Abstract
Purpose
Chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS) causes symptoms that include frequent and urgent need to urinate, pain or burning during urination, and pain radiating to the back, abdomen and/or colorectum. These urinary bladder symptoms suggest that CP/CPPS is associated with sensitization of adjacent organs, termed cross-organ sensitization. The objective of this study was to determine the extent of: 1] changes in immunomodulatory mediators in both the prostate and bladder after inflammation of the prostate and 2] bladder function and bladder afferent sensitization.
Materials/Methods
Prostate and bladder histology, immuno-histochemistry, and expression of immunomodulatory targets were examined weekly after either zymosan or vehicle was injected into the dorsal lobe of the mouse prostate. Cystometry, urinary bladder and bladder afferent sensitivity were also assessed weekly.
Results
Prostate inflammation induced a significant upregulation in pro- and anti-inflammatory cytokines (TNF-α and IL-10), growth factor NGF and T-lymphocyte markers (FoxP3, CD4, CD8) in both prostate and bladder. Notably, prostatitis significantly increased urinary voiding frequency, induced hypersensitivity to bladder distension, and sensitized bladder afferents. We also examined sensory (afferent) co-innervation by injecting different retrograde tracers into the bladder wall (DiI) and prostate (fast blue, FB), finding that a significant proportion (~ 17%) of dorsal root ganglion afferent somata contained tracers from both the bladder and prostate.
Conclusions
These observations support an afferent contribution to CP/CPPS and cross-organ sensitization from prostate to bladder.
Keywords: chronic pelvic pain syndrome, cross-organ sensitization, dichotomizing afferents, dual organ innervation, stretch-sensitive afferents, visceral organ cross-talk, viscero-visceral convergence
Introduction
Visceral pain is not typically felt at the source, but rather is referred to cutaneous/subcutaneous structures. In clinical practice, referred abdominal and pelvic visceral sensations are thus often confirmed by tenderness (hypersensitivity) to abdominal palpation. The mechanism underlying referred sensations has long been linked to the convergence of visceral and somatic (i.e., non-visceral) inputs onto second order neurons in the central nervous system, principally the spinal dorsal horn, formulated by Ruch1 as the “convergence–projection” theory of referred visceral sensation. Likewise, spinal second order neurons often receive convergent input from different visceral organs, contributing to cross-organ sensitization via viscero-visceral convergence [e.g.,2, 3, 4, 5]. In addition to a central mechanism, growing evidence suggests that a peripheral mechanism also contributes to cross-organ sensitization.
Clinically, cross-organ sensitization between lower gut and pelvic urinary or gynecologic organs is common, leading to significant difficulty in diagnosing and treating the diseased organ [e.g., 2, 4, 6, 7, 8]. The organs that appear most often involved, both in humans and animals, seem to be the colon/rectum, the urinary bladder/pelvic urethra, the uterus and the prostate3.
Chronic nonbacterial prostatitis, characterized by genitourinary pain in the pelvic region in the absence of an identifiable cause, is common in adult males. More than 90% of such patients report urologic pain or discomfort in the pelvic region, associated with urinary symptoms and/or sexual dysfunction9, [implicating bladder as the main organ affected by prostatitis. In an effort to probe the underlying mechanisms by which prostatitis alters bladder function and sensitivity, we employed a recently characterized mouse model of NIH category IIIA chronic prostatitis10 termed chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS), to determine the extent of: 1] immunomodulatory mediators in both organs and 2] bladder function and bladder afferent sensitization after prostate inflammation. We subsequently also examined possible sensory (afferent) co-innervation between the mouse prostate and urinary bladder and assess. Portions of these data have been reported in abstract form11.
Materials and Methods
Animals
Experiments were performed on male C57BL/6 mice, 6–11 weeks old (The Jackson Laboratory, Bar Harbor, ME), housed in the AAALAC accredited facility at the University of Pittsburgh. All protocols were approved by the Institutional Animal Care and Use Committee.
Surgical Procedures and Cell Labeling
All surgeries were aseptic; anesthesia was initiated and maintained with 4% and 2% isoflurane inhalation, respectively. Primary afferent neurons innervating the prostate or bladder were identified in dorsal root ganglion (DRG) tissue sections by content of the fluorescent retrograde tracer fast blue (FB; EMS-Chemie, Gross Umstadt, Germany) or 1,1'-Dioctadecyl-3,3,3',3'-Tetramethylindocarbocyanine Perchlorate (DiI; Molecular Probes, Eugene, OR), respectively. Tracer (DiI and FB) injection was performed 7 – 28 days prior to harvesting DRG. Mice were anesthetized with isoflurane, the prostate and bladder exposed by laparotomy, and the dorsal lobe of the prostate injected with FB (1 mg/100µl in sterile saline; 10µl total distributed into 2–4 sites) and the bladder wall/trigone injected with DiI (2 mg/100µl in DMSO; 10µl total distributed into 3–4 sites). Any leakage from injection sites was removed using a cotton-tipped applicator and the peritoneal cavity was rinsed with sterile saline before suturing muscle and skin separately. Buprenorphine (0.1 mg/kg, Bedford Labs, Bedford, OH) was given for postoperative analgesia.
Induction of Prostatitis
Vehicle (sterile saline) or zymosan (0.1 mg in 10µI in a single injection, and also containing FB) was injected into the dorsal lobe of the prostate. In different groups of mice, prostates and bladders were removed 7, 14, 21 or 28 days after injection and sectioned for histology and immunohistochemistry.
Real-Time qRT-PCR
Tissue qRT-PCR was performed as described previously12, 13. Prostate and bladder tissue from each animal was processed separately. RNA was isolated by homogenizing frozen tissue in 2 ml (bladder) or 1 ml (prostate) of Trizol reagent (Invitrogen, Carlsbad, CA) followed by isopropanol precipitation. Pellets were washed with 70% ethanol, suspended in RNase-free water and the concentration determined using a GeneQuant RNA/DNA calculator. 5 µg of RNA was treated with DNase (Invitrogen, Grand Island, NY) to remove genomic DNA and then 1 µg was reverse-transcribed using Superscript II reverse transcriptase (Invitrogen). The gene expression of cytokines, growth factors, and T cell mRNA in the bladder and prostate following zymosan-induced prostatitis was measured using SYBR® Green mastermix (Bio-Rad, Hercules, CA) in a CFX Connect real time cycler (Bio-Rad). Gene transcripts were quantified by linear regression of individual amplification curves using LinReg PCR13, 14 and normalized to the quantity of internal reference standard gene transcript GAPDH.
Histology
Histological assessments were made on frozen cross-sections of prostate or bladder stained with hematoxylin and eosin (H&E) from vehicle- and zymosan-treated mice; evaluations were performed a person blinded as to treatment.
Mast Cell Typtase
Mast cell typtase was measured in proteins extracted from prostates (200 µg) and bladders (200 µg) from zymosan- and vehicle-treated mice 7, 14, 21 or 28 days after treatment. Mast cell tryptase was measured using the mast cell degranulation kit (EMD Millipore, Billerica, MA) according to the manufacturer’s protocol.
Immunohistochemistry, Western blotting
Immunohistochemistry was performed as described previously12, 13, 14. Sections of prostates or bladder were incubated with rabbit anti-calcitonin gene-related peptide (CGRP) antibody (1:2000; Sigma-Aldrich, St. Louis, MO) followed by Cy3-conjugated anti-rabbit IgG (1:200, Jackson Immunoresearch, West Grove,PA) to immunostain peptidergic afferent fibers. Macrophages were stained in other prostate or bladder tissue sections using rat anti-F4/80 (1:1000; Abcam, Cambridge, MA) and Cy3-conjugated anti-rat IgG (1:200).
In other mice, prostates and bladders were collected after intravascular cold saline perfusion and homogenized; the supernatants were collected and immediately stored at −80°C. Protein concentration was determined by the BCA method (Pierce BCA assay kit, Life Technologies, Grand Island, NY). 40 µg of solubilized proteins were loaded in Nupage Bis-Tris 4–12% gels (Life Technologies). After separation, proteins were transferred electrophoretically to nitrocellulose membranes (Bio-Rad) and western blots were performed for CD4 (1:1000; Novus, Little, CO) and β-tubulin (1:2000; Sigma-Aldrich) for the loading control. Mouse spleen was used as a positive control.
Bladder cystometry, visceromotor responses and single fiber recording
At different times after intra-prostate zymosan or vehicle injection, mice were anesthetized (urethane, 1.2 g/kg) and the bladder exposed through a suprapubic midline incision. A PE 50 catheter was inserted through the apex into the bladder, fixed in place with cyanoacrylate, and connected to an infusion pump and pressure transducer. The bladder was drained and 37° C saline infused at a constant rate of 20 µl/min via a syringe pump for 20 min (0.4 ml total volume). Detrusor contraction frequency and amplitude, voiding frequency and intervals, and intravesical pressure was displayed and recorded continuously (Spike2 software, Cambridge Electronic Design, Cambridge, UK). Micturition onset, frequency and contraction/pressure amplitudes were analyzed by a person blinded as to treatment.
The visceromotor response (VMR) is a contraction of abdominal skeletal muscle, recorded electromyographically, in response to hollow organ distension, produced here by constant-pressure, graded (20, 40 and 60 mmHg, 10 sec duration) inflation of the bladder in urethane-anesthetized mice. For single fiber recordings, mice were deeply anesthetized, and the bladder and major pelvic ganglion and pelvic nerves (PN) removed en bloc and placed in a tissue chamber perfused with ice cold Krebs solution. After dissection on ice cold Krebs solution, the bladder with nerve attached was placed in a tissue chamber of oxygenated Krebs solution at 34°C. The pelvic nerve was extended from the tissue chamber into a recording compartment filled with warm mineral oil. The PN was extended from the tissue chamber into a recording compartment filled with warm mineral oil and action potentials from single PN afferents were recorded extracellularly (standard teased fiber method;15, 16 in response to graded bladder distension (20, 40, 60, and 80 mmHg, 10 sec duration).
Statistical analyses
Data are presented as mean ± SEM and were analyzed using SigmaStat (Version 3.1, Systat Software, San Jose, CA). Statistical analyses for differences in changes over time in multiple groups were performed using two-way ANOVAs followed by the Holm-Sidak multiple comparisons test. Differences between groups were tested using Student's t test (for two groups) or one-way ANOVA (for more than two groups) followed by Bonferroni-protected pairwise comparisons. Significance was set at p ≤ 0.05.
Results
Organ histology and inflammation
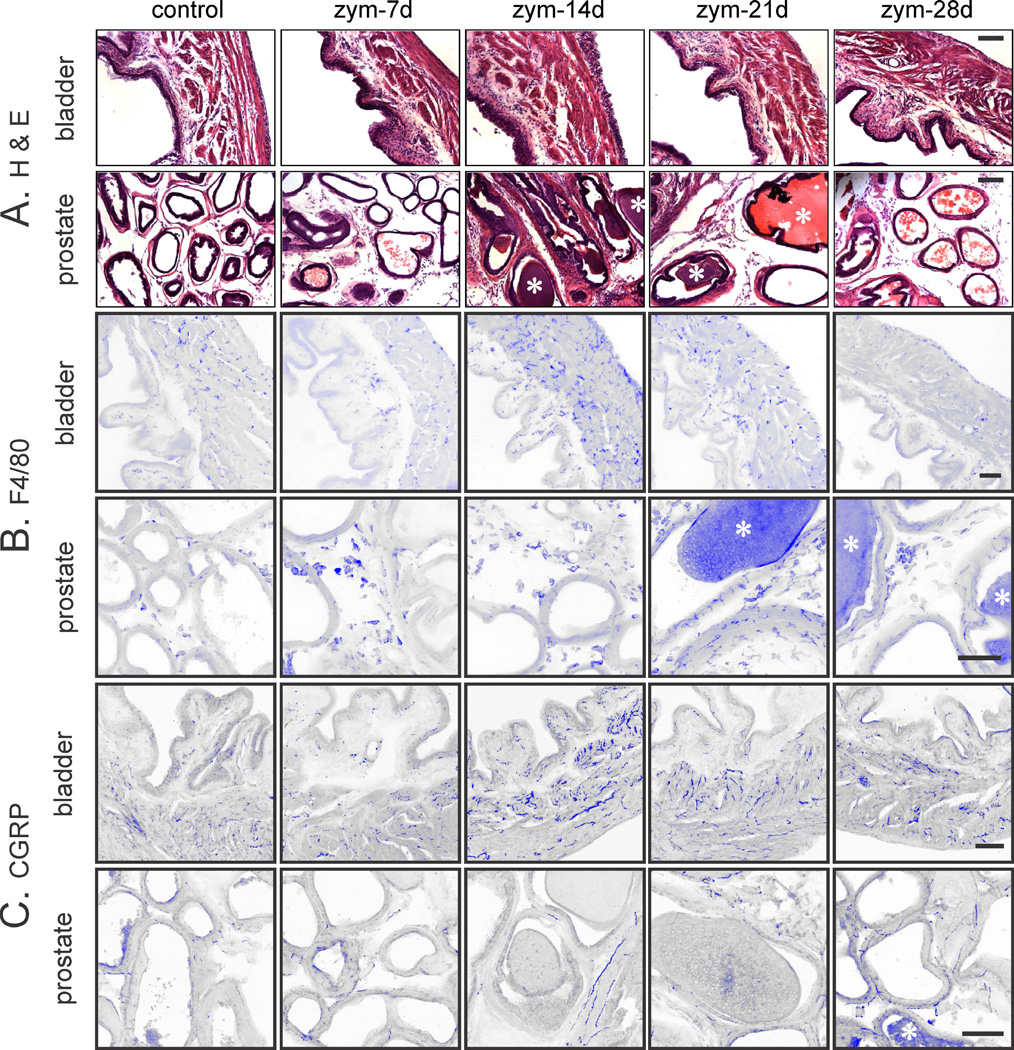
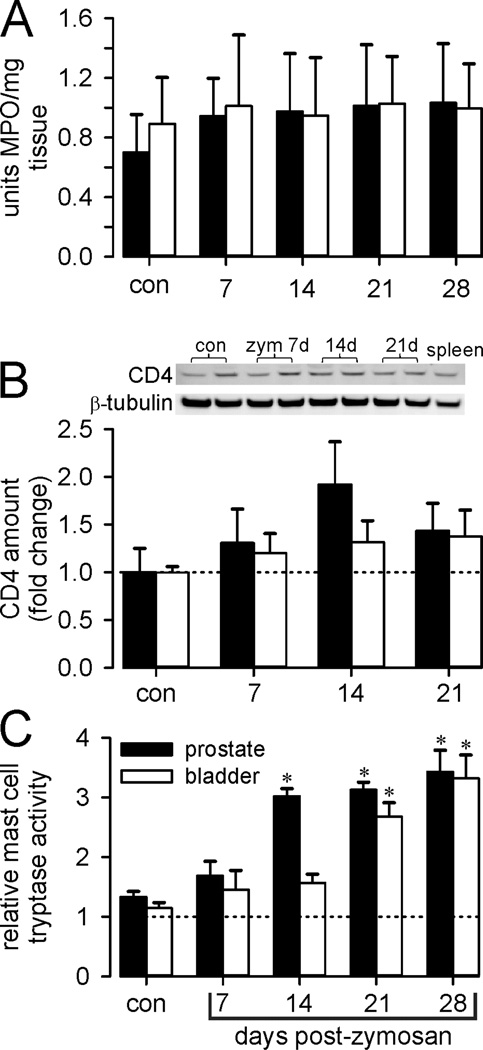
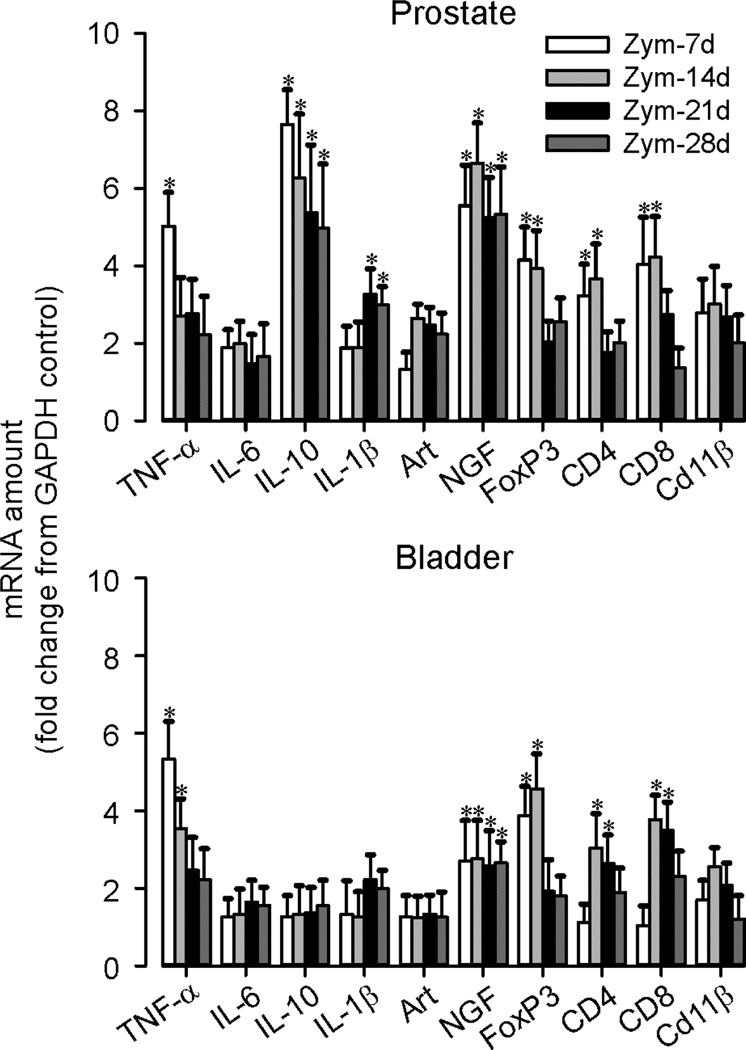
Prostate and bladder inflammation was evaluated histologically (H&E-stained sections) and immunohistochemically (Figure 1). Neither prostates nor urinary bladders of vehicle-treated mice were inflamed (Figure 1A), whereas intra-prostate injection of zymosan produced prostatitis of ≥4 weeks duration. Consistent with previous findings10, the prostate epithelial layer and fibromuscular stroma were thickened and infiltration of mononuclear inflammatory cells was evident. Changes were greatest at 14 days and persisted for 28 days. F4/80 immunostaining (Figure 1B) revealed infiltration of macrophages, and CGRP immunostaining (Figure 1C) revealed prostate hyperinnervation that peaked at 14 days, resolving by 28 days. Myeloperoxidase activity was unaffected (Figure 2A), revealing an inflammatory profile not mediated by polymorphonuclear cells (neutrophils), as supported by an increase in T-cell marker CD4 Western blots (Figure 2B) and qRT-PCR (Figure 3).
Figure 1.
Photomicrographs of mouse prostate and bladder in vehicle (control) and intra-prostate injection of zymosan (zym). A. Hematoxylin and Eosin (H&E) staining reveals inflammation in prostate; prostatic glandular lumen (*). B. H &E of urinary bladder inflammation and C. Immunohistochemical staining for the macrophage marker F4/80 (B) and the afferent fiber marker CGRP. The images were inverted and pseudo-colored blue to enhance contrast. n=8 mice/group. Scale bar = 100µm. Note that the magnification is less for bladder sections to include all layers in the bladder wall, giving a false impression of greater staining in the bladder.
Figure 2.
Assessment of organ inflammation. Data are presented as mean ± SEM from control and zymosan (zym) treated mice after intra-prostate injection of zym. Filled bars, prostate; unfilled bars, bladder. A: Myeloperoxidase activity (MPO) was not increased after intra-prostate injection of zymosan in either prostate or bladder tissue. B: CD4 protein, presented as fold vehicle treatment, was quantified from Western blots of prostate and bladder tissue taken from two vehicle and two zymosan-treated mice. C: Mast cell tryptase activity (units/mg tissue) increased significantly after intra-prostate injection of zymosan in both prostate and bladder. * p≤ 0.05 vs. vehicle, two-way ANOVA with Bonferroni posthoc test.
Figure 3.
Expression of cytokines, growth factors, and inflammatory markers in prostate and urinary bladder. The time course and magnitude of changes in pro- and anti-inflammatory cytokine and growth factor mRNA content in the prostate and bladder during zymosan-induced prostatitis was measured by real-time PCR, normalized to internal reference gene GAPDH, and is presented as fold change from GAPDH control. *p <0.05 vs. control; two-way ANOVA with Bonferroni posthoc test; n=8 mice/group.
Bladder inflammation was also evident (edema and mucosal/urothelial thickening) as were increases in F4/80 and CGRP immunostaining, all peaking at day 14 and largely resolving by day 28 (Figure 1). Prostatitis also significantly increased mast cell tryptase activity in both organs, activity in the prostate peaking earlier (day 14) than in bladder (day 21) (Figure 2C). With the exception of IL-10 and IL-1β, which were significantly upregulated only in the prostate, changes in cytokine, growth factor and T-cell marker expression were qualitatively similar in both organs after prostate inflammation (Figure 3). Quantitatively, upregulation (fold change from the amount of corresponding gene transcript in vehicle-treated mice) was typically greater in the prostate than bladder, with CD4 and CD8 in bladder lagging one week behind their upregulation in the prostate.
Bladder function and sensitization
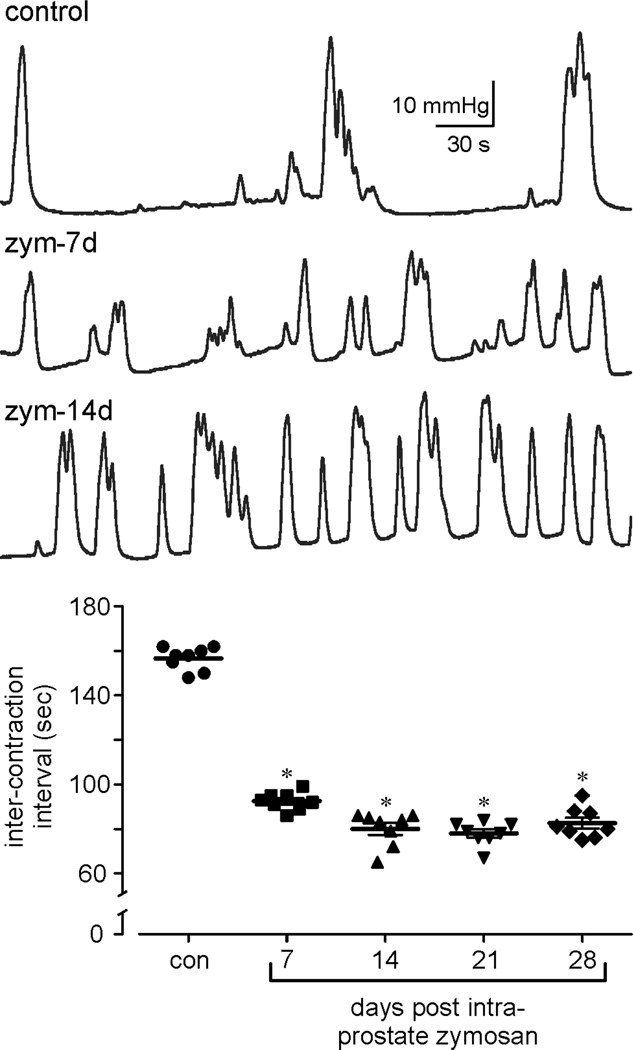
Bladder function (cystometry), hypersensitivity (VMR) and afferent sensitization were significantly affected by prostate inflammation. Bladder function assessed by cystometry was significantly affected across the period of testing through day 28 (Table 1). Figure 4 shows representative cystometric records illustrating increased frequency of contraction (reduced inter-contraction intervals) in prostate-inflamed relative to control mice.
Table 1.
Effect of prostatitis on urinary bladder cystometric assessment.
| saline | zym 7d | zym-14d | zym-21d | zym-28d | |
|---|---|---|---|---|---|
| Mean peak pressure (mmHg) during micturition |
20.0 ± 2.1 | 28.4 ± 2.2* | 29.9 ± 3.9* | 28.5 ± 2.2* | 24.8 ± 2.4 |
| Micturition pressure threshold (mmHg) |
26.6 ± 2.3 | 14.4 ± 3.4* | 16.7 ± 4.1* | 18.2 ± 4.2* | 17.7 ± 4.7* |
| Intercontraction interval (sec) |
156.2 ± 1.9 | 92.5 ± 1.4* | 80.0 ± 2.74* | 78.0 ± 1.9* | 82.6 ± 2.4* |
| Time to first micturition (sec) |
283.8 ± 31.1 | 215.6 ± 33.5* | 238.2 ± 28.1 | 221.6 ± 26.5* | 228.2 ± 26.1 |
| # Micturition events/20 min |
8.6 ± 1.6 | 13.6 ± 1.9* | 16.2 ± 2.4* | 14.0 ± 2.1* | 13.7 ± 2.4 |
| Baseline pressure (prior to infusion) |
1.4 ± 0.4 | 2.5 ± 0.8 | 1.9 ± 0.5 | 2.6 ± 0.4 | 1.9 ± 0.1 |
Data are presented as mean ± SEM from control (saline)- and zymosan (zym)- treated mice 7 – 28 days after intra-prostate injection of zym. n=8 mice/treatment group.
p≤0.05 vs. control, two-way ANOVA with Bonferroni post-hoc test.
Figure 4.
Bladder function (cystometry) was significantly affected during zymosan-induced prostatitis. A: representative cystometric records from control and zymosan (zym)-treated mice (7 and 14 days after intra-prostate injection of zym). B: quantification of micturition events showing inter-contraction intervals in control and zymosan-treated mice. *p <0.05 vs. control; two-way ANOVA with Bonferroni posthoc test; n=8 mice/group.
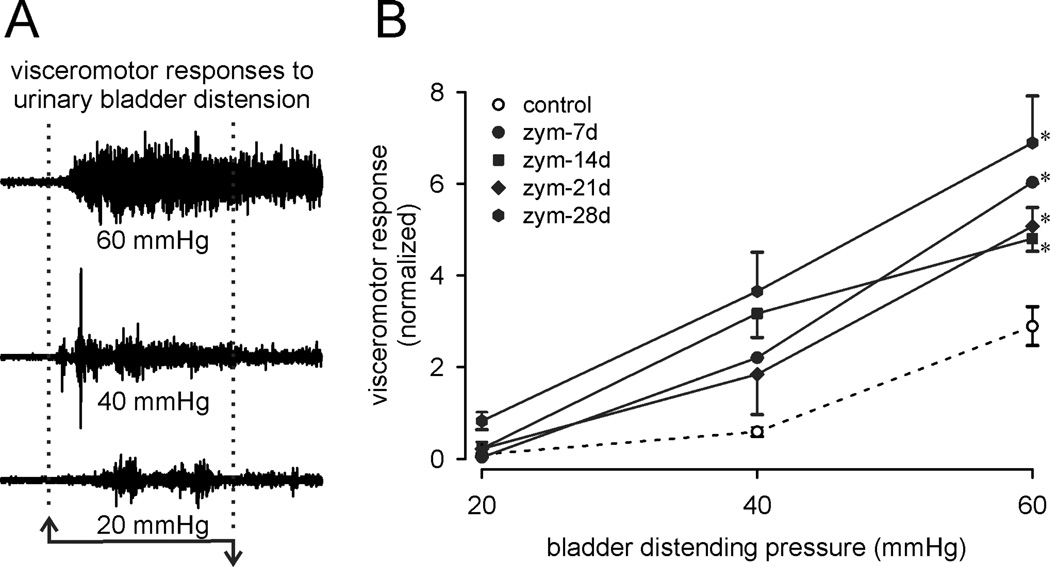
Representative VMRs to bladder distension, employed to assess bladder nociception and hypersensitivity17, 18, are shown in Figure 5A and summarized in Figure 5B as stimulus-response functions, confirming significant and persistent bladder hypersensitivity associated with prostatitis.
Figure 5.
Bladder sensitivity (visceromotor responses) was significantly increased during after intra-prostate injection of zymosan. A: representative electromyographic (EMG) recordings to graded intensities of bladder distension. EMG records were rectified and quantified as total activity (minus baseline activity) during bladder distension (10 sec). B: visceromotor responses presented as stimulus-response functions reveal significant bladder hypersensitivity during zymosan-induced prostatitis. *p <0.05 vs. control; two-way ANOVA with Bonferroni posthoc test; n=8 mice/group.
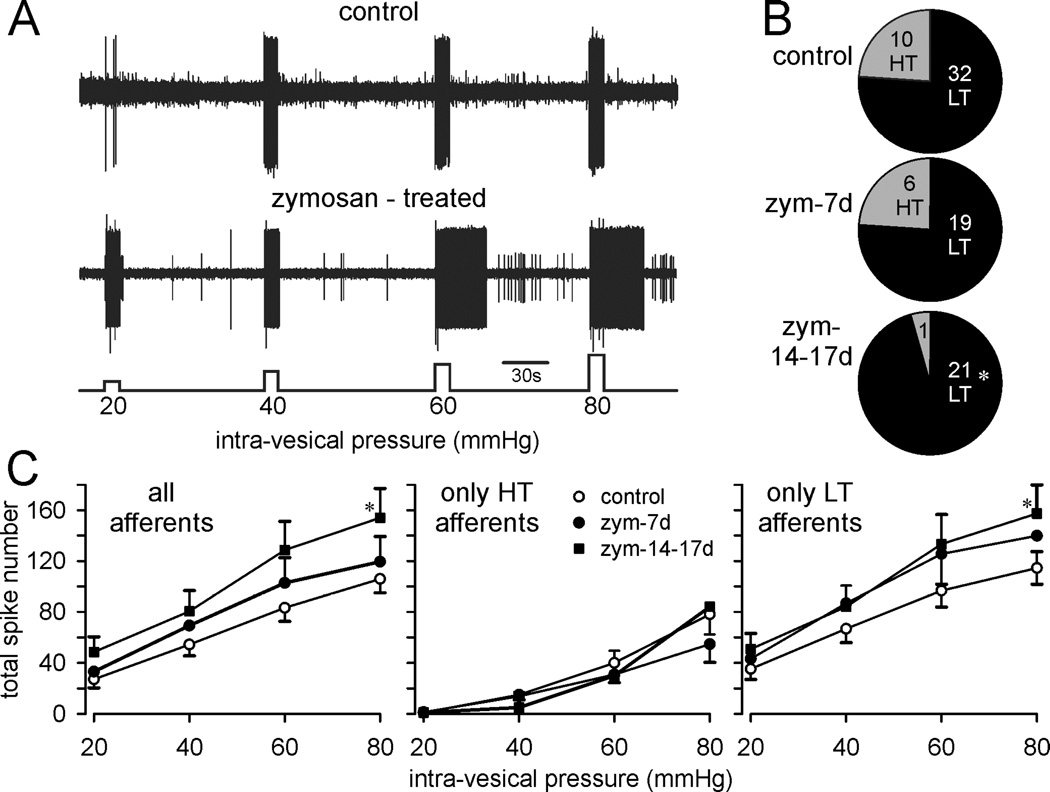
Prostatitis also sensitized PN afferents innervating the bladder. Figure 6A shows representative examples of single fiber responses to graded bladder distension, illustrating an increase in response magnitude in the bladder afferent from a prostate-inflamed relative to a control mouse, including significant after-discharge at greater intensities of bladder distension. Typically, 20–25% of stretch-sensitive bladder afferents have high thresholds for response (Figure 6B), the proportion of which significantly decreased during the course of prostatitis. Afferent sensitization is characterized by an increase in response magnitude and a decrease in response threshold, both of which are apparent in bladder afferents studied 14–17 days after prostate inflammation, summarized in Figure 6C.
Figure 6.
Pelvic nerve bladder afferents are sensitized during zymosan-induced prostatitis. A: representative teased fiber recordings of stretch-sensitive bladder afferents from a control (saline-treated) and zymosan-treated mouse in response to graded bladder distension B: Proportions of low threshold (LT, black) and high threshold (HT, gray) stretch-sensitive bladder afferents from control (saline) and zymosan-treated mice. The numbers in the pie plots indicate the number of HT and LT afferent fibers. C. Stimulus-response functions of LT and HT stretch-sensitive bladder afferents from control (saline-treated, n=42) and zymosan-treated mice (n=47 total). Afferent responses (quantified only during the duration of bladder inflation) were increased during prostatitis, significantly 14–17 days after intra-prostate injection of zymosan. *p <0.05 vs. control; two-way ANOVA with Bonferroni posthoc test.
Organ innervation
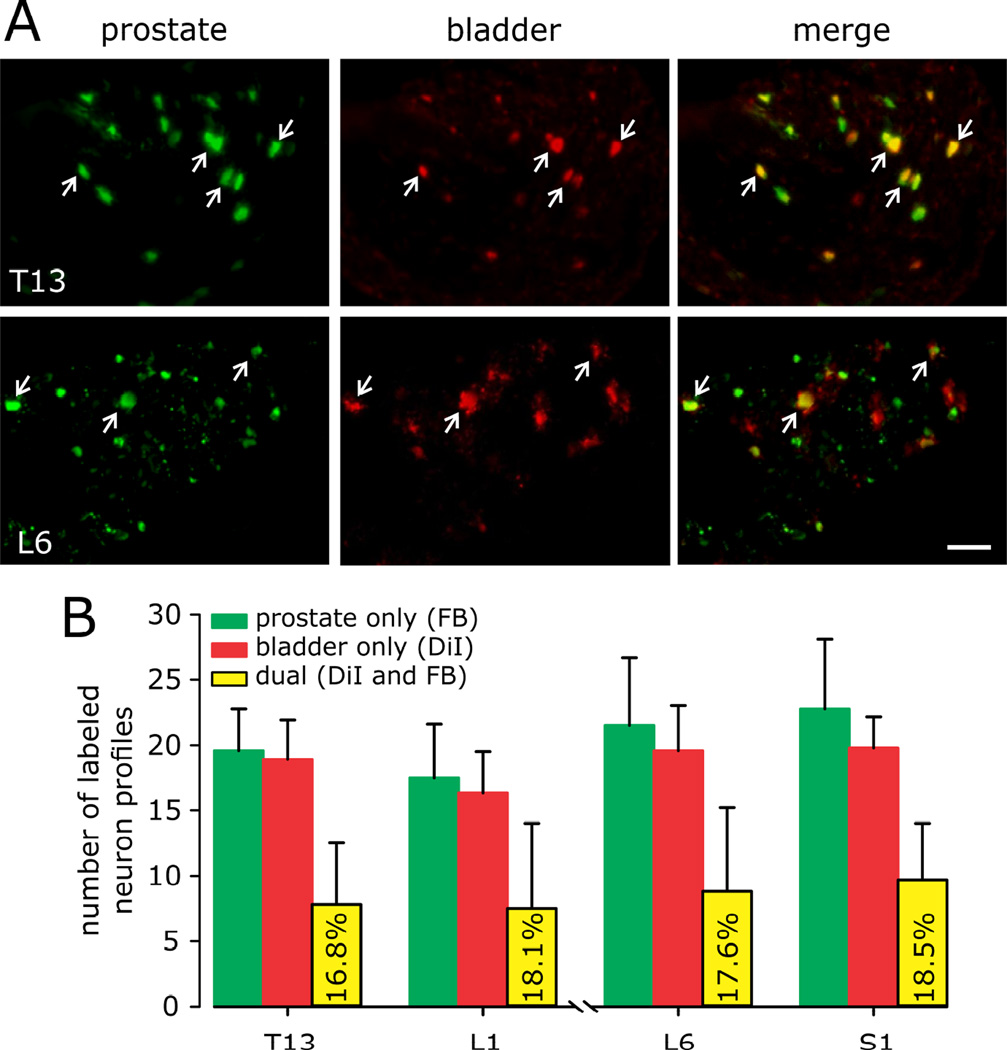
T13-L1 and L6-S1 DRG are the principal locations of somata innervating mouse prostate10 and urinary bladder16. Figure 7A shows representative examples of DRG somata retrogradely labeled from the prostate, bladder and merged images of somata containing both tracers. The density of innervation was similar for both organs (~15–20 neuron profiles/organ in each section/ganglion) as was the proportion of somata that contained both tracers (16.8 – 18.5%) (Figure 7B).
Figure 7.
Quantification of neuron profiles in dorsal root ganglia (DRG) retrogradely labeled from prostate (FB) and bladder wall (with DiI). Labeling in T13-L1 DRG represents the hypogastric/lumbar splanchnic nerve innervation; labeling in L6-S1 DRG represents the pelvic nerve innervation. Photomicrographs (A) of FB-positive (green), DiI-positive (red), and FB and DiI-positive (yellow) retrogradely labeled somata in T13 and L6 DRG from control mice. Positive profiles are indicated by white arrows (scale bar= 50µm). B: Number of retrogradely labeled DRG neurons by counting neuron profiles in every fifth 12 µm thick cryostat section (i.e., 60 µm between counted sections) in five sections/ganglion to avoid double counting of cells. Ganglia were taken bilaterally and a mean of counted neuron profiles was determined for each pair of ganglia. n=6 mice/group.
Discussion
This report documents functional changes in and sensitization of the urinary bladder, significantly in its afferent (sensory) innervation, in a mouse model of chronic nonbacterial prostatitis. These findings are consistent with complaints and symptoms of CP/CPPP patients – urinary symptoms (urge and frequency) and urologic pain – and are supported by inflammatory changes in both prostate and bladder. The novel finding of bladder afferent sensitization contributes to enhanced understanding of underlying mechanisms of cross-organ sensitization. Previous studies in rodents documented that bladder function and bladder afferents are sensitized by colon inflammation5, 19, 20, 21 and visceromotor responses and colon afferent responses to colon distension are sensitized by bladder inflammation5, 22 establishing bidirectional colon-bladder cross-organ sensitization. In addition to direct assessment of organ afferent sensitivity, experimental inflammation of the colon leads to release of neuropeptides in the bladder23,implicating neurogenic inflammatory mechanisms in cross-organ sensitization. Consistent with this suggestion, we found that inflammation of the prostate increased macrophage infiltration and the expression of T cell markers CD4 and CD8 in the bladder wall. It is reasonable to conclude that these changes in bladder arise from an axon reflex-like consequence of prostate inflammation, generating dorsal root reflexes24 and neurogenic inflammation in the bladder. In addition, acute5 or chronic19 [irritation of the rodent colon leads to increased frequency of urinary bladder contractions, reduced inter-contraction intervals and altered micturition reflexes. Conversely, experimental urinary bladder inflammation in rodents leads to hypersensitivity to colon distension.5
Cross-organ sensitization is a clinical condition increasingly recognized in abdominal/pelvic visceral disorders and typically explained as arising from convergence of inputs from different organs onto the same second order spinal neurons. The present and other observations, however, also support a contribution from afferent mechanisms [see 3]. That peripheral mechanisms are engaged is supported by electrophysiological studies of DRG somata and single fiber recordings of visceral afferents, as in the present study. In cultured DRG neurons labeled from the urinary bladder and taken from rats with an experimental colitis4, urinary bladder neurons exhibited increases in a capsaicin-evoked inward current and in the peak amplitude of a tetrodotoxin-resistant sodium current, suggesting an increase in excitability of urinary bladder DRG neurons. Similarly, in vitro recordings of rat pelvic nerve bladder afferents after either acute25 or chronic21 colon inflammation in the rat showed sensitization of bladder afferents to both mechanical and chemical stimuli. Likewise, in vitro recordings of mouse colon pelvic nerve afferents after urinary bladder inflammation revealed sensitization of colon afferents to mechanical stimulation22. For both recordings, we saw a leftward shift of the stimulus-response functions. HT fiber response thresholds were significantly reduced (i.e. sensitized) and that all afferents and only LT afferents exhibited leftward shifts in response to bladder inflation. The findings that HT fibers did not similarly exhibit a leftward shift in response to bladder inflation is likely due, primarily, to their significant reduction in response threshold and also relatively low number.
By use of different retrograde tracers injected into the prostate and urinary bladder, we provide evidence for innervation of these organs by one DRG neuron. Such dual innervation of visceral organs, referred to in earlier literature as dichotomizing axons, has previously been reported for rat4, 25 and mouse26 colon and bladder, rat colon and uterus8, 27 and rat prostate and bladder28. Reported proportions of double-labeled DRG neurons in rats and mice range between 14 – 21% (colon and bladder17), 5 – 15% (colon and uterus 29), and 7.5 – 14% (prostate and bladder28), consistent with the mean 17.7% reported here in mouse bladder and prostate. We regard the present quantification of DRG somata that innervate both the prostate and bladder as only a reasonable estimate and with cautions. The bladder and prostate are in close proximity and that some leaked retrograde tracer may have been transported centrally from an adjacent organ cannot be entirely discounted, although we consider it unlikely given the precautions taken. This interpretation is supported by the similar proportions of double-labeled neurons in all four DRG examined. In addition, because the number of sites and volume of tracer that can be injected into an organ wall is limited, not all organ nerve terminals were exposed to tracers. If we accept the foregoing, it is thus more likely that the number of DRG somata innervating either or both organs is underestimated, raising the likelihood that the proportion of dichotomizing axons innervating internal organs comprise a greater percentage of the overall visceral innervation than discussed above.
Caveats aside, the key question is whether dichotomizing axons play a significant role in cross-organ sensitization? The innervation of the viscera represents a relatively small proportion of all spinal afferents (5–10%30) and the proportion of dichotomizing axons arising from DRG somata innervating the viscera comprise only one-fifth or less of that small proportion of spinal afferents, suggesting a limited role given existing knowledge. The present results suggest a contribution by peripheral mechanisms to cross-organ sensitization, but the changes in bladder function and afferent sensitivity could have arisen from central consequences of prostate inflammation, particularly in consideration of the persistence of inflammation in the prostate. However, histological and immunomodulatory molecule changes in the urinary bladder indicate that peripheral mechanisms contributed directly to bladder hypersensitivity and afferent sensitization. It is important to mention that systemic factors, i.e. immune mediators from the prostate, may enter the circulation and could have secondary effects on other organs that is not specific to jus the bladder. It is not possible, however, to know whether the urinary bladder afferents sensitized after prostate inflammation share a DRG soma with a dichotomizing axon innervating the prostate. Thus, despite the present and other supporting evidence, it cannot be denied that cross-organ sensitization is also (or perhaps principally?) contributed to by converging afferent input onto second or higher order neurons in addition to (or rather than) input from branching afferent endings (see 3 for review).
In summary, urinary bladder histology, function and sensitivity, including bladder afferent sensitivity, were all significantly affected after an acute experimental inflammation of the dorsal lobe of the mouse prostate. Prostate inflammation persisted for the duration of the experiment and changes in the urinary bladder and its innervation were time-dependent, typically following changes documented in the prostate. In previous work, we established mechanical hypersensitivity in the pelvic area of prostate referral. In the aggregate, these findings suggest that strategies for managing lower abdominal/pelvic organ cross sensitization should address both peripheral and central mechanisms of inflammation and sensitization.
Acknowledgments
This work was supported by grants from the US National Institutes of Health (NIH award R01 DK093525 to GFG and K01 DK100460 to BF) and an American Pain Society Future Leaders in Pain Research Grant (ESS). We thank Michael Burcham for preparing the figures.
Abbreviations
- CP/CPPS
Chronic prostatitis/chronic pelvic pain syndrome
- DRG
Dorsal Root Ganglion
- Zym
Zymosan
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no competing financial interests.
References
- 1.Ruch TC. Pathophysiology of pain. In: Ruch TC, Patton HD, Woodbury IW, Lowe AL, editors. Neurophysiology Philadelphia. Saunders; 1961. pp. 350–368. [Google Scholar]
- 2.Berkley KJ. A life of pelvic Pain. Physiol Behav. 2005;86:272–280. doi: 10.1016/j.physbeh.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 3.Brumovsky PR, Gebhart GF. Visceral organ cross-sensitization- an integrated perspective. Auton Neurosci. 2010;153:106–115. doi: 10.1016/j.autneu.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Malykhina AP, Qin C, Foreman RD, et al. Colonic inflammation increases Na+ currents in bladder sensory neurons. Neuroreport. 2004;15:2601–2605. doi: 10.1097/00001756-200412030-00008. [DOI] [PubMed] [Google Scholar]
- 5.Pezzone MA, Liang R, Fraser MO. A model of neural cross-talk and irritation in the pelvis: implications for the overlap of chronic pelvic pain disorders. Gastroenterology. 2005;128:1953–1964. doi: 10.1053/j.gastro.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 6.Baranowski AP, Abrams P, Berger RE, et al. Urogenital pain - time to accept a new approach to phenotyping and, as a consequence, management. Eur Urol. 2008;53:33–36. doi: 10.1016/j.eururo.2007.10.010. [DOI] [PubMed] [Google Scholar]; alykhina AP. Neural mechanisms of pelvic organ cross-sensitization. Neuroscience. 2007;149:660–672. doi: 10.1016/j.neuroscience.2007.07.053. [DOI] [PubMed] [Google Scholar]
- 7.Saini R, Gonzalez RR, Te AE. Chronic pelvis pain syndrome and the overactive bladder: the inflammatory link. Curr Urol Rep. 2008;9:314–319. doi: 10.1007/s11934-008-0054-8. [DOI] [PubMed] [Google Scholar]
- 8.van de Merwe JP, Nordling J, Bouchelouche P, et al. Diagnostic criteria, classification, and nomenclature for painful bladder syndrome/interstitial cystitis: an ESSIC proposal. Eur Urol. 2008;53:60–67. doi: 10.1016/j.eururo.2007.09.019. [DOI] [PubMed] [Google Scholar]
- 9.Cohen JM, Fagin AP, Hariton E, et al. Therapeutic intervention for chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS): a systematic review and meta-analysis. PLoS ONE. 2012;7:e41941. doi: 10.1371/journal.pone.0041941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwartz ES, Xie A, La JH, et al. Nociceptive and Inflammatory mediator upregulation in a mouse model of chronic prostatitis. PAIN. 2015;156:1537–1544. doi: 10.1097/j.pain.0000000000000201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwartz ES, Young E, Feng B, et al. Society for Neuroscience Poster # 628. Washington DC: 2014. Prostatitis induces bladder hypersensitivity via neural cross-talk. [Google Scholar]
- 12.Schwartz ES, Christianson JA, Chen X, et al. Synergistic role of TRPV1 and TRPA1 in pancreatic pain and inflammation. Gastroenterology. 2011;140:1283–1291. doi: 10.1053/j.gastro.2010.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwartz ES, La JH, Scheff NN, et al. TRPV1 and TRPA1 antagonists prevent the transition of acute to chronic inflammation and pain in chronic pancreatitis. J Neurosci. 2013;27:5603–5611. doi: 10.1523/JNEUROSCI.1806-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.La JH, Schwartz ES, Gebhart GF. Differences in the expression of transient receptor potential channel V1, transient receptor potential channel A1 and mechanosensitive two pore-domain K+ channels between the lumbar splanchnic and pelvic nerve innervations of mouse urinary bladder and colon. Neuroscience. 2011;186:179–187. doi: 10.1016/j.neuroscience.2011.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feng B, Gebhart GF. Characterization of silent afferents in the pelvic and splanchnic innervations of the mouse colorectum. Am J Physiol Gastrointest Liver Physiol. 2011;300:G170–G180. doi: 10.1152/ajpgi.00406.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu L, Gebhart GF. Characterization of mouse lumbar splanchnic and pelvic nerve urinary bladder mechanosensory afferents. J Neurophysiol. 2008;99:244–253. doi: 10.1152/jn.01049.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Castroman P, Ness TJ. Vigor of visceromotor response to urinary bladder distension in rats increases with repeated trials and stimulus intensity. Neurosci Lett. 2001;22:97–100. doi: 10.1016/s0304-3940(01)01886-9. [DOI] [PubMed] [Google Scholar]
- 18.Ness TJ, Elhefni H. Reliable visceromotor responses are evoked by noxious bladder distension in mice. J Urol. 2004;171:1704–1708. doi: 10.1097/01.ju.0000116430.67100.8f. [DOI] [PubMed] [Google Scholar]
- 19.Lamb K, Zhong F, Gebhart GF, et al. Experimental colitis in mice and sensitization of converding visceral and somatic afferent pathways. Am J Physiol Gastrointest Liver Physiol. 2006;290:G451–G457. doi: 10.1152/ajpgi.00353.2005. [DOI] [PubMed] [Google Scholar]
- 20.Li J, Micevych P, McDonald J, et al. Inflammation in the uterus induces phosphorylated extracellular signal-regulated kinase and substance P immunoreactivity in dorsal root ganglia neurons innervating both uterus and colon in rats. J Neurosci Res. 2008;86:2746–2752. doi: 10.1002/jnr.21714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ustinova EE, Fraser MO, Pezzone MA. Cross-talk and sensitization of bladder afferent nerves. Neurourol Urodyn. 2010;29:77–81. doi: 10.1002/nau.20817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brumovsky P, Feng B, Xu L, et al. Cystitis increases colorectal afferent sensitivity in the mouse. Am J Physiol Gastrointest Liver Physiol. 2009;297:G1250–G1258. doi: 10.1152/ajpgi.00329.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pan XQ, Gonzalez JA, Chang S, et al. Experimental colitis triggers the release of substance P and calcitonin gene-related peptide in the urinary bladder via TRPV1 signaling pathways. Exp Neurol. 2010;225:262–273. doi: 10.1016/j.expneurol.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Willis WD., Jr Dorsal root potentials and dorsal root reflexes: a double-edged sword. Exp Brain Res. 1999;124:395–421. doi: 10.1007/s002210050637. [DOI] [PubMed] [Google Scholar]
- 25.Ustinova EE, Fraser MO, Pezzone MA. Colonic irritation in the rat sensitizes urinary bladder afferents to mechanical and chemical stimuli: an afferent origin of pelvic organ cross-sensitization. Am J Physiol Renal Physiol. 2006;290:F1478–F1487. doi: 10.1152/ajprenal.00395.2005. [DOI] [PubMed] [Google Scholar]
- 26.Chaban V, Christensen A, Wakamatsu M, et al. The same dorsal root ganglion neurons innervate uterus and colon in the rat. Neuroreport. 2007;18:209–212. doi: 10.1097/WNR.0b013e32801231bf. [DOI] [PubMed] [Google Scholar]
- 27.Qin C, Malykhina AP, Thompson AM, et al. Cross-organ sensitization of thoracic spinal neurons receiving noxious cardiac input in rats with gastroesophageal reflux. Am J Physiol Gastrointest Liver Physiol. 2010;296:G934–G942. doi: 10.1152/ajpgi.00312.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen Y, Wu X, Liu J, et al. Distribution of convergent afferents innervating bladder and prostate at dorsal root ganglia in rats. Urology. 2010;76:764e1–764e6. doi: 10.1016/j.urology.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 29.Christianson JA, Liang R, Ustinova EE, et al. Convergence of bladder and colon sensory innervation occurs at the primary afferent level. PAIN. 2007;128:235–243. doi: 10.1016/j.pain.2006.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cervero F, Connell LA, Lawson SN. Somatic and visceral primary afferents in the lower thoracic dorsal root ganglia of the cat. J Comp Neurol. 1984;228:422–431. doi: 10.1002/cne.902280309. [DOI] [PubMed] [Google Scholar]