Abstract
Context:
Cancer patients’ participation in social, recreational, and civic activities is strongly associated with quality of life (QOL), but these activities are not well integrated into cancer survivorship research or interventions.
Objective:
Test the hypothesis that for long-term (≥ 5 years) survivors of rectal cancer, clinical factors (type of surgery and bowel function) are associated with long-term participation in activities and that participation in activities is associated with long-term QOL.
Design:
Observational study with longitudinal and cross-sectional components.
Main Outcome Measures:
Participation in activities and QOL. Tumor registry records were used to identify patients and obtain clinical data; surveys assessed participation and QOL. Using general linear models, we analyzed participation in activities in relation to type of surgery and bowel function after adjustment for potential confounders. We analyzed overall QOL relative to participation in activities after adjustment.
Results:
A total of 567 rectal cancer survivors completed a mailed questionnaire. Overall response rate was 61%. The type of operation (p < 0.0001), receipt of radiation therapy (p = 0.002), and bowel function (p < 0.0001) were associated with participation in activities. Participation in activities was the strongest predictor of QOL (p < 0.0001), explaining 20% of the variance (R2) in QOL, with all other variables together accounting for another 18% of the variance.
Conclusion:
The importance of participation in activities on rectal cancer survivors’ QOL is underappreciated. We recommend revising QOL instruments used in cancer care and research to include questions about participation in activities. Interventions should address maintenance of preferred activities and adoption of new, fulfilling activities.
INTRODUCTION
Cancer treatment often results in long-term disabilities and decrements in quality of life (QOL). Patients’ and clinicians’ understanding of these effects can influence treatment choices. Researchers also must understand how treatment affects QOL to design measures and interventions to improve QOL.
Participation in activities, a well-established concept in rehabilitation,1,2 is less clearly conceptualized and measured in relation to QOL,2,3 a critical outcome for measuring the effects of cancer treatment. Participation, defined as “involvement in life situations,” includes ability to work, engage in family and other social interactions and relationships, pursue recreation, and participate in civic and community life.4
Advances in rehabilitation science show promise for clarifying concepts and measures of the long-term effects of cancer treatment. Ultimately, these improved concepts and measures could translate to better treatment decisions, supportive interventions, and long-term outcomes for cancer survivors.5,6 This opportunity is highlighted for patients with cancer of the low and midrectum, who often face a decision between sphincter-sparing surgery (anterior resection resulting in anastomosis with or without temporary ostomy) and complete excision of the rectum (abdominoperineal excision with permanent ostomy).
Approximately 40,000 new rectal cancer cases are diagnosed annually in the US, with 68% of these patients surviving at least 5 years.7 Even though sphincter-sparing surgery is more common than permanent ostomy, the decision to undergo sphincter-sparing surgery or permanent ostomy has vexed surgeons and patients alike.8 Although these surgeries have equivalent oncologic outcomes, they result in very different defecation practices and bodily appearance. Preoperatively, most patients choose sphincter-sparing surgery in the hopes of maintaining bowel function and avoiding the stigma of a permanent ostomy.9 However, bowel control after sphincter-sparing surgery can be poor. Although the prevailing wisdom is that sphincter-sparing surgery results in better QOL, this belief was refuted by a 2012 evidence synthesis that found no difference in QOL outcomes between surgery types.10
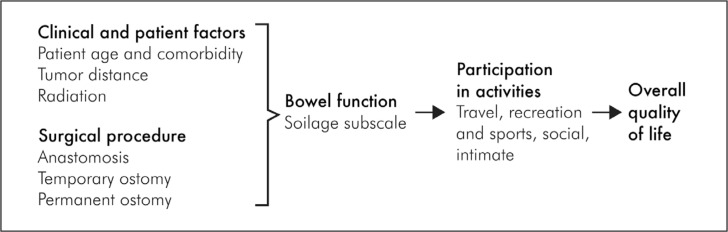
We assessed the roles of clinical and patient factors in relation to participation in activities as well as the role of participation in activities with overall QOL. We specifically hypothesized that 1) surgical approach and bowel function (as measured through soilage) interfere with participation in activities, and 2) participation in activities reduces overall QOL (see Figure 1 for our conceptual model).1,11
Figure 1.
Conceptual model.1
1 Wilson IB, Cleary PD. Linking clinical variables with health-related quality of life. A conceptual model of patient outcomes. JAMA 1995 Jan 4;273(1):59–65. DOI: https://doi.org/10.1001/jama.1995.03520250075037.
METHODS
This cross-sectional study included Kaiser Permanente members in Northern California, Northern Oregon, and Southwestern Washington. The institutional review boards at Kaiser Permanente and the University of Arizona coordinating center approved this study. Tumor registry data were used to identify eligible patients and their diagnosis dates. Surgery dates and comorbidity data were extracted from Health Plan data about procedures and diagnoses. We assessed soilage, activity participation, and overall QOL through mailed questionnaires. Smaller numbers in all our measures represented worse outcomes.
Study Population
The population included adults who were Health Plan members at the time of diagnosis and at the time of the survey (2010–2011). All had received a diagnosis of rectal cancer (International Classification of Diseases, Ninth Revision Codes C20.9 and C19.9) at least 5 years before the survey and had undergone surgery as part of their treatment. Patients with a temporary ostomy were considered separately because they had undergone additional surgery to reverse the ostomy and because they were likely to have lower anastomosis or other factors that elevate the risk of postoperative complications or deleterious long-term effects of treatment. We excluded patients with a diagnosis of severe mental illness, cognitive impairment, or local resections of rectal tumors. For this analysis, we excluded individuals with a permanent ostomy who did not regularly wear an ostomy bag.
Data Collection
In 2010 and 2011, we mailed questionnaires to eligible Health Plan members.12 Electronic medical record data were used to determine each patient’s surgery type (sphincter-sparing surgery, ostomy, temporary ostomy) and other clinical information. Abstractors reviewed participants’ charts to ascertain the distance between the tumor and the anal verge. We used a mailed survey13 to confirm surgery type and ascertain patient-reported bowel function, participation in activities, and overall QOL. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. Informed consent was obtained from each patient through mailed surveys.
We measured bowel function using an adaptation of the Memorial Sloan Kettering Cancer Center bowel function index, which was developed and validated with rectal cancer survivors who had sphincter-sparing surgery.14 The 18-item questionnaire contains 3 subscales—soilage, dietary, and frequency—with respective test-retest reliabilities of 0.87, 0.62, and 0.74 (0.84 overall). We adapted the bowel function index to measure bowel function in rectal cancer survivors with ostomy, established its reliability and validity, and found that the soilage subscale has the best comparability across patients with ostomy and sphincter-sparing surgery.12
For the soilage subscale we asked, “Over the past 4 weeks, how often have you had soilage (leakage of stool) of your garments during the day?,” “… how often have you had soilage of your garments when you go to bed?,” and “… did you use a tissue, napkin and/or pad in your garments in case of stool leakage?” Responses to each item could range from 1 (all the time) to 5 (never). We used the mean of each participant’s responses to obtain a summary score ranging from 1 (worst) to 5 (best). The fourth item in the bowel function index asks, “How often have you had to alter your activities because of your bowel function [or, for people with an ostomy, because of the number of times you changed or emptied your bag]?” We did not use this item because it conflated two constructs we were trying to analyze separately (bowel function and activity limitation).
Participation in activities was ascertained using the modified City of Hope Quality of Life questionnaire,12 which we adapted to use for both patients with anastomosis and those with ostomy.13 The questionnaire assesses the impact of rectal cancer surgery on well-being: we selected 4 items about participation that corresponded well with the World Health Organization’s International Classification of Functioning, Disability and Health (ICF)4,13: “At this time, how much does [your operation] interfere with your ability to travel?,” “… recreational/sports activities?,” “… social activities?,” and “… ability to be intimate?” Responses to each item could range from 0 (not at all) to 10 (completely). We created a summary measure of participation in activities by calculating the mean value of the 4 responses and then inverting it to obtain a score ranging from 0 (worst) to 5 (best).
Overall QOL was ascertained from the following question: “How good is your overall quality of life?,” with 0 being “extremely poor” and 10 being “excellent.” We also asked survey respondents to write about the greatest challenge they experienced related to their cancer surgery. We coded responses using previously reported theme analysis techniques15 to understand the type of activity limitations that survey respondents found most challenging.
Statistical Analysis
All quantitative data analyses were performed using SAS Version 9.13 software (SAS Institute Inc, Cary, NC). We used general linear models to estimate associations of patient demographic and clinical factors with 2 outcomes: 1) participation in activities and 2) overall QOL. We tested whether a variable improved the model’s fit with the F test comparing nested models with and without that variable.
Table 1 shows 14 demographic and clinical variables that were considered potential confounders. Because information on tumor distance was missing for 17.6% of patients, we conducted sensitivity analysis comparing multivariable models that did and did not include tumor distance, to assess the magnitude of potential confounding by this variable among the subset of patients who did have this information. We removed chemotherapy from the models because it was strongly correlated with radiotherapy. We compared nested models to estimate the proportion of variance (R2) in overall QOL that was attributable to participation in activities.
Table 1.
Demographic and clinical characteristics of rectal cancer survivors (N = 567)a
| Characteristics | Permanent ostomy (n= 176) | Sphincter-sparing (n = 324) | Temporary ostomy (n = 67) | p value |
|---|---|---|---|---|
| Sex, women | 34.1 | 44.8 | 37.3 | 0.21 |
| Mean age at survey, years | 74.9 | 73.1 | 69.6 | 0.003 |
| Race | ||||
| White | 88.1 | 81.5 | 88.1 | 0.55 |
| African American | 2.8 | 3.4 | 1.5 | |
| Asian, Pacific Islander | 6.3 | 9.9 | 6.0 | |
| Other | 0.6 | 1.9 | 3.0 | |
| Unknown | 2.3 | 3.4 | 1.5 | |
| Education level | ||||
| Less than high school | 4.6 | 7.1 | 1.5 | 0.0006 |
| High school | 32.4 | 16.4 | 22.4 | |
| Some college | 33.0 | 25.4 | 35.8 | |
| College graduate | 13.1 | 22.5 | 7.5 | |
| Graduate school | 11.9 | 18.3 | 21.0 | |
| Unknown | 5.1 | 10.5 | 11.9 | |
| Household income, $US | ||||
| < 30,000 | 33.0 | 25.3 | 25.4 | 0.34 |
| 30,000 to 75,000 | 38.6 | 39.8 | 47.8 | |
| > 75,000 | 21.0 | 25.9 | 19.4 | |
| Unknown | 7.4 | 9.0 | 7.5 | |
| Body mass index at survey completion, kg/m2 | ||||
| ≤ 26 | 57.4 | 54.0 | 49.3 | 0.17 |
| 27–29 | 19.9 | 17.9 | 16.4 | |
| ≥ 30 | 21.6 | 25.9 | 34.3 | |
| Data missing | 1.1 | 2.2 | 0 | |
| Charlson-Deyo comorbidity index | ||||
| 0 | 51.7 | 63.6 | 59.7 | 0.07 |
| 1 | 19.3 | 20.4 | 11.9 | |
| ≥ 2 | 29.0 | 16.1 | 28.4 | |
| Year of first rectal cancer-directed surgery | ||||
| 1989 or earlier | 12.5 | 7.7 | 4.5 | 0.82 |
| 1990–1994 | 14.8 | 15.1 | 9.0 | |
| 1995–1999 | 21.0 | 27.5 | 23.9 | |
| 2000–2001 | 10.2 | 15.7 | 14.9 | |
| 2002–2009 | 30.7 | 30.9 | 44.8 | |
| Data missing | 10.8 | 3.1 | 3.0 | |
| Tumor stage | ||||
| Localized | 44.9 | 51.5 | 50.8 | 0.06 |
| Regional | 48.9 | 45.4 | 46.3 | |
| Metastatic | 1.1 | 0.6 | 1.5 | |
| Unknown | 5.1 | 2.5 | 1.5 | |
| Tumor distance to anal verge, cm | ||||
| 0–4 | 31.8 | 2.8 | 6.0 | < 0.0001 |
| 5–9 | 34.1 | 18.5 | 47.8 | |
| 10–14 | 8.5 | 36.1 | 22.4 | |
| 15–19 | 3.4 | 20.4 | 6.0 | |
| ≥ 20 | 0.6 | 6.5 | 1.5 | |
| Data missing | 21.6 | 15.7 | 16.4 | |
| Other clinical characteristics | ||||
| Chemotherapy | 56.8 | 45.7 | 53.7 | 0.78 |
| History of radiation therapy | 47.2 | 31.5 | 49.3 | 0.85 |
Data are percentages except for mean age.
RESULTS
An initial search of the electronic medical record followed by further review identified 1063 potentially eligible patients, of whom we reached 1002. Of the 1002, a total of 913 were eligible, but 336 declined, resulting in participation by 577 individuals (183 ostomy recipients and 394 patients with sphincter-sparing surgery). The participation rate was 60.5%. Information needed for the present analysis was complete for 567 participants (Figure 2).
Figure 2.
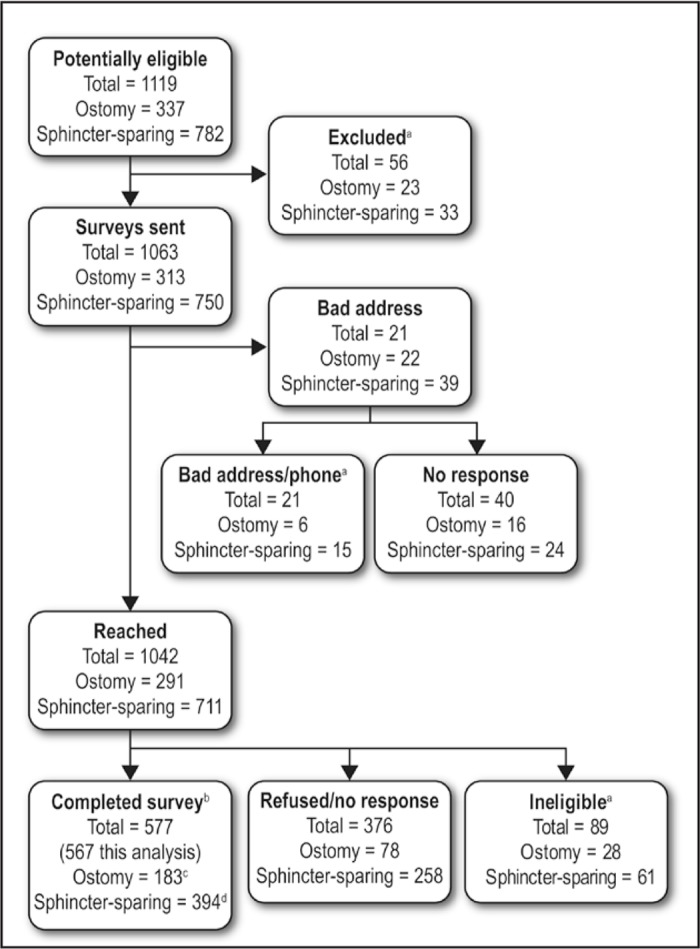
Flow diagram of participant recruitment.
aIneligible (dead, not rectal cancer, etc) after initial search, after wrong address and phone number, or after return of survey with patient reporting no rectal cancer or no intraabdominal surgery.
bResponse rates (completed/eligible): 66.8% ostomy; 58.6% sphincter-sparing; 60.5% total.
cSeven patients of the 182 with ostomy were excluded because they reported not regularly wearing ostomy bag.
dThree patients of the 394 with sphincter-sparing surgery were excluded because information they provide about the type of surgery was ambiguous.
Differences among rectal cancer survivors with ostomy, sphincter-sparing surgery, or temporary ostomy were most pronounced in relation to age at survey, education, Charlson-Deyo comorbidity index, and tumor distance to the anal verge (Table 1).
Associations with Participation in Activities
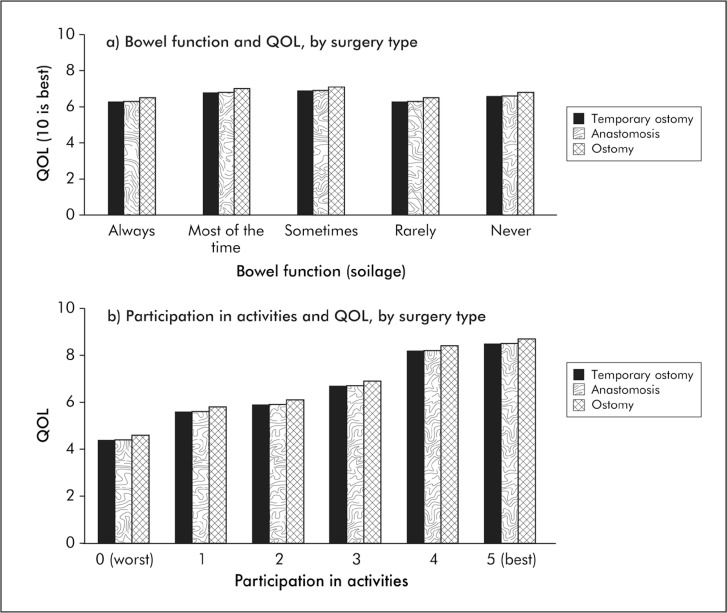
After multivariable adjustment, participation in activities was associated with surgery type (p < 0.0001; Table 2). The adjusted mean level of participation on a scale of 0 to 5 was 2.0 for ostomy, 2.9 for sphincter-sparing surgery, and 2.9 for temporary ostomy.
Table 2.
Adjusted associations with participation in activities and overall quality of life (N = 567)
| Factors | Association with participation in activities (0-worst to 5-best) | Association with overall quality of life (0-worst to 10-best) | ||
|---|---|---|---|---|
| Adjusted valuea | p value | Adjusted valuea | p value | |
| Operation | ||||
| Sphincter-sparing surgery (n = 324) | 2.9 | < 0.0001 | 6.5 | 0.79 |
| Ostomy (n = 176) | 2.0 | 6.7 | ||
| Temporary ostomy (n = 67) | 2.9 | 6.5 | ||
| Soilage | ||||
| Always (n = 18) | 1.5 | < 0.0001 | 6.3 | 0.07 |
| Most of the time (n = 61) | 2.0 | 6.8 | ||
| Sometimes (n = 114) | 2.7 | 6.9 | ||
| Rarely (n = 186) | 3.2 | 6.3 | ||
| Never (n = 179) | 3.6 | 6.6 | ||
| Participation in activitiesb | ||||
| 0 (interferes a great deal) | 4.4 | < 0.0001 | ||
| 1 | 5.6 | |||
| 2 | 5.9 | |||
| 3 | 6.7 | |||
| 4 | 8.2 | |||
| 5 (interferes not at all) | 8.5 | |||
| Sex | ||||
| Men | 2.4 | 0.01 | 6.6 | 0.94 |
| Women | 2.8 | 6.6 | ||
| Mean age at survey, years | ||||
| ≤ 64 | 2.4 | 0.48 | 6.7 | < 0.0001 |
| 65–74 | 2.6 | 7.2 | ||
| 75–84 | 2.7 | 6.3 | ||
| ≥ 85 | 2.7 | 6.1 | ||
| Household income, $US | ||||
| ≤ 15,000 | 2.2 | 0.007 | 6.1 | 0.15 |
| 15,000 to 30,000 | 2.3 | 6.6 | ||
| 30,001 to 50,000 | 2.9 | 6.6 | ||
| 50,001 to 75,000 | 2.7 | 6.8 | ||
| 75,001 to 100,000 | 2.7 | 6.2 | ||
| ≥ 100,000 | 3.1 | 7.0 | ||
| Not reported | 2.6 | 6.6 | ||
| Time since surgery, years | ||||
| 5–9 | 2.6 | 0.95 | 6.4 | 0.02 |
| 10–14 | 2.6 | 7.0 | ||
| ≥ 15 | 2.7 | 6.6 | ||
| Data missing | 2.6 | 6.2 | ||
| Body mass index at survey completion, kg/m2 | ||||
| ≤ 26 | 2.9 | 0.17 | 6.6 | 0.18 |
| 27–29 | 2.6 | 6.2 | ||
| ≥ 30 | 2.6 | 6.4 | ||
| Data missing | 2.4 | 7.0 | ||
| Charlson-Deyo comorbidity index | ||||
| 0 | 2.5 | 0.05 | 6.8 | 0.17 |
| 1 | 2.5 | 6.5 | ||
| ≥ 2 | 2.9 | 6.4 | ||
| History of radiation therapy | ||||
| No | 2.8 | 0.002 | 6.7 | 0.01 |
| Yes | 2.4 | 7.2 | ||
| Data missing | 2.8 | 5.7 | ||
Values were adjusted for potential confounders.
Data in this category for the “Association with participation in activities” columns are the dependent variables for this table.
Among the patients with sphincter-sparing surgery, participation in activities was associated with soilage (p < 0.0001; 2.1 and 4.1 in those with the most vs least soilage, respectively) and radiation therapy (p = 0.0006; 2.6 and 3.2 in those with and without radiation therapy, respectively). These and other covariates included in our model explained 34% of the variance in participation among patients with sphincter-sparing surgery.
For patients with an ostomy, participation in activities was associated with income (p = 0.01), comorbidity (p = 0.06), and soilage (p = 0.06). The adjusted participation level was 0.7 and 3.0 in those with the worst and best bowel function, respectively. Income, comorbidity, soil-age, and the other covariates included in our model explained only 15% of the variance in reported participation among patients with an ostomy.
Associations with Overall Quality of Life
After multivariable adjustment, overall QOL was associated with age (p < 0.0001) and participation in activities (p < 0.0001; Table 2). The adjusted mean QOL (scale = 0–10) ranged from 4.4 for those whose operation interfered with their participation “a great deal” to 8.5 for those whose operation “did not at all” interfere with participation, representing a 4.1-point difference on an 11-point scale (Figure 3). In adjusted analyses, overall QOL was not associated with operation type (p = 0.79) or soilage (p = 0.07). The R2 in QOL explained in the complete model was 37.8%. The R2 explained by participation was 20.0%. We repeated the analysis among patients with data on distance to the anal verge (n = 467) and found that including tumor distance in the model did not change the parameter estimates in our model to an important degree.
Figure 3.
Quality of life (QOL) of long-term rectal cancer survivors is related to participation in activities (p < 0.0001), not bowel function (p = 0.07) or surgery type (p = 0.79).a
aPredicted values from multivariate model (Table 2).
Responses about Constraints on Valued Activities
Thirty-nine of 440 respondents who answered the open-ended question about the greatest challenge experienced after cancer surgery provided a response that was coded under the theme “interference with valued activities.” The types of reported barriers to participation were similar across surgery types. They included
barriers to returning to work (caused by bowel dysfunction, depression, fatigue, pain, and postoperative recovery problems)
social activities away from home, such as eating at a restaurant or traveling (caused by needing to control diet, access to bathrooms, or ostomy self-care routines)
unpredictability about being home-bound during bouts of severe constipation or diarrhea
sexual or intimate contact (because of pain, impotence, embarrassment, or partner’s reactions after surgery)
physical activities (because of neuropathy, weakness, and poor balance).
DISCUSSION
We assessed the relationship of participation in activities with QOL and took into consideration the type of rectal cancer surgery (sphincter-sparing, ostomy) and level of soilage. Our major findings were that surgery type and soilage were largely associated with participation in activities but were only indirectly associated with overall QOL, through their effect on participation in activities. The strength and consistency of the association of participation on overall QOL were large, with 20.0% of variance in overall QOL attributable to participation and 17.8% attributable to many other variables. Patients with ostomy reported greater interference with participation than did patients with sphincter-sparing surgery, but after accounting for participation, we observed no differences in overall QOL. Thus, our results demonstrate that patients with ostomy and those with sphincter-sparing surgery have similar QOL after activity limitations are taken into account.10
QOL frameworks seek to elucidate patient-centered outcomes in a manner that supports measurement, prediction, decision support, and intervention. These frameworks may not capture what matters most to patients, particularly with respect to drivers of impairment and disability. The QOL model used most widely in the US1 is useful for health outcomes research because it denotes causal pathways of influence on QOL. This model, however, emphasizes symptoms and physical functioning while deemphasizing participation in activities.
Our study suggests that the ability to participate in life activities, as opposed to bowel function or surgery type, has a strong association with QOL among rectal cancer survivors and should be evaluated as a causal factor in reduced QOL. The consistency and strength of the relationship were striking. Although few reports have addressed the relationship of participation and QOL among cancer survivors, activity limitation has been shown to be strongly associated with QOL among patients with breast cancer and lymph-edema16,17 and patients with soft-tissue sarcoma.18 Moreover, cancer survivors experience more disability than do their healthy peers,19 and physical disability is a primary driver of emotional distress among cancer survivors.20
The widespread adoption of the ICF model21 has brought attention to activity and participation as drivers of patients’ well-being. For cancer rehabilitation, the ICF model productively moves inquiry and intervention away from the cause of impairments (difficult to change) to the impact of impairments (amenable to intervention).22 Interventions targeting physical function and participation have been developed for survivors of breast cancer, but participation has not been sufficiently addressed as a component of cancer survivorship care.23
Because impairment limits participation in complex ways, factoring participation into treatment decisions can be complicated. Although sphincter-sparing reconstructions aim to preserve bowel function, 52% of recipients of sphincter-sparing surgery in our study reported soilage always or most of the time. In contrast, although less than 5% of ostomy recipients reported soilage always or most of the time, 28% nonetheless restricted participation in activities because of their surgery, with issues such as body image and embarrassment perhaps playing a role.15,24
This study’s strengths include the community-based sample, confirmation of surgical and medical characteristics through self-report, electronic data and chart review, and systematic collection of patient-reported outcomes. The key limitation of our study was a lack of detailed information on activity participation, although patients specifically reported changes in various activities because of their surgery or ostomy. Although our cross-sectional design has some limitations, it gives us valuable information for subsequent prospective research. Future studies should also explore a wider range of activities, and our findings should be confirmed among other populations of cancer survivors.
CONCLUSION
Patients with rectal cancer should be made aware during preoperative discussions that, to a large degree, surgery type and mode of defecation affect overall QOL through their effects on participation in activities. Our findings also suggest new avenues for surveillance and supportive care interventions related to maintenance of participation. Multidimensional cancer survivor rehabilitation programs have shown benefit and cost-effectiveness for survivors of breast cancer.6 Our findings suggest the need to build the evidence for such interventions in other cancer survivor populations as well. Given the tremendous influence of participation in activities on rectal cancer survivors’ QOL, interventions to help patients maintain their activities and adopt new, fulfilling activities should be developed. In addition, routine assessment of participation in activities should become part of patients’ follow-up care.
Acknowledgments
This research was performed by the University of Arizona/Kaiser Permanente Collaborative Research Group and was made possible by Grant Number R01 CA106912, HRQOL in Colorectal Cancer Survivors with Stomas, from the National Cancer Institute, National Institutes of Health in collaboration with resources and the use of facilities provided at the Southern Arizona Veterans Affairs Health Care System, Tucson, AZ, and Kaiser Permanente. Dr Herrinton received support from Centocor (January 2008 to September 2011), Procter & Gamble Co (August 2006 to April 2012), Cincinnati, OH; Genentech (August 2008 to May 2012), South San Francisco, CA; and MedImmune (March 2013 to present), Gaithersburg, MD; she reports that this support does not pose a conflict of interest for this research.
Earlier versions of these findings were presented in part at the 7th Biennial Cancer Survivorship Research Conference, Atlanta, GA, June 18 to 20, 2014, and at the American Psychosocial Oncology Society 10th Annual Conference, February 14 to 16, 2013, Huntington Beach, CA.
We thank Stephen Fortmann, MD, and Lynn DeBar, PhD, MPH, for their thoughtful comments on the manuscript and Kevin Lutz, MFA, for his editorial assistance.
Kathleen Louden, ELS, of Louden Health Communications provided editorial assistance.
Footnotes
Disclosure Statement
The author(s) have no conflicts of interest to disclose.
Cure
Hippocrates in his aphorisme, as Galen writeth sure, Saith, foure things are needful to every kinde of cure. The first, saith he, to God belongeth the chiefest part,
The second to the Surgeon, who doth apply the art. The third unto the medicine, that is dame Natures friend, The fourth unto the patient, with whom I heere will end. How then may a Surgeon appoint a time, a day or houre, when three parts of the cure are quite without his power.
— William Clowes the Elder, 1543–1604, English surgeon
References
- 1.Ferrans CE, Zerwic JJ, Wilbur JE, Larson JL. Conceptual model of health-related quality of life. J Nurs Scholarsh. 2005;37(4):336–42. doi: 10.1111/j.1547-5069.2005.00058.x. DOI: https://doi.org/10.1111/j.1547-5069.2005.00058.x. [DOI] [PubMed] [Google Scholar]
- 2.Sullivan KJ, Cen SY. Model of disablement and recovery: Knowledge translation in rehabilitation research and practice. Phys Ther. 2011 Dec;91(12):1892–904. doi: 10.2522/ptj.20110003. DOI: https://doi.org/10.2522/ptj.20110003. [DOI] [PubMed] [Google Scholar]
- 3.van der Mei SF, Dijkers MP, Heerkens YF. Participation as an outcome measure in psychosocial oncology: Content of cancer-specific health-related quality of life instruments. Qual Life Res. 2011 Dec;20(10):1617–27. doi: 10.1007/s11136-011-9900-0. DOI: https://doi.org/10.1007/s11136-011-9900-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.International classification of functioning, disability and health (ICF) [Internet] Geneva, Switzerland: World Health Organization; 2001. [cited 2013 Oct 1]. Available from: www.who.int/classifications/icf/en/. [Google Scholar]
- 5.Stubblefield MD, Hubbard G, Cheville A, Koch U, Schmitz KH, Dalton SO. Current perspectives and emerging issues on cancer rehabilitation. Cancer. 2013 Jun 1;119(Suppl 11):2170–8. doi: 10.1002/cncr.28059. DOI: https://doi.org/10.1002/cncr.28059. [DOI] [PubMed] [Google Scholar]
- 6.Mewes JC, Steuten LM, Ijzerman MJ, van Harten WH. Effectiveness of multidimensional cancer survivor rehabilitation and cost-effectiveness of cancer rehabilitation in general: A systematic review. Oncologist. 2012;17(12):1581–93. doi: 10.1634/theoncologist.2012-0151. DOI: https://doi.org/10.1634/theoncologist.2012-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cancer facts & figures 2013 [Internet] Atlanta, GA: American Cancer Society; 2013. [cited 2013 Oct 1]. Available from: www.cancer.org/Research/CancerFactsFigures/CancerFactsFigures/2013-cancer-facts-and-figures.pdf. [Google Scholar]
- 8.Mulsow J, Winter DC. Sphincter preservation for distal rectal cancer—a goal worth achieving at all costs? World J Gastroenterol. 2011 Feb 21;17(7):855–61. doi: 10.3748/wjg.v17.i7.855. DOI: https://doi.org/10.3748/wjg.v17.i7.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cornish JA, Tilney HS, Heriot AG, Lavery IC, Fazio VW, Tekkis PP. A meta-analysis of quality of life for abdominoperineal excision of rectum versus anterior resection for rectal cancer. Ann Surg Oncol. 2007 Jul;14(7):2056–68. doi: 10.1245/s10434-007-9402-z. DOI: https://doi.org/10.1245/s10434-007-9402-z. [DOI] [PubMed] [Google Scholar]
- 10.Pachler J, Wille-Jørgensen P. Quality of life after rectal resection for cancer, with or without permanent colostomy. Cochrane Database Syst Rev. 2012 Dec;12:12. doi: 10.1002/14651858.CD004323.pub4. CD004323. DOI: https://doi.org/10.1002/14651858.CD004323.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilson IB, Cleary PD. Linking clinical variables with health-related quality of life. A conceptual model of patient outcomes. JAMA. 1995 Jan 4;273(1):59–65. DOI: https://doi.org/10.1001/jama.1995.03520250075037. [PubMed] [Google Scholar]
- 12.Wendel CS, Grant M, Herrinton L, et al. Reliability and validity of a survey to measure bowel function and quality of life in long-term rectal cancer survivors. Qual Life Res. 2014 Dec;23(10):2831–40. doi: 10.1007/s11136-014-0724-6. DOI: https://doi.org/10.1007/s11136-014-0724-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mohler MJ, Coons SJ, Hornbrook MC, et al. The health-related quality of life in long-term colorectal cancer survivors study: Objectives, methods and patient sample. Curr Med Res Opin. 2008 Jul;24(7):2059–70. doi: 10.1185/03007990802118360. DOI: https://doi.org/10.1185/03007990802118360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Temple LK, Bacik J, Savatta SG, et al. The development of a validated instrument to evaluate bowel function after sphincter-preserving surgery for rectal cancer. Dis Colon Rectum. 2005 Jul;48(7):1353–65. doi: 10.1007/s10350-004-0942-z. DOI: https://doi.org/10.1007/s10350-004-0942-z. [DOI] [PubMed] [Google Scholar]
- 15.McMullen CK, Hornbrook MC, Grant M, et al. The greatest challenges reported by long-term colorectal cancer survivors with stomas. J Support Oncol. 2008 Apr;6(4):175–82. [PubMed] [Google Scholar]
- 16.Tsauo JY, Hung HC, Tsai HJ, Huang CS. Can ICF model for patients with breast-cancer-related lymphedema predict quality of life? Support Care Cancer. 2011 May;19(5):599–604. doi: 10.1007/s00520-010-0857-2. DOI: https://doi.org/10.1007/s00520-010-0857-2. [DOI] [PubMed] [Google Scholar]
- 17.Dawes DJ, Meterissian S, Goldberg M, Mayo NE. Impact of lymphoedema on arm function and health-related quality of life in women following breast cancer surgery. J Rehabil Med. 2008 Aug;40(8):651–8. doi: 10.2340/16501977-0232. DOI: https://doi.org/10.2340/16501977-0232. [DOI] [PubMed] [Google Scholar]
- 18.Schreiber D, Bell RS, Wunder JS, et al. Evaluating function and health related quality of life in patients treated for extremity soft tissue sarcoma. Qual Life Res. 2006 Nov;15(9):1439–46. doi: 10.1007/s11136-006-0001-4. DOI: https://doi.org/10.1007/s11136-006-0001-4. [DOI] [PubMed] [Google Scholar]
- 19.Hewitt M, Rowland JH, Yancik R. Cancer survivors in the United States: Age, health, and disability. J Gerontol A Biol Sci Med Sci. 2003 Jan;58(1):82–91. doi: 10.1093/gerona/58.1.m82. DOI: https://doi.org/10.1093/gerona/58.1.m82. [DOI] [PubMed] [Google Scholar]
- 20.Silver JK, Baima J, Mayer RS. Impairment-driven cancer rehabilitation: An essential component of quality care and survivorship. CA Cancer J Clin. 2013 Sep;63(5):295–317. doi: 10.3322/caac.21186. DOI: https://doi.org/10.3322/caac.21186. [DOI] [PubMed] [Google Scholar]
- 21.ICF checklist Version 21a, clinician form: For International Classification of Functioning, Disability and Health [Internet] Geneva, Switzerland: World Health Organization; 2003. Sep, [cited 2013 Oct 1]. Available from: www.who.int/classifications/icf/training/icfchecklist.pdf. [Google Scholar]
- 22.Gilchrist LS, Galantino ML, Wampler M, Marchese VG, Morris GS, Ness KK. A framework for assessment in oncology rehabilitation. Phys Ther. 2009 Mar;89(3):286–306. doi: 10.2522/ptj.20070309. DOI: https://doi.org/10.2522/ptj.20070309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Supplement: A prospective surveillance model for rehabilitation for women with breast cancer. Cancer. 2012 Apr 15;118(8 Suppl):2187–334. doi: 10.1002/cncr.27476. [DOI] [PubMed] [Google Scholar]
- 24.Krouse RS, Herrinton LJ, Grant M, et al. Health-related quality of life among long-term rectal cancer survivors with an ostomy: Manifestations by sex. J Clin Oncol. 2009 Oct 1;27(28):4664–70. doi: 10.1200/JCO.2008.20.9502. DOI: https://doi.org/10.1200/JCO.2008.20.9502. [DOI] [PMC free article] [PubMed] [Google Scholar]