Abstract
We hypothesized that maintaining a normal electrocardiogram (ECG) status over time is associated with low cardiovascular (CV) disease in a dose-response fashion and subsequently could be used to monitor programs aimed at promoting CV health. This analysis included 4,856 CV disease-free participants from the ARIC study who had a normal ECG at baseline [1987–1989] and complete ECG data in subsequent three visits [1990–1992, 1993–1995, 1996–1998]. Participants were classified based on maintaining their normal ECG status during these four visits into “maintained”, “not-maintained” or “inconsistent” normal ECG status as defined by the Minnesota ECG classification. CV disease events [coronary heart disease, heart failure and stroke] were adjudicated from ARIC visit-4 through 2010. Over a median follow up of 13.2 years, 885 CV disease events occurred. The incidence rate of CV disease events was lowest among study participants who maintained a normal ECG status, followed by those with an inconsistent pattern, then those who did not maintain their normal ECG status (trend p-value<0.001). Similarly, the greater the number of visits with a normal ECG status, the lower was the incidence rate of CV disease events (trend p-value<0.001). Maintaining (vs. not-maintaining) a normal ECG status was associated with a lower risk of CV disease, which was lower than that observed in those with inconsistent normal ECG pattern (trend p-value<0.01). In conclusion, maintaining a normal ECG status over time is associated with low risk of CV disease in a dose-response fashion, suggesting its potential use as a monitoring tool for programs promoting CV health.
Keywords: Cardiovascular Health, Normal Electrocardiogram, ARIC study
INTRODUCTION
An abnormal ECG could be triggered by a wide variety of diseases that are not limited to structural and functional abnormalities of the cardiac muscle, but also neurohormonal abnormalities and electrolyte imbalance. Although presence of an abnormal ECG regardless of its cause has been associated with poor outcomes (1–13), the heterogeneity of the pathophysiological basis of an abnormal ECG requires considering certain ECG abnormalities and not others when it comes to accurate prediction of outcomes. This is unlike a normal ECG which simply means no deleterious effect on the heart by any factor i.e. good CV health. Assessment of CV health using an objective simple tool, such as the ECG, not only could help appropriately allocating resources to high risk groups but also could help assessing and monitoring the success of programs and interventions aimed to maintain CV health. That is to say, a normal ECG over time could be considered as a byproduct of CV health and hence could be used as a monitoring tool for the success of the actions implemented by CV health programs. Hence, we hypothesized that maintaining a normal ECG status over time is associated with a low risk of CV disease [i.e. CV health] in a dose-response fashion. We tested our hypothesis using data from the Atherosclerosis Risk in Communities [ARIC] Study, a community-based predominantly biracial cohort study.
METHODS
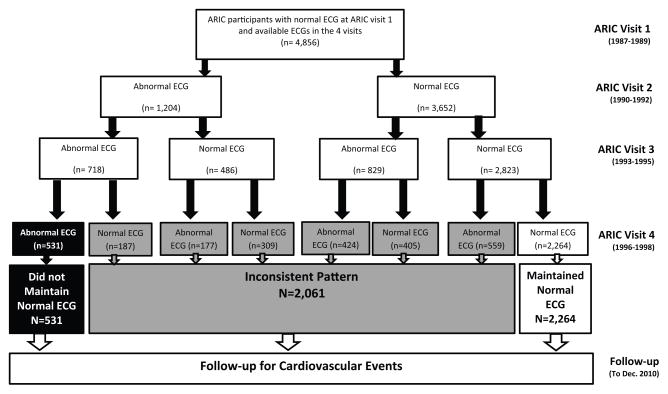
The ARIC study was designed to investigate causes of atherosclerosis and its clinical outcomes, as well as variation in cardiovascular risk factors, medical care, and disease by race and sex [14]. A total of 15,792 adults from Washington County, Maryland; suburbs of Minneapolis, Minnesota; Jackson, Mississippi; and Forsyth County, North Carolina were enrolled. After baseline visit [1987 to 1989, ARIC visit 1], three additional examinations were conducted in 1990–1992 [visit 2], 1993–1995 [visit 3], and 1996–1998 [visit 4]. The study was approved by each study site’s institutional review board. All participants provided written informed consent. For the purpose of this study we restricted the analysis sample to black and white ARIC participants with a normal ECG at baseline [visit 1], complete ECG data in all of the four ARIC visits and available follow up data after visit 4. Therefore, the following exclusions were made: 5,047 participants with incomplete ECG data in any of the four ARIC visits, 1,628 participants with history of CV disease prior or at visit 4, and 4,232 participants with any minor or major ECG abnormality at baseline. Similar to prior ARIC publications, we also excluded non-white and non-black individuals, as well as non-whites from the Minnesota and Washington sites [n=29]. The remaining sample [n=4,856] were then classified based on maintaining a normal ECG during all of the four ARIC visits into either “maintained”, “not maintained” or “inconsistent” normal ECG status, and were followed for outcomes until December 31, 2010 (Figure 1).
Figure 1.
Selection of various subgroups of the study population
Identical electrocardiographs [MAC PC, Marquette Electronics Inc., Milwaukee, Wisconsin] were used at all ARIC clinical sites, and resting standard 12-lead ECGs were recorded in all participants using strictly standardized procedures. All ECGs were processed in a central ECG laboratory [EPICARE Center, Wake Forest School of Medicine, Winston-Salem, North Carolina, USA]. ECGs were automatically processed using GE Marquette 12-SL 2001 [GE, Milwaukee, Wisconsin] with visual quality control by the central ECG laboratory staff. Presence of ECG abnormalities was defined using the standards of Minnesota ECG Classification (15). Major ECG abnormalities included major ventricular conduction defects [complete left or right bundle branch block, major ventricular conduction delay with QRS≥120 ms], definite and possible myocardial infarction, isolated major ST/T wave abnormalities, left ventricular hypertrophy with strain pattern, advanced atrioventricular conduction abnormalities, pacemaker, atrial fibrillation/flutter, and others. Minor ECG abnormalities included minor isolated Q/QS wave abnormalities, minor isolated ST/T abnormalities, high R waves/increased QRS voltage denoting left or right ventricular hypertrophy without strain pattern, non-ischemic ST segment elevation, incomplete [left or right] bundle branch block, short PR interval, left axis deviation, right axis deviation, atrial and ventricular premature beats, and others. A normal ECG was defined as absence of any minor or major ECG abnormality as defined by Minnesota ECG Classification (15).
The main outcome in this study was adjudicated incident CV disease, a composite of coronary heart disease, heart failure and stroke. An incident CV disease event was defined as the first occurrence of a fatal or non-fatal coronary heart disease, heart failure or stroke event from visit 4 [1996–1998] through December 31, 2010. Details on ascertainment of CV disease outcomes have been published before (16–19). Briefly, the components of the CV disease composite outcome were determined by physicians using validated adjudication protocols and review of ICD codes of hospital discharge records. Stroke was defined as sudden neurologic insult of > 24 hour duration or a neurologic insult associated with death without evidence of a non-stroke cause of death. Stroke events were ascertained from surveillance of ARIC participant hospitalizations using ICD-9 codes 430–438 through 1997 and codes 430–436 thereafter. Strokes were classified by physician review and computer algorithm with standardized criteria to confirm the diagnosis and type of stroke (16). Heart failure was ascertained by review of hospitalization records and death certificates for a heart failure diagnosis. Specifically, incident cases with an ICD-9 code of 428 [428.0–428.9] or ICD-10 Revision I50 were classified as heart failure (17). Coronary heart disease was determined using study surveillance as previously described (18, 19). Symptoms, biomarkers, and ECGs were incorporated into a computerized algorithm. Disagreement between discharge coding and computer algorithm were adjudicated by the ARIC Mortality and Morbidity Classification Committee. For the present analysis, coronary heart disease was defined as definite or probable myocardial infarction or definite fatal coronary heart disease.
Baseline characteristics were tabulated and compared by the status of maintaining a normal ECG status during the four ARIC visits [maintained, not maintained and inconsistent]. Age-adjusted incidence rate of CV disease events per 1000 person-years in each group of participants was calculated, and Kaplan-Meier survival curves were plotted to compare event-free survival curves across these levels starting from ARIC visit 4. Cox proportional hazards analysis was used to examine the association between maintaining a normal ECG and an inconsistent pattern, separately [vs. not maintaining normal ECG status] with the risk of CV disease in a series of models with incremental adjustments as follows: Model 1 adjusted for baseline [visit 1] age, sex, race, study site, and education level; Model 2 adjusted for model 1 covariates plus baseline [visit 1] body mass index, diabetes, hypertension, dyslipidemia, and smoking status. Additional analyses included: 1) Examining the association between the number of visits with a normal ECG status and risk of CV disease. Having a normal ECG in only 1 visit [which is eventually the baseline visit 1 since the entire sample had initially a normal ECG] was used as the reference group. The aim of this analysis was to examine the dose-response relationship between years with a normal ECG status and adverse outcomes; 2) Subgroup analysis stratified by median age, sex, race and ideal CV disease risk profile defined as never smoked, body mass index <25 kg/m2, untreated total cholesterol <200 mg/dL, untreated blood pressure <120/<80 mmHg, and untreated fasting blood glucose <100 mg/dL (20). Interactions were examined in model 2; 3) Using each of the components of the composite CV disease outcome separately [coronary heart disease, heart failure, and stroke] in models similar to those used for the main outcome and 4) Examining the association between a normal [vs. an abnormal] ECG at visit 4 [i.e. single time point] with CV disease events adjusting for participants’ characteristics at that time. This analysis serves two purposes: First, to confirm results from prior studies that an abnormal ECG is associated with poor CV disease outcome. Second, showing that the favorable association between a normal ECG and CV disease risk at a single point of time is less than that observed with maintaining a normal ECG over time is another indication of dose-response relationship.
RESULTS
This analysis included 4,856 participants [mean age 53 years, 66% women, 16% blacks] who had a normal ECG at the time of enrollment in the ARIC study [ARIC visit 1; 1987–1989]). Out of those, 2,264 participants maintained their normal ECG status until ARIC visit 4 [1996–1998], 531 participants developed and maintained their abnormal ECG status after visit 1, and 2,061 had an inconsistent pattern. Table 1 shows the baseline characteristics of the study population stratified by the status of maintaining normal ECG during the four ARIC visits. Participants who maintained their normal ECG status were more likely to be young, women, whites and with more favorable cardiovascular risk profile compared to those who did not maintain their normal ECG status or those with an inconsistent pattern.
Table 1.
Baseline characteristics stratified by maintaining a normal electrocardiogram*
| Characteristic | Maintained Normal ECG Status (N=2,264) | Inconsistent Pattern (N=2,061) | Did Not Maintain Normal ECG Status (N=531) | p-value† |
|---|---|---|---|---|
| Mean ± SD or % | ||||
| Age (years) | 53±5.5 | 54±5.6 | 54±5.9 | <.001 |
| Women | 71.9 % | 63.5% | 55.2% | <.001 |
| Black | 13.8% | 17.3% | 21.3% | <.001 |
| Education ≥ high school | 51.9% | 48.6% | 46.7% | 0.028 |
| Body mass index(kg/m2) | 26 ±4.6 | 27 ±5.1 | 28±5.1 | <.0001 |
| Current smoker | 19.4% | 21.5% | 21.5% | 0.093 |
| Diabetes Mellitus | 5.6% | 6.8% | 7.9% | 0.073 |
| Hypertension | 16.9% | 25.4% | 32.4% | <.001 |
| Antihypertensive medication use | 16.1% | 21.2% | 26.4% | <.001 |
| Systolic blood pressure (mmHg) | 114 ±14.7 | 118±16.0 | 121±16.8 | <.0001 |
| Diastolic blood pressure (mmHg) | 71±9.4 | 72±9.9 | 74±10.7 | <.001 |
| Total cholesterol (mg/dL) | 212±39.3 | 213±39.6 | 213±39.3 | 0.980 |
| HDL- cholesterol (mg/dl) | 56 ±17.1 | 53±17.5 | 52±39.3 | <.001 |
| Statin use | 0.4% | 0.6% | 0.4% | 0.640 |
| Ideal cardiovascular risk profile‡ | 5.7% | 4.3% | 3.2% | 0.014 |
Defined based on the presence of a normal ECG from ARIC baseline visit (ARIC visit 1; 1987–1989) to visit ARIC visit 4 (1996–1998)
p-value for differences in the three groups using ANOVA for continuous variables or Chi2 for categorical variables
Ideal cardiovascular health metrics defined as never smoked, body mass index <25 kg/m2, untreated total cholesterol <200 mg/dL, untreated blood pressure <120/<80 mmHg, and untreated fasting blood glucose <100 mg/dL
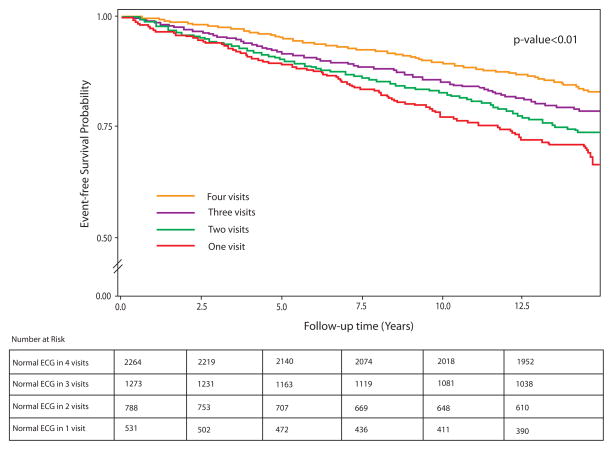
Over a median follow up of 13.2 years, 885 cardiovascular events occurred. The incidence rate of cardiovascular events was lowest among study participants who maintained a normal ECG status, followed by those with an inconsistent pattern, then those who did not maintain their normal ECG status [incidence rates 6.8, 10.0, and 13.7 per 1000 person-years, respectively, p<0.001 for trend). Similarly, the greater the number of visits with a normal ECG status, the lower was the incidence rate of cardiovascular events [incidence rates 6.8, 9.2, 11.4 and 13.7 per 1000 person-years in those who maintained their normal ECG status in 4, 3, 2 and 1 visits, respectively, p<0.001 for trend]. Figure 2 and Figure 3 show the event free survival curves stratified by maintaining a normal ECG status and by number of visits with a normal ECG status, respectively.
Figure 2.
Kaplan Meier event-free survival curves for cardiovascular events stratified by maintaining normal ECG status
Figure 3.
Kaplan Meier event-free survival curves for cardiovascular events stratified by number of visits with normal electrocardiogram status
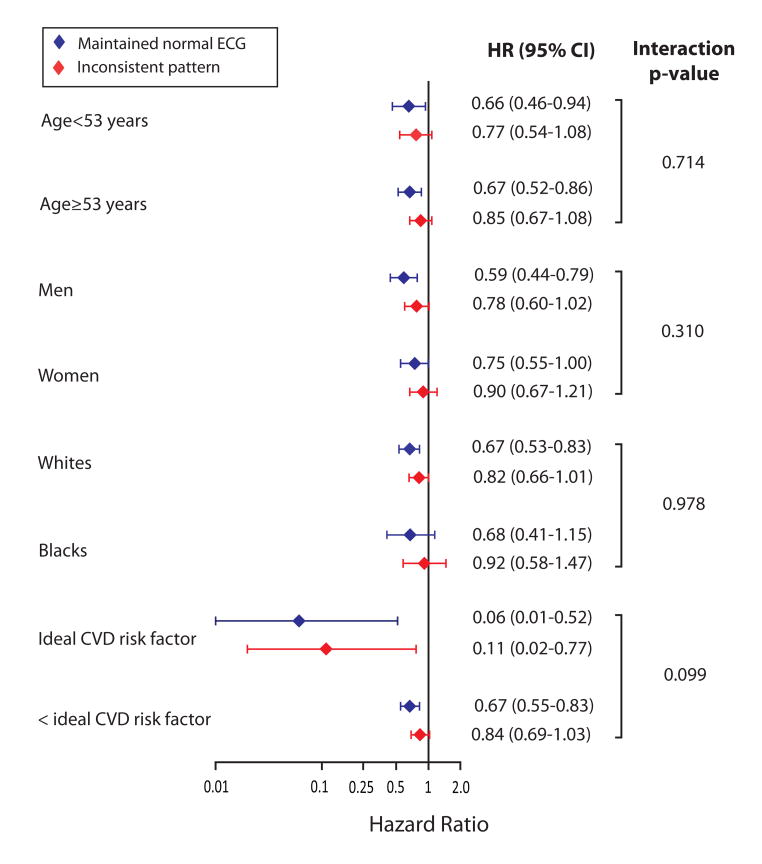
In multivariable-adjusted Cox proportional hazard model, maintaining [compared to not maintaining] a normal ECG status was associated with 41% [p<0.001] lower risk of CV disease events in the demographic-adjusted model which was slightly attenuated to 34% [p<0.001] after adjustment for baseline CV disease risk factors [Table 2]. Also, the results were consistent in subgroups stratified by median age [57 years], sex and race. However, the low risk of CV disease events associated with maintaining a normal ECG status was much more pronounced in the study participants with ideal [compared to those with less than ideal] cardiovascular risk factor profile at baseline, 94% vs. 33% lower risk, interaction p=0.098 [Figure 4].
Table 2.
Association between maintaining normal electrocardiogram and incident cardiovascular disease events
| Variable | Participants (n) | Events† (n) | Event Rate /1000 P-Year | Model 1‡ HR (95% CI) | Model 2# HR (95% CI) | |
|---|---|---|---|---|---|---|
| Normal ECG status* | Not maintained | 531 | 144 | 13.7 | Reference | Reference |
| Inconsistent Pattern | 2061 | 420 | 10.0 | 0.79 (0.65–0.95) | 0.83 (0.68–1.01) | |
| Maintained | 2264 | 321 | 6.8 | 0.59 (0.48–0.71) | 0.66 (0.54–0.81) | |
|
| ||||||
| Visits with normal ECG* | Only one visit | 531 | 144 | 13.7 | Reference | Reference |
| Two visits | 788 | 181 | 11.4 | 0.87 (0.70–1.08) | 0.88 (0.65–1.10) | |
| Three visits | 1273 | 239 | 9.2 | 0.74 (0.60–0.91) | 0.80 (0.65–0.99) | |
| Four visits | 2264 | 321 | 6.8 | 0.58 (0.48–0.71) | 0.66 (0.54–0.81) | |
Defined based on the presence of a normal electrocardiogram from ARIC baseline visit (ARIC visit 1; 1987–1989) to visit ARIC visit 4 (1996–1998)
Ascertained after ARIC visit 4 (1996–1998) through 2010
Adjusted for baseline (ARIC visit 1) age, sex, race, study field center, and education level.
Adjusted for Model 1 covariates plus baseline (ARIC visit 1) body mass index, diabetes, hypertension, dyslipidemia, and smoking status.
Figure 4.
Association between maintaining normal electrocardiogram status and incident cardiovascular disease events in subgroup analyses
The dose-response relationship between follow up time on a normal ECG [i.e. maintaining a normal ECG] with favorable CV disease risk was evident in different analyses. First, the hazard ratio for CV disease in those with an inconsistent normal pattern was mid-way between those who maintained and those who did not maintain a normal ECG status [Table 2]. Second, the greater the number of visits with a normal ECG status, the lower was the observed risk of CV disease in the Cox proportional hazard models [Table 2]. Third, a normal [compared to an abnormal] ECG status at visit 4 as a single time point was associated with a lower risk of CV disease [multivariable adjusted HR (95%CI): 0.81 (0.68–0.97], this favorable association was much less than that observed with maintaining a normal ECG in all of the four ARIC visits [HR (95%CI): 0.66 (0.54–0.81)]. Similar patterns of associations were observed when each of the components of the composite CV disease outcome [coronary heart disease, heart failure, and stroke] was used individually in models adjusted in a similar fashion to those used for the main outcome. That is to say, maintaining [compared to not maintaining] a normal ECG status over time was associated with a lower risk of coronary heart disease, heart failure and stroke in a dose-response fashion [Table 3].
Table 3.
Association between maintaining normal electrocardiogram and coronary heart disease, heart failure and stroke
| ECG status * | Subgroup* | Event Rate/1000 P-Year | HR (95% CI) ‡ | |
|---|---|---|---|---|
|
| ||||
| Coronary Heart Disease† | Normal ECG status* | Not maintained | 7.5 | Reference |
| Inconsistent Pattern | 5.6 | 0.86 (0.66–1.11) | ||
| Maintained | 4.1 | 0.74 (0.57–0.97) | ||
|
| ||||
| Visits with normal ECG* | Only one visit | 7.5 | Reference | |
| Two visits | 6.4 | 0.94 (0.70–1.27) | ||
| Three visits | 5.1 | 0.80 (0.61–1.07) | ||
| Four visits | 4.1 | 0.74 (0.57–0.97) | ||
|
| ||||
| Heart Failure† | Normal ECG status* | Not maintained | 6.1 | Reference |
| Inconsistent Pattern | 4.3 | 0.82 (0.61–1.10) | ||
| Maintained | 2.7 | 0.62 (0.45–0.85) | ||
|
| ||||
| Visits with normal ECG* | Only one visit | 6.0 | Reference | |
| Two visits | 5.1 | 0.86 (0.62–1.21) | ||
| Three visits | 3.8 | 0.79 (0.57–1.09) | ||
| Four visits | 2.7 | 0.62 (0.45–0.85) | ||
|
| ||||
| Stroke† | Normal ECG status* | Not maintained | 3.2 | Reference |
| Inconsistent Pattern | 2.2 | 0.88 (0.59–1.30) | ||
| Maintained | 1.4 | 0.64 (0.42–0.98) | ||
|
| ||||
| Visits with normal ECG* | Only one visit | 3.2 | Reference | |
| Two visits | 2.4 | 0.86 (0.54–1.36) | ||
| Three visits | 2.1 | 0.89 (0.58–1.38) | ||
| Four visits | 1.4 | 0.64 (0.42–0.98) | ||
Defined based on the presence of normal electrocardiogram from ARIC baseline visit (ARIC visit 1; 1987–1989) to visit ARIC visit 4 (1996–1998)
Ascertained after ARIC visit 4 (1996–1998) through 2010
Adjusted for baseline (ARIC visit 1) age, sex, race, study field center, and education level, body mass index, diabetes, hypertension, dyslipidemia, and smoking status.
DISCUSSION
In this analysis from the ARIC study we examined the association between maintaining a normal ECG status over time with incident CV disease events. Our hypothesis was that maintaining a normal ECG status over time is associated with low risk of CV disease in a dose-response relationship i.e. the greater the time with normal ECG status, the lower the risk of CV disease. Indeed, the key findings from our study support this hypothesis: 1) In individual with initially normal ECG status and no history of CV disease, maintaining [compared to not maintaining] a normal ECG status over time was associated with significantly lower risk of CV disease [coronary heart disease, heart failure, stroke and a composite of the three]; 2) The lower risk of CV disease associated with maintaining a normal ECG status was positively correlated with the number of follow-up visits with a normal ECG status in a dose-response relationship; 3) The lower risk associated with maintaining a normal ECG status was greater in those who had ideal cardiovascular risk profile. These findings suggest that maintaining a normal ECG status over time is associated with cardiovascular health, and hence, it could be used as a monitoring tool for programs aimed at promoting cardiovascular health as well as identifying individuals at low risk for CV disease.
The American Heart Association [AHA] has set forth a goal “to improve the cardiovascular health of all Americans by 20% while reducing deaths from CV disease by 20%” by 2020 (21). Traditionally, there has been a dominant focus on treating disease, which inevitably drives healthcare costs higher. However, in order to achieve the goal defined by the AHA, there is a need for a paradigm shift allowing for more emphasis on comprehensive assessment of CV health and implementation of health promoting measures (22). The Goals and Metrics Committee of the Strategic Planning Task Force of the AHA has outlined seven metrics to achieve ideal CV health [nonsmoking, body mass index <25 kg/m2, physical activity at goal levels, diet consistent with current guideline recommendations, untreated total cholesterol <200 mg/dL, untreated blood pressure <120/80 mm Hg, and fasting blood glucose <100 mg/dL] (21). These metrics provide a blueprint for achieving CV health. Nevertheless, genetic and environmental factors are known to impact and modify individuals’ responses to CV disease risk factors [or the aforementioned AHA CV health metrics for that matter] (23). This requires an objective intermediate endpoint, such as maintaining a normal ECG, to monitor the favorable impact of implementing these health metrics, and not simply depending on reaching certain levels of these factors. Therefore, given our results, it may be reasonable to suggest maintaining normal ECG status as an additional tool to assess the success of programs aimed to promote CV health.
One of the key applications of the AHA simple seven health metrics is their use as tools to risk stratify individuals as having poor, intermediate, or ideal CV health (21). This classification could help allocating resources and interventions to those at risk of CV disease. As we showed, maintaining a normal ECG status was associated with a remarkably lower risk of future CV disease in individuals with compared to those with less than ideal cardiovascular risk profile [94% vs. 33% lower risk]. This means that maintaining a normal ECG could also be used as a complementary component to tools used to for risk stratification of CV disease.
As could be expected, the prevalence of traditional CV disease risk factors was lower and the prevalence of ideal cardiovascular risk profile was higher in our study participants who maintained a normal compared to those with an inconsistent normal ECG or those who did not maintain a normal ECG status. However, there was a significant proportion of participants with prevalent CV disease risk factors who maintained a normal ECG status; 16.9% had hypertension and 5.6% had diabetes at baseline [ARIC visit 1]. It is not clear why those individuals managed to maintain a normal ECG for almost a decade [from ARIC visit 1 to visit 4] despite having major CV disease risk factors. Whether this could be explained by genetic or environmental factors that prevent the harmful impact of CV disease risk factors in certain individuals, or whether this could be due to better management of these risk factors is something that requires further studies. Examining the relationship between maintaining a normal ECG status and cardiac function and structure as assessed by imaging could also shed light on this observation, as well as the reasons for favorable outcomes associated with maintaining a normal ECG.
Our results should be read in the context of certain limitations. Per design we had to exclude a large proportion of the original ARIC cohort, which might have caused selection bias. Another potential limitation is the heterogeneity of ECG abnormalities when lumped together as minor/major. However, such heterogeneity would impact the analysis if we are using major/minor abnormalities as the exposure variable; instead we used a normal ECG which should provide a homogenous group. Finally, similar to other observational studies, residual confounding remains a possibility despite adjusting for several potentially important confounders. Our study has several notable strengths as well. This includes large sample with good representation of women and blacks as well as long follow up with well-ascertained outcomes identified by independent adjudication committee. The ECG data were systemically collected using standardized protocol including the use of an electrode locator to standardize the location of chest electrodes. Also, the ECG reading was conducted in a central core laboratory using standard ECG classification of abnormalities; the Minnesota ECG classification.
Acknowledgments
The authors thank the staff and participants of the ARIC study for their important contributions. The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute (NHLBI) contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C).
Footnotes
Conflict of Interest: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chou R, Arora B, Tracy D, Fu R, Walker M, Humphrey L. Screening asymptomatic adults with resting or exercise electrocardiography: a review of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2011;155:375–385. doi: 10.7326/0003-4819-155-6-201109200-00006. [DOI] [PubMed] [Google Scholar]
- 2.Denes P, Larson JC, Lloyd-Jones DM, Prineas RJ, Greenland P. Major and minor ECG abnormalities in asymptomatic women and risk of cardiovascular events and mortality. JAMA. 2007;297:978–985. doi: 10.1001/jama.297.9.978. [DOI] [PubMed] [Google Scholar]
- 3.Auer R, Bauer DC, Marques-Vidal P, Butler J, Min LJ, Cornuz J, Satterfield S, Newman AB, Vittinghoff E, Rodondi N. Association of major and minor ECG abnormalities with coronary heart disease events. JAMA. 2012;307:1497–1505. doi: 10.1001/jama.2012.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Denes P, Lloyd-Jones D, Garside DB, Gouskova N, Soliman EZ, Ostfeld R, Zhang ZM, Camacho A, Prineas R, Raij L, Daviglus ML. Major and minor electrocardiogram abnormalities and their association with underlying cardiovascular disease and risk factors in Hispanics/Latinos (From the Hispanic Community Health Study/Study of Latinos [HCHS/SOL]) Am J Cardiol. 2013;112:1667–1675. doi: 10.1016/j.amjcard.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soliman EZ, Prineas RJ, Boccara F, Duprez D, Roediger M, Stein J, Lundgren J, Boesecke C, Stephan C, Hodder S, Neaton J. Prevalence and prognostic significance of ECG abnormalities in HIV-infected patients: Results from The Strategies for Management of Antiretroviral Therapy (SMART) trial. J Electrocardiol. 2011;44:779–785. doi: 10.1016/j.jelectrocard.2010.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prineas RJ, Le A, Soliman EZ, Zhang ZM, Howard VJ, Ostchega Y, Howard G. US National prevalence of electrocardiographic abnormalities in black and white middle aged (45–64 Years) and older ≥ 65 Years) adults (From the Reasons For Geographic and Racial Differences In Stroke Study) Am J Cardiol. 2012;109:1223–1228. doi: 10.1016/j.amjcard.2011.11.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sellers MB, Divers J, Lu L, Xu J, Smith SC, Bowden DW, Herrington DH, Freedman BI, Soliman EZ. Prevalence and determinants of electrocardiographic abnormalities in African Americans with type 2 diabetes. J Epidemiol Glob Health. 2014;4:289–296. doi: 10.1016/j.jegh.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moyer VA. Screening for coronary heart disease with electrocardiography: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;157:512–518. doi: 10.7326/0003-4819-157-7-201210020-00514. [DOI] [PubMed] [Google Scholar]
- 9.Bakhoya VN, Kurl S, Laukkanen JA. T-wave inversion on electrocardiogram is related to the risk of acute coronary syndrome in the general population. Eur J Prev Cardiol. 2014;21:500–506. doi: 10.1177/2047487312460022. [DOI] [PubMed] [Google Scholar]
- 10.Inohara T, Kohsaka S, Okamura T, Watanabe M, Nakamura Y, Higashiyama A, Kadota A, Okuda N, Murakami Y, Ohkubo T, Miura K, Okayama A, Ueshima H for the NIPPON DATA 80/90 Research Group. Cumulative impact of axial, structural, and repolarization ECG findings on long-term cardiovascular mortality among healthy individuals in Japan: National Integrated Project for Prospective Observation of Non-Communicable Disease and its Trends in the Aged, 1980 and 1990. Eur J Prev Cardiol. 2014;21:1501–1508. doi: 10.1177/2047487313500568. [DOI] [PubMed] [Google Scholar]
- 11.Schröder K, Wegscheider K, Wenger NK, Vettorazzi E, Schröder R. Resting electrocardiogram predicts mortality in postmenopausal women with coronary heart disease or with risk factors for coronary heart disease. Eur J Prev Cardiol. 2012;21:749–757. doi: 10.1177/2047487312454022. [DOI] [PubMed] [Google Scholar]
- 12.Zhang ZM, Prineas RJ, Soliman EZ, Baggett C, Heiss G ARIC Research Group. Prognostic significance of serial Q/ST-T changes by the Minnesota Code and Novacode in the Atherosclerosis Risk in Communities (ARIC) study. Eur J Prev Cardiol. 2012;19:1430–1436. doi: 10.1177/1741826711426091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greenland P, Alpert JS, Beller GA. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2010;56:e50–103. doi: 10.1016/j.jacc.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 14.The ARIC Investigators. The Atherosclerosis Risk in Communities (ARIC) study: design and objectives. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 15.Prineas RJ, Crow RS, Blackburn H. The Minnesota Code Manual of Electrocardiographic Findings: Standards and Procedures for Measurement and Classification. Boston, MA: John Wright—PSG, Inc; 1982. [Google Scholar]
- 16.Rosamond WD, Folsom AR, Chambless LE, Wang CH, McGovern PG, Howard G, Copper LS, Shahar E. Stroke incidence and survival among middle-aged adults: 9-year follow-up of the atherosclerosis risk in communities (ARIC) cohort. Stroke. 1999;30:736–743. doi: 10.1161/01.str.30.4.736. [DOI] [PubMed] [Google Scholar]
- 17.Loehr LR, Rosamond WD, Chang PP, Folsom AR, Chambless LE. Heart failure incidence and survival (from the atherosclerosis risk in communities study) Am J Cardiol. 2008;101:1016–1022. doi: 10.1016/j.amjcard.2007.11.061. [DOI] [PubMed] [Google Scholar]
- 18.Soliman EZ, Lopez F, O'Neal WT, Chen LY, Bengtson L, Zhang ZM, Loehr L, Cushman M, Alonso A. Atrial fibrillation and risk of STsegment-elevation versus non-ST-segment-elevation myocardial infarction: The atherosclerosis risk in communities (ARIC) study. Circulation. 2015;131:1843–1850. doi: 10.1161/CIRCULATIONAHA.114.014145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.White AD, Folsom AR, Chambless LE, Sharret AR, Yang K, Conwill D, Higgins M, Williams OD, Tyroler HA. Community surveillance of coronary heart disease in the atherosclerosis risk in communities (ARIC) study: Methods and initial two years' experience. J Clin Epidemiol. 1996;49:223–233. doi: 10.1016/0895-4356(95)00041-0. [DOI] [PubMed] [Google Scholar]
- 20.Stamler J, Stamler R, Neaton JD, Wentworth D, Daviglus ML, Garside D. Low risk-factor profile and long-term cardiovascular and noncardiovascular mortality and life expectancy: findings for 5 large cohorts of young adult and middle-aged men and women. JAMA. 1999;282:2012–2018. doi: 10.1001/jama.282.21.2012. [DOI] [PubMed] [Google Scholar]
- 21.Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF, Arnett DK, Fonarow GC, Ho PM, Lauer MS, Masoudi FA, Robertson RM, Roger V, Schwamm LH, Sorlie P, Yancy CW, Rosamond WD American Heart Association Strategic Planning Task Force and Statistics Committee. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association's strategic Impact Goal through 2020 and beyond. Circulation. 2010;12:586–613. doi: 10.1161/CIRCULATIONAHA.109.192703. [DOI] [PubMed] [Google Scholar]
- 22.Knapper JT, Ghasemzadeh N, Khayata M, Patel SP, Quyyumi AA, Mendis S, Mensah GA, Taubert K, Sperling LS. Time to Change Our Focus: Defining, Promoting, and Impacting Cardiovascular Population Health. J Am Coll Cardiol. 2015;66:960–971. doi: 10.1016/j.jacc.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 23.Sing CF, Stengård JH, Kardia SL. Genes, environment, and cardiovascular disease. Arterioscler Thromb Vasc Biol. 2003;23:1190–1196. doi: 10.1161/01.ATV.0000075081.51227.86. [DOI] [PubMed] [Google Scholar]