This is the first study to demonstrate that habitual aerobic exercise may not protect against age/menopause-related whole forearm microvascular endothelial dysfunction in healthy nonobese estrogen-deficient postmenopausal women, consistent with recent findings regarding macrovascular endothelial function. This is in contrast to what is observed in healthy middle-aged and older aerobic exercise-trained men.
Keywords: women, aging, aerobic exercise, endothelium-dependent dilation
Abstract
Aging causes micro- and macrovascular endothelial dysfunction, as assessed by endothelium-dependent dilation (EDD), which can be prevented and reversed by habitual aerobic exercise (AE) in men. However, in estrogen-deficient postmenopausal women, whole forearm microvascular EDD has not been studied, and a beneficial effect of AE on macrovascular EDD has not been consistently shown. We assessed forearm blood flow in response to brachial artery infusions of acetylcholine (FBFACh), a measure of whole forearm microvascular EDD, and brachial artery flow-mediated dilation (FMD), a measure of macrovascular EDD, in 12 premenopausal sedentary women (Pre-S; 24 ± 1 yr; V̇o2max = 37.5 ± 1.6 ml·kg−1·min−1), 25 estrogen-deficient postmenopausal sedentary women (Post-S; 62 ± 1 yr; V̇o2max = 24.7 ± 0.9 ml·kg−1·min−1), and 16 estrogen-deficient postmenopausal AE-trained women (Post-AE; 59 ± 1 yr; V̇o2max = 40.4 ± 1.4 ml·kg−1·min−1). FBFACh was lower in Post-S and Post-AE compared with Pre-S women (135 ± 9 and 116 ± 17 vs. 193 ± 21 AUC, respectively, both P < 0.008), whereas Post-S and Post-AE women were not different (P = 0.3). Brachial artery FMD was 34% (5.73 ± 0.67%) and 45% (4.79 ± 0.57%) lower in Post-S and Post-AE, respectively, vs. Pre-S women (8.69 ± 0.95%, both P ≤ 0.01), but not different between Post-S and Post-AE women (P = 0.3). Post-AE women had lower circulating C-reactive protein and oxidized low-density lipoprotein compared with Post-S women (0.5 ± 0.1 vs. 1.1 ± 0.2 mg/l and 40 ± 4 vs. 55 ± 3 U/l, respectively, both P = 0.01), but these markers were not correlated to FBFACh (P = 0.3) or brachial artery FMD (P = 0.8). These findings are consistent with the idea that habitual AE does not protect against age/menopause-related whole forearm micro- and macrovascular endothelial dysfunction in healthy nonobese estrogen-deficient postmenopausal women, despite being associated with lower systemic markers of inflammation and oxidative stress.
NEW & NOTEWORTHY This is the first study to demonstrate that habitual aerobic exercise may not protect against age/menopause-related whole forearm microvascular endothelial dysfunction in healthy nonobese estrogen-deficient postmenopausal women, consistent with recent findings regarding macrovascular endothelial function. This is in contrast to what is observed in healthy middle-aged and older aerobic exercise-trained men.
cardiovascular diseases (CVDs) remain the leading cause of morbidity and mortality in developed societies in both men and women (32, 58), with aging as the primary risk factor for CVD (32, 60). Antecedent and contributing to increased CVD risk with age is the development of micro- and macrovascular endothelial dysfunction, as indicated by a decline in endothelium-dependent dilation (EDD) (29).
Microvascular (resistance artery) and macrovascular (conduit artery) EDD are most commonly assessed by increases in forearm blood flow to acetylcholine infusion (FBFACh) and brachial artery flow-mediated dilation (FMD), respectively (49). Both of these measures are reduced with advancing age (8, 10, 15, 17, 52–54) and independently predict CVD risk in men and women (30, 40, 61, 62). However, micro- and macrovascular EDD are not related to one another in healthy men (19) and are differentially affected by age. Specifically, the onset of the decline in microvascular EDD with age appears to precede that for macrovascular EDD by more than a decade in both sexes (8, 25, 52). Furthermore, the pattern of decline with age in each vascular bed differs between men and women (8, 52), being delayed and associated with the onset of menopause in women (8, 37, 52). These differences, as well as those related to vascular wall structure (41), indicate that the putative influence of environmental factors on endothelial dysfunction with aging needs to be assessed in both the micro- and macrovasculature.
Aerobic exercise (AE) is the first-line lifestyle recommendation for age-related vascular dysfunction, primarily since it consistently has been associated with preserved micro- and macrovascular endothelial function in men (10, 17, 21, 22, 44–46, 48). However, the data regarding vascular endothelial function and AE in women is inconsistent. Cross-sectional and intervention studies in healthy nonobese estrogen-deficient postmenopausal women have reported both beneficial effects (2, 63) and no impact (7, 39, 45) of AE on macrovascular endothelial function, with our laboratory previously finding no effect (39, 45). Importantly, there is no present information on the effects of habitual AE on whole forearm microvascular endothelial function in this group.
Accordingly, we tested the hypothesis that microvascular (FBFACh) endothelial function would be lower in healthy nonobese estrogen-deficient postmenopausal women compared with young sedentary women, regardless of training status. Furthermore, we took the opportunity to confirm our recent observation that impairments in macrovascular endothelial function (brachial artery FMD) in healthy nonobese estrogen-deficient postmenopausal women are not influenced by habitual AE status (39, 45).
MATERIALS AND METHODS
Participants.
In this cross-sectional analysis, participant data from our database was included, and additional participants were recruited to increase group sample sizes as needed. Fifty-three healthy, normally menstruating premenopausal sedentary (Pre-S; aged 18–33 yr) and estrogen-deficient postmenopausal (aged 50–71 yr) sedentary (Post-S) and AE-trained (Post-AE) women from Boulder, CO, and the surrounding communities were studied in the University of Colorado Boulder Clinical and Translational Research Center. All women but one (Pre-S, Asian) reported as White and non-Hispanic/Latina. Inclusion criteria were nonsmoking; body mass index <30 kg/m2; fasted plasma glucose concentrations <126 mg/dl; and absence of chronic disease, including peripheral arterial disease (ankle-brachial index >0.90), as assessed by medical history, physical examination, blood chemistries, and electrocardiogram and blood pressure at rest and during incremental treadmill exercise testing. Additionally, premenopausal women were not pregnant, as assessed by a pregnancy test, and had a ≥1-yr history of regular menstruation. Postmenopausal women were amenorrheic ≥1 yr and if ≤56 yr of age had a follicular stimulating hormone concentration ≥40 IU/l. Participants had not taken any hormone treatment (including birth control) ≥1 yr or cardiovascular or lipid-altering medications ≥6 mo. Participants were characterized as sedentary if they had not exercised regularly >2 days/wk or AE trained if they performed vigorous AE (primarily running, but also cycling and swimming) >3 days/wk for >10 yr. All procedures were reviewed and approved by the Institutional Review Board at the University of Colorado Boulder. The nature, risks, and benefits of all study procedures were explained to volunteers, and their written informed consent was obtained before participation in the study.
Measurements.
All testing was conducted following an overnight >12-h fast from food (water only), caffeine, and dietary supplements, and >24-h abstention from exercise, prescription medications, and alcohol (27). All testing on premenopausal women was performed 1 to 5 days after menses onset (early follicular phase).
Participant characteristics.
Arterial blood pressure was measured in triplicate over the nondominant brachial artery during rest in the seated position by using a semiautomated device (Dinamap XL, Johnson and Johnson). Waist and hip circumferences and body mass index were measured by anthropometry (31), and body fat percentage was measured by dual-energy X-ray absorptiometry (GE Lunar Prodigy Advance). Maximal oxygen consumption (V̇o2max) was assessed by indirect calorimetry during incremental treadmill exercise testing performed to exhaustion (Balke protocol) (20). Leisure time physical activity was determined by the Modifiable Activity Questionnaire (43).
Circulating blood factors.
All blood chemistries were assayed at the Colorado Clinical Translational Sciences Institute Clinical and Translational Research Center Core Laboratory or Boulder Community Hospital Clinical Laboratory, as described previously (12). Briefly, human chorionic gonadotropin urine pregnancy tests were performed at the University of Colorado Boulder Clinical and Translational Research Center. Fasting serum blood lipid concentrations were measured by standard assays. Fasting plasma glucose was determined by reflective spectrophotometry (Ortho Clinical Diagnostics), and fasting plasma insulin by radioimmunoassay (Millipore). Insulin resistance was estimated with the homeostasis model of insulin resistance as [fasting plasma glucose (mg/dl) × fasting plasma insulin (µU/ml)]/405 (33). Serum follicular stimulating hormone (Ortho Clinical Diagnostics) and serum estradiol (Beckman Coulter) were measured by chemiluminescence. Plasma oxidized low-density lipoprotein was assessed by ELISA (Mercodia), serum high-sensitivity C-reactive protein by immunoturbidimetry (Beckman Coulter), and plasma norepinephrine by high-performance liquid chromatography (BioRad).
Microvascular endothelial function.
Whole forearm microvascular (resistance artery) EDD was assessed by strain guage venous occlusion plethysmography (D.E. Hokanson; Windaq Data Acquisition Software, DATAQ Instruments), as previously described (10, 14). Briefly, a mercury-silica strain gauge was fitted to each forearm, and inflatable cuffs were placed around the upper arms and wrists. Wrist cuffs remained inflated at 250 mmHg during all FBF measures to exclude hand circulation, and upper arm cuffs cycled between 0 and 60 mmHg in 7-s cycles to occlude venous outflow. Forearm volume was determined by the water displacement method, and drug infusion rates were normalized per 100 ml forearm tissue. The nondominant brachial artery was catheterized for drug infusions for all participants. Whole forearm microvascular EDD was assessed by FBF responses to incremental intra-arterial infusion of acetylcholine (FBFACh; 1, 2, 4, and 8 μg/100 ml forearm volume/min for 3.5–4 min/dose; Bausch and Lomb). Whole forearm microvascular endothelium-independent dilation, a measure of vascular smooth muscle sensitivity to nitric oxide, was assessed by FBF responses to incremental intra-arterial infusion of sodium nitroprusside (FBFSNP; 0.5, 1, and 2 μg/100 ml forearm volume/min for 3.5–4 min/dose; Marathon Pharmaceuticals). FBF was calculated during the last 1.5 min of each infusion period. FBF values are reported as individual dose responses and area under the dose-response curve (AUC). Forearm vascular conductance was calculated as FBF/mean arterial pressure.
Macrovascular endothelial function.
Macrovascular (conduit artery) EDD was assessed by brachial artery FMD using high-resolution ultrasonography (Toshiba Xario XG and Power Vision 6000), as previously described (9, 18, 27). Briefly, the right brachial artery diameter change was measured after a 5-min FBF occlusion with a blood pressure cuff distal to the ultrasound probe. Brachial artery FMD was reported as a percentage and absolute (mm) change from baseline diameter (27). Brachial artery baseline and FMD peak shear rate was calculated as [8 × mean velocity (m/s)]/baseline diameter (m), from 10 baseline velocity envelopes, and [8 × mean velocity (m/s)]/occlusion diameter (m), from the first 10 velocity envelopes after cuff release, respectively (27). Brachial artery diameters and blood velocities were captured and analyzed by Vascular Research Tools 5.10.9 (Medical Imaging Applications). Brachial artery endothelium-independent dilation was assessed only in a small subset of participants (n = 1 to 18/group) because the resting blood pressures of many of these healthy women were in the low normal range, and the administration of sublingual nitroglycerin for the endothelium-independent dilation test may have lowered blood pressure to unsafe levels.
Data analysis.
Statistical analysis was performed with IBM SPSS 23 and G*Power 3.1. Data normality was assessed with the Shapiro-Wilk test, and nonnormal variables were log base 10 transformed for statistical analysis. Outliers (≥3 standard deviations) were replaced with the group mean. Group differences were determined by one-way analysis of variance. In the case of a significant group difference, least significant differences post hoc analysis was performed for between-group contrasts. Analysis of covariance was used to determine the effects of brachial artery baseline diameter on group differences in brachial artery FMD. Pearson product-moment correlation analysis was used to assess the linear relation between relevant variables. The study achieved >80% power at an α of 0.05 to find between-group differences in our primary outcome, FBFACh AUC, with an effect size of 0.46. Data are expressed as means ± SE. Statistical significance was set at P < 0.05.
RESULTS
Participant characteristics.
Post-S women were more years postmenopausal compared with Post-AE women (10 ± 2 vs. 6 ± 1 yr, respectively, P = 0.03), and 40% Post-S women were previous hormone replacement users compared with 6% of their AE-trained counterparts. Table 1 presents selected participant characteristics. Post-S women had higher body mass index, body fat percentage, waist-to-hip ratio, and systolic blood pressure (all P < 0.05), and lower maximal oxygen consumption (V̇o2max; both P < 0.001) compared with both other groups. Post-AE women had performed regular AE for ~28 yr on average (range 15–47 yr) and had lower body fat percentage (both P < 0.05) and resting heart rate (both P < 0.001), and higher leisure time physical activity (both P < 0.001) compared with both other groups. Post-AE women also had lower body mass compared with Post-S women (P = 0.008).
Table 1.
Participant characteristics
| Premenopausal Sedentary |
Postmenopausal Sedentary |
Postmenopausal AE-Trained |
|
|---|---|---|---|
| N | 12 | 25 | 16 |
| Age, yr | 24 ± 1* | 62 ± 1 | 59 ± 1 |
| Body mass, kg | 62.7 ± 2.5 | 66.2 ± 1.4 | 59.4 ± 1.9† |
| Body mass index, kg/m2 | 22.6 ± 1.0 | 24.5 ± 0.5* | 21.4 ± 0.5 |
| Body fat, % | 32.2 ± 1.9 | 38.3 ± 1.4* | 26.4 ± 1.9* |
| Waist-to-hip ratio, U | 0.72 ± 0.01 | 0.76 ± 0.01* | 0.72 ± 0.01 |
| Systolic blood pressure, mmHg | 107 ± 5 | 118 ± 3* | 105 ± 2 |
| Diastolic blood pressure, mmHg | 67 ± 3 | 71 ± 2 | 65 ± 1 |
| Resting heart rate, beats/min | 65 ± 3 | 64 ± 2 | 52 ± 2* |
| V̇o2max, ml·kg−1·min−1 | 37.5 ± 1.6 | 24.7 ± 0.9* | 40.4 ± 1.4 |
| LTPA, MET-hr/wkL | 23.6 ± 6.2 | 10.9 ± 2.0 | 105.2 ± 10.1* |
Data are means ± SE. LTPA, leisure time physical activity; L, data log transformed for statistical analysis.
P < 0.05 vs. all other groups;
P < 0.05 vs. postmenopausal sedentary women.
Circulating blood factors.
Table 2 presents selected circulating blood factors. Pre-S women had lower circulating total cholesterol and higher estradiol compared with both postmenopausal groups (both P < 0.001). Post-S women had higher low-density lipoprotein cholesterol compared with Pre-S women (P = 0.0003) and higher C-reactive protein compared with both other groups (both P < 0.05). Post-AE women had higher high-density lipoprotein cholesterol compared with both other groups (both P < 0.05) and lower plasma oxidized low-density lipoprotein compared with Post-S women (P = 0.01).
Table 2.
Circulating blood factors
| Premenopausal Sedentary | Postmenopausal Sedentary | Postmenopausal AE-Trained | |
|---|---|---|---|
| Total cholesterol, mg/dl | 160 ± 8* | 207 ± 7 | 205 ± 6 |
| HDL cholesterol, mg/dl | 58 ± 4 | 68 ± 3 | 78 ± 5* |
| LDL cholesterol, mg/dl | 83 ± 6 | 121 ± 7‡ | 104 ± 6 |
| Triglycerides, mg/dlL | 93 ± 12 | 90 ± 6 | 84 ± 8 |
| Glucose, mg/dl | 85 ± 2 | 87 ± 1 | 85 ± 2 |
| Insulin, µU/mlL | 9 ± 2 | 6 ± 1 | 7 ± 1 |
| HOMA-IR, UL | 1.8 ± 0.3 | 1.2 ± 0.1 | 1.5 ± 0.2 |
| Estradiol, pg/mlL | 59 ± 20 (n = 7)* | 17 ± 2 (n = 20) | 13 ± 1 (n = 13) |
| C-reactive protein, mg/lL | 0.4 ± 0.1 | 1.1 ± 0.2* | 0.5 ± 0.1 (n = 12) |
| Oxidized LDL, U/l | 48 ± 6 | 55 ± 3 | 40 ± 4 (n = 11)† |
| Norepinephrine, pg/ml L | 274 ± 43 | 358 ± 27 | 345 ± 54 (n = 11) |
Data are means ± SE. HDL, high-density lipoprotein; LDL, low-density lipoprotein; HOMA-IR, homeostasis model assessment-insulin resistance; L, data log transformed for statistical analysis.
P < 0.05 vs. all other groups;
P < 0.05 vs. postmenopausal sedentary;
P < 0.05 vs. premenopausal sedentary women.
Microvascular endothelial function.
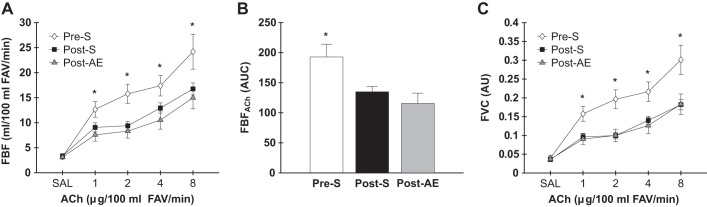
Post-S and Post-AE women had lower FBFACh at each dose infused and AUC compared with Pre-S women (doses, all P < 0.05; AUC, both P < 0.008; Fig. 1, A and B). Controlling FBFACh for mean arterial pressure did not change these group differences, as both postmenopausal women groups demonstrated lower forearm vascular conductance at all doses compared with Pre-S women (all P < 0.01; Fig. 1C). No differences were observed in the FBFACh dose response (all P > 0.2), AUC (P = 0.3), or forearm vascular conductance dose responses (all P > 0.5) between the Post-S and Post-AE groups (Fig. 1). One outlier (≥3 standard deviations) for Post-AE women for FBFACh at 8 μg/100 ml dose was removed; when included (Post-AE: 17.7 ± 1.6 ml/100 ml forearm volume/min) all results remained unchanged. FBF and forearm vascular conductance dose responses to SNP are shown in Fig. 2. No group differences were observed in FBFSNP (all P > 0.2) or AUC (P = 0.3); controlling FBFSNP for mean arterial pressure did not change these results with the exception that Pre-S women had higher forearm vascular conductance at the 1 μg/100 ml dose compared with Post-S women (P = 0.01), but not to the Post-AE women (P = 0.2).
Fig. 1.
Forearm blood flow (FBF) [A: increasing doses; B: area under the dose-response curve (AUC)] and forearm vascular conductance (FVC) (C) responses to acetylcholine (ACh) in premenopausal sedentary (Pre-S), postmenopausal sedentary (Post-S), and postmenopausal AE-trained (Post-AE) women. Data are means ± SE; *P < 0.05 vs. all other groups. FAV, forearm volume.
Fig. 2.
Forearm blood flow (FBF) [A: increasing doses; B: area under the dose-response curve (AUC)] and forearm vascular conductance (FVC) (C) responses to sodium nitroprusside (SNP) in premenopausal sedentary (Pre-S), postmenopausal sedentary (Post-S), and postmenopausal AE-trained (Post-AE) women. Data are means ± SE; †P < 0.05 vs. Post-S; FAV, forearm volume.
Macrovascular endothelial function.
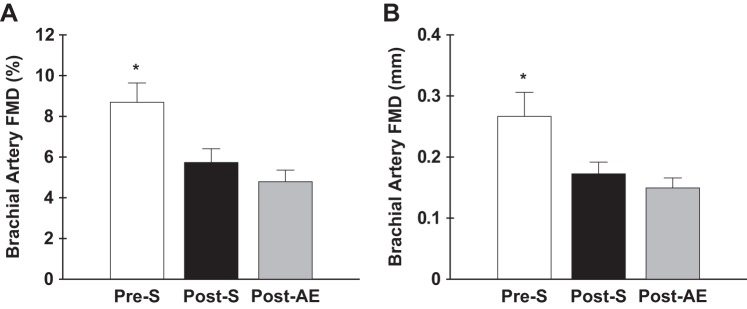
Brachial artery FMD and peak shear rate was assessed in a subset of participants (Pre-S: n = 8; Post-S: n = 20; and Post-AE: n = 16). Brachial artery FMD was reduced in Post-S and Post-AE women when expressed as percent (5.73 ± 0.67 and 4.79 ± 0.57%, respectively) and absolute (0.17 ± 0.02 and 0.15 ± 0.02 mm, respectively) change compared with Pre-S women (8.69 ± 0.95%, both P ≤ 0.01; 0.27 ± 0.04 mm, both P ≤ 0.01). Post-S and Post-AE women were not different in brachial artery FMD percentage (P = 0.3) or millimeter (P = 0.4) change (Fig. 3). When baseline diameter was added as a covariate for assessing brachial artery FMD group differences, it had no significant effect (P = 0.1). Post-AE women had a lower brachial artery FMD peak shear rate (both P < 0.01; Table 3) compared with both the other groups. After normalizing brachial artery FMD percent change to FMD peak shear rate, Post-S and Post-AE women still had reduced responses compared with Pre-S women (0.002 ± 0.0001 and 0.002 ± 0.0002 vs. 0.004 ± 0.0001% per second; both P < 0.05), and no differences were observed between the postmenopausal groups (P = 0.3).
Fig. 3.
Brachial artery flow-mediated dilation (FMD) expressed as percent (A) and absolute (B) change in a subset of premenopausal sedentary (Pre-S), postmenopausal sedentary (Post- S), and postmenopausal AE-trained (Post-AE) women. Data are means ± SE; *P < 0.05 vs. all other groups.
Table 3.
Brachial artery parameters
| Premenopausal Sedentary |
Postmenopausal Sedentary |
Postmenopausal AE-Trained |
|
|---|---|---|---|
| Baseline diameter, mm | 2.98 ± 0.12 | 3.08 ± 0.08 | 3.21 ± 0.09 |
| Baseline shear rate, s−1 | 444 ± 57 | 347 ± 87 | 343 ± 31 |
| Peak diameter, mm | 3.25 ± 0.16 | 3.25 ± 0.08 | 3.36 ± 0.09 |
| FMD peak shear rate, s−1 | 2,679 ± 751 | 2,807 ± 681 | 1,803 ± 97* |
Data are means ± SE. FMD, flow-mediated dilation.
P < 0.05 vs. all other groups.
Relations between circulating markers of inflammation/oxidative stress and vascular endothelial function.
C-reactive protein, a marker of systemic inflammation, and oxidized low-density lipoprotein, a marker of circulating oxidative stress, were not significantly related to FBFACh AUC (r = −0.17, P = 0.3 and r = −0.05, P = 0.7, respectively) or brachial artery FMD (r = 0.04, P = 0.8 and r = 0.18, P = 0.3, respectively) in the overall study group.
DISCUSSION
This is the first study to assess the association between habitual AE status and whole forearm microvascular endothelial function in postmenopausal women compared with premenopausal controls. We also assessed macrovascular endothelial function in subsets of participants from these groups to characterize both microvascular and macrovascular function in the same women. The present findings suggest that regular AE, despite being associated with lower systemic markers of inflammation and oxidative stress, does not protect healthy nonobese estrogen-deficient postmenopausal women from age/menopause-related whole forearm microvascular endothelial dysfunction, as assessed by FBFACh. In addition, we show that similar to their sedentary peers, estrogen-deficient postmenopausal AE-trained women who demonstrate microvascular dysfunction also display age/menopause-related macrovascular endothelial dysfunction, as assessed by brachial artery FMD.
Microvascular endothelial function with age/menopause and AE.
Whole forearm microvascular EDD, as assessed by FBFACh, is reduced with advancing age in both men and women (10, 52–54). Cross-sectional studies suggest that the age-related decline in microvascular endothelial function precedes macrovascular dysfunction by over a decade (8, 25, 52). In addition, the onset of the decline in microvascular EDD differs between men and women: FBFACh declines at a constant rate (1.8% per yr) throughout life in men but more modestly (0.5% per yr) in women between ages 40 and 50, after which a steeper decline (2.1% per yr) is observed (52). Moreover, whole forearm microvascular endothelial function, as assessed by FBFACh, is independently predictive of future cardiovascular events and mortality in men and women (30), and not correlated to macrovascular endothelial function (19). Collectively, these and other observations emphasize the importance of assessing putative factors that may influence endothelial function in both the micro- and macrovasculature.
To our knowledge, all previous research assessing the effects of AE on FBFACh with age have been in healthy men (10) or men and women combined (51). A study by our laboratory demonstrated that AE both prevented decline and improved whole forearm microvascular EDD with age in healthy men, with older sedentary men having 25% lower FBFACh compared with young sedentary and middle-aged/older habitually AE-trained men. In addition, 12 wk of AE improved FBFACh in previously sedentary late middle-aged and older men to the level observed in young sedentary and older AE-trained men (10). Furthermore, Taddei et al. (51) reported that a combined group of middle-aged and older AE-trained men and women had higher whole forearm microvascular EDD compared with their sedentary counterparts; however, sex differences were not examined, with only 30% of the participants being women.
In the present cross-sectional study, estrogen-deficient Post-AE women had significantly lower FBFACh compared with Pre-S women (both dose response and AUC) independent of group differences in blood pressure. Importantly, the FBFACh responses of the Post-AE women were not significantly different than those of their sedentary peers. In contrast, AE intervention studies assessing cutaneous microvascular endothelial function have demonstrated an increased blood flow response to ACh after 8 wk (4) or in response to heating after 48 wk (55) in healthy nonobese estrogen-deficient postmenopausal women. Different effects of AE in studies assessing cutaneous vs. whole forearm microvascular function may be due to a number of factors, including differences in location and function of these microvascular regions (i.e., skin vs. primarily skeletal muscle) and thermoregulatory adaptations to AE occurring selectively in the cutaneous microvasculature. Additionally, a recent study by Nyberg et al. (42) demonstrated that 12 wk of high-intensity AE modestly improved leg microvascular blood flow to ACh in early estrogen-deficient postmenopausal women (~3 yr after last menses), suggesting that timing of an AE intervention after menopause (timing hypothesis) may also play a role, with early postmenopausal women potentially being more responsive to AE interventions compared with later postmenopausal women.
These considerations aside, it is important to emphasize that our cross-sectional study in women who have habitually exercised for ~28 yr offers the greatest possibility to observe whole forearm microvascular endothelial adaptations, if present. As such, our study suggests that healthy nonobese estrogen-deficient postmenopausal women engaged in vigorous and habitual AE are not protected against whole forearm microvascular endothelial dysfunction with aging, in contrast to what is observed in older AE-trained men. Importantly, whole forearm microvascular endothelial function (FBFACh) is predictive of future CVD risk (30), while this predictive relevance has not yet been established for cutaneous or leg microvasculature. Further studies are needed to examine the role of different AE regimens, AE intervention timing after menopause, and the responsiveness of different microvascular beds.
Macrovascular endothelial function with age/menopause and AE.
Cross-sectional studies demonstrate that men and women both undergo an age-related decline in macrovascular EDD, assessed by brachial artery FMD (8, 11, 15–18). Additionally, young women typically display higher brachial artery FMD compared with their age-matched male counterparts. However, brachial artery FMD declines progressively (0.21% per year) after age 40 in normotensive men and after age 50 in women, but with a steeper (0.49% per year) decline compared with men, suggesting a sex-related difference in the timing and rate of progression of macrovascular endothelial dysfunction with age (8).
Habitual AE prevents and/or ameliorates the age-related decline in macrovascular endothelial function in men (17, 18, 22, 44–46, 48). However, the results of studies in postmenopausal women have been inconsistent, suggesting sex-related differences in macrovascular responses to AE. A previous cross-sectional study by our laboratory demonstrated that brachial artery FMD was 50% greater in middle-aged/older AE-trained men compared with their sedentary counterparts, but, in contrast, no difference was observed between estrogen-deficient AE-trained and sedentary postmenopausal women (45). Overall, studies assessing the effect of AE on age-related macrovascular EDD in healthy nonobese estrogen-deficient postmenopausal women, free of clinical diseases, are limited, with some showing no clear influence (7, 39, 45), whereas others have reported beneficial effects (2, 6, 63) of AE. An interventional study by our laboratory demonstrated that 8 wk of AE significantly improved brachial artery FMD in middle-aged/older, previously sedentary men, but that the same “dose” of AE had no effect in estrogen-deficient, previously sedentary postmenopausal women (45). These studies, along with our current findings, support the concept that AE, either viewed as a habitual lifestyle behavior or as an intervention, may have beneficial effects on macrovascular endothelial function with aging in men, but the effects of AE are inconsistent in estrogen-deficient postmenopausal women. In contrast to the previous (7, 39, 45) and current studies’ findings, other intervention (2, 63) and cross-sectional (6, 26) studies have shown AE improves or preserves brachial artery FMD in healthy nonobese estrogen-deficient postmenopausal women. Small sample sizes (<10) and differences between studies’ AE regimen (type, intensity, duration, and frequency), brachial artery FMD cuff placement (proximal vs. distal), previous history of hormone therapy supplementation, and years postmenopausal at time of AE intervention are likely among the factors that contribute to differing outcomes.
Mechanism of impaired endothelial response to habitual AE in estrogen-deficient postmenopausal women.
Cross-sectional studies support sex-related differences in age-related vascular endothelial dysfunction timing and rate of decline with the onset of menopause as a key event in women (8, 37, 52). Although the exact mechanisms remain unknown, one hypothesis for sex-related differences in the beneficial effects of AE with age on the vascular endothelium is the reduction in sex hormones in women after menopause. In support of this idea, Moreau et al. (37) observed a progressive decline in brachial artery FMD across the stages of menopause in healthy women that correlated with the corresponding declines in circulating sex hormones, suggesting a link between the development of macrovascular endothelial dysfunction and estrogen concentrations. These results are consistent with those from studies in which premenopausal women were assessed serially over the menstrual cycle (28, 59), and findings of acute and chronic estrogen supplementation trials in previously estrogen-deficient postmenopausal women (24, 36, 38, 47).
To determine the influence of estrogen levels on the ability of the vascular endothelium of conduit arteries to respond to AE, Moreau et al. (39) recently conducted a complex intervention study. They first demonstrated that brachial artery FMD was not improved by 12 wk of AE in previously sedentary, estrogen-deficient postmenopausal women, confirming the previous observations of Pierce et al. (45). However, after 12 wk of estrogen supplementation, which restored circulating concentrations to premenopausal levels, previously estrogen-deficient sedentary women demonstrated improvements in brachial artery FMD in response to a 12-wk AE intervention (39). Taken together, these findings suggest a permissive role for circulating estrogen concentrations in transducing the beneficial effects of AE in postmenopausal women.
Estrogen supplementation status was not assessed in the present cross-sectional study because of insufficient numbers of available estrogen-supplemented sedentary and AE-trained postmenopausal women. This is because chronic hormone replacement is now contraindicated as a chronic preventive strategy for postmenopausal women as a result of the findings of the Women’s Health Initiative study (5). However, based on the recent findings of Moreau et al. described above, it is likely that, as is the case with macrovascular endothelial function, the ability of habitual AE to preserve whole forearm microvascular endothelial function with aging/menopause in healthy women may depend on the presence of “premenopausal-like” circulating concentrations of estrogen. Indeed, estrogen status may be the dominant influence affecting vascular endothelial function in healthy postmenopausal women (30, 35). This notion is supported by our findings of a similar degree of vascular dysfunction in Post-AE women compared with their sedentary counterparts, despite lower circulating systemic markers of inflammation and oxidative stress, as well as the absence of significant inverse relations between these circulating markers and measures of vascular endothelial function.
Previous studies have shown that circulating markers may not reflect the state of the vascular endothelium (13, 44) in healthy nonobese adults, a population in which circulating biomarkers of oxidative stress and inflammation are only modestly elevated compared with diseased individuals (1, 56). This may explain the lack of relation between circulating markers (oxidized low-density lipoprotein and C-reactive protein) and vascular endothelial function observed in the present study. Local vascular endothelial oxidative stress and inflammation may contribute to vascular endothelial dysfunction in estrogen-deficient postmenopausal AE-trained women, as we have previously shown (39) that acute suppression of oxidative stress via vitamin C infusion improved brachial artery FMD in estrogen-deficient postmenopausal women who were habitually AE trained or underwent an AE intervention, but not in estrogen-supplemented postmenopausal women who underwent an AE intervention. Additional studies are needed to examine the role of vascular endothelial inflammation and further investigate these mechanisms in estrogen-deficient and supplemented postmenopausal women.
Although other sex hormones such as progesterone (with estrogen) may contribute to sex differences observed in vascular endothelial dysfunction with age, most literature supports that progesterone either has no effect (23, 24, 34) or blunts (3, 35, 50, 57) the beneficial effects of estrogen on vascular endothelial function in postmenopausal women. More studies are needed to determine the interactive effects of estrogen and progesterone on the responsiveness of the vascular endothelium to AE in healthy postmenopausal women. However, the available data support the hypothesis that estrogen plays an essential permissive role in transducing the AE stimulus in the vascular endothelium of postmenopausal women.
Limitations.
The primary goal of the present study was to gain initial insight into the effects of habitual AE on microvascular endothelial function with aging in healthy nonobese estrogen-deficient postmenopausal women, and compare this association to that observed for macrovascular endothelial function. As a result, the mechanisms involved in the lack of protective effects of AE in women were not extensively assessed. In addition, because of the limited number of women previously on hormone replacement therapy in the estrogen-deficient postmenopausal AE-trained group (6% vs. 40% of Post-S women), we were not able to determine if previous hormone replacement therapy use was related to micro- and/or macrovascular endothelial function. Additionally, we were not able to assess macrovascular endothelium-independent dilation (via nitroglycerin) because of safety restrictions (low baseline blood pressure). However, our laboratory and others have previously shown that brachial artery endothelium-independent dilation does not change with age in healthy women (8, 37, 45), as observed in healthy aging men (8, 15, 17, 44). Furthermore, the study was performed with primarily healthy Caucasian women; therefore, it is unknown if AE may be protective in other populations, including different ethnicities and/or women with major risk factors for CVD or clinically documented CVD. These are important questions for future investigation building on results of the present study.
Conclusions.
In conclusion, our study is the first to demonstrate that habitual AE may not be protective of age/menopause-related whole forearm microvascular endothelial dysfunction in healthy nonobese estrogen-deficient postmenopausal women, consistent with recent findings concerning macrovascular endothelial function. This finding has important clinical implications given that based largely on data from previous studies in men, AE is currently a first-line lifestyle recommendation for preventing and improving both micro- and macrovascular endothelial dysfunction with aging. Additional studies are needed to resolve differing findings on the effects of AE on vascular endothelial function in healthy estrogen-deficient postmenopausal women, to determine the mechanisms involved in the sex-specific differences in endothelial responsiveness to AE-training, to identify alternative therapies to preserve micro- and macrovascular endothelial function with aging/menopause in women, and to determine if AE-training has beneficial effects on endothelial function with aging in women with major risk factors for CVD or clinical CVD.
GRANTS
Support for this work was provided by National Institute on Aging Grants R37 AG-013038, T32 AG-000279-14S1, R37 AG-013038-17S1, and R21 AG-042795-01A1S1; and Colorado CTSA UL1 TR001082. Contents are the authors’ sole responsibility and do not necessarily represent official National Institutes of Health views.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
J.R.S.-P., T.R.S., and G.L.P. performed experiments; J.R.S.-P., T.R.S., V.M.V., and G.L.P. analyzed data; J.R.S.-P. and D.R.S. interpreted results of experiments; J.R.S.-P. prepared figures; J.R.S.-P. drafted manuscript; J.R.S.-P., T.R.S., V.M.V., G.L.P., and D.R.S. edited and revised manuscript; J.R.S.-P., T.R.S., V.M.V., G.L.P., and D.R.S. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank the staff of the University of Colorado Boulder Clinical and Translational Research Center for technical assistance.
REFERENCES
- 1.Abdelmouttaleb I, Danchin N, Ilardo C, Aimone-Gastin I, Angioï M, Lozniewski A, Loubinoux J, Le Faou A, Guéant JL. C-Reactive protein and coronary artery disease: additional evidence of the implication of an inflammatory process in acute coronary syndromes. Am Heart J 137: 346–351, 1999. doi: 10.1053/hj.1999.v137.92052. [DOI] [PubMed] [Google Scholar]
- 2.Akazawa N, Choi Y, Miyaki A, Tanabe Y, Sugawara J, Ajisaka R, Maeda S. Curcumin ingestion and exercise training improve vascular endothelial function in postmenopausal women. Nutr Res 32: 795–799, 2012. doi: 10.1016/j.nutres.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 3.Akman L, Duygu H, Akercan F, Ulukus M, Ozerkan F, Akin M. The effects of different hormone treatment on endothelial function in healthy postmenopausal women. Gynecol Endocrinol 29: 867–872, 2013. doi: 10.3109/09513590.2013.813471. [DOI] [PubMed] [Google Scholar]
- 4.Alkhatib A, Klonizakis M. Effects of exercise training and Mediterranean diet on vascular risk reduction in post-menopausal women. Clin Hemorheol Microcirc 57: 33–47, 2014. doi: 10.3233/CH-131770. [DOI] [PubMed] [Google Scholar]
- 5.Anderson GL, Limacher M, Assaf AR, Bassford T, Beresford SA, Black H, Bonds D, Brunner R, Brzyski R, Caan B, Chlebowski R, Curb D, Gass M, Hays J, Heiss G, Hendrix S, Howard BV, Hsia J, Hubbell A, Jackson R, Johnson KC, Judd H, Kotchen JM, Kuller L, LaCroix AZ, Lane D, Langer RD, Lasser N, Lewis CE, Manson J, Margolis K, Ockene J, O’Sullivan MJ, Phillips L, Prentice RL, Ritenbaugh C, Robbins J, Rossouw JE, Sarto G, Stefanick ML, Van Horn L, Wactawski-Wende J, Wallace R, Wassertheil-Smoller S, Women’s Health Initiative Steering Committee . Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA 291: 1701–1712, 2004. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 6.Black MA, Cable NT, Thijssen DH, Green DJ. Impact of age, sex, and exercise on brachial artery flow-mediated dilatation. Am J Physiol Heart Circ Physiol 297: H1109–H1116, 2009. doi: 10.1152/ajpheart.00226.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Casey DP, Pierce GL, Howe KS, Mering MC, Braith RW. Effect of resistance training on arterial wave reflection and brachial artery reactivity in normotensive postmenopausal women. Eur J Appl Physiol 100: 403–408, 2007. doi: 10.1007/s00421-007-0447-2. [DOI] [PubMed] [Google Scholar]
- 8.Celermajer DS, Sorensen KE, Spiegelhalter DJ, Georgakopoulos D, Robinson J, Deanfield JE. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J Am Coll Cardiol 24: 471–476, 1994. doi: 10.1016/0735-1097(94)90305-0. [DOI] [PubMed] [Google Scholar]
- 9.Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, Deanfield J, Drexler H, Gerhard-Herman M, Herrington D, Vallance P, Vita J, Vogel R, International Brachial Artery Reactivity Task Force . Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol 39: 257–265, 2002. doi: 10.1016/S0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- 10.DeSouza CA, Shapiro LF, Clevenger CM, Dinenno FA, Monahan KD, Tanaka H, Seals DR. Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation 102: 1351–1357, 2000. doi: 10.1161/01.CIR.102.12.1351. [DOI] [PubMed] [Google Scholar]
- 11.DeVan AE, Eskurza I, Pierce GL, Walker AE, Jablonski KL, Kaplon RE, Seals DR. Regular aerobic exercise protects against impaired fasting plasma glucose-associated vascular endothelial dysfunction with aging. Clin Sci (Lond) 124: 325–331, 2013. doi: 10.1042/CS20120291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeVan AE, Johnson LC, Brooks FA, Evans TD, Justice JN, Cruickshank-Quinn C, Reisdorph N, Bryan NS, McQueen MB, Santos-Parker JR, Chonchol MB, Bassett CJ, Sindler AL, Giordano T, Seals DR. Effects of sodium nitrite supplementation on vascular function and related small metabolite signatures in middle-aged and older adults. J Appl Physiol (1985) 120: 416–425, 2016. doi: 10.1152/japplphysiol.00879.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donato AJ, Black AD, Jablonski KL, Gano LB, Seals DR. Aging is associated with greater nuclear NF kappa B, reduced I kappa B alpha, and increased expression of proinflammatory cytokines in vascular endothelial cells of healthy humans. Aging Cell 7: 805–812, 2008. doi: 10.1111/j.1474-9726.2008.00438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Donato AJ, Eskurza I, Jablonski KL, Gano LB, Pierce GL, Seals DR. Cytochrome P-450 2C9 signaling does not contribute to age-associated vascular endothelial dysfunction in humans. J Appl Physiol (1985) 105: 1359–1363, 2008. doi: 10.1152/japplphysiol.90629.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Donato AJ, Eskurza I, Silver AE, Levy AS, Pierce GL, Gates PE, Seals DR. Direct evidence of endothelial oxidative stress with aging in humans: relation to impaired endothelium-dependent dilation and upregulation of nuclear factor-kappaB. Circ Res 100: 1659–1666, 2007. doi: 10.1161/01.RES.0000269183.13937.e8. [DOI] [PubMed] [Google Scholar]
- 16.Eskurza I, Kahn ZD, Seals DR. Xanthine oxidase does not contribute to impaired peripheral conduit artery endothelium-dependent dilatation with ageing. J Physiol 571: 661–668, 2006. doi: 10.1113/jphysiol.2005.102566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eskurza I, Monahan KD, Robinson JA, Seals DR. Effect of acute and chronic ascorbic acid on flow-mediated dilatation with sedentary and physically active human ageing. J Physiol 556: 315–324, 2004. doi: 10.1113/jphysiol.2003.057042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eskurza I, Myerburgh LA, Kahn ZD, Seals DR. Tetrahydrobiopterin augments endothelium-dependent dilatation in sedentary but not in habitually exercising older adults. J Physiol 568: 1057–1065, 2005. doi: 10.1113/jphysiol.2005.092734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eskurza I, Seals DR, DeSouza CA, Tanaka H. Pharmacologic versus flow-mediated assessments of peripheral vascular endothelial vasodilatory function in humans. Am J Cardiol 88: 1067–1069, 2001. doi: 10.1016/S0002-9149(01)01997-X. [DOI] [PubMed] [Google Scholar]
- 20.Evans SL, Davy KP, Stevenson ET, Seals DR. Physiological determinants of 10-km performance in highly trained female runners of different ages. J Appl Physiol (1985) 78: 1931–1941, 1995. [DOI] [PubMed] [Google Scholar]
- 21.Franzoni F, Ghiadoni L, Galetta F, Plantinga Y, Lubrano V, Huang Y, Salvetti G, Regoli F, Taddei S, Santoro G, Salvetti A. Physical activity, plasma antioxidant capacity, and endothelium-dependent vasodilation in young and older men. Am J Hypertens 18: 510–516, 2005. doi: 10.1016/j.amjhyper.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 22.Galetta F, Franzoni F, Virdis A, Ghiadoni L, Taddei S, Salvetti A, Santoro G. Endothelium-dependent vasodilation and carotid artery wall remodeling in athletes and sedentary subjects. Atherosclerosis 186: 184–192, 2006. doi: 10.1016/j.atherosclerosis.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 23.Gambacciani M, Monteleone P, Vitale C, Silvestri A, Fini M, Genazzani AR, Rosano GM. Dydrogesterone does not reverse the effects of estradiol on endothelium-dependent vasodilation in postmenopausal women: a randomised clinical trial. Maturitas 43: 117–123, 2002. doi: 10.1016/S0378-5122(02)00184-6. [DOI] [PubMed] [Google Scholar]
- 24.Gerhard M, Walsh BW, Tawakol A, Haley EA, Creager SJ, Seely EW, Ganz P, Creager MA. Estradiol therapy combined with progesterone and endothelium-dependent vasodilation in postmenopausal women. Circulation 98: 1158–1163, 1998. doi: 10.1161/01.CIR.98.12.1158. [DOI] [PubMed] [Google Scholar]
- 25.Gutterman DD, Chabowski DS, Kadlec AO, Durand MJ, Freed JK, Ait-Aissa K, Beyer AM. The human microcirculation: regulation of flow and beyond. Circ Res 118: 157–172, 2016. doi: 10.1161/CIRCRESAHA.115.305364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hagmar M, Eriksson MJ, Lindholm C, Schenck-Gustafsson K, Hirschberg AL. Endothelial function in post-menopausal former elite athletes. Clin J Sport Med 16: 247–252, 2006. doi: 10.1097/00042752-200605000-00011. [DOI] [PubMed] [Google Scholar]
- 27.Harris RA, Nishiyama SK, Wray DW, Richardson RS. Ultrasound assessment of flow-mediated dilation. Hypertension 55: 1075–1085, 2010. doi: 10.1161/HYPERTENSIONAHA.110.150821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hashimoto M, Akishita M, Eto M, Ishikawa M, Kozaki K, Toba K, Sagara Y, Taketani Y, Orimo H, Ouchi Y. Modulation of endothelium-dependent flow-mediated dilatation of the brachial artery by sex and menstrual cycle. Circulation 92: 3431–3435, 1995. doi: 10.1161/01.CIR.92.12.3431. [DOI] [PubMed] [Google Scholar]
- 29.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a “set up” for vascular disease. Circulation 107: 139–146, 2003. doi: 10.1161/01.CIR.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- 30.Lind L, Berglund L, Larsson A, Sundström J. Endothelial function in resistance and conduit arteries and 5-year risk of cardiovascular disease. Circulation 123: 1545–1551, 2011. doi: 10.1161/CIRCULATIONAHA.110.984047. [DOI] [PubMed] [Google Scholar]
- 31.Lohman TG. Roche AF, Martorell R. Anthropometric Standardization Reference Manual. Champaign, IL: Human Kinetics, 1988. [Google Scholar]
- 32.Lonn E, Bosch J, Teo KK, Pais P, Xavier D, Yusuf S. The polypill in the prevention of cardiovascular diseases: key concepts, current status, challenges, and future directions. Circulation 122: 2078–2088, 2010. doi: 10.1161/CIRCULATIONAHA.109.873232. [DOI] [PubMed] [Google Scholar]
- 33.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28: 412–419, 1985. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 34.McCrohon JA, Adams MR, McCredie RJ, Robinson J, Pike A, Abbey M, Keech AC, Celermajer DS. Hormone replacement therapy is associated with improved arterial physiology in healthy post-menopausal women. Clin Endocrinol (Oxf) 45: 435–441, 1996. doi: 10.1046/j.1365-2265.1996.8070816.x. [DOI] [PubMed] [Google Scholar]
- 35.Miner JA, Martini ER, Smith MM, Brunt VE, Kaplan PF, Halliwill JR, Minson CT. Short-term oral progesterone administration antagonizes the effect of transdermal estradiol on endothelium-dependent vasodilation in young healthy women. Am J Physiol Heart Circ Physiol 301: H1716–H1722, 2011. doi: 10.1152/ajpheart.00405.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moreau KL, Deane KD, Meditz AL, Kohrt WM. Tumor necrosis factor-α inhibition improves endothelial function and decreases arterial stiffness in estrogen-deficient postmenopausal women. Atherosclerosis 230: 390–396, 2013. doi: 10.1016/j.atherosclerosis.2013.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moreau KL, Hildreth KL, Meditz AL, Deane KD, Kohrt WM. Endothelial function is impaired across the stages of the menopause transition in healthy women. J Clin Endocrinol Metab 97: 4692–4700, 2012. doi: 10.1210/jc.2012-2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moreau KL, Meditz A, Deane KD, Kohrt WM. Tetrahydrobiopterin improves endothelial function and decreases arterial stiffness in estrogen-deficient postmenopausal women. Am J Physiol Heart Circ Physiol 302: H1211–H1218, 2012. doi: 10.1152/ajpheart.01065.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moreau KL, Stauffer BL, Kohrt WM, Seals DR. Essential role of estrogen for improvements in vascular endothelial function with endurance exercise in postmenopausal women. J Clin Endocrinol Metab 98: 4507–4515, 2013. doi: 10.1210/jc.2013-2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Newcomer SC, Leuenberger UA, Hogeman CS, Proctor DN. Heterogeneous vasodilator responses of human limbs: influence of age and habitual endurance training. Am J Physiol Heart Circ Physiol 289: H308–H315, 2005. doi: 10.1152/ajpheart.01151.2004. [DOI] [PubMed] [Google Scholar]
- 41.Nichols WW, O’Rourke MF, and McDonald DA. McDonald's Blood Flow in Arteries: Theoretical, Experimental, and Clinical Principles. New York: Oxford University Press, 2005. [Google Scholar]
- 42.Nyberg M, Egelund J, Mandrup CM, Nielsen MB, Mogensen AS, Stallknecht B, Bangsbo J, Hellsten Y. Early postmenopausal phase is associated with reduced prostacyclin-induced vasodilation that is reversed by exercise training: The Copenhagen Women Study. Hypertension 68: 1011–1020, 2016. doi: 10.1161/HYPERTENSIONAHA.116.07866. [DOI] [PubMed] [Google Scholar]
- 43.Pereira MA, FitzerGerald SJ, Gregg EW, Joswiak ML, Ryan WJ, Suminski RR, Utter AC, Zmuda JM. A collection of Physical Activity Questionnaires for health-related research. Med Sci Sports Exerc 29, Suppl: S1–S205, 1997. [PubMed] [Google Scholar]
- 44.Pierce GL, Donato AJ, LaRocca TJ, Eskurza I, Silver AE, Seals DR. Habitually exercising older men do not demonstrate age-associated vascular endothelial oxidative stress. Aging Cell 10: 1032–1037, 2011. doi: 10.1111/j.1474-9726.2011.00748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pierce GL, Eskurza I, Walker AE, Fay TN, Seals DR. Sex-specific effects of habitual aerobic exercise on brachial artery flow-mediated dilation in middle-aged and older adults. Clin Sci (Lond) 120: 13–23, 2011. doi: 10.1042/CS20100174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rinder MR, Spina RJ, Ehsani AA. Enhanced endothelium-dependent vasodilation in older endurance-trained men. J Appl Physiol (1985) 88: 761–766, 2000. [DOI] [PubMed] [Google Scholar]
- 47.Saitta A, Altavilla D, Cucinotta D, Morabito N, Frisina N, Corrado F, D’Anna R, Lasco A, Squadrito G, Gaudio A, Cancellieri F, Arcoraci V, Squadrito F. Randomized, double-blind, placebo-controlled study on effects of raloxifene and hormone replacement therapy on plasma no concentrations, endothelin-1 levels, and endothelium-dependent vasodilation in postmenopausal women. Arterioscler Thromb Vasc Biol 21: 1512–1519, 2001. doi: 10.1161/hq0901.095565. [DOI] [PubMed] [Google Scholar]
- 48.Schaun MI, Dipp T, Rossato JS, Wilhelm EN, Pinto R, Rech A, Plentz RD, Homem de Bittencourt PI, Reischak-Oliveira A. The effects of periodized concurrent and aerobic training on oxidative stress parameters, endothelial function and immune response in sedentary male individuals of middle age. Cell Biochem Funct 29: 534–542, 2011. doi: 10.1002/cbf.1781. [DOI] [PubMed] [Google Scholar]
- 49.Seals DR, Jablonski KL, Donato AJ. Aging and vascular endothelial function in humans. Clin Sci 120: 357–375, 2011. doi: 10.1042/CS20100476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sherwood A, Bower JK, McFetridge-Durdle J, Blumenthal JA, Newby LK, Hinderliter AL. Age moderates the short-term effects of transdermal 17beta-estradiol on endothelium-dependent vascular function in postmenopausal women. Arterioscler Thromb Vasc Biol 27: 1782–1787, 2007. doi: 10.1161/ATVBAHA.107.145383. [DOI] [PubMed] [Google Scholar]
- 51.Taddei S, Galetta F, Virdis A, Ghiadoni L, Salvetti G, Franzoni F, Giusti C, Salvetti A. Physical activity prevents age-related impairment in nitric oxide availability in elderly athletes. Circulation 101: 2896–2901, 2000. doi: 10.1161/01.CIR.101.25.2896. [DOI] [PubMed] [Google Scholar]
- 52.Taddei S, Virdis A, Ghiadoni L, Mattei P, Sudano I, Bernini G, Pinto S, Salvetti A. Menopause is associated with endothelial dysfunction in women. Hypertension 28: 576–582, 1996. doi: 10.1161/01.HYP.28.4.576. [DOI] [PubMed] [Google Scholar]
- 53.Taddei S, Virdis A, Ghiadoni L, Salvetti G, Bernini G, Magagna A, Salvetti A. Age-related reduction of NO availability and oxidative stress in humans. Hypertension 38: 274–279, 2001. doi: 10.1161/01.HYP.38.2.274. [DOI] [PubMed] [Google Scholar]
- 54.Taddei S, Virdis A, Mattei P, Ghiadoni L, Gennari A, Fasolo CB, Sudano I, Salvetti A. Aging and endothelial function in normotensive subjects and patients with essential hypertension. Circulation 91: 1981–1987, 1995. doi: 10.1161/01.CIR.91.7.1981. [DOI] [PubMed] [Google Scholar]
- 55.Tew GA, George KP, Cable NT, Hodges GJ. Endurance exercise training enhances cutaneous microvascular reactivity in post-menopausal women. Microvasc Res 83: 223–228, 2012. doi: 10.1016/j.mvr.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 56.Toshima S, Hasegawa A, Kurabayashi M, Itabe H, Takano T, Sugano J, Shimamura K, Kimura J, Michishita I, Suzuki T, Nagai R. Circulating oxidized low density lipoprotein levels. A biochemical risk marker for coronary heart disease. Arterioscler Thromb Vasc Biol 20: 2243–2247, 2000. doi: 10.1161/01.ATV.20.10.2243. [DOI] [PubMed] [Google Scholar]
- 57.Wakatsuki A, Okatani Y, Ikenoue N, Fukaya T. Effect of medroxyprogesterone acetate on endothelium-dependent vasodilation in postmenopausal women receiving estrogen. Circulation 104: 1773–1778, 2001. doi: 10.1161/hc4001.097035. [DOI] [PubMed] [Google Scholar]
- 58.Weintraub WS, Daniels SR, Burke LE, Franklin BA, Goff DC Jr, Hayman LL, Lloyd-Jones D, Pandey DK, Sanchez EJ, Schram AP, Whitsel LP, American Heart Association Advocacy Coordinating Committee, Council on Cardiovascular Disease in the Young, Council on the Kidney in Cardiovascular Disease, Council on Epidemiology and Prevention, Council on Cardiovascular Nursing, Council on Arteriosclerosis, Thrombosis and Vascular Biology, Council on Clinical Cardiology, and Stroke Council . Value of primordial and primary prevention for cardiovascular disease: a policy statement from the American Heart Association. Circulation 124: 967–990, 2011. doi: 10.1161/CIR.0b013e3182285a81. [DOI] [PubMed] [Google Scholar]
- 59.Williams MR, Westerman RA, Kingwell BA, Paige J, Blombery PA, Sudhir K, Komesaroff PA. Variations in endothelial function and arterial compliance during the menstrual cycle. J Clin Endocrinol Metab 86: 5389–5395, 2001. doi: 10.1210/jcem.86.11.8013. [DOI] [PubMed] [Google Scholar]
- 60.Writing Group Members, Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Després JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jiménez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER III, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB, American Heart Association Statistics Committee, Stroke Statistics Subcommittee . Heart Disease and Stroke Statistics—2016 Update: A Report From the American Heart Association. Circulation 133: e38–e360, 2016. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 61.Yeboah J, Crouse JR, Hsu FC, Burke GL, Herrington DM. Brachial flow-mediated dilation predicts incident cardiovascular events in older adults: the Cardiovascular Health Study. Circulation 115: 2390–2397, 2007. doi: 10.1161/CIRCULATIONAHA.106.678276. [DOI] [PubMed] [Google Scholar]
- 62.Yeboah J, Folsom AR, Burke GL, Johnson C, Polak JF, Post W, Lima JA, Crouse JR, Herrington DM. Predictive value of brachial flow-mediated dilation for incident cardiovascular events in a population-based study: the multi-ethnic study of atherosclerosis. Circulation 120: 502–509, 2009. doi: 10.1161/CIRCULATIONAHA.109.864801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yoshizawa M, Maeda S, Miyaki A, Misono M, Choi Y, Shimojo N, Ajisaka R, Tanaka H. Additive beneficial effects of lactotripeptides intake with regular exercise on endothelium-dependent dilatation in postmenopausal women. Am J Hypertens 23: 368–372, 2010. doi: 10.1038/ajh.2009.270. [DOI] [PubMed] [Google Scholar]