Abstract
Fish skeletal muscle is often used to monitor mercury concentrations and is used by regulatory agencies to develop fish consumption advisories. However, the distribution of mercury species (MeHg+ and THg) in muscle tissue and other organs is not well understood in a number of fish species. Here we evaluate the spatial distribution of THg and MeHg+ in skeletal muscle and internal organs (heart, liver, and kidney) of 19 sculpin representing three species: Myoxocephalus scorpius (shorthorn sculpin n = 13), Myoxocephalus jaok (plain sculpin, n = 4), and Megalocottus platycephalus (belligerent sculpin, n = 2). Four subsamples of muscle were taken along the lateral aspect of each fish, from muscle A (cranial) to muscle D (caudal). Using Games–Howell post hoc procedure to compare mean concentrations of all tissues, muscle samples were significantly different from internal organs, although there was no difference between muscle-sampling locations. THg concentrations (ww) were higher in muscle (muscle A through D mean ± SD, 0.30 ± 0.19 mg/kg) than that in heart (0.06 ± 0.05 mg/kg), kidney (0.08 ± 0.06 mg/kg), and liver (0.09 ± 0.08 mg/kg). Percent MeHg+ decreased with age in both skeletal muscle and organs (p < 0.05). In contrast to some previous reports for other fish species, this study found significantly higher THg concentrations in muscle than in the liver. This study highlights the importance of using muscle samples when evaluating potential Hg exposure in risk assessments for piscivorous wildlife and human populations, and assumptions related to organ mercury concentrations should be examined with care.
Keywords: Fish, Mercury, Methylmercury, Sculpin, Muscle
Introduction
Mercury (Hg) is detectable in nearly all commercial and subsistence-harvested fish species. Monitoring of Hg concentrations is important for both human health risk assessment and ecological monitoring. There are a number of chemical species of Hg, but environmental Hg falls into two general groupings—inorganic mercury (Hg0, Hg2+, Hg+) and organic mercury, which consists of one or two methyl groups (MeHg+, Me2Hg). Inorganic Hg is poorly absorbed in the vertebrate gut and is excreted fairly rapidly in urine and feces following ingestion (Clarkson 1997). However, in humans and other predators that consume a fish-based diet, monomethylmercury (henceforth: methylmercury, or MeHg+) is of concern because it bioaccumulates and biomagnifies in aquatic ecosystems, is easily absorbed from the gut, and distributes to tissues such as muscle and liver (Wolfe et al. 1998). Transport throughout the body is facilitated by complexes formed with cysteine groups (MeHg–S–R) which are able to cross the blood–brain barrier and accumulate in brain tissue (Roos et al. 2010). Methylmercury is a well-known neurotoxicant, and it has been implicated in poor neurological developmental outcomes in children in a number of longitudinal studies (Oken et al. 2005; Debes et al. 2006).
Concern over health effects has led governmental agencies to set action levels and tolerances limiting the amount of MeHg+ that can be present in commercially available fish. These limits include 1 μg/g for retail fish in the USA, and certain predatory fish in Australia, Canada, and the European Union; and 0.5 μg/g for the majority of retail fish in Australia, Canada, and the European Union (Canadian Food Inspection Agency 1998; The Commission of the European Communities 2001; United Nations Environment Programme Chemicals 2002; US Food and Drug Administration 2007). Additionally, there are many government agencies that have recommended fish consumption limits for Hg. In Alaska, the presence of contaminants in fish has prompted specific recommendations for consumption limits for different fish species (Hamade 2014). These recommendations are of particular concern to people who rely on fish and marine mammals as part of a subsistence diet. As many parts of some fish are commonly consumed, understanding how THg and MeHg+ are distributed across different tissue types and in different species is important in assessing the risk of fish consumption and the development of consumption recommendations.
There have been numerous studies examining the species of Hg found in fish muscle, most of which support the conclusion that MeHg+ is the primary species (i.e.,>80 %) in muscle tissue (Jewett et al. 2003). Some studies have suggested that [THg] can vary according to spatial location of muscle sampled by as much as 36 % (mean = 0.35 mg/kg vs. mean = 0.23 mg/kg) and that this variation may be driven by lipid content (Balshaw et al. 2008). Since inorganic and organic species of Hg have different octanol-water partitioning dynamics, it is conceivable that differences in [THg] related to lipid content may also drive differences in percent methylmercury of THg (%MeHg+). Another explanation is that lipid composition of muscle tissue can affect available binding sites for Hg species on proteins. A decrease in protein concentration in muscle may reflect lower THg concentrations due to decreased binding site availability, such as cysteine residues.
In a number of vertebrate species (including mammals, birds, and fishes), [THg] are highest in the liver (Wagemann et al. 1998; Dietz et al. 2000; Moses et al. 2009). The liver and kidney tissues are important for metal sequestration, biotransformation, and/or elimination and are often evaluated for evidence of damage due to Hg (Sonne et al. 2007). Because liver is generally considered to have the highest concentration of Hg within an organism and because of the importance of liver (as well as kidney) in metal toxicokinetics, many Hg-monitoring studies focus exclusively on the liver and kidney (Riget et al. 2005; Mieiro et al. 2009). However, the %MeHg+ in liver appears to be more variable than in other tissues, and low %MeHg+ in livers of some fish species may be indicative of demethylation processes which are known to occur in livers of a number of species (Martoja and Berry 1980; Palmisano et al. 1995; Eagles-Smith et al. 2009).
Species of sculpin (superfamily Cottoidea) have been advocated as ecosystem sentinels for heavy metal exposure for a number of reasons: (1) their circumpolar distribution allows for comparisons across the Arctic region, (2) individual sculpins generally have high site fidelity, potentially reflecting point sources of contamination (Dorts et al. 2011; Sonne et al. 2014), and (3) sculpins comprise a dietary component of a number of piscivorous mammals including humans (Burger et al. 2007a), Steller sea lions (Womble and Sigler 2006), bearded seals (Dehn et al. 2006), and belugas (Seaman et al. 1982). In addition there is evidence that sculpin consumption has been increasing in bearded seals in the 2000s (Quakenbush et al. 2011).
The focus of this study is to characterize the distribution of Hg across different tissues (muscle, liver, kidney, and heart) and to determine whether Hg concentration differs by location within marine sculpin skeletal muscle. Specimens were collected opportunistically, and differences in Hg concentrations were examined between species. Because of the potential adverse effects of MeHg+ specifically, the percent of THg that was MeHg+ was also determined.
Materials and methods
Sculpin collection and species identification
Nineteen sculpin were collected in summer 2009 via hook and line sampling from subsistence fishers off the northern side of Nunivak Island, AK, near the harbor for the village of Mekoryuk (60°23′N; 167°48′W, see Fig. 1). Fish length was measured from the tip of the mandible to the tip of the tail, and fish were subsequently frozen whole until subsampling in the laboratory. Muscle samples were taken from four different locations (Fig. 2). Muscle sample A was taken from the anterior end of the body (just caudal to the gill openings), anterior and posterior of the pleural ribs (muscle B and C, respectively), and caudal peduncle (muscle D). Due to the morphology of these fish, varying amounts of skeletal muscle tissue above and below the lateral line were collected based on the size of the fish. Samples weighed on average 10 g (range 3–25 g each). After subsampling for muscle, the abdomen was opened and the entire liver, both kidneys, and the heart were collected. Skin and underlying connective tissue was removed from muscle samples with the aid of a scalpel. Samples were freeze-dried for 48–72 h in Whirl–Paks and homogenized by breaking up and mixing the freeze-dried material within a Whirl–Pak bag. Weight of each sample before and after freeze-drying was used to calculate the percent water in each tissue.
Fig. 1.
Location of the sampling site on Nunivak Island, AK. Sculpin were subsistence-harvested from the harbor in the village of Mekoryuk. Image of Nunivak Island is from Google Earth
Fig. 2.
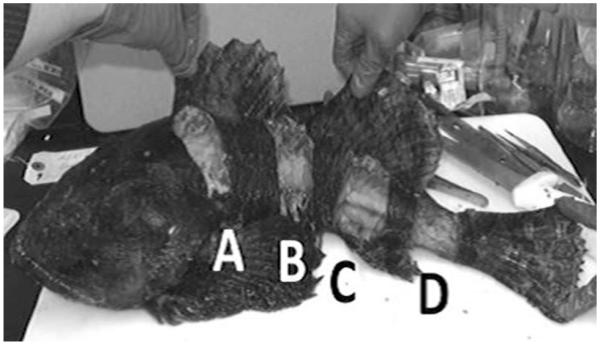
Locations of the longitudinal muscle-sampling sites. Site A is at the anterior end of the trunk and site D is on the caudal peduncle
The remainder of the carcass was examined at the University of Alaska Museum of the North for species determination. A dichotomous key for species of the family Cottidae (sculpin) was used for species assignment (Mecklenburg et al. 2002). Thirteen specimens were identified as shorthorn sculpin (Myoxocephalus scorpius) based on size, configuration of dorsal fins, and shape of dorsal scales. Four specimens were identified as plain sculpin (Myoxocephalus jaok) relying on the presence and distribution of head ornamentation. Finally, based on a projecting lower jaw and configuration of posterior head spines, two specimens were identified as belligerent sculpin (Megalocottus platycephalus). All but two fish, both shorthorn sculpin, were female.
Aging
Specimen ages were estimated from otolith growth rings. Transverse sections of otoliths were mounted on glass slides and annuli-counted using a light microscope (Campana and Neilson 1985; Ennis 1970). Annuli were counted separately by three different technicians. Ages determined by each technician were within 1 year of each other. The median of the three ages was used for further analysis. Age estimates were obtained for all fish except two plain sculpin.
Total mercury analysis
Total Hg (THg) concentrations were determined using a direct Hg analyzer (DMA-80, Milestone, Shelton, Conneticut). Instruments were calibrated using an 11-point square function calibration curve from 0.75 to 25 ng and a seven-point square function calibration curve from 30 to 450 ng. Samples (11–35 mg of dried material) were considered below detection limit (BDL) when the THg detected in the sample was below 1 ng for at least two of the repeated samples. All samples were analyzed in duplicate. Additional samples were analyzed when results of the duplicates differed by more than 10 %. The mean value for all replicates was used in the statistical analysis. At the beginning of each run, blanks, aqueous standards, and DORM3 (National Research Council Canada, Ottawa ON, Canada) were analyzed for quality assurance and control. Liver from same shorthorn sculpin were also analyzed with each run as a quality assurance method. Blanks were below 0.1 ng THg, standards were all within 10 % of expected values, and the total Hg concentration in the reference material was within the certified range (0.382 ± 0.060 μg/ g). Wet weight [THg] for each sample was calculated using the percent water content by taking [THg] dry weight and multiplying by the proportion dry mass to total mass.
Methyl mercury analysis
A rapid method for MeHg+ extraction was used (Nam and Basu 2011), and the extract was analyzed on the DMA-80. Because [MeHg+] after extraction was relatively low, the DMA-80 was calibrated using a six-point linear calibration curve from 0.25 to 7 ng. Samples were considered BDL when the Hg detected in the sample was below 0.25 ng for at least two of the repeated samples. In addition to the quality control samples described for the analysis of total Hg, certified reference materials (DORM-3 and DOLT-4) were extracted and analyzed with each run. Certified references were within an average of 11.6 ± 9.14 % of the mean certified value for DORM 3 (mean ± SD certified concentration 0.355 ± 0.056 μg/g; rapid extraction 0.314 ± 0.033 μg/g) and 13.7 ± 9.3 % of the mean certified value for DOLT-4 (mean ± SD certified concentration 1.33 ± 1.12 μg/g; rapid extraction 1.14 ± 0.12 lg/g). All tissues (except two hearts that had insufficient mass) were subsampled and extracted on at least three different days. Each run included at least one sample that had been previously extracted and analyzed. All extracts were analyzed in duplicate. A third measurement was made when results of the duplicates differed by more than 10 %. For quality assurance of the rapid technique, results were compared to those obtained with cold vapor atomic fluorescence detection (CVAFS; Bloom 1989) for muscle sample C based on previous methods in our laboratory (e.g., Moses et al. 2009). Three subsamples of muscle for all 13 shorthorn sculpin and three of four plain sculpin were extracted and analyzed using EPA Method 1630 with a Brooks Rand Model III CVAFS system. To compare measurements produced by the two methods, a paired Wilcoxon signed-rank test was performed. No significant difference was found in MeHg+ concentrations determined by the two methods (p = 0.40). The average percent difference between the rapid technique and CVAFS was 1.6 ± 6.6 %. Only data from the rapid extraction on the DMA-80 are presented here.
Statistical analysis
The plain (n = 4) and belligerent sculpin (n = 2) measurements were excluded from statistical analyses due to low samples sizes for these species. Percent MeHg+ was calculated for each individual and tissue as mean [MeHg+]/mean [THg]. Because [THg] and [MeHg+] were non-normally distributed, log transformations were applied where appropriate. Similar to Wagemann et al. (1997), we found that using mean values to calculate %MeHg+ resulted in an overestimation of this parameter. Therefore, average tissue %MeHg+ was determined by the slope of the regression comparing [MeHg+]–[THg] for each tissue. To assess differences in tissue [THg], [MeHg+], and %MeHg+, we utilized a repeated-measures analysis of variance (ANOVA) which accounts for variability within individual fish, since organs within a single fish cannot be considered independent with respect to Hg distribution. We followed this analysis with a comparison of mean values (post hoc Games–Howell). Age and length were highly correlated (r = 0.74), and thus both variables could not be included together in further analyses. Because age is a more direct measure of potential time of exposure to contaminants than length, age was used for further comparisons. In order to investigate the relationship between age and concentrations of Hg species, linear regressions were performed using data grouped according to the results of the Games–Howell. Statistical analyses were performed using R Programming Language (R Core Team 2012) including the ‘car’ package (Fox and Sanford 2011). Figures were produced with the ‘ggplot2’ package (Wickham 2009). A p value<0.05 (after multiple test corrections) was considered significant for all comparisons.
Results
Age and length
As expected, age and length are highly correlated (r = 0.74, p < 0.01) for shorthorn sculpin. Age ranged from 4 to 21 years and length ranged from 28 to 65 cm (Table 1). Belligerent and plain sculpin fell within the range of lengths determined for the shorthorn sculpin (42–50 cm for plain, 29–36 cm for belligerent).
Table 1.
Species, age, length, sex, and ratios of mean muscle [THg]–internal organs [THg]. Species were identified using dichotomous key (Mecklenburg et al. 2002)
| Species | Age (years) | Length (cm) | Sex | Ratio average [THg] in muscle to | ||
|---|---|---|---|---|---|---|
|
|
||||||
| Liver | Kidney | Heart | ||||
| Myoxocephalus scorpius (shorthorn sculpin) | 4 | 28 | F | 3.7 | 3.5 | 3.9 |
| 6 | 41 | F | 3.4 | 3.2 | 3.6 | |
| 6 | 37 | F | 6.0 | 4.8 | 7.5 | |
| 6 | 38 | M | 8.5 | 7.4 | 9.2 | |
| 7 | 36.5 | M | 14.0 | 5.5 | 7.2 | |
| 9 | 43 | F | 5.8 | 6.2 | 12.4 | |
| 9 | 55 | F | 4.1 | 5.0 | 6.5 | |
| 10 | 44 | F | 6.7 | 5.3 | 7.1 | |
| 13 | 58 | F | 4.0 | 4.4 | 6.6 | |
| 17 | 54 | F | 5.0 | 5.2 | 6.5 | |
| 19 | 65 | F | 3.1 | 3.3 | 5.1 | |
| 19 | 43 | F | 2.9 | 4.7 | 5.8 | |
| 21 | 54 | F | 4.2 | 3.2 | 4.8 | |
| Average | 5.5 ± 3.0 | 4.8 ± 1.3 | 6.6 ± 2.3 | |||
| Myoxocephalus jaok (plain sculpin) | 4 | 43 | F | 2.8 | 2.6 | 3.3 |
| 5 | 42 | F | 3.7 | 4.8 | 5.3 | |
| – | 46 | F | 3.4 | 3.0 | 5.1 | |
| – | 50 | F | 4.5 | 5.0 | 5.0 | |
| Average | 3.6 ± 0.7 | 3.9 ± 1.2 | 4.7 ± 0.9 | |||
| Megalocottus platycephalus (belligerent sculpin) | 4 | 29 | F | 9.5 | 9.2 | 9.5 |
| 8 | 36 | F | 10.0 | 7.8 | 5.5 | |
| Average | 9.7 ± 0.3 | 8.5 ± 1.0 | 7.5 ± 2.9 | |||
Ages were assigned by counting annuli of Table 1
Hg species in tissues
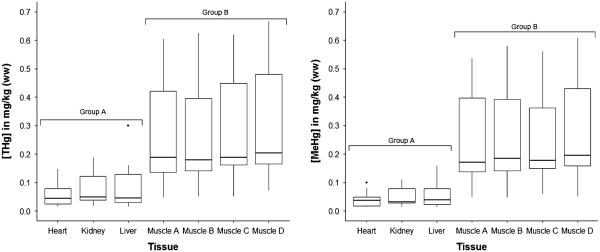
Mean concentrations of Hg species are presented in Table 2. Mean muscle [THg] and [MeHg+] were higher than internal organs. Ratios of muscle [THg] to internal organ [THg] ranged from 3:1 up to approximately 14:1 (Table 1). Within individual fish, the internal organs had lower %MeHg+ (calculated as [MeHg+]/[THg] × 100) than muscle samples (Table 1). The variation in %MeHg+ for internal organs was higher, as measured by percent relative standard deviation (%RSD, data not shown). Average tissue MeHg+ as determined by the slope of the regression comparing [MeHg+]–[THg] was 50–60 % for the internal organs and 81–94 % for the muscle-sampling sites (Table 2).
Table 2.
Concentrations (mg/kg wet weight) of total Hg ([THg]), methylmercury ([MeHg +]), and mean %MeHg ([THg]/[MeHg +] × 100) for shorthorn sculpin
| Shorthorn sculpin tissue | Mean concentration (mg/kg) ± SD | Mean percent MeHg+a | Mean percent water ± SD | |
|---|---|---|---|---|
|
|
||||
| THg | MeHg+a | |||
| Muscle A | 0.29 ± 0.19 | 0.26 ± 0.16 | 86 | 80 ± 1.4 |
| Muscle B | 0.29 ± 0.19 | 0.28 ± 0.18 | 94 | 80 ± 1.5 |
| Muscle C | 0.30 ± 0.20 | 0.26 ± 0.16 | 81 | 79 ± 1.1 |
| Muscle D | 0.33 ± 0.21 | 0.29 ± 0.18 | 88 | 80 ± 1.0 |
| Liver | 0.09 ± 0.08 | 0.06 ± 0.04 | 50 | 69 ± 7.0 |
| Heart | 0.06 ± 0.05 | 0.04 ± 0.03 | 60 | 84 ± 2.6 |
| Kidney | 0.08 ± 0.06 | 0.05 ± 0.03 | 55 | 82 ± 1.3 |
Mean percent MeHg calculated as the percent value of the slope of regression between [THg] and [MeHG]
Using a repeated-measures ANOVA, we found that tissue type was a significant variable in predicting con centrations of THg (F = 18.2, p < 0.01), MeHg+ (F = 15.9, p < 0.01), and percent MeHg+ (F = 24.5, p < 0.01). Using post hoc Games–Howell test, we found that individual muscle sections (A–D) were not statistically different from each other for either [THg] or [MeHg+]. Similarly, internal organs (heart, liver, and kidney) were not statistically different from each other. Muscle samples from the four sites (A–D) were all statistically different from each internal organ (p < 0.01), resulting in the grouping presented in Fig. 3. Muscle samples had significantly higher [THg] and [MeHg+] than internal organs.
Fig. 3.
Concentrations of total Hg ([THg]) and methylmercury ([MeHg+]) in different tissues of shorthorn sculpin. Groups were assigned using post hoc Games–Howell, where each tissue was compared against all others at an adjusted alpha level of 0.05. Individual points represent outliers
Hg and age
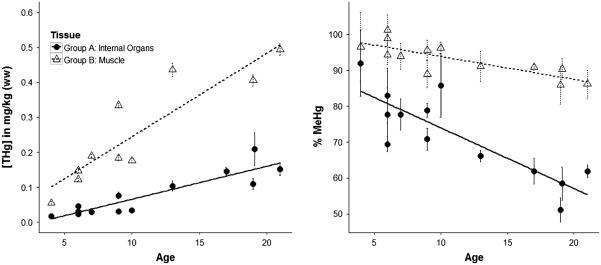
Based on the grouping suggested by Games–Howell, we performed a regression using averaged grouped data (muscle and internal organs). Both [THg] and [MeHg+] increased significantly with age (p < 0.01 for both). Using log-transformed data, the equation for the relationship between [THg] and age for muscle tissue is log[THg in muscle] = 0.12 × (age) − 3.51. Similarly, the equation for the relationship between [THg] and age for internal organs is log[THg in organs] = 1.13 × (age) − 4.36. For both [MeHg+] and [THg], the interaction between age and tissue was not significant.
Percent MeHg+ appeared to decrease with age for both muscle and internal organs (Fig. 4). The interaction term between age and tissue (muscle vs. internal organs) as a predictor of %MeHg+ was not significant.
Fig. 4.
Concentration of total Hg ([THg]) and percent methylmercury of THg (%MeHg+) in two groups of tissues of shorthorn sculpin. Groups were assigned using post hoc Games–Howell as in Fig. 3, where Group A represents the mean values for muscle A, B, C, and D, while Group B represents the mean value for heart, kidney, and liver. Error bars represent mean ± standard error
Discussion
The shorthorn sculpin in this study were generally older and larger than those reported from Norway (1–6 years and 6–30 cm; Luksenburg and Pedersen 2002) and included fish older and larger than the maximum age and size reported elsewhere (15 years and 50 cm; Ennis 1970). The belligerent sculpin in this study were also larger than had been previously published (Ball et al. 2007) for belligerent sculpin from the eastern Bering Sea (13.6 ± 0.62 cm, ages not reported). Plain sculpin were within the age and size range reported for the eastern Bering Sea (TenBrink and Hutchinson 2009).
The livers analyzed in this study had a broader range for [THg] (0.01–0.30 mg/kg) than the [THg] in livers from shorthorn sculpin (measuring >27 cm) reported in the literature (0.054–0.093 mg/kg in northwest Greenland and 0.015–0.061 mg/kg in central Greenland; Rigét et al. 2007). Exact lengths in that study were not reported, so it is possible that the broader range found in this study is due to the broad age and length range of the specimens we examined.
It is interesting to note the much higher concentrations of Hg in the muscle tissues compared with liver detected in this study. For many fish species, [THg] has been reported to be higher in liver than in muscle (Shultz and Crear 1976; Havelková et al. 2008; Mieiro et al. 2009; Guilherme et al. 2010). A study that evaluated Hg tissue distribution in 12 freshwater fish species in the Amazonian Basin found that only two species (both in the characid genus Leporinus) had muscle [Hg] greater than liver [Hg], and one additional species (the auchenipterid catfish Aegeneiosus inermis) was found to have muscle and liver concentrations of close to 1:1 ratio (Régine et al. 2006). The 1:1 ratio has been reported in several other studies, both in freshwater and marine fish (Jewett et al. 2003; Burger et al. 2007a). Although not included in statistical analyses, ratios of [THg] in muscle–liver for belligerent sculpin and plain sculpin are presented in Table 2. These ratios (especially for [THg] in muscle–liver) are higher than those reported in other sculpin species. For instance, great sculpin (Myoxocephalus polyacanthocephalus) caught off Adak, Alaska, were found to have ratios of 1:1 (muscle–liver) and 3:1 (muscle– kidney) (Burger et al. 2007b). A muscle–liver ratio of >1 for [Hg] has been reported in estuarine fish species and elasmobranchs (Mathieson et al. 1996; Coelho et al. 2010). In adult marine mammals including whales, pinnipeds, and polar bears (Hyvärinen et al. 1998; Wagemann et al. 1998; Dietz et al. 2000; Frodello et al. 2000; Moses et al. 2009), liver and/or kidney generally have the highest [THg]. Our study indicates that, at least in some fish, skeletal muscle may play an important role in Hg bioaccumulation within this specific tissue (i.e., not other muscle types such as heart) and contribute more significantly to body burden since muscle tissue is a relatively large compartment by mass. It has been previously shown (Giblin and Massaro 1973) that the rate of elimination of Hg from muscle is much slower than the rate of elimination from liver, kidney or heart in rainbow trout (Oncorhynchus mykiss) where most of the Hg distributed to the muscle was still present after 100 days. Additionally, muscle represents a higher percentage of the body weight (larger compartment) than liver or kidneys and is more likely to be preferentially consumed by humans. Even when the [THg] is lower in muscle than in liver, muscle may have a greater proportion of the body burden of Hg (Giblin and Massaro 1973; Mathieson et al. 1996; Brookens et al. 2008). Thus, if there is concern about the risk of Hg exposure through piscivory, it is critical to include muscle samples in any assessment, and this is particularly important in species where differential tissue-specific distribution of Hg has not been examined.
When tissues were compared using post hoc Games– Howell test (which has been done in other studies to compare tissue concentrations of Hg, for instance Brookens et al. 2008), muscle samples were not significantly different from each other (Fig. 3). This is in contrast to findings in other species, for instance bluefin tuna in Balshaw et al. (2008), where the authors found up to 63 % difference in [THg] between two muscle sections. Their study noted an inverse relationship with lipid content of the muscle tissue, and while we did not measure lipid content directly, the differences in %H2O of muscle tissue, as measured by weighing samples before and after freeze-drying, did not display large variation among sampling location (Table 1). In other fish species, percent H2O can be used as an effective proxy for percent lipid content in muscle since the two measures are highly correlated (Zaboukas et al. 2006). Thus the lipid content of muscle tissue and spatial distribution of [THg] are important considerations when taking muscle samples for comparison among individuals within a species or between species.
In order to investigate the associations of Hg species with age, we constructed a linear regression with tissues combined according to the results of the Games–Howell test (Fig. 4). Both Group A (internal organs) and Group B (muscle) had relationships between [THg] and age and [MeHg+] and age that were significantly different from 0 (p < 0.01). The interaction term between group and age was significant for both [THg] and [MeHg+], indicating that the relationships between Hg species and age were different between these groups of tissues. This is likely the result of tissue-specific dynamics of MeHg+ demethylation, transport, and storage.
In this study, we found that %MeHg+ was inversely associated with age in both internal organs and muscle. In the internal organs, the decrease in %MeHg+ with increasing [THg] likely represents demethylation of MeHg+ and storage as inorganic forms of Hg (Komsta-Szumska et al. 1983; Wagemann et al. 1998, 2000). The interaction term age– group was not a significant predictor of %MeHg+, indicating that %MeHg+ decreased similarly with age in both muscle (%MeHg+ = −0.58 × (Age) + 99.31) and internal organs (%MeHg+ = −1.13 × (Age) + 80.81). The lack of significance of the interaction term, while possibly reflecting a true similar relationship, is potentially driven by the large variation in %MeHg+ in internal organs, especially for young fish. There are several possible explanations for the large variation in %MeHg+ in young fish including the uncertainty of the age estimation process in general. The first is that our small sample size led to a poor distribution of ages, and the variation seen in young fish might represent the variation across the entire population. It has also been suggested that demethylation of MeHg+ occurs only when THg concentrations (mostly MeHg+) have reached a critical threshold (for waterfowl, reported dry weight thresholds were between 7.48 and 9.91 mg/kg dry weight, Eagles-Smith et al. 2009). While concentrations in liver in our study were not approaching the concentrations noted in Eagles-Smith et al. (2009), it is possible that a similar demethylation threshold exists in sculpin.
In conclusion, our study highlights the importance of assessing variability in Hg concentrations among different fish species, as some previously held assumptions for Hg accumulation do not appear to hold true for the species of sculpin we evaluated. If sculpin are to continue to be used as a species for biomonitoring, species-specific dynamics of THg and MeHg+ need to be considered. Since concentrations of THg and MeHg+ were significantly higher in muscle than in liver and since muscle is the tissue preferentially consumed by humans, this supports the importance of including muscle samples in addition to liver samples for biomonitoring rather than focusing exclusively on the liver. Mercury contamination of marine species is a topic of increasing concern for subsistence hunters and fish consumers, who often consume only select tissue types. It is important to have a thorough understanding of the dynamics of Hg in different tissues to support development of technically sound management decisions and risk assessments.
Acknowledgments
The research described was supported through the Center for Alaska Native Health Research by Award Number P20RR016430 from the National Center for Research Resources. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health. The authors would like to thank Randy Brown of the National Fish and Wildlife Service and Gabrielle Johnson for assistance with mounting otoliths and aging the fish; Rachel Witter and Joel Pierson for their work in developing the rapid methyl mercury technique in our laboratory; Andrea Price Repetto and Bonita Dainowski for their help with the CVAFS MeHg+ determination; Sara Moses for assistance with editing the manuscript; the residents of Mekoryuk, Alaska for their generosity in providing time, skill, and fish; and the YukonKuskokwim Health Consortium for their support for our research.
References
- Ball J, Esler D, Schmutz J. Proximate composition, energetic value, and relative abundance of prey fish from the inshore eastern Bering Sea: implications for piscivorous predators. Polar Biol. 2007;30:699–708. doi:10.1007/s00300-006-0227-1. [Google Scholar]
- Balshaw S, Edwards JW, Ross KE, Daughtry BJ. Mercury distribution in the muscular tissue of farmed southern bluefin tuna (Thunnus maccoyii) is inversely related to the lipid content of tissues. Food Chem. 2008;111:616–621. doi:10.1016/j.foodchem.2008.04.041. [Google Scholar]
- Bloom N. Determination of picogram levels of methylmercury by aqueous phase ethylation, followed by cryogenic gas chromatography with cold vapour atomic fluorescence. Can J Fish Aquat Sci. 1989;46:1131–1140. [Google Scholar]
- Brookens TJ, O’Hara TM, Taylor RJ, et al. Total mercury body burden in Pacific harbor seal, Phoca vitulina richardii, pups from central California. Mar Pollut Bull. 2008;56:27–41. doi: 10.1016/j.marpolbul.2007.08.010. doi:10.1016/j.marpolbul.2007.08.010. [DOI] [PubMed] [Google Scholar]
- Burger J, Gochfeld M, Jeitner C, et al. Mercury levels and potential risk from subsistence foods from the Aleutians. Sci Total Environ. 2007a;384:93–105. doi: 10.1016/j.scitotenv.2007.05.004. doi:10.1016/j.scitotenv.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Burger J, Gochfeld M, Jeitner C, et al. Metal levels in flathead sole (Hippoglossoides elassodon) and great sculpin (Myoxocephalus polyacanthocephalus) from Adak Island, Alaska: potential risk to predators and fishermen. Environ Res. 2007b;103:62–69. doi: 10.1016/j.envres.2006.02.005. doi:10.1016/j.envres.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Campana SE, Neilson JD. Microstructure of fish otoliths. Can J Fish Aquat Sci. 1985;42:1014–1032. [Google Scholar]
- Canadian Food Inspection Agency Appendix 3: Canadian guidelines for chemical contaminants and toxins in fish and fish products. Fish products standards and methods manual. 1998 [Google Scholar]
- Clarkson TW. The toxicology of mercury. Crit Rev Clin Lab Sci. 1997;34:369–403. doi: 10.3109/10408369708998098. [DOI] [PubMed] [Google Scholar]
- Coelho JP, Santos H, Reis AT, et al. Mercury bioaccumulation in the spotted dogfish (Scyliorhinus canicula) from the Atlantic Ocean. Mar Pollut Bull. 2010;60:1372–1375. doi: 10.1016/j.marpolbul.2010.05.008. doi:10.1016/j.marpolbul.2010.05.008. [DOI] [PubMed] [Google Scholar]
- Debes F, Budtz-Jørgensen E, Weihe P, et al. Impact of prenatal methylmercury exposure on neurobehavioral function at age 14 years. Neurotoxicol Teratol. 2006;28:363–375. doi: 10.1016/j.ntt.2006.02.004. doi:10.1016/j.ntt.2006.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehn L-A, Sheffield GG, Follmann EH, et al. Feeding ecology of phocid seals and some walrus in the Alaskan and Canadian Arctic as determined by stomach contents and stable isotope analysis. Polar Biol. 2006;30:167–181. doi:10.1007/s00300-006-0171-0. [Google Scholar]
- Dietz R, Riget F, Born EW. Geographical differences of zinc, cadmium, mercury and selenium in polar bears (Ursus maritimus) from Greenland. Sci Total Environ. 2000;245:25–47. doi: 10.1016/s0048-9697(99)00431-3. [DOI] [PubMed] [Google Scholar]
- Dorts J, Kestemont P, Dieu M. Proteomic response to sublethal cadmium exposure in a sentinel fish species, Cottus gobio. J Proteome Res. 2011;10:470–478. doi: 10.1021/pr100650z. [DOI] [PubMed] [Google Scholar]
- Eagles-Smith CA, Ackerman JT, Yee J, Adelsbach TL. Mercury demethylation in waterbird livers: dose-response thresholds and differences among species. Environ Toxicol Chem. 2009;28:568–577. doi: 10.1897/08-245.1. doi:10.1897/08-245.1. [DOI] [PubMed] [Google Scholar]
- Ennis GP. Age, growth, and sexual maturity of the shorthorn sculpin, Myoxocephalus scorpius, in Newfoundland waters. J Fish Res Board Canada. 1970;27:2155–2158. doi:10.1139/f70-244. [Google Scholar]
- Fox J, Weisberg S. An {R} companion to applied regression. 2nd Sage; Thousand Oaks: 2011. [Google Scholar]
- Frodello JP, Roméo M, Viale D. Distribution of mercury in the organs and tissues of five toothed-whale species of the Mediterranean. Environ Pollut. 2000;108:447–452. doi: 10.1016/s0269-7491(99)00221-3. doi:10.1016/S0269-7491(99)00221-3. [DOI] [PubMed] [Google Scholar]
- Giblin FJ, Massaro EJ. Pharmacodynamics of methyl mercury in the rainbow trout (Salmo gairdneri): tissue uptake, distribution and excretion. Toxicol Appl Pharmacol. 1973;24:81–91. doi: 10.1016/0041-008x(73)90183-x. doi:10.1016/0041-008X(73)90183-X. [DOI] [PubMed] [Google Scholar]
- Guilherme S, Pereira ME, Santos MA, Pacheco M. Mercury Distribution in Key Tissues of Caged Fish (Liza aurata) along an Environmental Mercury Contamination Gradient. Interdiscip Stud Environ Chem. 2010;3:165–173. doi: 10.1016/j.ecoenv.2007.08.016. [DOI] [PubMed] [Google Scholar]
- Hamade AK. Fish consumption advice for Alaskans: a risk management strategy to optimize the public’s health. Alaska Scientific Advisory Committee for Fish Consumption. 2014 Available at http://www.epi.alaska.gov/eh/fish/. Accessed 13 Feb 2015.
- Havelková M, Dusšek L, Némethová D, et al. Comparison of mercury distribution between liver and muscle—a biomonitoring of fish from lightly and heavily contaminated localities. Sensors. 2008;8:4095–4109. doi: 10.3390/s8074095. doi:10.3390/s8074095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyvärinen H, Sipilä T, Kunnasranta M, Koskela JT. Mercury pollution and the Saimaa ringed seal (phoca hispida saimensis) Mar Pollut Bull. 1998;36:76–81. doi:10.1016/S0025-326X(98)90037-6. [Google Scholar]
- Jewett SC, Zhang X, Naidu AS, et al. Comparison of mercury and methylmercury in northern pike and Arctic grayling from western Alaska rivers. Chemosphere. 2003;50:383–392. doi: 10.1016/s0045-6535(02)00421-6. [DOI] [PubMed] [Google Scholar]
- Komsta-Szumska E, Reuhl KR, Miller DR. The effect of methylmercury on the distribution and excretion of selenium by the guinea pig. Arch Toxicol. 1983;54:303–310. doi: 10.1007/BF01234483. [DOI] [PubMed] [Google Scholar]
- Luksenburg JA, Pedersen T. Sexual and geographical variation in life history parameters of the shorthorn sculpin. J Fish Biol. 2002;61:1453–1464. doi:10.1111/j.1095-8649.2002.tb02489.x. [Google Scholar]
- Martoja R, Berry J-P. Identification of tiemannite as a probable product of demethylation of mercury by selenium in cetaceans. A complement to the scheme of the biological cycle of mercury. Vie Milieu. 1980;30:7–10. [Google Scholar]
- Mathieson S, George S, McLusky D. Temporal variation of total mercury concentrations and burdens in the liver of eelpout Zoarces viviparus from the Forth Estuary, Scotland: implications for mercury biomonitoring. Mar Ecol Prog Ser. 1996;138:41–49. [Google Scholar]
- Mecklenburg CW, Mecklenburg TA, Thorsteinson LK. Fishes of Alaska. American Fisheries Society; Bethesda: 2002. [Google Scholar]
- Mieiro CL, Pacheco M, Pereira ME, Duarte AC. Mercury distribution in key tissues of fish (Liza aurata) inhabiting a contaminated estuary-implications for human and ecosystem health risk assessment. J Environ Monit. 2009;11:1004–1012. doi: 10.1039/b821253h. doi:10.1039/b821253h. [DOI] [PubMed] [Google Scholar]
- Moses SK, Whiting AV, Bratton GR, et al. Inorganic nutrients and contaminants in subsistence species of Alaska: linking wildlife and human health. Int J Circumpolar Health. 2009;68:53–74. doi: 10.3402/ijch.v68i1.18294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam D-H, Basu N. Rapid methods to detect organic mercury and total selenium in biological samples. Chem Cent J. 2011;5:1–5. doi: 10.1186/1752-153X-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oken E, Wright RO, Kleinman KP, et al. Maternal fish consumption, hair mercury, and infant cognition in a US cohort. Environ Health Perspect. 2005;113:1376–1380. doi: 10.1289/ehp.8041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmisano F, Cardellicchio N, Zambonin PG. Speciation of mercury in dolphin liver: a two-stage mechanism for the demethylation accumulation process and role of selenium. Mar Environ Res. 1995;40:109–121. [Google Scholar]
- Quakenbush L, Citta J, Crawford J. Biology of the ringed seal (Phoca hispida) in Alaska, 1960–2010. Final Report to the National Marine Fisheries Service. 2011 [Google Scholar]
- Régine M-B, Gilles D, Yannick D, Alain B. Mercury distribution in fish organs and food regimes: significant relationships from twelve species collected in French Guiana (Amazonian basin) Sci Total Environ. 2006;368:262–270. doi: 10.1016/j.scitotenv.2005.09.077. [DOI] [PubMed] [Google Scholar]
- Riget F, Muir D, Kwan M, et al. Circumpolar pattern of mercury and cadmium in ringed seals. Sci Total Environ. 2005;351–352:312–322. doi: 10.1016/j.scitotenv.2004.05.032. doi:10.1016/j.scitotenv.2004.05.032. [DOI] [PubMed] [Google Scholar]
- Rigét F, Dietz R, Born EW, et al. Temporal trends of mercury in marine biota of west and northwest Greenland. Mar Pollut Bull. 2007;54:72–80. doi: 10.1016/j.marpolbul.2006.08.046. doi:10.1016/j.marpolbul.2006.08.046. [DOI] [PubMed] [Google Scholar]
- Roos DH, Puntel RL, Lugokenski TH, et al. Complex methylmercury-cysteine alters mercury accumulation in different tissues of mice. Basic Clin Pharmacol Toxicol. 2010;107:789–792. doi: 10.1111/j.1742-7843.2010.00577.x. doi:10.1111/j.1742-7843.2010.00577.x. [DOI] [PubMed] [Google Scholar]
- Seaman GA, Lowry LF, Frost KJ. Foods of belukha whales (Delphinapterus leucas) in Western Alaska. Cetology. 1982;44:1–19. [Google Scholar]
- Shultz C, Crear D. The distribution of total and organic mercury in seven tissues of the Pacific blue marlin, Makaira nigricans. Pacific Sci. 1976;30:101–107. [Google Scholar]
- Sonne C, Dietz R, Leifsson PS, et al. Are liver and renal lesions in East Greenland polar bears (Ursus maritimus) associated with high mercury levels? Environ Health. 2007;6:11. doi: 10.1186/1476-069X-6-11. doi:10.1186/1476-069X-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonne C, Bach L, Søndergaard J, et al. Evaluation of the use of common sculpin (Myoxocephalus scorpius) organ histology as bioindicator for element exposure in the fjord of the mining area Maarmorilik, West Greenland. Environ Res. 2014;133:304–311. doi: 10.1016/j.envres.2014.05.031. doi:10.1016/j.envres.2014.05.031. [DOI] [PubMed] [Google Scholar]
- TenBrink TT, Hutchinson CE. Age, growth, and mortality of the plain sculpin, Myoxocephalus jaok, in the eastern Bering Sea. North Pacific Research Board Project Final Report. 2009 [Google Scholar]
- The Commission of the European Communities EC No 466/20061Setting Maximum levels for cetain contaminants in foodstuffs. 2001 Section 3. [Google Scholar]
- United Nations Environment Programme Chemicals . Chapter 4: Current mercury exposure and risk evaluations for humans. Global Mercury Assesment; Geneva: 2002. [Google Scholar]
- US food and drug administration CPG Sec 540.600 Fish, shellfish, crustaceans, and other aquatic animals-fresh, frozen or processed-methyl mercury. 2007 [Google Scholar]
- Wagemann R, Trebacz E, Hunt R, Boila G. Percent methylmercury and organic mercury in tissues of marine mammals and fish using different experimental and calculation methods. Environ Toxicol Chem. 1997;16:1859–1866. [Google Scholar]
- Wagemann R, Trebacz E, Boila G, Lockhart WL. Methylmercury and total mercury in tissues of arctic marine mammals. Sci Total Environ. 1998;218:19–31. doi: 10.1016/s0048-9697(98)00192-2. [DOI] [PubMed] [Google Scholar]
- Wagemann R, Trebacz E, Boila G, Lockhart WL. Mercury species in the liver of ringed seals. Sci Total Environ. 2000;261:21–32. doi: 10.1016/s0048-9697(00)00592-1. doi:10.1016/S0048-9697(00)00592-1. [DOI] [PubMed] [Google Scholar]
- Wickham H. ggplot2: elegant graphics for data analysis. Springer; New York: 2009. [Google Scholar]
- Wolfe MF, Schwarzbach S, Sulaiman RA. Effects of mercury on wildlife: a comprehensive review. Environ Toxicol Chem. 1998;17:146–160. [Google Scholar]
- Womble JN, Sigler MF. Seasonal availability of abundant, energy-rich prey influences the abundance and diet of a marine predator, the Steller sea lion Eumetopias jubatus. Mar Ecol Prog Ser. 2006;325:281–293. [Google Scholar]
- Zaboukas N, Miliou H, Megalofonou P, Moraitou-Apostolopoulou M. Biochemical composition of the Atlantic bonito Sarda sarda from the Aegean Sea (eastern Mediterranean Sea) in different stages of sexual maturity. J Fish Biol. 2006;69:347–362. doi:10.1111/j.1095-8649.2006.01090.x. [Google Scholar]