A common response to DNA damage, such as DNA double strand breaks (DSBs), is the inhibition of cell division to provide time for repair and prevent propagation of mutations. DSBs are sensed by the kinase Ataxia telangiectasia-mutated (Atm) that initiates a regulatory cascade resulting in the inactivation of cyclin-dependent kinases (CDKs; Fig. 1).1 However, CDK activity has been found to be required for homology-dependent repair (HR), also called homologous recombination repair, giving rise to the question how this prominent DNA repair pathway can be triggered at times of mitotic arrest.
Figure 1.
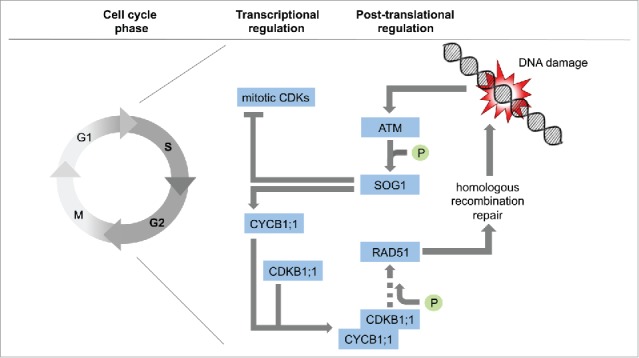
DNA damage triggers the checkpoint kinase ATM, which activates the transcription factor SOG1. SOG1 directly promotes the expression of CYCB1;1 while repressing mitotic CDKs. CYCB1;1 together with the plant-specific CDKB1 kinases build an active complex, needed for HR and the recruitment of the central HR mediator RAD51 to DNA lesions. (Importance of RAD51 phosphorylation by CDKB1 not clear yet).
In the recent issue of The EMBO Journal, we present data that address this question and describe how this apparent conflict of CDK regulation in the DNA damage response is tackled in Arabidopsis thaliana.2 Here, we briefly summarize these findings and raise some questions for future work.
Following up earlier observations that CYCLIN B1;1 (CYCB1;1), a mitotic cyclin in the model plant Arabidopsis thaliana, is often upregulated under DNA damage, e.g. after ionizing radiation,3 we tested the growth of mutants of CYCB1;1 and its 3 sister genes in the B1-cyclin class, i.e. CYCB1;2, CYCB1;3 and CYCB1;4, on different DNA-damage inducing media. While mutants in all 4 cyclin genes grew indistinguishably from the wildtype on the S-phase stress-triggering substance hydroxy urea and the DSB-inducing drug Bleomycin, all mutants were hypersensitive to cisplatin, which induces DNA crosslinks that require homology dependent repair for dissolution. This growth reduction was associated with high levels of DNA damage as revealed by a large number of yH2AX foci and an increased tail in comet assays. Subsequently, we found that homologous recombination is severely reduced in all cycb1 mutants.
In search for the kinase partner of the B1-type cyclins, we tested growth of mutants in CDKA;1, the combined Cdk1 and Cdk2 homolog of Arabidopsis, on cisplatin. CDKA;1 is constitutively expressed during the cell cycle and represents the major cell-cycle promoting kinase in Arabidopsis.4 However, plants with reduced CDKA;1 activity levels were not hypersensitive to cisplatin or other DNA damage-inducing drugs. Plants contain a specific class of B-type CDKs whose transcription accumulates from S-phase till mitosis when a sister chromatid is available for repair. Double mutants of both B1-type CDKs (referred to here as cdkb1) in Arabidopsis have been previously analyzed and only showed mild developmental defects under unperturbed conditions.5 In contrast, when grown on cisplatin and bleomycin, cdkb1 mutants were much more affected than the wildtype. Quantification of the DNA damage in cdkb1 mutants revealed that they accumulated as much damage as atm mutants, placing them in the group of major DNA damage response regulators in plants. Since the triple mutant cycb1;1 cdkb1;1 cdkb1;2 showed no additional sensitivity on cisplatin, we concluded that indeed CDKB1, but not other Cdks, are the major partners of CYCB1s during DNA damage response.
In search of targets of CDKB1-CYCB1 action, we found that this complex could efficiently phosphorylate RAD51 in vitro, the central HR protein.6 Conversely, we found that the number of RAD51 foci was strongly reduced in cycb1 and cdkb1 mutants, consistent with their low levels of recombination activity and strong hypersensitivity to cisplatin. However, it remains to be seen whether phosphorylation is directly required for proper RAD51 accumulation at DNA damage sites. In addition, it is likely that the CDKB1-CYCB1 complex has other, yet to be identified targets during HR.
As a last question, we addressed how CYCB1 accumulates upon DNA damage. To this end, we first confirmed that CYCB1, as well as CDKB1;1, are transcriptionally upregulated in plants treated with cisplatin. While a p53 homolog is not present in Arabidopsis and other plants analyzed so far, plants do contain a transcriptional regulator, called SUPRESSOR OF GAMMA RESPONSE 1 (SOG1) that appears to act similarly to p53 in animals.7 SOG1 is a target of ATM action and previous work has shown that CYCB1;1 is not upregulated in sog1 mutants when exposed to ionizing radiation.7 At the same time, core mitotic regulators, which are usually downregulated in the wildtype upon DNA damage, remain strongly expressed in sog1 mutants indicating a failure to arrest cell proliferation.7 Here, we found that SOG1 directly binds to the promoter region of CYCB1;1 indicating that SOG1 activates CYCB1;1 expression upon DNA damage.
In Weimer et al,2 we conclude that, while the major mitotic force, including mitosis-specific CDKs, is inactivated upon DNA damage in a SOG1-dependent manner, SOG1 directly induces CYCB1 expression and activates CDKB1-CYCB1 complexes, which can then mediate HR (Fig. 1). The mild developmental defects of cdkb1 mutants suggest that active CDKB1s by themselves cannot efficiently trigger cell proliferation and hence under stress conditions, plants can tolerate their activation to promote HR while still staying in an arrested cell cycle phase.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- [1].Yata K, Esashi F. Dual role of CDKs in DNA repair: to be, or not to be. DNA Repair (Amst) 2009; 8:6-18; PMID:18832049; http://dx.doi.org/ 10.1016/j.dnarep.2008.09.002 [DOI] [PubMed] [Google Scholar]
- [2].Weimer AK, Biedermann S, Harashima H, Roodbarkelari F, Takahashi N, Foreman J, Guan Y, Pochon G, Heese M, Van Damme D, et al.. The plant-specific CDKB1-CYCB1 complex mediates homologous recombination repair in Arabidopsis. EMBO J 2016; 35(19):2068-2086; PMID:27497297; http://dx.doi.org/19010080 10.15252/embj.201593083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Cools T, De Veylder L. DNA stress checkpoint control and plant development. Curr Opin Plant Biol 2009; 12:23-8; PMID:19010080; http://dx.doi.org/ 10.1016/j.pbi.2008.09.012 [DOI] [PubMed] [Google Scholar]
- [4].Nowack MK, Harashima H, Dissmeyer N, Zhao X, Bouyer D, Weimer AK, De Winter F, Yang F, Schnittger A. Genetic framework of cyclin-dependent kinase function in Arabidopsis. Dev Cell 2012; 22:1030-40; PMID:22595674; http://dx.doi.org/ 10.1016/j.devcel.2012.02.015 [DOI] [PubMed] [Google Scholar]
- [5].Gutierrez C. 25 years of cell cycle research: What’s ahead. Trends Plant Sci 2016; 21(10):823–833; PMID:27401252; http://dx.doi.org/19549833 10.1016/j.tplants.2016.06.007 [DOI] [PubMed] [Google Scholar]
- [6].Kowalczykowski SC. An overview of the molecular mechanisms of recombinational DNA repair. Cold Spring Harb Perspect Biol 2015; 7(11):1–36; PMID:26525148; http://dx.doi.org/19549833 10.1101/cshperspect.a016410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Yoshiyama K, Conklin PA, Huefner ND, Britt AB. Suppressor of gamma response 1 (SOG1) encodes a putative transcription factor governing multiple responses to DNA damage. Proc Natl Acad Sci U S A 2009; 106:12843-8; PMID:19549833; http://dx.doi.org/ 10.1073/pnas.0810304106 [DOI] [PMC free article] [PubMed] [Google Scholar]


