Abstract
The continued emergence and spread of infectious agents is of great concern, and systems biology approaches to infectious disease research can advance our understanding of host-pathogen relationships and facilitate the development of new therapies and vaccines. Molecular characterization of infectious samples outside of appropriate biosafety containment can take place only subsequent to pathogen inactivation. Herein, we describe a modified Folch extraction using chloroform/methanol that facilitates the molecular characterization of infectious samples by enabling simultaneous pathogen inactivation and extraction of proteins, metabolites, and lipids for subsequent mass spectrometry-based multi-omics measurements. This single-sample metabolite, protein and lipid extraction (MPLEx) method resulted in complete inactivation of clinically important bacterial and viral pathogens with exposed lipid membranes, including Yersinia pestis, Salmonella Typhimurium, and Campylobacter jejuni in pure culture, and Yersinia pestis, Campylobacter jejuni, and West Nile, MERS-CoV, Ebola, and influenza H7N9 viruses in infection studies. In addition, >99% inactivation, which increased with solvent exposure time, was also observed for pathogens without exposed lipid membranes including community-associated methicillin-resistant Staphylococcus aureus, Clostridium difficile spores and vegetative cells, and adenovirus type 5. The overall pipeline of inactivation and subsequent proteomic, metabolomic, and lipidomic analyses was evaluated using a human epithelial lung cell line infected with wild-type and mutant influenza H7N9 viruses, thereby demonstrating that MPLEx yields biomaterial of sufficient quality for subsequent multi-omics analyses. Based on these experimental results, we believe that MPLEx will facilitate systems biology studies of infectious samples by enabling simultaneous pathogen inactivation and multi-omics measurements from a single specimen with high success for pathogens with exposed lipid membranes.
Infectious disease research is of global interest since the emergence and spread of infectious agents represent ongoing challenges due to population growth and associated increased livestock production to meet food demands, increased urbanization and land-use changes, and greater travel1–5. Emerging infectious diseases are often zoonotic and can be transmitted to humans from animals (e.g., Middle Eastern Respiratory Syndrome coronavirus (MERS-CoV); West Nile virus, influenza A viruses, and Ebola)3. As recently demonstrated by the 2014 Ebola virus infection diagnoses in the United States, the ease of world travel and increased global interdependence have added complexity to containing these infectious diseases6. Similarly, as new infectious diseases evolve and emerge, preexisting infectious diseases re-emerge with new genetic adaptations. For example, novel antigenically distinct subtypes of influenza A viruses that are not recognized by neutralizing antibodies and cause pandemic outbreaks7. Overuse of antimicrobial drugs and decreased compliance with vaccination policies has led to the development of resistant pathogens (e.g., drug-resistance Staphylococcus and Campylobacter)8, 9 and re-emergence of diseases that were previously under control (e.g., Pertussis and Measles)10, 11. In addition, uncontrolled neglected tropical diseases such as Dengue fever and West Nile encephalitis that are endemic in developing countries are now emerging in the United States12–14.
In response to these global threats, the National Institute of Allergy and Infectious Diseases (NIAID) Systems Biology for Infectious Disease Research Program supports research focusing on host-pathogen interactions that are characterized using combined multi-omics approaches and dataset integration to “develop and validate predictive models of infectious disease initiation, progression, and outcomes”15. Infectious disease research using a systems biology approach is imperative to understanding host-pathogen relationships and allows for development of new therapies and vaccines16–18. Specifically, proteomics, metabolomics, and lipidomics measurements can assist in unraveling host-pathogen relationships. Proteins are the major effectors of cellular pathways and represent the dynamic expression of information encoded within the genome during infection. Metabolites are intermediates and products of cellular pathways and represent the level at which most pharmaceuticals exert their effects19. In addition to having key functions in signaling pathways, energy storage and the structural integrity of cell membranes, lipids also function in host-pathogen interactions and immunomodulation since they act in first line recognition and host cell signaling during pathogen docking, invasion and intracellular trafficking20. However, proteomic, metabolomic, and lipidomic characterizations of infectious biological specimens outside of appropriate biosafety level (BSL) containment laboratories can take place only subsequent to pathogen inactivation.
Non-enveloped viruses such as adenoviruses, noroviruses and bacterial spores are resistant to most disinfectants and require alternative methods for inactivation21, 22. Pathogens with exposed lipid membranes are more susceptible to disinfectants including detergents and other solvents23–25. Organic solvents render many pathogens non-infectious by solubilizing and disrupting their lipid membranes or envelopes26–28, and inactivation of pathogens by organic solvents has been leveraged for vaccine development29–32, transfusion fluids33–36, and sanitation37–39. In systems biology studies of pathogenic bacteria and viruses, it would be highly efficient to both inactivate pathogens and extract the molecular components needed for proteomic, metabolomic, and lipidomic analyses in a single step. Two of the most highly cited publications for lipid-based extraction methods, Folch et al.40 and Dyer et al.41, use high ratios of organic solvents to sample (e.g., 4:1), illustrating the broad utility of this technique in cell and tissue extraction. Recently, variations of the Folch technique have had broader utility in the extraction of metabolites and proteins42–45.
Here, we demonstrate that a modified Folch40 technique using chloroform/methanol for simultaneous protein, metabolite, and lipid extraction and subsequent mass spectrometry (MS)-based multi-omics analyses also results in concurrent pathogen inactivation. Specifically, we investigated if the metabolite, protein and lipid extraction (MPLEx) protocol46 could inactivate a diversity of infectious agents with exposed and embedded lipid bilayers including three Gram-negative bacteria (Yersinia pestis, Salmonella enterica subspecies enterica serovar Typhimurium, and Campylobacter jejuni), Gram-positive community-associated methicillin-resistant Staphylococcus aureus (MRSA) isolate USA300, Clostridium difficile strain 630 spores and vegetative cells, four RNA viruses (West Nile [WNV-New York 1999], MERS-CoV [icMERS], Ebola [Ebola-Zaire delta-VP30] and Influenza H7N9 [wild-type, A/Anhui/1/13 and mutant A/Anhui/103F-106M]). We also tested if MPLEx could inactivate adenovirus type 5, which does not contain an outer lipid bilayer.
MPLEx uses chloroform, methanol, and water (8:4:3) to induce a tri-phasic partitioning of the sample into metabolite, protein, and lipid fractions. Metabolites are located in the upper aqueous layer, whereas lipids are located in the lower organic layer after centrifugation, with a protein disc situated between the two phases (Fig. 1). In this study, MPLEx and subsequent assessment of pathogen inactivation was performed across multiple laboratories, where each laboratory has unique expertise and experience studying the pathogens in question. All studies presented in this manuscript were conducted in accordance with appropriate biosafety guidelines performed in BSL containment laboratories at the University of Wisconsin-Madison, Ohio State University, Washington State University, University of North Carolina at Chapel Hill, University of Texas Medical Branch, and Washington University School of Medicine, all of which are approved for such use by the Centers for Disease Control and Prevention and by the US Department of Agriculture.
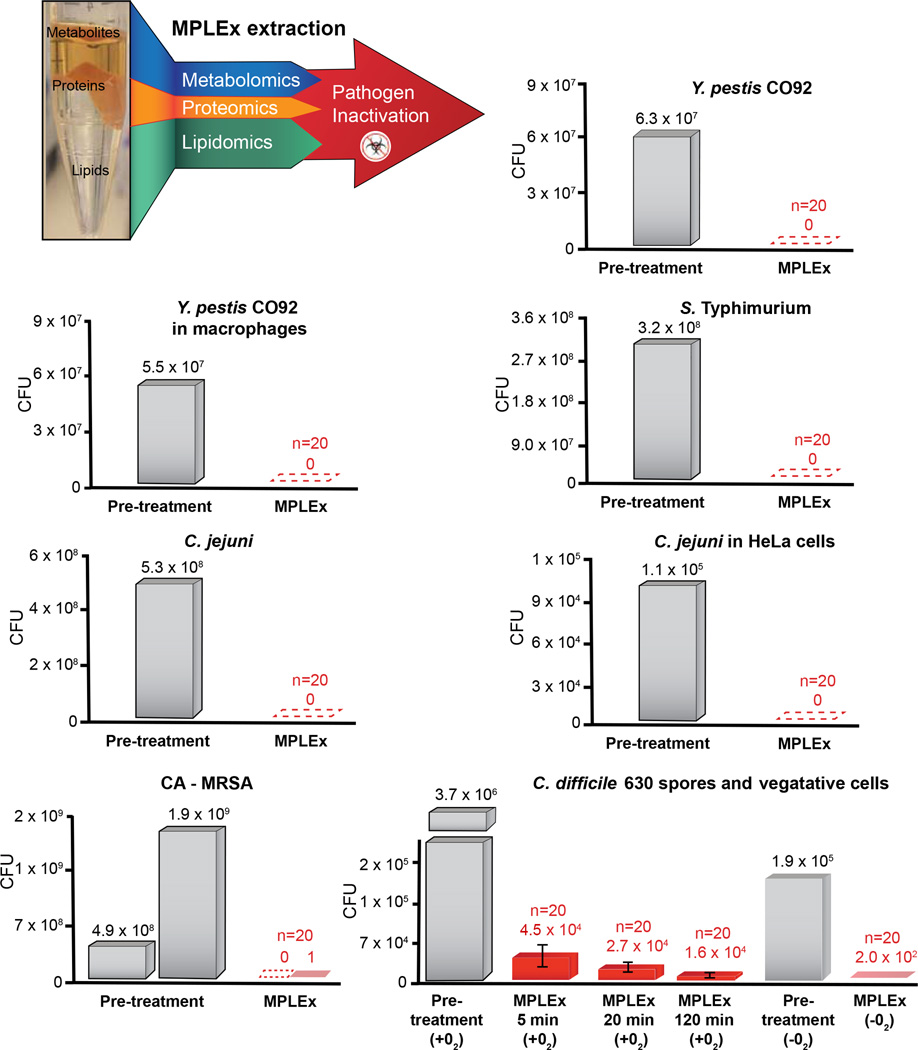
Figure 1. MPLEx Inactivation of Bacterial Pathogens.
(Top, Left) Schematic of our MPLEx extraction method which uses chloroform, methanol, and water (8:4:3) to induce simultaneous pathogen inactivation and tri-phasic liquid partitioning into metabolite, protein, and lipid fractions. The bargraphs depict the level of pathogen inactivation after MPLEx extraction by quantifying the number of Colony Forming Units (CFU) in pre-treatment control samples (grey) and MPLEx-treated samples (red) in pure cultures of Yersinia pestis strain CO92 (Y. pestis CO92), Salmonella enterica subsp. enterica serovar Typhimurium (S. Typhimurium), Campylobacter jejuni (C. jejuni), Methicillin-resistant Staphylococcus aureus USA300 (CA-MRSA), and Clostridium difficile strain 630 (C. difficile 630) spores and vegetative cells and in Y. Pestis CO92 and C. jejuni infection studies in macrophages and Hela cells, respectively.
The ability of MPLEx to inactivate pathogens was determined by comparing pathogen presence and abundance in either pre-treatment or positive controls vs. MPLEx-treated samples. S. Typhimurium, C. jejuni, MRSA, and C. difficile spore inactivation experiments (pre-treatment and MPLEx-treated) were replicated 20 times. Y. pestis and viral inactivation experiments were replicated 20 times for MPLEx treatment and a single sample was used for assessment of viral activity in the pre-treatment or positive control. We describe below the basic pathogen inactivation and simultaneous multi-omics extraction protocol. Because methods for culturing bacteria and viruses and for establishing infections vary depending on the model pathogen, we include those relevant experimental details in the description of each study in the Supplemental Material.
MPLEx inactivation protocol
In a biosafety cabinet (BSC), remove media from infected cells; immediately wash cells with a suitable buffer. The authors recommend a buffer that can be used to quench cell metabolism, such as a solution maintained at ~−40°C and consisting of 60% methanol and 0.85% ammonium bicarbonate in water47; remove rapid quenching solution
Add 150 µL of ice-cold 150 mM ammonium bicarbonate solution; scrape cells off of plates or wells for cells grown on agar or in well plate format. Collect cells and buffer into a 1.5 mL Sorenson microcentrifuge tube (or glass vials); if screw cap tops are required (e.g. for BSL-3+ level pathogens) use Fisher Screw Cap microcentrifuge tubes (Cat # 3468)
Add 600 µL of MPLEx solution (~-40°C chloroform/methanol [CHCl3/MeOH; 2:1, v/v and a 4-fold excess to sample volume])
Vortex for 10 s, leaving the samples on ice for 5 min, and vortex again for 10 s
Remove a 100 µl aliquot of mixed solution for inactivation assay
Centrifuge the remaining solution at 13,000 g for 10 min
Remove top phase to fresh tube, remove bottom phase to second tube leaving protein disc behind. Rinse the protein disc with 200 µL methanol and pellet at 9,000 g for 5 min. Decant the methanol solution and allow pellet to dry in BSC for 5 min
All tubes are then removed from BSC and BSL containment laboratories after appropriate disinfection and either stored at −80°C or dried in vacuo (for safe shipping of metabolite and lipid phases) and then stored at −80°C
Some of the bacterial pathogens and all of the viral pathogens were assessed in infection studies where infected host cells were treated with MPLEx and inactivation was evaluated via inoculation of the resulting cell lysates into fresh cells. For these infection studies, the MPLEx-treated cell lysates were diluted to retain host cell viability, as undiluted samples can induce toxicity. Some validations of the inactivation procedures were carried out with samples that had been dried in the speedvac, rather than the liquid/diluted extract prior to drying.
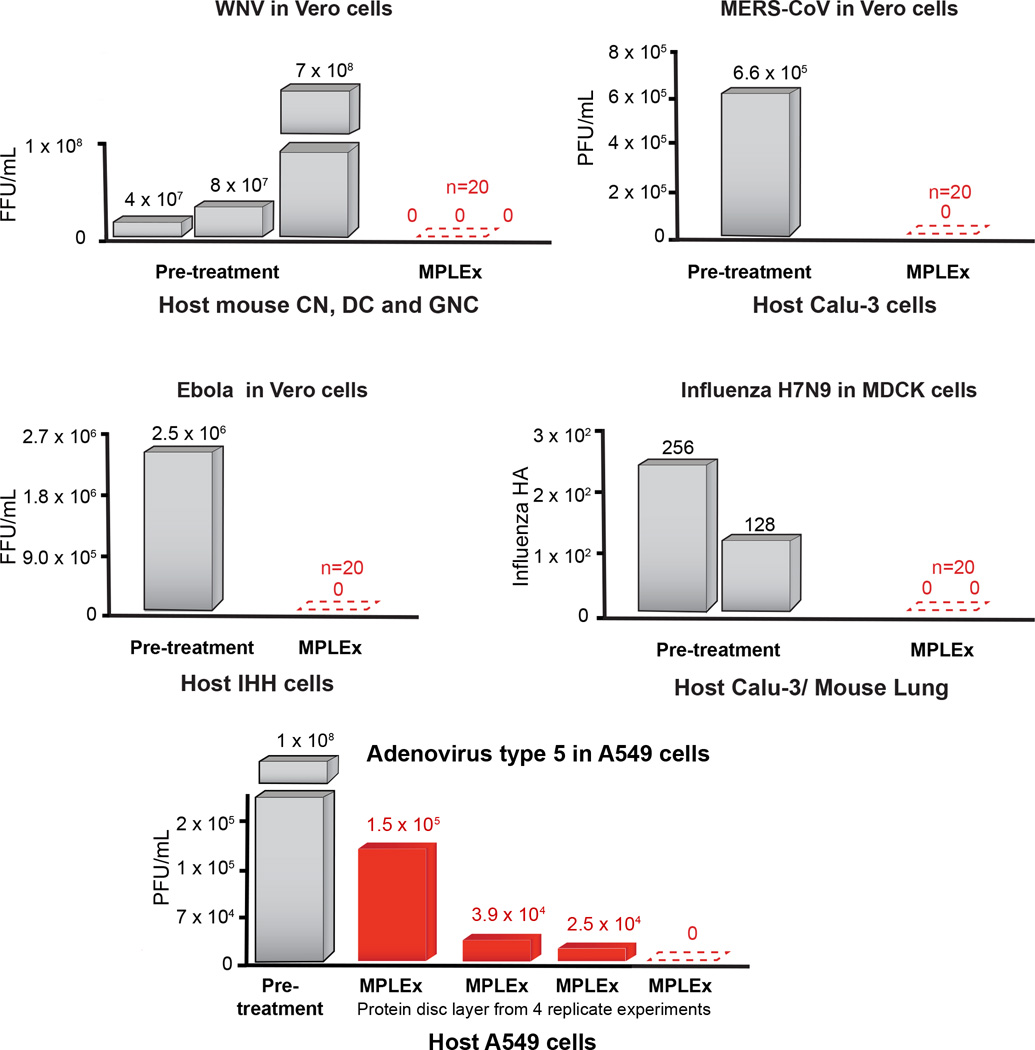
As depicted in Figure 1 and Figure 2, the MPLEx method for extraction of proteins, metabolites and lipids from a single sample for MS-based multi-omics profiling also simultaneously inactivates a number of clinically important bacterial and viral pathogens with exposed lipid membranes, including Y. pestis CO92, S. Typhimurium, and C. jejuni in pure culture and Y. pestis CO92, C. jejuni, WNV, MERS-CoV, Ebola and influenza viruses in infection studies. Near complete inactivation (>99%) was observed for pathogens without exposed lipid membranes including CA-MRSA, C. difficile 630 spores and vegetative cells after ≥ 20 min MPLEx exposure time, and Ad5 (see Supplemental Material and Table S1 for details).
Figure 2. MPLEx Inactivation of Viral Pathogens.
The bargraphs depict the level of pathogen inactivation after MPLEx extraction by quantifying the number of Focus Forming Units (FFU) or Plaque Forming Units (PFU) in pre-treatment control samples (grey) and MPLEx-treated samples (red) in infection studies of West Nile (WNV, New York 1999), MERS-CoV (icMERS), Ebola (Ebola-Zaire delta-VP30) and Avian Influenza (H7N9).
In addition, to demonstrating that MPLEx yields biomaterial of sufficient quality for subsequent multi-omics analyses, Figure 3 highlights the reproducibility of proteomics, metabolomics and lipidomics data from human epithelial lung cells that were infected with either wild-type, A/Anhui/1/2013 (H7N9), or A/Anhui/1/2013 (H7N9) possessing two mutations in the NS1 gene (L103F and I106M) that reduce virus pathogenicity in mice (A. Eisfeld, S. Fan and Y. Kawaoka, unpublished results). To demonstrate the utility of the MPLEx protocol for both inactivating pathogens and for obtaining high quality metabolite, protein, and lipid fractions for respective omics analyses, we implemented the protocol in a systems biology study of influenza infection in a human lung epithelial cell line. The MPLEx protocol was implemented to extract proteins, metabolites and lipids from Calu-3 cells infected with wild-type (AH1) and mutant (FM) influenza viruses, or mock-infected controls for each of 6 timepoints; 0, 3, 7, 12, 18 and 24 h, and using 5 biological replicates per sample (treatment and timepoint). From this experiment, we quantified 23,688 peptides, 81 metabolites (50 were identified through matching of experimental mass spectra and retention indices to entries in the Agilent Fiehn Metabolomics Retention Time Locked Library)48, and 251 and 245 lipids from analyses in negative and positive electrospray mode analyses, respectively; therefore as previously reported46 the number of detected analytes with MPLEx provides comparable or more identifications compared to other methods. To assess reproducibility, we calculated the coefficient of variation (CV) of the 5 biological replicates within each of the datatypes (protein, metabolite, and lipid). For proteomics, metabolomics, and lipidomics, the maximum CV across features within each set of biological replicates was <0.24 (proteomics), < 0.17 (metabolomics), <0.22 (lipidomics data collected under negative electrospray ionization (ESI)), and <0.19 (lipidomics data collected under positive ESI) (Table S2). Figure 3 shows viral peptides, ribosomal proteins, metabolites and lipids significantly increasing in infected samples compared to matched mock controls due to infection duration across both viral strains (Table S2). Peptide, metabolite, and lipid statistics were performed by comparing data from matched virus and mock-infected controls using Analysis of Variance (ANOVA) with a Dunnett multiple test correction within time point and for qualitative changes via a G-test with a Bonferroni multiple test correction within time point49. Peptides, metabolites, and lipids with a p-value less than 0.05 were identified as significantly different (Table S2). The top of Figure 3 depicts the 123 viral peptides belonging to viral proteins; hemagglutinin (HA), matrix protein 1 (M1), neuraminidase (NA), nucleoprotein (NP), NS1, NS2, and RNA polymerase complex (PA, PB1, and PB2) significantly increased by G-test (only observed in infected samples) as early as 7 h with the number of significant peptides increasing from 7–24 h. Statistical analysis of our proteomic data also found significant quantitative increases in the relative abundance of ribosomal subunits 40S and 60S with infection duration across both viral strains. During the influenza life cycle, single-stranded viral RNAs migrate to the nucleus where they are copied into mRNA by the viral RNA polymerase complex. Invading viruses do not harbor functional ribosomes in their virions; therefore, viral mRNAs hijack host cell ribosomes and use sophisticated mechanisms50 to enable selective translation of viral mRNAs. Free fatty acid metabolites myristic acid (14:0) and oleic acid (18:1) significantly increased at 18 h post infection across all 3 viral strains (Fig. 3). Ceramides (Cer) containing stearic acid (18:0) and tetracosanoic acid (24:0) significantly increased at 18 and 24 h in both AH1 and FM and at 12 h in AH1; likewise, Cer containing stearic acid (18:0) and nervonic acid (24:1) significantly increased at 18 h in both AH1 and FM and at 24 h in FM (Fig. 3). It has been previously reported that pathogenic avian influenza viruses, such as H7N9, decrease expression of lipid metabolism genes51. A NS1 mutation in the FM strain reduces overall pathogenicity; at the early 7 hour time point of infection the wild-type AH1 strain shows over a 30% increase in viral peptide compared to the FM mutant (Fig. 3). Correspondingly at this 7 h time point ribosome expression is only significantly increased in the AH1 virus and at the late 24 hour time point significant ribosomal expression is increased by over 40% in the AH1 compared to FM.
Figure 3. Reliable multi-omic measurements resulting from MPLEx extraction of influenza infected Calu-3 cells.
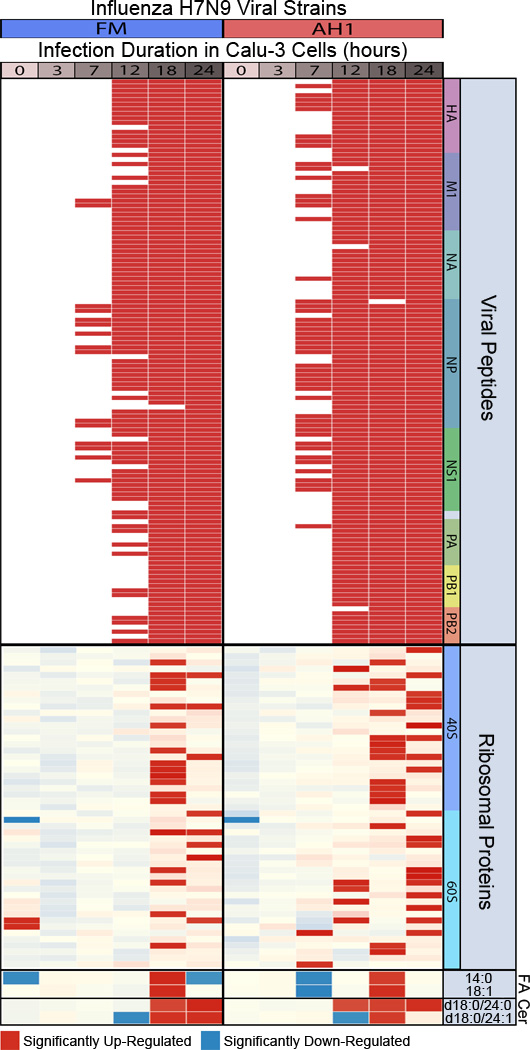
The heatmap depicts significant abundant changes between Calu-3 cells treated with wild-type (A/Anhui/1/13 [AH1]) and mutant (A/Anhui/103F-106M [FM]) Influenza H7N9 viral strains and time-matched (0, 3, 7, 12, 18 and 24 hours) mock-infected controls. Red color, significantly increase in virus vs. time matched mock (p-value < 0.05). The viral peptides labeled at the top of the heatmap include, hemagglutinin (HA), matrix protein 1 (M1), neuraminidase (NA), nucleoprotein (NP), non-structural (NS1), non-structural (NS2) (corresponds to the two unlabeled viral peptides), and polymerase complex proteins (PA, PB1, and PB2); fatty acids, FA; Ceramide lipids, Cer.
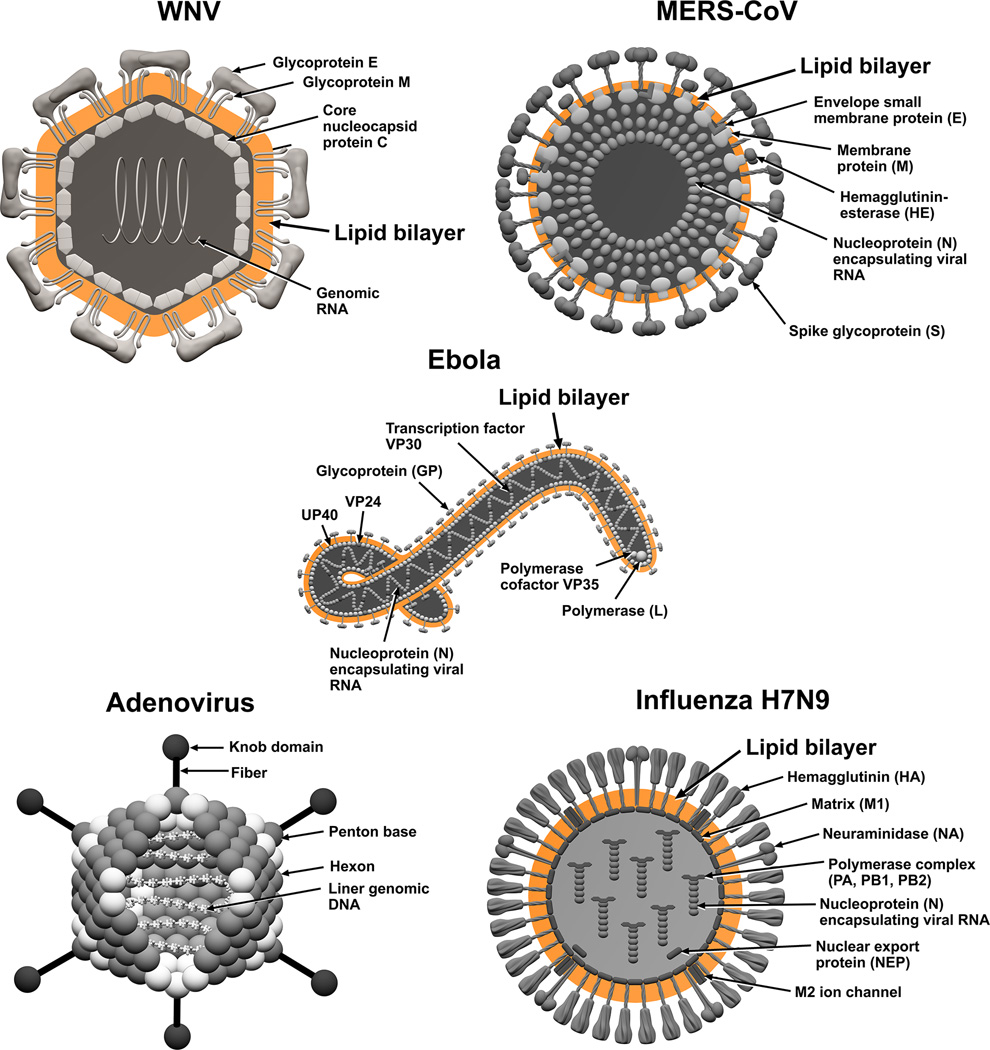
Based on these results we believe that MPLEx will benefit and facilitate systems biology studies of infectious samples by enabling both pathogen inactivation and multi-omics measurements from a single specimen with high success for pathogens with exposed lipid membranes. Figures 4 and 5 illustrate the bacterial and viral pathogens from this study that contain exposed and embedded lipid membranes; these membranes are denoted with orange color. Of the bacterial pathogens characterized in this study, Y. pestis CO92, S. Typhimurium, and C. jejuni are Gram-negative bacteria, CA-MRSA is a Gram-positive bacterium, and C. difficile 630 is a spore forming Gram-positive bacterium (Fig. 4). The exposed walls for Gram-positive bacteria have a higher peptidoglycan (PG) and lower lipid content than Gram-negative bacteria. This bacterial classification is based on the Gram staining method, which differentiates bacteria by the chemical and physical properties of their cell walls; it detects PG, which is present in a thick layer in Gram-positive bacteria52. The inner membrane of dormant spores is composed of a bilayer of immobile phospholipids, which has similar phospholipid composition to growing bacteria but exhibits low permeability to small molecules including water53, 54. This inner membrane surrounds the spore core which contains the genome. The basic spore architecture is conserved55–57; outside of the inner membrane is a layer of PG, known as the germ cell wall that is encircled in a thick layer of a modified form of PG, the cortex, which is essential for the acquisition and maintenance of heat resistance. The cortex is surrounded by an outer membrane derived from the mother cell that is essential for spore formation but confers no resistance properties 58. A proteinaceous coat surrounds the outer membrane. In C. difficile 630 spores, the coat is further enclosed within a structure known as the exosporium. The partial inactivation results presented for CA-MRSA (where only 2 out of 20 experimental replicates still retained a small level of bacterial activity) and C. difficile 630 spores reveals that MPLEx can dissolve protective PG layers encircling lipid bilayers and with long enough exposure times could lead to complete pathogen inactivation. The level of inactivation of C. difficile 630 spores due to MPLEx treatment exposure time in min was 120 > 20 > 5 (Fig. 1), in addition in the two replicate experiments of CA-MRSA the experiment with the lower initial bacterial concentration (pre-treatment, 4.9 × 108) did show complete inactivation due to MPLEx. WNV, MERS-CoV, Ebola, and Influenza H7N9 virus all contain lipid bi-layered envelopes (Fig. 5). WNV virions acquire their lipid envelope by budding into the endoplasmic reticulum of the host cell; Coronaviruses such as MERS-CoV acquire their membranes in the ER-Golgi intermediate compartment (ERGIC) and use normal cellular processes to leave the cell; and Ebola and Influenza H7N9 virions acquire their lipid envelopes by budding from host plasma membranes often at lipid raft microdomains that are enriched in sphingomyelin and cholesterol59–61. Ad5 was the only virus that was not completely inactivated by MPLEx; Ad5 is a non-enveloped virus and therefore does not contain any lipid membranes (Fig. 5).
Figure 4. The structural layers of Gram-positive and -negative Bacteria and Bacterial Spores.
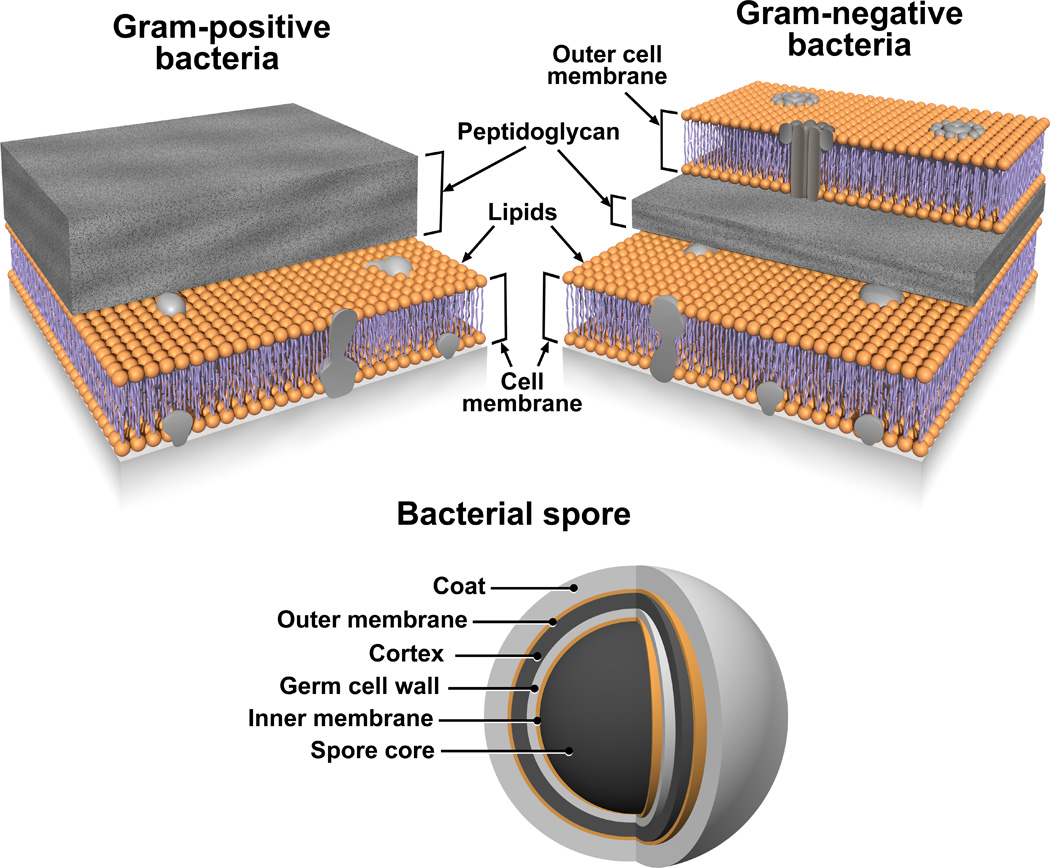
Lipid membranes are denoted with orange color.
Figure 5. The structure of lipid enveloped viruses; West Nile Virus (WNV), Middle East Respiratory Syndrome Coronavirus (MERS-CoV), Ebola, and Avian Influenza Virus.
Lipid bilayers are denoted with orange color.
In conclusion, we show that MPLEx completely inactivates pathogens with exposed lipid membranes, such as enveloped viruses and Gram-negative bacteria, thereby facilitating multi-omics measurements from a single specimen in clinically important pathogen studies. Pathogens with internal or protected lipid membranes, such as Gram-positive bacteria and bacterial spores still show a significant decrease in activity (>99% reduction in the number of viable microorganisms after MPLEx treatment) related to both MPLEx solution exposure time and pre-treatment pathogen levels. Even Ad5, a virus without a lipid membrane, showed >99% decreased activity after MPLEx exposure, presumably since non-enveloped viruses can still be susceptible to organic solvent, in particular CHCl3, due to the denaturation of proteins that are solvent sensitive28, 62. In summary, we believe our MPLEx method for concurrent pathogen inactivation and extraction of samples for multi-omics profiling will be broadly applicable to samples containing clinically important bacterial and viral pathogens with exposed lipid membranes since molecular characterization of infectious samples outside of appropriate biosafety containment can take place only subsequent to complete pathogen inactivation. We note that, for biosafety reasons, this protocol should be evaluated in each investigator’s laboratory for efficacy in pathogen inactivation, prior to implementation and particularly for pathogens that were not evaluated in our study.
Supplementary Material
Acknowledgments
This project was funded in part by the Systems Biology Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health, via grant U19AI106772. Portions of the work utilized capabilities developed under National Institute of General Medical Sciences grant GM103493. Multi-omics measurements were performed in the Environmental Molecular Science Laboratory, a U.S. DOE national scientific user facility at PNNL in Richland, WA. Battelle operates PNNL for the DOE under contract DE-AC05-76RLO01830. We would like to thank PNNL Graphic Designer Michael Perkins for assistance in preparing the figures.
REFERENCES
- 1.Heesterbeek H, Anderson RM, Andreasen V, Bansal S, De Angelis D, Dye C, Eames KTD, Edmunds WJ, Frost SDW, Funk S, Hollingsworth TD, House T, Isham V, Klepac P, Lessler J, Lloyd-Smith JO, Metcalf CJE, Mollison D, Pellis L, Pulliam JRC, Roberts MG, Viboud C I.N.I. I. Collaboration. Science. 2015;347 doi: 10.1126/science.aaa4339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Daszak P, Cunningham AA, Hyatt AD. Science. 2000;287:443–449. doi: 10.1126/science.287.5452.443. [DOI] [PubMed] [Google Scholar]
- 3.Jones KE, Patel NG, Levy MA, Storeygard A, Balk D, Gittleman JL, Daszak P. Nature. 2008;451:990–993. doi: 10.1038/nature06536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lloyd-Smith JO, George D, Pepin KM, Pitzer VE, Pulliam JRC, Dobson AP, Hudson PJ, Grenfell BT. Science. 2009;326:1362–1367. doi: 10.1126/science.1177345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keesing F, Belden LK, Daszak P, Dobson A, Harvell CD, Holt RD, Hudson P, Jolles A, Jones KE, Mitchell CE, Myers SS, Bogich T, Ostfeld RS. Nature. 2010;468:647–652. doi: 10.1038/nature09575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morens DM, Fauci AS. PLoS Pathogens. 2013;9:e1003467. doi: 10.1371/journal.ppat.1003467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Jong JC, Rimmelzwaan GF, Fouchier RAM, Osterhaus ADME. Journal of Infection. 2000;40:218–228. doi: 10.1053/jinf.2000.0652. [DOI] [PubMed] [Google Scholar]
- 8.Ruiz-Palacios GM. Clinical Infectious Diseases. 2007;44:701–703. doi: 10.1086/509936. [DOI] [PubMed] [Google Scholar]
- 9.Grundmann H, Aires-De-Sousa M, Boyce J, Tiemersma E. Lancet. 2006;368:874–885. doi: 10.1016/S0140-6736(06)68853-3. [DOI] [PubMed] [Google Scholar]
- 10.Cherry JD. New England Journal of Medicine. 2012;367:785–787. doi: 10.1056/NEJMp1209051. [DOI] [PubMed] [Google Scholar]
- 11.Omer SB, Salmon DA, Orenstein WA, deHart MP, Halsey N. New England Journal of Medicine. 2009;360:1981–1988. doi: 10.1056/NEJMsa0806477. [DOI] [PubMed] [Google Scholar]
- 12.Gubler DJ. Clinical Infectious Diseases. 2007;45:1039–1046. doi: 10.1086/521911. [DOI] [PubMed] [Google Scholar]
- 13.Bouri N, Sell TK, Franco C, Adalja AA, Henderson DA, Hynes NA. Public Health Reports. 2012;127:259–266. doi: 10.1177/003335491212700305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hotez PJ, Bottazzi ME, Franco-Paredes C, Ault SK, Periago MR. PLoS Negl Trop Dis. 2008;2:e300. doi: 10.1371/journal.pntd.0000300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aderem A, Adkins JN, Ansong C, Galagan J, Kaiser S, Korth MJ, Law GL, McDermott JG, Proll SC, Rosenberger C, Schoolnik G, Katze MG. mBio. 2011;2 doi: 10.1128/mBio.00325-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Forst CV. Drug Discovery Today. 2006;11:220–227. doi: 10.1016/S1359-6446(05)03735-9. [DOI] [PubMed] [Google Scholar]
- 17.Zak DE, Tam VC, Aderem A. Annual Review of Immunology. 2014;32:547–577. doi: 10.1146/annurev-immunol-032713-120254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Josset L, Tisoncik-Go J, Katze MG. Virus Research. 2013;178:151–167. doi: 10.1016/j.virusres.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fontana JM, Alexander E, Salvatore M. Translational Research. 2012;159:430–453. doi: 10.1016/j.trsl.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wenk MR. FEBS Letters. 2006;580:5541–5551. doi: 10.1016/j.febslet.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 21.Rutala WA, Weber DJ. Clinical Infectious Diseases. 2004;39:702–709. doi: 10.1086/423182. [DOI] [PubMed] [Google Scholar]
- 22.Maillard JY. Journal of Hospital Infection. 2011;77:204–209. doi: 10.1016/j.jhin.2010.06.028. [DOI] [PubMed] [Google Scholar]
- 23.Favero M, Bond W. Chemical disinfection of medical and surgical materials. In: Block SS, editor. Disinfection, sterilization, and preservation. Philadelphia: Lippincott Williams & Wilkins; 2001. [Google Scholar]
- 24.McMurry LM, Oethinger M, Levy SB. Nature. 1998;394:531–532. doi: 10.1038/28970. [DOI] [PubMed] [Google Scholar]
- 25.Feldman H, Wang S. Proc Soc Exp Biol Med. 1961;106:736–738. doi: 10.3181/00379727-106-26459. [DOI] [PubMed] [Google Scholar]
- 26.Vidaver AK, Koski RK, Van Etten JL. Journal of Virology. 1973;11:799–805. doi: 10.1128/jvi.11.5.799-805.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Olsen RH, Siak JS, Gray RH. Journal of Virology. 1974;14:689–699. doi: 10.1128/jvi.14.3.689-699.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feinstone SM, Mihalik KB, Kamimura T, Alter HJ, London WT, Purcell RH. Infection and Immunity. 1983;41:816–821. doi: 10.1128/iai.41.2.816-821.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fries LF, Waag DM, Williams JC. Infect Immun. 1993;61:1251–1258. doi: 10.1128/iai.61.4.1251-1258.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parisius W, Macmorine HG. Applied Microbiology. 1969;17:379–383. doi: 10.1128/am.17.3.379-383.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Evans RK, Nawrocki DK, Isopi LA, Williams DM, Casimiro DR, Chin S, Chen M, Zhu D-M, Shiver JW, Volkin DB. Journal of Pharmaceutical Sciences. 2004;93:2458–2475. doi: 10.1002/jps.20157. [DOI] [PubMed] [Google Scholar]
- 32.Cham BE, Vickery K, Tohidi-Esfahani R, Cossart Y. Journal of Virological Methods. 2006;137:160–163. doi: 10.1016/j.jviromet.2006.05.027. [DOI] [PubMed] [Google Scholar]
- 33.Horowitz B, Wiebe ME, Lippin A, Stryker MH. Transfusion. 1985;25:516–522. doi: 10.1046/j.1537-2995.1985.25686071422.x. [DOI] [PubMed] [Google Scholar]
- 34.Pelletier JPR, Transue S, Snyder EL. Best Practice & Research Clinical Haematology. 2006;19:205–242. doi: 10.1016/j.beha.2005.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dichtelmüller HO, Flechsig E, Sananes F, Kretschmar M, Dougherty CJ. Results in Immunology. 2012;2:19–24. doi: 10.1016/j.rinim.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hellstern P, Solheim BG. Transfusion Medicine and Hemotherapy. 2011;38:65–70. doi: 10.1159/000323552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Du W-X, Danyluk MD, Harris LJ. Journal of Food Science. 2010;75:M7–M13. doi: 10.1111/j.1750-3841.2009.01393.x. [DOI] [PubMed] [Google Scholar]
- 38.Macinga DR, Sattar SA, Jaykus L-A, Arbogast JW. Applied and Environmental Microbiology. 2008;74:5047–5052. doi: 10.1128/AEM.00487-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Turner RB, Fuls JL, Rodgers ND. Antimicrobial Agents and Chemotherapy. 2010;54:1363–1364. doi: 10.1128/AAC.01498-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Folch J, Lees M, Stanley GHS. Journal of Biological Chemistry. 1957;226:497–509. [PubMed] [Google Scholar]
- 41.Bligh EG, Dyer WJ. Canadian Journal of Biochemistry and Physiology. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 42.Fay D, Bowman BU. J Virol. 1978;27:432–435. doi: 10.1128/jvi.27.2.432-435.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Belle JEL, Harris NG, Williams SR, Bhakoo KK. NMR in Biomedicine. 2002;15:37–44. doi: 10.1002/nbm.740. [DOI] [PubMed] [Google Scholar]
- 44.Ferraz TPL, Fiúza MC, dos Santos MLA, Pontes de Carvalho L, Soares NM. Journal of Biochemical and Biophysical Methods. 2004;58:187–193. doi: 10.1016/j.jbbm.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 45.Jiang L, He L, Fountoulakis M. Journal of Chromatography A. 2004;1023:317–320. doi: 10.1016/j.chroma.2003.10.029. [DOI] [PubMed] [Google Scholar]
- 46.Nakayasu ES, Nicora CD, Sims AC, Burnum-Johnson KE, Kim Y-M, Kyle JE, Matzke MM, Shukla AK, Chu RK, Schepmoes AA, Jacobs JM, Baric RS, Webb-Robertson B-J, Smith RD, Metz TO. mSystems. 2016;1 doi: 10.1128/mSystems.00043-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sellick CA, Hansen R, Maqsood AR, Dunn WB, Stephens GM, Goodacre R, Dickson AJ. Analytical Chemistry. 2009;81:174–183. doi: 10.1021/ac8016899. [DOI] [PubMed] [Google Scholar]
- 48.Kind T, Wohlgemuth G, Lee DY, Lu Y, Palazoglu M, Shahbaz S, Fiehn O. Analytical Chemistry. 2009;81:10038–10048. doi: 10.1021/ac9019522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Webb-Robertson B-JM, McCue LA, Waters KM, Matzke MM, Jacobs JM, Metz TO, Varnum SM, Pounds JG. Journal of Proteome Research. 2010;9:5748–5756. doi: 10.1021/pr1005247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bushell M, Sarnow P. The Journal of Cell Biology. 2002;158:395–399. doi: 10.1083/jcb.200205044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Morrison J, Josset L, Tchitchek N, Chang J, Belser JA, Swayne DE, Pantin-Jackwood MJ, Tumpey TM, Katze MG. Journal of Virology. 2014;88:10556–10568. doi: 10.1128/JVI.00570-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bergey D, Holt J, Krieg N, Sneath P. 1994 [Google Scholar]
- 53.Cowan AE, Olivastro EM, Koppel DE, Loshon CA, Setlow B, Setlow P. Proc Natl Acad Sci U S A. 2004;101:7733–7738. doi: 10.1073/pnas.0306859101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Barra-Carrasco J, Olguin-Araneda V, Plaza-Garrido A, Miranda-Cardenas C, Cofre-Araneda G, Pizarro-Guajardo M, Sarker MR, Paredes-Sabja D. J Bacteriol. 2013;195:3863–3875. doi: 10.1128/JB.00369-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Henriques AO, Moran CPJr. Annu Rev Microbiol. 2007;61:555–588. doi: 10.1146/annurev.micro.61.080706.093224. [DOI] [PubMed] [Google Scholar]
- 56.McKenney PT, Driks A, Eichenberger P. Nat Rev Microbiol. 2013;11:33–44. doi: 10.1038/nrmicro2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.de Hoon MJ, Eichenberger P, Vitkup D. Curr Biol. 2010;20:R735–R745. doi: 10.1016/j.cub.2010.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Paredes-Sabja D, Shen A, Sorg JA. Trends Microbiol. 2014;22:406–416. doi: 10.1016/j.tim.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Millet JK, Whittaker GR. Proc Natl Acad Sci U S A. 2014;111:15214–15219. doi: 10.1073/pnas.1407087111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Adu-Gyamfi E, Johnson KA, Fraser ME, Scott JL, Soni SP, Jones KR, Digman MA, Gratton E, Tessier CR, Stahelin RV. J Virol. 2015 doi: 10.1128/JVI.01087-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Takeda M, Leser GP, Russell CJ, Lamb RA. Proc Natl Acad Sci U S A. 2003;100:14610–14617. doi: 10.1073/pnas.2235620100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ackermann HW. Classification of bacteriophages, in The Bacteriophages. New York, NY: Oxford University Press; 2006. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.