Abstract
Objective. Total knee arthroplasty is a painful procedure. No studies have evaluated modifiable predictors of acute postoperative pain trajectories during hospitalization.
Methods. Consecutive patients (N = 188) were enrolled in a longitudinal cohort study and completed a demographic questionnaire, as well as the Brief Pain Inventory, Hospital Depression and Anxiety Scale, Lee Fatigue Scale, Fatigue Severity Scale, and Brief Illness Perception Questionnaire on the day before surgery. Clinical data were extracted from medical records.
Setting and Patients. Each patient completed a pain diary that assessed pain at rest and with activity, and hours per day in pain every evening from day of surgery until postoperative day 3. Using hierarchical linear modeling, we investigated which demographic, clinical, symptom, and psychological characteristics predicted initial levels as well as the trajectories of acute pain at rest and with activity, and hours per day in pain.
Results. Higher levels of all three acute pain characteristics on the day of surgery resulted in worse trajectories. Higher pain scores with rest and with activity on the day of surgery were associated with more days with femoral block, higher average dose of opioids, and higher emotional response to osteoarthritis. Higher number of comorbidities, higher average dose of opioids, and lower perceived control predicted more hours per day in pain on the day of surgery.
Conclusions. This study identified several potentially modifiable predictors of worsening pain trajectories following total knee arthroplasty. Optimal pain management warrants identification of these high-risk patients and treatment of modifiable risk factors.
Keywords: Total Knee Arthroplasty, Acute Postoperative Pain, Pain at Rest, Pain with Activity, Hierarchical Linear Modeling
Introduction
Total knee arthroplasty (TKA) for osteoarthritis (OA) is an effective, albeit painful [1,2] procedure to relieve pain and increase function. Despite improvements in pain management [3], 58% of patients report moderate to severe pain at rest immediately following TKA [4]. Moderate to intolerable pain in the first week following TKA was associated with a three to 10 times higher risk for the development of chronic pain [5]. These findings underline the importance of identifying modifiable predictors that potentially may relieve inadequately managed postoperative pain. In addition, recent studies emphasize the need to evaluate acute pain at rest and with activity following surgical procedures [6].
To date, only two longitudinal studies evaluated risk factors for acute postoperative pain (i.e., from the day of surgery (DOS) to hospital discharge) [7,8] with rest and activity after TKA. In terms of pain at rest, the following predictors were identified: younger age [8], higher preoperative resting pain [8], and depression [8]. In terms of pain with activity, predictors associated with higher pain scores included higher preoperative pain with activity [7,8], higher pain intensity with mechanical stimuli [8], higher anxiety [7], as well as higher catastrophizing [7]. While both of these studies were longitudinal, neither reported predictors of the trajectories of acute postoperative pain at rest and with activity following TKA. Therefore, as noted in a systematic review [9], additional longitudinal studies are needed to evaluate modifiable predictors of acute postoperative pain at rest and with activity following TKA. Knowledge of modifiable risk factors would enable clinicians to identify patients at higher risk and initiate appropriate interventions to decrease acute postoperative pain.
The large amount of inter-individual variability in patients’ pain experiences is a major challenge in acute pain management. In addition, acute pain is a dynamic process that changes over time and can be influenced by a number of factors. An analysis of predictors of acute postoperative pain trajectories [10] acknowledges pain as a dynamic process, takes inter-individual variability into account, and may identify modifiable risk factors for more severe pain.
To our knowledge, no studies have used hierarchical linear modeling (HLM) to identify modifiable and non-modifiable predictors of the trajectories of acute postoperative pain with and without activity following TKA. HLM is a statistical approach that allows one to evaluate trajectories of pain as well as characteristics that influence these trajectories. The purposes of this study using HLM were to describe the trajectories of acute pain at rest and with activity, and hours per day in pain from DOS until postoperative day (POD) 3, and to identify demographic, clinical, symptom, and psychological characteristics that predicted each of these outcomes.
Methods
Patients and Study Procedures
This study is part of a longitudinal study of pain, symptoms, and health-related quality of life in patients who underwent a TKA for osteoarthritis (OA) at a surgical clinic in Oslo, Norway. Patients (N = 188) were included if they were >18 years; were able to read, write, and understand Norwegian; and were scheduled for unilateral primary TKA. Patients were excluded if they underwent unicompartmental or revision surgery.
Patients received written information about the study either by mail prior to admission or on the day of admission. Most patients were admitted to the hospital the day before surgery. Patients who met the inclusion criteria were invited to participate by a nurse on the day of admission. After obtaining written informed consent, patients completed a questionnaire that assessed demographic characteristics, preoperative pain, preoperative symptoms, and psychological factors. Preoperative clinical tests, comorbidities, body mass index (BMI), and information on medications were obtained from the medical records.
After surgery, patients completed a pain diary that evaluated acute pain intensity with and without activity and hours per day in pain, every night from the DOS until POD3. The completed questionnaires and the pain diaries were collected by the nursing staff in sealed envelopes.
A power analysis with an alpha level of 0.05, power of 0.80 and a medium effect size (f = 0.25) for multiple regression yielded a sample size estimation of 180. A total of 200 patients were recruited which allowed for 10% attrition rate. The study was approved by the Regional Medical Research Ethics Committee of Health South East of Norway (# 2011/1755).
Surgical Procedures
The anesthesia, surgery, postoperative pain management, and physical therapy procedures were standardized. All patients received the same cruciate-retaining implant for the TKA. A tourniquet was used during surgery and drains were placed and removed on POD1. Spinal block with bupivacaine and sedation were the first choice for anesthesia. Epidural analgesia (EDA) with continuous infusion of bupivacaine 1 milligram/milliliter (mg/ml), adrenaline 2 micrograms (µg)/ml, and fentanyl 2 µg/ml (5–12 ml/hour) was the first choice for acute postoperative pain management.
If spinal blockade and epidural analgesia was contraindicated, patients received total intravenous anesthesia and a continuous femoral nerve block (CFNB) with bupivacaine 2.5 mg/ml at 4–10 ml/hour for postoperative pain management. In most cases, the regional block (i.e., CFNB, EDA) was removed on POD2. Oral acetaminophen 1 gram was given every 6 hours and celecoxib 200 mg and controlled release oxycodone 5–20 mg was given every 12 hours unless contraindicated. Immediate release oxycodone 5 mg tablets or intravenous ketobemidone 2.5–5 mg were available as rescue medications. If pain control was not satisfactory, low dose ketamine 1.5 µg/kg/minute was administered as a short-term intravenous infusion (usually on the DOS).
Patients were mobilized out of bed and allowed full weight bearing on the operated knee on POD1. No continuous passive motion was used. Patients received standardized physical therapy on a daily basis with walking, flexion, and extension of the knee beginning on POD1. Patients were usually discharged on POD3 or POD4.
Clinical and Perioperative Characteristics
Data on type of implant, American Society of Anesthesiologists (ASA) physical status classification [11], length of surgery, tourniquet use, infections (i.e., deep prosthetic, wound), as well as comorbidities, BMI, preoperative blood pressure, hemoglobin, C-reactive protein, and creatinine levels were obtained from medical records. Data on anesthesia regimen, number of days with epidural or femoral block, and doses of postoperative pain medications were obtained from patients’ medical records and were included in this analysis as covariates. All opioid analgesics were converted to intravenous (IV) morphine equivalents using the European Association for Palliative Care recommendations for opioid conversion [12] and were collapsed into single time-invariant composite variables for the purpose of the analysis.
Pain Measures
The Brief Pain Inventory
The Brief Pain Inventory (BPI) [13] was used to measure OA pain prior to surgery and pain’s impact on function. The BPI consists of four items that measure pain intensity on a 0 to 10 numeric rating scale (NRS), one item that measures pain relief, a body map to assess pain locations, and seven items that measure interference with function. The BPI is sensitive to changes in pain intensity. A change of two units on the 11-point scale is considered to be clinically meaningful [14]. The Norwegian version of the BPI has well-established validity and reliability [15].
Pain Diary
Patients rated their acute pain at rest and with activity using a 0 to 10 NRS. In addition, the patients were asked to indicate in the past 24 hours how many hours they had experienced pain of ≥ 4. The patients completed the pain diary every night from the DOS until POD3.
Symptom Measures
Fatigue Severity
The five-item Lee Fatigue Scale (LFS) was used to evaluate fatigue severity prior to surgery. Each item was rated on a 0 to 10 NRS. A total score was calculated as the mean of the five items, with higher scores indicating higher fatigue severity. The LFS has satisfactory validity and reliability [16,17]. In this study, its Cronbach’s alpha was 0.90.
Fatigue Interference
The seven-item Fatigue Severity Scale (FSS-7) was used to measure fatigue interference during the past week prior to surgery. Patients rated their agreement with seven statements, using a seven-point Likert scale that ranged from disagree to agree. FSS-7 has better psychometric properties than the original nine-item questionnaire [18–20]. A total score can range from 1 to 7. In this study, its Cronbach’s alpha was 0.93.
Mood States
The Hospital Anxiety and Depression Scale (HADS) [21] was used to measure preoperative depression and anxiety. The scale consists of 14 items (seven for depression and seven for anxiety). On each subscale, scores can range from 0 to 21 with higher scores indicating higher levels of anxiety and depression. Psychometric properties of the Norwegian version of HADS were excellent in a large population-based study in Norway [22]. In this study, the Cronbach’s alphas for the depression and the anxiety subscales were 0.79 and 0.85, respectively.
Psychological Measure
The Brief Illness Perception Questionnaire
The Brief Illness Perception Questionnaire (BIPQ) [23] was used to measure self-reported illness perception prior to surgery. The scale consists of eight items that measure different dimensions of self-reported illness perception: consequences, timeline, personal control, treatment control, identity, illness concern, coherence, and emotional response. Each item was rated on a 0 to 10 NRS. For this study, patients rated their illness perception in relation to their OA knee. Five items from the BIPQ (consequences, personal control, identity, concern, and emotional response) were used in the statistical analyses because these specific items were sensitive to change over time in patients with traumatic injuries [24].
Statistical Analyses
Descriptive statistics were performed to characterize the sample, using SPSS version 22 (IBM, Armonk, NY). For acute pain at rest and with activity, HLM based on full maximum likelihood estimation was performed using the software developed by Raudenbush and Bryk [25].
As previously described by Miaskowski and colleagues [26]: “Compared with other methods for analyzing change, HLM has two major advantages. First, it can accommodate unbalanced designs, which allows for the analysis of data when the number and spacing of assessments vary across respondents. Second, HLM has the ability to model individual change, which helps to identify more complex patterns of change that are often overlooked by other methods. With HLM, repeated measures of the outcome variable (e.g., pain intensity at rest) are conceptualized as being nested within individuals and the analysis of change in the outcome variable is at 2 levels: within persons (level 1) and between persons (level 2). At level 1, the outcome is conceptualized as varying within individuals and is a function of person-specific change parameters plus error. At level 2, the person-specific change parameters are multivariate outcomes that vary across individuals. Level 2 outcomes can be modeled as a function of a number of predictors that vary between individuals, plus an error associated with the individual. Combining level 1 and level 2 results in a mixed model with fixed and random effects.”
Separate HLM analyses were done to evaluate for changes over time in acute pain intensity scores at rest and with movement. During level 1, intra-individual variability in pain intensity over time was examined. Three level 1 models were compared to determine whether pain intensity scores did not change over time (i.e., no time effect), changed at a constant rate (i.e., linear time effect), or changed at a rate that accelerated or decelerated over time (i.e., quadratic effect). At this point, the level 2 model was constrained to be unconditional (i.e., no predictors) and likelihood ratio tests were used to determine the best model.
At the second level of the HLM analysis, inter-individual differences in the trajectories of pain intensity were examined by modeling the individual change parameters (i.e., intercept and the linear and the quadratic components of the slope) as a function of proposed predictors. Table 1 presents a list of the proposed predictors that was developed based on a review of the literature from cross sectional and longitudinal studies of pain in patients undergoing TKA [7,8,27–31]. To improve estimation efficiency and construct a parsimonious model, exploratory level 2 analyses were performed. Each potential predictor was assessed to determine whether it would result in a better fitting model if it alone was added as a level 2 predictor. Predictors with a t value of < 2.0, which indicates a lack of significant effect, were excluded from further model testing. All significant predictors from the exploratory analysis were entered into the model to predict each individual change parameter. Only predictors that maintained a statistically significant contribution in conjunction with other variables were retained in the final model. A P value of < 0.05 indicates statistical significance.
Table 1.
Potential predictors of the intercept, linear coefficient, and quadratic coefficient for pain at rest, pain with activity, and hours per day in pain
| Pain at rest (N = 188) | Pain with activity (N = 188) | Hours in pain (N = 185) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Demographic characteristics | I | LC | QC | I | LC | QC | I | LC | QC |
| Age | x | x | x | x | |||||
| Sex | |||||||||
| Education level | |||||||||
| Cohabitation status | |||||||||
| Employment status | x | x | |||||||
| Preoperative clinical characteristics | |||||||||
| Body mass index | |||||||||
| Number of comorbidities | x | x | x | ||||||
| American Society of Anesthesiologists’ physical status classification | x | ||||||||
| Systolic blood pressure | |||||||||
| Diastolic blood pressure | |||||||||
| C-reactive protein | |||||||||
| Hemoglobin | |||||||||
| Pain characteristics | |||||||||
| Average pain prior to surgery | |||||||||
| Worst pain prior to surgery | x | x | |||||||
| Pain at rest, day of surgery | n/a | x | x | n/a | n/a | n/a | n/a | n/a | n/a |
| Pain with activity, day of surgery | n/a | n/a | n/a | n/a | x | x | n/a | n/a | n/a |
| Hours per day in pain, day of surgery | n/a | n/a | n/a | n/a | n/a | n/a | n/a | x | x |
| Pain interference with function | x | x | x | ||||||
| Perioperative characteristics: | |||||||||
| Side of knee surgery | x | x | |||||||
| Type of anesthesia | x | x | x | ||||||
| Length of surgery (minutes) | |||||||||
| Pain management characteristics | |||||||||
| Number of days with epidural analgesia | x | x | x | x | x | ||||
| Number of days with continuous femoral nerve block | x | x | x | x | x | ||||
| Number of days with ketamine | x | x | x | ||||||
| Average dose of opioids over 4 days | x | x | x | ||||||
| Symptoms | |||||||||
| Fatigue severity | x | x | x | ||||||
| Fatigue interference | x | ||||||||
| Depression | |||||||||
| Anxiety | x | x | x | ||||||
| Psychological characteristics from the BIPQ | |||||||||
| Consequences | x | x | |||||||
| Personal control | x | ||||||||
| Identity | x | x | x | ||||||
| Concern | x | ||||||||
| Emotional response | x | x | x | ||||||
I = intercept; LC = linear coefficient; QC = quadratic coefficient; BIPQ = Brief Illness Perception questionnaire; x = t values >2.0 in exploratory analyses.
Number of hours per day in pain was not normally distributed, as is common for count variables. Poisson regression for cross-sectional data [32–35] or multilevel Poisson regression for longitudinal data [36,37] are often used to evaluate a right-skewed count variable. However, the use of Poisson regression assumes that the variance of the distribution is equal to the mean, which was not true for number of hours per day in pain. When the variance is greater than the mean as with these data, the distribution is called “overdispersed,” and a negative binomial regression model is used [38]. Therefore, the change in the number of hours per day in pain was examined with multilevel negative binomial regression using Stata Release 13 (StataCorp, College Station, TX) [39,40]. Multilevel negative binomial regression models were estimated using maximum likelihood estimation with mean and variance-adjusted adaptive Gauss-Hermite quadrature, the best method of estimation for random effects for Poisson and negative binomial regression models [41]. While the figures for number of hours per day in pain are based on estimated values in log scales, Table 2 presents the exponents of the estimated values. The expected value for the intercept in a negative binomial model, in exponential terms (the incidence rate ratio) is 1.0, not the customary value of zero.
Table 2.
Hierarchical linear models of the trajectories for pain at rest, pain with activity, and hours per day in pain
| Pain at rest (N = 188) | Coefficient (SE) |
|
|---|---|---|
| Unconditional model | Final model | |
| Fixed effects | ||
| Intercept | 2.140 (0.147)* | 2.153 (0.136)* |
| Linear rate of change | 1.028 (0.186)* | 1.012 (0.214)* |
| Quadratic rate of change | −0.358 (0.055)* | −0.354 (0.061)* |
| Time invariant covariates | ||
| Intercept | ||
| Number of days with continuous femoral nerve block | 0.323 (0.102)** | |
| Average dose of opioid over 4 days | 0.060 (0.010)* | |
| Emotional response | 0.097 (0.027)* | |
| Change over time | ||
| Pain at rest, DOS | ||
| Linear rate of change | 0.369 (0.075)* | |
| Quadratic rate of change | −0.115 (0.026)* | |
| Variance component | ||
| In intercept | 2.388* | 1.720* |
| In linear rate | 1.750*** | 3.750 |
| In quadratic rate | 0.077 | 0.192 |
| Goodness of fit deviance (df) | 2606.372 (10) | 2526.377 (15) |
| Model comparison (χ2) | 79,995 (5)* | |
| Pain with activity (N = 188) |
Coefficient (SE) |
|
| Unconditional model | Final model | |
| Fixed effects | ||
| Intercept | 3.071 (0.189)* | 3.064 (0.175)* |
| Linear rate of change | 1.765 (0.200)* | 1.778 (0.235)* |
| Quadratic rate of change | −0.492 (0.059)* | −0.495 (0.065)* |
| Time invariant covariates | ||
| Intercept | ||
| Number of days with continuous femoral nerve block | 0.349 (0.123)** | |
| Average dose of opioid over 4 days | 0.060 (0.012)* | |
| Emotional response | 0.118 (0.032)* | |
| Change over time | ||
| Pain with activity, DOS | ||
| Linear rate of change | 0.330 (0.065)* | |
| Quadratic rate of change | −0.082 (0.023)* | |
| Variance component | ||
| In intercept | 4.311* | 3.290* |
| In linear rate | 1.347 | 3.982* |
| In quadratic rate | 0.028 | 0.380 |
| Goodness of fit deviance (df) | 2728.181 (10) | 2655.759 (15) |
| Model comparison (χ2) | 72.422 (5)* | |
| Hours per day in pain (N = 185) |
Incidence risk ratio (SE) |
|
| Unconditional model | Final model | |
| Fixed effects | ||
| Intercept | 1.382 (0.157)** | 1.174 (0.144) |
| Linear rate of change | 2.435 (0.326)* | 2.720 (0.368)* |
| Quadratic rate of change | 0.766 (0.033)* | 0.753 (0.032)* |
| Time invariant covariates | ||
| Intercept: | ||
| Number of comorbidities | 1.250 (0.110)** | |
| Average dose of opioid over 4 days | 1.078 (0.012)* | |
| Personal control | 1.114 (0.041)** | |
| Change over time | ||
| Hours per day in pain, DOS | ||
| Linear rate of change | 0.902 (0.003)** | |
| Quadratic rate of change | 1.020 (0.011) | |
| Variance component | ||
| In intercept | 0.815 | 0.843 |
| In linear rate | 0.103*** | 0.058*** |
| Akaike’s information criterion (df) | 2807.471 (6) | 2748.577 (11) |
df = degrees of freedom; SE = standard error; DOS = day of surgery. Time was coded as zero on the day of surgery. The coefficients reported for hours in pain are incidence rate ratios. Null hypothesized value for the intercept of hours in pain = 1. Significance for variance components for hours in pain is interpreted from the confidence intervals.
*P < 0.001.
**P < 0.01.
***P < 0.05.
A trajectory requires an initial point (i.e., DOS) with a shape defined by a mathematical function, including an end point of interest (i.e., POD 3). This definition does not require a specific number of observations as long as it is a theoretically justified trajectory that fits the data in a statistical sense [10].
The terminology “predictor” within the HLM context does not necessarily imply causality but simply implies an association. While the individual coefficients for the linear and quadratic components of the curve are reported in Table 2, these coefficients cannot be interpreted individually to understand the effect of a predictor variable. Thus, the effect of each predictor variable on a patient’s pain trajectory is shown in figures. The figures provide the reader with the clinical meaning of changes in pain experience for two persons with higher versus lower scores on a predictor variable.
Results
Patient Characteristics
Of the 245 patients who were invited to participate, six had their surgery cancelled and 33 declined to participate. A total of 206 patients agreed to participate and were enrolled into the study. Two patients were excluded after surgery because of postoperative disorientation, one patient died from postoperative complications, and 15 had incomplete data on a number of predictors. The patients with incomplete data were significantly older (mean age: 74 years [SD 7.45], P = 0.009) than those with complete data. A total of 188 (77%) patients are included in this analysis.
The demographic, clinical, symptom, and psychological characteristics of the patients are shown in Table 3. The majority of the patients were female (68.6%), were married or partnered (61.2%), retired or unemployed (63.3%), and had completed higher education (52.7%). The mean age of the patients was 67.7 years (SD = 9.1). The average opioid consumption per day is shown in Table 3. The number of patients who received ketobemidone as rescue medication decreased during the 4 days (i.e., DOS: N = 107, 57%; POD1: N = 28, 15%; POD2: N = 19, 10%; POD3: N = 5, 3%). In addition, a total of 27 patients received ketamine as a rescue pain medication.
Table 3.
Demographic, clinical, symptom, and psychological characteristics of patients (N = 188) at enrollment
| Characteristic | |||
|---|---|---|---|
| Demographic characteristics | Mean | SD | |
| Age | Years | 67.7 | 9.1 |
| N | % | ||
| Sex | Female | 129 | 68.6 |
| Cohabitation status | Married/partnered | 115 | 61.2 |
| Employment status | Unemployed/retired | 119 | 63.3 |
| Education level | College/university level | 99 | 52.7 |
| Preoperative clinical characteristics | Mean | SD | |
| Body mass index | 29.3 | 4.8 | |
| Number of comorbidities (0–5) | 1.1 | 1.0 | |
| American Society of Anesthesiologists’ physical status classification score (1–3) | 2.0 | 0.5 | |
| Systolic blood pressure | 138.2 | 15.3 | |
| Diastolic blood pressure | 81.8 | 11.0 | |
| C-reactive protein | 3.2 | 2.9 | |
| Hemoglobin | 13.9 | 1.1 | |
| Creatinine | 75.4 | 15.7 | |
| Preoperative pain characteristics | Mean | SD | |
| Average pain | 5.3 | 1.7 | |
| Worst pain | 5.5 | 2.1 | |
| Pain interference with function | 4.5 | 2.0 | |
| Perioperative characteristics | N | % | |
| Surgical side | Right side | 98 | 52.1 |
| Anesthesia | Neuraxial block | 163 | 86.7 |
| Total intravenous anesthesia | 25 | 13.3 | |
| Mean | SD | ||
| Length of surgery (minutes) | 65.1 | 13.6 | |
| Pain management characteristics | |||
| Number of days with epidural analgesia (N = 160) | 2.1 | 0.4 | |
| Number of days with continuous femoral nerve block (N = 29) | 2.1 | 0.4 | |
| Number of days with ketamine (N = 27) | 1.3 | 0.4 | |
| Average dose of opioids (mg)* | 13.3 | 7.5 | |
| Opioid dose, day of surgery | 12.5 | 13.6 | |
| Opioid dose, postoperative day 1 | 13.7 | 9.0 | |
| Opioid dose, postoperative day 2 | 15.9 | 8.0 | |
| Opioid dose, postoperative day 3 | 11.1 | 8.6 | |
| Symptoms | Mean | SD | |
| Fatigue severity | 2.7 | 2.1 | |
| Fatigue interference | 4.0 | 1.5 | |
| Depression | 3.5 | 3.1 | |
| Anxiety | 4.6 | 3.5 | |
| Psychological characteristics** | |||
| Consequences | 6.4 | 1.8 | |
| Personal control | 5.4 | 2.4 | |
| Identity | 6.7 | 1.6 | |
| Concern | 5.1 | 2.7 | |
| Emotional response | 4.5 | 2.6 | |
HAD-S = Hospital Anxiety and Depression Scale; mg = milligram; SD = standard deviation.
*All opioids were converted to intravenous morphine equivalents. Value is the average dose of opioids over 4 days.
**Single item scores from the Brief Illness Perception Questionnaire.
Individual and Mean Change in Pain Characteristics
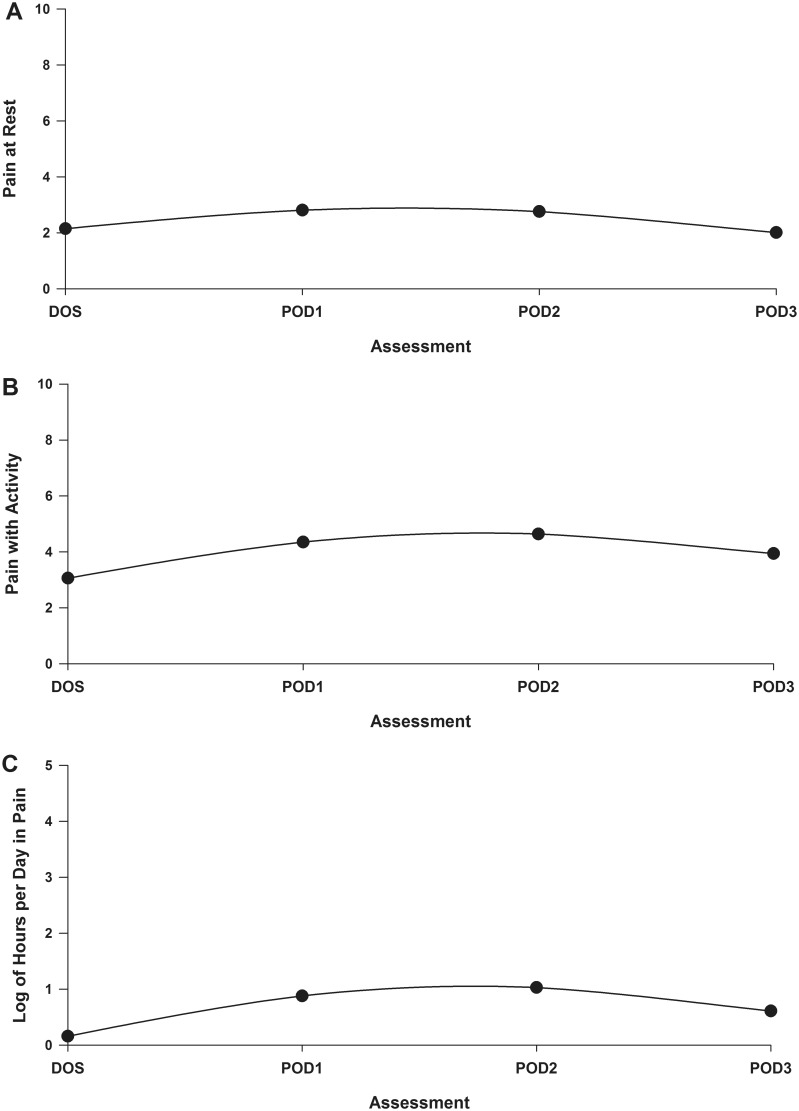
The goodness of fit tests of the deviance among the models indicated that a quadratic model fit the data best for all three pain characteristics. Table 2 presents the estimates of the unconditional quadratic change models for the level 1 analysis. Because the models have no covariates, the intercepts represent the estimated levels of acute pain at rest (2.140), acute pain with activity (3.071), and hours per day in pain (1.382). Figures 1A through 1C present the quadratic trajectories for the three pain characteristics. The trajectories for all three pain characteristics display the same pattern with increasing trends reaching a peak on POD2, before decreasing from POD2 to POD3. The pain scores presented in the figures are estimated values based on the HLM and binomial regression analyses.
Figure 1.
Trajectories of pain at rest (A), pain with activity (B), and log of hours per day in pain (C) using an unconditional model.
Inter-Individual Differences in the Trajectories of Acute Pain at Rest
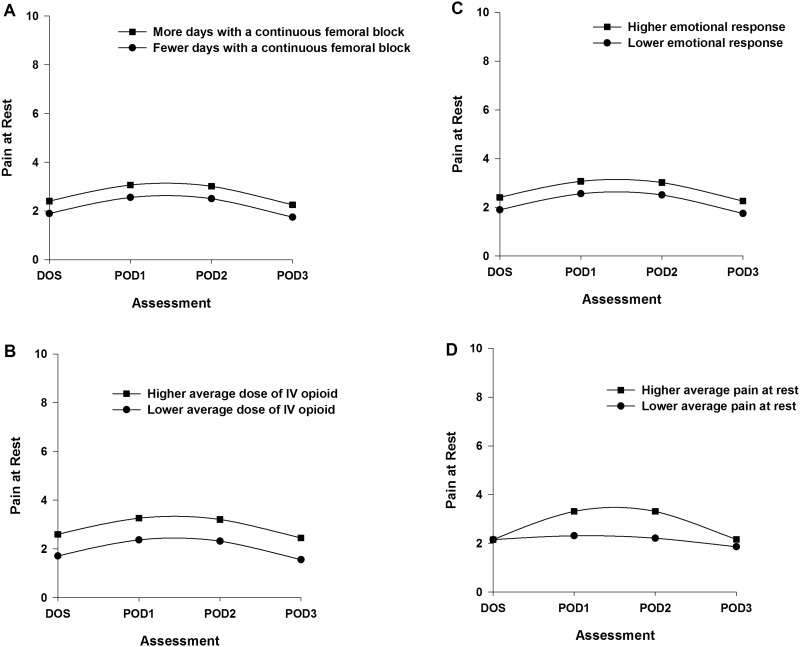
Two postsurgical (i.e., number of days with a CFNB, average dose of opioid over 4 days) and one presurgical (i.e., emotional response to OA) characteristics were associated with initial levels of pain at rest. Figures 2A through 2C display the estimated effects of each of these characteristics on pain at rest based on differences in number of days with CFNB (higher/lower number based on ± 1 standard deviation (SD) of the number of days, Figure 2A); average dose of opioid over 4 days (higher/lower based on ± 1 SD of the mean dose, Figure 2B); and emotional response (higher/lower based on ± 1 SD of the mean score, Figure 2C). One postsurgical characteristic (i.e., pain at rest on the DOS) was associated with the trajectory of pain at rest. Figure 2D displays the effect of pain at rest on the DOS on the trajectory of pain at rest.
Figure 2.
Trajectories of pain at rest by number of days with a continuous femoral nerve block (A), average dose of intravenous opioid equivalents (B), emotional response to osteoarthritis (C), and average pain at rest on the day of surgery (D) from the day of surgery until postoperative day 3. Higher/lower differences in Figures 2A–C were calculated based on 1 standard deviation above/below the predicted value.
Inter-Individual Differences in the Trajectories of Acute Pain with Activity
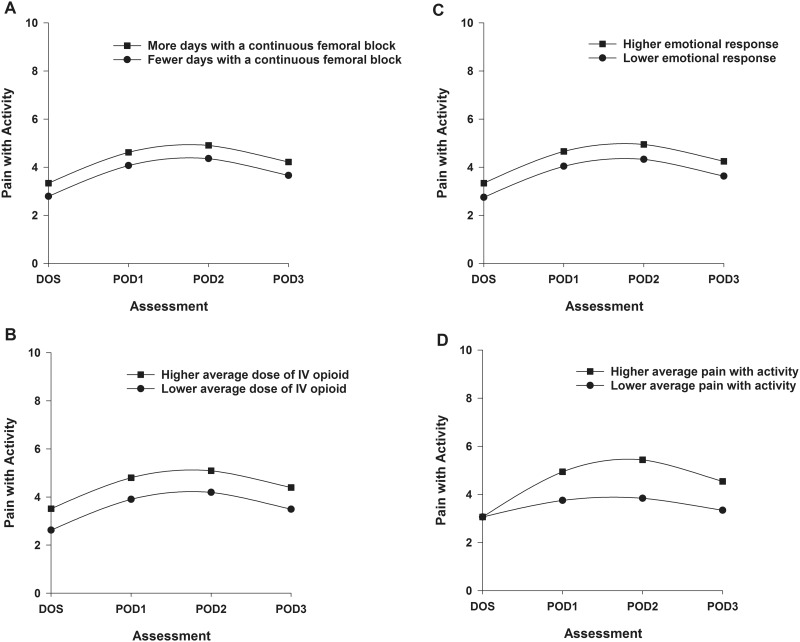
Two postsurgical (i.e., number of days with a CFNB, average dose of opioid over 4 days) and one presurgical (i.e., emotional response to OA) characteristics were associated with initial levels of pain with activity. Figures 3A through 3C display the estimated effects of each of these characteristics on pain with activity based on differences in number of days with CFNB (higher/lower number based on ± 1 standard deviation (SD) of the number of days, Figure 3A); average dose of opioid over 4 days (higher/lower based on ± 1 SD of the mean dose, Figure 3B); and emotional response (higher/lower based on ± 1 SD of the mean score, Figure 3C). One postsurgical characteristic (i.e., pain with activity on the DOS) was associated with the trajectory of pain with activity. Figure 3D displays the effect of pain with activity on the DOS on the trajectory of pain with activity.
Figure 3.
Trajectories of pain with activity by number of days with a continuous femoral nerve block (A), average dose of intravenous opioid equivalents (B), emotional response to osteoarthritis (C), and average pain with activity on the day of surgery (D) from the day of surgery until postoperative day 3. Higher/lower differences in Figures 3A–C were calculated based on 1 standard deviation above/below the predicted value.
Inter-Individual Differences in the Trajectories of Hours Per Day in Pain
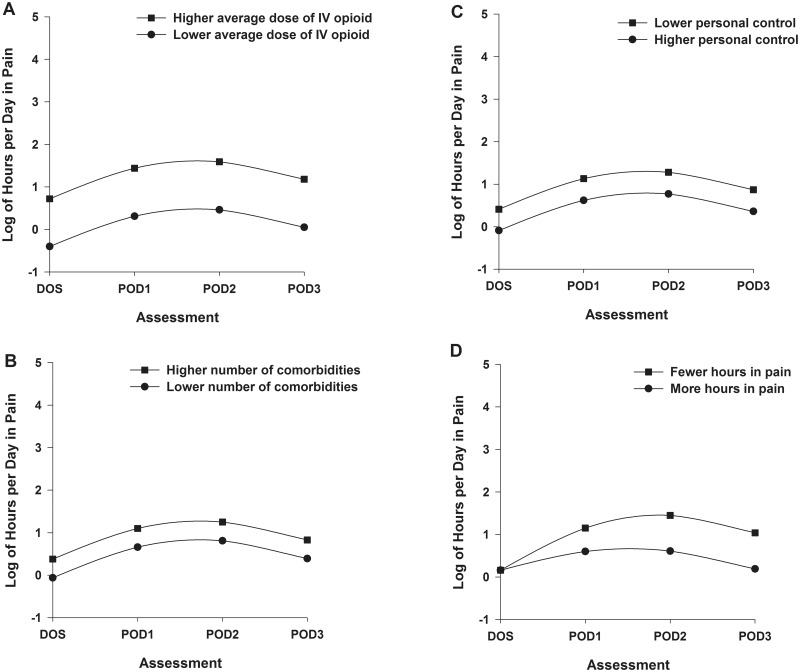
One postsurgical (i.e., average dose of opioid over 4 days) and two presurgical (i.e., number of comorbidities, personal control) characteristics were associated with the initial number of hours per day in pain. Figures 4A through 4C display the effects of each of these characteristics on number of hours per day in pain estimated based on differences in average dose of opioid over 4 days (higher/lower based on ± 1 SD of the mean dose, Figure 4A); number of comorbidities (higher/lower number based on ± 1 SD of the number of comorbidities, Figure 4B); and perceived control (higher/lower based on ± 1 SD of the mean score, Figure 4C). One postsurgical characteristic (i.e., hours per day in pain on the DOS) was associated with the trajectory of hours per day in pain. Figure 4D displays the effect of hours per day in pain on the DOS on the trajectory of hours per day in pain.
Figure 4.
Trajectories of hours per day in pain by average dose of intravenous opioid equivalents (A), number of comorbidities (B), perceived control of osteoarthritis (C), and hours per day in pain on the day of surgery (D) from the day of surgery until postoperative day 3. Higher/lower differences in Figures 4A–C were calculated based on 1 standard deviation above/below the predicted value.
Discussion
This study is the first to use HLM to evaluate for inter-individual differences in the trajectories of acute pain at rest, acute pain with activity, and hours per day in pain following TKA, as well as to identify potentially modifiable risk factors for these outcomes. From a comprehensive list of potential predictors, a number of modifiable clinical and psychological characteristics were identified, which clinicians can use to identify patients at risk for more severe postoperative pain and initiate appropriate interventions to improve postoperative pain management.
Postsurgical Risk Factors
Consistent with a previous report [42], higher scores for pain at rest and pain with activity, as well as more hours per day in pain on the DOS, were associated with worse pain trajectories for each of these outcomes (Figures 2D–4D). Thus, our findings suggest that the pain levels immediately after surgery are associated with the patient’s pain experience for the next three days. Aggressive early pain management might both reduce pain on the DOS and result in lower pain scores in the first days after surgery. While patients in this study received a standardized, multimodal acute postoperative pain management regimen [43], these findings suggest that some patients still need higher doses of analgesics on the DOS. Careful preoperative evaluation is necessary to identify patients who may not respond to standard treatment, in order to adjust their pain management plan [43]. After surgery, systematic assessment of pain at rest and with activity using standardized tools may help clinicians to identify patients who continue to experience insufficient pain relief [6].
Both number of days with EDA and number of days with CFNB were evaluated as being potentially associated with all three pain characteristics. Patients with a higher number of days with a CFNB reported higher pain intensity scores at rest and with activity on the DOS and this association was moderate to strong (d = 0.64 to 0.69, respectively). While a higher number of days with EDA was associated with lower pain scores, it was not retained in the final model because CFNB and EDA were highly correlated. Contrary to our results, findings from meta-analyses [44,45] suggest that femoral nerve block (FNB) is superior to EDA with lower morphine consumption and better pain scores both at rest and with activity. Currently, femoral block is considered to be the procedure of choice for pain management after TKA surgery [46]. CFNB alone may not be sufficient for pain management after TKA because some patients may still experience posterior knee pain. Therefore, the addition of a sciatic nerve block (SNB) to the FNB is standard procedure in many surgical centers. However, the evidence for this approach is inconclusive [47–49].
It should be noted that only 15% of the patients in our study received CFNB. EDA was the first choice for perioperative pain management (85%) and physicians chose the most appropriate approach for each patient. Therefore, the difference between CFNB and EDA found in this study may be confounded and should be interpreted with caution. In addition, patients who received CFNB were more likely to receive total intravenous anesthesia and to report higher worst pain scores prior to surgery than those without a CFNB (P < 0.001 and P = 0.02, respectively). While the pain management was standardized, the regimen was individualized based on a variety of patient and clinical characteristics [43]. Therefore, our results may reflect the complexity of clinical pain management and not the comparative effectiveness of CFNB versus EDA.
In this study, CFNBs were placed after surgery, while EDAs were placed prior to surgery and the infusions were started as the spinal block diminished. Therefore, the CFNBs may have been placed after the onset of pain. However, studies that used nerve blocks prior compared to after surgery have failed to show any benefit on postoperative pain intensity [50].
Patients who reported higher acute pain intensity scores at rest and with movement and greater number of hours per day in pain on the DOS had a higher average opioid consumption than those with lower pain scores. Of note, opioid consumption was the only characteristic that was associated with all three pain characteristics. While not a surprising finding, higher initial pain intensity and perioperative opioid consumption should be an early warning signal that prompts clinicians to modify and individualize a patient’s pain management plan [3,43].
Presurgical Risk Factors
Patients who prior to surgery reported that their knee OA affected them emotionally to a greater degree (e.g., caused anger, fear, worry) reported higher postoperative acute pain scores at rest and with activity on the DOS. A similar trend was found for hours per day in pain. Patients who prior to surgery reported less perceived control of their knee OA reported more hours per day in pain on the DOS. These findings suggest that preoperative psychological factors may contribute to patients’ perioperative pain experiences. Our findings are consistent with a recent study [51] that found negative correlations between higher emotional representations, illness coherence, consequences, and function at 6 weeks and 12 months after TKA. Illness coherence and consequences explained 7.9% of the variance in function after 6 weeks. Illness perceptions that were evaluated in this study, using the BIPQ, are considered beliefs and therefore are considered modifiable [52]. Of note, in one meta-analysis [53], significant correlations were found between illness perceptions and coping strategies. For example, viewing one’s illness as controllable was associated with a greater use of problem-solving coping strategies. These beliefs could be considered markers that indicate greater difficulty handling pain. Whether these beliefs can be modified and the potential effect on postoperative pain needs to be addressed in future studies.
As summarized in Table 4, the same predictors were associated with higher acute pain at rest and pain with activity on the DOS. This finding contrasts with a previous prospective study of TKA patients [8] that found different predictors for pain at rest (i.e., higher preoperative pain with rest, depression and younger age) and for pain with activity (i.e., higher preoperative pain with activity, lower heat pain threshold). The reasons for these differences may be that the measurement of pain trajectories over time captures different aspects of the pain experience than the conventional measurement of pain at one single time point.
Table 4.
Overview of predictors of the intercept and slope for pain at rest, pain with activity, and hours per day in pain
| Predictors | Pain at rest | Pain with activity | Hours per day in pain |
|---|---|---|---|
| Number of comorbidities | I | ||
| Number of days with continuous femoral nerve block | I | I | |
| Average dose of opioids over 4 days | I | I | I |
| Personal control | I | ||
| Emotional response | I | I | |
| Pain at rest, day of surgery | S | ||
| Pain with activity, day of surgery | S | ||
| Hours per day in pain, day of surgery | S |
I = intercept; S = slope.
Number of comorbidities was associated with hours per day in pain only. Of note, in a previous study [54], number of comorbidities was the only characteristic that was associated with a higher risk of chronic pain two years after TKA. The most common comorbidities in our sample were hypertension (41%), followed by back or head injuries (38%), cardiac disease (31%), and pulmonary disease (17%). While hypertension was associated with a lower prevalence of chronic musculoskeletal pain [55], no associations were found between preoperative systolic blood pressure and any of the pain characteristics evaluated in this study. Although not a modifiable risk factor, findings from this study suggest that patients with a higher number of comorbidities need to be identified before surgery. They should be monitored carefully after TKA and receive individualized perioperative pain management, especially on the DOS.
Sex and age were not associated with any of the pain characteristics in the present study. While age correlated with pain at rest and with activity in the exploratory analysis, it was not retained in the final model. While female gender was a risk factor for early postoperative pain after arthroscopy in three randomized controlled trials [56], it was not a significant predictor of any of the pain characteristics evaluated in this study.
Several limitations need to be acknowledged. While reflective of the population [57], more women than men participated in this study. Patients with incomplete data were slightly older which may have affected our ability to find any associations between age and pain characteristics. Because patients were recruited from only one hospital, these findings may not generalize to other settings. It is interesting to note that pain scores at rest and with activity were relatively similar. Future studies should evaluate pain with activity at the time the activity is performed. Finally, we do not have data on the incidence of utilization of oxycodone and ketobemidone rescue doses and side effects that may potentially have influenced our findings.
Several study strengths warrant consideration. First, the prospective design is an advantage. Second, the study had a relatively large sample size and a low attrition rate. Third, only patients who underwent TKA for OA using the same elective procedure with the same surgical technique and the same implant were included in the study. Fourth, a comprehensive list of predictors was evaluated using updated statistical methods with high precision. Finally, patients from a wide age range were included to increase the generalizability of our results. Patients over the age of 70 tend to be excluded from studies and are under-represented [9].
In summary, higher pain scores on the day of TKA surgery were associated with higher acute postoperative pain trajectories and more hours per day in pain. Therefore, effective pain management is imperative, especially on the DOS. Clinicians should be attentive to patients’ psychological status prior to surgery and to patients with higher opioid consumption on the DOS. These patients are at increased risk for higher acute pain scores and more hours per day in pain on the DOS and may need an individualized pain management plan. Additional research is needed to evaluate the effect of reducing acute pain on the DOS on changes in pain intensity over time.
Acknowledgments
The authors would like to thank the Orthopedic Research Group at Lovisenberg Diakonale Hospital for their cooperation. Special thanks to Einar Amlie MD, general surgeon, for assistance with electronic data extraction from the medical records using Qlik View, and to Nina Solheim, MD, anesthesiologist, for her help describing the anesthetic and analgesic regimes. The authors would also like to thank Merete Egeland, RN, Tove Granheim, RN, and Turid Undebakke Schweitz, RN, for their assistance in planning the study and with data collection.
Funding sources: The study was funded by Lovisenberg Diakonale Hospital. PhD fellowships for Maren Falch Lindberg were provided by Lovisenberg Diakonale Hospital, the Norwegian Nursing Organisation, and The US Norway Fulbright Foundation.
Disclosure and conflicts of interest: None of the funding sources were involved in any stage of the study. All authors state that they have no conflicts of interest to report.
References
- 1.Dalury DF, Lieberman JR, Macdonald SJ. Current and innovative pain management techniques in total knee arthroplasty. Instr Course Lect 2011;61:383–8. [PubMed] [Google Scholar]
- 2.Andersen LO, Gaarn-Larsen L, Kristensen BB, et al. Subacute pain and function after fast-track hip and knee arthroplasty. Anaesthesia 2009;64(5):508–13. [DOI] [PubMed] [Google Scholar]
- 3.Ranawat AS, Ranawat CS. Pain management and accelerated rehabilitation for total hip and total knee arthroplasty. J Arthroplasty 2007;22(7 Suppl 3):12–5. [DOI] [PubMed] [Google Scholar]
- 4.Wylde V, Rooker J, Halliday L, Blom A. Acute postoperative pain at rest after hip and knee arthroplasty: Severity, sensory qualities and impact on sleep. Orthop Traumatol Surg Res 2011;97(2):139–44. [DOI] [PubMed] [Google Scholar]
- 5.Puolakka PA, Rorarius MG, Roviola M, et al. Persistent pain following knee arthroplasty. Eur J Anaesthesiol 2010;27(5):455–60. [DOI] [PubMed] [Google Scholar]
- 6.Breivik H, Borchgrevink PC, Allen SM, et al. Assessment of pain. Br J Anaesth 2008;101(1):17–24. [DOI] [PubMed] [Google Scholar]
- 7.Lunn TH, Gaarn-Larsen L, Kehlet H. Prediction of postoperative pain by preoperative pain response to heat stimulation in total knee arthroplasty. Pain 2013;154(9):1878–85. [DOI] [PubMed] [Google Scholar]
- 8.Rakel BA, Blodgett NP, Bridget Zimmerman M, et al. Predictors of postoperative movement and resting pain following total knee replacement. Pain 2012;153(11):2192–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ip HY, Abrishami A, Peng PW, et al. Predictors of postoperative pain and analgesic consumption: A qualitative systematic review. Anesthesiology 2009;111(3):657–77. [DOI] [PubMed] [Google Scholar]
- 10.Henly SJ, Wyman JF, Findorff MJ. Health and illness over time: The trajectory perspective in nursing science. Nurs Res 2011;60(suppl 3):S5–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Owens WD, Felts JA, Spitznagel EL. ASA physical status classifications: A study of consistency of ratings. Anesthesiology 1978;49(4):239–43. [DOI] [PubMed] [Google Scholar]
- 12.Caraceni A, Hanks G, Kaasa S, et al. Use of opioid analgesics in the treatment of cancer pain: Evidence-based recommendations from the EAPC. Lancet Oncol 2012;13(2):e58–68. [DOI] [PubMed] [Google Scholar]
- 13.Cleeland CS. Measurement and prevalence of pain in cancer. Semin Oncol Nurs 1985;1(2):87–92. [DOI] [PubMed] [Google Scholar]
- 14.Mathias SD, Crosby RD, Qian Y, et al. Estimating minimally important differences for the worst pain rating of the Brief Pain Inventory-Short Form. J Support Oncol 2011;9(2):72–8. [DOI] [PubMed] [Google Scholar]
- 15.Klepstad P, Loge JH, Borchgrevink PC, et al. The Norwegian brief pain inventory questionnaire: Translation and validation in cancer pain patients. J Pain Symptom Manage 2002;24(5):517–25. [DOI] [PubMed] [Google Scholar]
- 16.Lee KA, Hicks G, Nino-Murcia G. Validity and reliability of a scale to assess fatigue. Psychiatry Res 1991;36(3):291–8. [DOI] [PubMed] [Google Scholar]
- 17.Lerdal A, Kottorp A, Gay CL, Lee KA. Development of a short version of the Lee Visual Analogue Fatigue Scale in a sample of women with HIV/AIDS: A Rasch analysis application. Qual Life Res 2013;22(6):1467–72. [DOI] [PubMed] [Google Scholar]
- 18.Lerdal A, Wahl A, Rustoen T, et al. Fatigue in the general population: A translation and test of the psychometric properties of the Norwegian version of the fatigue severity scale. Scand J Public Health 2005;33(2):123–30. [DOI] [PubMed] [Google Scholar]
- 19.Lerdal A, Johansson S, Kottorp A, von Koch L. Psychometric properties of the Fatigue Severity Scale: Rasch analyses of responses in a Norwegian and a Swedish MS cohort. Mult Scler 2010;16(6):733–41. [DOI] [PubMed] [Google Scholar]
- 20.Lerdal A, Kottorp A, Gay C, et al. A 7-item version of the fatigue severity scale has better psychometric properties among HIV-infected adults: An application of a Rasch model. Qual Life Res 2011;20(9):1447–56. [DOI] [PubMed] [Google Scholar]
- 21.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983;67(6):361–70. [DOI] [PubMed] [Google Scholar]
- 22.Mykletun A, Stordal E, Dahl AA. Hospital Anxiety and Depression (HAD) scale: Factor structure, item analyses and internal consistency in a large population. Br J Psychiatry 2001;179:540–4. [DOI] [PubMed] [Google Scholar]
- 23.Broadbent E, Petrie KJ, Main J, Weinman J. The brief illness perception questionnaire. J Psychosom Res 2006;60(6):631–7. [DOI] [PubMed] [Google Scholar]
- 24.Lee BO, Chaboyer W, Wallis M. Illness representations in patients with traumatic injury: A longitudinal study. J Clin Nurs 2010;19(3-4):556–63. [DOI] [PubMed] [Google Scholar]
- 25.Raudenbush SW. Comparing personal trajectories and drawing causal inferences from longitudinal data.pdf. Annu Rev Psychol 2001;52:501–25. [DOI] [PubMed] [Google Scholar]
- 26.Miaskowski C, Paul SM, Cooper BA, et al. Predictors of the trajectories of self-reported sleep disturbance in men with prostate cancer during and following radiation therapy. Sleep 2011;34(2):171–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu SS, Buvanendran A, Rathmell JP, et al. Predictors for moderate to severe acute postoperative pain after total hip and knee replacement. Int Orthop 2012;36(11):2261–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pinto PR, McIntyre T, Ferrero R, Almeida A, Araujo-Soares V. Predictors of acute postsurgical pain and anxiety following primary total hip and knee arthroplasty. J Pain 2013;14(5):502–15. [DOI] [PubMed] [Google Scholar]
- 29.Roth ML, Tripp DA, Harrison MH, Sullivan M, Carson P. Demographic and psychosocial predictors of acute perioperative pain for total knee arthroplasty. Pain Res Manag 2007;12(3):185–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kalkman CJ, Visser K, Moen J, et al. Preoperative prediction of severe postoperative pain. Pain 2003;105(3):415–23. [DOI] [PubMed] [Google Scholar]
- 31.Brander VA, Stulberg SD, Adams AD, et al. Predicting total knee replacement pain: A prospective, observational study. Clin Orthop Relat Res 2003;Nov(416):27–36. [DOI] [PubMed] [Google Scholar]
- 32.Gardner W, Mulvey EP, Shaw EC. Regression analyses of counts and rates: Poisson, overdispersed Poisson, and negative binomial models. Psychol Bull 1995;118(3):392–404. [DOI] [PubMed] [Google Scholar]
- 33.Hardin JW,, Hilbe JM. Generalized Linear Models and Extentions. 3rd edition College Station, TX: Stata Press; 2012. [Google Scholar]
- 34.Hutchinson MK, Holtman MC. Analysis of count data using poisson regression. Res Nurs Health 2005;28(5):408–18. [DOI] [PubMed] [Google Scholar]
- 35.Vittinghoff E, Glidden DV, Shiboski SC, McCulloch CE. Regression Methods in Biostatistics: Linear, Logistic, Survival, and Repeated Measures Models. 2nd edition. New York: Springer; 2012. [Google Scholar]
- 36.Hedeker D, Gibbons RD. Longitudinal Data Analysis. Hoboken: Wiley-Interscience; 2006. [Google Scholar]
- 37.Hox JJ. Multilevel Analysis: Techniques and Applications. 2nd edition New York: Routledge; 2010. [Google Scholar]
- 38.Hilbe J. Negative Binomial Regression. 2nd edition. Cambridge: Cambridge University Press; 2011. [Google Scholar]
- 39.Rabe-Hesketh S, Skrondal A. Multilevel and Longitudinal Modeling Using Stata. 3rd edition. College Station, TX: Stata Press; 2012. [Google Scholar]
- 40.Swislocki A, Orth M, Bales M, et al. A randomized clinical trial of the effectiveness of photon stimulation on pain, sensation, and quality of life in patients with diabetic peripheral neuropathy. J Pain Symptom Manage 2010;39(1):88–99. [DOI] [PubMed] [Google Scholar]
- 41.Rabe-Hesketh S, Skrondal A, Pickles A. Reliable estimation of generalized linear models using adaptive quadrature. The Stata Journal 2002;2(1):21. [Google Scholar]
- 42.Lavand'homme PM, Grosu I, France MN, Thienpont E. Pain trajectories identify patients at risk of persistent pain after knee arthroplasty: An observational study. Clin Orthop Relat Res 2014;472(5):1409–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baratta JL, Gandhi K, Viscusi ER. Perioperative pain management for total knee arthroplasty. Journal of Surgical Orthopaedic Advances 2014;23(1):22–36. [DOI] [PubMed] [Google Scholar]
- 44.Paul JE, Arya A, Hurlburt L, et al. Femoral nerve block improves analgesia outcomes after total knee arthroplasty: A meta-analysis of randomized controlled trials. Anesthesiology 2010;113(5):1144–62. [DOI] [PubMed] [Google Scholar]
- 45.Chan EY, Fransen M, Parker DA, Assam PN, Chua N. Femoral nerve blocks for acute postoperative pain after knee replacement surgery. Cochrane Database Syst Rev 2014;5:Cd009941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Danninger T, Opperer M, Memtsoudis SG. Perioperative pain control after total knee arthroplasty: An evidence based review of the role of peripheral nerve blocks. World J Orthop 2014;5(3): 225–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Abdallah FW, Brull R. Is sciatic nerve block advantageous when combined with femoral nerve block for postoperative analgesia following total knee arthroplasty? A systematic review. Reg Anesth Pain Med 2011;36(5):493–8. [DOI] [PubMed] [Google Scholar]
- 48.Abdallah FW, Chan VW, Gandhi R, et al. The analgesic effects of proximal, distal, or no sciatic nerve block on posterior knee pain after total knee arthroplasty: A double-blind placebo-controlled randomized trial. Anesthesiology 2014;121(6):1302–10. [DOI] [PubMed] [Google Scholar]
- 49.Abdallah FW, Brull R. Sciatic nerve block for analgesia after total knee arthroplasty: The jury is still out. Reg Anesth Pain Med 2012;37(1):122–3. [DOI] [PubMed] [Google Scholar]
- 50.Katz J, Clarke H, Seltzer Z. Review article: Preventive analgesia: Quo vadimus? Anesth Analg 2011;113(5):1242–53. [DOI] [PubMed] [Google Scholar]
- 51.Hanusch BC, O'Connor DB, Ions P, Scott A, Gregg PJ. Effects of psychological distress and perceptions of illness on recovery from total knee replacement. Bone Joint J 2014;96-b(2):210–6. [DOI] [PubMed] [Google Scholar]
- 52.Leventhal H, Cameron LD. The Self-Regulation of Health and Illness Behaviour. London: Routledge; 2003. [Google Scholar]
- 53.Hagger MS, Orbell S. A meta-analytic review of the common-sense model of illness representations. Psychol Health 2003;18(2):141–84. [Google Scholar]
- 54.Forsythe ME, Dunbar MJ, Hennigar AW, Sullivan MJ, Gross M. Prospective relation between catastrophizing and residual pain following knee arthroplasty: Two-year follow-up. Pain Res Manag 2008;13(4):335–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hagen K, Zwart JA, Holmen J, et al. Does hypertension protect against chronic musculoskeletal complaints? The Nord-Trondelag Health Study. Arch Intern Med 2005;165(8):916–22. [DOI] [PubMed] [Google Scholar]
- 56.Rosseland LA, Stubhaug A. Gender is a confounding factor in pain trials: Women report more pain than men after arthroscopic surgery. Pain 2004;112(3):248–53. [DOI] [PubMed] [Google Scholar]
- 57.The Norwegian Arthroplasty Register. Annual Report, 2013. Available at: http://nrlweb.ihelse.net/rapporter/rapport2013.pdf (accessed April 2016)