Abstract
Importance
Cerebral amyloid angiopathy (CAA) is characteristically associated with MRI biomarkers of small vessel brain injury, including strictly lobar cerebral microbleeds, cortical superficial siderosis, centrum semiovale perivascular spaces, and white matter hyperintensities. Although these neuroimaging markers reflect distinct pathophysiological aspects in CAA, no studies to date have combined these structural imaging features to gauge total brain small vessel disease burden in CAA.
Objectives
To develop a composite score to capture the total brain MRI burden of small vessel disease in CAA, based on the most salient imaging signatures of the disease. Furthermore, to explore whether this score contributes independent and complementary information about CAA severity, defined as intracerebral hemorrhage (ICH) during life or bleeding-related neuropathologic changes.
Design
MRI-pathological study.
Setting
Single centre neuropathological CAA cohort based on eligible patients from the Massachusetts General Hospital (MGH) (1997–2012).
Participants
Patients with both pathological evidence of CAA (i.e. any presence of CAA from routinely collected brain biopsy, biopsy at hematoma evacuation or autopsy) and available brain MRI sequences of adequate quality including T2-weighted, T2*-weighted gradient-recalled echo (T2*-GRE) and/or SWI and FLAIR sequences.
Main outcomes and measures
Brain MRIs were rated for lobar cerebral microbleeds, cortical superficial siderosis, centrum semiovale perivascular spaces and white matter hyperintensities. All four MRI lesions were incorporated into a pre-specified ordinal total small vessel disease score ranging from 0–6 points. Associations with severity of CAA-associated vasculopathic changes (fibrinoid necrosis and concentric splitting of the wall), clinical presentation, ICH number and other imaging markers not included in the score were explored using logistic and ordinal regression.
Results
In multivariable ordinal regression analysis, severity of CAA-associated vasculopathic changes (OR: 2.40; 95%CI: 1.06–5.45; p=0.035) and CAA presentation with symptomatic ICH (OR: 2.23; 95%CI: 1.07–4.64; p=0.033) were independently associated with the total MRI small vessel disease score. The score was associated with small acute diffusion-weighted imaging lesions and posterior white matter hyperintensities in adjusted analyses.
Conclusions and Relevance
Our study provides evidence for concept validity of a total MRI small vessel disease score in CAA. After further validation, this approach can be potentially used in prospective clinical studies.
Keywords: Intracerebral hemorrhage, MRI, cerebral amyloid angiopathy, cerebral small vessel disease
Introduction
Sporadic cerebral amyloid angiopathy (CAA) is the most common cause of symptomatic lobar intracerebral haemorrhage (ICH) in the elderly and results from cerebrovascular amyloid-β deposition, preferentially involving cortical and leptomeningeal small vessels.1–4 CAA is frequently present in the brain of patients with Alzheimer’s disease, but can also have an independent contribution in age-related cognitive decline.5,6
CAA is characteristically associated with magnetic resonance imaging (MRI) biomarkers of small vessel brain injury including multiple strictly lobar cerebral microbleeds (CMBs),7 cortical superficial siderosis (cSS),8 centrum semiovale perivascular spaces (CSO-PVS)9,10 and white matter hyperintensities (WMH),1,11 Although these neuroimaging markers probably reflect distinct pathophysiological aspects or steps in CAA,4,12 they are often closely related. No studies to date have combined these structural imaging features to gauge total brain small vessel disease burden in CAA. A comprehensive approach might have potential advantages over individual markers, providing a practical framework to better assess the impact of CAA-related damage on clinical outcomes.13
Recently, the approach of assessing total MRI small vessel disease burden has been developed and applied in patients at high risk for ischaemic small vessel damage, including lacunar or non-disabling cortical stroke.14–16 By summing different MRI features of ischaemic small vessel disease in one measure (with one point assigned for each: lacunar infarct, CMBs, basal ganglia perivascular spaces and WMH), the authors demonstrated that a higher score is independently related to lacunar stroke subtype and certain vascular risk factors (age, hypertension, smoking etc.).14 In patients with lacunar stroke, this score was associated with blood pressure15 and cognition.16 Furthermore, the score was associated with lower general cognitive ability in 680 older participants.17
In this study we pre-specified and developed a total MRI small vessel disease score specifically tailored for CAA patients, based on the four major imaging signatures of the disease as described in a consensus report on CAA neuroimaging biomarkers. In a cohort with pathological evidence of CAA, we investigated whether this is a valid construct by examining: (a) whether the total MRI CAA score is associated with symptomatic CAA-related intraparenchymal bleeding events during life and severe CAA on neuropathologic examination; and (b) the association between the total MRI CAA score and other characteristic imaging findings in CAA including small positive lesions on diffusion-weighted imaging (DWI)18 and occipital-predominant WMH.19,20
Methods
Case selection and clinical data collection
We used data from a neuropathological defined CAA cohort based on eligible patients from the Massachusetts General Hospital (MGH) (1997–2012), who were systematically identified using overlapping methods as described.12,21 Patients with both pathological evidence of CAA (i.e. any presence of CAA from routinely collected brain biopsy, biopsy at hematoma evacuation or autopsy) and available in vivo brain MRI sequences of adequate quality including T2-weighted, T2*-weighted gradient-recalled echo (T2*-GRE) and/or SWI and FLAIR sequences, were considered for the study, as previously described.12,21
The clinical presentation of included patients was ascertained based on all available data, and was classified as: (a) symptomatic lobar ICH (confirmed on neuroimaging); or (b) presentation without ICH (including cognitive impairment, transient focal neurological episodes, or other neurological symptoms) at the time of the MRI.12
Standard Protocol Approvals, Registrations, and Patient Consents
The study received ethical approval by the Institutional Review Board of MGH.
Pathological data collection
Morphological assessment was performed in routine haematoxylin-eosin staining and the presence or absence and severity of vascular amyloid-β deposition was confirmed by immunohistochemical detection (anti–Ab 6E10 (1:200; Signet Laboratories, Dedham, MA) and congo red staining. Cases were considered positive for CAA when they had at least one leptomeningeal or cortical vessel with amyloid-β reported by an experienced neuropathologist, providing enough information to reliably classify CAA severity according to modified Vonsattel grading system.22,23 We systematically extracted information on the presence of CAA-related vasculopathic changes, defined as vessel-within-vessel appearance (sometimes called “double-barrelling”) and fibrinoid necrosis, corresponding to modified Vonsattel grade 3 and grade 4 respectively and hence severe CAA.23
Neuroimaging data and analysis
Imaging for all patients included T2-weighted, FLAIR, T2*-GRE (field strength: 1.5T, slice thickness: 5mm, slice gap: 1mm, echo time: 24 msec) and/or susceptibility-weighted imaging (field strength: 3T, slice thickness: 1.2mm, slice gap: 0mm, echo time: 21 msec).21 MR images were reviewed blinded to all clinical and histopathological by trained observers, according to STandards for ReportIng Vascular changes on nEuroimaging (STRIVE).24 For patients with multiple MRIs available, the MRI closest to the neuropathological assessment was examined.
CMBs presence and number were evaluated according to current consensus criteria.11 The presence and number of “macro” ICHs (>5 mm in diameter)25 was also noted. cSS was defined as linear residues of chronic blood products in the superficial layers of the cerebral cortex showing a characteristic “gyriform” pattern of low signal on blood-sensitive sequences as previously described.26 The distribution and severity of cSS was classified as focal (restricted to ≤3 sulci) or disseminated (≥4 sulci).8 cSS contiguous or potentially anatomically connected with any lobar ICH were not included in the aforementioned categories.
PVS were assessed in line with STRIVE definitions24 and rated on axial T2-weighted MR images, using a previously described validated 4-point visual rating scale (0=no PVS, 1=<10 PVS, 2=11–20 PVS, 3=21–40 PVS and 4=>40 PVS) in the basal ganglia and CSO.9,27 Deep and periventricular WMH were assessed according to the four-point Fazekas rating scale.28 WMH in the frontal and occipital lobe were further evaluated separately on axial FLAIR as described by Zhu et al.19 The frontal-occipital gradient (the WMH score in the frontal lobe minus that in the occipital lobe) was calculated (ranging −6 to 6; >0 implies frontal dominance and <0 implies occipital dominance).19
All available diffusion-weighted images were reviewed for the presence of small (generally <4mm) hyperintensities by trained raters.29
Total MRI burden of small vessel disease in CAA
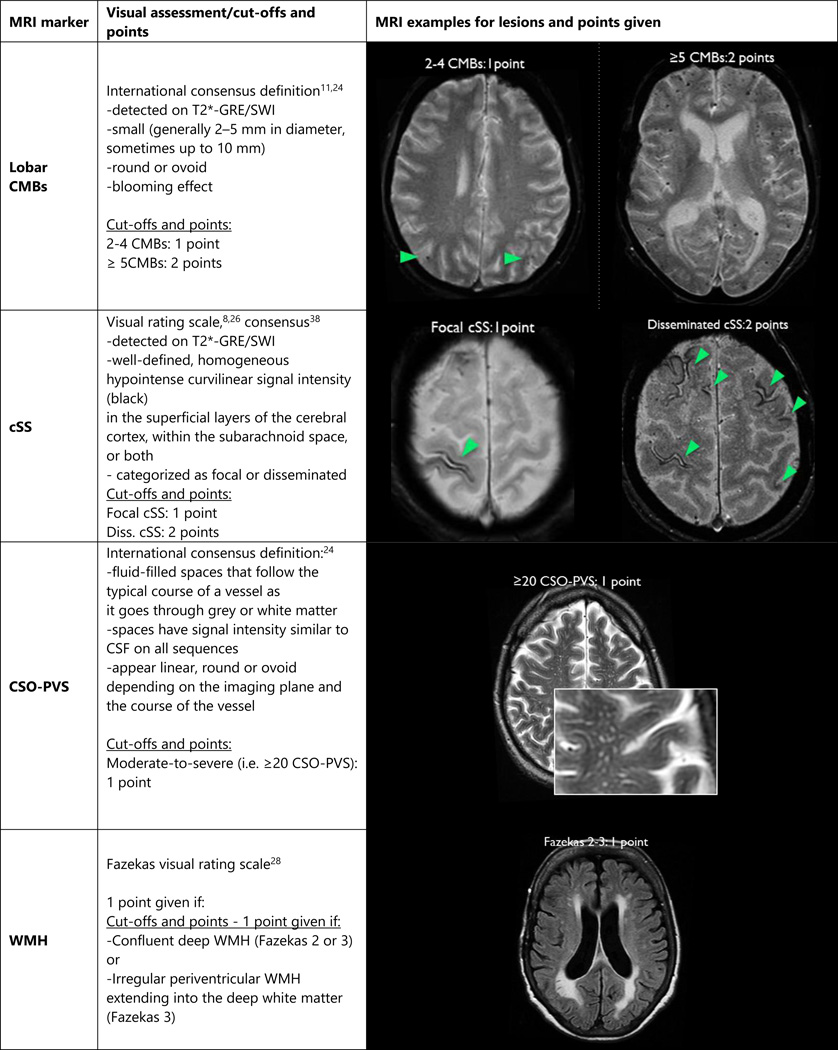
We pre-specified an ordinal scale representing the total burden of small vessel disease in CAA by incorporating the four most characteristic MRI markers of the disease (lobar CMBs, cSS, CSO-PVS and WMH). For lobar CMBs a point was awarded if 2–4 CMBs were present and two points for ≥5 CMBs. Presence of cSS was awarded with one point if focal and two points if disseminated. Presence of CSO-PVS was counted if there were moderate-to-severe (grade 3–4, i.e. >20) PVS (one point if present). Presence of WMH was defined as either (early) confluent deep (i.e. the region between juxtacortical and ventricular areas) WMH (Fazekas score ≥2) or irregular periventricular WMH extending into the deep white matter (Fazekas score 3) (one point if either present), as recently described.14 Hence, the score ranged from a minimum of 0 to a maximum of 6 points, creating an ordinal scale.
In defining these cut-offs we took into account current evidence from cross-sectional and longitudinal studies in CAA. We aimed to distinguish the weights of haemorrhagic imaging markers of CAA (lobar CMBs, cSS), from other non-haemorrhagic markers (CSO-PVS, WMH) which might also occur with high frequency in other diseases. For example, multiple (≥2) strictly lobar CMBs are the most common and characteristic imaging feature of CAA and are part of the Boston criteria;8,30 the presence of ≥5 CMBs is associated with recurrent lobar ICH.31 cSS, particularly disseminated, is a common haemorrhagic marker of CAA,26 associated with future lobar ICH risk.32 The prespecified definition of CSO-PVS (i.e. >20, grade 3) was found to be more strongly related to CAA.9,10,33 The WMH cutoffs chosen are also in line with the scores used in the suggested total MRI scale for ischaemic small vessel disease14–17 based on those Fazekas scores related to small vessel disease in a histopathological study.34
As an exploratory analysis, we tested the effect of using two alternative scores: (a) an expanded score additionally including the presence of occipital predominant WMH19 as another element (1 extra point–hence a total score ranging 0–7); and (b) a simplified version of the CAA score in line with the original MRI score suggested for ischaemic small vessel disease.14–16 For this simplified score, we rated the presence (yes vs. no) of each of the four MRI features above and assigned one point for each (i.e. presence of any lobar CMBs, any cSS, moderate-to-severe CSO-PVS and extensive WMH), resulting in an ordinal scale of 0–4.
Statistical analysis
To investigate the construct validity of this score, our statistical approach was to use the total MRI score as the dependent variable against clinical and neuropathologic markers of CAA severity. We performed univariable ordinal logistic regression to investigate the association between the MRI small vessel disease score with clinical characteristics, presence of CAA-associated vasculopathic changes and CAA clinical presentation with ICH. We performed multivariable ordinal logistic regression exploring the independent association between the presence of vasculopathic changes and CAA clinical presentation with the small vessel disease score (as dependent variable), controlling for age, sex and hypertension. To further account for potential biases of the size of pathology specimen (biopsy vs. autopsy), with full autopsy being more likely to demonstrate both some CAA as well as severe CAA pathology than biopsy, we adjusted for this in a sensitivity analysis.
Separate univariable and multivariable ordinal regression analyses were used to assess the association between the small vessel disease score and diffusion-weighted imaging lesions, occipital predominant WMH presence and symptomatic ICH number, adjusting for age and sex, and additionally for CAA-related vasculopathic changes and clinical presentation.
We tested the effect of the occipital predominant WMH as an additional score element and the alternative simplified total MRI small vessel disease score using a similar approach.
Significance level was set at 0.05. We used Stata software (Version 11.2, StataCorp.). In the ordinal regression models the proportional odds assumption was checked using relevant tests (‘omodel’ command). Collinearity was tested using the variance inflation factor (vif <10 for all variables). The manuscript was prepared with reference to the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) guidelines.35
Results
In total, 104 patients with pathologically-defined CAA were included: 52 with autopsies, 22 with brain biopsies and 30 with pathological samples from hematoma evacuations. Fifty-three patients presented with symptomatic, spontaneous lobar ICH, while 51 patients presented without any symptomatic ICH at baseline. Patients without ICH presented either with cognitive impairment (n=42, median Clinical Dementia Rating score 1; interquartile range [IQR] 0.5–2), transient focal neurological episodes (n=3), or a combination of other symptoms (n=6; including altered mental status, or seizures consistent with inflammatory CAA). Clinical and imaging characteristics are shown in Table 1. As we have previously reported mild (Vonsattel grade 1) or moderate to severe (Vonsattel grades 2–4) CAA were equally represented in the groups (p>0.2).12 Among the 52 patients with autopsy data, 39 (75%) had evidence of arteriolosclerosis (i.e. concentric hyaline thickening of small arteries) in deep (basal ganglia or deep white matter) perforating arterioles, which was moderate-to-severe in 31 cases.
Table 1.
Clinical and imaging and genetic characteristics of the CAA study cohort.
| Whole CAA cohort*¥ (N=105) |
CAA with ICH (N=54) |
CAA without ICH (N=51) |
p-value | |
|---|---|---|---|---|
| Age, mean (95% CI), years | 72.7 (71.1–74.2) | 71.6 (69.4–73.7) | 73.9 (71.5–76.2) | 0.148 |
| Sex, female n (%) | 55 (52.4) | 33 (63.1) | 22 (43.1) | 0.065 |
| Hypertension, n (%) | 66 (64.7) | 30 (57.8) | 36 (72) | 0.131 |
| Antithrombotic drug use, n (%) | 40 (44.4) | 15 (36.6) | 25 (51) | 0.170 |
|
Time from MRI-pathology, months, median (IQR) |
5.3 (0.3–31.4) | 1.3 (0.2–11) | 23.3 (1.4–50.7) | 0.0002 |
| High grade CSO-EPVS (>20), n (%) | 57 (54.8) | 29 (54.7) | 28 (54.9) | 0.985 |
| High grade BG-EPVS (>20), n (%) | 25 (24) | 11 (20.8) | 14 (27.5) | 0.424 |
| Lobar CMBs presence, n (%) | 63 (60) | 36 (66.7) | 27 (52.9) | 0.151 |
| Lobar CMBs count, median (IQR) | 2 (0–27) | 3 (0–23) | 2 (0–31) | 0.411 |
| Presence of cSS, n (%) | 38 (39.2) | 28 (51.9) | 10 (19.6) | 0.001 |
| Focal cSS, n (%) | 17 (16.2) | 10 (18.5) | 7 (13.7) | 0.505 |
| Disseminated cSS, n (%) | 21 (20) | 18 (33.3) | 3 (5.9) | <0.0001 |
| Small acute DWI lesions, n (%) | 15 (17.4) | 9 (20.9) | 6 (14) | 0.394 |
| Frontal-Occipital gradient, n (%) | 50 (49) | 26 (51) | 24 (47.1) | 0.689 |
We used Pearson’s χ2 or Fisher exact test and 2-sample t test (for normal distributions) or Wilcoxon rank sum (for non-normal distributions) to compare characteristics between CAA patients presenting with versus without ICH.
CAA pathological samples: 52 from autopsies, 22 from brain biopsies and 30 with pathological samples from hematoma evacuations.
Two different MRI scanners were used during the study period: a Siemens Trio at 3T (used in 25% of the patients) and a GE Signa at 1.5T. CMBs were detected on T2*-GRE (field strength: 1.5T, slice thickness: 5mm, slice gap: 1mm, echo time: 24 msec) and/or susceptibility-weighted imaging (field strength: 3T, slice thickness: 1.2mm, slice gap: 0mm, echo time: 21 msec), respectively.
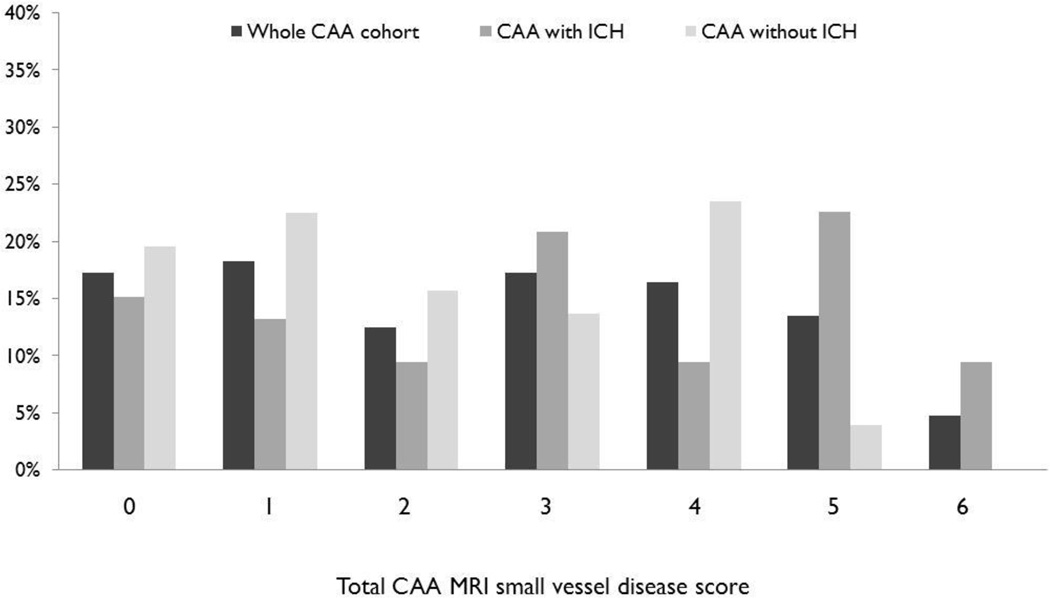
Eighteen (17.3%) patients had none of the MRI markers of small vessel disease included in the score. The majority of these patients (15/18) had only mild CAA pathology. The distribution of total small vessel disease score severity is shown in Figure 2. CAA patients with ICH had higher ratings of small vessel disease burden compared to those presenting without ICH (median score, IQR: 3 (1–5) vs. 2 (1–4) respectively; p=0.015). In univariable analysis, total small vessel disease score was associated with the presence of CAA-related vasculopathic changes on pathology (OR: 2.21; 95%CI: 1.02–4.78; p=0.045) and CAA presentation with ICH (OR: 2.37; 95%CI: 1.19–4.72; p=0.015), but not with age, hypertension, sex, or antithrombotic drug use. None of the different MRI markers comprising the score were individually associated with vasculopathic changes in univariable or multivariable logistic regression analyses including all four markers (all p>0.1).
Figure 2.
Total MRI small vessel disease score distribution for all CAA patients and separately for patients presenting with and without ICH. Mann–Whitney test CAA with ICH vs CAA without ICH: p=0.015.
In multivariable ordinal regression, the total score was associated both with the presence of vasculopathic changes and CAA presentation with ICH (Table 2). The results remained consistent and of similar effect size when hypertension was not included in the model, when the size of the pathology specimen (biopsy vs. autopsy), time from MRI to pathology and if different blood-sensitive MRI sequences were included in sensitivity analyses (data not shown). There was an increasing MRI score with increasing macrobleed count on MRI (median no: 1; range: 1–6) in the patient group with symptomatic ICH (Coef. 1.77; 95%CI: 0.08–3.45; p=0.04).
Table 2.
Multivariable ordinal logistic regression model of the total MRI small vessel disease score associations in the whole CAA cohort.
| Variable | OR (95% CI) | p-value |
|---|---|---|
| Age (per year) | 1.01 (0.97–1.05) | 0.672 |
| Sex, female | 1.78 (0.87–3.66) | 0.115 |
| Hypertension, Yes vs. No | 0.89 (0.42–1.88) | 0.763 |
| CAA-associated vasculopathic changes* | 2.40 (1.06–5.45) | 0.035 |
| CAA with symptomatic ICH, Yes vs. No | 2.23 (1.07–4.64) | 0.033 |
CAA-associated vasculopathic changes were defined as23 (a) fibrinoid necrosis in CAA-laden vessels, recognized as homogeneous discrete foci or segments of the vascular wall that contain smudgy eosinophilic material obscuring the cytoarchitecture; and (b) Concentric splitting of the wall of amyloid-laden vessels (‘double-barrelling’).
Small acute diffusion-weighted imaging lesions and occipital predominant WMH were associated with small vessel disease score in unadjusted ordinal regression, and remained so in fully adjusted models (Table 3).
Table 3.
Univariable and multivariable ordinal regression analyses of the association between total MRI small vessel disease score and DWI lesions as well as symptomatic ICH number. The association with ICH number was also explored using linear regression analyses.
| Unadjusted OR (95%CI); p-value | Age/sex adjusted OR (95%CI); p-value | Fully adjusted† OR (95%CI); p-value | |
|---|---|---|---|
|
Acute small DWI lesions* (yes vs. no) |
4.15 (1.58–10.92); p=0.004 | 3.94 (1.48–10.48); p=0.006 | 2.96 (1.10–7.95); p=0.032 |
|
Occipital predominant WMH (yes vs. no) |
1.94 (0.85–4.39); p=0.114 | 2.02 (0.88–4.64); p=0.096 | 2.30 (1–5.27); p=0.050 |
n=86 for the DWI analysis, because of missing data
Adjusted for: age, sex, vasculopathic changes and CAA clinical presentation
Alternative scores: Inclusion of occipital predominant WMH as a score element and simplified total MRI small vessel disease score
In the same multivariable regression model as above, the alternative score including occipital predominant WMH was associated with CAA-related vasculopathic changes (OR: 2.64; 95%CI: 1.13–6.14; p=0.025) and symptomatic ICH presentation (OR: 2.28; 95%CI: 1.09–4.79; p=0.029). However, while the simplified total MRI small vessel disease score was associated with CAA presentation with ICH (OR: 2.51; 95% CI: 1.19–5.30; p=0.016), there was only a trend for an association with vasculopathic changes presence (OR: 1.99; 95% CI: 0.88–4.48; p=0.099).
Discussion
In this study we developed an MRI small vessel disease score based on the four most characteristic neuroimaging signatures of sporadic CAA, to compile the overall brain burden of the disease. Using a neuropathologically-defined CAA cohort of patients presenting with and without ICH, we provide evidence of construct validity of our approach. The total small vessel disease score was found to be independently associated with CAA-related vasculopathic changes on pathology (a marker of CAA-related microangiopathy severity) and clinical presentation with symptomatic lobar ICH.
Studies have suggested pathology-based staging schemes for the extent of cerebral small vessel disease in ageing brains.36,37 In the Oxford Project to Investigate Memory and Ageing (OPTIMA) cohort, small vessel disease severity assessed using one of these pathology schemes, was correlated with cognitive impairment.37 Approaches to integrate a range of imaging manifestations of small vessel disease into one score have been recently advocated by an international working group (STRIVE).24 Some studies have presented a first attempt to capture the total MRI burden in ischaemic small vessel disease, validating this mainly in lacunar stroke populations.14–17 An assessment of total small vessel disease load on MRI has several potential advantages, as it avoids over-reliance on any one individual marker of small vessel disease. Hence, a more complete evaluation of the total burden may be important to understand the impact of small vessel disease on clinical outcomes such as disability and cognition.
In CAA, studies have shown that the combination of two different MRI markers of the disease (lobar CMBs and cSS; lobar CMBs and high grade CSO-PVS) increase diagnostic sensitivity.8,9 Our score provides a practical and easily applied structural MRI visual tool to comprehensively evaluate small vessel disease in CAA, using validated rating systems for each feature.24 We acknowledge that the assessment of the total MRI small vessel disease burden is complex, and different imaging features probably reflect distinct pathophysiological mechanisms in CAA.4,12 For example, cSS likely represents blood leaking episodes into the subarachnoid space from CAA-affected leptomeningeal vessels38 (as opposed to lobar CMBs which may arise from CAA-laden parenchymal vessels), whereas CSO-PVS might be related to cerebrovascular amyloid burden and drainage impairment10,39,40 and WMH to chronic ischaemia.41 The pathological substrates of small vessel disease imaging markers are however heterogenenous42,43 and direct histopathological-imaging correlations studies are notably underutilised in the field.44 Despite different mechanisms, these features are often related and co-occur in CAA patients.6 We also note that the chosen cut-offs and weightings in our CAA score might not be optimal and to a certain extent are arbitrary, although we did take into account the totality of current clinical, neuroimaging and neuropathological evidence from cross-sectional and longitudinal studies in defining different weights for these markers. Supporting our approach, none of the individual MRI lesions included in the score seem to be significantly driving the associations with CAA severity in isolation.
Clinically silent small areas of restricted diffusion on DWI MRI, thought to represent acute microinfarcts, are sometimes considered an additional marker of CAA.18 Their transient nature combined with the lack of neuropathological confirmation and their occurrence in hypertensive arteriopathy as well as in normal subjects45 may render it difficult to reliably use them as a specific biomarker of the disease. Based on these considerations, we did not include DWI lesions in the MRI small vessel disease score. We did however find an association between DWI lesions and total MRI score, indicating that they may be an additional marker of disease severity. Similarly, a more posterior distribution of WMH may be an additional promising marker for CAA,19,20 potentially associated with more severe disease.46
Our study has limitations. The fact that our score was tested on a clinical-pathology series of CAA, including patients with the most salient clinical manifestations of the disease, makes the current analysis powerful and informative for construct validity. However, this could also represent a constraint because of selection bias, as only symptomatic patients with both pathologic evaluation and brain MRI were included, likely representing patients at the more severe end of the disease spectrum. The differences in pathology sampling between autopsied brains and biopsies may have contributed to bias in that some CAA patients with negative brain biopsies may have been excluded. Another limitation of the current study is the retrospective (which means not all MRI sequence parameters were standardised) and cross-sectional design, which precludes any analysis on the prognostic significance of the small vessel disease scores. T1-sequences were not systematically obtained as part of the MRI protocol, precluding any assessment of lacunes and atrophy. Finally, the tacit implication of the current and previous scores is that there are only two major types of cerebral microvascular disease in the brain - CAA and 'everything else' (so called ‘hypertensive arteriopathy’, ranging from arteriolosclerosis, lipohyalinosis, fibrinoid necrosis, microaneurysm etc.) which is an oversimplification.44
Despite limitations, the suggested scale for CAA performed reasonably well in the current study and raises several questions as well as opportunities for further testing and development in large scale studies. Longitudinal cohorts should investigate whether this system, with or without modifications, can be used to assess the impact of CAA on clinical outcomes in different settings, including cognitive impairment, ICH risk and disability.13,47
Figure 1.
CAA total small vessel disease score: MRI signatures, categories and points.
Acknowledgments
"Dr Andreas Charidimou and Dr Anand Viswanathan had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis"
Funding
Funding organizations or sponsor did not have any role in: design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Authors and their individual contributions to the manuscript
Statistical analysis was conducted by Dr A. Charidimou
A. Charidimou: study concept and design, data collection, imaging analysis, data analysis, write up
S. Martinez-Ramirez: data collection, imaging analysis, critical revisions
Y. D. Reijmer: critical revisions
J. Oliveira-Filho: data collection, imaging analysis, critical revisions
M. Frosch: data collection, pathological analysis, critical revisions
A. Vashkevich: data collection and management
A. Ayres: data collection and management
J. Rosand: data collection, critical revisions, supervision
M. E. Gurol: data collection, critical revisions, supervision
S. M. Greenberg: funding, study concept and design, data collection, critical revisions
A. Viswanathan: funding, study concept and design, data collection, write up, critical revisions
Disclosures
The authors report no disclosures relevant to this work.
References
- 1.Charidimou A, Gang Q, Werring DJ. Sporadic cerebral amyloid angiopathy revisited: recent insights into pathophysiology and clinical spectrum. J Neurol Neurosurg Psychiatry. 2012 Feb;83(2):124–137. doi: 10.1136/jnnp-2011-301308. [DOI] [PubMed] [Google Scholar]
- 2.Viswanathan A, Greenberg SM. Cerebral amyloid angiopathy in the elderly. Annals of neurology. 2011 Dec;70(6):871–880. doi: 10.1002/ana.22516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vinters HV. Cerebral amyloid angiopathy. A critical review. Stroke. 1987 Mar-Apr;18(2):311–324. doi: 10.1161/01.str.18.2.311. [DOI] [PubMed] [Google Scholar]
- 4.Mandybur TI. Cerebral amyloid angiopathy: the vascular pathology and complications. J Neuropathol Exp Neurol. 1986 Jan;45(1):79–90. [PubMed] [Google Scholar]
- 5.Arvanitakis Z, Leurgans SE, Wang Z, Wilson RS, Bennett DA, Schneider JA. Cerebral amyloid angiopathy pathology and cognitive domains in older persons. Ann Neurol. 2011 Feb;69(2):320–327. doi: 10.1002/ana.22112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reijmer YD, van Veluw SJ, Greenberg SM. Ischemic brain injury in cerebral amyloid angiopathy. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2015 May 6; doi: 10.1038/jcbfm.2015.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dierksen GA, Skehan ME, Khan MA, et al. Spatial relation between microbleeds and amyloid deposits in amyloid angiopathy. Ann Neurol. 2010 Oct;68(4):545–548. doi: 10.1002/ana.22099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Linn J, Halpin A, Demaerel P, et al. Prevalence of superficial siderosis in patients with cerebral amyloid angiopathy. Neurology. 2010 Apr 27;74(17):1346–1350. doi: 10.1212/WNL.0b013e3181dad605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Charidimou A, Jaunmuktane Z, Baron JC, et al. White matter perivascular spaces: An MRI marker in pathology-proven cerebral amyloid angiopathy? Neurology. 2014 Jan 7;82(1):57–62. doi: 10.1212/01.wnl.0000438225.02729.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martinez-Ramirez S, Pontes-Neto OM, Dumas AP, et al. Topography of dilated perivascular spaces in subjects from a memory clinic cohort. Neurology. 2013 Apr 23;80(17):1551–1556. doi: 10.1212/WNL.0b013e31828f1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greenberg SM, Vernooij MW, Cordonnier C, et al. Cerebral microbleeds: a guide to detection and interpretation. Lancet Neurol. 2009 Feb;8(2):165–174. doi: 10.1016/S1474-4422(09)70013-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Charidimou A, Martinez-Ramirez S, Shoamanesh A, et al. Cerebral amyloid angiopathy with and without hemorrhage: Evidence for different disease phenotypes. Neurology. 2015 Mar 24;84(12):1206–1212. doi: 10.1212/WNL.0000000000001398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greenberg SM, Salman RA, Biessels GJ, et al. Outcome markers for clinical trials in cerebral amyloid angiopathy. Lancet Neurol. 2014 Feb 26;13(4):419–428. doi: 10.1016/S1474-4422(14)70003-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Staals J, Makin SD, Doubal FN, Dennis MS, Wardlaw JM. Stroke subtype, vascular risk factors, and total MRI brain small-vessel disease burden. Neurology. 2014 Sep 30;83(14):1228–1234. doi: 10.1212/WNL.0000000000000837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klarenbeek P, van Oostenbrugge RJ, Rouhl RP, Knottnerus IL, Staals J. Ambulatory blood pressure in patients with lacunar stroke: association with total MRI burden of cerebral small vessel disease. Stroke. 2013 Nov;44(11):2995–2999. doi: 10.1161/STROKEAHA.113.002545. [DOI] [PubMed] [Google Scholar]
- 16.Huijts M, Duits A, van Oostenbrugge RJ, Kroon AA, de Leeuw PW, Staals J. Accumulation of MRI Markers of Cerebral Small Vessel Disease is Associated with Decreased Cognitive Function. A Study in First-Ever Lacunar Stroke and Hypertensive Patients. Frontiers in aging neuroscience. 2013;5:72. doi: 10.3389/fnagi.2013.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Staals J, Booth T, Morris Z, et al. Total MRI load of cerebral small vessel disease and cognitive ability in older people. Neurobiology of aging. 2015 Oct;36(10):2806–2811. doi: 10.1016/j.neurobiolaging.2015.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Auriel E, Gurol ME, Ayres A, et al. Characteristic distributions of intracerebral hemorrhage-associated diffusion-weighted lesions. Neurology. 2012 Dec 11;79(24):2335–2341. doi: 10.1212/WNL.0b013e318278b66f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu YC, Chabriat H, Godin O, et al. Distribution of white matter hyperintensity in cerebral hemorrhage and healthy aging. Journal of neurology. 2012 Mar;259(3):530–536. doi: 10.1007/s00415-011-6218-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thanprasertsuk S, Martinez-Ramirez S, Pontes-Neto OM, et al. Posterior white matter disease distribution as a predictor of amyloid angiopathy. Neurology. 2014 Aug 26;83(9):794–800. doi: 10.1212/WNL.0000000000000732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martinez-Ramirez S, Romero JR, Shoamanesh A, et al. Diagnostic value of lobar microbleeds in individuals without intracerebral hemorrhage. Alzheimer's & dementia : the journal of the Alzheimer's Association. 2015 Dec;11(12):1480–1488. doi: 10.1016/j.jalz.2015.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vonsattel JP, Myers RH, Hedley-Whyte ET, Ropper AH, Bird ED, Richardson EP., Jr Cerebral amyloid angiopathy without and with cerebral hemorrhages: a comparative histological study. Ann Neurol. 1991 Nov;30(5):637–649. doi: 10.1002/ana.410300503. [DOI] [PubMed] [Google Scholar]
- 23.Greenberg SM, Vonsattel JP. Diagnosis of cerebral amyloid angiopathy. Sensitivity and specificity of cortical biopsy. Stroke. 1997 Jul;28(7):1418–1422. doi: 10.1161/01.str.28.7.1418. [DOI] [PubMed] [Google Scholar]
- 24.Wardlaw JM, Smith EE, Biessels GJ, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013 Aug;12(8):822–838. doi: 10.1016/S1474-4422(13)70124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kidwell CS, Greenberg SM. Red meets white: do microbleeds link hemorrhagic and ischemic cerebrovascular disease? Neurology. 2009 Nov 17;73(20):1614–1615. doi: 10.1212/WNL.0b013e3181c17fa1. [DOI] [PubMed] [Google Scholar]
- 26.Charidimou A, Jager RH, Fox Z, et al. Prevalence and mechanisms of cortical superficial siderosis in cerebral amyloid angiopathy. Neurology. 2013 Aug 13;81(7):626–632. doi: 10.1212/WNL.0b013e3182a08f2c. [DOI] [PubMed] [Google Scholar]
- 27.Doubal FN, MacLullich AM, Ferguson KJ, Dennis MS, Wardlaw JM. Enlarged perivascular spaces on MRI are a feature of cerebral small vessel disease. Stroke. 2010 Mar;41(3):450–454. doi: 10.1161/STROKEAHA.109.564914. [DOI] [PubMed] [Google Scholar]
- 28.Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA. MR signal abnormalities at 1.5 T in Alzheimer's dementia and normal aging. AJR. American journal of roentgenology. 1987 Aug;149(2):351–356. doi: 10.2214/ajr.149.2.351. [DOI] [PubMed] [Google Scholar]
- 29.Kimberly WT, Gilson A, Rost NS, et al. Silent ischemic infarcts are associated with hemorrhage burden in cerebral amyloid angiopathy. Neurology. 2009 Apr 7;72(14):1230–1235. doi: 10.1212/01.wnl.0000345666.83318.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Knudsen KA, Rosand J, Karluk D, Greenberg SM. Clinical diagnosis of cerebral amyloid angiopathy: validation of the Boston criteria. Neurology. 2001 Feb 27;56(4):537–539. doi: 10.1212/wnl.56.4.537. [DOI] [PubMed] [Google Scholar]
- 31.Greenberg SM, Eng JA, Ning M, Smith EE, Rosand J. Hemorrhage burden predicts recurrent intracerebral hemorrhage after lobar hemorrhage. Stroke. 2004 Jun;35(6):1415–1420. doi: 10.1161/01.STR.0000126807.69758.0e. [DOI] [PubMed] [Google Scholar]
- 32.Charidimou A, Peeters AP, Jager R, et al. Cortical superficial siderosis and intracerebral hemorrhage risk in cerebral amyloid angiopathy. Neurology. 2013 Nov 5;81(19):1666–1673. doi: 10.1212/01.wnl.0000435298.80023.7a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Charidimou A, Hong YT, Jager HR, et al. White matter perivascular spaces on magnetic resonance imaging: marker of cerebrovascular amyloid burden? Stroke. 2015 Jun;46(6):1707–1709. doi: 10.1161/STROKEAHA.115.009090. [DOI] [PubMed] [Google Scholar]
- 34.Fazekas F, Kleinert R, Offenbacher H, et al. Pathologic correlates of incidental MRI white matter signal hyperintensities. Neurology. 1993 Sep;43(9):1683–1689. doi: 10.1212/wnl.43.9.1683. [DOI] [PubMed] [Google Scholar]
- 35.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007 Oct 20;370(9596):1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 36.Deramecourt V, Slade JY, Oakley AE, et al. Staging and natural history of cerebrovascular pathology in dementia. Neurology. 2012 Apr 3;78(14):1043–1050. doi: 10.1212/WNL.0b013e31824e8e7f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smallwood A, Oulhaj A, Joachim C, et al. Cerebral subcortical small vessel disease and its relation to cognition in elderly subjects: a pathological study in the Oxford Project to Investigate Memory and Ageing (OPTIMA) cohort. Neuropathology and applied neurobiology. 2012 Jun;38(4):337–343. doi: 10.1111/j.1365-2990.2011.01221.x. [DOI] [PubMed] [Google Scholar]
- 38.Charidimou A, Linn J, Vernooij MW, et al. Cortical superficial siderosis: detection and clinical significance in cerebral amyloid angiopathy and related conditions. Brain. 2015 Aug;138(Pt 8):2126–2139. doi: 10.1093/brain/awv162. [DOI] [PubMed] [Google Scholar]
- 39.Charidimou A, Meegahage R, Fox Z, et al. Enlarged perivascular spaces as a marker of underlying arteriopathy in intracerebral haemorrhage: a multicentre MRI cohort study. J Neurol Neurosurg Psychiatry. 2013 Jun;84(6):624–629. doi: 10.1136/jnnp-2012-304434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Veluw SJ, Biessels GJ, Bouvy WH, et al. Cerebral amyloid angiopathy severity is linked to dilation of juxtacortical perivascular spaces. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2015 Dec 7; doi: 10.1177/0271678X15620434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gurol ME, Viswanathan A, Gidicsin C, et al. Cerebral amyloid angiopathy burden associated with leukoaraiosis: a positron emission tomography/magnetic resonance imaging study. Ann Neurol. 2013 Apr;73(4):529–536. doi: 10.1002/ana.23830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Veluw SJ, Biessels GJ, Klijn CJ, Rozemuller AJ. Heterogeneous histopathology of cortical microbleeds in cerebral amyloid angiopathy. Neurology. 2016 Feb 3; doi: 10.1212/WNL.0000000000002419. [DOI] [PubMed] [Google Scholar]
- 43.Fisher M. Cerebral microbleeds: where are we now? Neurology. 2014 Oct 7;83(15):1304–1305. doi: 10.1212/WNL.0000000000000871. [DOI] [PubMed] [Google Scholar]
- 44.Charidimou A, Pantoni L, Love S. The concept of sporadic cerebral small vessel disease: A road map on key definitions and current concepts. International journal of stroke : official journal of the International Stroke Society. 2016 Jan;11(1):6–18. doi: 10.1177/1747493015607485. [DOI] [PubMed] [Google Scholar]
- 45.Batool S, O'Donnell M, Sharma M, et al. Incidental magnetic resonance diffusion-weighted imaging-positive lesions are rare in neurologically asymptomatic community-dwelling adults. Stroke. 2014 Jul;45(7):2115–2117. doi: 10.1161/STROKEAHA.114.005782. [DOI] [PubMed] [Google Scholar]
- 46.Biffi A, Halpin A, Towfighi A, et al. Aspirin and recurrent intracerebral hemorrhage in cerebral amyloid angiopathy. Neurology. 2010 Aug 24;75(8):693–698. doi: 10.1212/WNL.0b013e3181eee40f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jickling GC, Chen C. Rating total cerebral small-vessel disease: Does it add up? Neurology. 2014 Sep 30;83(14):1224–1225. doi: 10.1212/WNL.0000000000000843. [DOI] [PubMed] [Google Scholar]