+ TALs and TILs frequency (Fig. 5A, left two panels). Strikingly, triple blockade significantly enhanced the percentage of CD8+ TALs in the TME at early time points as compared with that of any dual blockade therapy (Fig. 5A). This was also true in the CD4+ TALs, albeit the effect of triple blockade on the frequency of CD4+ TIL population was not as dramatic. The effect of triple blockade on the frequency of both CD8+ and CD4+ TALs was persistent till the endpoint (Fig. S1A). Surprisingly, after blocking all three pathways these CD8+ TALs did not significantly produce more cytokines than those after the dual blockade of PD-1/LAG-3 or PD-1/CTLA-4 (Fig. 5B). Specifically, CD8+ TALs from the mice with dual blockade of PD-1/LAG-3 maintained the highest functionality at the early time point as determined by the percentage of IFNγ+ (Fig. 5B, left panel) and IFNγ+TNFα+ cells (Fig. 5B, far-right panel). However, CD8+ TALs from mice treated with PD-1/CTLA-4 dual blockade were more functional than those treated with the other dual blockades and the triple blockade at endpoint using the same criteria (Fig. S1B). The lasting effect of dual blockade of PD-1/CTLA-4 on the functionality of CD8+ TALs indeed correlated with its significant effect on the survival of the tumor-bearing mice (Fig. 4C). Collectively, our results show that the major effect of combinatorial blockade of PD-1, LAG-3, and CTLA-4 is increased infiltration and, to a lesser extent, effector function of CD8+ TALs/TILs.
Figure 5.
Combinatorial blockade of PD-1, CTLA-4, and LAG-3 significantly enhance lymphocyte infiltration and function at early time point. (A) Frequency of CD8+ and CD4+ TALs (ascites) and TILs (tumor) from ovarian tumor-bearing mice after checkpoint blockade at early time point. Both CD8+ and CD4+ TALs were significantly elevated in triple antibody-treated group. (B) Frequency of cytokine-producing CD8+ TALs is enhanced after antibody blockade treatment. T effector function was assessed by percentage of cytokine-producing cells (IFNγ, TNFα, and IL2). Dual IFNγ- and TNFα-producing cells represent polyfunctionality of the population. Mice were treated every other day with total six doses of antibodies on days 10–20. TALs and TILs were isolated at 25 d posttumor implantation. For cytokine production TALs were stimulated with PMA/Ionomycin in the presence of BFA for 5 h. Cytokine producing cells were analyzed as described in Material and Methods. Data are representative of two independent experiments with 3–5 animals per group. Error bars represent SD. Statistical significance was determined by Student's t-test. *p < 0.05; **p < 0.01; ***p < 0.001.
Blockade of PD-1-LAG-3 and CTLA-4 differentially reduces Tregs and myeloid-derived suppressive cell (MDSC) populations
The TME is often enriched in Treg and myeloid-derived suppressor cells (MDSCs), which contribute to a highly immunosuppressive milieu that lead to reduced CD8+ T-cell effector function. We observed that blockade of PD-1 and LAG-3 additively reduced the suppressive Tregs (Fig. 6A). In comparison, dual blockade with PD-1/CTLA-4 or LAG-3/CTLA-4 did not significantly reduce the frequency of CD25+FoxP3+ Treg cells whereas blocking CTLA-4 alone had a significant, albeit transient, impact at early time points only following intervention (Fig. S2). Triple blockade of PD-1, LAG-3, and CTLA-4 further reduced the percentage of the Treg cells in TALs, as compared with that of the different dual blockades (Fig. 6A, left panel). This effect was not as prominent in the TILs (Fig. 6A, right panel).
Figure 6.
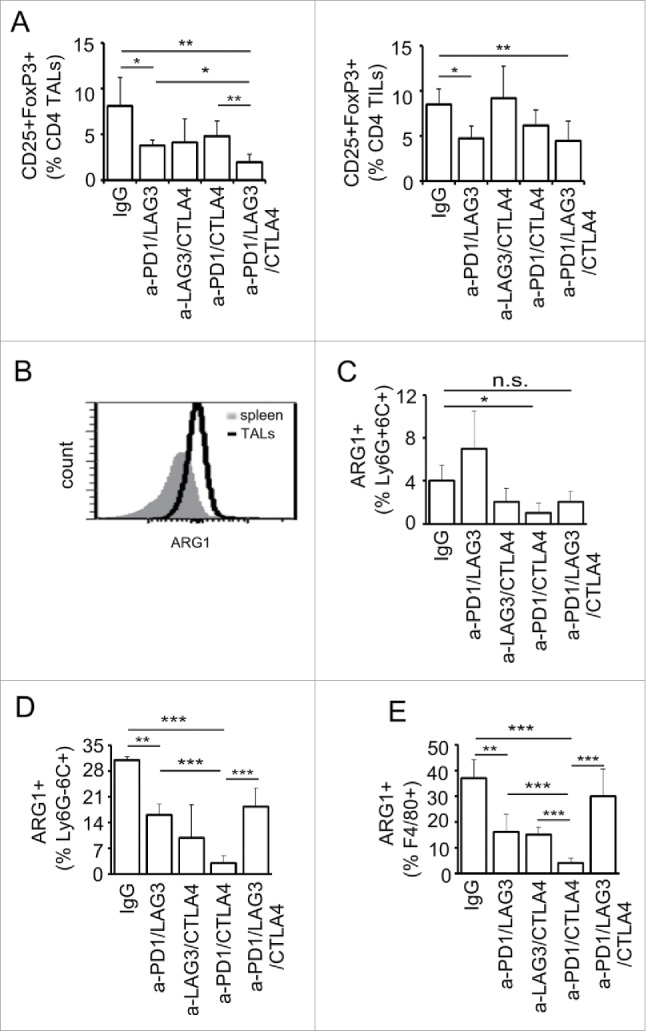
Effects of combinatorial blockade of PD-1, CTLA-4, and LAG-3 on Treg and MDSC population in ovarian TME. (A) Dual and triple antibody blockade treatment decreased the frequency of CD4+CD25+FoxP3+ cells at early time point of ovarian tumor progression. TALs and TILs were isolated as described above and stained for the expression of CD4+, CD25, and FoxP3 protein and analyzed using flow cytometry. (B) Arginase 1 (ARG1)-expressing population was increased in the ovarian TME as compared with that in spleen. Splenocytes and TALs were isolated as described in Fig. 5. Frequency of ARG1+ population in the CD45+CD11b+ cells was determined based on the gating shown in Fig. S3. (C–E) Various checkpoint blockade combinations reduced the frequencies of ARG1+-MDSCs. The frequency of ARG1+-Ly6G+6C+ cells was significantly reduced by PD-1/CTLA-4 dual blockade (C). All three dual blockades of PD-1/LAG-3, PD-1/CTLA-4, and LAG-3/CTLA-4 significantly reduced the frequency of ARG1+-Ly6G−6C+ (D) and ARG1+-F4/80+ cells (E). Data were obtained from 5 mice per group, analyzed using GraphPad Prism 6, and are representative of two independent experiments. Error bars represent SD. Statistical significance was determined by Student's t-test. *p < 0.05; **p < 0.01; ***p < 0.001.
It has been shown previously that blockade of PD-1 and CTLA-4 in melanoma reduces MDSC populations.16,26 Therefore, the effect of combinatorial blockade of PD-1/CTLA-4/LAG-3 pathways on the MDSC populations in the ovarian TME was investigated. Based on the relative expression levels of CD11b, Ly6G, and Ly6G (gating strategy shown in Fig. S3), we defined CD11b+Ly6G+Ly6C+ cells as granulocytic myeloid-derived suppressor cells (G-MDSC) and CD11b+Ly6G−Ly6C+ cells as monocytic MDSCs (M-MDSC).27,28 In addition, the expression of F4/80 was analyzed as the overall macrophage population in the CD11b+ cells. Furthermore, we used expression of arginase 1 (ARG1) as an indicator for the immune suppressive activity of MDSCs and macrophages, as ARG1 catalyzes the conversion of l-arginine to ornithine and urea, which deprives T cells of this amino acid and renders them functionally unresponsive.29 In wild-type tumor-bearing mice, the percentage of CD11b+ARG+ cells in CD45+ leukocytes was dramatically increased compared to the spleen (Fig. 6B). Combinatorial blockade of PD-1 and CTLA-4 significantly reduced the percentage of the ARG1-expressing Ly6G+6C+ (G-MDSC) cells, whereas, surprisingly, dual blockade PD-1 and LAG-3 had an opposite effect (Fig. 6C). Note that only small percentage (2–7%) of the CD11b+Ly6G+6C+ cells expressed ARG1, indicating that most of the Ly6G+6C+ cells are probably inflammatory DCs or neutrophils. In contrast, approximately 35% of the CD11b+Ly6G−Ly6C+ (M-MDSC) cells were ARG1-expressing M-MDSC in IgG-treated TALs. All the blockade treatments had a trend of reducing the percentage of ARG1-expressing Ly6G−Ly6C+ cells (Fig. 6D). In addition, all the dual blockades significantly reduced the ARG1-expressing F4/80+ cells (Fig. 6E), whereas the triple blockade did not have effect on this population. Most strikingly, dual blockade of PD-1 and CTLA-4 significantly decrease the ARG1-expressing G-MDCS, M-MDSC, and F4/80+ macrophages. These results are highly correlated with the significant effect of enhanced survival by combinatorial blockade of these two pathways.
Genetic knockout of PD-1 or LAG-3 enhances the impact of combination antibody
The results described above indicate that dual blockade of PD-1/CTLA-4 or triple blockade (including LAG-3) was only curative in a small percentage of treated animals (20%), raising the possibility that either the blockade was not complete in the majority of the mice, or the timing/duration or the doses of the treatment was not adequate, or that additional pathways and other mechanisms of immune suppression were being activated. To investigate this further, we tested treatment strategies involving blockade of the PD-1 ligand, PD-L1, which was upregulated in the TME (data not shown), along with combined blockade of PD-1, CTLA-4, and LAG-3. However, the treatment with a combination of four antibodies crossed the threshold of inducing acute toxicity, as 40% of the treated mice showed deleterious health condition soon following treatment (data not shown). Since blockade using antibodies may not be completely effective and we had the capacity to completely block the highly potent (PD-1) versus less potent (LAG-3) pathway with knockout animals, we tested triple combinations in these settings to understand how the response would differ. As observed in the dual blockade in the wild-type mice, additional blockade of CTLA-4 in PD-1KO mice show synergistic effect on controlling tumor growth (p = 0.001). Additional blocking of the LAG-3 pathway in PD-1KO mice had a minimal benefit. In contrast, dual blockade with anti-CTLA-4 and anti-LAG-3 antibodies in PD-1KO mice significantly increased the rates of tumor rejection (40%) as compared with treatment using anti-CTLA-4 antibody alone (25%, p = 0.001) (Fig. 7A). The tumor rejection rate by blocking the three pathways in this setting was significantly better than the triple blockade with antibodies (20%, Fig. 4B). In addition, no sever cytotoxicity was observed in the dual blockade of CTLA4/LAG3 in the PD-1KO mice. These data suggest that if one of the immune checkpoints is completely abrogated, additional blockade with two other major pathways may provide additional therapeutic benefit not observed following triple blockade strategies. To further corroborate this finding, we next tested whether dual blockade of PD-1/CTLA-4 in the context of LAG-3KO mice, where LAG-3 pathway is completely abrogated, would have a similar impact. Although blocking PD-1 in the LAG-3KO mice (Fig. 7B, p = 0.001) has a similar effect as that of dual blockade of PD-1-LAG-3 in wild-type BL6 mice (Fig. 4B), blocking CTLA-4 did not have significant effect in delaying tumor growth in the LAG-3KO mice. Importantly, antibody-mediated dual blockade of PD-1 and CTLA-4 pathways in LAG-3KO mice had an additive effect in delaying tumor growth as compared with blocking PD-1 or CTLA-4 alone (20% tumor rejection rate, Fig. 7B). However, the efficacy of triple blockade in LAG-3KO mice setting was diminished compared to observations in PD-1KO mice (Fig. 7A). Taken together, these data suggest that combinatorial blockade of LAG-3-CTLA-4 has significant benefit in controlling ovarian tumor growth when PD-1 pathway is completely blocked.
Figure 7.
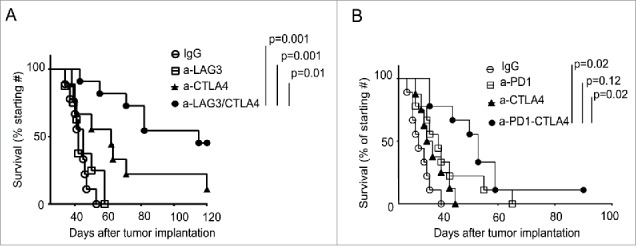
Genetic knockout of PD-1 or LAG-3 enhances the impact of combination antibody treatment. (A) Combinatorial blockade of LAG-3 and CTLA-4 significantly enhanced the survival of ovarian tumor-bearing PD-1KO mice. Antibody treatment and data analysis were performed as described in Fig. 4. Survival data were derived from 10 to 12 mice per group and are representative of two independent experiments. (B) Combinatorial blockade of PD-1 and CTLA-4 significantly enhanced survival of LAG-3KO mice bearing ovarian tumors. Survival data were derived from 8 to 10 mice per group and are representative of two independent experiments.
Discussion
We show here that multiple inhibitory checkpoints are elevated on the TALs and TILs in the human and murine ovarian TME, preventing durable tumor attack by infiltrating T cells. Combinatorial blockade against PD-1, CTLA-4, and LAG-3 pathways showed an additive efficacy in controlling murine ovarian tumor growth. The predominant effect observed following blockade of the three targeted pathways was increased infiltration of CD8+ T cells, as well as improved effector function, which correlated with antitumor activity and improved survival. In addition, dual antibody blockade combinations, to a lesser extent, also had differential effects on reducing the Tregs and MDSC populations which may act to limit T-cell efficacy. Our data suggest that blocking the PD-1 axis is the major factor in mediating an effective checkpoint blockade strategy in immunotherapy in murine ovarian cancer. This is consistent with recent clinical data in melanoma where blocking PD-1 pathway is more potent than inhibiting the CTLA-4.30 However, single checkpoint blockade against PD-1 leads to a compensatory induction of LAG-3 and CTLA-4 and other checkpoint pathways which contributes to a feedback loop that acts to mediate local immune suppression. This may have limited the therapeutic efficacy of single-agent PD-1 or PD-L1 blockade in ovarian cancer patients.13,15 On the other hand, the compensatory upregulation of other checkpoints also leads to an opportunity for choosing an appropriate second agent(s) for combinatorial treatment.
Our data suggest that the relative contribution of immune co-inhibitory receptors to antitumor immunity includes both hierarchical order and compensatory mechanisms that need to be considered in designing combination treatments. Data shown here suggest that PD-1 is at the top of this hierarchy, supported by the observation that dual blockade of CTLA-4 and LAG-3 in PD-1KO mice results in a 40% survival rate (Fig. 7A) as compared to 20% from the dual blockade of PD-1 and CTLA-4 in LAG-3KO mice (Fig. 7B). It is important to point out, however, that the optimal timing and dosing of checkpoint blockade with antibodies is difficult to determine and this may play a factor in the improved outcomes observed in the context of complete genetic ablation. This notion is reflected in the study using an ID8 ovarian tumor model that treatment of anti-PD-L1 antibody at early stage tumor progression is superior to the late stage intervention.31 Interestingly, the impact of PD-1/PD-L1 blockade in this ID8 model (25–65% tumor clearance rate) was more striking than the data shown in current study (10% tumor clearance, Fig. 4B). In addition, the same group31 demonstrated that blocking both PD1 and CTLA4 pathways in mice achieve a 50% tumor clearance rate in a subcutaneous ID8 ovarian tumor model.18 In contrast, however, another group reported that anti-PD-1/CTLA-4 combination had no benefit in rejecting intraperitoneal ID8 ovarian tumors.32 The discrepancy among these studies and ours may be due to difference in cell lines, dosing/schedules, and/or methods of implantation used. The hierarchical preference for PD-1-mediated immunity has also been previously suggested in viral infection.3 However, each checkpoint clearly has unique attributes and relative impact on T-cell function and each pathway may execute distinct and non-redundant actions of immune regulation. This is reflected in their blocking effect in modulating T-cell responses (Fig. 5) and delaying tumor growth shown here (Fig. 4) and elsewhere.16 One of the possible determining factors is that these checkpoint receptors are expressed in different cell populations beyond the T-cell compartment. Here, we have focused our analysis largely on T cells, and although CTLA-4 is mainly expressed on T cells,33 PD-1 and LAG-3 are also expressed by B cells, NK cells, dendritic cells, and macrophages,34,35 thereby, having the potential to contribute broadly in the context of antitumor immune responses. In addition, these checkpoint receptors are differentially expressed on CD8+ and CD4+ T cells (Figs. 1 and 2), which may influence checkpoint blockade effects. The fact that dual blockade of LAG-3 and CTLA-4 in PD-1KO mice had a more profound effect in tumor rejection than triple blockade in wild-type mice also points to PD-1 being at the top of this hierarchy order of checkpoint actions in the control of ovarian cancer. Our previous observation that CD8+ T cells from non-tumor-bearing PD-1KO mice produce higher levels of IFNγ, TNFα, and IL2 than those from the wild-type and LAG-3KO mice when activated in vitro with anti-CD3 antibody19 also support this notion. Thus, data shown here in the ovarian TME and those demonstrated in a chronic viral infection model suggest that PD-1 is the major inhibitory receptor controlling T-cell exhaustion,3,36 with additional receptors having the ability to further modulate T-cell activity and having the potential to also be exploited therapeutically.
Our data and recent work by others using mouse models of lymphocytic choriomeningitis virus (LCMV) infection37 suggest that in the absence of PD-1, other immune checkpoints, such as LAG-3 or CTLA-4, can compensate for its inhibitory function, with removal of any single checkpoint receptor not preventing T-cell exhaustion (data not shown,19,37). The compensatory mechanism seems to be common across different tumor types, as it has been recently demonstrated in lung cancer that treatment with anti-PD-1 antibody results in upregulation of TIM-3 on T cells, both in a mouse model and in the treated patients.38 We also observed upregulation of TIM-3 in the genetic ablation of PD-1 and LAG-3 pathways (Fig. 3A and B), albeit this gene was not significantly affected using antibody blockade against PD-1 or LAG-3. The compensatory upregulation of the additional checkpoints may also explain the synergistic effect of combinatorial blockade of LAG-3 and CTLA-4 in the PD-1KO mice. Interestingly, CTLA-4 is upregulated only in the PD-1KO but not LAG-3KO mice, and in the anti-PD-1 but not anti-LAG3 antibody treatment in WT mice. It is possible that the level of CTLA-4 expression in PD-1KO mice contributed to the treatment outcome. Another group has also demonstrated using a different murine ovarian cancer cell line in a subcutaneous model that PD-1 and CTLA-4 combine treatment cured 50% of the mice as compared with a ∼25% with single treatment.18 The triple blockade of PD-1/CTLA-4/LAG-3 also increased the frequency of CD8+ TALs, but had a similar effect to the dual PD-1/CTLA-4 blockade in controlling tumor growth (Fig. 4B). Of interest, dual blockade appears to benefit a majority of mice, although briefly (like targeted therapy), whereas triple blockade has little additional benefit except in a minority of mice who show long-term benefit (like immune therapy).
The mechanisms of compensatory upregulation of immune inhibitory receptors on T cells are currently unclear. It is possible that the compensation arises during various stages of adaptive immune responses and that these checkpoints function in both distinctive and overlapping manners.2 For example, although PD-1 and CTLA-4 interact with different ligands and act through different mechanisms, both interactions lead to suppression.22,23 LAG-3, on the other hand, is thought to act through binding of MHC class II molecules, which present antigens to the T-cell receptor to initiate T-cell signaling.39 All of these actions lead to recruitment of certain phosphatases and inhibition of downstream signaling in T cells. Blockade of either PD-1, LAG-3, or CTLA-4 can activate T cells and promote their proliferation, and at the same time, reduce the suppressive activity of Tregs.9,40 The degree of reinvigoration of the TILs and TALs resulting from different checkpoint blockade varies as seen in the percentage of functional T cells, Tregs, and ARG1+ MDSCs (Figs. 3–6). Alternatively, each checkpoint blockade may target different populations of T cells as suggested by our data that PD-1, but not CTLA-4, is increased after anti-LAG3 antibody blockade. For example, it is well known that CTLA4 plays a major role in Treg function.41 However, it has also been shown that both Teff and Treg cells are relevant targets for the therapeutic efficacy of anti-CTLA-4 antibodies.42 Furthermore, it is possible that the kinetics of upregulation of additional checkpoints by a given checkpoint blockade antibody differs. With the limited time points analyzed (early and late), we may have missed the period where CTLA-4 was also upregulated after anti-LAG3 blockade.
In future studies, it will be of great interest to identify the potential factors that regulate the compensatory induction of checkpoint expression. It has been reported in the LCMV system that monomeric NFAT (nuclear factors of activated T cell) binding in the absence of a transcriptional partner induces a gene expression program involved in T-cell exhaustion.43 It is not known whether the NFAT factors and/or their transcriptional partners co-regulate the compensatory expression of checkpoints under the blockade regimen in the ovarian TME described here. The identification of the potential regulators would lead to strategies that could prevent other checkpoint receptors from being upregulated. In addition, the current study does not address whether the compensatory events occur on the same cells as those that have been blocked by the checkpoint antibody or randomly in the whole population of cells. Future study using single-cell-based gene/protein analysis should shed light on this issue.
Of the dual antibody blockade, blocking CTLA-4 and LAG-3 had the least effect in delaying OVC growth in wild-type mice; this may have resulted from the increased number of suppressive Treg cells in TILs (Fig. 6A, right panel). In contrast, combinatorial blockade with PD-1 and CTLA-4 had a significantly better efficacy than that of either single agent. Although triple blockade did not significantly improve the survival contributed by the dual blockade of PD-1-CTLA-4, the treatment additively increased infiltration of CD8+ T cells and maintained the polyfunctionality of these T cells (Fig. 4B), and the effect on the percentage of T cell numbers persisted to endpoint (Fig. S1). Therefore, it seems that triple blockade with appropriate dosing and schedules may have significant efficacy in controlling ovarian tumor growth. Our data also indicate that checkpoint blockade, single or combined, results in substantial reprogramming of the TME. Both effector and helper T cell populations and the MDSC repertoire all undergo progressive changes regarding their number and function. Although the major effect of checkpoint blockade is to reinvigorate T-cell function, the changes in other immune population can provide a window of opportunity for treatment with checkpoint inhibitors to combine with intervention of other immune suppressive molecules in the TME.
In conclusion, our data indicate that blocking PD-1 alone is not sufficient to control murine ovarian tumor growth; however, dual blockade of PD-1-LAG-3 or PD-1-CTLA-4 pathways can delay murine ovarian tumor growth and that triple blockade of PD-1-CTLA-4-LAG-3 pathways is superior if PD-1 pathway is completely blocked. Future studies should aim at identifying the ideal schedule, dose, and duration of administering these antibodies combinatorially in order to enhance antitumor immunity in ovarian cancer. Of special note, our data show that LAG-3 and TIM-3 are also highly expressed in human ovarian cancer and, in contrast to TALs from mice, TALs from patients contain large population of PD-1+TIM3+ CD8+ or CD4+ cells. These results are highly relevant to the future design of combined checkpoint inhibition regimens. At the same time, attention should be paid to a careful balance between generating durable antitumor immunity and the potential for autoimmune pathology that could result from exploiting these pathways.
Materials and methods
Human TILs and TALs
Tumor specimens and ascites fluids were obtained from patients undergoing surgery for EOC at the Roswell Park Cancer Institute, Buffalo, NY, under an approved protocol (I215512) from the Institutional Review Board. Tumor specimens were dissociated using GentleMACS Dissociator (Miltenyi) and tissue clumps were removed via filtration through 100 μm mesh. Lymphocytes were isolated using lymphocyte separation medium.
Mice
C57BL/6 (BL6, or Wild-type, WT) mice were purchased from The Jackson Laboratory (Bar Harbor, ME) and bred in our facility (Roswell Park Cancer Institute, RPCI) under an approved Animal Committee protocol (#1145). LAG-3KO (LAG-3−/−) mice4 were a kind gift from Dario Vignali (St. Jude Children's Research Hospital). PD-1KO (Pdcd1−/−) mice were provided by Tasuku Honjo (Osaka University). All mice were maintained under specific pathogen-free conditions in the RPCI animal facility according to approved institutional guidelines.
Ovarian tumor model
The IE9mp1 cell line was generated by in vitro expansion of the IE9-OVA orthotopic tumor44 explant from a C57BL/6 mouse as described previously19 and was performed following the approved protocol (#1127). To generate tumors in a syngeneic mouse model, 6- to 8-week-old female mice were injected intraperitoneally (I.P.) with 1 × 107 IE9mp1 cells suspended in 350 μL of PBS. Wild-type C57BL/6 and PD-1KO and LAG-3KO mice bearing the IE9mp1 tumors became moribund at approximately day 35–45, after tumor cell implantation. For antibody blockade studies, mice were randomized and I.P. injected (200 μg per antibody, per mouse) with IgG control, anti-LAG-3 (C9B7W, m-IgG1 or rat-IgG1, BE0174), anti-PD-1 (4H2, mIgG1 or RMP1-14, rat-IgG, BE0146) or anti-CTLA-4 (9D9, mIgG, or rat-IgG, BE0164 ), anti-PD-L1 (10F.9G2, rat-IgG, BE0101), or the combination every other day (from days 10 to 20). Blockade treatments were six times for survival study and endpoint immune cell analysis and four times for early time point TILs and TALs study. The dose and frequency were adapted based on published reports18,45, and the frequency and timing of treatment were empirically determined. Due to the aggressiveness of the IE9mp1 cells, the efficacy of antibody blockade was not significant when treatment was performed at a later time point or using 4–5 doses. All blocking antibodies were either gifts from Bristol-Myers Squibb or purchased from BIOXCell. Tumor progression was monitored by the development of abdominal ascites, and analysis of long-term survival. Following institutional guidelines, mice were euthanized when they developed ascites and their abdominal circumference reached 12 cm in diameter or when moribund. Survival estimates were calculated with the Kaplan–Meier method and statistics performed with the Mantel–Cox log rank test using GraphPad Prism 6.
Cell culture
IE9mp1 cells were cultured in RPMI complete media supplemented with 10% fetal bovine serum, 2 mM l-glutamine, 1% minimal essential amino acid, 100 μM sodium pyruvate, 50 μM ß-mercaptoethanol, 15 mM HEPES, 100 μg/mL penicillin, and 100 μg/mL streptomycin.
Isolation of murine TALs and TILs
Mice were euthanized at 25 d (for early time point) or when their abdominal circumference reached 12 cm in diameter or when moribund (late time point) after IE9mp1 tumor challenge. TALs were derived from peritoneal wash and/or ascites and TILs were isolated by dissociating tumor tissue in the presence of liberase (25 μg/mL, Roche, 05-401119001) and DNase (100 unit/mL, Roche, 10104159001) for 30 min. Tissue clumps were removed via filtration through a 100 μm mesh. Lymphocytes were also isolated from spleens of tumor-bearing mice via homogenization and strained through the 100 μm mesh. Red blood cells from all the preparations were removed using ACK lysis buffer.
Cytokine analysis
For intracellular staining of IL2, IFNγ, and TNFα production from TALs and TILs, cells were incubated with phorbol 12-myristate 13-acetate (PMA, 100 ng/mL, Sigma, P8139) and ionomycin (500 ng/mL, Sigma, I0634) as indicated in the presence of brefeldin (10 μg/mL, Sigma, B7651-5) for 5 h. Intracellular staining was performed as previously described.24
Flow cytometry
TILs and TALs were stained with Fc Block and respective antibodies (see below). The samples were run on a FACS LSRII flow cytometer (BD Biosciences) and analyzed using FCS Express (De Novo Software). The following antibodies were used for human samples: Alexafluo-700-CD8a (Biolegend, 300920), FITC-CD4+ (eBioscience, 25-0041-82 ), Brilliant violet-421-PD-1 (Biolegend, 329919), phycoerythrin (PE)-LAG-3 (R&D systems, FAB2319P), APC-TIM-3 (Biolegend, 345011), and PE-Cy7-CTLA-4 (Biolegend, 349913), The following antibodies were used for mouse study: PE-LAG-3 (Biolegend, 125208), Brilliant violet-421-PD-1 (Biolegend, 135218), Alexafluor-700-CD8a (Biolegend, 100730), PE-Cy7-CD4 (eBioscience, 25-0041-82), APC-TIM-3 (eBioscience, 17-5871-82), and FITC-CTLA-4 (Southern Biotechnology, 1790-02), PE-CD160 (Biolegend, 143004), FITC-CD244 (2B4, eBioscience, 11-2441-82), APC-BTLA (eBioscience, 17-5956-80), FITC-KLRG1 (eBioscience, 11-5893-82), PE-Cy7-TNF-alpha (BD Biosciences, 557644), APC-IFNγ (BD Biosciences, 557644), FITC-IL2 (BD Biosciences, 554427), APC-CD25 (eBioscience, 17-0251-82) PE-FOXP3 (eBioscience, 12-5773-82), Live-dead stain (Near IR fluorescent reactive dye; Molecular probe, L10119), V450-CD45 (BD Biosciences, 560501), CD11b (BD Biosciences, 557397), FITC-Ly-6G (BD Biosciences, 551460), Alexa Fluor 700-Ly-6C (BD Biosciences 581237), PerCP-Cyanine5.5-F4/80 Antigen (Biolegend, 123128), anti-human/mouse Arginase1 APC-conjugated (R&D System, IC5868A).
Supplementary Material
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgements
We thank Ms. Adeiyewunmi Osinubi and Cheryl Eppolito for their excellent technical support.
Funding
This work was supported by the Roswell Park Alliance Foundation, NCI Cancer Center Support Grant P30 CA016056, NIH 1R01CA158318-01A1, and RPCI-UPCI Ovarian Cancer SPORE P50CA159981-01A1.
References
- 1.Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, Jungbluth AA, Frosina D, Gnjatic S, Ambrosone C et al.. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci U S A 2005; 102(51):18538-43; PMID:16344461; http://dx.doi.org/ 10.1073/pnas.0509182102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012; 12(4):252-64; PMID:22437870; http://dx.doi.org/ 10.1038/nrc3239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wherry EJ. T cell exhaustion. Nat Immunol 2011; 12(6):492-9; PMID:21739672; http://dx.doi.org/ 10.1038/ni.2035 [DOI] [PubMed] [Google Scholar]
- 4.Baitsch L, Legat A, Barba L, Fuertes Marraco SA, Rivals JP, Baumgaertner P, Christiansen-Jucht C, Bouzourene H, Rimoldi D, Pircher H et al.. Extended co-expression of inhibitory receptors by human CD8 T-cells depending on differentiation, antigen-specificity and anatomical localization. PLoS One 2012; 7(2):e30852; PMID:22347406; http://dx.doi.org/ 10.1371/journal.pone.0030852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnston RJ, Comps-Agrar L, Hackney J, Yu X, Huseni M, Yang Y, Park S, Javinal V, Chiu H, Irving B et al.. The immunoreceptor TIGIT regulates antitumor and antiviral CD8(+) T cell effector function. Cancer Cell 2014; 26(6):923-37; PMID:25465800; http://dx.doi.org/ 10.1016/j.ccell.2014.10.018 [DOI] [PubMed] [Google Scholar]
- 6.Liu J, Yuan Y, Chen W, Putra J, Suriawinata AA, Schenk AD, Miller HE, Guleria I, Barth RJ, Huang YH et al.. Immune-checkpoint proteins VISTA and PD-1 nonredundantly regulate murine T-cell responses. Proc Natl Acad Sci USA 2015; 112(21):6682-7; PMID:25964334; http://dx.doi.org/ 10.1073/pnas.1420370112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K et al.. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012; 366(26):2455-65; PMID:22658128; http://dx.doi.org/ 10.1056/NEJMoa1200694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA et al.. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012; 366(26):2443-54; PMID:22658127; http://dx.doi.org/ 10.1056/NEJMoa1200690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Callahan MK, Wolchok JD, Allison JP. Anti-CTLA-4 antibody therapy: immune monitoring during clinical development of a novel immunotherapy. Semin Oncol 2010; 37(5):473-84; PMID:21074063; http://dx.doi.org/ 10.1053/j.seminoncol.2010.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 2006; 439(7077):682-7; PMID:16382236; http://dx.doi.org/ 10.1038/nature04444 [DOI] [PubMed] [Google Scholar]
- 11.Mangsbo SM, Sandin LC, Anger K, Korman AJ, Loskog A, Totterman TH. Enhanced tumor eradication by combining CTLA-4 or PD-1 blockade with CpG therapy. J Immunother 2010; 33(3):225-35; PMID:20445343; http://dx.doi.org/ 10.1097/CJI.0b013e3181c01fcb [DOI] [PubMed] [Google Scholar]
- 12.Postow MA, Callahan MK, Wolchok JD. Immune checkpoint blockade in cancer therapy. J Clin Oncol 2015; 33(17):1974-82; PMID:25605845; http://dx.doi.org/ 10.1200/JCO.2014.59.4358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Felice F, Marchetti C, Palaia I, Musio D, Muzii L, Tombolini V, Panici PB. Immunotherapy of ovarian cancer: the role of checkpoint inhibitors. J Immunol Res 2015; 2015:191832; PMID:26236750; http://dx.doi.org/ 10.1155/2015/191832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hannani D, Vetizou M, Enot D, Rusakiewicz S, Chaput N, Klatzmann D, Desbois M, Jacquelot N, Vimond N, Chouaib S et al.. Anticancer immunotherapy by CTLA-4 blockade: obligatory contribution of IL-2 receptors and negative prognostic impact of soluble CD25. Cell Res 2015; 25(2):208-24; PMID:25582080; http://dx.doi.org/ 10.1038/cr.2015.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamanishi J, Mandai M, Ikeda T, Minami M, Kawaguchi A, Murayama T, Kanai M, Mori Y, Matsumoto S, Chikuma S et al.. Safety and antitumor activity of Anti-PD-1 antibody, nivolumab, in patients with platinum-resistant ovarian cancer. J Clin Oncol 2015; 33(34):4015-22; PMID:26351349; http://dx.doi.org/ 10.1200/JCO.2015.62.3397 [DOI] [PubMed] [Google Scholar]
- 16.Curran MA, Montalvo W, Yagita H, Allison JP. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci USA 2010; 107(9):4275-80; PMID:20160101; http://dx.doi.org/ 10.1073/pnas.0915174107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Woo SR, Turnis ME, Goldberg MV, Bankoti J, Selby M, Nirschl CJ, Bettini ML, Gravano DM, Vogel P, Liu CL et al.. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res 2012; 72(4):917-27; PMID:22186141; http://dx.doi.org/ 10.1158/0008-5472.CAN-11-1620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duraiswamy J, Kaluza KM, Freeman GJ, Coukos G. Dual blockade of PD-1 and CTLA-4 combined with tumor vaccine effectively restores T-cell rejection function in tumors. Cancer Res 2013; 73(12):3591-603; PMID:23633484; http://dx.doi.org/ 10.1158/0008-5472.CAN-12-4100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang RY, Eppolito C, Lele S, Shrikant P, Matsuzaki J, Odunsi K. LAG3 and PD1 co-inhibitory molecules collaborate to limit CD8+ T cell signaling and dampen antitumor immunity in a murine ovarian cancer model. Oncotarget 2015; 6(29):27359-77; PMID:26318293; http://dx.doi.org/ 10.18632/oncotarget.4751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, Segal NH, Ariyan CE, Gordon RA, Reed K et al.. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med 2013; 369(2):122-33; PMID:23724867; http://dx.doi.org/ 10.1056/NEJMoa1302369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fourcade J, Sun Z, Benallaoua M, Guillaume P, Luescher IF, Sander C, Kirkwood JM, Kuchroo V, Zarour HM. Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen-specific CD8+ T cell dysfunction in melanoma patients. J Exp Med 2010; 207(10):2175-86; PMID:20819923; http://dx.doi.org/ 10.1084/jem.20100637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harris NL, Ronchese F. The role of B7 costimulation in T-cell immunity. Immunol Cell Biol 1999; 77(4):304-11; PMID:10457196; http://dx.doi.org/ 10.1046/j.1440-1711.1999.00835.x [DOI] [PubMed] [Google Scholar]
- 23.Park JJ, Omiya R, Matsumura Y, Sakoda Y, Kuramasu A, Augustine MM, Yao S, Tsushima F, Narazaki H, Anand S et al.. B7-H1/CD80 interaction is required for the induction and maintenance of peripheral T-cell tolerance. Blood 2010; 116(8):1291-8; PMID:20472828; http://dx.doi.org/ 10.1182/blood-2010-01-265975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsuzaki J, Gnjatic S, Mhawech-Fauceglia P, Beck A, Miller A, Tsuji T, Eppolito C, Qian F, Lele S, Shrikant P et al.. Tumor-infiltrating NY-ESO-1-specific CD8+ T cells are negatively regulated by LAG-3 and PD-1 in human ovarian cancer. Proc Natl Acad Sci U S A 2010; 107(17):7875-80; PMID:20385810; http://dx.doi.org/ 10.1073/pnas.1003345107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wherry EJ, Ha SJ, Kaech SM, Haining WN, Sarkar S, Kalia V, Subramaniam S, Blattman JN, Barber DL, Ahmed R. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity 2007; 27(4):670-84; PMID:17950003; http://dx.doi.org/ 10.1016/j.immuni.2007.09.006 [DOI] [PubMed] [Google Scholar]
- 26.Pico de Coana Y, Poschke I, Gentilcore G, Mao Y, Nystrom M, Hansson J, Masucci GV, Kiessling R. Ipilimumab treatment results in an early decrease in the frequency of circulating granulocytic myeloid-derived suppressor cells as well as their Arginase1 production. Cancer Immunol Res 2013; 1(3):158-62; PMID:24777678; http://dx.doi.org/ 10.1158/2326-6066.CIR-13-0016 [DOI] [PubMed] [Google Scholar]
- 27.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol 2009; 9(3):162-74; PMID:19197294; http://dx.doi.org/ 10.1038/nri2506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Youn JI, Nagaraj S, Collazo M, Gabrilovich DI. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J Immunol 2008; 181(8):5791-802; PMID:18832739; http://dx.doi.org/ 10.4049/jimmunol.181.8.5791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gallina G, Dolcetti L, Serafini P, De Santo C, Marigo I, Colombo MP, Basso G, Brombacher F, Borrello I, Zanovello P et al.. Tumors induce a subset of inflammatory monocytes with immunosuppressive activity on CD8+ T cells. J Clin Invest 2006; 116(10):2777-90; PMID:17016559; http://dx.doi.org/ 10.1172/JCI28828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mahoney KM, Freeman GJ, McDermott DF. The next immune-checkpoint inhibitors: PD-1/PD-L1 blockade in melanoma. Clin Ther 2015; 37(4):764-82; PMID:25823918; http://dx.doi.org/ 10.1016/j.clinthera.2015.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duraiswamy J, Freeman GJ, Coukos G. Therapeutic PD-1 pathway blockade augments with other modalities of immunotherapy T-cell function to prevent immune decline in ovarian cancer. Cancer Res 2013; 73(23):6900-12; PMID:23975756; http://dx.doi.org/ 10.1158/0008-5472.CAN-13-1550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wei H, Zhao L, Li W, Fan K, Qian W, Hou S, Wang H, Dai M, Hellstrom I, Hellstrom KE et al.. Combinatorial PD-1 blockade and CD137 activation has therapeutic efficacy in murine cancer models and synergizes with cisplatin. PLoS One 2013; 8(12):e84927; PMID:24367702; http://dx.doi.org/ 10.1371/journal.pone.0084927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huard B, Tournier M, Hercend T, Triebel F, Faure F. Lymphocyte-activation gene 3/major histocompatibility complex class II interaction modulates the antigenic response of CD4+ T lymphocytes. Eur J Immunol 1994; 24(12):3216-21; PMID:7805750; http://dx.doi.org/ 10.1002/eji.1830241246 [DOI] [PubMed] [Google Scholar]
- 34.Workman CJ, Wang Y, El Kasmi KC, Pardoll DM, Murray PJ, Drake CG, Vignali DA. LAG-3 regulates plasmacytoid dendritic cell homeostasis. J Immunol 2009; 182(4):1885-91; PMID:19201841; http://dx.doi.org/ 10.4049/jimmunol.0800185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Agata Y, Kawasaki A, Nishimura H, Ishida Y, Tsubata T, Yagita H, Honjo T. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int Immunol 1996; 8(5):765-72; PMID:8671665; http://dx.doi.org/ 10.1093/intimm/8.5.765 [DOI] [PubMed] [Google Scholar]
- 36.Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A, Betts MR, Freeman GJ, Vignali DA, Wherry EJ. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol 2009; 10(1):29-37; PMID:19043418; http://dx.doi.org/ 10.1038/ni.1679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Odorizzi PM, Pauken KE, Paley MA, Sharpe A, Wherry EJ. Genetic absence of PD-1 promotes accumulation of terminally differentiated exhausted CD8+ T cells. J Exp Med 2015; 212(7):1125-37; PMID:26034050; http://dx.doi.org/ 10.1084/jem.20142237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koyama S, Akbay EA, Li YY, Herter-Sprie GS, Buczkowski KA, Richards WG, Gandhi L, Redig AJ, Rodig SJ, Asahina H et al.. Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nat Commun 2016; 7:10501; PMID:26883990; http://dx.doi.org/ 10.1038/ncomms10501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bruniquel D, Borie N, Hannier S, Triebel F. Regulation of expression of the human lymphocyte activation gene-3 (LAG-3) molecule, a ligand for MHC class II. Immunogenetics 1998; 48(2):116-24; PMID:9634475; http://dx.doi.org/ 10.1007/s002510050411 [DOI] [PubMed] [Google Scholar]
- 40.Walker LS, Sansom DM. The emerging role of CTLA4 as a cell-extrinsic regulator of T cell responses. Nat Rev Immunol 2011; 11(12):852-63; PMID:22116087; http://dx.doi.org/ 10.1038/nri3108 [DOI] [PubMed] [Google Scholar]
- 41.Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z, Nomura T, Sakaguchi S. CTLA-4 control over Foxp3+ regulatory T cell function. Science 2008; 322(5899):271-5; PMID:18845758; http://dx.doi.org/ 10.1126/science.1160062 [DOI] [PubMed] [Google Scholar]
- 42.Peggs KS, Quezada SA, Chambers CA, Korman AJ, Allison JP. Blockade of CTLA-4 on both effector and regulatory T cell compartments contributes to the antitumor activity of anti-CTLA-4 antibodies. J Exp Med 2009; 206(8):1717-25; PMID:19581407; http://dx.doi.org/ 10.1084/jem.20082492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martinez GJ, Pereira RM, Aijo T, Kim EY, Marangoni F, Pipkin ME, Togher S, Heissmeyer V, Zhang YC, Crotty S et al.. The transcription factor NFAT promotes exhaustion of activated CD8(+) T cells. Immunity 2015; 42(2):265-78; PMID:25680272; http://dx.doi.org/ 10.1016/j.immuni.2015.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roby KF, Taylor CC, Sweetwood JP, Cheng Y, Pace JL, Tawfik O, Persons DL, Smith PG, Terranova PF. Development of a syngeneic mouse model for events related to ovarian cancer. Carcinogenesis 2000; 21(4):585-91; PMID:10753190; http://dx.doi.org/ 10.1093/carcin/21.4.585 [DOI] [PubMed] [Google Scholar]
- 45.Woo SR, Li N, Bruno TC, Forbes K, Brown S, Workman C, Drake CG, Vignali DA. Differential subcellular localization of the regulatory T-cell protein LAG-3 and the coreceptor CD4. Eur J Immunol 2010; 40(6):1768-77; PMID:20391435; http://dx.doi.org/ 10.1002/eji.200939874 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.