Abstract
Background
The aim of this study was to investigate the effects of aqueous extract of alfalfa on blood glucose and serum lipids in alloxan-induced diabetic rats.
Materials and methods
Thirty-two adult male Wistar rats weighing 210–250 g were selected and divided randomly into four groups of eight animals each for 21 days as follows: (1) control group, (2) diabetic control group, (3) diabetic group plus aqueous extract of alfalfa (250 mg/l), and (4) diabetic group plus aqueous extract of alfalfa (500 mg/l). Serum concentrations of total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), triglycerides (TG), very low-density lipoprotein (VLDL), glucose, and the liver enzymes such as aspartate transaminase (AST) and alanine transaminase (ALT) were measured at the end of period in all studied groups.
Results
Administration of 250 and 500 mg/l aqueous alfalfa extract resulted in a significantly decreased glucose, TC, TG, LDL-C, VLDL, ALT, and AST levels and increased HDL levels as compared with the control group and diabetic control group (p < 0.05). Histological examination showed that the aqueous alfalfa extract caused reconstruction of damaged liver and pancreas.
Conclusion
These results suggest that aqueous alfalfa extract revealed significant effects on blood lipids and glucose levels in diabetic rats and might be useful in prevention and treatment of diabetes. However, further studies are needed to determine the exact impacts of those effects.
Keywords: aqueous extract alfalfa, diabetes, alloxan, blood sugar, plant extracts, alfalfa, lipid profile, antidiabetic property, Wistar rats
Introduction
Diabetes mellitus is the most common chronic disease that affects the endocrine system. It is a metabolic disorder of carbohydrate, fat, and protein, affecting a large number of populations in the world. Diabetes mellitus is not a single disorder, but it is a group of metabolic disorders that are characterized by chronic hyperglycemia, resulting from defects in insulin secretion, insulin action, or both. Increased thirst, increased urinary output, ketonemia, and ketonuria are the common symptoms of diabetes mellitus, which occur due to the abnormalities in carbohydrate, fat, and protein metabolism [1, 2]. Diabetes mellitus has caused significant morbidity and mortality due to microvascular (retinopathy, neuropathy, and nephropathy) and macrovascular (heart attack, stroke, and peripheral vascular disease) complications [3].
Considering the great developments in human knowledge about diabetes mellitus diversity and knowing that it is one of the major health, social, and economic problems in the world, a need for finding effective compounds, with fewer side effects, to treat diabetes and its complications has been arisen [4]. Although medicinal herbs and their derivatives have been used as a remedy for diabetes mellitus for a long time, in most cases their effectiveness has not yet been proven by any valid research [5]. Recent decades have seen a rapid rise in reports indicating that botanical dietary supplements can help and prevent disease with various steps [6, 7].
Alfalfa (Medicago sativa) or green gold is one of the medicinal plants that are used in traditional medicine due to being high in protein, calcium, and vitamins, and low in percentage of cellulose. It contains many enzymes, including amylase, invertase, and pectinase, and so it can be used as digestive aids. More than 20% of the dry weight of alfalfa is protein and it is the best source of amino acids, such as arginine (Arg), histidine (His), aspartic acid (Asp), phenylalanine (Phe), and cysteine (Cys). Alfalfa has an extremely high-nutritive value, which includes vitamins A, B1, B6, B12, C, D, E, and K, niacin, pantothenic acid, biotin, folic acid, minerals, protein, and beneficial saponins [8, 9]. Previous studies showed that adding of alfalfa seed in human diet reduced triglycerides (TG) and decreased blood sugar levels [10, 11]. Alfalfa leaves are traditionally used in South Africa as an effective treatment for diabetes [12]. Alfalfa causes stimulation of insulin secretion, and also improves insulin function by reducing the plasma glucose [12]. Increasing of cholesterol and TG levels is due to lack of insulin, resulting in fat storage in the liver [13]. Insulin deficiency in diabetic patients leads to increased levels of amino acids in the blood, and it will lead to an increase in transaminase activity, and its eventual result is the increasing of ketogenesis and gluconeogenesis [14, 15].
Therefore, the aim of this study is to evaluate the effects of aqueous extract of alfalfa on serum lipids, blood glucose, and liver enzymes in alloxan-induced diabetic rats.
Materials and Methods
Thirty-two adult male Wistar rats of about 3–5 months of age and weighing 210–250 g were purchased from the Razi Institute (Karaj, Iran). They were fed with rat laboratory chow diet during 2 weeks of adaptation, and then were divided randomly into four groups of eight animals each for 21 days as follows: (1) control group, fed by usual water and food, (2) diabetic control group, (3) diabetic group by gavage 250 mg/l dose of aqueous extract of alfalfa, and (4) diabetic group by gavage 500 mg/l dose of aqueous extract of alfalfa. Rats selected for the intervention group, were injected with 120 mg/kg dose of alloxan monohydrate, 7 days after injecting alloxan monohydrate to confirm the diabetic state in rats [16], blood glucose level of rats was measured, and rats with blood glucose concentration more than 200 mg/100 cm3 that were diabetics were selected [17].
Identification and preparation of the aqueous extract of alfalfa
M. sativa was collected before sprouting from Semirum District, Isfahan Province. The plant specimen was authenticated and deposited at the Herbarium of the College of Sciences, Isfahan University. The aerial part of the plant was dried in shade.
For preparing the treatment sample, alfalfa plants were collected after the approval of agricultural experts, dried in the shade, and then grinded. Resulting powder was dissolved in physiological serum, and the solution was filtered and used for oral administration after 48 h.
Prepared aqueous solution of alfalfa was administrated orally or intraperitoneally in 250 mg/l dose to third diabetic group and 500 mg/l dose to fourth group (only to alloxan monohydrate-induced diabetics). For investigation of treatment effect 21 days after the alfalfa solution administration, blood samples from all animals were prepared and serum concentrations of total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), TG, very low-density lipoprotein (VLDL), glucose, and the liver enzymes such as aspartate transaminase (AST) and alanine transaminase (ALT) levels were measured at the end of supplementation period in all studied groups.
Histological studies
After a 3-week experimental period and the last blood sampling, the whole pancreas and liver was removed after sacrificing the animal and was immersed in 10% formalin for histopathological examination. Sections were cut and stained with hematoxylin and eosin for histological examination [18].
Statistical analysis
Statistical analyses were conducted using SPSS software version 13.0 (SPSS Inc., Chicago, IL, USA). Between-group comparisons of biochemical factors were carried out using Kruskal–Wallis test. Post-hoc multiple comparisons were made using Tukey’s test. p < 0.05 was considered as statistically significant.
Results
Measurement of blood glucose, AST, and ALT levels in rats showed that the diabetic control group that were treated by alloxan monohydrate has increased level of blood glucose, AST, and ALT as expected. Glucose, AST, and ALT concentrations of blood in two diabetic groups that were treated by alfalfa solution (250 and 500 mg/l aqueous alfalfa extract) showed significant decrease as compared to diabetic controls without alfalfa treatment (p < 0.05) (Table I). As shown in Table I, the decrease of blood glucose, AST, and ALT levels in rats that were treated with 500 mg/l dose is more than in rats with 250 mg/l dose treatment.
Table I.
Effects of alfalfa on serum concentrations of blood glucose and liver enzymes in experimental groups
| Parameters | Control group | Diabetic control group | Treated with 250 mg/l dose | Treated with 500 mg/l dose |
| Glucose (mg/dl) | 56 ± 16.17a | 48.80 ± 2.38b | 55.50 ± 4.23a,b | 47.57 ± 6.57a,b,c |
| AST (IU/l) | 127 ± 3.47a | 199 ± 14.22b | 178.25 ± 8.08a,b | 138.45 ± 2.37a,b,c |
| ALT (IU/l) | 53.55 ± 1.43a | 84 ± 2.33b | 62.37 ± 2.09a,b | 54 ± 3.1a,b,c |
All values are expressed as mean ± SD (n = 8), AST: aspartate transaminase, ALT: alanine transaminase
Significant difference with the diabetic control group (p < 0.05)
Significant difference with the normal control group (p < 0.05)
Significant difference with the treated by 250 mg/l
Table II shows TC, TG, LDL-C, and VLDL levels of rats blood comparison of all groups confirmed diabetic rats (that treated with alloxan monohydrate) have higher in blood rather than intact control group. Rats treated with the extract of aqueous alfalfa (250 and 500 mg/l) showed a significant decrease in TC, TG, LDL-C, and VLDL levels compared with untreated diabetic control. As shown in Table II, the decrease of blood glucose, TC, TG, LDL-C, and VLDL levels in rats that were treated with 500 mg/l dose is more than in rats with 250 mg/l dose treatment.
Table II.
Effects of alfalfa on serum concentrations of lipid profile parameters in experimental groups
| Parameters | Intact control group | Diabetic control group | Treated with 250 mg/l dose | Treated with 500 mg/l dose |
| TG (mg/dl) | 104.87 ± 7.39a | 221.87 ± 13.37b | 175.37 ± 12.43a,b | 153.5 ± 10.90a,b,c |
| TC (mg/dl) | 81.52 ± 5.65a | 131.12 ± 11.36b | 100.72 ± 2.62a,b | 90.77 ± 2.47a,b,c |
| HDL-C (mg/dl) | 15.05 ± 3.61a | 9.85 ± 2.03b | 22.65 ± 5.26a,b | 31.87 ± 7.50a,b,c |
| LDL-C (mg/dl) | 17 ± 3.02a | 19.62 ± 2.61b | 18.37 ± 3.71a,b | 15.25 ± 3.19a,b,c |
| VLDL (mg/dl) | 34.37 ± 7.79a | 78.87 ± 9.34b | 53.25 ± 8.11a,b | 42.12 ± 11.45a,b,c |
All values are expressed as the mean ± SD (n = 8), TG: triglycerides, TC: total cholesterol, HDL-C: high-density lipoprotein cholesterol, LDL-C: low-density lipoprotein cholesterol, VLDL: very low-density lipoprotein
Significant difference with the diabetic control group (p < 0.05)
Significant difference with the normal control group (p < 0.05)
Significant difference with the treated by 250 mg/l
Table II shows the HDL level of rat’s blood. Rats treated with the extract of aqueous alfalfa (250 and 500 mg/l) showed a significant increase in the HDL level as compared to untreated diabetic control. As shown in Table II, the increase in HDL level in rats that were treated with 500 mg/l dose is more than in rats with 250 mg/l dose treatment.
Pancreas and liver cells were highly organized in the normal (control) rats with intact cells. The pancreas and liver cells were disorganized in the diabetic rats with impaired cells. However, the pancreas and liver cells in the groups of treatment with alfalfa aqueous were improved compared with the diabetes group (Figs 1 and 2).
Fig. 1.
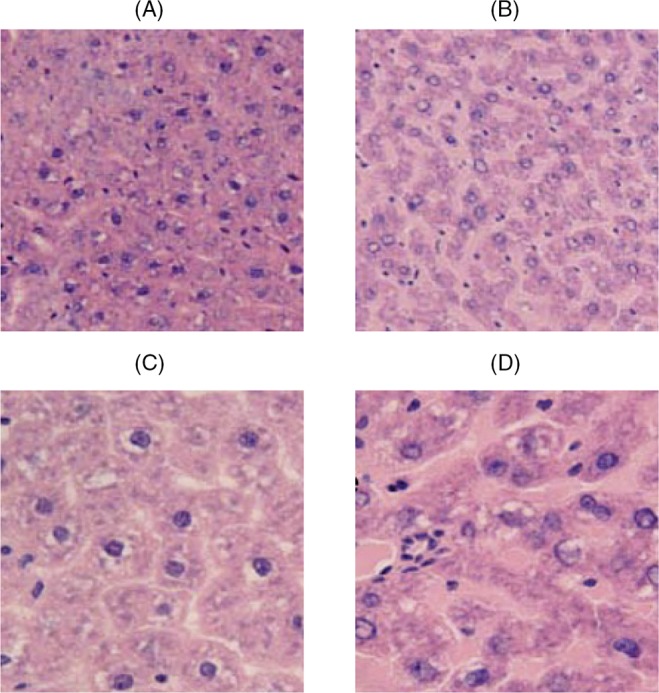
Liver cross-section in the studied groups at the end of 21 days. The histological studies revealed a significant reduction in the treated diabetic groups. Aqueous alfalfa extract caused reconstruction of damaged liver. (A) Liver of control rats that shows normal arrangement of hepatocytes, (B) liver slice of diabetic control group, (C) liver of rats treated with 250 mg/l dose, and (D) liver of rats treated with 500 mg/l dose. All panels were stained with hematoxylin and eosin, 400× magnification
Fig. 2.
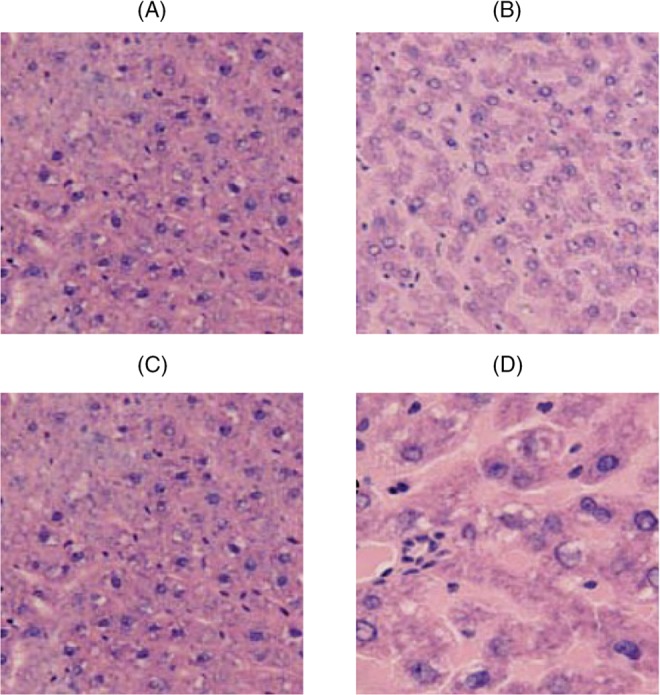
Histopathological effect of aqueous extract of alfalfa on the changes of pancreatic islets of alloxan-induced diabetic rats at the end of 21 days. (A) Negative control – pancreas, (B) diabetic control – shows depleted islets, (C) diabetic rats treated with 250 mg/l of extract – shows preserved islets, and (D) diabetic rats treated with 500 mg/l of extracts – shows full regeneration of islets and exocrine. All panels were stained with hematoxylin and eosin, 400× magnification
The histology of the pancreas (Fig. 2) reveals that the normal (control) rats had intact pancreatic islets. However, alloxan-induced diabetic rats (diabetic control rats) showed depleted islet cells. Diabetic rats that had been treated with the 250 mg/l extract showed preserved islet cells, which is an improvement from what occurred in the untreated alloxan-induced diabetic rats. Further improvements were observed in rats that had been treated with 500 mg/l of extract, such as more prominent islet cells, which indicated an improvement in the architecture of the pancreas as the concentration of the extract increased.
Discussion
This study investigated the effects of alfalfa extract as a possible cure for diabetes disease, so we used diabetic rats induced by alloxan monohydrate injection. Diabetic rats were under oral administration of aqueous extract of alfalfa in different doses. Twenty-one days after oral administration, the TG, cholesterol, and glucose levels of blood were measured, and the results were analyzed.
Results showed that diabetics rats that were treated with alfalfa extract introduced blood factors improved compared with diabetic control that did not receive treatment. This improvement was enhanced with administration of high alfalfa dose.
Comparison of diabetic control and intact control rats showed an increase of cholesterol level in diabetic control rats. Both treatment groups compared with control diabetic group showed a significant decrease in cholesterol level, which may suggest the positive effect of alfalfa in cure.
Diabetic control group showed decreased level of HDL compared with intact control group. Reduced HDL level is one of the signs of diabetes disease and application of alfalfa extract caused compensation of this decrease in diabetic rats that were treated by alfalfa.
Previous studies confirmed that alloxan-induced diabetes caused tissue lipid peroxidation in liver, kidney, and heart. It also caused secretion and release of liver enzymes to circulation [19, 20]. In other words, diabetes and hyperlipidemia result in destruction of cells by altering membrane structure by which the activity of liver enzymes in diabetic rat is increased [15]. Therefore, it appears that alfalfa extraction causes reduction of liver enzymes that may be due to improved diabetes and lipid peroxidation.
In patients with diabetes, increased levels of liver enzymes in the blood indicate liver damage. Under normal circumstances, these enzymes are within the liver cells but in hepatic disorders that liver cells are damaged, enzymes are released into the bloodstream. Oral administration of alfalfa extract with 250 mg/l dose decreased liver enzyme concentration in blood and this decrease was more significant in 500 mg/l dose, so alfalfa extract caused reduction of liver damage.
Overall, the results suggest that the aqueous extract of alfalfa significantly reduces blood sugar, TG, cholesterol, and LDL levels and increases HDL levels and liver enzymes (AST and ALT) in blood. AST and ALT are good indicators for liver function [21].
The histological studies revealed a significant reduction in the size of Langerhans islets in the diabetic group, and this group showed significantly increased liver inflammation compared with intact control group. Therefore, aqueous alfalfa extract caused reconstruction of damaged liver and pancreas.
Alfalfa stimulates insulin secretion and improves insulin function resulting in the reduction of plasma glucose concentration [12]. A previous study reported the increase of insulin secretion up to 3-fold in the presence of alfalfa extraction [22]. Oral administration of alfalfa seeds triggered the reduction of blood lipoprotein level in diabetics that this factor was very high. Alfalfa is a phytoestrogen source [23]. Phytoestrogens are non-steroidal plant-derived compounds with biological activity similar to estrogen [24]. Phytoestrogens increase liver lipid biosynthesis from glucose [25].
Alfalfa consists of saponins that have heart-protective effects due to cholesterol reduction, and these agents can absorb in digestive system in the body [26]. Saponins inhibit cholesterol esterase and acetyl coenzyme A carboxylase enzymes, so inhibit fatty acid synthesis, which increases the ratio of HDL-C to LDL-C. Therefore, they can be effective in reducing cardiovascular complications of diabetes. Saponins decrease the intestinal absorption and increase the defecation of cholesterol [27]. Alfalfa is a rich source of vitamins and phytoestrogens, and so it is used as a food additive in many developed countries [28, 29]. The liver is one of the organs that is affected by diabetes [26]. When liver enzyme activity increases in serum, it reflects liver damage and increased activity of liver enzymes ALT and AST is because of leakage from the cytosol into the blood stream [30, 31]. Inflammation that showed in liver slice of untreated diabetic rats was very slight in rats that were cured with alfalfa extraction. This may show curable effect of alfalfa in liver disease improvement.
Diabetes is associated with increased oxidative stress, and antioxidants are able to reduce the oxidative stress and ameliorate diabetes mellitus complications [28–30]. It should be noted that antioxidants are also able to reduce the plasma lipid level [31, 32] and ameliorate liver damage [33]. Hence, alfalfa that has antioxidant activity may have these positive effects, at least in part, by its antioxidant activity. Antioxidants also reduce other complications of diabetes including renal damage [33–35]. Therefore, alfalfa may have more benefits on diabetic and hyperlipidemic patients.
Conclusion
The results of this study indicate that the diameter and number of pancreatic islets were significantly increased in diabetic rats treated with the extract compared with diabetic controls. According to biochemical and histological results, we can conclude that the mechanism of alfalfa extract for hypoglycemia is islets repair.
Authors’ contribution
MKF, EA, PK, and MK prepared the manuscript and MK edited it.
Conflict of interest
The authors declared no competing interests.
Ethics
Ethical issues (including plagiarism, misconduct, data fabrication, falsification, double publication or submission, and redundancy) have been completely observed by the authors.
Funding Statement
Funding sources: None.
References
- 1.Pareek H, Sharma S, Khajja BS, Jain K, Jain G: Evaluation of hypoglycemic and anti-hyperglycemic potential of Tridax procumbens (Linn.). BMC Complement Altern Med 9, 48 (2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Craig ME, Jefferies C, Dabelea D, Balde N, Seth A, Donaghue KC: Definition, epidemiology, and classification of diabetes in children and adolescents. Pediatr Diabetes 15(Suppl 20), 4–17 (2014) [DOI] [PubMed] [Google Scholar]
- 3.Thévenod F. (2008): Pathophysiology of diabetes mellitus type 2: Roles of obesity, insulin resistance and β-cell dysfunction In: Diabetes and Cancer, eds Masur K, Thévenod F, Zänker KS, Karger, Basel, pp. 1–18 [Google Scholar]
- 4.Suji G, Sivakami S: Approaches to the treatment of diabetes mellitus: An overview. Cell Mol Biol (Noisy-le-grand) 49, 635–639 (2003) [PubMed] [Google Scholar]
- 5.Shapiro K, Gong WC: Natural products used for diabetes. J Am Pharm Assoc 42, 217–226 (2002) [DOI] [PubMed] [Google Scholar]
- 6.Mahmood ZA, Sualeh M, Mahmood S, Karim MA: Herbal treatment for cardiovascular disease the evidence based therapy. Pak J Pharm Sci 23, 119–124 (2010) [PubMed] [Google Scholar]
- 7.Nasri H, Rafieian-Kopaei M: Protective effects of herbal antioxidants on diabetic kidney disease. J Res Med Sci 19, 82–83 (2014) [PMC free article] [PubMed] [Google Scholar]
- 8.Zargari A. (1996): Therapeutic Plants (1st ed.). Tehran University Press, Tehran, pp. 642–646 [Google Scholar]
- 9.Hong Y-H, Chao W-W, Chen M-L, Lin B-F: Ethyl acetate extracts of alfalfa (Medicago sativa L.) sprouts inhibit lipopolysaccharide-induced inflammation in vitro and in vivo. J Biomed Sci 16, 64 (2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Asgary S, Moshtaghian J, Hosseini M, Siadat H: Effects of alfalfa on lipoproteins and fatty streak formation in hypercholesterolemic rabbits. Pak J Pharm Sci 21, 460–464 (2008) [PubMed] [Google Scholar]
- 11.Mehranjani M, Shariatzadeh M, Desfulian A, Noori M, Abnosi M, Moghadam Z: Effects of Medicago sativa on nephropathy in diabetic rats. Indian J Pharm Sci 69, 768–772 (2007) [Google Scholar]
- 12.Gray AM, Abdel-Wahab YH, Flatt PR: The traditional plant treatment, Sambucus nigra (elder), exhibits insulin-like and insulin-releasing actions in vitro. J Nutr 130, 15–20 (2000) [DOI] [PubMed] [Google Scholar]
- 13.Dhandapani S, Subramanian VR, Rajagopal S, Namasivayam N: Hypolipidemic effect of Cuminum cyminum L. on alloxan-induced diabetic rats. Pharmacol Res 46, 251–255 (2002) [DOI] [PubMed] [Google Scholar]
- 14.Gokce G, Haznedaroglu MZ: Evaluation of antidiabetic, antioxidant and vasoprotective effects of Posidonia oceanica extract. J Ethnopharmacol 115, 122–130 (2008) [DOI] [PubMed] [Google Scholar]
- 15.Udayakumar R, Kasthurirengan S, Mariashibu TS, Rajesh M, Anbazhagan VR, Kim SC, Ganapathi A, Choi CW: Hypoglycaemic and hypolipidaemic effects of Withania somnifera root and leaf extracts on alloxan-induced diabetic rats. Int J Mol Sci 10, 2367–2382 (2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matkovics B, Sasvari M, Kotorman M, Varga IS, Hai DQ, Varga C: Further prove on oxidative stress in alloxan diabetic rat tissues. Acta Physiol Hung 85, 183–192 (1996) [PubMed] [Google Scholar]
- 17.Takasu N, Asawa T, Komiya I, Nagasawa Y, Yamada T: Alloxan-induced DNA strand breaks in pancreatic islets. Evidence for H2O2 as an intermediate. J Biol Chem 266, 2112–2114 (1991) [PubMed] [Google Scholar]
- 18.Nagappa A, Thakurdesai P, Rao NV, Singh J: Antidiabetic activity of Terminalia catappa Linn fruits. J Ethnopharmacol 88, 45–50 (2003) [DOI] [PubMed] [Google Scholar]
- 19.Stanely P, Prince M, Menon VP: Hypoglycaemic and other related actions of Tinospora cordifolia roots in alloxan-induced diabetic rats. J Ethnopharmacol 70, 9–15 (2000) [DOI] [PubMed] [Google Scholar]
- 20.Amraie E, Farsani MK, Sadeghi L, Khan TN, Babadi VY, Adavi Z: The effects of aqueous extract of alfalfa on blood glucose and lipids in alloxan-induced diabetic rats. Interv Med Appl Sci 7, 124–128 (2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Devi PU, Sharada A, Solomon F: Antitumor and radiosensitizing effects of Withania somnifera (Ashwagandha) on a transplantable mouse tumor, Sarcoma-180. Indian J Exp Biol 31, 607–611 (1993) [PubMed] [Google Scholar]
- 22.McClenaghan NH, Barnett CR, Ah-Sing E, Abdel-Wahab YH, O’Harte FP, Yoon T-W, Swanston-Flatt SK, Flatt PR: Characterization of a novel glucose-responsive insulin-secreting cell line, BRIN-BD11, produced by electrofusion. Diabetes 45, 1132–1140 (1996) [DOI] [PubMed] [Google Scholar]
- 23.Basch E, Ulbricht C, Harrison M, Sollars D, Smith M, Dennehy C, Szapary P: Alfalfa (Medicago sativa L.): A clinical decision support tool. J Herb Pharmacother 3, 69–90 (2003) [DOI] [PubMed] [Google Scholar]
- 24.Lorand T, Vigh E, Garai J: Hormonal action of plant derived and anthropogenic non-steroidal estrogenic compounds: Phytoestrogens and xenoestrogens. Curr Med Chem 17, 3542–3574 (2010) [DOI] [PubMed] [Google Scholar]
- 25.Wang J-F, Guo Y-X, Niu J-Z, Liu J, Wang L-Q, Li P-H: Effects of Radix Puerariae flavones on liver lipid metabolism in ovariectomized rats. World J Gastroenterol 10, 1967–1970 (2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fan W, Du H, Zhou L, Shi P, Wang C: Digital gene-expression of alfalfa saponin extract on laying hens. Genomics Data 3, 97–99 (2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jackson IM: Abundance of immunoreactive thyrotropin-releasing hormone-like material in the alfalfa plant. Endocrinology 108, 344–346 (1981) [DOI] [PubMed] [Google Scholar]
- 28.Seguin P, Zheng W, Souleimanov A: Alfalfa phytoestrogen content: Impact of plant maturity and herbage components. J Agronomy Crop Sci 190, 211–217 (2004) [Google Scholar]
- 29.Barens DK, Sheaffer C: Alfalfa. Agriculture 2, 205–216 (1995) [Google Scholar]
- 30.Kazemi S, Asgary S, Moshtaghian J, Rafieian M, Adelnia A, Shamsi F: Liver-protective effects of hydroalcoholic extract of allium hirtifolium boiss. In rats with alloxan-induced diabetes mellitus. ARYA Atheroscler 6, 11 (2010) [PMC free article] [PubMed] [Google Scholar]
- 31.Navarro MC, Montialla M, Martín A, Jiménez J, Utrilla MP: Free radical scavenger and antihepatotoxic activity of Rosmarinus tomentosus. Planta Med 59, 312–314 (1993) [DOI] [PubMed] [Google Scholar]
- 32.Udayakumar R, Kasthurirengan S, Mariashibu TS, Rajesh M, Anbazhagan VR, Kim SC, Ganapathi A, Choi CW: Hypoglycaemic and hypolipidaemic effects of Withania somnifera root and leaf extracts on alloxan-induced diabetic rats. Int J Mol Sci 10, 2367–2382 (2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nasri H, Rafieian-Kopaei M: Tubular kidney protection by antioxidants. Iran J Public Health 42, 1194–1196 (2013) [PMC free article] [PubMed] [Google Scholar]
- 34.Baradaran A, Nasri H, Rafieian-Kopaei M: Protection of renal tubular cells by antioxidants: Current knowledge and new trends. Cell J 16, 568–571 (2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Asgary S, Kazemi S, Moshtaghian SJ, Rafieian M, Bahrami M, Adelnia A: The protective effect of Cucurbita pepo L. on liver damage in alloxan-induced diabetic rats. J Shahrekord Univ Med Sci 11, 59–65 (2010) [Google Scholar]


