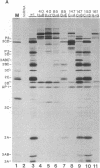
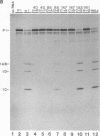
Abstract
The picornavirus 3C proteinases are substrate-specific thiol proteases that have been shown by secondary structure predictions and protein modeling studies to be similar to the trypsin-like serine proteases. We have examined several mutations of the 3C proteinase at putative active site and non-active site residues. The effect on 3C-mediated protein processing supports the model of serine protease similarity. In particular, we have shown that 3C can utilize a serine at position 147, which is predicted to supply the nucleophilic residue of the catalytic triad. We suggest that picornavirus 3C proteinases may represent a class of enzymes that have maintained the catalytic mechanism characteristic of a proposed enzyme ancestral to the highly divergent class of serine proteases.
Full text
PDF

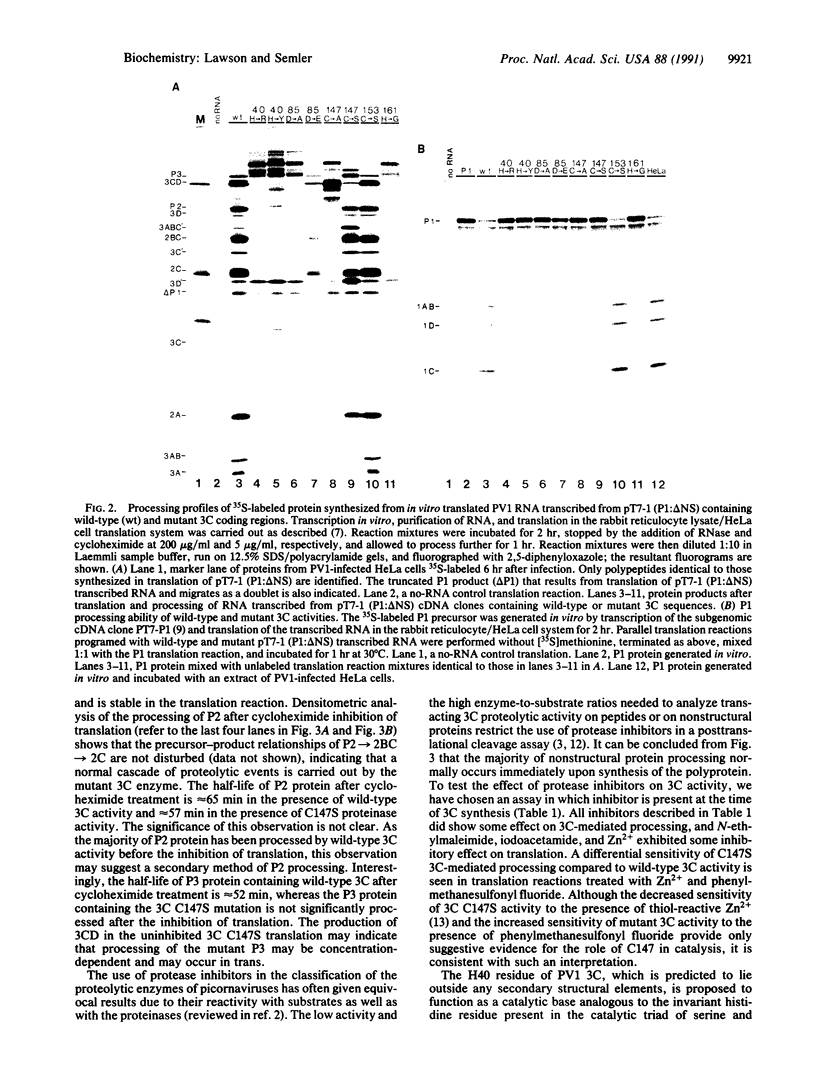

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Argos P., Kamer G., Nicklin M. J., Wimmer E. Similarity in gene organization and homology between proteins of animal picornaviruses and a plant comovirus suggest common ancestry of these virus families. Nucleic Acids Res. 1984 Sep 25;12(18):7251–7267. doi: 10.1093/nar/12.18.7251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazan J. F., Fletterick R. J. Viral cysteine proteases are homologous to the trypsin-like family of serine proteases: structural and functional implications. Proc Natl Acad Sci U S A. 1988 Nov;85(21):7872–7876. doi: 10.1073/pnas.85.21.7872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner S. The molecular evolution of genes and proteins: a tale of two serines. Nature. 1988 Aug 11;334(6182):528–530. doi: 10.1038/334528a0. [DOI] [PubMed] [Google Scholar]
- Carter P., Wells J. A. Dissecting the catalytic triad of a serine protease. Nature. 1988 Apr 7;332(6164):564–568. doi: 10.1038/332564a0. [DOI] [PubMed] [Google Scholar]
- Cheah K. C., Leong L. E., Porter A. G. Site-directed mutagenesis suggests close functional relationship between a human rhinovirus 3C cysteine protease and cellular trypsin-like serine proteases. J Biol Chem. 1990 May 5;265(13):7180–7187. [PubMed] [Google Scholar]
- Craik C. S., Roczniak S., Largman C., Rutter W. J. The catalytic role of the active site aspartic acid in serine proteases. Science. 1987 Aug 21;237(4817):909–913. doi: 10.1126/science.3303334. [DOI] [PubMed] [Google Scholar]
- Dewalt P. G., Lawson M. A., Colonno R. J., Semler B. L. Chimeric picornavirus polyproteins demonstrate a common 3C proteinase substrate specificity. J Virol. 1989 Aug;63(8):3444–3452. doi: 10.1128/jvi.63.8.3444-3452.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty W. G., Parks T. D., Cary S. M., Bazan J. F., Fletterick R. J. Characterization of the catalytic residues of the tobacco etch virus 49-kDa proteinase. Virology. 1989 Sep;172(1):302–310. doi: 10.1016/0042-6822(89)90132-3. [DOI] [PubMed] [Google Scholar]
- Gorbalenya A. E., Donchenko A. P., Blinov V. M., Koonin E. V. Cysteine proteases of positive strand RNA viruses and chymotrypsin-like serine proteases. A distinct protein superfamily with a common structural fold. FEBS Lett. 1989 Jan 30;243(2):103–114. doi: 10.1016/0014-5793(89)80109-7. [DOI] [PubMed] [Google Scholar]
- Hahn C. S., Strauss J. H. Site-directed mutagenesis of the proposed catalytic amino acids of the Sindbis virus capsid protein autoprotease. J Virol. 1990 Jun;64(6):3069–3073. doi: 10.1128/jvi.64.6.3069-3073.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higaki J. N., Gibson B. W., Craik C. S. Evolution of catalysis in the serine proteases. Cold Spring Harb Symp Quant Biol. 1987;52:615–621. doi: 10.1101/sqb.1987.052.01.070. [DOI] [PubMed] [Google Scholar]
- Hämmerle T., Hellen C. U., Wimmer E. Site-directed mutagenesis of the putative catalytic triad of poliovirus 3C proteinase. J Biol Chem. 1991 Mar 25;266(9):5412–5416. [PubMed] [Google Scholar]
- Ivanoff L. A., Towatari T., Ray J., Korant B. D., Petteway S. R., Jr Expression and site-specific mutagenesis of the poliovirus 3C protease in Escherichia coli. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5392–5396. doi: 10.1073/pnas.83.15.5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamphuis I. G., Drenth J., Baker E. N. Thiol proteases. Comparative studies based on the high-resolution structures of papain and actinidin, and on amino acid sequence information for cathepsins B and H, and stem bromelain. J Mol Biol. 1985 Mar 20;182(2):317–329. doi: 10.1016/0022-2836(85)90348-1. [DOI] [PubMed] [Google Scholar]
- Kean K. M., Teterina N. L., Marc D., Girard M. Analysis of putative active site residues of the poliovirus 3C protease. Virology. 1991 Apr;181(2):609–619. doi: 10.1016/0042-6822(91)90894-h. [DOI] [PubMed] [Google Scholar]
- Kräusslich H. G., Wimmer E. Viral proteinases. Annu Rev Biochem. 1988;57:701–754. doi: 10.1146/annurev.bi.57.070188.003413. [DOI] [PubMed] [Google Scholar]
- Lawson M. A., Dasmahapatra B., Semler B. L. Species-specific substrate interaction of picornavirus 3C proteinase suballelic exchange mutants. J Biol Chem. 1990 Sep 15;265(26):15920–15931. [PubMed] [Google Scholar]
- Nicklin M. J., Harris K. S., Pallai P. V., Wimmer E. Poliovirus proteinase 3C: large-scale expression, purification, and specific cleavage activity on natural and synthetic substrates in vitro. J Virol. 1988 Dec;62(12):4586–4593. doi: 10.1128/jvi.62.12.4586-4593.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprang S., Standing T., Fletterick R. J., Stroud R. M., Finer-Moore J., Xuong N. H., Hamlin R., Rutter W. J., Craik C. S. The three-dimensional structure of Asn102 mutant of trypsin: role of Asp102 in serine protease catalysis. Science. 1987 Aug 21;237(4817):905–909. doi: 10.1126/science.3112942. [DOI] [PubMed] [Google Scholar]
- Ypma-Wong M. F., Dewalt P. G., Johnson V. H., Lamb J. G., Semler B. L. Protein 3CD is the major poliovirus proteinase responsible for cleavage of the P1 capsid precursor. Virology. 1988 Sep;166(1):265–270. doi: 10.1016/0042-6822(88)90172-9. [DOI] [PubMed] [Google Scholar]
- Ypma-Wong M. F., Filman D. J., Hogle J. M., Semler B. L. Structural domains of the poliovirus polyprotein are major determinants for proteolytic cleavage at Gln-Gly pairs. J Biol Chem. 1988 Nov 25;263(33):17846–17856. [PubMed] [Google Scholar]
- Ypma-Wong M. F., Semler B. L. In vitro molecular genetics as a tool for determining the differential cleavage specificities of the poliovirus 3C proteinase. Nucleic Acids Res. 1987 Mar 11;15(5):2069–2088. doi: 10.1093/nar/15.5.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ypma-Wong M. F., Semler B. L. Processing determinants required for in vitro cleavage of the poliovirus P1 precursor to capsid proteins. J Virol. 1987 Oct;61(10):3181–3189. doi: 10.1128/jvi.61.10.3181-3189.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]