Abstract
Purpose
Human amniotic epithelial cells (hAECs) are promising tools for endothelial repair in vascular regenerative medicine. We hypothesized that these epithelial cells are capable of repairing the damaged endothelial layer following balloon injury of the carotid artery in adult male rats.
Results
Two days after injury, the transplanted hAECs were observed at the luminal side of the arterial wall. Then, 4 weeks after the injury, significant intimal thickening was observed in both untreated and cell implanted vessels. Constriction was decreased in both implanted and control animals. Immunohistochemical analysis showed a few surviving cells in the intact arterial wall, but no cells were observed at the site of injury. Interestingly, acetylcholine-induced dilation was preserved in the intact side and the sham-transplanted injured arteries, but it was a trend toward decreased vasodilation in the hAECs’ transplanted vessels.
Conclusion
We conclude that hAECs were able to incorporate into the arterial wall without immunosuppression, but failed to improve vascular function, highlighting that morphological implantation does not necessarily result in functional benefits and underscoring the need to understand other mechanisms of endothelial regeneration.
Keywords: amniotic epithelial cells, amnion, endarterectomy, vascular injury, BrdU
Introduction
Vascular injury leads to pathological repair and remodeling that involve vascular smooth muscle cell migration and proliferation, resulting in neointimal hyperplasia [1]. Endothelial cell loss is a major contributing factor to the pathological repair of the injured blood vessel [2]. The disruption of endothelial integrity leads to a concomitant reduction in the production of vasculoprotective mediators, such as nitric oxide and prostacyclin, and increased vasoconstrictor and growth-promoting factors resulting in elevated vascular tone, platelet adhesion, enhanced inflammation, and medial smooth muscle cell proliferation [3, 4]. The resultant neointimal hyperplasia is the pathological basis for restenosis after revascularization procedures, such as angioplasty, stenting, and bypass grafting [1, 2]. Carbon monoxide, thalidomide, and tamoxifen have been shown to reduce the neointimal formation [5, 6], and stem cells and interleukin-1 receptor antagonist were also applied in several investigations to decrease the intimal hyperplasia [7–11]. Hybrid biodegradable stents can accelerate re-endothelialization [12], while endoglin antibody-coated stents can inhibit neointimal formation of porcine coronary arteries [13].
Because endothelial cell loss plays a pivotal role in the pathogenesis of intimal hyperplasia after vascular injury, we postulated that a therapeutic cell transplantation strategy that promotes early re-endothelialization of the injured vessels would inhibit intimal lesion development, facilitate vascular repair, and improve long-term vessel patency. Progenitor cells originating from the bone marrow have previously been isolated from the mononuclear cell fraction of peripheral blood [14, 15]. These cells have high proliferative potential [14] and differentiate into endothelial cells under specific growth conditions [16], suggesting that they may be suitable sources for the re-endothelialization of damaged vessels.
It has been recently shown that transplantation of autologous circulating endothelial progenitor cells into balloon-denuded arteries led to rapid re-endothelialization of the injured artery [17]. Recently, the multipotent differentiation ability of amnion-derived cells has been reported [18, 19]. In the present study, we sought to determine the viability of integrated human amniotic epithelial cells (hAECs) in an animal model of arterial injury by evaluating the effects of exogenous amniotic cell transplantation in promoting re-endothelialization and inhibition of neointimal hyperplasia after 4 weeks.
Methods
The authors of this manuscript have certified that they comply with the principles of ethical publishing in Interventional Medicine & Applied Science: Szél Á, Merkely B, Hüttl K, Gál J, Nemes B, Komócsi A: Statement on ethical publishing and scientific authorship [20].
Animal studies were carried out under the guidelines of the ethical committee of Semmelweis University.
hAECs
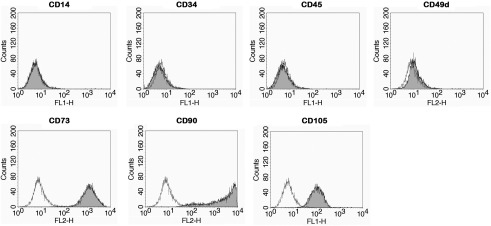
Placentas were provided by the gynecological and pediatric hospital in Linz and prepared as previously described [21, 22]. The local ethical board approved the collection of placentas. After cryopreservation in Cryo-SFM, hAECs derived from 64 human amniotic membranes were expanded in endothelial growth medium-2. The identity of cells was confirmed by analyzing specific cell surface markers (CD14, CD34, CD45, CD49d, CD73, CD90, and CD105) by flow cytometry [23]. These markers confirm the lineage of hAEC86, but this marker profile is concordant for all hAECs including those used in the present study. Furthermore, for the applied hAEC, we evaluated the expression of CD31, CD54, CD106, CD133, CD146, VEGFR2, and vWF (Fig. 1).
Fig. 1.
Characterization of cells. The identity of cells was confirmed by the presence of lineage-specific cell surface markers with flow cytometry. hAEC86 are negative for CD14, CD34, CD45, and CD49d, and positive for CD73, CD90, and CD105
Cell staining
Two weeks before the experiments, the cells were washed twice with phosphate-buffered saline (PBS), trypsinized, and centrifuged in endothelial basal medium-2 (EBM-2) at 1,200 rpm for 8 min. Then, the cells were counted and 500,000 cells/animal were transplanted as described below (n = 3 animals). For short-term in vivo detection, hAECs were labeled with the lipophilic membrane dye Vybrant DiL (excitation 549 nm, emission 565 nm, Molecular Probes) in a dilution of 1:200 for 30 min at 37 °C. After labeling, the cells were washed, centrifuged, and injected immediately into three male Sprague-Dawley rats. For long-term in vivo detection, injected cells labeling the hAECs were cultured in EBM-2 containing 10 mM 5-bromo-2-deoxyuridine (BrdU) (Sigma-Aldrich, B5002) (Fig. 2).
Fig. 2.
BrdU labeled hAECs in vitro. Red color represents the nuclear Hoechst staining (Panel A). Green color represents the BrdU (Panel B). Panel C shows the transmitted light. Co-localization of the red and green colors represents the successful BrdU labeling (white arrows, Panel D). The image was taken at 20× magnification with ZEISS LSM 510 Meta confocal laser scanning microscope
Rat carotid artery balloon angioplasty
Male Sprague-Dawley rats (400–450 g) were anesthetized with 2-bromo-2-chloro-1,1,1-trifluoroethane dinitrogen-monoxide (Halothane) in a mixture of O2 and N2O (50%–50%). The right common, the right external, and the right internal carotid arteries were isolated through a cervical midline incision. After heparinization through the internal jugular vein (150 IU/kg), hemostatic controls were placed in the internal and external carotid arteries. A 2F Fogarty embolectomy catheter (Edwards Lifesciences Corporation, Irvine, CA, USA) was inserted through the external branch into the common carotid artery to the aortic arch. The balloon was withdrawn and rotated slowly while inflated to 1.6–1.8 atm three times. After balloon denudation, 5 × 105 hAECs in 200 μl suspension were incubated in the common carotid artery of six male Sprague-Dawley rats for 30 min [24]. The external carotid was ligatured, and blood flow was restored through the internal carotid artery. Animals were killed after 2 days to perform confocal microscopic study and after 4 weeks to perform the measurement of vascular reactivity on isolated carotid arteries and histologic studies.
Histology
Vessels were fixed in situ by constant pressure fixation at 100 mmHg with 10% formalin through a 22-gauge butterfly angiocatheter placed in the abdominal aorta. Three cell implanted rats and five control rats were used in this experiment. Each carotid artery was embedded in paraffin, and eight cross-sections were continuously cut from all eight animals: four sections from the intact side and four sections from the injured side. Parallel sections were subjected to standard hematoxylin and eosin staining as well as to elastica Van Gieson staining and immunohistochemistry. The formation of neointima was expressed in the ratio of intima/media.
Immunohistochemistry
After deparaffinization, the sections were washed three times with PBS. DNAse I (Sigma-Aldrich, Poole, UK) was applied for DNA digestion. After the antigen retrieval with Retrivagen A (BD Pharmingen, Basel, Switzerland) and washing with PBS, the sections were blocked in normal goat serum (Vector Laboratories, Burlingame, CA, USA) for 1 h. The primary mouse anti-BrdU antibody (BD Pharmingen, Franklin Lakes, NJ) was solubilized in blocking serum in a dilution of 1:200 and applied overnight at 4 °C. After washing with PBS, Alexa Fluor 488 goat anti-mouse secondary antibody was used to visualize the labeling (Molecular Probes, Invitrogen, Carlsbad, CA, USA). Hoechst 33342 (Molecular Probes, Eugene, Oregon, USA) was applied to counterstain nuclei. To visualize the implanted hAECs in the blood vessels, a Zeiss LSM 510 Meta confocal laser scanning microscope (Carl Zeiss, Jena, Germany) was used with a 63× oil immersion objective.
Measurement of vascular reactivity on isolated carotid arteries
Nine male Sprague-Dawley rats were used in sham group (n = 3), in control group (n = 3), and in cell implanted group (n = 3). The freshly harvested segments of arteries were cut into 3–4 mm long ring segments and mounted in organ baths filled with warmed (37 °C) and oxygenated (95% O2 and 5% CO2) Krebs’ solution (CaCl2 – 5 mM; MgSO4 – 1.17 mM; NaCl – 119 mM; NaHCO3 – 20 mM; KCl – 4.7 mM; KH2PO4 – 1.18 mM; Glucose – 11 mM; EDTA – 0.035 mM) as previously described [25]. A maximum of three segments were isolated from each artery and on the whole, 37 segments were measured in this experiment. The state of arterial smooth muscle was tested with K+-Krebs’ solution (NaCl – 0 mM; KCl – 123.7 mM). Isometric tension was measured with isometric transducers (Hottinger Baldwin Messtechnik, Germany) and displayed on a data acquisition recorder (Kipp & Zonen, Holland). A tension of 1.5 g was applied and the rings were equilibrated for 60 min, followed by measurements of dose-dependent contractility to epinephrine (10−10 to 3 × 10−6 M). After another 60 min of washing and equilibrating, concentration-dependent relaxation to acetylcholine (10−9 to 3 × 10−4 M) and sodium nitroprusside (10−8–10−5 M) was determined after precontraction with epinephrine (10−6 M). Experiments were conducted in rings from 5–6 animals in each experimental group.
Statistical analysis
Results are reported as mean ± SEM. Statistical significance was determined by t-test, two-way ANOVA, and Newman–Keuls post-hoc tests, using GraphPad Prism statistical software. Probability values of p < 0.0001, p < 0.01, and p < 0.05 were considered significant.
Results
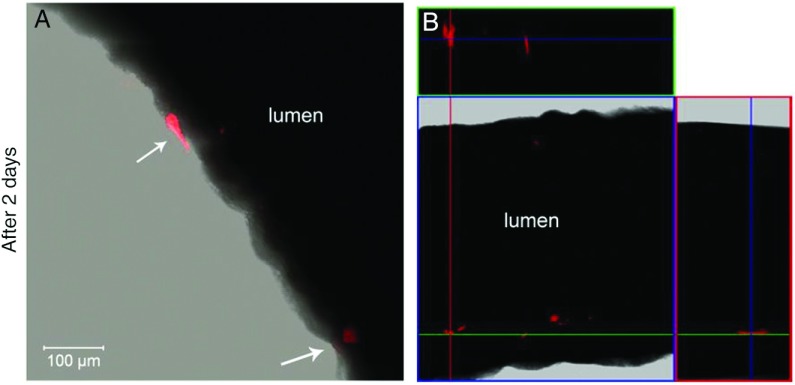
Two days after balloon injury, the transplanted Vybrant DiL-labeled hAECs were visible at the luminal side of the arterial wall at the injured side (Fig. 3A), and no cells were found in the intact contralateral side. Cells adhered to the damaged intima, which were visible in three-dimensional rendered microscopic images (Fig. 3B).
Fig. 3.
Incorporation of hAECs into the vessel walls after 2 days of injury. Panel A shows an acute infiltration at the luminal side of the arterial wall at the injured side. Red color represents the incorporated cells. Transplanted hAECs were stained with Vybrant DiL. Fresh vessel preparations were used and were evaluated with confocal microscopy. The image was taken at 63× magnification. Panel B shows a 3D structure Z-Stack image that confirms the incorporation of transplanted cells (red)
Four weeks after injury, significant intimal thickening was observed at the injured site in both study groups. The percentage of intima thickness in the control group was 61.2 ± 4.1% compared to the intact side with 1.4 ± 0.1% thickness (n = 41, p < 0.0001). Similar results were found in the transplanted groups (cell implanted injured side: 53.1 ± 2.9%, cell implanted intact side 2.9 ± 1.0%, n = 40, p < 0.0001). There was a significant difference between the injured and intact sides, but not between the control and the transplanted groups.
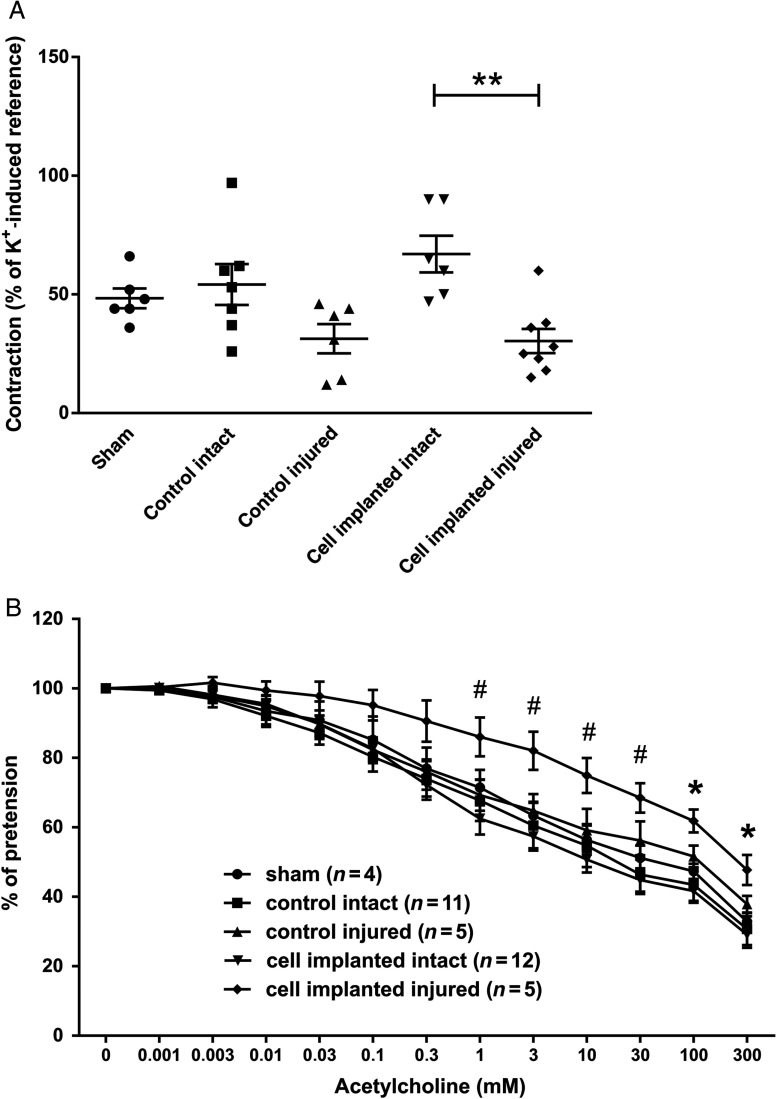
Four weeks after transplantation, the injured arteries showed a decreased constriction that was similar in implanted and control animals. The uninjured contralateral arteries had a similar constrictional capacity to sham-operated animals (Fig. 4A).
Fig. 4.
Effects of cell transplantation on the contraction and relaxation capacity of carotid arteries. (Panel A) The extent of contraction was similar in the sham group and on the intact side of both cell transplanted and control groups. Contraction was greater on the intact side compared to the injured side in both cell transplanted and control groups. Four weeks after injury, there was a significant difference between the intact and injured side in the transplanted groups. (Panel B) The injured endothelial layer had a decreased response to acetylcholine stimulus in both groups that had angioplasty. The implantation of stem cells had significant effect on the NO-dependent relaxation of the injured vessels. Data are presented in mean ± SEM (#), **: p < 0.01 and *: p < 0.05 cell implanted intact vs. cell implanted injured
Dilational capacity of the arteries was comparable in each experimental group, with only the implanted segments showing a trend toward decreased vasodilation. There were significant differences between cell implanted intact and cell implanted injured groups (Fig. 4B).
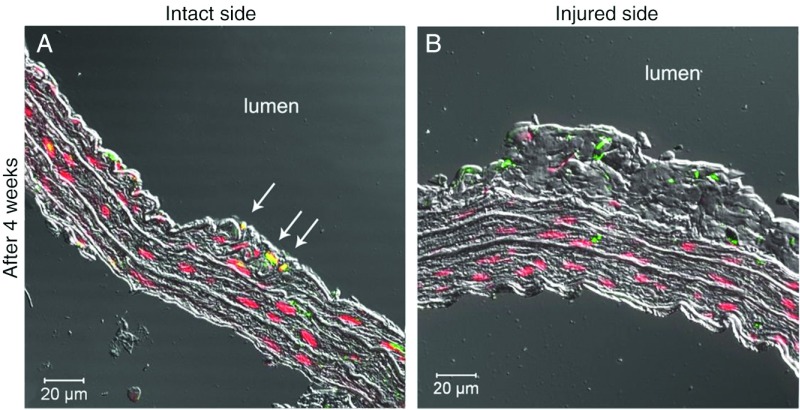
Four weeks after transplantation, cells labeled with anti-BrdU antibody were observed at the intact arterial side, where they incorporated into the walls of the blood vessels (Fig. 5). Surprisingly, no incorporated cells were found at the injured side at this time point.
Fig. 5.
Long-term incorporation of hAECs into the vessel walls after balloon injury. Long-term cell survival and incorporation was observed on the intact side in the cell transplanted group (Panel A). Red color represents the nuclear Hoechst staining and green color represents the BrdU positive cells. Yellow color represents that Hoechst stained and BrdU positive cells are present in the intact side. This phenomenon is not seen at the injured side (Panel B)
Discussion
We successfully implanted hAECs into the vessel wall after endothelial injury of carotid arteries. hAECs have several beneficial characteristics, such as low immunogenicity and anti-inflammatory functions [26]; moreover, they do not require the sacrifice of human embryos for their isolation.
Although short-term implantation at 48 h was promising, the cells did not persist at the site of the injury and the vascular dilational capacity was hardly modified. Our hypothesis that hAECs can rebuild the endothelial layer and decrease the thickening of neointima was not supported by the present results.
Remodeling of the arterial wall after injury to the endothelial layer is a well-known process that leads to thickening of the intima and a consequent decrease of the inner diameter of the artery. Although one may hypothesize that implantation of an epithelial cell lineage would improve re-endothelialization, another line of reasoning would be that implantation of a multipotent cell worsens the situation because intima hyperplasia and transplantation are both anabolic processes. Although our field of regenerative medicine seeks to take advantage of the first hypothesis, we must be aware that sometimes the results are not supporting the hypothesis, as in the present case. Since injuring the carotid arteries causes significant intima thickening and reduce vascular function with only a mild difference between the study groups, we cannot conclude any correlation between cell transplantation and vascular function in the present study model.
According to the recent data, muscle-derived stem cells promote angiogenesis and improve perfusion on the side of ischemia [27]. In addition, mesenchymal stem cells reduce the formation of intima hyperplasia [28]. Although these adult stem cells are successfully used to treat vascular injury, the main action of stem cells is not through integration, differentiation, or tissue augmentation, but rather through paracrine mechanisms [29]. Transplanted hAECs, for instance, secrete proangiogenic cytokines, and this paracrine effect could regenerate myocardial tissue after myocardial infarction [30]. Immunosuppressive factors, which inhibit both the innate and adaptive immune systems and are secreted by hAECs, were also investigated in ocular surface inflammation [31] showing the importance of paracrine mechanisms in tissue regeneration.
Vascular biology is gaining attention in regenerative medicine because the revascularization of the graft is the bottleneck of implanting sizeable tissue constructs. The presence of vessels canalizing the structures is the first step in the process; however, ample experimental and clinical evidence show the active tone of the arteries is the main determining factor in actual blood flow. Although experimental methods of the measurement of vascular function are well established [32, 33], a few authors have used these in a tissue engineering setting. Advancements in the understanding of hAECs suggest that long-term survival of the implanted cells, which were observed in intact vessel walls, does not alter vascular function. This was investigated and confirmed in the present experiment.
We conclude that survival of the implanted cells and its functional effectiveness are independent from each other in this model. Although hAECs can incorporate into the endothelial wall of artery without immunosuppression and can also adhere far from damaged tissues, these cells cannot use their positive effects to prevent intimal thickening, showing that successful cell transplantation does not necessarily improve functionality as well.
Funding sources
This study was supported by TÉT-SIN-CELLTHER, TÁMOP-4.2.1/B09/1/KMR-2010-0001, OTKA 83803. Dr. Lacza was supported by Bolyai and Öveges Fellowships.
Authors’ contribution
GV, AC, ZC, RB, ZL: study design, experiments, data evaluation, and manuscript preparation. EK, EP, AL, SW: experiments and data evaluation. CMS, DBH, IH, LK: study design and manuscript preparation.
Conflict of interest
The authors declare that they do not have any conflict of interest.
References
- 1.Pauletto P, Sartore S, Pessina AC: Smooth-muscle-cell proliferation and differentiation in neointima formation and vascular restenosis. Clin Sci (Lond) 87, 467–479 (1994) [DOI] [PubMed] [Google Scholar]
- 2.Schwartz RS: Pathophysiology of restenosis: Interaction of thrombosis, hyperplasia, and/or remodeling. Am J Cardiol 81, 14E–17E (1998) [DOI] [PubMed] [Google Scholar]
- 3.Cines DB, Pollak ES, Buck CA, Loscalzo J, Zimmerman GA, McEver RP, Pober JS, Wick TM, Konkle BA, Schwartz BS: Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood 91, 3527–3561 (1998) [PubMed] [Google Scholar]
- 4.Behrendt D, Ganz P: Endothelial function. From vascular biology to clinical applications. Am J Cardiol 90, 40L–48L (2002) [DOI] [PubMed] [Google Scholar]
- 5.Tulis DA, Keswani AN, Peyton KJ, Wang H, Schafer AI, Durante W: Local administration of carbon monoxide inhibits neointima formation in balloon injured rat carotid arteries. Cell Mol Biol 51, 441–446 (2005) [PMC free article] [PubMed] [Google Scholar]
- 6.Santana AC, Marinotto DBE, Dellę H, Cordeiro AC, Noronha IL: Effectiveness of thalidomide and tamoxifen in preventing neointimal hyperplasia in experimental vascular injury in rats. Transplant Proc 42, 585–588 (2010) [DOI] [PubMed] [Google Scholar]
- 7.Wang CH, Cherng WJ, Yang NI, Kuo LT, Hsu CM, Yeh HI, Lan YJ, Yeh CH, Stanford WL: Late-outgrowth endothelial cells attenuate intimal hyperplasia contributed by mesenchymal stem cells after vascular injury. Arterioscler Thromb Vasc Biol 28, 54–60 (2007) [DOI] [PubMed] [Google Scholar]
- 8.Melo LG, Gnecchi M, Pachori AS, Kong D, Wang K, Liu X, Pratt RE, Dzau VJ: Endothelium-targeted gene and cell-based therapies for cardiovascular disease. Arterioscler Thromb Vasc Biol 24, 1761–1774 (2004) [DOI] [PubMed] [Google Scholar]
- 9.Xiao Q, Zeng L, Zhang Z, Margariti A, Ali ZA, Channon KM, Xu Q, Hu Y: Sca-1+ progenitors derived from embryonic stem cells differentiate into endothelial cells capable of vascular repair after arterial injury. Arterioscler Thromb Vasc Biol 26, 2244–2251 (2006) [DOI] [PubMed] [Google Scholar]
- 10.De Boer HC, Verseyden C, Ulfman LH, Zwaginga JJ, Bot I, Biessen EA, Rabelink TJ, Vanzonneveld AJ: Fibrin and activated platelets cooperatively guide stem cells to a vascular injury and promote differentiation towards an endothelial cell phenotype. Arterioscler Thromb Vasc Biol 26, 1653–1659 (2006) [DOI] [PubMed] [Google Scholar]
- 11.Isoda K, Akita K, Isobe S, Niida T, Adachi T, Iwakura Y, Daida H: Interleukin-1 receptor antagonist originating from bone marrow-derived cells and non-bone marrow-derived cells helps to suppress arterial inflammation and reduce neointimal formation after injury. J Atheroscler Thromb 21, 1208–1218 (2014) [DOI] [PubMed] [Google Scholar]
- 12.Lee CH, Chang SH, Lin YH, Liu SJ, Wang CJ, Hsu MY, Hung KC, Yeh YH, Chen WJ, Hsieh IC, Wen MS: Acceleration of re-endothelialization and inhibition of neointimal formation using hybrid biodegradable nanofibrous rosuvastatin-loaded stents. Biomaterials 35, 4417–4427 (2014) [DOI] [PubMed] [Google Scholar]
- 13.Cui S, Liu JH, Song XT, Ma GL, Du BJ, Lv SZ, Meng LJ, Gao QS, Li K: A novel stent coated with antibodies to endoglin inhibits neointimal formation of porcine coronary arteries. Biomed Res Int 2014, 428619 (2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM: Isolation of putative progenitor endothelial cells for angiogenesis. Science 275, 964–967 (1997) [DOI] [PubMed] [Google Scholar]
- 15.Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, Kearne M, Magner M, Isner JM: Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res 85, 221–228 (1999) [DOI] [PubMed] [Google Scholar]
- 16.Kawamoto A, Gwon HC, Iwaguro H, Yamaguchi JI, Uchida S, Masuda H, Silver M, Ma H, Kearney M, Isner JM, Asahara T: Therapeutic potential of ex vivo expanded endothelial progenitor cells for myocardial ischemia. Circulation 103, 634–637 (2001) [DOI] [PubMed] [Google Scholar]
- 17.Griese DP, Ehsan A, Melo LG, Kong D, Zhang L, Mann MJ, Pratt RE, Mulligan RC, Dzau VJ: Isolation and transplantation of autologous circulating endothelial cells into denuded vessels and prosthetic grafts: Implications for cell-based vascular therapy. Circulation 108, 2710–2715 (2003) [DOI] [PubMed] [Google Scholar]
- 18.Ilancheran S, Michalska A, Peh G, Wallace EM, Pera M, Manuelpillai U: Stem cells derived from human fetal membranes display multilineage differentiation potential. Biol Reprod 77, 577–588 (2007) [DOI] [PubMed] [Google Scholar]
- 19.Shi M, Li W, Li B, Li J, Zhao C: Multipotency of adult stem cells derived from human amnion. Chin J Biotechnol 25, 754–760 (2009) [PubMed] [Google Scholar]
- 20.Szél Á, Merkely B, Hüttl K, Gál J, Nemes B, Komócsi A: Statement on ethical publishing and scientific authorship. Interv Med Appl Sci 2, 101–102 (2010) [Google Scholar]
- 21.Stadler G, Hennerbichler S, Lindenmair A, Peterbauer A, Hofer K, Van Griensven M, Gabriel C, Redl H, Wolbank S: Phenotypic shift of human amniotic epithelial cells in culture is associated with reduced osteogenic differentiation in vitro. Cytotherapy 10, 743–752 (2008) [DOI] [PubMed] [Google Scholar]
- 22.Wolbank S, Peterbauer A, Fahrner M, Hennerbichler S, van Griensven M, Stadler G, Redl H, Gabriel C: Dose-dependent immunomodulatory effect of human stem cells from amniotic membrane: A comparison with human mesenchymal stem cells from adipose tissue. Tissue Eng 13, 1173–1183 (2007) [DOI] [PubMed] [Google Scholar]
- 23.Parolini O, Alviano F, Bagnara GP, Bilic G, Buhring HJ, Evangelista M, Hennerbichler S, Liu B, Magatti M, Mao N, Miki T, Marongiu F, Nakajima H, Nikaido T, Portmann-Lanz CB, Sankar V, Soncini M, Stadler G, Surbek D, Takahashi TA, Redl H, Sakuragawa N, Wolbank S, Zeisberger S, Zisch A, Strom SC: Concise review: Isolation and characterization of cells from human term placenta: Outcome of the first international workshop on placenta derived stem cells. Stem Cells 26, 300–311 (2008) [DOI] [PubMed] [Google Scholar]
- 24.Griese DP, Achatz S, Batzlsperger CA, Strauch UG, Grumbeck B, Weil J, Riegger GAJ: Vascular gene delivery of anticoagulants by transplantation of retrovirally-transduced endothelial progenitor cells. Cardiovasc Res 58, 469–477 (2003) [DOI] [PubMed] [Google Scholar]
- 25.Chen YT, Li W, Hayashida Y, He H, Chen SY, Tseng DY, Kheirkhah A, Tseng SCG: Human amniotic epithelial cells as novel feeder layers for promoting ex vivo expansion of limbal epithelial progenitor cells. Stem Cells 25, 1995–2005 (2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Solomon A, Wajngarten M, Alviano F, Anteby I, Elchalal U, Pe’er J, Levi Schaffer F: Suppression of inflammatory and fibrotic responses in allergic inflammation by the amniotic membrane stromal matrix. Clin Exp Allergy 35, 941–948 (2005) [DOI] [PubMed] [Google Scholar]
- 27.Park HS, Hahn S, Choi GH, Yoo YS, Lee JY, Lee T: Muscle-derived stem cells promote angiogenesis and attenuate intimal hyperplasia in different murine vascular disease models. Stem Cells Dev 22, 866–877 (2013) [DOI] [PubMed] [Google Scholar]
- 28.Kim AK, Kim MH, Kim DH, Go HN, Cho SW, Um SH, Kim DI: Inhibitory effects of mesenchymal stem cells in intimal hyperplasia after balloon angioplasty. J Vasc Surg 63, 510–517 (2016) [DOI] [PubMed] [Google Scholar]
- 29.Baraniak PR, McDevitt TC: Stem cell paracrine actions and tissue regeneration. Regen Med 5, 121–143 (2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Song YS, Joo HW, Park IH, Shen GY, Lee Y, Shin JH, Kim H, Shin IS, Kim KS: Transplanted human amniotic epithelial cells secrete paracrine proangiogenic cytokines in rat model of myocardial infarction. Cell Transplant 24, 2055–2064 (2015) [DOI] [PubMed] [Google Scholar]
- 31.Li H, Niederkorn JY, Neelam S, Mayhew E, Word RA, McCulley JP, Alizadeh H: Immunosuppressive factors secreted by human amniotic epithelial cells. Invest Ophthalmol Vis Sci 46, 900–907 (2005) [DOI] [PubMed] [Google Scholar]
- 32.Liu R, Lang MG, LĂĽscher TF, Kaufmann M: Propofol-induced relaxation of rat mesenteric arteries: Evidence for a cyclic GMP-mediated mechanism. J Cardiovasc Pharmacol 32, 709–713 (1998) [DOI] [PubMed] [Google Scholar]
- 33.Horvath B, Lenzser G, Benyo B, Nemeth T, Benko R, Iring A, Herman P, Komjati K, Lacza Z, Sandor P, Benyó Z: Hypersensitivity to thromboxane receptor mediated cerebral vasomotion and CBF oscillations during acute NO-deficiency in rats. PLoS One 5, e14477 (2010) [DOI] [PMC free article] [PubMed] [Google Scholar]