We have demonstrated that intermittent parathyroid hormone administration can rescue age-related vascular dysfunction by improving endothelial-dependent dilation in the aorta of older rodents. This demonstrates a novel potential benefit of parathyroid hormone administration in aging.
Keywords: parathyroid hormone, aging, endothelial, nitric oxide
Abstract
Aging is an independent risk factor for cardiovascular disease and is characterized by a decline in endothelial function. Parathyroid hormone (PTH) administration has been shown to increase endothelial nitric oxide synthase (eNOS) expression. The purpose of this investigation was to determine the effect of intermittent PTH administration on aortic endothelial function in old rodents. We hypothesized that intermittent PTH administration would improve endothelial function in older rodents. Old (24-mo-old) and young (4-mo-old) Fischer-344 rats were given 10 injections of PTH 1–34 (43 μg·kg−1·day−1) or phosphate-buffered saline (100 μl/day) over 15 days. Endothelium-dependent relaxation of aortic rings in response to acetylcholine (10−9 to 10−5 M) was significantly impaired in old control (OC) compared with young control (YC) as indicated by a reduced area under the curve (AUC, 100 ± 6.28 vs. 54.08 ± 8.3%; P < 0.05) and impaired maximal relaxation (Emax, 70.1 ± 4.48 vs. 92.9 ± 4.38%; P < 0.05). Emax was improved in old animals treated with PTH (OPTH) (OC, 70.1 ± 4.48 vs. OPTH, 85 ± 7.48%; P < 0.05) as well as AUC (OC, 54.08 ± 8.3 vs. OPTH, 82.5 ± 5.7%; P < 0.05) while logEC50 was not different. Endothelial-independent relaxation in response to sodium nitroprusside was not different among groups. Aortic eNOS protein expression was significantly decreased in OC compared with YC (P < 0.05). PTH treatment restored eNOS expression in OPTH animals (P < 0.05). These data suggest that PTH may play a role in attenuating age-related impairments in aortic endothelial function.
NEW & NOTEWORTHY We have demonstrated that intermittent parathyroid hormone administration can rescue age-related vascular dysfunction by improving endothelial-dependent dilation in the aorta of older rodents. This demonstrates a novel potential benefit of parathyroid hormone administration in aging.
advancing age increases the risk for cardiovascular disease (CVD) (17). Endothelial dysfunction is considered a hallmark of aging (6, 11, 24) and is associated with an increased risk for CVD (11). Vascular dysfunction occurs during aging and is associated with an imbalance between vasodilating and vasoconstricting substances that are produced by the endothelium (11). This disruption in balance is largely the result of reduced bioavailability and/or production of the endothelium-derived vasodilator nitric oxide (NO) (24), a largely accepted mechanism responsible for impaired endothelial function in aging (8). In addition to vasodilation, NO plays an important role in protecting the vessel from inflammation, leukocyte adhesion, and platelet aggregation (32). Hence, a decrease in the bioavailability of NO may contribute to the development of atherosclerosis (32). Age-related reductions in NO have been attributed to a lower expression or activity of the enzyme endothelial nitric oxide synthase (eNOS) (1, 7, 27, 30), a reduction in the cofactor tetrahydrobiopterin (12), a reduction in the NO precursor l-arginine (3), or an increase in the endogenous inhibitor asymmetric dimethyl arginine (23). These factors may contribute to the reductions observed in aortic endothelial-dependent vasodilation in old rodents compared with young rodents (10, 15). Because of the advancing age of the world’s population, investigating interventions that target the age-related declines in endothelial function is of importance.
Although parathyroid hormone (PTH) has been used to treat individuals with low bone mass, it has been shown to impact the vasculature as well. Acute exogenous treatment with PTH can evoke relaxation in a variety of vessels (5, 20, 35). This relaxation appears to be partially mediated through an increase in NO production (14, 22). NO production is elevated in vitro in human umbilical vein endothelial cells (HUVEC) with acute treatment of PTH and PTH-related peptide (14, 22). Furthermore, eNOS expression and activity was increased when HUVECs were treated with PTH 1–34 (22). Most recently, 15 days of intermittent PTH 1–84 administration increased endothelium-dependent vasodilation in the femoral principal nutrient artery (PNA) of male Wistar rats via augmented NO-mediated participation (21).
There are two PTH analogs (i.e., 1–34 and 1–84) that are approved for use in humans for the treatment of osteoporosis. Biologically, they differ in their amino acid length. PTH 1–34 is synthetic and consists of the first 34 amino acids of parathormone or the active part, whereas PTH 1–84 consists of 84 amino acids, is the complete parathormone, and is released by the parathyroid glands (33). To date, neither analog has been tested in the aorta. We chose to use PTH 1–34 in this investigation since it has approval for clinical use in the United States (25).
In this study, we hypothesized that intermittent PTH 1–34 administration would improve endothelial function in the aorta of old rodents. To test this hypothesis, we administered PTH 1–34 to young and old rodents over 15 days and performed measures of endothelial-dependent relaxation upon death. Whereas the role of intermittent PTH administration on bone has been well established, less is known regarding the effects of intermittent PTH administration on other physiological systems. Because of the reported ability of PTH to increase vasodilation via NO-mediated mechanisms in the PNA (21), PTH may be a novel intervention to rescue age-related declines in endothelial function in the aorta.
MATERIALS AND METHODS
Animal care and use.
This experimental protocol was approved by the University of Delaware Institutional Animal Care and Use Committee and was conducted in accordance with the National Institutes of Health policy regarding the Humane Care and Use of Laboratory Animals.
Male Fisher-344 rats were purchased from Harlan Laboratories (Indianapolis, IN). Animals were individually housed in standard caging and kept on a 12:12-h light-dark cycle. Water and rat chow were provided ad libitum. Thirty-four young (~5-mo-old) and 34 old (~24-mo-old) animals were randomly assigned to one of the following four experimental groups: young control (YC), young PTH (YPTH), old control (OC), and old PTH (OPTH). All groups were given subcutaneous injections of either phosphate-buffered saline as a control (100 μl/day) or PTH 1–34 (43 µg·kg−1·day−1; ProSpec Bio). All groups received 10 injections over a 15-day period at the same time of day, and the final injection was administered at least 18 h before death. This protocol has been used previously (21).
Tissue preparation.
Animals were anesthetized using isoflurane (3% to oxygen balance) and were killed by excision of the heart. Following death, the thoracic aorta was dissected and placed in ice-cold physiological saline solution (PSS; 118.99 mmol NaCl, 4.69 mmol KCl, 2.50 mmol CaCl2-2H2O, 1.17 mmol MgSO4-7H2O, 1.18 mmol KH2PO4, 0.03 mmol EDTA, 1.091 g/l glucose, and 2.100 g/l NaHCO3; pH, 7.4). Aortas were cut into 3-mm sections for assessment of vascular function as described below. Two sections of aorta were denuded by gently scraping away the endothelium with a pair of forceps to assess vascular smooth muscle function. Remaining tissue was snap-frozen in liquid nitrogen and stored at −80°C for later analysis.
Assessment of vascular function.
Vascular relaxation was assessed in vitro by performing isometric ring studies using aortic ring segments. Rings were prepared as described above and mounted on wire force transducers (DMT 610M; Danish Myotechnology) within individual organ baths containing PSS at 37°C and pH 7.4. Vessels were oxygenated with carbogen gas (5% CO2 and 95% O2), and resting tension was set at 30 mN following normalization of the length-tension curve. Aortic rings underwent a 1-h period of equilibration followed by an assessment of vessel viability. This involved constricting the rings with phenylephrine (3 × 10−7 M) and relaxing them using a single dose of Acetylcholine (ACh; 10−4) as previously reported (18, 19).
Following the initial assessment of vessel viability, rings were washed every 10 min with PSS for 1 h. Following the initial washout period, basal tension was calculated as the passive tension of the rings before assessment of endothelial function. Rings were then preconstricted using a single dose of phenylephrine (3 × 10−7 M) and relaxed using ACh (10−9 to 10−5 M) or sodium nitroprusside (SNP; 10−10 to 10−6 M) in a dose-dependent manner to assess both endothelium-dependent relaxation (EDR) and endothelium-independent relaxation (EIR), respectively. Preconstriction values were measured as the steady-state tension calculated following a submaximal dose of phenylephrine (3 × 10−7 M).
Western blotting.
Protein expression of eNOS and phosphorylated eNOS (Ser1177) was assessed using Western blot. Aortic tissue samples were homogenized using a Bullet blender (Next Advance). Tissue homogenates were centrifuged at 10,000 g for 10 min at 4°C, and supernatant was extracted and prepared for analysis. Protein concentration was assessed using the Bradford method (4). Samples were loaded in an 8% Tris·HCl gel (Thermo Scientific) and electrophoresed for 60 min at 100 volts. Gels were transferred to a nitrocellulose membrane, blocked, and immunoblotted with the primary antibody for the protein of interest, eNOS (1:500; BD 610297), and phosphorylated eNOS Ser1177 (1:500; BD 612393). Membranes were washed and incubated with the appropriate recommended secondary antibody. Membranes were incubated with enhanced chemiluminescence detection reagents (Thermo Scientific) for development. Protein abundance was expressed as relative units normalized to β-actin (1:1,000, sc-81178; Santa Cruz Biotechnology) and quantified using Image J software (version 1.43; National Institutes of Health).
Statistical analysis.
Data were analyzed using a 2 × 2 (age × treatment) ANOVA. When a significant interaction was found, Tukey’s post hoc testing was performed (IBM SPSS 21.0). Dose-response curves were generated for all vascular function data using GraphPad Prism 5.0 software and normalized to percent relaxation from each individual vessel’s phenylephrine (3 × 10−7 M) constriction value that was set to 0%. Dose-response curves were fit with a nonlinear regression, and maximal relaxation (Emax), area under the curve (AUC), and the logEC50 were calculated. LogEC50 was calculated as the concentration of the agonist needed to elicit a 50% response. The alpha level was set at P < 0.05 a priori, and all data are presented as means ± SE.
RESULTS
Animal characteristics.
Both groups of older rodents had a significantly higher body mass than the young rodents (YC, 362.4 ± 31.3 g; YPTH, 370.1 ± 31.3 g; OC, 444.1 ± 13.1 g; OPTH, 431.7 ± 34.2 g; P < 0.05). PTH treatment had no effect on body mass, since mass did not differ between the control groups and their respective PTH groups.
Effect of aging and PTH on EDR and EIR.
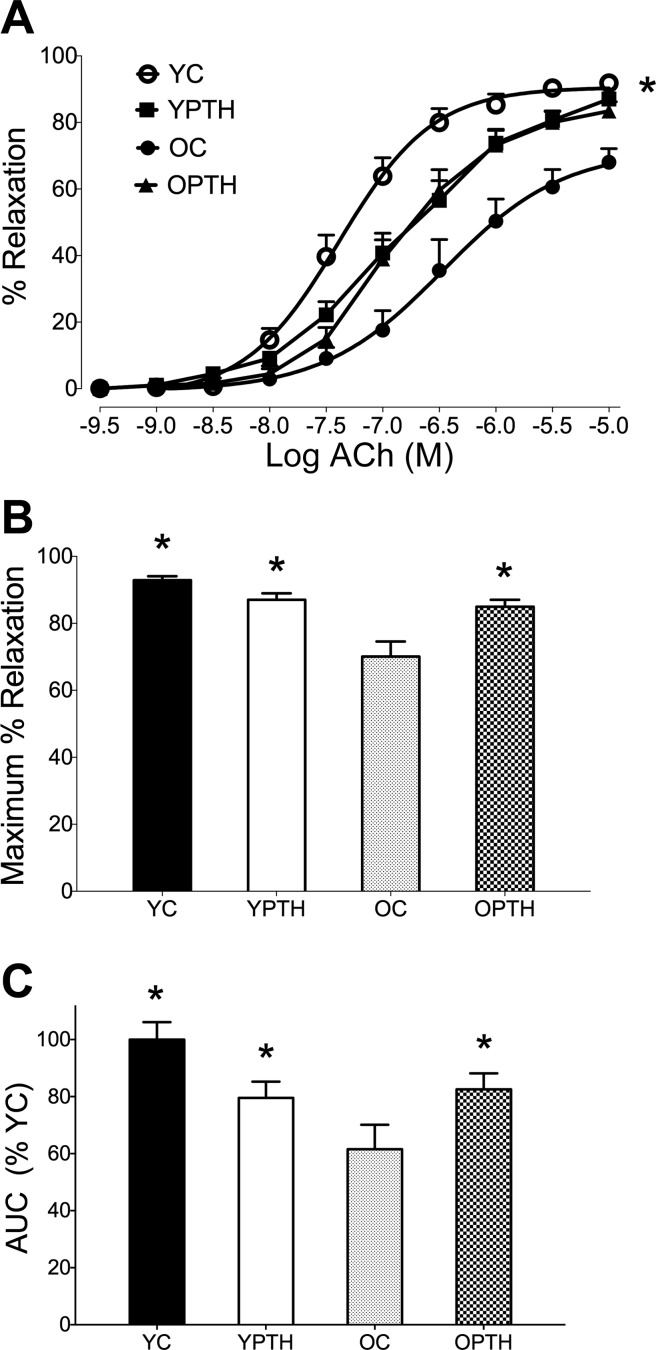
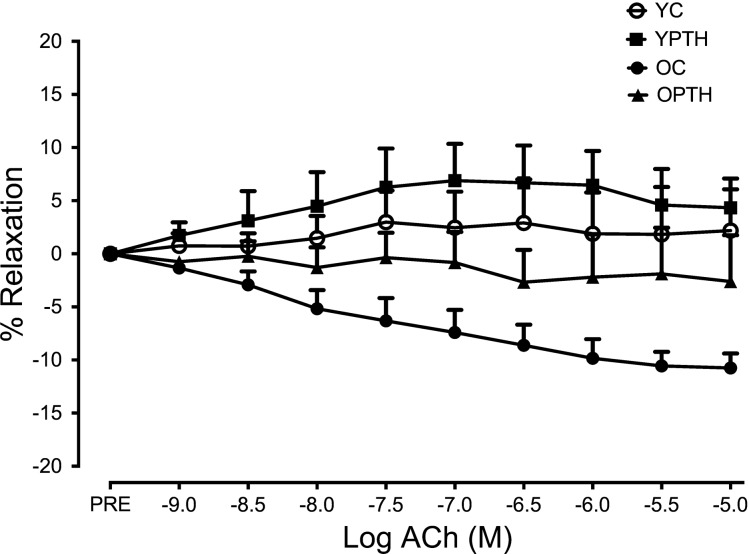
The complete EDR dose response to ACh in aortic rings is presented in Fig. 1A, showing a reduction in relaxation in the OC group compared with all other groups. PTH treatment augmented Emax by 15% in the OPTH group compared with the OC group (P < 0.05, Fig. 1B), resulting in no difference between OPTH and YC or YPTH. AUC was significantly impaired in the OC group compared with the YC group as indicated by a 46% reduction and was rescued with PTH treatment (P < 0.05, Fig. 1C). Finally, there were no significant differences in logEC50. However, logEC50 trended downward (YC, −7.33 ± 0.14 vs. YPTH, −5.83 ± 0.93; P = 0.087) in young PTH-treated animals compared with young control. Furthermore, when vessels were denuded to assess relaxation in the absence of the endothelium, relaxation was impaired for all groups, but no differences in relaxation were observed among groups (Fig. 2). Finally, there was no difference in basal tone or PE preconstriction between groups (data not shown; P > 0.05).
Fig. 1.
Endothelial-dependent relaxation (EDR) in young and old control and PTH-treated rats. Dose responses of aortic rings to acetylcholine (ACh, A), maximal relaxation (Emax) to ACh (B), and area under the curve (AUC, C). *Difference from old control, P < 0.05. OC, old control (n = 11); OPTH, old animals treated with parathyroid hormone (PTH, n = 13); YC, young control (n = 14); YPTH, young animals treated with PTH (n = 14).
Fig. 2.
EDR in denuded vessels from young and old control and PTH-treated rats. Dose responses of the aortic rings to ACh are shown. OC, n = 6; OPTH, n = 6; YC, n = 7; YPTH, n = 7.
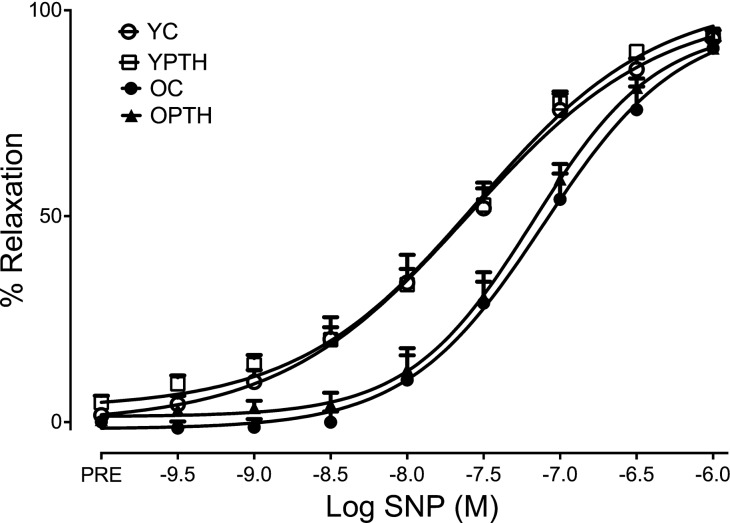
The EIR response to SNP is shown in Fig. 3. There were no group differences (P > 0.05) in response to SNP as assessed by AUC (%YC: YC, 100 ± 0.07; YPTH, 80 ± 0.08; OC, 82 ± 0.07; OPTH, 90 ± 0.08%), Emax (YC, 93.1 ± 2.0; YPTH, 94.1 ± 1.33; OC, 90.8 ± 4.4; OPTH, 90.8 ± 1.8%), and logEC50 (YC, −7.8 ± 0.2; YPTH, −7.49 ± 0.1; OC, −7.35 ± 0.2; OPTH, −7.3 ± 0.1%) indicating no age- or treatment-related changes to EIR.
Fig. 3.
Endothelial-independent relaxation (EIR) of aortic rings to sodium nitroprusside (SNP). OC, n = 11; OPTH, n = 13; YC, n = 14; YPTH, n = 14.
Effect of PTH on aortic eNOS expression.
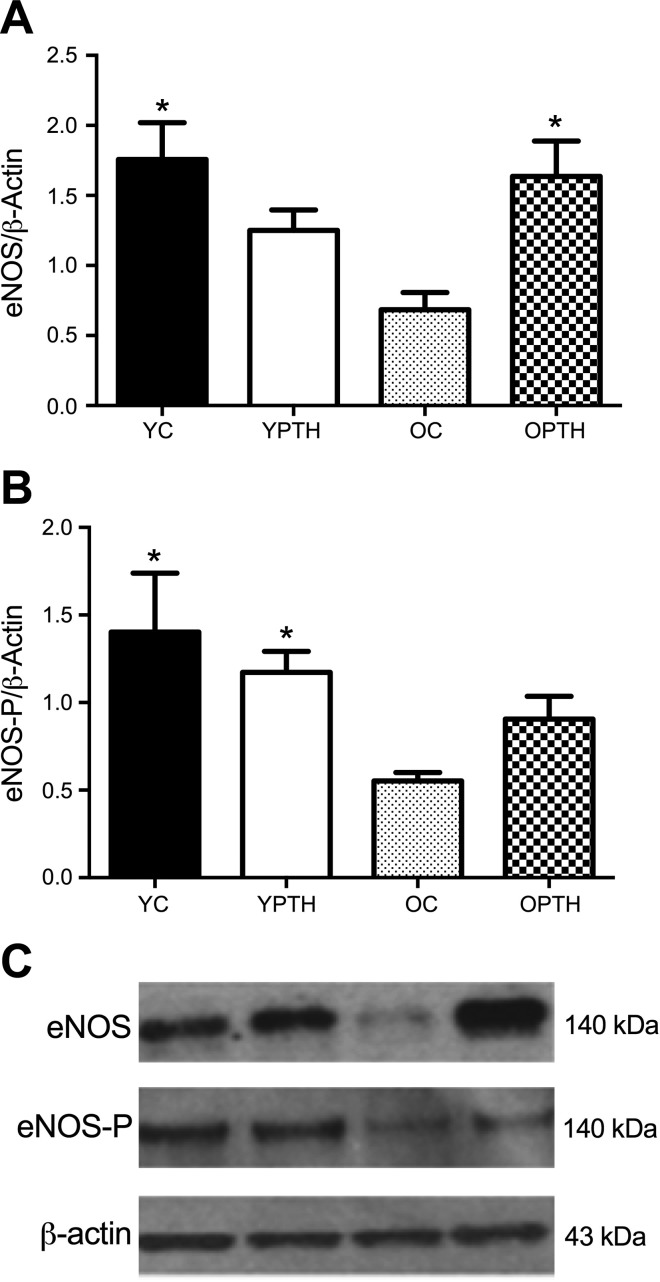
Aortic eNOS protein content was decreased by 61% in the OC group compared with YC (P < 0.05, Fig. 4A). PTH treatment restored eNOS in the OPTH group by 59% compared with OC (P < 0.05, Fig. 4A), resulting in no difference between OPTH and YC. Furthermore, the YPTH group was not significantly different from any group. However, when phosphorylated eNOS (Ser1177) expression was normalized to total eNOS expression (Fig. 4B), there were no differences among groups.
Fig. 4.
Total endothelial nitric oxide synthase (eNOS) protein expression in aorta normalized to β-actin (A), phosphorylated eNOS relative to total eNOS (B), and representative Western blots (C) are shown. Total eNOS protein expression was significantly greater in YC and OPTH compared with OC. There was a main effect of treatment for the ratio of phosphorylated eNOS/total eNOS. *Difference from OC, P < 0.05; n = 7 for each group.
DISCUSSION
Intermittent PTH administration is a commonly used treatment for osteoporosis because of its ability to directly stimulate osteoblasts. Osteoporosis is a disease that typically occurs later in life when vascular function is also declining. The novel finding of the present study is that intermittent PTH treatment restored endothelial function in the aortas of old rodents. PTH treatment did not alter the response to SNP, indicating that PTH’s influence on vascular relaxation in the aorta is mediated primarily through the endothelium. Furthermore, PTH was able to restore the age-related loss in eNOS protein expression similar to levels seen in young control rats. From a translational perspective, this demonstrates a potential role for PTH treatment in improving both bone and vascular health in the aging population.
Acute administration of PTH has previously been shown to have a relaxing effect on the vasculature (5, 20, 35). However, more recently, 15 days of intermittent PTH 1–84 administration augmented ACh-mediated endothelium-dependent vasodilation of the femoral PNA by 33% in young Wistar rats compared with untreated controls. This is important, since the PNA is the primary source of blood flow to long bones. This improved dilation of the PNA was attenuated when the vessel was incubated with the NOS inhibitor NG-nitro-l-arginine methyl ester (l-NAME), suggesting that intermittent administration of PTH improves vascular function via a NO-dependent pathway (21). We extend these findings to the aorta of aged rats using 15 days of intermittent PTH 1–34 administration.
In the current study, 15 days of intermittent PTH administration improved maximal EDR by 15% in the aortas of aged rodents. The decline in EDR in our old control animals is consistent with prior research (2, 16). We also provide evidence that this effect was specific to the endothelium, since aging did not alter relaxation to SNP. To verify these results, we next tested the response to ACh in denuded vessels and found impaired relaxation in all groups but no differences in relaxation among groups. Our finding that PTH treatment can improve EDR as indicated by improved maximal relaxation to ACh suggests that intermittent PTH administration has similar actions in the aorta of old rodents as previously reported in the PNA of young rodents (21). Whereas PTH treatment did not significantly alter relaxation in our young rats, this may be attributable to differences in the structural properties and vasodilatory response between the femoral PNA and the aorta as well as differences in rodent strains. Also, our study used the 1–34 amino acid NH2-terminal peptide (PTH 1–34) as opposed to the intact 1–84 amino acid peptide (PTH 1–84). We chose to use PTH 1–34 because it has clinical approval in the United States.
The effect of aging on eNOS protein expression has been inconsistent, with eNOS content shown to either increase (9, 31), decrease (1, 7, 30), or result in no change (29). In the current study, we observed a decline in eNOS protein expression in the aorta of aged rodents. In addition to changes in eNOS content, aging may decrease the phosphorylation of eNOS in old rodents when measured at the serine residue 1177 (27).
When given acutely, PTH has been shown to increase eNOS protein expression and activity in cultured endothelial cell lines, including bovine pulmonary artery and HUVECs (14, 22). The subsequent increase in eNOS production and/or activity may augment the bioavailability of NO, leading to improved vascular function in adult and senescent rodents (28, 34). Therefore, daily PTH administration may increase eNOS activity and in turn attenuate the negative effects aging has on eNOS production. Our data suggest that intermittent PTH administration restored eNOS protein expression in old aorta to similar levels as in young aorta. Previously, Soucy et al. (27) found age-related reductions in aortic eNOS phosphorylation when measured relative to total eNOS content in Wistar rats. We were unable to detect significant changes when phosphorylated eNOS was normalized to total eNOS protein expression. Contrary to what was found in that study, we found that age-related changes were a result of an overall reduction in eNOS content rather than a reduction in eNOS phosphorylation. Last, our study demonstrates that, when given intermittently, PTH administration does not increase eNOS phosphorylation (Ser1177), suggesting that any improvements in NO production/bioavailability are related to an increase in eNOS or potentially other PTH-mediated mechanisms.
PTH administration was beneficial in the older rats; however, we observed no significant differences in the ACh response between YC and YPTH for Emax, AUC, or the logEC50. Indeed, Emax was similar in the young groups; however, there was a trend for a difference in the logEC50 response. Although this did not reach statistical significance, we had anticipated a similar response between the young groups. At this time, we can only speculate on this response, which suggests that PTH administration in young healthy rodents may negatively impact endothelial relaxation as related to a reduction in eNOS. While eNOS expression was not significantly different from YC, it was not different from OC either, suggesting a possible decline in expression. However, when phosphorylated eNOS was expressed relative to total, the reduction was less pronounced.
Although the upregulation of eNOS may be a principal mediator for improvements in vascular function, it is possible other factors contribute as well. Acute treatment with PTH has been shown to act as an antioxidant, scavenging hydrogen peroxide (H2O2) in cultured osteoblast cells (13). Intermittent doses of PTH (28 days) also attenuated the rise of Alox15, a factor known to increase oxidative stress through lipid peroxidation (13). Whereas PTH has been shown to have an antioxidant effect in bone tissue, it remains unknown if it has a similar effect in the vasculature. In addition, there was a downward trend in relaxation seen in young PTH-treated rodents. PTH’s potential ability to scavenge H2O2 in young animals with a normal redox balance may negatively affect signaling in the aorta, as previously reported in exercise-trained skeletal muscle arterioles (26). A reduction in both H2O2 and peroxynitrate hindered relaxation, suggesting they both play a key role in endothelium-dependent relaxation. However, future studies are needed to elucidate whether PTH has an antioxidant role.
Limitations and future directions.
We chose to study the aorta to evaluate endothelial function in the context of PTH in an aged model. Whereas resistance vessels do contribute significantly to overall vascular resistance compared with the aorta, evaluation of aortic endothelial function provides information relative to conduit artery function.
In the present study, we sought to gain insight into the potential of intermittent PTH therapy to attenuate age-related declines in endothelial function. Experiments using NO synthase inhibitors such as l-NAME need to be performed to examine the overall contribution of NO to EDR. Based on prior literature, we focused on the contribution of the NO pathway via an upregulation of eNOS. However, PTH is also capable of indirect NO upregulation by means of augmented vascular endothelial growth factor production (21), which may have contributed to our findings. Additionally, further experiments need to be conducted to examine which factors may have contributed to the downward shift in endothelial function in young animals with PTH treatment. Finally, it would be useful to examine transcriptional factors that mediate changes in eNOS protein expression.
Perspectives and Significance
In conclusion, we demonstrated that 15 days of intermittent PTH administration improves endothelial function in old rodents but does not appear to alter smooth muscle function. Furthermore, intermittent PTH treatment increased eNOS protein expression. This suggests that PTH improves age-related vascular dysfunction by increasing NO bioavailability, potentially by increasing eNOS content. Finally, these data demonstrate that intermittent administration of PTH to manage bone loss may also play a dual role in the protection of the vasculature. Future studies should explore the mechanisms responsible for the increase in eNOS protein expression in older rodents in response to PTH administration.
GRANTS
This study was supported in part by National Institute of Arthritis and Musculoskeletal and Skin Diseases Grant 7R15-AR-062882–02 (R. D. Prisby).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.D.P., D.G.E., and S.L.E. conception and study design; J.J.G. performed experiments; J.J.G., S.L.E. data analysis; S.L.E., D.G.E., J.J.G., R.D.P data interpretation; J.J.G. prepared figures; J.J.G., S.L.E. drafted manuscript; S.L.E., D.G.E., J.J.G., R.D.P. edited and revised manuscript; and S.L.E., D.G.E., J.J.G., R.D.P. approved final version of manuscript.
REFERENCES
- 1.Barton M, Cosentino F, Brandes RP, Moreau P, Shaw S, Lüscher TF. Anatomic heterogeneity of vascular aging: role of nitric oxide and endothelin. Hypertension 30: 817–824, 1997. doi: 10.1161/01.HYP.30.4.817. [DOI] [PubMed] [Google Scholar]
- 2.Blackwell KA, Sorenson JP, Richardson DM, Smith LA, Suda O, Nath K, Katusic ZS. Mechanisms of aging-induced impairment of endothelium-dependent relaxation: role of tetrahydrobiopterin. Am J Physiol Heart Circ Physiol 287: H2448–H2453, 2004. doi: 10.1152/ajpheart.00248.2004. [DOI] [PubMed] [Google Scholar]
- 3.Bode-Böger SM, Scalera F, Martens-Lobenhoffer J. Asymmetric dimethylarginine (ADMA) accelerates cell senescence. Vasc Med 10, Suppl 1: S65–S71, 2005. doi: 10.1177/1358836X0501000110. [DOI] [PubMed] [Google Scholar]
- 4.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254, 1976. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 5.Bukoski RD, Kremer D. Calcium-regulating hormones in hypertension: vascular actions. Am J Clin Nutr 54, Suppl: 220S–226S, 1991. [DOI] [PubMed] [Google Scholar]
- 6.Celermajer DS, Sorensen KE, Spiegelhalter DJ, Georgakopoulos D, Robinson J, Deanfield JE. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J Am Coll Cardiol 24: 471–476, 1994. doi: 10.1016/0735-1097(94)90305-0. [DOI] [PubMed] [Google Scholar]
- 7.Csiszar A, Ungvari Z, Edwards JG, Kaminski P, Wolin MS, Koller A, Kaley G. Aging-induced phenotypic changes and oxidative stress impair coronary arteriolar function. Circ Res 90: 1159–1166, 2002. doi: 10.1161/01.RES.0000020401.61826.EA. [DOI] [PubMed] [Google Scholar]
- 8.El Assar M, Angulo J, Vallejo S, Peiró C, Sánchez-Ferrer CF, Rodríguez-Mañas L. Mechanisms involved in the aging-induced vascular dysfunction. Front Physiol 3: 132, 2012. doi: 10.3389/fphys.2012.00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goettsch W, Lattmann T, Amann K, Szibor M, Morawietz H, Münter K, Müller SP, Shaw S, Barton M. Increased expression of endothelin-1 and inducible nitric oxide synthase isoform II in aging arteries in vivo: implications for atherosclerosis. Biochem Biophys Res Commun 280: 908–913, 2001. doi: 10.1006/bbrc.2000.4180. [DOI] [PubMed] [Google Scholar]
- 10.Gong X, Ma Y, Ruan Y, Fu G, Wu S. Long-term atorvastatin improves age-related endothelial dysfunction by ameliorating oxidative stress and normalizing eNOS/iNOS imbalance in rat aorta. Exp Gerontol 52: 9–17, 2014. doi: 10.1016/j.exger.2014.01.015. [DOI] [PubMed] [Google Scholar]
- 11.Herrera MD, Mingorance C, Rodríguez-Rodríguez R, Alvarez de Sotomayor M. Endothelial dysfunction and aging: an update. Ageing Res Rev 9: 142–152, 2010. doi: 10.1016/j.arr.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 12.Higashi Y, Sasaki S, Nakagawa K, Kimura M, Noma K, Hara K, Jitsuiki D, Goto C, Oshima T, Chayama K, Yoshizumi M. Tetrahydrobiopterin improves aging-related impairment of endothelium-dependent vasodilation through increase in nitric oxide production. Atherosclerosis 186: 390–395, 2006. doi: 10.1016/j.atherosclerosis.2005.07.025. [DOI] [PubMed] [Google Scholar]
- 13.Jilka RL, Almeida M, Ambrogini E, Han L, Roberson PK, Weinstein RS, Manolagas SC. Decreased oxidative stress and greater bone anabolism in the aged, when compared to the young, murine skeleton with parathyroid hormone administration. Aging Cell 9: 851–867, 2010. doi: 10.1111/j.1474-9726.2010.00616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalinowski L, Dobrucki LW, Malinski T. Nitric oxide as a second messenger in parathyroid hormone-related protein signaling. J Endocrinol 170: 433–440, 2001. doi: 10.1677/joe.0.1700433. [DOI] [PubMed] [Google Scholar]
- 15.Kim SY, Park JT, Park JK, Lee JS, Choi JC. Aging impairs vasodilatory responses in rats. Korean J Anesthesiol 61: 506–510, 2011. doi: 10.4097/kjae.2011.61.6.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Küng CF, Lüscher TF. Different mechanisms of endothelial dysfunction with aging and hypertension in rat aorta. Hypertension 25: 194–200, 1995. doi: 10.1161/01.HYP.25.2.194. [DOI] [PubMed] [Google Scholar]
- 17.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a “set up” for vascular disease. Circulation 107: 139–146, 2003. doi: 10.1161/01.CIR.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- 18.Martens CR, Kuczmarski JM, Kim J, Guers JJ, Harris MB, Lennon-Edwards S, Edwards DG. Voluntary wheel running augments aortic l-arginine transport and endothelial function in rats with chronic kidney disease. Am J Physiol Renal Physiol 307: F418–F426, 2014. doi: 10.1152/ajprenal.00014.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martens CR, Kuczmarski JM, Lennon-Edwards S, Edwards DG. Impaired L-arginine uptake but not arginase contributes to endothelial dysfunction in rats with chronic kidney disease. J Cardiovasc Pharmacol 63: 40–48, 2014. doi: 10.1097/FJC.0000000000000022. [DOI] [PubMed] [Google Scholar]
- 20.Pang PK, Tenner TE Jr, Yee JA, Yang M, Janssen HF. Hypotensive action of parathyroid hormone preparations on rats and dogs. Proc Natl Acad Sci U S A 77: 675–678, 1980. doi: 10.1073/pnas.77.1.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prisby R, Menezes T, Campbell J. Vasodilation to PTH (1-84) in bone arteries is dependent upon the vascular endothelium and is mediated partially via VEGF signaling. Bone 54: 68–75, 2013. doi: 10.1016/j.bone.2013.01.028. [DOI] [PubMed] [Google Scholar]
- 22.Rashid G, Bernheim J, Green J, Benchetrit S. Parathyroid hormone stimulates the endothelial nitric oxide synthase through protein kinase A and C pathways. Nephrol Dial Transplant 22: 2831–2837, 2007. doi: 10.1093/ndt/gfm269. [DOI] [PubMed] [Google Scholar]
- 23.Schulze F, Maas R, Freese R, Schwedhelm E, Silberhorn E, Böger RH. Determination of a reference value for N(G), N(G)-dimethyl-L-arginine in 500 subjects. Eur J Clin Invest 35: 622–626, 2005. doi: 10.1111/j.1365-2362.2005.01561.x. [DOI] [PubMed] [Google Scholar]
- 24.Seals DR, Jablonski KL, Donato AJ. Aging and vascular endothelial function in humans. Clin Sci (Lond) 120: 357–375, 2011. doi: 10.1042/CS20100476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Silverman SL, Nasser K. Teriparitide update. Rheum Dis Clin North Am 37: 471–477, 2011. doi: 10.1016/j.rdc.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 26.Sindler AL, Reyes R, Chen B, Ghosh P, Gurovich AN, Kang LS, Cardounel AJ, Delp MD, Muller-Delp JM. Age and exercise training alter signaling through reactive oxygen species in the endothelium of skeletal muscle arterioles. J Appl Physiol (1985) 114: 681–693, 2013. doi: 10.1152/japplphysiol.00341.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soucy KG, Ryoo S, Benjo A, Lim HK, Gupta G, Sohi JS, Elser J, Aon MA, Nyhan D, Shoukas AA, Berkowitz DE. Impaired shear stress-induced nitric oxide production through decreased NOS phosphorylation contributes to age-related vascular stiffness. J Appl Physiol (1985) 101: 1751–1759, 2006. doi: 10.1152/japplphysiol.00138.2006. [DOI] [PubMed] [Google Scholar]
- 28.Sun D, Huang A, Koller A, Kaley G. Short-term daily exercise activity enhances endothelial NO synthesis in skeletal muscle arterioles of rats. J Appl Physiol (1985) 76: 2241–2247, 1994. [DOI] [PubMed] [Google Scholar]
- 29.Sun D, Huang A, Yan EH, Wu Z, Yan C, Kaminski PM, Oury TD, Wolin MS, Kaley G. Reduced release of nitric oxide to shear stress in mesenteric arteries of aged rats. Am J Physiol Heart Circ Physiol 286: H2249–H2256, 2004. doi: 10.1152/ajpheart.00854.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tschudi MR, Barton M, Bersinger NA, Moreau P, Cosentino F, Noll G, Malinski T, Lüscher TF. Effect of age on kinetics of nitric oxide release in rat aorta and pulmonary artery. J Clin Invest 98: 899–905, 1996. doi: 10.1172/JCI118872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van der Loo B, Labugger R, Skepper JN, Bachschmid M, Kilo J, Powell JM, Palacios-Callender M, Erusalimsky JD, Quaschning T, Malinski T, Gygi D, Ullrich V, Lüscher TF. Enhanced peroxynitrite formation is associated with vascular aging. J Exp Med 192: 1731–1744, 2000. doi: 10.1084/jem.192.12.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vanhoutte PM, Shimokawa H, Tang EH, Feletou M. Endothelial dysfunction and vascular disease. Acta Physiol (Oxf) 196: 193–222, 2009. doi: 10.1111/j.1748-1716.2009.01964.x. [DOI] [PubMed] [Google Scholar]
- 33.Verhaar HJ, Lems WF. PTH-analogs: comparable or different? Arch Gerontol Geriatr 49: e130–e132, 2009. doi: 10.1016/j.archger.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 34.Woodman CR, Price EM, Laughlin MH. Shear stress induces eNOS mRNA expression and improves endothelium-dependent dilation in senescent soleus muscle feed arteries. J Appl Physiol (1985) 98: 940–946, 2005. doi: 10.1152/japplphysiol.00408.2004. [DOI] [PubMed] [Google Scholar]
- 35.Yang MC, Kuo JS, Pang PK. Mechanisms of the vascular action of parathyroid hormone. J Pharmacol Exp Ther 252: 840–844, 1990. [PubMed] [Google Scholar]