Abstract
Background
Haemophilia care is commonly provided via multidisciplinary specialized management. To date, there has been no systematic assessment of the impact of haemophilia care delivery models on patient-important outcomes.
Objective
To conduct a systematic review of published studies assessing the effects of the integrated care model for persons with haemophilia (PWH).
Search methods
We searched MEDLINE, EMBASE and CINAHL up to April 22, 2015, contacted experts in the field, and reviewed reference lists.
Selection criteria
Randomized and non-randomized studies of PWH or carriers, focusing mainly on the assessment of care models on delivery.
Data collection and analysis
Two investigators independently screened title, abstract, and full text of retrieved articles for inclusion. Risk of bias and overall quality of evidence was assessed using Cochrane’s ACROBAT-NRSI tool and GRADE respectively. Relative risks, mean differences, proportions, and means and their variability were calculated as appropriate.
Results
27 non-randomized studies were included: eight comparative and 19 non-comparative studies. We found low- to very low-quality evidence that in comparison to other models of care, integrated care may reduce mortality, hospitalizations and emergency room visits, may lead to fewer missed days of school and work, and may increase knowledge seeking.
Conclusion
Our comprehensive review found low- to very low-quality evidence from a limited number of non-randomized studies assessing the impact of haemophilia care models on some patient-important outcomes. While the available evidence suggests that adoption of the integrated care model may provide benefit to PWH, further high-quality research in the field is needed.
Keywords: care model, delivery of health care, haemophilia, health care team, integrated care, review
Introduction
Haemophilia care is complex, often requiring health management beyond the prevention and treatment of bleeding. Ad hoc trained haematologists and nurses have the knowledge and experience to address a wide range of haemophilia-specific needs [1,2]. Musculoskeletal experts, such as physical therapists, can help manage recovery from bleeding into joints and prevent chronic joint damage. Psychosocial support can be delivered by social workers and psychologists, as persons with haemophilia (PWH) often experience social stigma, vocational challenges and decreased quality of life [3]. Infectious disease specialists and gastroenterologists have been required to manage viral infections, such as HIV and hepatitis C.
As a result, care for PWH is often multidisciplinary and specialized. This model of care can be defined as ‘integrated care’, and is the most largely represented of the four principle care models for PWH available for adoption in the Western world. The integrated care model is usually delivered by Comprehensive Care Centers (‘comprehensive care’ is used as an alternative definition for integrated care), which provide all components of care (including supervision of home-based treatment) via coordinated and geographically co-located multidisciplinary teams. Comprehensive Care Centers coordinate care, secure and administer funding, provide technical assistance, organize professional education and training, and engage in data collection and analysis.
There are three alternative models of care. In a specialist-based care model, a haematologist, who may or may not have specialized training in haemophilia, provides care in a non-specialized centre, such as a hospital or medical office. Care delivered by a non-specialist in a non-specialist setting takes the form of a family physician delivering care in their practice, or an emergency room physician delivering care in an emergency room setting. The ‘no care’ model, with a complete absence of dedicated care, does not appear to be operating in the Western world, but exists in other areas of the world where PWH do not have access to care due to profound resource constraints [4,5].
While the integrated care model has been strongly supported by physician and patient organizations since its introduction in the United Kingdom in the late 1940s [6–9] and increased uptake in the 1960s and 1970s, and is currently widely accepted as the ‘de facto’ standard of care, there have been no attempts to systematically assess the evidence comparing the different care models for PWH. The aim of this paper is to report the results of a systematic review of the literature to assess the impact of haemophilia care models on patient-important outcomes.
Materials and methods
This systematic review was performed according to methods in the Cochrane Collaboration Handbook [10] and reported according to the PRISMA statement [11], and was used to provide the evidence base for a guideline addressing the choice of care models for the management of haemophilia [12].
Inclusion and exclusion criteria
Inclusion and exclusion criteria were defined a priori. Included articles had to report on PWH or carriers, and had to focus on haemophilia care models, defined as: integrated care; care delivered by a specialist in a non-specialist setting; care delivered by a non-specialist in a non-specialized setting; and no care. Included articles had to report on a health care model (or models) of interest, or report on a health care model of interest and an add-on care delivery option (such as home care). Since it was anticipated that there would be few studies comparing different models of care, randomized and non-randomized studies were included. Non-randomized studies could compare models or describe a single model of care.
Outcomes
Articles had to report on at least one of the following outcomes: mortality, missed days of school or work, emergency room visits, length of in-patient stay, quality of life, joint damage or disease (and other measures of functional status), educational attainment, patient adherence, and patient knowledge. These outcomes were identified through a survey given to a panel of clinicians, programme managers, researchers, patients with haemophilia and caregivers as critical or important to patients [12].
Search strategies
We searched OVID MEDLINE, OVID MEDLINE In-Process and Other Non-Indexed Citations, OVID EMBASE, and EBSCO Cumulative Index to Nursing & Allied Health Literature (CINAHL) databases up to April 22, 2015. We also contacted clinical experts in the field and used snowballing through references to identify studies. The search strategies consisted of MeSH headings, keywords and text words related to haemophilia, models of care and health services, and were not restricted by language or study design. Complete search strategies are provided in Appendix S1.
Study selection and data extraction
Two investigators (SM and BY) independently screened title, abstract and full text of relevant articles for inclusion. All disagreements were adjudicated by a third investigator (TN or CHTY). Authors of abstracts were contacted by email if necessary, to retrieve full text articles. Electronic data extraction forms were developed and pilot tested. Data extraction was independently performed by two investigators (SM and BY), and all discrepancies were adjudicated by a third investigator (TN or CHTY).
Assessment of study quality
Two investigators (CHTY and NS) independently assessed the risk of bias for each study using the ACRO-BAT-NRSI tool [13]. Using the GRADE (Grading of Recommendations, Assessment, Development and Evaluation) approach, two investigators (MP and AI) evaluated the quality/certainty of the evidence for each outcome, and a third investigator resolved any discrepancies (NS or CHTY) [14]. The quality of the evidence was assessed as high, moderate, low or very low. The effect estimates and quality of evidence were summarized in a GRADE evidence profile [15,16].
Statistical analysis
Analysis was conducted using RevMan 5.3 (RevMan, Computer Program, Copenhagen: The Nordic Cochrane Center, The Cochrane Collaboration, 2012) [17]. Results are expressed as risk ratios (RR) or mean differences (MD) with corresponding 95% confidence intervals (95% CI), or are presented narratively. For studies that described a single model of care, either the risk of an event (or proportion) was calculated, or the mean (or median) and standard deviation of the outcome point estimate were obtained.
It was determined a priori that a meta-analysis would be performed when estimates were clinically and statistically sufficiently homogenous. The intent was to conduct subgroup analyses by disease severity (severe: factor levels <1%; and non-severe: factor levels ≥1%), carriers of haemophilia, comorbidities (HIV, hepatitis), differing access to care (i.e. urban vs. rural) and age (paediatric: ≤18 years of age; older population: ≥65 years of age).
Results
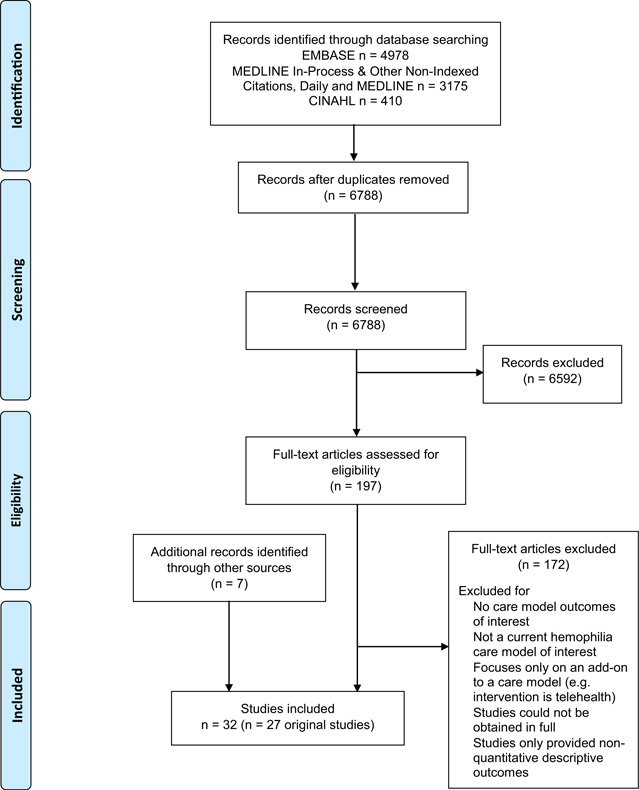
The literature search identified 6789 non-duplicate records. After title and abstract screening, 197 articles were assessed for eligibility with full-text review, and subsequently, 172 articles were excluded. Seven additional articles eligible for inclusion were retrieved during the process. As a result, 27 unique non-randomized studies (which were reported in 32 published articles) were included. A PRISMA diagram of the selection flow is provided in Fig. 1. Eight studies were comparative and 19 studies were single-arm non-comparative studies.
Fig. 1.
PRISMA flow diagram for care models in the management of haemophilia.
Characteristics of the included comparative and non-comparative studies are described in Table 1 and Appendix S2 respectively. The risk of bias of the comparative studies assessed using the ACROBAT-NRSI tool is presented in Table 2. We present a summary of the results from comparative studies in Table 3, and present the data from the non-comparative studies in Appendix S3. We reported the results with a study estimate or narrative, and did not perform a formal meta-analysis due to heterogeneity in the study designs and outcome definitions.
Table 1.
Description of comparative non-randomized studies.
| Study | Design | n | Country, sites | Population | Intervention | Control | Outcome(s) of interest |
|---|---|---|---|---|---|---|---|
| Arnold 2014 [48] |
Cross-sectional Surveyed individuals identify their existing knowledge levels and gaps |
104 | Canada, three HTCs (from Eastern, Central and Western parts of Canada) |
All patients were ≥18 years old 49% mild 13% moderate 29% severe (similar to Canadian distribution) |
HTC attendance within the past 12 months |
No HTC attendance within the past 12 months |
Patient knowledge: Knowledge seeking over the past 12 months |
| Lazerson 1972 [26] |
Before-after Patients compared prior to and after development of a comprehensive care centre |
20 before 20 after |
United States, NR (presumably, the Children’s Hospital at Stanford) |
10 patients were 5–9 years old; 10 patients were 10- 17 years old All severe No inhibitors |
The year 1970–1971 is the period during which all children were well established in the comprehensive care program |
The year 1968 to 1969 was prior to the establishment of a comprehensive care program |
Missed days of school or work: Number of days lost from school or work |
| Monahan 2011 [27] |
Cross-sectional Universal Data Collection (UDC) data |
6420 | United States, −130 HTCs | All patients were ≤18 years old 50.2% severe 24.3% moderate 25.4% mild 14% reported having inhibitors |
Patients who were frequent users (one or more visits per year) |
Patients who were infrequent users (less than one visit per year, excluding the first visit) or only had a 1st visit to the HTC |
Missed days of school or work: >11 days lost from school or work per year Joint damage or disease (and other measures of functional status): Decreased activity (work, school, recreational, and self-care) per year |
| Smith 1982 [39] |
Before-after Patients compared prior to and after development of a Comprehensive Hemophilia Center |
23 before 43 after |
Hemophilia Center of Rhode Island, Rhode Island Hospital, Rhode Island, US |
49% ≤16 years old 51% >16 years old 70% severe 30% moderately severe |
Three years following the initiation of the Comprehensive Hemophilia Centre program |
The year preceding the Comprehensive Hemophilia Centre program |
Emergency room visits: Number of visits to the emergency room and walk-in clinic |
| Smith 1984 [27] |
Before-after Patients compared prior to and after development of a Comprehensive Hemophilia Center |
2112 before 4742 after |
United States, 11 federally funded Comprehensive Hemophilia Centers |
67% severe 33% mild or moderate |
The fifth year following the initiation of the Comprehensive Hemophilia Centre program |
The year preceding the Comprehensive Hemophilia Centre program |
Missed days of school or work: Number of days lost from school or work Length of in-patient stay: Number of days spent as in-patient |
| Soucie 2000 [18] |
Prospective cohort Hemophilia Surveillance System (HSS) data |
2950 | United States, HTCs in Colorado, Georgia, Louisiana, Massachusetts, New York, and Oklahoma |
46% 0–19 years old 49% 20–59 years old 5% 60–70+ years old 42% severe 24% moderate 31% mild 5% reported having inhibitors 2% reporting having liver disease 25% reported having a positive HIV serostatus; 7% AIDS |
Patients receiving care in HTCs |
Patients received care primarily from private physicians or haematologists, hospital- and nonhospital-based clinics, only from hospitals or emergency rooms, or care from a variety of other sources |
Mortality or survival: Mortality adjusted |
| Soucie 2001 [38] |
Prospective cohort Hemophilia Surveillance System (HSS) data |
2546 | United States, HTCs in Colorado, Georgia, Louisiana, Massachusetts, New York, and Oklahoma |
0–24 years old 41% 25–44 years old 45.4% ≥45 years old 13.7% 47% severe 23% moderate 28% mild 5.3% reported having inhibitors |
Patients receiving care in HTCs |
Patients received care primarily from private physicians or haematologists, hospital- and nonhospital-based clinics, only from hospitals or emergency rooms, or care from a variety of other sources |
Emergency room visits: Number of people with at least one hospitalization over four years adjusted |
| Soucie 2004 [46] |
Cross-sectional Universal Data Collection (UDC) data |
4343 | United States, −130 HTCs | All patients were ≤19 years old 21% mild 24% moderate 55% severe 10.8% reported having inhibitors |
Patients who were frequent users (one or more visits per year) |
Infrequent users (less than one visit per year) |
Joint damage or disease (and other measures of functional status): Overall joint ROM |
HTC, Hemophilia Treatment Center; ROM, range-of-motion.
Table 2.
Risk of bias summary by non-randomized comparative study assessed by the ACROBAT-NRSI tool.
| Study | Bias due to confounding |
Bias in selection of participants into the study |
Bias in measurement of interventions |
Bias due to departures intended interventions |
Bias due to missing data |
Bias in measurement of outcomes |
Bias in selection of reported results |
|---|---|---|---|---|---|---|---|
| Arnold 2014 [48] | Moderate risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Lazerson 1972 [26] | Serious risk | Low risk | Low risk | Low risk | Serious risk | Low risk | Low risk |
| Monahan 2011 [28] | Moderate risk | Moderate risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Smith 1982 [39] | Serious risk | Moderate risk | Low risk | Low risk | Unclear risk | Unclear risk | Low risk |
| Smith 1984 [27] | Serious risk | Low risk | Low risk | Low risk | Unclear risk | Unclear risk | Low risk |
| Soucie 2000 [18] | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Soucie 2001 [38] | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Soucie 2004 [46] | Moderate risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
Table 3.
GRADE evidence profile for summary of findings from non-randomized comparative studies.
| Quality assessment |
No of patients |
Effect |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No of studies (participants) | Study design | Risk of bias |
Inconsistency | Indirectness | Imprecision | Other considerations |
Integrated care model |
Non-integrated care model |
Relative (95% CI) |
Absolute (95% CI) |
Quality |
| Mortality | |||||||||||
| 1 (2950) | Non-randomized comparative study |
Not Serious* |
Not serious | Not serious | Not serious | None | 149/1979 (7.5%) |
86/971 (8.9%) | RR0.6 (0.5–0.8) |
35 fewer per 1000 (from 18 fewer to 44 fewer) |
 |
| Missed days of school or work | |||||||||||
| 3 (3032 without and 10 282 with integrated care) |
Non-randomized comparative study |
Serious† | Not serious | Not serious† | Serious† | None | 4742 | 2112 | – | MD 10.2 lower (not reported) |
 |
| Emergency room visits | |||||||||||
| 2 (662 without and 1950 with integrated care) |
Non-randomized comparative study |
Not serious* |
Not serious | Not serious‡ | Not serious | None | 557/1907 (29.2%) |
225/639 (35.2%) | RR0.6 (0.5–0.7) |
141 fewer per 1000 (from 106 fewer to 176 fewer) |
 |
| Length of in-patient stay | |||||||||||
| 1 (4742 without and with integrated care) |
Non-randomized comparative study |
Serious† | Not serious | Not serious† | Serious† | None | 4742 | 2112 | – | MD 7.6 fewer (not reported) |
 |
| Quality of life - not measured | |||||||||||
| Joint damage or disease (and other measures of functional status) | |||||||||||
| 2 (10 763) | Non-randomized comparative study |
Not serious§ |
Not serious | Serious§ | Not serious | None | Severe disease: frequent HTC users had less ROM limitation than infrequent users (unadjusted analysis). Moderate disease: infrequent HTC users has less ROM limitation than frequent users (adjusted analysis). Mild disease: infrequent HTC users has less ROM limitation than frequent users (adjusted analysis). |
 |
|||
| Educational attainment – not measured | |||||||||||
| Patient knowledge | |||||||||||
| 1 (104) | Non-randomized comparative study |
Not serious¶ |
Not serious | Serious¶ | Serious¶ | None | Significantly fewer people who did not attend HTC in past 12 months sought information |
 |
|||
| Patient adherence - not measured | |||||||||||
CI, confidence interval; RR, risk ratio; MD, mean difference; HTC, Hemophilia Treatment Center; ROM, range-of-motion.
Potential for bias related to definition of integrated care as an HTC user with at least one visit to the centre but not downgraded.
Overall, the results were not adjusted for confounding factors, and the 95% confidence intervals were not calculated. There is also some indirectness as the integrated care model in 1970s/1980s has changed.
Not downgraded, although the number of hospitalizations was used as a surrogate for number of emergency room visits.
Overall, downgraded once for unadjusted analysis depending on severity of disease and differences in definition of integrated care and non-integrated care by frequency of HTC use.
Overall, the results were not adjusted for confounding, integrated care was defined as attendance at an HTC in last 12 months, and there were few events.
Mortality
One comparative non-randomized study by Soucie et al. [18] with 2950 participants, reported results on adjusted mortality over a three-year period. The risk of death was reduced in PWH receiving care at a Haemophilia Treatment Center (HTC) compared to not receiving care at an HTC (RR 0.60; 95% CI, 0.50–0.80), with an absolute overall death rate of 40.4 deaths/1000 person-years, reflective of the burden of HIV mortality [18]. Six unique non-comparative studies (which were reported in seven published articles) reported mortality as an event rate [19–25]. The number of deaths in the study population over 1–8 years, ranged from 6 to 100 deaths per 1000 persons (Appendix S3). Overall, the quality of evidence for a reduction in mortality with integrated care was low based on data from the non-randomized comparative study.
Missed days of school or work
Three studies measured days lost from work or school. Two before–after studies measured the mean number of days missed prior to and after implementation of an HTC (Table 3). Lazerson [26] with 20 patients before and after the implementation of integrated care, found a reduction in days [MD −50.20 (95% CI, −61.68, −38.72) days per year]. Smith and Levine [27] with 2112 participants before and 4742 after the adoption of integrated care, found a reduction of 10.2 days per year. The third study [28] compared participants with >11 days lost from work who had frequent HTC use to infrequent or first time use. We reanalyzed the data from 6420 participants and found a risk ratio of 1.01 (95% CI, 0.75–1.36) (Table 3). Seven unique non-comparative studies (which were reported in twelve published articles) reported a range of results with some reporting low rates in absenteeism, but others reporting greater than 15 missed days per year [22–24,29–37] (Appendix S3). Overall, there was very low-quality evidence for a reduction in missed days of work or school per year of approximately 10 days. This was primarily due to lack of adjustment for confounding factors in the comparative studies from Smith and Levine [27] and our reanalysis of Monahan et al. [28], and to the potential for little to no difference in days missed (Table 3).
Emergency room visits
Two comparative non-randomized studies measured the number of emergency room visits or hospitalizations. Soucie et al. [38] with 2546 participants, described the number of people with at least one hospitalization over 4 years. They reported that PWH who had received care at an HTC any time during the study period were hospitalized less than those who did not receive care at an HTC [RR 0.60 (95% CI, 0.50–0.70)] (Table 3). In agreement with Soucie et al. [38], a small study by Smith, Keyes and Forman [39] of 43 participants before and 23 participants after the implementation of integrated care, reported a mean difference of 23.3 emergency room and walk-in clinic visits favouring integrated care (Table 2). Three non-comparative studies reported the mean number of emergency room visits [32,33,40] and ranged from 0.9 to 500 per 1000 persons per year (Appendix S3). Overall, the quality of evidence from the non-randomized comparative studies was low, although we did not downgrade for indirectness based on the use of hospitalizations to represent emergency room visits (Table 3).
Length of in-patient stay
One comparative non-randomized study by Smith and Levine [27] of 2112 participants before and 4742 participation after the adoption of integrated care, reported number of days spent in hospital. The mean difference in length of stay after implementation of integrated care was −7.6 days spent in the hospital per year (95% CI not reported) (Table 3). Thirteen non-comparative studies reported the mean number of days per patient per year [20–24,34,35,40–45]. The mean number of visits ranged from 0.4 to 14.5 per person per year (Appendix S3). Overall, the quality of evidence was very low from the non-randomized comparative study which had a high risk of bias due to non-adjustment for potential confounding and potential bias from patients lost to follow-up and non-participation. These factors were considered with indirectness (study was from the 1980s and current treatment modalities have changed) and the few hospitalizations that occurred (Table 3).
Joint damage or disease (and other measures of functional status)
Two comparative non-randomized studies reported on the progression of joint damage or decreased activity (another measure of functional status) per year. Soucie et al. [46] with 4343 participants, found that for PWH with severe disease, frequent HTC users (one or more visits per year) had less ROM limitation than infrequent users (less than one visit per year). In contrast, for PWH with moderate and mild disease, frequent HTC use was associated with higher ROM limitation, even when the association was tested in a model adjusted for age and BMI. The authors appropriately suggest that for mild and moderate patients, frequent bleeds may drive both frequency of HTC visits and limitation in ROM, creating a spurious (confounded) association (Table 3). From Monahan et al. [28], we reanalyzed the data from 6420 participants to compare participants with decreased activity (actions related to work, school, recreation and self-care) who had frequent HTC use to infrequent or first time use and calculated RR 1.20 (95% CI, 0.98–1.46). Two unique non-comparative studies (which were reported in three published articles) reported [25,29,30] a range from 234 to 333 joints damaged or diseased per 1000 persons (Appendix S3). Four non-comparative studies (which were reported in six published articles) reported [23,24,31,36,37,47] that the proportion of patients with joint damage or disease ranged from 44 to 429 per 1000 persons (Appendix S3). Overall, there was very low-quality evidence for a reduction in joint damage or disease from the two comparative studies due to lack of adjustment for confounding factors in combination with indirectness (using decreased activity per year to define joint damage or disease) (Table 3).
Patient knowledge
The comparative non-randomized study by Arnold et al. [48] of 104 participants found that HTC attendance within the past 12 months was significantly associated (P < 0.05) with increased knowledge seeking (e.g. recognizing and treating a bleed, knowledge of the genetics of haemophilia, physical activity selections) in an unadjusted analysis (Table 3). The overall quality of evidence was very low due to potential bias from unadjusted confounding factors, indirectness from defining integrated care as attendance at an HTC in the last 12 months, and few participants (Table 3).
Other outcomes
We did not find comparative studies reporting on quality of life, educational attainment and patient adherence. However, two non-comparative studies reported that 83–494 per 1000 persons adhered to their treatment regimens [31,49] (Appendix S3).
Discussion
We found low- to very low-quality evidence that integrated care reduces mortality, emergency room and walk-in clinic visits, hospitalizations (and length of stay), missed days of school and work, and increases knowledge seeking. The evidence for the effects of integrated care on functional status, measured by joint damage or joint disease was less clear, and the analysis is likely confounded by disease severity. This means that the true effects of integrated care may be substantially different from what we found (i.e. overestimated or under-estimated). We also did not find evidence to compare the effects of integrated care to other models for quality of life, educational attainment and patient adherence.
This systematic review has two clear strengths: its rigorous methodology and its comprehensive scope. Our systematic search of electronic databases was supplemented with snowballing and broad expert consultation (useful strategies when addressing a rare disease). We did not restrict by study design and therefore believe that we have captured the current available evidence for care models in PWH. We used best-in-class tools to assess risk of bias and evaluate the quality of the body of evidence to interpret and present the current best evidence. Despite the comprehensive search, we found few comparative studies yielding a limited body of evidence, but allowing us to suggest several considerations for future research.
Limitations of the current body of evidence clearly indicate targets for improvement. The conduct of randomized controlled trials in health care services and different care models is possible but very challenging, as evident from systematic reviews of randomized controlled trials of health care models in other chronic conditions, such as asthma and chronic obstructive pulmonary disease [50–53]. The challenges would be even greater in haemophilia, where the rarity of disease, the long-term nature of the most patient-important outcomes (e.g. mortality or joint disease), and the widespread adoption of the integrated care model as standard of care would make a randomized trial almost impossible to perform. However, even where integrated care models are already in place, non-randomized studies comparing PWH attending integrated or non-integrated care are still feasible.
First, future attempts to more clearly define integrated and non-integrated care groups could be made. We found three studies by Soucie et al. [18,38,46] and another by Monahan et al. [28] that included large populations of PWH and compared the risk of many important patient outcomes between different uses of HTC and integrated care. Use and attendance at HTC were however, defined differently across these studies. Soucie et al. [18,38] defined their integrated and non-integrated care arms as patients attending HTC and non-HTC respectively. In contrast, the other studies did not define integrated and non-integrated care arms, and thus we reanalyzed their data to provide comparisons. Soucie et al. [46] and Monahan et al. [28] compared the frequency of HTC visits rather than HTC and non-HTC arms. To reanalyzed this data, we defined integrated care as equivalent to frequent HTC use (one or more visits per year) and non-integrated care as equivalent to infrequent HTC use (less than one visit per year) or first time visits. These were, admittedly, indirect measures of integrated and non-integrated care. Stemming from the literature we reviewed, integrated care would include supervision of the PWH by a coordinated and centralized multidisciplinary team. The integrated care model would include some or all of the following: provide home-based treatment, coordinated care and technical assistance; secure and administer funding; organize professional education and training; and engage in data collection and analysis. Therefore, in future studies, it will be important to provide details on which components and functions the specific integrated care model under study entails. The non-integrated care group would be further defined as the specialist-based care model that centres on a haematologist, who may or may not have specialized training in haemophilia, providing care in a non-specialized centre, such as a hospital or medical office; care delivered by a non-specialist in a non-specialist setting with a family physician delivering care in their practice, or an emergency room physician delivering care in a hospital; or the ‘no care’ model, in which there is a complete absence of care. Of course, integrated care models with different compositions or function could also be used as comparators.
Second, we found that the selected outcomes, and how they were defined and measured were very heterogeneous across studies, which essentially prevented us from pooling data across studies. Few to no studies reported on some of our set of outcomes, which were identified as important by key stakeholders [12]. Additionally, reported outcomes were measured differently from study to study. For example, missed days of school or work was measured continuously in days in some studies, but dichotomized as the number of people who had greater or less than a threshold value of missed days in others. In future research, we suggest that data should be collected and analyzed for standardized measures of missed days from work or school, length of in-patient stay, quality of life, joint damage and other functional outcomes, educational attainment, patient adherence, and patient knowledge. Reference to standardized international definitions can be of value [54].
Third, we had hypothesized that integrated care may have different effects in different populations with haemophilia, but we were unable to incorporate subgroup analyses into this review. There were a paucity of data from the studies to group by disease severity, age (in particular paediatric and geriatric groups), inhibitor status, ethnicity, comorbidities and access to care (i.e. insurance status). We found one large study showing that effects on joint damage or disease may be related to severity of haemophilia, but were unable to explore this further in other studies. In the future, studies addressing these principle subgroups would allow for analyses to determine the benefits or harms of integrated care models in specific populations.
Conclusions
To the best of our knowledge, this is the first systematic review to assess the impact of models of care delivery on important outcomes for patients with haemophilia. From its results, it is clear that care models in the management of haemophilia is a research topic that has been relatively neglected in the past, which is likely amplified by the rare disease setting. While the paucity of supportive evidence has not had a negative impact on the provision of integrated care, it should be noted that, at least in the US, approximately two-thirds of the haemophilia population receive care from an HTC [18], a figure that is certainly much lower in developing countries. Should further research reinforce integrated care as an important component of haemophilia management on patient-important outcomes, there may be important implications for future research to address and overcome barriers to HTC utilization, including the allocation of resources to areas that need this care. On the other hand, if further research changes the current favourable effects of integrated care, then it may be worth considering providing less costly and resource-intensive care models to specific patient populations, perhaps starting with those with low disease burden. Coordinated, well-funded, large-scale research initiatives to better understand the effect of care models in the management of haemophilia are worth being pursued and are urgently needed. On this note, this review provides a starting point and many suggestions for future research on integrated health care models in PWH.
Supplementary Material
Acknowledgments
AI, HJS, MP and NS led the question development and refinement, and the development of the list and rating of importance of outcomes. CHTY and TN performed the systematic reviews. CHTY and NS performed the quality of evidence assessment. AI and MP contributed to and provided oversight to the systematic review process. CHTY wrote the paper and all the authors revised it critically. We thank Norma Brown, Chris Cotoi, Nicholas Hobson, Sunil Mammen, Naushin Sholapur, Rebecca Smith and Basil Yang for their contributions. This manuscript is one of six reporting about the NHF-McMaster Guideline on Care Models for Haemophilia Management. The NHF-McMaster Guideline on Care Models for Haemophilia Management have been endorsed by the American Society of Hematology (May 27, 2016), the International Society for Thrombosis and Haemostasis (May 28, 2016), the World Federation of Hemophilia (May 20, 2016).
Sources of support
This work was funded by the National Hemophilia Foundation.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Footnotes
Disclosures
CHTY, NS, TN, JMS, and HJS have stated that they had no interests which might be perceived as posing a conflict or bias. MP has received honoraria for speaking engagements from Bayer, and consulting income from Bayer, BMS-Pfizer and Sanofi. CK has received consulting income from Alnylam, Bayer, Baxter, Grifols, NovoNordisk, Octapharma, Pfizer and Roche; research funding from Bayer, Grifols, Octapharma, and NovoNordisk; non-monetary research support from Bayer, Baxter, Grifols, NovoNordisk, Octapharma, and Pfizer; works at a US HTC; and directs a 340B Program. NSK’s institution has received research funding from Baxter; consulting income from Bayer and Novo Nordisk; and works at a US HTC. MM’s institution has received project-based funding from Bayer, Biogen, Baxter, Biotest, BPL, CSL Behring, LFB, Grifols, Kedrion, Octapharma, Pfizer, SOBI/Biogen and NovoNordisk; works at a non-US HTC. AI has received consulting income from Bayer and Biogen Idec; research support from NovoNordisk, Biogen Idec, and Pfizer; and works at a non-US HTC.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Appendix S1 Care models for haemophilia management search strategies in MEDLINE, EMBASE and CINAHL
Appendix S2 Description of single-arm non-comparative studies
Appendix S3 Outcome data from non-comparative studies
References
- 1.Nuss R, Hedegaard H, Riske B, Hoffman R, Michael S, Manco-Johnson M. Medical care for haemophilia. Haemophilia. 1998;4:806–811. doi: 10.1046/j.1365-2516.1998.00196.x. [DOI] [PubMed] [Google Scholar]
- 2.Hacker MR, Primeaux J, Manco-Johnson MJ. A patient satisfaction survey for haemophilia treatment centres. Haemophilia. 2006;12:163–168. doi: 10.1111/j.1365-2516.2006.01199.x. [DOI] [PubMed] [Google Scholar]
- 3.Cassis FRMY, Querol F, Forsyth A, Iorio A HERO International Advisory Board. Psychosocial aspects of haemophilia: asystematic review of methodologies and findings. Haemophilia. 2012;18:e101–e114. doi: 10.1111/j.1365-2516.2011.02683.x. [DOI] [PubMed] [Google Scholar]
- 4.De Kleijn P, Odent T, Berntorp E, et al. Differences between developed and developing countries in paediatric care in haemophilia. Haemophilia. 2012;18(Suppl 4):94–100. doi: 10.1111/j.1365-2516.2012.02875.x. [DOI] [PubMed] [Google Scholar]
- 5.Srivastava A, Chuansumrit A, Chandy M, Duraiswamy G, Karagus C. Management of haemophilia in the developing world. Haemophilia. 1998;4:474–480. doi: 10.1046/j.1365-2516.1998.440474.x. [DOI] [PubMed] [Google Scholar]
- 6.Hoots WK. Comprehensive care for hemophilia and related inherited bleeding disorders: why it matters. Curr Hematol Rep. 2003;2:395–401. [PubMed] [Google Scholar]
- 7.Bonser GM, Biggs RP, Willis R. William Goldie, 31 December 1907–13 February 1970. J Pathol. 1971;105:69–74. doi: 10.1002/path.1711050111. [DOI] [PubMed] [Google Scholar]
- 8.Evans D. Haemophilia centres. BMJ. 285:1420. [Google Scholar]
- 9.Biggs R. Haemophilia treatment in the United Kingdom from 1969 to 1974. Br J Haematol. 1977;35:487–504. doi: 10.1111/j.1365-2141.1977.tb00615.x. [DOI] [PubMed] [Google Scholar]
- 10.Higgins J, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions [Internet] [Accessed February 23, 2016];The Cochrane Collaboration, 2011. 2011 Available at: www.cochrane-handbook.org.
- 11.Shamseer L, Moher D, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;349:g7647. doi: 10.1136/bmj.g7647. [DOI] [PubMed] [Google Scholar]
- 12.Pai M, Key NS, Skinner M, et al. NHF-McMaster guideline on care models for haemophilia management. Haemophilia. 2016;22(Suppl 3):6–16. doi: 10.1111/hae.13008. [DOI] [PubMed] [Google Scholar]
- 13.Sterne J, Higgins J, Reeves BC on behalf of the development group for ACROBAT-NRSI. [Accessed February 23, 2016];A Cochrane Risk Of Bias Assessment Tool: for Non-Randomized Studies of Interventions (ACROBAT-NRSI) [Internet] 2014 Available at: http://www.riskof-bias.info.
- 14.Balshem H, Helfand M, Schünemann HJ, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64:401–406. doi: 10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 15.Guyatt GH, Oxman AD, Santesso N, et al. GRADE guidelines: 12. Preparing summary of findings tables-binary outcomes. J Clin Epidemiol. 2013;66:158–172. doi: 10.1016/j.jclinepi.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 16.Guyatt GH, Thorlund K, Oxman AD, et al. GRADE guidelines: 13. Preparing summary of findings tables and evidence profiles-continuous outcomes. J Clin Epidemiol. 2013;66:173–183. doi: 10.1016/j.jclinepi.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 17.The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan) Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration; 2012. [Google Scholar]
- 18.Soucie JM, Nuss R, Evatt B, et al. Mortality among males with hemophilia: relations with source of medical care. The Hemophilia Surveillance System Project Investigators. Blood. 2000;96:437–442. [PubMed] [Google Scholar]
- 19.Mahlangu JN. Medical and Scientific Council of the South African Haemophilia Foundation. Haemophilia care in South Africa: 2004–2007 look back. Haemophilia. 2009;15:135–141. doi: 10.1111/j.1365-2516.2008.01807.x. [DOI] [PubMed] [Google Scholar]
- 20.Chuansumrit A, Isarangkura P, Chantanakajornfung A, Panthangkool W, Hathirat P. Home treatment for patients with congenital bleeding disorders in a developing country. J Med Assoc Thai. 1999;82(Suppl 1):S57–S62. [PubMed] [Google Scholar]
- 21.Kennelly JM, Tolley KH, Ghani AC, Sabin CA, Maynard AK, Lee CA. Hospital costs of treating haemophilic patients infected with HIV. AIDS. 1995;9:787–793. doi: 10.1097/00002030-199507000-00017. [DOI] [PubMed] [Google Scholar]
- 22.Strawczynski H, Stachewitsch A, Morgenstern G, Shaw ME. Delivery of care to hemophilic children: home care versus hospitalization. Pediatrics. 1973;51:986–991. [PubMed] [Google Scholar]
- 23.Levine PH, Britten AF. Supervised patient-management of hemophilia. A study of 45 patients with hemophilia A and B. Ann Intern Med. 1973;78:195–201. doi: 10.7326/0003-4819-78-2-195. [DOI] [PubMed] [Google Scholar]
- 24.Levine PH. The home therapy program at the New England area hemophilia center. Scand J Haematol Suppl. 1977;31:37–51. doi: 10.1111/j.1600-0609.1977.tb01543.x. [DOI] [PubMed] [Google Scholar]
- 25.Isarangkura P, Chuansumrit A, Panthangkura W, Hathirat P, Pandhawong S. Home therapy for hemophilia in Thailand. Southeast Asian J Trop Med Public Health. 1987;18:552–557. [PubMed] [Google Scholar]
- 26.Lazerson J. Hemophilia home transfusion program: effect on school attendance. J Pediatr. 1972;81:330–332. doi: 10.1016/s0022-3476(72)80304-4. [DOI] [PubMed] [Google Scholar]
- 27.Smith PS, Levine PH. The benefits of comprehensive care of hemophilia: a five-year study of outcomes. Am J Public Health. 1984;74:616–617. doi: 10.2105/ajph.74.6.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Monahan PE, Baker JR, Riske B, Soucie JM. Physical functioning in boys with hemophilia in the U.S. Am J Prev Med. 2011;41(Suppl 4):S360–S368. doi: 10.1016/j.amepre.2011.09.017. [DOI] [PubMed] [Google Scholar]
- 29.Ekert H, Moorehead M, Williamson G. Home treatment of haemophilia. A follow-up study. Med J Aust. 1981;2:21–23. [PubMed] [Google Scholar]
- 30.Ekert H, Smibert E. “Home care” for haemophilia. Med J Aust. 1974;2:803–806. doi: 10.5694/j.1326-5377.1974.tb71198.x. [DOI] [PubMed] [Google Scholar]
- 31.Hilgartner MW. Home care for hemophilia: current state of the art. Scand J Haematol Suppl. 1977;30:58–64. doi: 10.1111/j.1600-0609.1977.tb01521.x. [DOI] [PubMed] [Google Scholar]
- 32.Panicucci F, Baicchi U, Sagripanti A. Three years’ experience in haemophilia home treatment. Scand J Haematol Suppl. 1977;30:81–84. doi: 10.1111/j.1600-0609.1977.tb01529.x. [DOI] [PubMed] [Google Scholar]
- 33.Panicucci F. Hemophilia home treatment in Italy. Scand J Haematol Suppl. 1977;31:25–28. doi: 10.1111/j.1600-0609.1977.tb01541.x. [DOI] [PubMed] [Google Scholar]
- 34.Szucs TD, Offner A, Kroner B, Giangrande P, Berntorp E, Schramm W. Resource utilisation in haemophiliacs treated in Europe: results from the European Study on Socioeconomic Aspects of Haemophilia Care. Resource utilisation in haemophiliacs treated in Europe: results from the European Study on Socioeconomic Aspects of Haemophilia Care. The European Socioeconomic Study Group. Haemophilia. 1998;4:498–501. doi: 10.1046/j.1365-2516.1998.440498.x. [DOI] [PubMed] [Google Scholar]
- 35.Szucs TD, Öffner A, Schramm W. Socioeconomic impact of haemophilia care: results of a pilot study. Haemophilia. 1996;2:211–217. doi: 10.1111/j.1365-2516.1996.tb00139.x. [DOI] [PubMed] [Google Scholar]
- 36.Rabiner SF, Telfer MC, Fajardo R. Home transfusions of hemophiliacs. JAMA. 1972;221:885–887. [PubMed] [Google Scholar]
- 37.Rabiner SF, Telfer MC. Home transfusion for patients with hemophilia A. N Engl J Med. 1970;283:1011–1015. doi: 10.1056/NEJM197011052831902. [DOI] [PubMed] [Google Scholar]
- 38.Soucie JM, Symons J, Evatt B, et al. Home-based factor infusion therapy and hospitalization for bleeding complications among males with haemophilia. Haemophilia. 2001;7:198–206. doi: 10.1046/j.1365-2516.2001.00484.x. [DOI] [PubMed] [Google Scholar]
- 39.Smith PS, Keyes NC, Forman EN. Socioeconomic evaluation of a state-funded comprehensive hemophilia-care program. N Engl J Med. 1982;306:575–579. doi: 10.1056/NEJM198203113061004. [DOI] [PubMed] [Google Scholar]
- 40.Tencer T, Roberson C, Duncan N, Johnson K, Shapiro A. A haemophilia treatment centre-administered disease management programme in patients with bleeding disorders. Haemophilia. 2007;13:480–488. doi: 10.1111/j.1365-2516.2007.01495.x. [DOI] [PubMed] [Google Scholar]
- 41.Abdullaev GM, Efendiev ZI, Askerova FR. [A five-year experience with the management of a group of patients with hemophilia in a special boarding school] Gematol Transfuziol. 1983;28:49–51. [PubMed] [Google Scholar]
- 42.Carter F, Forbes CD, MacFarlane JD, Prentice CR. Cost of management of patients with haemophilia. Br Med J. 1976;2:465–467. doi: 10.1136/bmj.2.6033.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heemstra HE, Zwaan T, Hemels M, et al. Cost of severe haemophilia in Toronto. Haemophilia. 2005;11:254–260. doi: 10.1111/j.1365-2516.2005.01082.x. [DOI] [PubMed] [Google Scholar]
- 44.Martínez-Murillo C, Quintana S, Ambriz R, et al. An economic model of haemophilia in Mexico. Haemophilia. 2004;10:9–17. doi: 10.1046/j.1365-2516.2003.00811.x. [DOI] [PubMed] [Google Scholar]
- 45.McKenzie LL, Fie R, van Eys J. Medical, economic, and social impact of a home therapy program for hemophilia A on selected patients. South Med J. 1974;67:555–559. doi: 10.1097/00007611-197405000-00015. [DOI] [PubMed] [Google Scholar]
- 46.Soucie JM, Cianfrini C, Janco RL, et al. Joint range-of-motion limitations among young males with hemophilia: prevalence and risk factors. Blood. 2004;103:2467–2473. doi: 10.1182/blood-2003-05-1457. [DOI] [PubMed] [Google Scholar]
- 47.Ingram GI, Dykes SR, Creese AL, et al. Home treatment in haemophilia: clinical, social and economic advantages. Clin Lab Haematol. 1979;1:13–27. doi: 10.1111/j.1365-2257.1979.tb00586.x. [DOI] [PubMed] [Google Scholar]
- 48.Arnold E, Lane S, Webert KE, et al. What should men living with haemophilia need to know? The perspectives of Canadian men with haemophilia. Haemophilia. 2014;20:219–225. doi: 10.1111/hae.12297. [DOI] [PubMed] [Google Scholar]
- 49.Weiss HM, Simon R, Levi J, Forster A, Hubbard M, Aledort L. Compliance in a comprehensive hemophilia center and its implications for home care. Family Systems Medicine. 1991;9:111–120. [Google Scholar]
- 50.Peytremann-Bridevaux I, Staeger P, Bridevaux P-O, Ghali WA, Burnand B. Effectiveness of chronic obstructive pulmonary disease-management programs: systematic review and meta-analysis. Am J Med. 2008;121:433–443. doi: 10.1016/j.amjmed.2008.02.009. e4. [DOI] [PubMed] [Google Scholar]
- 51.Kruis AL, Smidt N, Assendelft WJJ, et al. Integrated disease management interventions for patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2013;10:CD009437. doi: 10.1002/14651858.CD009437.pub2. [DOI] [PubMed] [Google Scholar]
- 52.Baishnab E, Karner C. Primary care based clinics for asthma. Cochrane Database Syst Rev. 2012;4:CD003533. doi: 10.1002/14651858.CD003533.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lemmens KMM, Nieboer AP, Huijsman R. A systematic review of integrated use of disease-management interventions in asthma and COPD. Respir Med. 2009;103:670–691. doi: 10.1016/j.rmed.2008.11.017. [DOI] [PubMed] [Google Scholar]
- 54.Blanchette VS, Key NS, Ljung LR, et al. Definitions in hemophilia: communication from the SSC of the ISTH. J Thromb Haemost. 2014;12:1935–1939. doi: 10.1111/jth.12672. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.