Abstract
Purpose
This article is devoted to the investigation of the structural features of the bone marrow of mature rats.
Materials and methods
The investigation of the structural features of the bone marrow was performed on the femurs of the mature male rats. General structure of the organ was studied with hematoxylin–eosin and Van Gieson staining of samples. Certain features of the bone marrow structure were studied using immunohistochemical method (CD3, CD79α, S100, myeloperoxidase, and cyclin D1).
Results
We can state that stromal–parenchymal structure is typical for the bone marrow of rats as for any other organ. The stromal component is presented with bone tissue (48.8 ± 3.3% at epiphyses), the net of blood vessels (18.7 ± 2.1%), fat tissue (11 ± 2%), fibrous tissue (0.7 ± 0.2%), and the network of reticular fibers. Hematopoietic tissue covers 20.9 ± 3.7% at the femoral epiphyses and 69.6 ± 2.2% at diaphysis. Among these tissues, myelopoiesis occupies 74.2 ± 4.7%, erythropoiesis – 24.3 ± 4.7%, and lymphopoiesis – less than 5%. Megalokaryocytes take 0.1–0.3%.
Conclusion
Considering the lack of significant anatomical, morphological, and histological differences of red bone marrow of rats and humans, we can state that hematopoiesis in rats takes place on the basis of the same principles as in humans, although it has certain mechanisms.
Keywords: bone marrow, immunohistochemical method, myelopoiesis, erythropoiesis, lymphopoiesis, megalokaryocytes
Introduction
Bone marrow is the largest hematopoietic organ in mammals. It compounds from 3% to 5% of total body weight [1]. Hematopoiesis in bones begins at the prenatal period and continues throughout life [2]. Therefore, different endogenous and exogenous factors may constantly influence the quality of the process of hematopoiesis, which is reflected in violation of histoarchitectonics of the bone marrow [3].
We always need to compare the morphological structure of organs of experimental animals with those of intact tissue in the process of studying histological changes in tissues under the influence of various factors. Therefore, the availability of clear data of the structure of unaltered organs is a key element when conducting the experimental research. In recent decades, researchers obtained considerable data about histoarchitectonics and the quality of the bone marrow. These data include the topography of the bone marrow location and the age of animals [1, 4], but there is a constant need in improving the data due to the rapid scientific development. Routine histological methods do not allow to fully identify all the components of the bone marrow, so there is a demand to involve other (histochemical and immunohistochemical) techniques.
The aim of this study is the investigation of the structural features of the bone marrow of mature rats and comparing their indices with the data of normal human bone marrow structure.
Materials and Methods
The investigation of the structural features of the bone marrow was performed on the femurs of mature (4 months old) male rats of Wistar line (n = 12). All investigations were performed in accordance with “European Convention for the Protection of vertebrate animals used for experimental and other scientific purposes,” “General ethical animal experimentation,” and “Helsinki Declaration of the General Assembly of the World Medical Association.” Rats were on a standard diet indoors in the vivarium at 20–25 °C, with humidity not more than 50%, and they had day/night light mode. Access to water was free.
After the process of decapitation of animals under ether anesthesia, two femur bones were removed, and then they were immersed in 10% buffered formalin solution. Decalcification was conducted with the help of EDTA solution (pH – 7.0) for 14 days, provided daily replacement of the solution, and then studied tissue was embedded in paraffin. We made 4 mm slices to study histoarchitectonics of tissue in detail. General structure of the organ was studied with hematoxylin and eosin staining of samples. Qualitative composition of stromal component was investigated using Van Gieson staining of samples. Certain features of the bone marrow structure were studied using immunohistochemical method. To investigate functional and morphological features of various stages of hematopoiesis, we installed the expression of the following kinds of receptors: CD3, CD79α, S100, myeloperoxidase, and cyclin D1.
The measurement of the sizes of the constituent elements of micro specimen was conducted using “Digimizer” morphometric program. We obtained and stored the images of specimen using “SEO Scan” digital image output system (Ukraine). Mathematical calculations were done using Microsoft Excel 2010 with 12.0.5 Attestat option.
Results
In a comprehensive study of the morphological properties of the bone marrow structure we revealed that hematopoietic tissue is presented in all parts of the femur – medullary canal of diaphysis and intratrabecular space of trabecular bone epiphysis. Parenchyma (centers of hematopoietic cells) and stroma are differentiated in the bone marrow structure like in any other organ. Stromal component is represented by bone, fat and connective tissues, blood vessels, reticular fibers, and other types of cells (Fig. 1).
Fig. 1.
Longitudinal section of rat femur. 1 – stroma, 2 – parenchyma. (A) Diaphysis area and (B) epiphysis area. Hematoxylin and eosin staining. Magnification 100×
Compact bone tissue is an outside line of the bone marrow. As a part of trabecular bone, it is also presented in the form of beams (trabeculas), combined together in different directions. It has a typical Gavers system structure and is covered with a layer of osteogenic cells – endosteum (Fig. 2). The share of bone tissue accounts for 48.8 ± 3.3% of the total tissue in the composition of the trabecular bone.
Fig. 2.
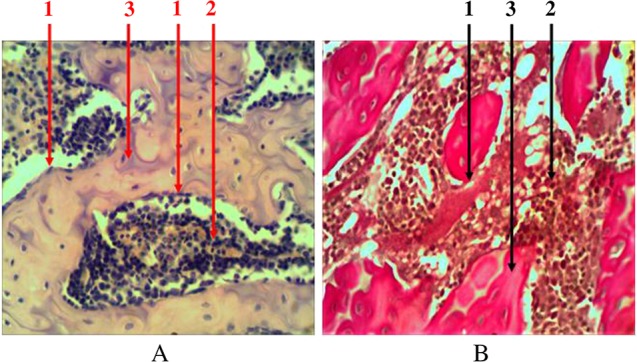
Longitudinal section of rat femur. 1 – endost (osteogenic cells), 2 – hematopoietic tissue, 3 – bone tissue. (A) Hematoxylin and eosin staining and (B) Van Gieson staining. Magnification 400×
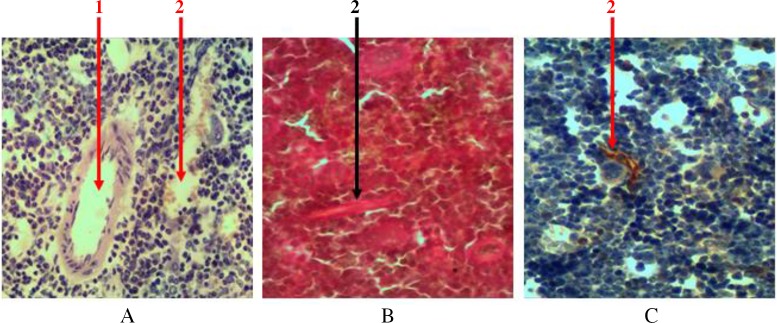
Hematopoietic tissue in all parts of the bone marrow is penetrated with a large number of blood vessels. These vessels are presented with ascending and descending branches of nutrient artery and vein with extensive network of arteriolas, which fall into venous sinuses. The network of radial arteries that depart from the periosteal artery penetrates through bone tissue and falls into these sinuses. Thick-walled vessels are easily noticeable at histological examination of samples. But a significant number of sinusoids represented by one layer of endothelium, with almost complete lack of basal membrane, can be seen only when using additional methods of tissue staining (Fig. 3). The total area occupied by a network of blood vessels is 18.7 ± 2.1%.
Fig. 3.
Cross section of rat femur. 1 – arteriola, 2 – sinusoid. (A) Hematoxylin and eosin staining, (B) Van Gieson staining, and (C) immunohistochemical study of cyclin D1 receptor. Magnification 400×
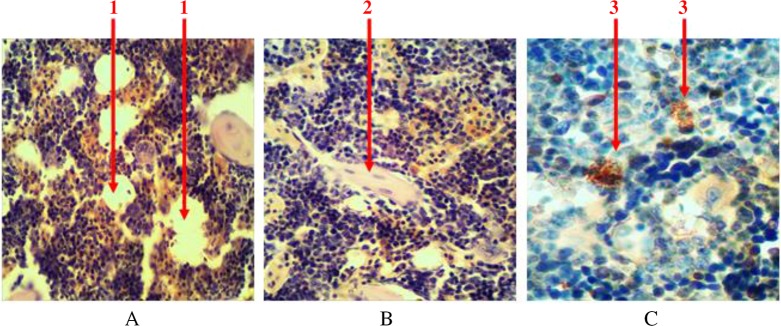
Single-scattered and grouped lipocytes (yellow bone marrow) are presented among the parenchyma of the bone marrow in the histological samples stained with hematoxylin and eosin. They can be seen in the form of lacunas with peripherally placed nuclei (in the form of a ring) (Fig. 4A). At the femoral diaphysis, they are located in the center and have the tendency to grouping. The share of adipose tissue accounts for 11 ± 2% of the bone marrow.
Fig. 4.
Longitudinal section of rat femur. 1 – liposites, 2 – fibrous fibers, 3 – mast cells. (A) and (B) Hematoxylin and eosin staining and (C) immunohistochemical study. Magnification 400×
All hematopoietic tissues are penetrated with connective fibers (collagen and elastic), which serve as an original frame to it (Fig. 4B). They are almost invisible during routine histological studies, but they are clearly evident when their number increases at histochemical and immunohistochemical studies. The share of these connective fibers accounts for 0.7 ± 0.2%.
Individual mast cells are always presented in the tissue of the bone marrow. They are in round or oval shape, with size ranging from 4.5 to 15 µm. There is a significant number of granules (with size of 0.25–2.5 µm) available in the cytoplasm. They are able to produce, store and secrete biologically active substances (heparin, serotonin, and others) that affect the morphological and functional state of hematopoietic tissue. At immunohistochemical study, intracellular granules bind with diaminobenzidine (Fig. 4C). The share of intracellular granules accounts for no more than 0.1% of the total number of cells.
Summarizing the abovementioned facts, we can state that the share of stroma at femoral epiphysis in mature rats accounts for 79.1 ± 3.7%, and its share at diaphysis accounts for 30.4 ± 2.2%.
Parenchymal component of the bone marrow of rats is submitted with precursors of erythropoiesis, leucopoiesis, and thrombocytopoiesis at all stages of differentiation (I–VI stages).
Granulocytopoiesis in the bone marrow is characterized with more endosteal localization of precursors. Leucopoiesis centers are located around sinusoids in diaphyseal part of the femur. They are presented with various maturing predictors (myeloblasts, progranulocytes, medullocells, metamyelocytes, and stab granulocytes) and mature granulocytes. With increasing differentiation of cells their size gradually decreases, specific granularity appears, and qualitative changes in the nucleus take place (its fragmentation enhances, basophilia of nuclei reduces, chromatin condenses). Most of eosinophiles and young and stab neutrophiles have ring-shaped nucleus because granulocyte development in rats occurs in annulated type. Granulosity of neutrophiles is very small, and their nuclei have 5–8 segments. It was found that predictors of granulocytopoiesis are located separately and in the form of clusters among hematopoietic tissue when using immunohistochemical studies to identify myeloperoxidase receptors, which are indicators of granulocyte sprout (Fig. 5). In cells that are on the higher step of differentiation, there is a more intense reaction at the immunohistochemical detection of myeloperoxidase receptors.
Fig. 5.
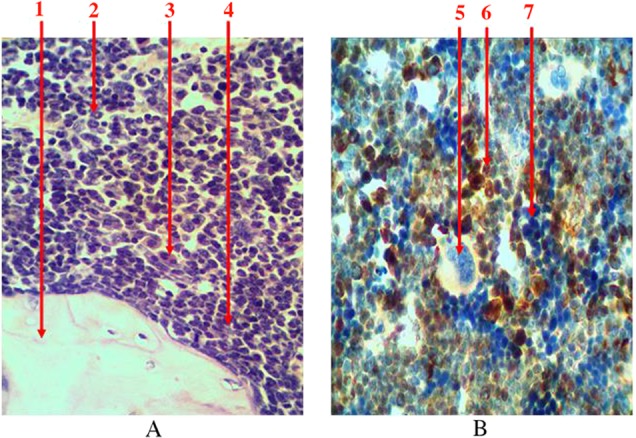
Longitudinal section of rat femur. 1 – bone tissue, 2 – ring-shaped neutrophiles, 3 – eosinophiles, 4 – periosteal growth of granulocytes precursors, 5 – megalokaryocyte, 6 – the center of leucopoiesis, 7 – erythrocytic insula. (A) Hematoxylin and eosin staining and (B) immunohistochemical study of receptors for myeloperoxidase. Magnification 400×
Among the total number of hematopoietic cells, granulocyte asprout accounts for 62–81% (average is 70.6 ± 2.3%) and occupies 13–17% (average is 14.8 ± 1.4%) of the total area of the bone marrow at epiphysis of femur. At the diaphysis, the area occupied by granulocytopoiesis is 46–58% (average is 51.2 ± 3.3%). Among the parenchyma, this figure is 67–84% (average is 74.2 ± 4.7%).
Group location of predictors in the bone marrow, which are located directly near sinusoids in the erythroblastic insulas, is typical for the process of erythropoiesis (Fig. 6). When we identified leucopoiesis tissue at immunohistochemical study of nuclei-containing cells that were receptor negative (CD3, CD79α, and myeloperoxidase), we considered them to be the precursors of red blood cells.
Fig. 6.
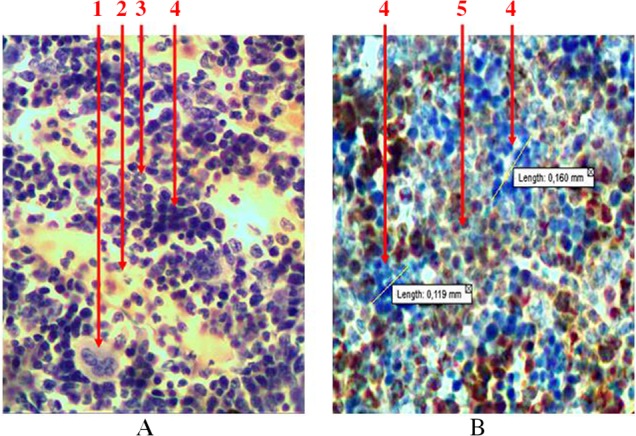
Longitudinal section of rat femur. 1 – megalokaryocyte, 2 – sinusoid, 3 – ring-shaped neutrophiles, 4 – erythroblastic insula, 5 – granulocytopoiesis area. (A) Hematoxylin and eosin staining and (B) immunohistochemical study of receptors for myeloperoxidase. Magnification 400×
Among the total number of hematopoietic cells, erythropoietic sprout at the femoral epiphysis accounts for 14–33% (average is 24.4 ± 6.3%) and occupies 3–7% (average is 5.3 ± 1.4%) of the total area of the bone marrow. At the diaphysis, the area occupied by precursors of red blood cells is 11–23% (average is 17.8 ± 3.3%). Among the parenchyma, this figure is 15–31% (average is 24.3 ± 4.7%).
Erythroblastic insulas, which are located in the central position in relation to the bone marrow channel, are presented with proerythroblasts, erythroblasts (basophilic, polychromatophilic, and oxyphilic), normocytes, and reticulocytes with discrete-located erythrocytes.
Gradual decrease in size, the condensation of chromatin in the nucleus, and the change of nucleus shape take place with increasing differentiation of cells. Also, the nucleus disappears from the cell, cytoplasm basophilia transits in its oxiphilia, and the hemoglobin accumulates when the differentiation of cells increases. The quantity of cellular precursors in erythroid part of the insula of red bone marrow is 30.6 ± 2.4. The average area of this mass is 0.014 mm2.
The ratio between the precursors of granulocytopoiesis and erythropoiesis is 2:1–5:1.
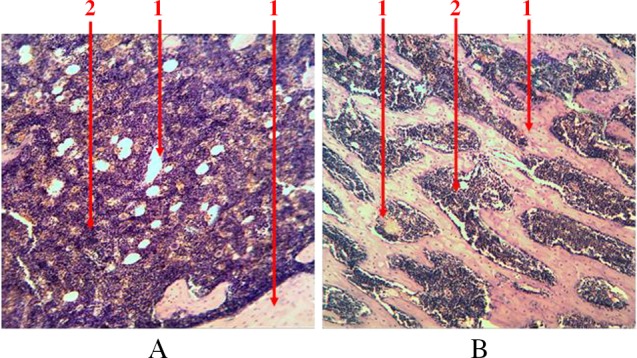
The precursors of thrombocytopoiesis in the bone marrow of rats are presented as megalokaryocytes – giant multinuclei cells. The diameter of megalokaryocytes varies within 70–110 µm. This is a mononuclear cell. Its nucleus consists of many different pieces, which have different chimeric form. The cytoplasm of platelets precursors of large size has azurophilia granulosity. On its periphery, there are separated parts (clasmatosis) that are mature platelets. Megalokaryocytes are irregularly dispersed in the reticulum net. They are situated among hematopoietic cells alone or form groups (no more than five cells) and clusters (more than five cells) near sinusoids (Fig. 7). Their number fluctuates around 0.1–0.3%.
Fig. 7.
Longitudinal section of rat femur. 1 – megalokaryocyte. (A) Single, (B) grouped, and (C) clustered location of megalokaryocytes. Hematoxylin and eosin staining. Magnification 400×
To detect precursors of lymphopoiesis, we performed immunohistochemical studies with detection of receptors to CD3 (a marker of T-cell precursors) and to CD79α (a marker of B lymphocytes) because of the absence of opportunities to identify them at histological examination (Fig. 8). There are both cell precursors and mature cells among the lymphoid cells. There is a sprout of B lymphocytes that is more common among cell precursors (lymphoblasts and prolymphocytes) and there are T lymphocytes that are more common among mature cells. They are located separately and scattered as small clusters among hematopoietic tissue. There is a gradual reduction of the cells size and chromatin condensation when differentiation of cells increases. The amount of cytoplasm that surrounds the nucleus of a mature cell in the form of a thin rim also reduces. In case of detection of the mature forms of B lymphocytes, there are isolated plasmocytes, which are presented with relatively large cells. These plasmocytes have a large amount of eccentrically located cytoplasm, and they also have trochal structure of chromatin in the nucleus. The total number of lymphocytes does not exceed 5% of all forms of hematopoietic cells in red bone marrow of rat’s femur.
Fig. 8.
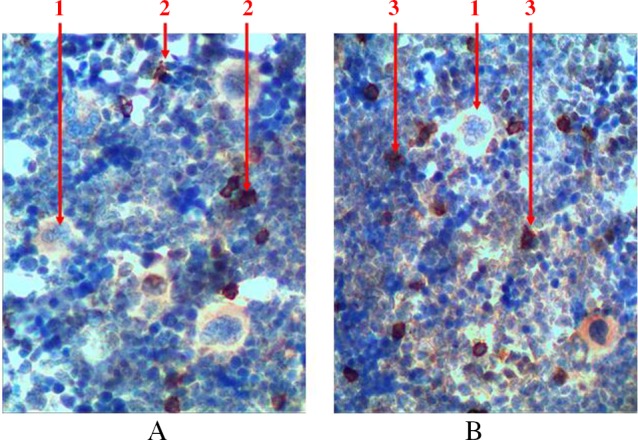
Longitudinal section of rat femur. 1 – megalokaryocytes, 2 – T lymphocytes, 3 – B lymphocytes. (A) immunohistochemical study of CD3 receptors and (B) immunohistochemical study of CD79α receptors. Magnification 400×
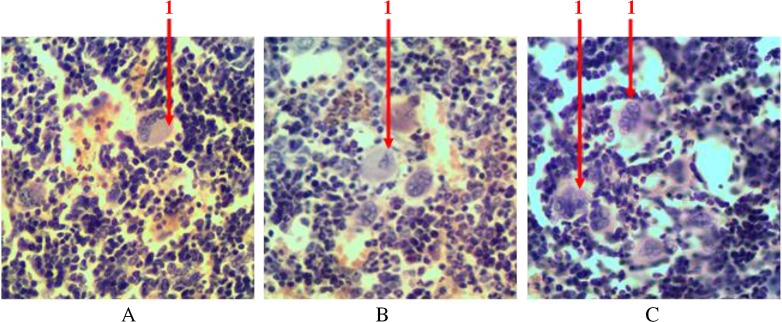
With the help of immunohistochemical studies which detected S100 receptors, we found that singly scattered macrophage cells are presented among hematopoietic tissue. They are available in the centers of erythropoiesis (in the structure of erythroblastic insulas) and among the areas of myelopoiesis. We detected S100-positive cells with different shape and size. Macrophages often formed processes that wrapped different types of hematopoietic cells (Fig. 9).
Fig. 9.
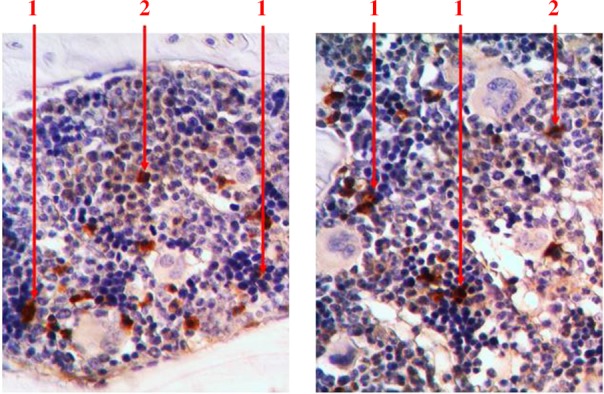
Longitudinal section of rat femur. 1 – S100-positive cells in the structure of erythroblastic insulas, 2 – S100-positive cells in the areas of myelopoiesis. Immunohistochemical study of S100 receptors. Magnification 400×
Discussion
Evaluating the research data, we can state that stromal–parenchymal structure is typical for the bone marrow of rats as for any other organ. The relationship between these components is different depending on the part of the femur. The predominance of stroma is typical for epiphysis, and the predominance of hematopoietic tissue is typical for diaphysis. Thus, the stromal component of the bone marrow is presented with bone tissue (48.8 ± 3.3% at epiphyses), the net of blood vessels (18.7 ± 2.1%), fat tissue (11 ± 2%), fibrous tissue (0.7 ± 0.2%), and the network of reticular fibers. Macrophages and mast cells are diffusely scattered in the fat and fibrous tissues. Hematopoietic tissue covers 20.9 ± 3.7% at the femoral epiphyses and 69.6 ± 2.2% at diaphysis. Among these tissues, myelopoiesis occupies 74.2 ± 4.7%, erythropoiesis – 24.3 ± 4.7%, and lymphopoiesis – less than 5%. Megalokaryocytes take 0.1–0.3% of the area of sanguification. There is a clear zonal location in various sprouts of sanguification. Precursors of granulocytes in most cases are placed in periosteal areas. Erythrocyte sprout is represented as insulas that are located more centrally and around capillaries. Dispersed type of location is typical for lymphocyte precursors. Megakaryocytes are detected singly, in groups, and clusters clearly at sinusoids with phenomena of clasmatosis in the lumen of blood vessels.
It was important to obtain data about the structural features of the bone marrow of rats and compare these data with the data of human bone marrow structure. These data can be used in the further modeling for the possible impact of various exogenous and endogenous factors on the process of hematopoiesis.
Overall, we have not detected any significant differences comparing quantitative and qualitative indicators of red bone marrow of rats and humans, although hematopoietic cells of rats were characterized by some features [1, 5]. Most of eosinophiles and young and stab neutrophiles have ring-shaped nucleus because granulocyte development in rats occurs in annulated type. Granulosity of neutrophilic granulocytes is very small, and their nuclei have 5–8 segments. Basophilic granulocytes (mast cells) have large size and are found as single cells. Lymphocytes are found in a slightly smaller quantity among parenchymatous component, if compared with human bone marrow (5% vs. 10–15% in humans).
Thus, these results allow to simulate different effects on rats considering large identity of morphological structure of their bone marrow to the central hematopoietic organ of a human. Then scientists will be able to use these results when studying the influence of different factors on people.
Conclusion
Bone marrow of mature rats’ femur, like any other organ, has specific stromal–parenchymal type of structure with distinct features of localization and location of each of its components.
Considering the lack of significant anatomical, morphological, and histological differences of red bone marrow of rats and humans, we can state that hematopoiesis in rats takes place on the basis of the same principles as in humans, although it has certain mechanisms.
Authors’ contribution
AR and ML made the critical review; YuL and VS prepared the article, prepared figures, and manuscript preparation; LK did literature review, prepared figures; OG did literature review; IM prepared figures. All authors read and approved the final form of the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Funding Statement
Funding sources: No financial support was received for this study.
References
- 1.Travlos GS: Normal structure, function, and histology of the bone marrow. Toxicol Pathol 34, 548–565 (2006) [DOI] [PubMed] [Google Scholar]
- 2.Tavian M, Péault B: Embryonic development of the human hematopoietic system. Int J Dev Biol 49, 243–250 (2005) [DOI] [PubMed] [Google Scholar]
- 3.Travlos GS: Histopathology of bone marrow. Toxicol Pathol 34, 566–598 (2006) [DOI] [PubMed] [Google Scholar]
- 4.Cline JM, Maronp RR: Variations in the histologic distribution of rat bone marrow cells with respect to age and anatomic site. Toxicol Pathol 13, 349–355 (1985) [DOI] [PubMed] [Google Scholar]
- 5.Levitsky A. (2012): Trepan biopsy of bone marrow: Guide to modern clinical and pathologic diagnosis. Levitsky Edouard, Zhytomyr, Polesye [Google Scholar]