Abstract
Purpose
The development of inflammatory periodontal disease in young people is an urgent problem of today's periodontology, and requires a development of new methods that would give an opportunity not only to diagnose but also for prognosis of periodontitis course in a given patients contingent.
Results
Cellular structure of periodontal pockets is presented by hematogenous and epithelial cells. Our results are confirmed by previous studies, and show that the penetration of periodontal pathogens leads to formation in periodontal tissue of a highly active complex compounds—cytokines that are able to modify the activity of neutrophils and reduce their specific antibacterial properties. Cytokines not only adversely affect the periodontal tissues, but also cause further activation of cells that synthesized them, and inhibit tissue repair and process of resynthesis of connective tissue by fibroblasts.
Conclusion
Neutrophilic granulocytes present in each of the types of smear types, but their functional status and quantitative composition is different. The results of our cytological study confirmed the results of immunohistochemical studies, and show that in generalized periodontitis, an inflammatory cellular elements with disorganized epithelial cells and connective tissue of the gums and periodontium, and bacteria form specific types of infiltration in periodontal tissues.
Keywords: generalized periodontitis, epithelial cells, periodontal pockets, cytokines, periodontal tissues
Introduction
The development of inflammatory periodontal disease in young people is an urgent problem in today’s periodontology, and it requires a development of new methods that would give an opportunity not only to diagnose but also in prognosis of periodontitis course in a given patients contingent [1–3].
In the future without timely intervention will lead to more severe damage of periodontal tissues, premature loss of teeth, and changes in temporomandibular joints. Results from the previous research indicate the role of periodontal pathological processes in the development of systemic inflammation [4–6].
Considering the mucous membrane of the gums, periodontium is strategically considered as an important organ that is capable to initiate an immune response under the conditions of inflammatory process [7–9]. For these reasons, a great interest arise to understand the characteristics of cellular structure of periodontal pockets of patients with generalized periodontitis, changes in cytological composition of the gingival epithelium, and reactions of the epithelial component of other anatomical sites for the presence of foci of chronic intoxication and chronic infection in periodontal tissues [2, 8, 10, 11].
The aim of our research is to determine the cytological criteria for prognosis of clinical course of generalized periodontitis and to examine the gums and buccal epithelium.
Materials and Methods
Material used for the study is gum epithelium taken from a marginal area of the gums in 55 young adults with generalized periodontitis for 3–5 years. The epithelium removed with a spatula was transferred to a glass plate and dried in the open air for 3–5 min. The staining of the smear was done according to Himza–Romanovsky method, followed by microscopic and morphological analyses, considering the percentage of different forms of epithelial cells in normal and different age group.
To standardize the epithelium and for a more in-depth study, cytological classification based on the presence of epithelium in the gum (basal, parabasal, intermediate, and superficial cells in the area of keratinized epithelium of gums and horny scales) was used in this study.
Statistical analysis of the results was carried out at the Department of Statistical Research, State Higher Educational Institution, Ministry of Health of Ukraine Ternopil State Medical University. Parametric methods are used for the indicators, the distribution of which meets the requirements of normality. To assess the nature of the distribution were determined coefficient of skewness and kurtosis. The test was performed on tests of normality asymmetry test conducted by Shapiro and Wilkie. Probability differences of the results for different groups were determined using Student’s t-test. The difference was considered likely in common in the medical and research error probability (p < 0.05). The probability of error evaluated the tables Student given the size of the experimental groups, where the law of distribution statistically significantly different from the expected normal nonparametric criterion (Mann–Whitney U nonparametric analog) of a Student’s t-test.
Results
Considering that it is a invasive and highly informative method for cytological study, a detailed morphological analysis of cytograms was performed to determine the cellular composition of periodontal pockets. Cytograms were stained by Gram, Romanovsky, and Himza and were further examined in three different fields of vision.
Cellular structures in the periodontal pockets present as hematogenous and epithelial cells are analyzed.
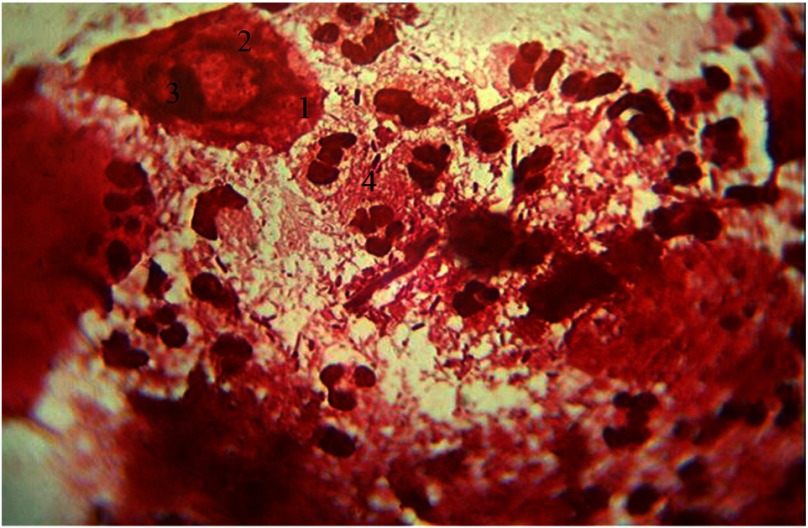
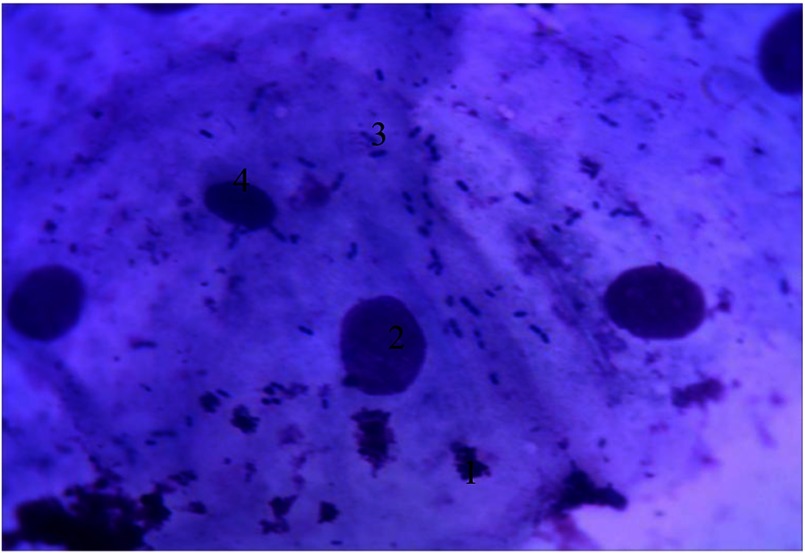
A significant number of basal cells are present, which are usually absent in normal cytogram preparations. These basal cells appear prismatic in shape with oval nucleus and nucleolus shifted toward the periphery and are characterized by sharp basophilic cytoplasm. Nuclear–cytoplasmic ratio (NCR) of these basal cells is high (0.49 ± 0.002), generally shifted toward the nucleus, which allows them to be included into the first stage of cell differentiation. The percentage of basal epithelial cells is 15% (of 100 cells) in the cytograms. The appearance of basal cells indicates the deep epithelial affection by inflammation and characterizes the severity of periodontitis in the given patients contingent. These visible signs present in the cells are indicative of the cytopathological condition.
The other characteristic feature of cytograms in the given patients contingent is the absence of parabasal cells, as a result, the second stage of cell differentiation is characterized by reduced NCR because of the increase in the cytoplasmic volume (Fig. 1).
Fig. 1.
Cellular composition of periodontal pockets. Gram staining. Magnification: 400×. 1, basal epithelial cells; 2, the nucleus of epithelial cells; 3, nucleolus; 4, neutrophilic granulocytes
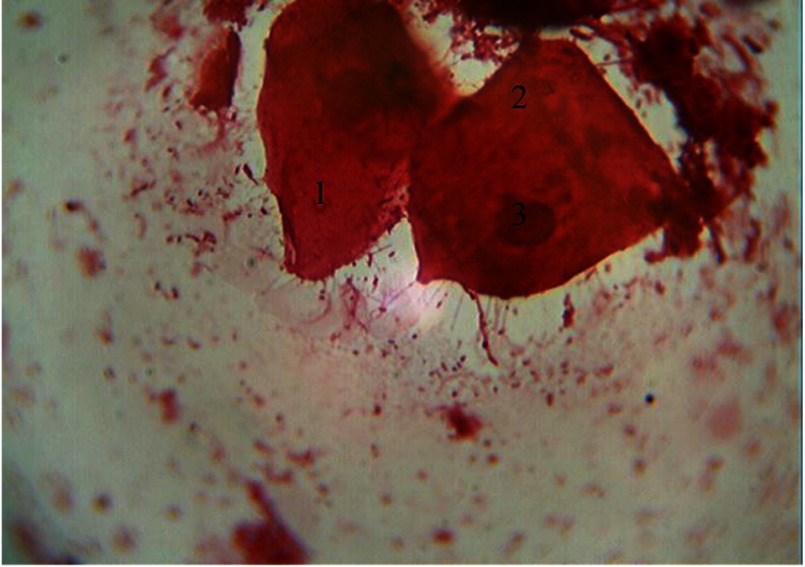
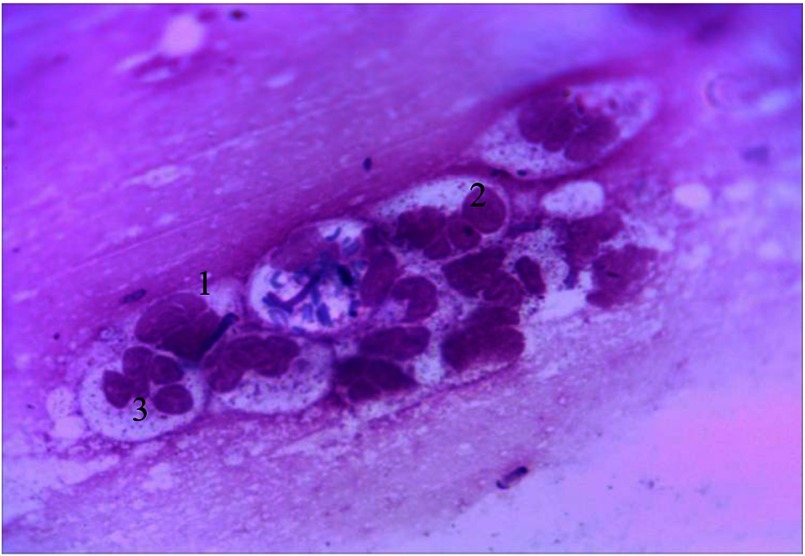
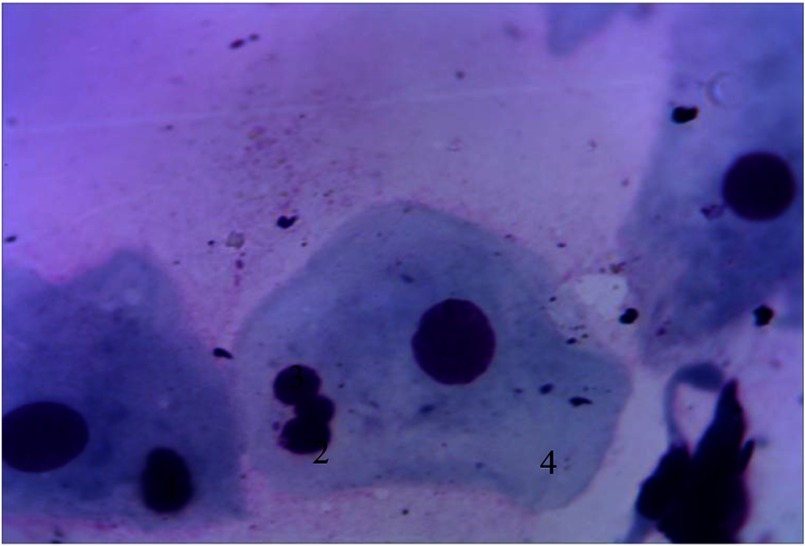
Another characteristic feature of cytograms from the periodontal pockets content is the presence of polygonal intermediate cells with optically bright cytoplasm and eccentrically placed oval-shaped vesicular nucleus. NCR decreases toward the nucleus (0.34 ± 0.001), which allows them to be included into the third stage of cell differentiation (Fig. 2).
Fig. 2.
Cellular composition of periodontal pockets. Himza–Romanovsky staining. Magnification: 150×. 1, intermediate cells; 2, superficial cells; 3, vesicular nucleus of intermediate cell
It is important to note that the presence of intermediate cells, which are prevalent in smears of patients with intact gums [12] and is an indicator of maturation and differentiation of epithelial cells, are in reduced quantities (35% of 100 cells) in smears from the given patients contingent. This indicates disturbed epithelial layer maturation in the gums because of inflammation.
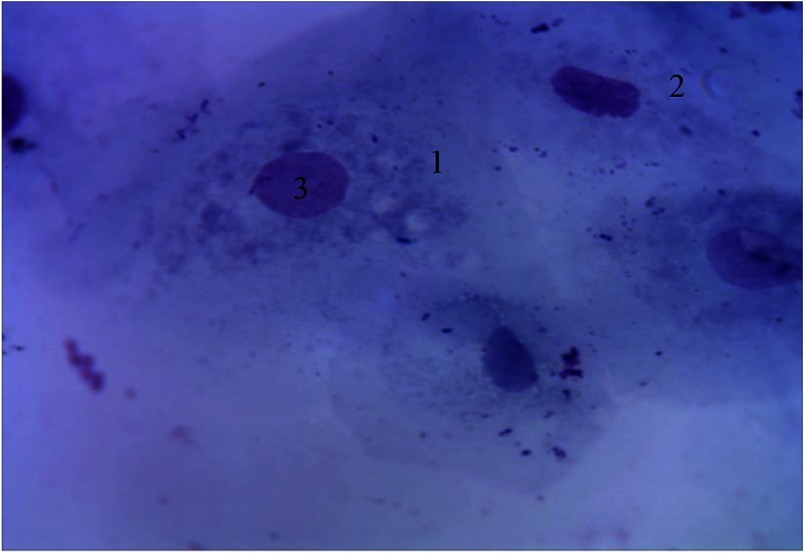
Based on the position of the nucleus in the cells, two types of superficial cells were observed in the cytograms from periodontal pockets (Fig. 3).
Fig. 3.
Cellular composition of periodontal pockets. Himza–Romanovsky staining. Magnification: 400×. 1, superficial cells of the first type; 2, nucleus of superficial cells; 3, segmented leukocytes
The first type of superficial epithelial cells have dimensions slightly larger than the intermediate cells, with clearly contoured nucleus of normal size located in the center of the cell. The NCR shifted toward the cytoplasm is 0.25 ± 0.001, which allows them to be included into the fourth stage of cell differentiation. The percentage content is 20% (of 100 cells) in the cytograms of given patients contingent.
The second type of superficial epithelial cells have dimensions similar to that of the first type: pyknotic nucleus with clear contours, often found as colored vacuoles, karyolysis, and fragmentation followed by elimination from cytoplasm. The NCR shifted toward the cytoplasm is 0.1 ± 0.001, which allows them to be included into the fourth stage of cell differentiation. The percentage content is 30% (of 100 cells) in the cytograms of given patients contingent (Fig. 4).
Fig. 4.
Cellular composition of periodontal pockets. Gram staining. Magnification: 400×. 1, superficial cells of the first type; 2, superficial cells of the second type (with pyknotic nucleus); 3, pyknotic nucleus
To prognose an inflammation in periodontal tissues, cytograms analysis obtained from periodontal pockets during exacerbation of periodontitis was carried out.
Results indicate that epithelial cell composition remains constant, but emphasize the change in the quantitative proportion of epithelial cells in periodontal tissues.
Cells (NCR 0.5 ± 0.002) corresponding to the basal epithelial cells is 25% (of 100 cells). Parabasal cells similar to the cytograms from chronic clinical course are not present. Number of intermediate cells (NCR 0.35 ± 0.001) remains constant, and the percentage content is 35%. Although the quantitative composition of cell fractions in the surface layer with NCR of 0.25 ± 0.001 and 0.1 ± 0.001 is changed by increase in the superficial cells, the percentage content is 35% for cells with centric-located nucleus and 5% for cells with pyknotic nucleus (Fig. 5).
Fig. 5.
Cellular composition of periodontal pockets. Himza–Romanovsky staining. Magnification: 400×. 1, superficial cells of the first type; 2, nucleus of the superficial cells; 3, superficial cells of the second type; 4, pyknotic nucleus
These results confirm that in prolonged presence of inflammation in the periodontal tissues, a violation of keratinization occurs, which is characterized by the absence of all epithelial cells and the change in the percentage of cell vanguard.
This study results have found histological confirmation on the stage of histological study of structure of periodontal pockets.
Among a number of hematogenous cells present in the periodontal pockets in cytograms, percentage content is determined by a large number of neutrophils at different stages of phagocytosis, with clear hypersegmented nucleus without septum between the segments. It should be noted that this type of cell was found in cytograms irrespective of generalized periodontitis course. But functional status was changed.
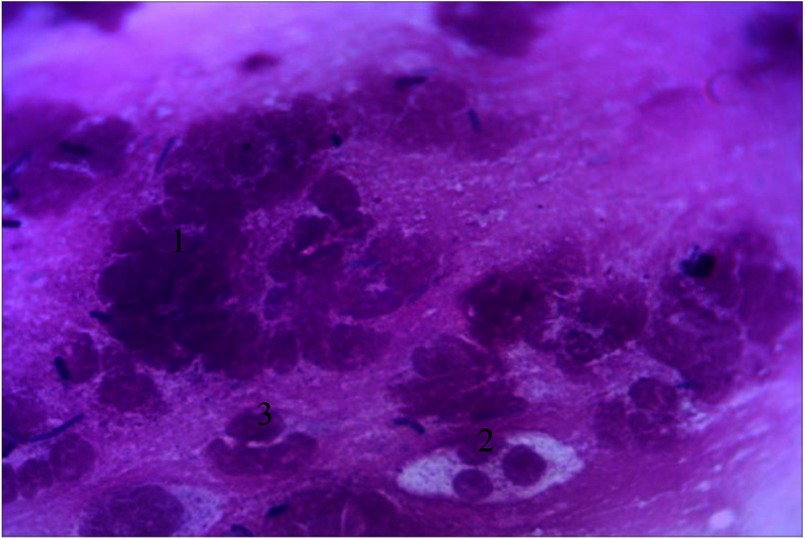
Thus, under conditions of chronic clinical course, neutrophilic granulocytes form cell clusters. At the same time, contours of plasmalemma are clearly visualized with mostly rounded and preserved nuclei segmentation (Fig. 6).
Fig. 6.
Hematogenous cells of periodontal pockets. Himza–Romanovsky staining. Magnification: 400×. 1, neutrophilic granulocytes; 2, segmented nucleus; 3, granularity
This type of smear obtained under conditions of chronic clinical course is prognostic criteria of high exacerbation rate.
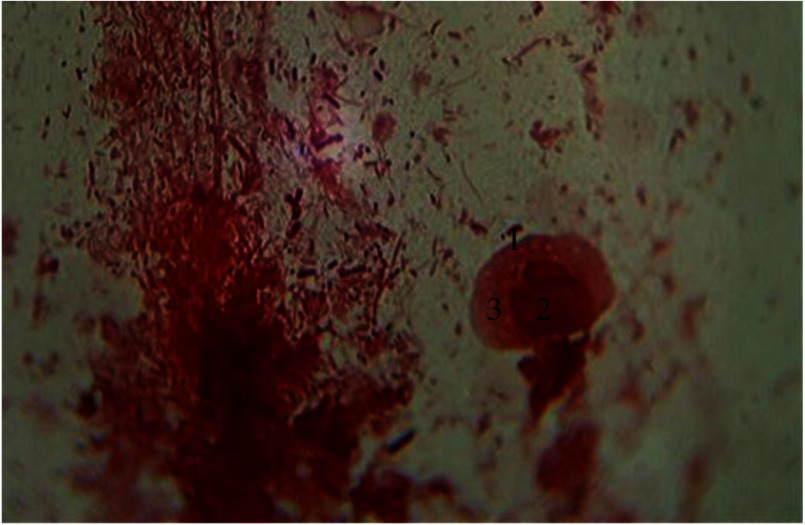
Large number of elongated macrophages with optically dense nucleus are visualized. In the cytoplasm of these macrophages, a well-developed lysosome is localized along with other moderately developed organelles. Their quantitative structure explains the constant need to phagocytose necrotic tissue components and intercellular substances and microorganisms in the periodontal foci of inflammation. Besides this function, these type of cells are involved in the initiation of immune responses through a process of synthesis and antigen presentation to lymphocytes and regulation of other cell-type activity, including fibroblasts. This type of smear obtained under conditions of chronic clinical course suggests a relatively lower incidence of exacerbation (Fig. 7).
Fig. 7.
Hematogenous cells of periodontal pockets. Gram staining. Magnification: 400×. 1, functionally active macrophage; 2, nucleus; 3, lysosomal apparatus
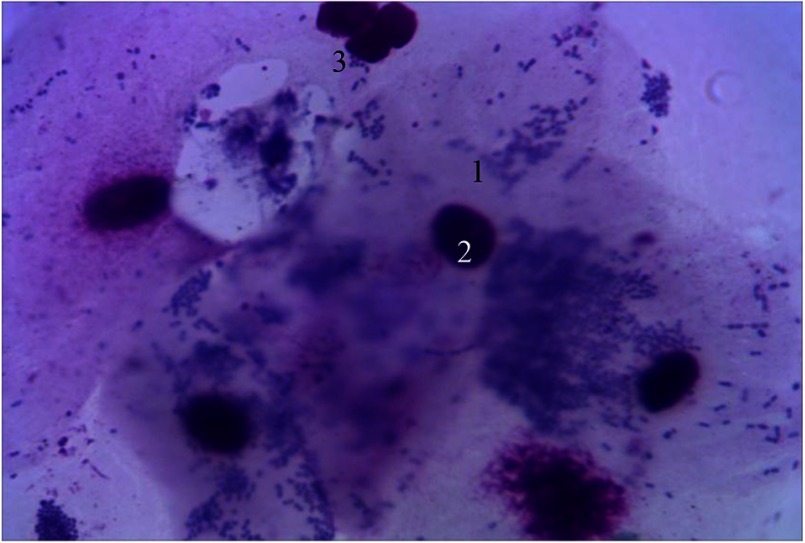
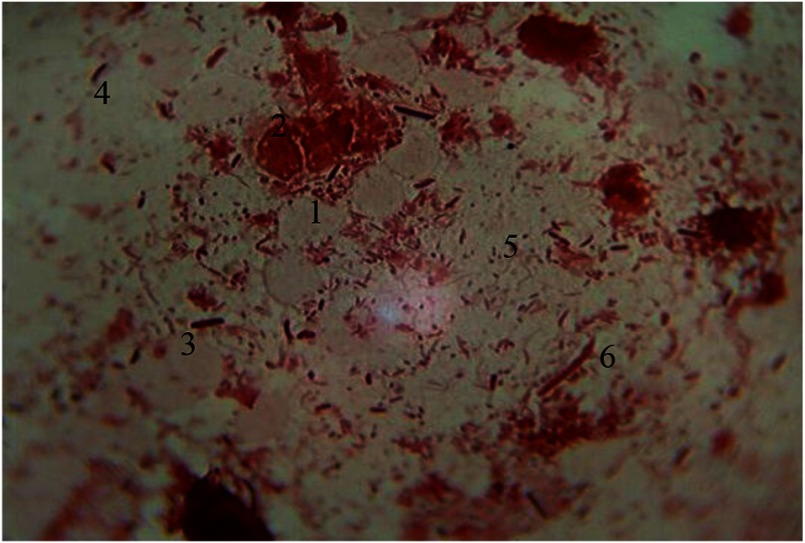
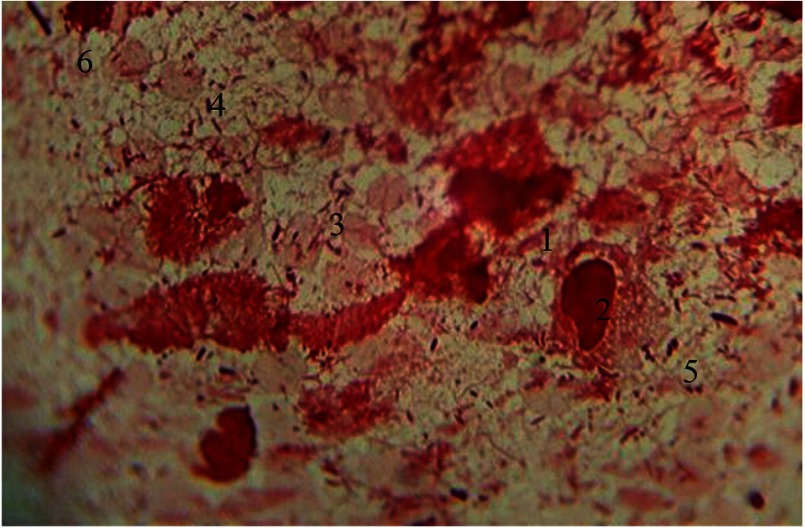
Along with functionally active macrophages in cytograms, lysed cells are seen. Contours of plasmalemma are violated. Besides macrophage cell system, Gram-stained erythrocytes were seen. These erythrocytes appear as double concave discs with nucleus lost in the process of phylogenesis. Their cytoplasm is poorly stained and is characterized by specific color. Among microbial occupancy complex systems of rods, cocci, and fungi of the genus Candida, spirilla are visualized. Under the conditions of prolonged inflammation, opportunistic strains are replaced by other more pathogenic and nonpathogenic bacteria that are closely associated to periodontal diseases (Fig. 8).
Fig. 8.
Hematogenous cells of periodontal pockets. Gram staining. Magnification: 400×. 1, lysed macrophage; 2, fragmented nucleus; 3, red blood cells; 4, rods; 5, cocci; 6. mycelium of fungi of the genus Candida
Studies [2, 8, 9] indicate the role of parodontopathogenic microflora such as Actinobacillus actinomycetemcomitans, Bacteroides forsythus, Prevotella intermedia, Porphyromonas gingivalis, Treponema denticola (by polymerase chain reaction), Chlamydia (Chlamydia pneumoniae and Chlamydia trachomatis), Helicobacter pylori, viruses (such as influenza virus, measles virus, cytomegalovirus, and herpes virus), Bordetella pertussis, and Bacillus anthracis in the initiation of pathogenesis of periodontal disease. These parodontopathogenic microflora are involved not only in the pathogenesis of periodontal disease but also in the development of atherosclerosis, stroke, insult, and other diseases.
There are two reasons by which pathogenic bacteria can cause tissue damage: (1) the actual bacterial toxic effects and (2) the periodontal tissues’ response to bacterial aggression. Reverse reaction of periodontal tissues to destruction initiates a cascade of cellular responses, primarily involving polymorphonuclear leukocytes, macrophages, and fibroblasts.
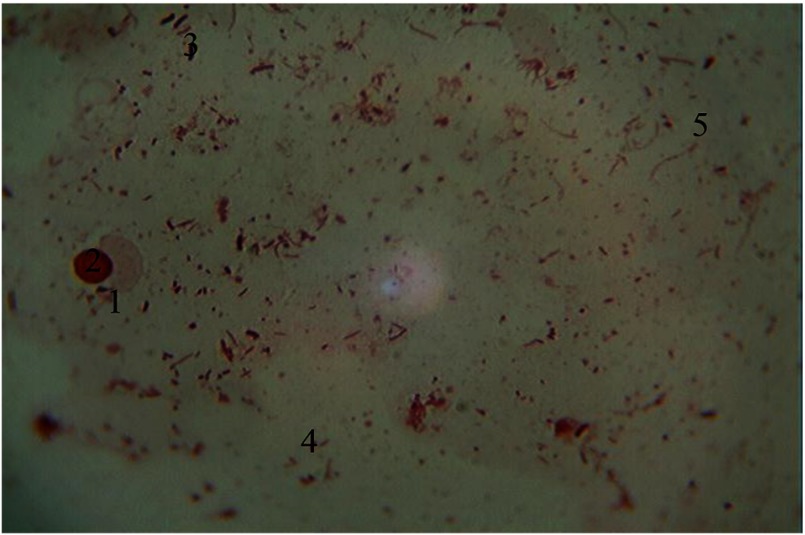
In many cytograms, the presence of monocytes with oval, kidney-shaped, nonsegmented large nucleus rich in chromatin has gained importance. Cytoplasm is of relatively large size, containing well-developed lysosomal components. The presence of these cells in smears indicates the functional activity of mesenchyma and characterizes a weak tendency to exacerbation of chronic clinic process (Fig. 9).
Fig. 9.
Hematogenous cells of periodontal pockets. Gram staining. Magnification: 400×. 1, monocyte; 2, nucleus of monocyte; 3, red blood cells; 4, rods; 5, cocci; 6, mycelium fungi of the genus Candida
In all the cytograms under conditions of chronic clinic periodontitis course, lymphocytes were observed, which by Gram staining showed large spherical nucleus located mainly in the center of the cell occupying almost all cell space. The nucleus contained a large amount of diffusely localized heterochromatin (Fig. 10).
Fig. 10.
Hematogenous cells of periodontal pockets. Gram staining. Magnification: 400×. 1, lymphocyte; 2, lymphocyte nucleus; 3, rods; 4, cocci; 5, mycelium fungi of the genus Candida
The cytological properties of lymphocytes in periodontal pockets were investigated using preparations stained by Romanovsky–Himza method. The cytoplasm of lymphocytes is characterized by basophilic properties, stained in blue color and the nucleus surrounded by a narrow border. The cytoplasm is rendered in the light perinuclear area (Fig. 11).
Fig. 11.
Cellular composition of periodontal pockets. Himza–Romanovsky staining. Magnification: 400×. 1, lymphocyte; 2, neutrophils; 3, segmented nucleus; 4, superficial cell
The presence of lymphocytes in the cytograms justifies the results obtained from complex histological and immunohistochemical investigations that deals with the involvement of immune system and initiation of chronic clinic process and presence of counteraction between foci of chronic infection of periodontal tissues and systemic diseases of organism.
All these results indicate that cellular and humoral immunities of periodontal pockets are closely related, primarily by polymorphonuclear leukocytes and macrophages. These cells play a key role in the inflammatory response and protection of the body from influence of xenogenic factors, including bacteria and their toxins.
Under the conditions of exacerbated clinical course of periodontitis, cellular composition of cytograms change and is characterized by a predominance of neutrophilic granulocytes. However, it should be noted that most of these changes are degenerative and can be visualized with hypersegmented nuclei without connections between them, and there is no specific granularity (Fig. 12).
Fig. 12.
Cellular composition of periodontal pockets under conditions of exacerbated clinical course of generalized periodontitis. Himza–Romanovsky staining. Magnification: 400× 1, lysed leukocytes; 2, segmented nucleus; 3, active phagocytic leukocytes
Discussion
The results obtained in this study are in agreement with previous studies and indicate that the penetration of periodontal pathogens leads to the formation in periodontal tissue of a highly active complex compounds—cytokines that are able to modify the activity of neutrophils and reduce their specific antibacterial properties [4, 7, 13].
Cytokines not only affect the periodontal tissues adversely but also cause further activation of cells that synthesized them and inhibit tissue repair and process of resynthesis of connective tissue by fibroblasts [5, 14].
Therefore, as shown by the results of this study, neutrophilic granulocytes are present in each of the types of smear types, but their functional status and quantitative composition are different.
Conclusion
The results of this cytological study confirmed the results of immunohistochemical studies and showed that in generalized periodontitis, inflammatory cellular elements with disorganized epithelial cells and connective tissue of the gums and periodontium and bacteria form specific types of infiltration in periodontal tissues.
Authors’ contribution
PH and NH made the critical review, prepared figures; DK and VI prepared the article and manuscript; DK did literature review; SZ prepared figures. All authors read and approved the final form of this manuscript.
Conflict of interest
The authors declare that there is no conflict of interest.
Funding Statement
Funding sources: No financial support was received for this study.
References
- 1.Kornman K, Van Dyke T: Inflammation and factors that may regulate inflammatory response. J Periodontol 79, 1313–1326 (2008) [DOI] [PubMed] [Google Scholar]
- 2.Van Dyke T, Kornman K: Bringing light to the heat: “Inflammation and periodontal diseases: A reappraisal”. J Periodontol 79, 1503–1507 (2008) [DOI] [PubMed] [Google Scholar]
- 3.Kornman K: Mapping the pathogenesis of periodontitis: A new look. J Periodontol 79, 1560–1568 (2008) [DOI] [PubMed] [Google Scholar]
- 4.Kuzenko Y, Romaniuk A, Politun A, Karpenko A: S100, bcl2 and myeloperoxid protein expirations during periodontal inflammation. BMC Oral Health 15, 93 (2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berglundh T, Liljenberg B, Tarkowski A, Lindhe J: The presence of local and circulating autoreactive B cells in patients with advanced periodontitis. J Periodontol 29, 281–286 (2002) [DOI] [PubMed] [Google Scholar]
- 6.Chappie I, Milward M, Dietrich T: The prevalence of inflammatory periodontitis is negatively associated with serum antioxidant concentrations. J Nutr 64, 657–673 (2007) [DOI] [PubMed] [Google Scholar]
- 7.Chatenoud L, Salomon B, Bluestone J: Suppressor T cells – They’re back and critical for regulation of autoimmunity! Immunol Rev 182, 149–163 (2001) [DOI] [PubMed] [Google Scholar]
- 8.Engebretson S, Grbic J, Singer R, Lamster I: GCF IL-lβ profiles in periodontal disease. J Periodontol 29, 48–53 (2002) [DOI] [PubMed] [Google Scholar]
- 9.Etz H, Minh D, Henics T, Dryla A, Winkler B, Triska C, Boyd AP, Söllner J, Schmidt W, von Ahsen U, Buschle M, Gill SR, Kolonay J, Khalak H, Fraser CM, von Gabain A, Nagy E, Meinke A: Identification of in vivo expressed vaccine candidate antigens from Staphylococcus aureus. Proc Natl Acad Sci U S A 99, 6573–6578 (2002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lamster I, Ahlo J: Analysis of gingival crevicular fluid as applied to the diagnosis of oral and systemic diseases. Ann N Y Acad Sci 1098, 216–229 (2007) [DOI] [PubMed] [Google Scholar]
- 11.Niederman R, Westernoff T, Lee C, Mark LL, Kawashima N, Ullman-Culler M, Dewhirst FE, Paster BJ, Wagner DD, Mayadas T, Hynes RO, Stashenko P: Infection-mediated early-onset periodontal disease in P/E-selectin-deficient mice. J Periodontol 28, 569–575 (2001) [DOI] [PubMed] [Google Scholar]
- 12.Fabricius L, Dahlen G, Halm S, Mooler A: Influence of combinations of oral bacteria of periapical tissues of monkeys. Scand J Dent Res 90, 200–206 (1982) [DOI] [PubMed] [Google Scholar]
- 13.Faggion C, Petersilka G, Lange D, Gerss J, Flemmig T: Prognostic model for tooth survival in patients treated for periodontitis. J Periodontol 31, 226–243 (2007) [DOI] [PubMed] [Google Scholar]
- 14.Evans R, Klausen B, Sojar H, Ramamurthy N, Evans M, Genco RJ: Molecular pathogenesis of periodontal disease. Am Soc Microbiol 38, 267–278 (1994) [Google Scholar]