Abstract
Hypertension is common in patients with chronic kidney disease (CKD) and is the most important modifiable risk factor for CKD progression and adverse cardiovascular events in these patients. Diagnosis and successful management of hypertension are critically dependent on accurate blood pressure (BP) measurement. This is most relevant to CKD patients, in whom BP control is difficult to achieve and in whom early antihypertensive treatment is imperative to prevent kidney and cardiovascular complications. Accumulated data indicate that ambulatory blood pressure monitoring (ABPM) is better in detecting hypertension than office BP measurement. ABPM is also a superior prognostic marker compared with office BP and has successfully identified hypertensive CKD patients at increased risk. Additionally, ABPM provides information on circadian BP variation and short-term BP variability, which is associated with cardiovascular and renal outcomes. This paper reviews the evidence for the usefulness of ABPM in detection and management of hypertension in CKD patients and discusses our current understanding of the pathophysiology of altered circadian BP rhythm and variability in CKD and the role of abnormal BP patterns detected by ABPM in relation to outcomes in CKD. In addition, this Review examines the emerging role of antihypertensive chronotherapy to tailor BP management to the circadian BP pattern abnormality detected by 24-hour ABPM.
Keywords: ambulatory blood pressure monitoring, cardiovascular disease, chronic kidney disease, circadian rhythm, hypertension
Hypertension is a common problem in patients with CKD, and its incidence and prevalence increase with declining glomerular filtration rate (GFR).1 Among individuals with hypertension, elevated systolic BP is associated with incident CKD and a more rapid decline in renal function.2 Based on data from the US Renal Data System, it is estimated that hypertension occurs in about 23.3% of individuals without CKD and 35.8% of stage 1, 48.1% of stage 2, 59.9% of stage 3, and 84.1% of stages 4 and 5 CKD patients.1 Hypertension is also the single most powerful risk factor for cardiovascular (CV) disease. In clinical practice, the diagnosis of hypertension as well as decisions regarding management of the elevated BP has been based on BP measurements made in the clinic.
Ambulatory blood pressure monitoring (ABPM) has become an established clinical tool for the evaluation and management of hypertension both in clinical practice and in the research setting. ABPM allows serial BP measurements at specific time intervals throughout a 24-hour period, thereby providing a better assessment of the normal fluctuations in BP levels associated with daily activities and sleep. In this regard, ABPM is particularly useful in evaluating the patient with highly variable BP with wide discrepancies between the BP readings obtained in the clinic and outside the clinician's office. In addition, accumulated evidence from longitudinal studies in the general population as well as in patients with hypertension has consistently shown that ambulatory BP (ABP) is superior to office BP for prediction of clinical outcome.3 It has also proved useful in the treatment of hypertensive patients, providing a better guide for monitoring adequacy of antihypertensive therapy in patients with CKD.
Physiology of BP Variability
BP may be viewed as a continuous variable that fluctuates throughout the day and night and follows a circadian rhythm with levels rising during daytime and falling during sleep. The physiological control of systemic arterial pressure is tightly regulated and involves a complex interplay of multiple mechanisms that act to maintain arterial pressure at a relatively constant level. Any inciting stimulus or activity that raises BP by 1 or more mechanisms is opposed by counterregulatory mechanisms that lower BP. Conversely, any intervention or agent that acts to lower BP is countered by mechanisms that oppose or limit the action of the primary agent.
Circadian BP variation has been recognized for decades and has been documented in several studies using either invasive or noninvasive BP recorders in unrestricted or ambulant subjects.4, 5, 6, 7, 8 Typically, BP is highest mid-morning at 10 AM and then falls progressively throughout the remainder of the day, and BP is lowest at 3 AM and begins to rise again during the early hours of the morning before waking.7 The daily BP variation appears to be mediated by the circadian rhythm of sympathetic tone, linked to changes in the waking–sleeping cycle.8 The morning surge in BP may be related to increased α-sympathetic vasoconstrictor activity in the morning.9 The fall in BP during sleep is associated with a decrease in sympathetic activity that corresponds to the stages of non–rapid eye movement (NREM) sleep.10 However, during REM sleep, which is associated with dreaming, BP and sympathetic tone is increased, returning to the levels observed during wakefulness.10
Cortisol secretion also follows a circadian rhythm with the plasma cortisol level rising during the early morning until the waking hours and then falling gradually during the day.11 Increased sensitivity of blood vessels to norepinephrine accompanies an increased plasma cortisol level, suggesting that cortisol is another contributory factor to the early-morning BP surge. A diurnal pattern for renin and aldosterone has also been described.12, 13 Plasma renin activity (PRA) increases during the early hours of the morning, peaking at 8 AM, and gradually decreases during the day, reaching its nadir at 4 PM, followed by a gradual increase overnight. Plasma levels of aldosterone follow a diurnal pattern similar to that of renin, with an increase during the early hours of the morning that peaks just before or during the waking hours.12 Of interest is the observation that PRA levels are closely and temporally related to the pattern of sleep cycle. When sleep cycles were complete, declining PRA levels coincided with REM sleep phases and increasing levels with NREM sleep phases. Spontaneous and provoked awakenings blunt the rise in PRA normally associated with NREM sleep.13 The nocturnal oscillations in PRA closely reflect the pattern of sleep stage distribution and are modified when sleep is interrupted. These observations suggest that a common regulatory mechanism within the central nervous system controls both PRA oscillations and the REM–NREM sleep alternation.
The diurnal BP rhythm is affected by the cycle of sleep–awake activity and adapts to a shifted phase of activity and sleep as has been observed in shift workers.14, 15, 16 For example, day workers show a normal circadian rhythm with a fall in both systolic and diastolic BP at night. This pattern was reversed in night workers. Among workers who rotated shifts, BP follows the cycle of rest and activity rather than the time of day.
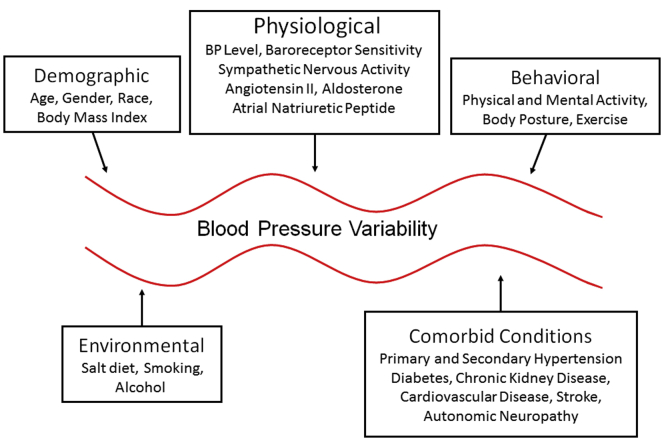
Other factors that affect BP variability include age, gender, ethnicity, baroreflex sensitivity, body mass index, sodium intake, smoking, and alcohol intake, as well as the level of BP itself.17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28 The overall degree of BP variability in a given individual is likely the result of the complex interaction of neural and humoral changes and the superimposed effects of biological and behavioral as well as environmental factors (Figure 1).
Figure 1.
Various factors affecting the overall degree of blood pressure variability in a given individual.
BP Variability and Altered Circadian BP Patterns in CKD Patients
Several cross-sectional and prospective studies using 24-hour ABPM have demonstrated alterations in BP variability and circadian BP patterns in patients with CKD (Table 1). In a study of 803 untreated hypertensive patients with varying degrees of kidney function, Manios et al.29 showed that the 24-hour rate of systolic BP variation (a measure of short-term BP variability) was significantly higher in patients with reduced kidney function (estimated GFR [eGFR] < 60 ml/min per 1.73 m2) than in those with an eGFR ≥ 60 ml/min per 1.73 m2. In a larger study of 10,271 Spanish hypertensive patients with and without CKD, Mojón and colleagues30 showed that those with CKD (defined by eGFR < 60 ml/min per 1.73 m2, urine albumin-to-creatinine ratio ≥ 30 mg/g, or both) had higher systolic ABP particularly at night but lower diastolic BP than those without CKD (eGFR > 60 ml/min per 1.73 m2). Furthermore, the prevalence of both non-dipping and rising nocturnal BP patterns increased with increasing severity of CKD.
Table 1.
Altered BP variability and ambulatory BP pattern in CKD patients
| Study design | Patients (n) | ABP profiles/outcomes | References |
|---|---|---|---|
| Cross-sectional | HTN pts (n = 803) | 24-hour rate of SBP variation higher in pts with eGFR <60 | Manios et al.29 |
| Cross-sectional | HTN pts (n = 10,271) | Higher prevalence of non-dipping in pts with CKD (eGFR <60) versus pts with no CKD (eGFR >60); higher nocturnal SBP and lower DBP in pts with CKD versus pts with no CKD | Mojón et al.30 |
| Cross-sectional | African Americans (n = 1022) with and without CKD | Higher average 24-hour BP variability in pts with CKD | Tanner et al.31 |
| Cross-sectional | HTN pts (n = 328) | Higher average 24-hour BP variability in pts with reduced eGFR | Mulè et al.32 |
| Cross-sectional | CKD pts (n = 14,382) (stages 1–5) | Higher daytime and nighttime SBP and DBP variability | Gorostidi et al.33 |
| Prospective (f/u 2 yr) | CRI pts (n = 68) | Blunted nocturnal BP fall; decline in creatinine clearance correlated with nocturnal DBP fall | Timio et al.34 |
| Prospective (f/u 3.2 yr) | CRI pts (n = 322) | Decline in eGFR in non-dippers; stable eGFR in dippers | Davidson et al.37 |
| Prospective (f/u 3.5 yr) | CKD pts (n = 217) | Non-dipping associated with increased risk of ESRD and total mortality | Agarwal and Andersen38 |
| Prospective | Hypertensive CKD African Americans (n = 617) | 24-hour SBP predicted both renal and CV outcomes | Gabbai et al.39 |
| Prospective (f/u 3.5 yr) | African Americans (n = 603) | 10% higher nocturnal dipping associated with decreased risk of CKD (eGFR <60) and lower annual rate of eGFR decline | McMullan et al.40 |
ABP, ambulatory blood pressure; BP, blood pressure; CKD, chronic kidney disease; CRI, chronic renal insufficiency; CV, cardiovascular; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; ESRD, end-stage renal disease; f/u, follow-up; HTN, hypertension; pts, patients; SBP, systolic blood pressure.
Other groups have reported similar results of increased 24-hour BP variability in patients with CKD31 and hypertensive patients with reduced kidney function and microalbuminuria.32 Gorostidi et al.33 conducted a cross-sectional analysis of a large sample of 14,382 Spanish hypertensive patients with no CKD and with stage 1 to stage 5 CKD and showed that BP variability assessed by SD of mean daytime and nighttime systolic BP (SBP) and diastolic blood pressure (DBP) was significantly higher in CKD patients than in those without CKD. This association was stronger for SBP than for DBP and for nighttime than for daytime BP.
Several prospective studies also demonstrate a close association between blunted nocturnal BP fall or non-dipping and reduced kidney function in CKD patients. Timio et al.34 prospectively studied 27 normotensive and 41 hypertensive patients with stable CKD, 28 matched healthy subjects, and 47 patients with essential hypertension without kidney disease. After a follow-up of 24 months, both the normotensive and the hypertensive patients with CKD showed an alteration in the 24-hour ABP with significantly blunted nocturnal BP reduction as compared with the respective control groups. The same investigators extended their observations in 48 hypertensive patients with chronic renal insufficiency who were followed for 3 years and observed that the non-dippers had a faster rate of creatinine clearance decline than the dippers.35 Moreover, the increase in urinary protein excretion was higher in the non-dipper group than in the dipper group, indicating that the non-dipping pattern of ABP can be associated with a faster progression of renal insufficiency in renal hypertensives. Kanaoka et al. also found significant positive relationships between urinary albumin excretion rate and 24-hour, daytime, and nighttime ambulatory SBP.36
Decreased diurnal BP variation has been shown by Davidson et al.37 to be associated with a subsequent decline in kidney function that is independent of SBP load and other known risk factors. In another prospective study of 217 veterans with CKD followed for a median of 3.5 years, Agarwal and Andersen38 showed that non-dipping is associated with increased risk of composite kidney end point of end-stage renal disease (ESRD) and total mortality. In a large cohort of 617 hypertensive CKD patients enrolled in the African American Study of Kidney Disease and Hypertension (AASK) who were followed for a median period of 5 years, Gabbai et al.39 also demonstrated that 24-hour SBP predicts both kidney and CV outcomes. Further analyses revealed that both daytime and nighttime SBP levels were predictive of renal and CV outcomes. A prospective study involving 603 African Americans enrolled in the Jackson Heart Study with normal baseline kidney function showed that loss of nocturnal BP dipping and not morning BP surge is associated with risk of CKD.40
Collectively, these studies suggest that nocturnal non-dipping is a common feature of altered circadian BP rhythm in CKD patients, which may partly contribute to the deterioration of kidney function in such patients.
Another important abnormal ABP pattern is “masked hypertension.” This phenomenon, also called “reverse white-coat hypertension” or “white-coat normotension” and described by Pickering,41 refers to individuals having normal clinic BP levels but elevated ABPM levels and is commonly found in CKD patients. In a cross-sectional study, Pogue et al.42 examined clinic BP and ABP patterns and their associations with target organ damage in 617 African Americans with hypertensive kidney disease. Masked hypertension was defined by elevated daytime (≥135/85 mm Hg) or elevated nighttime (≥120/70 mm Hg) ABP in those with controlled clinic BP (<140/90 mm Hg); non-dipping was defined by a ≤10% decrease in mean nighttime SBP; reverse dipping was defined by a higher nighttime than daytime SBP. Of the 617 participants with both clinic BP and ABP, 80% had a non-dipping or reverse dipping profile. Of the 377 participants with controlled clinic BP (61%), 70% had masked hypertension. Moreover, target organ damage such as proteinuria and left ventricular hypertrophy were more common in those with elevated nighttime BP, masked hypertension, or sustained hypertension, compared with those with controlled clinic BP or white-coat hypertension. A meta-analysis involving 980 CKD patients showed that the overall prevalence of masked hypertension was 8.3% and of white-coat hypertension was 18.3%.43 Of great prognostic importance is the masked hypertension, which, like nocturnal BP non-dipping, is also associated with increased risk of progression to ESRD and total mortality in CKD patients.38 In another study of 333 veterans with CKD, Agarwal et al.44 prospectively evaluated the prevalence of masked uncontrolled hypertension using 3 definitions of hypertension (daytime hypertension ≥135/85 mm Hg; either nighttime hypertension ≥120/70 mm Hg or daytime hypertension; and 24-hour hypertension ≥130/80 mm Hg) or by home BP monitoring (hypertension ≥135/85 mm Hg). In this study, the prevalence of masked uncontrolled hypertension was 26.7% by daytime ABP, 32.8% by 24-hour ABP, 56.1% by daytime or nighttime ABP, and 50.8% by home BP. Thus, masked uncontrolled hypertension is common in CKD patients, and its diagnosis by ABP is reproducible with 75% to 78% agreement between 2 repeated measurements.
ABPM and CV Events in CKD
Recent studies have evaluated target organ damage and CV events associated with ABP patterns in patients with CKD45, 46, 47, 48 (Table 2). In an observational ABPM study of 540 Chinese CKD patients, Wang and associates45 reported that patients with reversed dipper BP pattern had worse kidney function and CV damage compared with patients with a dipper BP pattern. These investigators46 further studied a larger cohort of 1219 patients with CKD, which included 432 nondiabetic CKD patients without hypertension, 565 nondiabetic CKD patients with hypertension, and 222 diabetic patients. They found that BP load and ABP levels both were independently correlated with left ventricular mass index (LVMI), eGFR, and proteinuria in all groups of CKD patients. Furthermore, nighttime SBP load correlated with LVMI in nondiabetic CKD patients with or without hypertension.
Table 2.
Altered ambulatory BP profile and cardiovascular events in patients with CKD
| Study design | Patients (n) | ABP profiles/outcomes | References |
|---|---|---|---|
| Cross-sectional | African Americans with CKD (n = 617) | Proteinuria and LVH more common in patients with elevated nighttime BP and masked hypertension | Pogue et al.42 |
| Cross-sectional | CKD pts (n = 540) | Reverse dipper BP pattern closely related to worse renal function and severe CV damage | Wang et al.45 |
| Cross-sectional | CKD pts (n = 1219) | BP load and ABP levels correlated with LVMI, eGFR, and proteinuria; nighttime SBP load correlated with TOD in pts with nondiabetic CKD | Wang et al.46 |
| Longitudinal (follow-up >4 yr) | Veterans with CKD (n = 274) | Rate of growth of LVMI was rapid in first 2 years and plateaued subsequently; clinic BP and 24-hour ABP were associated with LVMI and its growth over time | Agarwal47 |
| Cross-sectional | CKD pts (n = 1492) | Masked hypertension was independently associated with low eGFR, higher proteinuria, and higher LVMI and pulse wave | Drawz et al.48 |
ABP, ambulatory blood pressure; BP, blood pressure; CKD, chronic kidney disease; CV, cardiovascular; eGFR, estimated glomerular filtration rate; LV, left ventricular; LVH, left ventricular hypertrophy; LVMI, left ventricular mass index; pts, patients; SBP, systolic blood pressure; TOD, target organ damage.
Recent data also suggest an association between masked hypertension and CV target organ damage in CKD patients.47, 48 In a longitudinal study of 274 veterans with CKD followed for about 4 years, Agarwal47 showed that both clinic BP and 24-hour ABP were associated with LVMI and its rate of growth over time. Moreover, different categories of hypertension, namely, masked uncontrolled hypertension, controlled hypertension, and uncontrolled hypertension, were associated with increasing LVMI when diagnosed by 24-hour ambulatory and awake BP but not sleep BP. In another study, Drawz et al.48 performed 24-hour ABPM in 1492 men and women with CKD enrolled in the Chronic Renal Insufficiency Cohort (CRIC) Study, who were categorized into 4 groups—controlled BP and white-coat, masked, and sustained hypertension—on the basis of clinic and 24-hour ABP. In this study, masked hypertension was independently associated with low eGFR, higher proteinuria, and higher LVMI and pulse wave velocity compared with controlled BP. The association between masked hypertension and lower eGFR was observed only in those participants with elevated nighttime BP.
ABPM and CV Events in ESRD
As in patients with CKD, a number of studies have examined the BP variability and circadian BP patterns and their relationships to CV events or risks in patients with ESRD49, 50, 51, 52 (Table 3). In a prospective study of 57 treated hypertensive hemodialysis patients, Amar et al.49 reported that elevated nocturnal SBP and elevated pulse pressure were independently associated with CV mortality. Similarly, in 80 hemodialysis patients, Liu et al.50 have shown that nocturnal BP non-dipping was positively associated with CV events and CV mortality.
Table 3.
Altered ambulatory BP profile and cardiovascular events in patients with end-stage renal disease
| Study design | Patients (n) | ABP profiles/outcomes | References |
|---|---|---|---|
| Prospective | Hypertensive HD pts (n = 57) | Elevated nocturnal systolic BP and elevated PP were independently associated with CV mortality | Amar et al.49 |
| Prospective | HD patients (n = 80) | Nocturnal BP non-dipping positively associated with CV events and CV mortality | Liu et al.50 |
| Prospective | Nondiabetic HD pts (n = 168) | Night/day systolic BP ratio strongly predicts total and CV mortality | Tripepi et al.51 |
| Cross-sectional | ESRD pts undergoing APD (n = 20) versus CAPD (n = 28) | LVMI higher in diastolic non-dippers compared to dippers; non-dipper diastolic BP pattern associated with LVMI | Ataş et al.52 |
ABP, ambulatory blood pressure; APD, automated peritoneal dialysis; BP, blood pressure; CAPD, continuous ambulatory peritoneal dialysis; CV, cardiovascular; ESRD, end-stage renal disease; HD, hemodialysis; LVMI, left ventricular mass index; PP, pulse pressure; pts, patients.
In another prospective study of 168 nondiabetic hemodialysis patients without a prior history of CV events, Tripepi et al.51 have shown that the night/day SBP ratio was shown to be a stronger predictor of total mortality and CV mortality. As in hemodialysis patients, hypertension and non-dipping BP pattern have also been shown to be associated with left ventricular hypertrophy in peritoneal dialysis patients. Ataş et al.52 compared the ABPM profile and LVMI in 2 groups of ESRD patients undergoing either automated peritoneal dialysis or continuous ambulatory peritoneal dialysis and showed that LVMI was significantly higher in diastolic non-dippers compared to dippers.
Mechanisms of Altered BP Variability and Circadian Rhythm in CKD
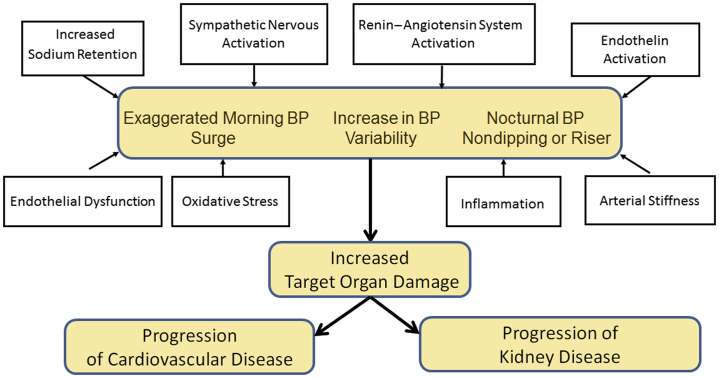
The mechanisms underlying the altered BP variability and circadian rhythm in CKD are incompletely understood. Several factors or mechanisms, including increased sodium and fluid volume retention, impaired baroreceptor sensitivity, altered sympathetic nervous system activity, activation of the renin–angiotensin system (RAS), endothelial dysfunction, oxidative stress, inflammation, and increased arterial stiffness, have been proposed to explain the altered BP circadian rhythm (Table 4 and Figure 2).
Table 4.
Putative mechanisms of altered BP variability in CKD
| 1. Increased sodium and fluid volume retention |
| 2. Impaired baroreceptor sensitivity |
| 3. Altered sympathetic nervous system activity |
| 4. Activation of the renin–angiotensin–aldosterone system |
| 5. Endothelial dysfunction |
| 6. Oxidative stress |
| 7. Inflammation |
| 8. Increased arterial stiffness |
Figure 2.
Mechanisms of altered blood pressure variability and circadian rhythm in chronic kidney disease.
Increased sodium and fluid retention due to impaired ability of the diseased kidney has been proposed as the major factor contributing to nocturnal hypertension and non-dipping BP pattern in CKD.53 Nocturnal hypertension is thought to compensate for the sodium retention during the daytime and enhanced pressure natriuresis during the night. Baroreflex dysfunction resulting in impaired BP regulation could contribute to increased BP variability in CKD.54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66 Sympathetic nervous system overactivity is well documented in patients with chronic kidney failure.57, 58, 59, 60, 61, 62 Increased sympathetic nervous system activity has been shown to be closely associated with blunted nocturnal BP reduction in hypertensive patients with diabetic nephropathy.62 Of note is the finding by Okuguchi et al.63 in a study of salt-sensitive patients and essential hypertensive patients, who showed that salt loading converted the circadian BP pattern from dippers to non-dippers, an effect that was associated with an increase in the nighttime/daytime ratio of urinary noradrenaline excretion. The failure of nocturnal BP to fall is thought to be related to the lack of nocturnal decrease in sympathetic nervous activity.
Circadian BP variability has also been linked to RAS activity, since it is known that renin and angiotensin II levels are influenced by circadian rhythms.64, 65 Local RAS activity in the kidneys has been suggested to contribute to BP variability in patients with primary hypertension.66 Intrarenal RAS activation has also been implicated in the abnormal circadian BP changes in CKD.67
Endothelial dysfunction is a common event in the development of hypertension,68 and in all stages of CKD,69, 70 and is closely associated with abnormal circadian BP variation and non-dipping pattern.71, 72 In hypertensive patients, nocturnal BP is elevated and endothelial function deteriorates in parallel with a decline in kidney function.73
There is evidence that suggests a role of the endothelin-1 (ET-1) system in diurnal BP variation. A circadian rhythm of plasma ET-1 has been observed in healthy volunteers74 and in patients with CKD.75 One study has shown that the circadian clock protein period 1 regulates ET-1 mRNA and protein in the kidney, and this contributes to the regulation of renal sodium transport and BP.76 Further support for the contribution of the ET-1 system in hypertension and loss of nocturnal BP dipping in CKD is provided by the recent studies of Dhaun and co-investigators77 showing that treatment with the selective ETA receptor antagonist sitaxentan for 6 weeks increased the nocturnal dip in both SBP and DBP in patients with CKD.
Oxidative stress with increased production of reactive oxygen species plays a causal role in the development of hypertension78 and contributes to kidney damage and progression of CKD.79, 80 Increased reactive oxygen species formation by peripheral blood mononuclear cells has been observed in hypertensive patients exhibiting exaggerated BP dipping and morning BP surge.81 Serum markers of oxidative stress, such as asymmetric dimethylarginine and nitrotyrosine, are increased in non-dipper hypertensive patients compared with dipper hypertensive patients.82
Inflammation has also been suggested to promote increased BP variability. Circulating inflammatory markers, such as C-reactive protein or tumor necrosis factor-α, have been shown to be positively correlated with 24-hour ABP variability in healthy adults83 and in African Americans,84 as well as in hypertensive patients, independent of absolute BP levels.85 Inflammatory mediators are frequently elevated in CKD,86, 87, 88 which could contribute to increased BP variability through accelerated peripheral artery disease observed in CKD.89, 90 Studies are needed to determine whether controlling inflammation could reduce BP variability and the adverse CV consequences in CKD.
Enhanced aortic stiffening is well documented in patients with CKD91, 92, 93 and is closely related to increased short-term or 24-hour BP.94, 95, 96 The mechanism for increased arterial stiffness in CKD and ESRD is likely multifactorial.97, 98, 99, 100 Vascular remodeling with changes in arterial wall composition and elasticity and arterial calcification, a pathological process linked to abnormal mineral metabolism in CKD, are major factors implicated in arterial stiffening.99 Other mechanisms or factors, such as RAS activation, ET-1, endothelial dysfunction, oxidative stress, and inflammation, as well as the anti-aging molecule Klotho,100 are also implicated in this process.
ABPM and Effects of Kidney Transplantation
Only a few studies have examined the effects of kidney transplantation (KTx) on circadian BP profile and BP control in ESRD patients. Gatzka et al.101 evaluated 24-hour ABP circadian profile in 45 patients after successful KTx and observed that the prevalence of dippers increased from 27% in the early phase (<7 months) to 73% in the late phase (≥1 year) after KTx. The longer the time after KTx, the more marked was the decrease of BP during sleep. The improvement in nocturnal BP dipping pattern effect did not appear to be related to the concomitant antihypertensive or immunosuppressive therapy. Covic et al.102 compared the ABP profiles recorded at 1 month before and 1 month and >1 year after successful KTx in 20 living kidney transplant recipients and observed that at 1 month post-transplant, KTx had little or no effect on abnormal circadian pattern in most recipients. However, at 1 year post-transplant, KTx resulted in a significant improvement of the circadian BP profile. This effect is mainly determined by the prevailing level of kidney function and by the initial pre-transplantation dipping profile.
Wadei et al.103 analyzed ABPM profile in relation to allograft function in 119 kidney transplant recipients after 1 year of transplantation. Nocturnal fall in SBP and GFR was related to worse allograft function in those with abnormal circadian BP change. Additional studies in a subgroup of 36 kidney recipients who were followed for 3 to 4 years showed that the patients with nocturnal non-dipping or reverse dipping patterns had significantly greater loss of kidney function compared with those with normal dipping pattern.104 These results indicate that kidney transplant patients with abnormal circadian BP pattern at 1 year are at increased risk for kidney function loss in subsequent years.
More recently, Lee et al.105 prospectively evaluated the impact of KTx on the status of hypertension and circadian rhythm in 48 ESRD patients before and 1 year after KTx. After KTx, the mean 24-hour ABP value did not change, but the proportion of patients taking antihypertensive drugs and the pill number significantly decreased. In contrast, the mean nocturnal fall in SBP significantly decreased and was associated with inferior allograft function during follow-up. Taken collectively, the results of these studies suggest that the overall impact of KTx on circadian BP pattern may vary depending on the time interval after transplantation, prevailing allograft function, and concomitant use of antihypertensive or immunosuppressive medications. Longer prospective studies involving a larger number of kidney transplant patients are needed to determine whether restoration of normal BP circadian pattern will confer better allograft outcome in the long term.
ABP and Effects of Antihypertensive Treatment in CKD
Most clinical trials on the effects of various classes of antihypertensive medications on 24-hour ABP have been conducted in hypertensive patients without CKD.106, 107, 108, 109, 110 Generally, calcium channel blockers such as nifedipine are more effective with bedtime than morning dosing.108 Given that the RAS follows a circadian rhythm and activates during nighttime sleep, a series of clinical studies by Hermida et al. have been conducted that show that bedtime dosing of angiotensin-converting enzyme inhibitors or angiotensin receptor blockers exerts a more marked effect on asleep than awake BP means in hypertensive patients.109
Only a few studies have evaluated the effects of daytime versus bedtime administration of antihypertensive agents in patients with CKD, and the results of these studies are mixed.110, 111, 112, 113, 114 In an 8-week uncontrolled trial of 32 CKD patients, Minutolo et al.111 showed that changing the timing of antihypertensive medication dosing from morning to evening decreased the night/day ratio of mean ABP in 93.7% of patients and restored normal circadian rhythm in 87.5%. Moreover, proteinuria also was reduced with evening administration of antihypertensive medications. In a randomized study of 661 hypertensive patients with CKD, Hermida et al.110 compared the effects on ABP control of changing 1 or more of the daily antihypertensive medications to bedtime versus giving all medications upon awakening. The subjects randomized to bedtime medication showed a better overall control of BP on ABPM, greater reduction in mean asleep SBP, and a lower proportion of non-dippers. Subjects randomized to bedtime therapy also had greater reduction in albuminuria compared to the control group. More importantly, the group receiving bedtime dosing had a significant reduction in the composite outcome of CV death, myocardial infarction, or stroke. The same group of investigators112 reported similar results in a randomized trial comparing bedtime treatment versus conventional therapy in 448 hypertensive patients with type 2 diabetes. After a median follow-up of 5.4 years, patients ingesting ≥1 hypertension medication at bedtime showed significantly lower CV events than subjects ingesting all medications upon awakening. The patients treated at bedtime showed significantly lower sleep time BP mean and higher prevalence of controlled ABP. However, one study in 147 former participants of the African American Study of Kidney Disease and Hypertension (AASK) with hypertensive kidney disease failed to show nocturnal SBP reduction with nightly antihypertensive drug dosing.113 The reasons for the discrepant results are not clear but may partly relate to differences in race, age, kidney function, nocturnal BP status, or duration of hypertension.
Another recent study by Wang et al.114 showed that bedtime dosing of an ARB once daily was significantly more effective than awakening dosing in reducing nighttime BP, proteinuria, and left ventricular mass in CKD patients with non-dipping pattern. Further studies are needed to determine whether other classes of antihypertensive medications or interventions are superior in controlling nocturnal hypertension in CKD patients.
Conclusion and Future Directions
In conclusion, a large body of evidence supports the use and superiority of ABPM over clinic BP measurements in making an accurate diagnosis and classification of hypertension, assessing target organ damage, predicting outcomes, and evaluating response to therapy in patients with CKD, in whom optimal BP control is often difficult to achieve. Numerous studies using 24-hour ABPM in hypertensive patients with and without CKD, in ESRD patients, and in kidney transplant patients have identified high-risk individuals with altered BP variability and circadian BP patterns, which have been shown to be associated with target organ damage or adverse kidney and CV outcomes. In hypertensive patients with and without CKD, several measures of BP variability are increased and have been shown to be closely associated with kidney injury (e.g., reduced eGFR or albuminuria as well as CV target organ damage). The most important circadian BP patterns frequently observed in these patients are blunted nocturnal BP fall (non-dipping) and elevated nighttime BP (nocturnal hypertension). Both non-dipping status and nocturnal hypertension are associated with target organ damage and CV risk. Another important abnormal ABP pattern commonly observed in CKD patients is masked hypertension, which has also been shown to be strongly associated with CV and target organ damage.
Recent work has focused on antihypertensive chronotherapy based on ABP profile in the management of hypertensive patients with CKD. Dosing 1 or more antihypertensive medications at bedtime has been shown to improve BP control, lower nocturnal BP, and reduce the risk of CV events in a few studies but not in others.110, 111, 113, 114 Therefore, until more data in CKD patients are available, caution is warranted in recommending antihypertensive chronotherapy in CKD patients. Management of hypertension in CKD patients should focus on choosing appropriate use of antihypertensive drugs to reduce the level of nocturnal BP and restore diurnal rhythm of BP. For example, one important strategy is the timely use of diuretic therapy alone or in combination with other antihypertensive agents to treat nocturnal hypertension. More clinical trials in CKD and ESRD patients are needed to determine whether chronotherapy targeting these ABPM findings improves CV outcomes in the long term.
Disclosure
All the authors declared no competing interests.
Acknowledgments
DSR is supported by National Institutes of Health grants 1R01-DK073665-01A1, 1U01-DK099924-01, and 1U01-DK099914-01.
References
- 1.US Renal Data System. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, 2010.
- 2.Hanratty R., Chonchol M., Havranek E.P. Relationship between blood pressure and incident chronic kidney disease in hypertensive patients. Clin J Am Soc Nephrol. 2011;6:2605–2611. doi: 10.2215/CJN.02240311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mancia G., Verdecchia P. Clinical value of ambulatory blood pressure: evidence and limits. Circ Res. 2015;116:1034–1045. doi: 10.1161/CIRCRESAHA.116.303755. [DOI] [PubMed] [Google Scholar]
- 4.Mueller S.C., Brown G.E. Hourly rhythms in blood pressure in persons with normal and elevated pressures. Ann Intern Med. 1930;3:1190–1200. [Google Scholar]
- 5.Bevan A.T., Honour A.J., Stott F.H. Direct arterial pressure recording in unrestricted man. Clin Sci. 1969;36:329–344. [PubMed] [Google Scholar]
- 6.Littler W.A., Honour A.J., Sleight P., Stott F. Continuous recording of direct arterial pressure and electrocardiogram in unrestricted man. Br Med J. 1972;3:76–78. doi: 10.1136/bmj.3.5818.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Millar-Craig M.W., Bishop C.N., Raftery E.B. Circadian variation of blood-pressure. Lancet. 1978;1:795–797. doi: 10.1016/s0140-6736(78)92998-7. [DOI] [PubMed] [Google Scholar]
- 8.Clark L.A., Denby L., Pregibon D. A quantitative analysis of the effects of activity and time of day on the diurnal variations of blood pressure. J Chronic Dis. 1987;40:671–681. doi: 10.1016/0021-9681(87)90103-2. [DOI] [PubMed] [Google Scholar]
- 9.Panza J.A., Epstein S.E., Quyyumi A.A. Circadian variation in vascular tone and its relation to alpha-sympathetic vasoconstrictor activity. N Engl J Med. 1991;325:986–990. doi: 10.1056/NEJM199110033251402. [DOI] [PubMed] [Google Scholar]
- 10.Somers V.K., Dyken M.E., Mark A.L., Abboud F.M. Sympathetic-nerve activity during sleep in normal subjects. N Engl J Med. 1993;328:303–307. doi: 10.1056/NEJM199302043280502. [DOI] [PubMed] [Google Scholar]
- 11.Weitzman E.D., Fukushima D., Nogeire C. Twenty-four hour pattern of the episodic secretion of cortisol in normal subjects. J Clin Endocrinol Metab. 1971;33:14–22. doi: 10.1210/jcem-33-1-14. [DOI] [PubMed] [Google Scholar]
- 12.Stern N., Sowers J.R., McGinty D. Circadian rhythm of plasma renin activity in older normal and essential hypertensive men: relation with inactive renin, aldosterone, cortisol and REM sleep. J Hypertens. 1986;4:543–550. doi: 10.1097/00004872-198610000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Brandenberger G., Follenius M., Simon C. Nocturnal oscillations in plasma renin activity and REM-NREM sleep cycles in humans: a common regulatory mechanism? Sleep. 1988;11:242–250. doi: 10.1093/sleep/11.3.242. [DOI] [PubMed] [Google Scholar]
- 14.Sundberg S., Kohvakka A., Gordin A. Rapid reversal of circadian blood pressure rhythm in shift workers. J Hypertens. 1988;6:393–396. [PubMed] [Google Scholar]
- 15.Chau N.P., Mallion J.M., Gaudemaris R.D. Twenty-four-hour ambulatory blood pressure in shift workers. Circulation. 1989;80:341–347. doi: 10.1161/01.cir.80.2.341. [DOI] [PubMed] [Google Scholar]
- 16.Sternberg H., Rosenthal T., Shamiss A., Green M. Altered circadian rhythm of blood pressure in shift workers. J Hum Hypertens. 1995;9:349–353. [PubMed] [Google Scholar]
- 17.Imai Y., Abe K., Munakata M. Circadian blood pressure variations under different pathophysiological conditions or diseases. J Hypertens Suppl. 1990;8:S125–S132. [PubMed] [Google Scholar]
- 18.Conway J., Boon N., Davies C. Neural and humoral mechanisms involved in blood pressure variability. J Hypertens. 1984;2:203–208. doi: 10.1097/00004872-198404000-00013. [DOI] [PubMed] [Google Scholar]
- 19.Mancia G., Parati G., Pomidossi G. Arterial baroreflexes and blood pressure and heart rate variabilities in humans. Hypertension. 1986;8:147–153. doi: 10.1161/01.hyp.8.2.147. [DOI] [PubMed] [Google Scholar]
- 20.Floras J.S., Hassan M.O., Jones J.V. Factors influencing blood pressure and heart rate variability in hypertensive humans. Hypertension. 1988;11:273–281. doi: 10.1161/01.hyp.11.3.273. [DOI] [PubMed] [Google Scholar]
- 21.Laitinen T., Hartikainen J., Niskanen L. Sympathovagal balance is a major determinant of short-term blood pressure variability in healthy subjects. Am J Physiol. 1999;276:H1245–H1252. doi: 10.1152/ajpheart.1999.276.4.H1245. [DOI] [PubMed] [Google Scholar]
- 22.James G.D., Toledano T., Datz G., Pickering T.G. Factors influencing the awake-sleep difference in ambulatory blood pressure: main effects and sex differences. J Hum Hypertens. 1995;9:821–826. [PubMed] [Google Scholar]
- 23.Stewart M.J., Jyothinagaram S., McGinley I.M., Padfield P.L. Cardiovascular effects of cigarette smoking: ambulatory blood pressure and BP variability. J Hum Hypertens. 1994;8:19–22. [PubMed] [Google Scholar]
- 24.Kotsis V., Stabouli S., Bouldin M. Impact of obesity on 24-hour ambulatory blood pressure and hypertension. Hypertension. 2005;45:602–607. doi: 10.1161/01.HYP.0000158261.86674.8e. [DOI] [PubMed] [Google Scholar]
- 25.Kagan A., Faibel H., Ben-Arie G. Gender differences in ambulatory blood pressure monitoring profile in obese, overweight and normal subjects. J Hum Hypertens. 2007;21:128–134. doi: 10.1038/sj.jhh.1002118. [DOI] [PubMed] [Google Scholar]
- 26.Itoh K., Kawasaki T., Cugini P. Effects of timing of salt intake to 24-hour blood pressure and its circadian rhythm. Ann N Y Acad Sci. 1996;783:324–325. doi: 10.1111/j.1749-6632.1996.tb26732.x. [DOI] [PubMed] [Google Scholar]
- 27.Ohira T., Tanigawa T., Tabata M. Effects of habitual alcohol intake on ambulatory blood pressure, heart rate, and its variability among Japanese men. Hypertension. 2009;53:13–19. doi: 10.1161/HYPERTENSIONAHA.108.114835. [DOI] [PubMed] [Google Scholar]
- 28.de la Sierra A., Redon J., Banegas J.R. Spanish Society of Hypertension Ambulatory Blood Pressure Monitoring Registry Investigators. Prevalence and factors associated with circadian blood pressure patterns in hypertensive patients. Hypertension. 2009;53:466–472. doi: 10.1161/HYPERTENSIONAHA.108.124008. [DOI] [PubMed] [Google Scholar]
- 29.Manios E., Tsagalis G., Tsivgoulis G. Time rate of blood pressure variation is associated with impaired renal function in hypertensive patients. J Hypertens. 2009;27:2244–2248. doi: 10.1097/HJH.0b013e328330a94f. [DOI] [PubMed] [Google Scholar]
- 30.Mojón A., Ayala D.E., Piñeiro L. Comparison of ambulatory blood pressure parameters of hypertensive patients with and without chronic kidney disease. Chronobiol Int. 2013;30:145–158. doi: 10.3109/07420528.2012.703083. [DOI] [PubMed] [Google Scholar]
- 31.Tanner R.M., Shimbo D., Dreisbach A.W. Association between 24-hour blood pressure variability and chronic kidney disease: a cross-sectional analysis of African Americans participating in the Jackson heart study. BMC Nephrol. 2015;16:84. doi: 10.1186/s12882-015-0085-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mulè G., Calcaterra I., Costanzo M. Relationship between short-term blood pressure variability and subclinical renal damage in essential hypertensive patients. J Clin Hypertens. 2015;17:473–480. doi: 10.1111/jch.12534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gorostidi M., Sarafidis P., Sierra Ade L. Blood pressure variability increases with advancing chronic kidney disease stage. A cross-sectional analysis of 14,382 hypertensive patients from Spain. J Hypertens. 2015;33(Suppl 1):e40. doi: 10.1097/HJH.0000000000001670. [DOI] [PubMed] [Google Scholar]
- 34.Timio M., Lolli S., Verdura C. Circadian blood pressure changes in patients with chronic renal insufficiency: a prospective study. Ren Fail. 1993;15:231–237. doi: 10.3109/08860229309046157. [DOI] [PubMed] [Google Scholar]
- 35.Timio M., Venanzi S., Lolli S. “Non-dipper” hypertensive patients and progressive renal insufficiency: a 3-year longitudinal study. Clin Nephrol. 1995;43:382–387. [PubMed] [Google Scholar]
- 36.Kanaoka T., Tamura K., Ohsawa M. Relationship of ambulatory blood pressure and the heart rate profile with renal function parameters in hypertensive patients with chronic kidney disease. Clin Exp Hypertens. 2012;34:264–269. doi: 10.3109/10641963.2012.681082. [DOI] [PubMed] [Google Scholar]
- 37.Davidson M.B., Hix J.K., Vidt D.G., Brotman D.J. Association of impaired diurnal blood pressure variation with a subsequent decline in glomerular filtration rate. Arch Intern Med. 2006;166:846–852. doi: 10.1001/archinte.166.8.846. [DOI] [PubMed] [Google Scholar]
- 38.Agarwal R., Andersen M.J. Prognostic importance of ambulatory blood pressure recordings in patients with chronic kidney disease. Kidney Int. 2006;69:1175–1180. doi: 10.1038/sj.ki.5000247. [DOI] [PubMed] [Google Scholar]
- 39.Gabbai F.B., Rahman M., Hu B., African American Study of Kidney Disease and Hypertension Study Group Relationship between ambulatory BP and clinical outcomes in patients with hypertensive CKD. Clin J Am Soc Nephrol. 2012;7:1770–1776. doi: 10.2215/CJN.11301111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McMullan C.J., Hickson D.A., Taylor H.A., Forman J.P. Prospective analysis of the association of ambulatory blood pressure characteristics with incident chronic kidney disease. J Hypertens. 2015;33:1939–1946. doi: 10.1097/HJH.0000000000000638. [DOI] [PubMed] [Google Scholar]
- 41.Pickering T.G., Davidson K., Gerin W., Schwartz J.E. Masked hypertension. Hypertension. 2002;40:795–796. doi: 10.1161/01.hyp.0000038733.08436.98. [DOI] [PubMed] [Google Scholar]
- 42.Pogue V., Rahman M., Lipkowitz M., African American Study of Kidney Disease and Hypertension Collaborative Research Group Disparate estimates of hypertension control from ambulatory and clinic blood pressure measurements in hypertensive kidney disease. Hypertension. 2009;53:20–27. doi: 10.1161/HYPERTENSIONAHA.108.115154. [DOI] [PubMed] [Google Scholar]
- 43.Bangash F., Agarwal R. Masked hypertension and white-coat hypertension in chronic kidney disease: a meta-analysis. Clin J Am Soc Nephrol. 2009;4:656–664. doi: 10.2215/CJN.05391008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Agarwal R., Pappas M.K., Sinha A.D. Masked uncontrolled hypertension in CKD. J Am Soc Nephrol. 2016;27:924–932. doi: 10.1681/ASN.2015030243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang C., Zhang J., Liu X. Reversed dipper blood-pressure pattern is closely related to severe renal and cardiovascular damage in patients with chronic kidney disease. PLoS One. 2013;8:e55419. doi: 10.1371/journal.pone.0055419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang C., Zhang J., Deng W. Nighttime systolic blood-pressure load is correlated with target-organ damage independent of ambulatory blood-pressure level in patients with non-diabetic chronic kidney disease. PLoS One. 2015;10:e0131546. doi: 10.1371/journal.pone.0131546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Agarwal R. Longitudinal study of left ventricular mass growth: comparative study of clinic and ambulatory systolic blood pressure in chronic kidney disease. Hypertension. 2016;67:710–716. doi: 10.1161/HYPERTENSIONAHA.115.07052. [DOI] [PubMed] [Google Scholar]
- 48.Drawz P.E., Alper A.B., Anderson A.H., Chronic Renal Insufficiency Cohort Study Investigators Masked hypertension and elevated nighttime blood pressure in CKD: prevalence and association with target organ damage. Clin J Am Soc Nephrol. 2016;7:642–652. doi: 10.2215/CJN.08530815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Amar J., Vernier I., Rossignol E. Nocturnal blood pressure and 24-hour pulse pressure are potent indicators of mortality in hemodialysis patients. Kidney Int. 2000;57:2485–2491. doi: 10.1046/j.1523-1755.2000.00107.x. [DOI] [PubMed] [Google Scholar]
- 50.Liu M., Takahashi H., Morita Y. Non-dipping is a potent predictor of cardiovascular mortality and is associated with autonomic dysfunction in haemodialysis patients. Nephrol Dial Transplant. 2003;18:563–569. doi: 10.1093/ndt/18.3.563. [DOI] [PubMed] [Google Scholar]
- 51.Tripepi G., Fagugli R.M., Dattolo P. Prognostic value of 24-hour ambulatory blood pressure monitoring and of night/day ratio in nondiabetic, cardiovascular events-free hemodialysis patients. Kidney Int. 2005;68:1294–1302. doi: 10.1111/j.1523-1755.2005.00527.x. [DOI] [PubMed] [Google Scholar]
- 52.Ataş N., Erten Y., Okyay G.U. Left ventricular hypertrophy and blood pressure control in automated and continuous ambulatory peritoneal dialysis patients. Ther Apher Dial. 2014;18:297–304. doi: 10.1111/1744-9987.12104. [DOI] [PubMed] [Google Scholar]
- 53.Fukuda M., Munemura M., Usami T. Nocturnal blood pressure is elevated with natriuresis and proteinuria as renal function deteriorates in nephropathy. Kidney Int. 2004;65:621–625. doi: 10.1111/j.1523-1755.2004.00419.x. [DOI] [PubMed] [Google Scholar]
- 54.Salman I.M., Hildreth C.M., Ameer O.Z., Phillips J.K. Differential contribution of afferent and central pathways to the development of baroreflex dysfunction in chronic kidney disease. Hypertension. 2014;63:804–810. doi: 10.1161/HYPERTENSIONAHA.113.02110. [DOI] [PubMed] [Google Scholar]
- 55.Tomiyama O., Shiigai T., Ideura T. Baroreflex sensitivity in renal failure. Clin Sci. 1980;58:21–27. doi: 10.1042/cs0580021. [DOI] [PubMed] [Google Scholar]
- 56.Johansson M., Gao S.A., Friberg P. Reduced baroreflex effectiveness index in hypertensive patients with chronic renal failure. Am J Hypertens. 2005;18:995–1000. doi: 10.1016/j.amjhyper.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 57.Converse R.L., Jr., Jacobsen T.N., Toto R.D. Sympathetic overactivity in patients with chronic renal failure. N Engl J Med. 1992;327:1912–1918. doi: 10.1056/NEJM199212313272704. [DOI] [PubMed] [Google Scholar]
- 58.Augustyniak R.A., Tuncel M., Zhang W. Sympathetic overactivity as a cause of hypertension in chronic renal failure. J Hypertens. 2002;20:3–9. doi: 10.1097/00004872-200201000-00002. [DOI] [PubMed] [Google Scholar]
- 59.Klein I.H., Ligtenberg G., Neumann J. Sympathetic nerve activity is inappropriately increased in chronic renal disease. J Am Soc Nephrol. 2003;14:3239–3244. doi: 10.1097/01.asn.0000098687.01005.a5. [DOI] [PubMed] [Google Scholar]
- 60.Grassi G., Quarti-Trevano F., Seravalle G. Early sympathetic activation in the initial clinical stages of chronic renal failure. Hypertension. 2011;57:846–851. doi: 10.1161/HYPERTENSIONAHA.110.164780. [DOI] [PubMed] [Google Scholar]
- 61.Schlaich M.P., Socratous F., Hennebry S. Sympathetic activation in chronic renal failure. J Am Soc Nephrol. 2009;20:933–939. doi: 10.1681/ASN.2008040402. [DOI] [PubMed] [Google Scholar]
- 62.Nielsen F.S., Hansen H.P., Jacobsen P. Increased sympathetic activity during sleep and nocturnal hypertension in Type 2 diabetic patients with diabetic nephropathy. Diabet Med. 1999;16:555–562. doi: 10.1046/j.1464-5491.1999.00127.x. [DOI] [PubMed] [Google Scholar]
- 63.Okuguchi T., Osanai T., Kamada T. Significance of sympathetic nervous system in sodium-induced nocturnal hypertension. J Hypertens. 1999;17:947–957. doi: 10.1097/00004872-199917070-00011. [DOI] [PubMed] [Google Scholar]
- 64.Kala R., Fyhrquist F., Eisalo A. Diurnal variation of plasma angiotensin II in man. Scand J Clin Lab Invest. 1973;31:363–365. doi: 10.3109/00365517309084318. [DOI] [PubMed] [Google Scholar]
- 65.Kawasaki T., Ueno M., Uezono K. Differences and similarities among circadian characteristics of plasma renin activity in healthy young women in Japan and the United States. Am J Med. 1980;68:91–96. doi: 10.1016/0002-9343(80)90177-1. [DOI] [PubMed] [Google Scholar]
- 66.Ozkayar N., Dede F., Akyel F. Relationship between blood pressure varıability and renal activity of the renin-angiotensin system. J Hum Hypertens. 2016;30:297–302. doi: 10.1038/jhh.2015.71. [DOI] [PubMed] [Google Scholar]
- 67.Isobe S., Ohashi N., Fujikura T. Disturbed circadian rhythm of the intrarenal renin-angiotensin system: relevant to nocturnal hypertension and renal damage. Clin Exp Nephrol. 2015;19:231–239. doi: 10.1007/s10157-014-0973-2. [DOI] [PubMed] [Google Scholar]
- 68.Félétou M., Vanhoutte P.M. Endothelial dysfunction: a multifaceted disorder. Am J Physiol Heart Circ Physiol. 2006;291:H985–H1002. doi: 10.1152/ajpheart.00292.2006. [DOI] [PubMed] [Google Scholar]
- 69.Bolton C.H., Downs L.G., Victory J.G. Endothelial dysfunction in chronic renal failure: roles of lipoprotein oxidation and pro-inflammatory cytokines. Nephrol Dial Transplant. 2001;16:1189–1197. doi: 10.1093/ndt/16.6.1189. [DOI] [PubMed] [Google Scholar]
- 70.Perticone F., Maio R., Perticone M. Endothelial dysfunction and subsequent decline in glomerular filtration rate in hypertensive patients. Circulation. 2010;122:379–384. doi: 10.1161/CIRCULATIONAHA.110.940932. [DOI] [PubMed] [Google Scholar]
- 71.Higashi Y., Nakagawa K., Kimura M. Circadian variation of blood pressure and endothelial function in patients with essential hypertension: a comparison of dippers and non-dippers. J Am Coll Cardiol. 2002;40:2039–2043. doi: 10.1016/s0735-1097(02)02535-4. [DOI] [PubMed] [Google Scholar]
- 72.Quinaglia T., Martins L.C., Figueiredo V.N. Non-dipping pattern relates to endothelial dysfunction in patients with uncontrolled resistant hypertension. J Hum Hypertens. 2011;25:656–664. doi: 10.1038/jhh.2011.43. [DOI] [PubMed] [Google Scholar]
- 73.Yamamoto K., Takeda Y., Yamashita S. Renal dysfunction impairs circadian variation of endothelial function in patients with essential hypertension. J Am Soc Hypertens. 2010;4:265–267. doi: 10.1016/j.jash.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 74.Elherik K., Khan F., McLaren M. Circadian variation in vascular tone and endothelial cell function in normal males. Clin Sci. 2002;102:547–552. [PubMed] [Google Scholar]
- 75.Maeda M., Kachi H., Takagi H., Kitajima Y. Is there circadian variation of plasma endothelin (ET-1) in patients with systemic scleroderma (SSc)? J Dermatol Sci. 1997;16:38–44. doi: 10.1016/s0923-1811(97)00619-1. [DOI] [PubMed] [Google Scholar]
- 76.Stow L.R., Richards J., Cheng K.Y. The circadian protein period 1 contributes to blood pressure control and coordinately regulates renal sodium transport genes. Hypertension. 2012;59:1151–1156. doi: 10.1161/HYPERTENSIONAHA.112.190892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dhaun N., Moorhouse R., MacIntyre I.M. Diurnal variation in blood pressure and arterial stiffness in chronic kidney disease: the role of endothelin-1. Hypertension. 2014;64:296–304. doi: 10.1161/HYPERTENSIONAHA.114.03533. [DOI] [PubMed] [Google Scholar]
- 78.Wilcox C.S. Oxidative stress and nitric oxide deficiency in the kidney: a critical link to hypertension? Am J Physiol Regul Integr Comp Physiol. 2005;289:R913–R935. doi: 10.1152/ajpregu.00250.2005. [DOI] [PubMed] [Google Scholar]
- 79.Terawaki H., Yoshimura K., Hasegawa T. Oxidative stress is enhanced in correlation with renal dysfunction: examination with the redox state of albumin. Kidney Int. 2004;66:1988–1993. doi: 10.1111/j.1523-1755.2004.00969.x. [DOI] [PubMed] [Google Scholar]
- 80.Schäufele T.G., Schlaich M.P., Delles C. Impaired basal NO activity in patients with glomerular disease and the influence of oxidative stress. Kidney Int. 2006;70:1177–1181. doi: 10.1038/sj.ki.5001745. [DOI] [PubMed] [Google Scholar]
- 81.Maeda K., Yasunari K., Watanabe T., Nakamura M. Oxidative stress by peripheral blood mononuclear cells is increased in hypertensives with an extreme-dipper pattern and/or morning surge in blood pressure. Hypertens Res. 2005;28:755–761. doi: 10.1291/hypres.28.755. [DOI] [PubMed] [Google Scholar]
- 82.Gönenç A., Hacışevki A., Tavil Y. Oxidative stress in patients with essential hypertension: a comparison of dippers and non-dippers. Eur J Intern Med. 2013;24:139–144. doi: 10.1016/j.ejim.2012.08.016. [DOI] [PubMed] [Google Scholar]
- 83.Abramson J.L., Lewis C., Murrah N.V. Relation of C-reactive protein and tumor necrosis factor-alpha to ambulatory blood pressure variability In healthy adults. Am J Cardiol. 2006;98:649–652. doi: 10.1016/j.amjcard.2006.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Veerabhadrappa P., Diaz K.M., Feairheller D.L. Enhanced blood pressure variability in a high cardiovascular risk group of African Americans: FIT4Life Study. J Am Soc Hypertens. 2010;4:187–195. doi: 10.1016/j.jash.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kim K.I., Lee J.H., Chang H.J. Association between blood pressure variability and inflammatory marker in hypertensive patients. Circ J. 2008;72:293–298. doi: 10.1253/circj.72.293. [DOI] [PubMed] [Google Scholar]
- 86.Stam F., van Guldener C., Schalkwijk C.G. Impaired renal function is associated with markers of endothelial dysfunction and increased inflammatory activity. Nephrol Dial Transplant. 2003;18:892–898. doi: 10.1093/ndt/gfg080. [DOI] [PubMed] [Google Scholar]
- 87.Oberg B.P., McMenamin E., Lucas F.L. Increased prevalence of oxidant stress and inflammation in patients with moderate to severe chronic kidney disease. Kidney Int. 2004;65:1009–1016. doi: 10.1111/j.1523-1755.2004.00465.x. [DOI] [PubMed] [Google Scholar]
- 88.Shankar A., Sun L., Klein B.E. Markers of inflammation predict the long-term risk of developing chronic kidney disease: a population-based cohort study. Kidney Int. 2011;80:1231–1238. doi: 10.1038/ki.2011.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.de Vinuesa S.G., Ortega M., Martinez P. Subclinical peripheral arterial disease in patients with chronic kidney disease: prevalence and related risk factors. Kidney Int. 2005;67(suppl):S44–S47. doi: 10.1111/j.1523-1755.2005.09310.x. [DOI] [PubMed] [Google Scholar]
- 90.Wattanakit K., Folsom A.R., Selvin E. Kidney function and risk of peripheral arterial disease: results from the Atherosclerosis Risk in Communities (ARIC) Study. J Am Soc Nephrol. 2007;18:629–636. doi: 10.1681/ASN.2005111204. [DOI] [PubMed] [Google Scholar]
- 91.Ford M.L., Tomlinson L.A., Chapman T.P. Aortic stiffness is independently associated with rate of renal function decline in chronic kidney disease stages 3 and 4. Hypertension. 2010;55:1110–1115. doi: 10.1161/HYPERTENSIONAHA.109.143024. [DOI] [PubMed] [Google Scholar]
- 92.Elias M.F., Davey A., Dore G.A. Deterioration in renal function is associated with increased arterial stiffness. Am J Hypertens. 2014;27:207–214. doi: 10.1093/ajh/hpt179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Briet M., Boutouyrie P., Laurent S. Arterial stiffness and pulse pressure in CKD and ESRD. Kidney Int. 2012;82:383–400. doi: 10.1038/ki.2012.131. [DOI] [PubMed] [Google Scholar]
- 94.Schillaci G., Bilo G., Pucci G. Relationship between short-term blood pressure variability and large-artery stiffness in human hypertension: findings from 2 large databases. Hypertension. 2012;60:369–377. doi: 10.1161/HYPERTENSIONAHA.112.197491. [DOI] [PubMed] [Google Scholar]
- 95.García-García Á., García-Ortiz L., Recio-Rodríguez J.I. Relationship of 24-h blood pressure variability with vascular structure and function in hypertensive patients. Blood Press Monit. 2013;18:101–106. doi: 10.1097/MBP.0b013e32835ebc58. [DOI] [PubMed] [Google Scholar]
- 96.Bahrainwala J., Patel A., Diaz K.M. Ambulatory Arterial Stiffness Index and circadian blood pressure variability. J Am Soc Hypertens. 2015;9:705–710. doi: 10.1016/j.jash.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 97.Gusbeth-Tatomir P., Covic A. Causes and consequences of increased arterial stiffness in chronic kidney disease patients. Kidney Blood Press Res. 2007;30:97–107. doi: 10.1159/000100905. [DOI] [PubMed] [Google Scholar]
- 98.London G.M. Mechanisms of arterial calcifications and consequences for cardiovascular function. Kidney Int Suppl (2011) 2013;3:442–445. doi: 10.1038/kisup.2013.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Briet M., Burns K.D. Chronic kidney disease and vascular remodelling: molecular mechanisms and clinical implications. Clin Sci (Lond) 2012;123:399–416. doi: 10.1042/CS20120074. [DOI] [PubMed] [Google Scholar]
- 100.Kitagawa M., Sugiyama H., Morinaga H. A decreased level of serum soluble Klotho is an independent biomarker associated with arterial stiffness in patients with chronic kidney disease. PLoS One. 2013;8:e56695. doi: 10.1371/journal.pone.0056695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gatzka C.D., Schobel H.P., Klingbeil A.U. Normalization of circadian blood pressure profiles after renal transplantation. Transplantation. 1995;59:1270–1274. [PubMed] [Google Scholar]
- 102.Covic A., Gusbeth-Tatomir P., Mardare N. Dynamics of the circadian blood pressure profiles after renal transplantation. Transplantation. 2005;80:1168–1173. doi: 10.1097/01.tp.0000167003.97452.a8. [DOI] [PubMed] [Google Scholar]
- 103.Wadei H.M., Amer H., Taler S.J. Diurnal blood pressure changes one year after kidney transplantation: relationship to allograft function, histology, and resistive index. J Am Soc Nephrol. 2007;18:1607–1615. doi: 10.1681/ASN.2006111289. [DOI] [PubMed] [Google Scholar]
- 104.Wadei H.M., Amer H., Griffin M.D. Abnormal circadian blood pressure pattern 1-year after kidney transplantation is associated with subsequent lower glomerular filtration rate in recipients without rejection. J Am Soc Hypertens. 2011;5:39–47. doi: 10.1016/j.jash.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 105.Lee M.H., Ko K.M., Ahn S.W. The impact of kidney transplantation on 24-hour ambulatory blood pressure in end-stage renal disease patients. J Am Soc Hypertens. 2015;9:427–434. doi: 10.1016/j.jash.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 106.Hermida R.C., Ayala D.E., Smolensky M.H., Portaluppi F. Chronotherapy in hypertensive patients: administration-time dependent effects of treatment on blood pressure regulation. Expert Rev Cardiovasc Ther. 2007;5:463–475. doi: 10.1586/14779072.5.3.463. [DOI] [PubMed] [Google Scholar]
- 107.Hermida R.C., Ayala D.E., Fernández J.R. Administration-time differences in effects of hypertension medications on ambulatory blood pressure regulation. Chronobiol Int. 2013;30:280–314. doi: 10.3109/07420528.2012.709448. [DOI] [PubMed] [Google Scholar]
- 108.Hermida R.C., Ayala D.E., Mojón A., Fernández J.R. Chronotherapy with nifedipine GITS in hypertensive patients: improved efficacy and safety with bedtime dosing. Am J Hypertens. 2008;21:948–954. doi: 10.1038/ajh.2008.216. [DOI] [PubMed] [Google Scholar]
- 109.Hermida R.C., Ayala D.E., Fernández J.R. Circadian rhythms in blood pressure regulation and optimization of hypertension treatment with ACE inhibitor and ARB medications. Am J Hypertens. 2011;24:383–391. doi: 10.1038/ajh.2010.217. [DOI] [PubMed] [Google Scholar]
- 110.Minutolo R., Gabbai F.B., Borrelli S. Changing the timing of antihypertensive therapy to reduce nocturnal blood pressure in CKD: an 8-week uncontrolled trial. Am J Kidney Dis. 2007;50:908–917. doi: 10.1053/j.ajkd.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 111.Hermida R.C., Ayala D.E., Mojón A., Fernández J.R. Bedtime dosing of antihypertensive medications reduces cardiovascular risk in CKD. J Am Soc Nephrol. 2011;22:2313–2321. doi: 10.1681/ASN.2011040361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hermida R.C., Ayala D.E., Mojon A., Fernandez J.R. Influence of time of day of blood pressure-lowering treatment on cardiovascular risk in hypertensive patients with type 2 diabetes. Diabetes Care. 2011;34:1270–1276. doi: 10.2337/dc11-0297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rahman M., Greene T., Phillips R.A. A trial of 2 strategies to reduce nocturnal blood pressure in blacks with chronic kidney disease. Hypertension. 2013;61:82–88. doi: 10.1161/HYPERTENSIONAHA.112.200477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wang C., Zhang J., Liu X. Effect of valsartan with bedtime dosing on chronic kidney disease patients with non-dipping blood pressure pattern. J Clin Hypertens. 2013;15:48–54. doi: 10.1111/jch.12021. [DOI] [PMC free article] [PubMed] [Google Scholar]