Using the single passive leg movement (PLM) technique, a variant of the vascular function assessment PLM, we have identified a novel peripheral vascular assessment method that is more easily performed than PLM, which, by not evoking potentially confounding central hemodynamic responses, may be more useful clinically.
Keywords: flow-mediated dilation, endothelial function, cardiovascular disease assessment
Abstract
Passive leg movement (PLM)-induced hyperemia is a novel approach to assess vascular function, with a potential clinical role. However, in some instances, the varying chronotropic response induced by PLM has been proposed to be a potentially confounding factor. Therefore, we simplified and modified the PLM model to require just a single PLM (sPLM), an approach that may evoke a peripheral hemodynamic response, allowing a vascular function assessment, but at the same time minimizing central responses. To both characterize and assess the utility of sPLM, in 12 healthy subjects, we measured heart rate (HR), stroke volume, cardiac output (CO), mean arterial pressure (MAP), leg blood flow (LBF), and calculated leg vascular conductance (LVC) during both standard PLM, consisting of passive knee flexion and extension performed at 1 Hz for 60 s, and sPLM, consisting of only a single passive knee flexion and extension over 1 s. During PLM, MAP transiently decreased (5 ± 1 mmHg), whereas both HR and CO increased from baseline (6.0 ± 1.1 beats/min, and 0.8 ± 0.01 l/min, respectively). Following sPLM, MAP fell similarly (5 ± 2 mmHg; P = 0.8), but neither HR nor CO responses were identifiable. The peak LBF and LVC response was similar for PLM (993 ± 189 ml/min; 11.9 ± 1.5 ml·min−1·mmHg−1, respectively) and sPLM (878 ± 119 ml/min; 10.9 ± 1.6 ml·min−1·mmHg−1, respectively). Thus sPLM represents a variant of the PLM approach to assess vascular function that is more easily performed and evokes a peripheral stimulus that induces a significant hyperemia, but does not generate a potentially confounding, chronotropic response, which may make sPLM more useful clinically.
NEW & NOTEWORTHY Using the single passive leg movement (PLM) technique, a variant of the vascular function assessment PLM, we have identified a novel peripheral vascular assessment method that is more easily performed than PLM, which, by not evoking potentially confounding central hemodynamic responses, may be more useful clinically.
the recent literature has underlined the clinical importance of endothelial dysfunction in the pathophysiology of patients with cardiovascular disease, Alzheimer’s disease, chronic obstructive pulmonary disease, type 2 diabetes, and even the mortality of subjects in the intensive care unit (5, 9, 11, 12, 22, 23, 36). Therefore, vascular function testing should have a key prognostic role in the clinical and preclinical phases of many disease states; however, not a single assessment of vascular function has been adopted as standard clinical care. Concerning flow-mediated dilation (FMD) evoked by the hyperemic response to ischemic cuff occlusion, first described by Celermajer and colleagues in 1992 (8), although not used clinically, the results of research with this test have been both the most long lived and accepted by the scientific and clinical community. Much of the appeal of FMD can be linked to the indication that this assessment represents nitric oxide (NO)-mediated endothelial function and seems to be a surrogate for coronary artery endothelial function (3). However, recent evidence questioning the role of NO in FMD testing, but perhaps more importantly concerns about the relatively complex methodology and analyses (25, 28, 33, 39), has encouraged the development of new approaches to assess vascular function that can be more easily incorporated into standard clinical care.
Passive leg movement (PLM)-induced hyperemia is a novel, noninvasive, and potentially clinically relevant assessment of vascular function, championed by our group (14, 32) and others (21). The PLM-induced vascular response also appears to be predominantly NO mediated, but, by not relying on the measurement of small changes in vessel diameter, has a methodological advantage over FMD. However, some investigations have suggested that the hyperemic response to PLM is not exclusively a consequence of the peripheral vasculature. Indeed, several studies have underlined the potential role of cardioacceleration (10, 24, 38) and, therefore, cardiac output (CO) in this hyperemic response (18). Further supporting a strong link to central hemodynamics, PLM, in the absence of hyperemia, achieved by occluding the passively moved leg with a cuff inflated to suprasystolic pressure, still induces cardioacceleration and an increase in CO (18). This phenomenon can be explained by afferent feedback from skeletal muscle (type III afferent fibers) that triggers the cardioacceleration (10, 18, 24). Recently, to better understand the contribution of these central hemodynamic responses to PLM, we studied young subjects with pharmacologically reduced neurological feedback from mechanoreceptors in the moving limb (31). This study revealed that the magnitude of peripheral hemodynamic response to PLM does, in fact, appear to be augmented by the central hemodynamic responses, with the partial block of the afferents attenuating the hyperemic response. The heart rate (HR)-driven increase in CO, which likely helps to offset the fall in mean arterial pressure (MAP), is probably the consequence of the PLM-induced vasodilation in the leg and partially directly triggered by mechanoreceptor feedback. Although not negating the use of PLM as a vascular assessment, it would, perhaps, in certain scenarios, be useful to minimize the impact of these potentially complicating central hemodynamic responses. Indeed, in a recent study from our group (37), the impact of quite different central hemodynamic responses to limb movement in patients with heart failure and healthy controls was parsed out by the use of both PLM and a modified PLM approach, single PLM (sPLM). With just a single movement, sPLM appears to generate a significant peripheral hemodynamic response, but at the same time minimizes afferent feedback, eliminating changes in central hemodynamics. However, although already demonstrating some potential in the clinical arena, the sPLM response in young, healthy subjects has yet to be thoroughly characterized.
Consequently, this study aimed to characterize the response to sPLM by evaluating the central and peripheral hemodynamics associated with both a standard continuous PLM and a sPLM assessment in young, healthy subjects. With the expectation of minimal afferent feedback, due the brevity of the sPLM and the smaller peripheral vascular stimulus, we hypothesized that 1) both HR and CO responses would be smaller with sPLM than PLM, and 2) the peripheral vascular response would be attenuated in sPLM compared with PLM, but the data attained from both methods would be qualitatively similar.
METHODS
Subjects.
Twelve young healthy men (28 ± 5 yr, 72 ± 8 kg, 178 ± 9 cm) participated in this study. None of the participants was a smoker, and most were physically active. All procedures conformed to the standards set forth by the Declaration of Helsinki and were approved by the Institutional Review Boards of the University of Utah, and the Salt Lake City VA Medical Center. Subjects gave written, informed consent before their participation. Subjects reported to the laboratory in the morning after an overnight fast and had not exercised for the past 24 h.
PLM protocols.
Subjects rested in the upright-seated position for 20 min before the start of data collection and remained in this position throughout the study. The PLM protocol consisted of 60 s of resting baseline central and peripheral hemodynamic data collection, followed by 60 s of passive knee extension and flexion with the same measures. PLM was performed by a member of the research team, who moved the subject’s lower leg through a 90° range of motion (180–90° knee joint angle) at 1 Hz. The sPLM protocol consisted of 60 s of resting baseline data collection followed by one passive knee flexion and extension, which took 1 s, after which the leg was maintained fully extended for the remaining 59 s of postmovement data collection.
Knee angle.
During each protocol, knee joint angle was continuously recorded using a Vishay Spectrol 360-degree Smart Position Sensor (Vashay Intertechnology, Malvern, PA) mounted on a BREG X2K knee brace (BREG, Vista, CA) worn by each subject.
LBF and LVC.
Measurements of arterial blood velocity and vessel diameter were performed in the common femoral artery of the passively moved leg, distal to the inguinal ligament and proximal to the deep and superficial femoral bifurcation with a Logiq-7 ultrasound Doppler system (General Electric Medical Systems, Milwaukee, WI). The ultrasound Doppler system was equipped with a 12- to 14-MHz linear array transducer. Artery diameter was determined at a 90° angle along the central axis of the scanned area. Blood velocity (Vmean) was measured using the same probe utilizing a frequency of 5 MHz. Measurements of Vmean were obtained with the probe positioned to maintain an insonation angle of 60° or less, and the sample volume was centered and maximized according to vessel size. Utilizing arterial diameter and Vmean, leg blood flow (LBF) was calculated second by second as:
where LBF is in milliliters per minute. Both anterograde and retrograde LBF were also calculated on a second-by-second basis. All scanning and blinded analyses were performed by experienced and skilled sonographers. Leg vascular conductance (LVC) was calculated as LBF/MAP.
Central hemodynamics.
HR, stroke volume (SV), CO, and MAP were determined using a Finometer (Finapres Medical Systems, Amsterdam, The Netherlands). The photoplethysmographic cuff of the finger pressure device was placed on the third finger of the left hand. The subject’s arm was supported by an armrest to avoid arm and finger movement. The Finometer signal was calibrated utilizing the procedure indicated by the manufacturer. The height adjustment sensor and reference were also positioned according to the manufacturer’s instructions. SV was estimated using the Modelflow algorithm (Beatscope version 1.1a; Finapres Medical Systems) (6). CO was then calculated as the product of HR and SV. The same method has been documented to accurately track CO during exercise (4, 30).
Data collection and analysis.
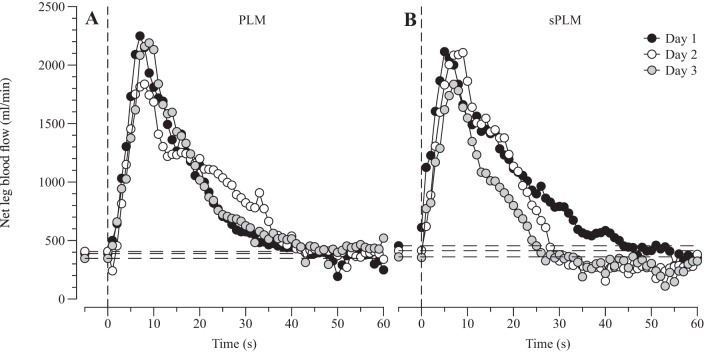
HR, SV, CO, MAP, ECG, and knee joint angle underwent A/D conversion and were simultaneously acquired (200 Hz) by commercially available data acquisition software (AcqKnowledge, Biopac Systems, Goleta, CA). This software allowed second-by-second analysis of HR, SV, CO, and MAP throughout the PLM protocols. Vmean was analyzed with 1-Hz resolution on the Doppler ultrasound system (GE Logiq-7) for 60 s at rest and the first 60 s following the initiation of both PLM and sPLM. Maximal absolute (peak), s (Δpeak), and the area under the curve (AUC) were determined for each subject in all measured variables. In a subset of these subjects (n = 4), the between-day (3 days) coefficient of variation for LBF Δpeak for both sPLM and PLM was determined to be 15%. The blood flow response to PLM and sPLM of a representative subject is illustrated in Fig. 1.
Fig. 1.
Between-day (3 days) reproducibility of the blood flow response to continuous passive leg movement (PLM; A) and single PLM (sPLM; B) in a representative subject. Horizontal dashed lines represent mean baseline values.
Statistical analysis.
Two-way ANOVA was used to establish differences between PLM and sPLM at rest as a result of the passive movement. Following these analyses, where indicated, a Tukey post hoc was used to define differences. Potential relationships between variables were then analyzed using linear regression, and the corresponding strength was assessed using Pearson correlation. The limits of agreement between peak LBF, Δpeak LBF, and blood flow evoked by PLM and sPLM were investigated by plotting the individual differences against their respective means (Bland-Altman Plot). Significance was set at an α-level of 0.05, and data are presented as means ± SE throughout the paper.
RESULTS
Central hemodynamics.
The central responses to both PLM and sPLM are summarized in Table 1. During PLM, all central hemodynamic responses were transient (HR, SV, CO, and MAP), changing shortly after the onset of PLM and returning to baseline within 30 s. In contrast, the only central hemodynamic response to sPLM was a transient fall in MAP (Fig. 2A). Specifically, MAP was decreased from the 10th to 25th s (ΔMAP: −5 ± 1 mmHg) and from the 9th to 22nd s (ΔMAP: −5 ± 2 mmHg) for PLM and sPLM, respectively (Fig. 2A), with no difference between either form of passive movement. HR during PLM was significantly elevated, compared with baseline, within 4 s, increased from 60 ± 3 beats/min to a maximum of 67 ± 3 beats/min, and remained elevated for 22 s (Fig. 2D). As sPLM did not stimulate any degree of cardioacceleration, peak, Δpeak, and AUC for HR were significantly greater during PLM than as a result of sPLM. SV did not change remarkably with either PLM or sPLM (Fig. 2C). CO increased significantly above baseline after 4 s of PLM, reaching a maximum of 7.0 ± 0.9 l/min (ΔCO: +0.77 ± 0.01 l/min), and then in 22 s decreased back to resting values. As there was no distinguishable CO response to sPLM (Fig. 2B), peak, Δpeak, and AUC for CO were all significantly greater during PLM than as a result of sPLM.
Table 1.
Central and peripheral hemodynamics during continuous passive leg movement and single PLM
| Peak |
ΔPeak |
AUC |
||||
|---|---|---|---|---|---|---|
| PLM | sPLM | PLM | sPLM | PLM | sPLM | |
| Leg blood flow, ml/min | 993 ± 189 | 878 ± 119 | 609 ± 115 | 522 ± 98 | 232 ± 82* | 116 ± 65 |
| Cardiac output, l/min | 7.0 ± 0.9* | 6.1 ± 0.7 | 0.77 ± 0.01* | 0.01 ± 0.01 | 0.13 ± 0.03* | 0.01 ± 0.01 |
| Heart rate, beats/min | 67 ± 3* | 61 ± 3 | 6 ± 1* | 1 ± 1 | 2 ± 0* | 0 ± 0 |
| Stroke volume, ml | 109 ± 5 | 105 ± 4 | 5 ± 2 | 4 ± 2 | 1 ± 1 | −1 ± 1 |
| MAP, mmHg | 81 ± 3 | 83 ± 2 | −5 ± 1 | −5 ± 2 | −2 ± 0 | −2 ± 1 |
Values are means ± SE. PLM, passive leg movement; sPLM, single PLM; MAP, mean arterial pressure; AUC, area under the curve.
Significant difference between methods.
Fig. 2.
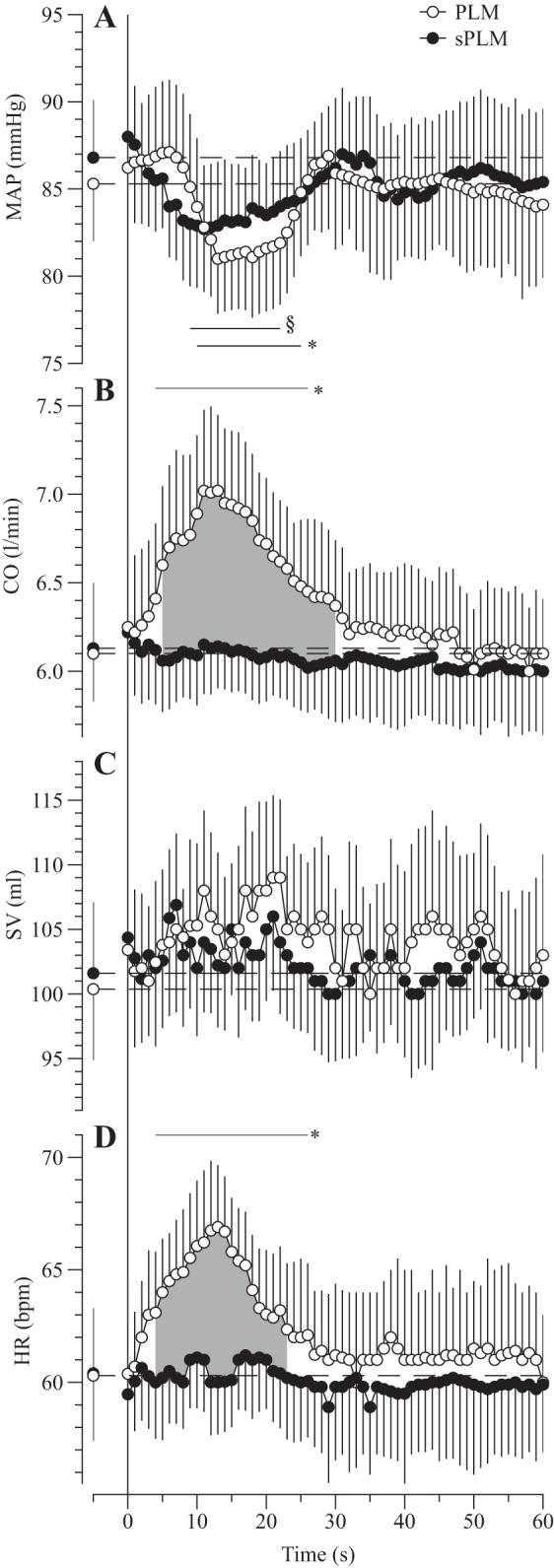
Central hemodynamic responses to continuous passive leg movement (PLM) and single PLM (sPLM) over time: mean arterial pressure (MAP; A), cardiac output (CO; B), stroke volume (SV; C), and heart rate (HR; D). Values are means ± SE. Horizontal dashed lines represent mean baseline values. *Significantly different from PLM baseline. §Significantly different from sPLM baseline. Shaded area indicates a significant difference between PLM and sPLM.
LBF and LVC.
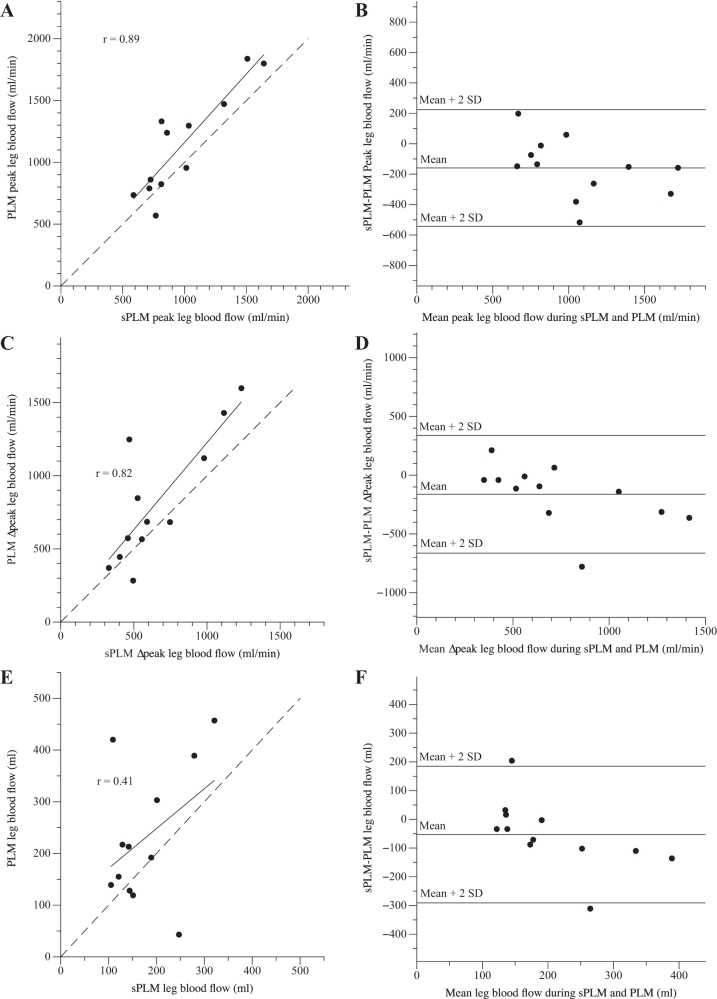
The LBF response to PLM and sPLM is summarized in Table 1 and illustrated in Fig. 3A. Baseline LBF was similar before PLM (391 ± 68 ml/min) and sPLM (376 ± 45 ml/min) and increased at the onset of both PLM and sPLM. Specifically, during PLM, net LBF became significantly elevated, compared with baseline, 5 s after the start of the passive movement, reached a maximum value of 993 ± 189 ml/min (ΔLBF: 609 ± 115 ml/min), and within 35 s had greatly decreased, yet remained elevated above baseline for the duration of the passive movement (Fig. 3A). Similarly, in response to sPLM net LBF became elevated at 3 s after the start of the passive movement, reached a peak value of 878 ± 119 ml/min (ΔLBF: 522 ± 98 ml/min), but, in contrast to PLM, returned to baseline levels within 19 s (Fig. 3A). Thus net LBF was significantly elevated during the last 35 s of PLM compared with sPLM. Furthermore, the AUC for the LBF response was significantly greater for PLM (232 ± 82 ml) than as a result of sPLM (116 ± 65 ml). However, the PLM and sPLM LBF responses were reasonably well correlated, and a strong agreement between the two protocols was apparent for peak and Δpeak LBF (Fig. 4). Despite transient changes in MAP, LVC essentially reflected the LBF responses, as illustrated in Fig. 3B.
Fig. 3.
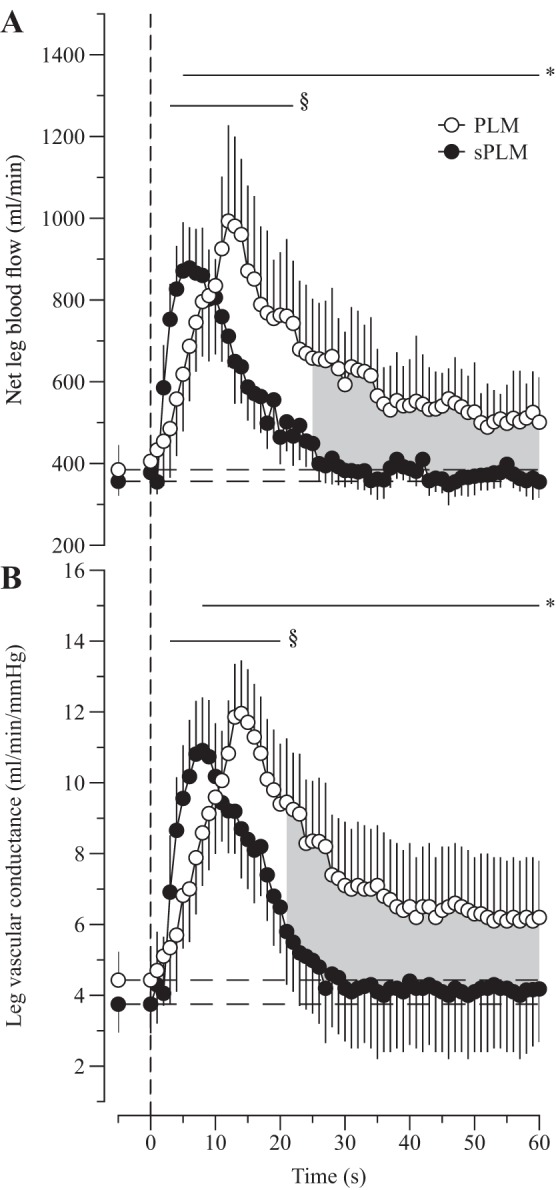
The leg blood flow (A) and leg vascular conductance (B) response to continuous passive leg movement (PLM) and single PLM (sPLM). Values are means ± SE. Horizontal dashed lines represent mean baseline values. *Significantly different from PLM baseline. §Significantly different from sPLM baseline. Shaded area indicates a significant difference between PLM and sPLM.
Fig. 4.
The correlations and agreement between peak leg blood flow (A and B), the change in peak leg blood flow (Δpeak; C and D), and blood flow (E and F) evoked by continuous passive limb movement (PLM) and single PLM (sPLM). The relationships and agreement (Bland-Altman plots) between PLM and sPLM achieved statistical significance for peak and Δpeak leg blood flow, while leg blood flow, area under the curve, did not.
Anterograde and retrograde LBF.
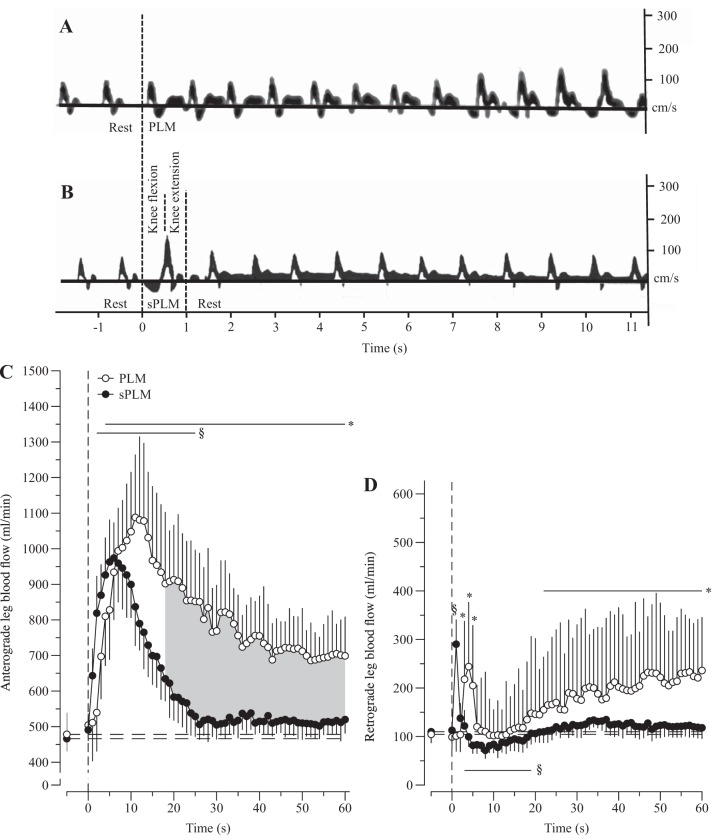
The anterograde and retrograde LBF responses to PLM and sPLM are illustrated in Fig. 5. Interestingly, the change in net LBF during PLM comprised a significant increase in both anterograde and retrograde LBF, whereas, in contrast, sPLM-induced hyperemia was predominantly characterized by an increase in anterograde LBF. Specifically, the blood velocity spectra during PLM revealed a transient increase in the anterograde blood velocity, the signal above the zero velocity line in Fig. 5A, and, with no change in vessel diameter, this translated into an increase in anterograde LBF (Fig. 5C). Additionally, after an initially rapid, but transient, increase of ~5 s, retrograde blood velocity progressively increased the signal below the zero velocity line in Fig. 5A, and, with no change in vessel diameter, this translated into an increase in retrograde LBF (Fig. 5D). Somewhat similarly to PLM, anterograde blood velocity and LBF were significantly increased 2 s after the sPLM and remained elevated for 25 s (Fig. 5, B and C). However, as noted, in contrast to PLM, retrograde blood velocity and LBF increased significantly during the sPLM, but, immediately after the movement was completed, and for the subsequent 20-s retrograde, LBF was below prepassive movement baseline values (Fig. 5, B and D).
Fig. 5.
Exemplar Doppler blood velocity spectra collected in the passively moved leg during continuous passive leg movement (PLM; A) and single PLM (sPLM; B). Anterograde (C) and retrograde (D) blood flow in the passively moved leg during PLM and sPLM are shown. Values are means ± SE. Horizontal dashed lines represent mean baseline values. *Significantly different from PLM baseline. §Significantly different from sPLM baseline. Shaded area indicates a significant difference between PLM and sPLM.
DISCUSSION
With the ever-growing recognition that vascular dysfunction is a precursor to the onset of many common pathologies, and that not a single method to determine vascular function has been adopted clinically, this study sought to thoroughly characterize sPLM, a variant of PLM. It was anticipated that sPLM would minimize central hemodynamic responses, but still facilitate the assessment of peripheral vascular function. Indeed, and in line with our first hypothesis, sPLM, in comparison to PLM, did not evoke a measurable increase in either HR or CO, likely as a consequence of the minimal afferent feedback due to the brevity of the sPLM. In our second hypothesis, we predicted that, due to the reduced central hemodynamic responses and smaller peripheral stimulus, sPLM would yield attenuated peripheral hemodynamic responses. Interestingly, however, the variables that reflect the initial magnitude of the peripheral hemodynamic response, such as peak LBF and Δpeak LBF, were not statistically significantly different between PLM and sPLM, whereas the LBF AUC, which reflects both the duration and magnitude of the response, was attenuated. These data, in combination with the similar drop in MAP with both PLM and sPLM, suggest a somewhat minimal impact of the central hemodynamic response on the initial peripheral hemodynamic response. Furthermore, as anticipated, the PLM and sPLM LBF responses were reasonably well correlated. These findings reveal that sPLM is a variant of the PLM approach to assess vascular function that is more easily performed and does not evoke potentially confounding central hemodynamic responses, which may make sPLM more useful clinically.
FMD, PLM, and sPLM.
Vascular dysfunction is a key prognostic factor in many clinical and preclinical pathophysiological processes (5, 9, 11, 12, 22, 36). Therefore, the identification of progressive vascular dysfunction in the population as a whole could have significant health care consequences by alerting health care providers to the need to instigate interventions and guide rehabilitation. However, there is currently not a single vascular function assessment that has mustered widespread clinical use. The method that has come the closest to attaining this accolade is FMD, but the somewhat complex nature of the data acquisition and, perhaps, recent reports questioning the link between FMD and NO-related endothelial function have kept this method on the fringe of clinical medicine (25, 28, 33, 39). Therefore, lately (7, 13, 20, 32), there has been renewed interest in developing novel and potentially clinically relevant approaches to assess vascular function.
PLM-induced hyperemia is a noninvasive approach to determine NO-dependent vascular function with potential clinical relevance that has been recognized by our group (14, 32) and others (21). Compared with FMD, PLM is a significant methodological improvement, requiring only the relatively easy assessment of the passive movement-induced change in LBF and not depending on small changes in conduit vessel diameter as the major outcome variable. Therefore, PLM is a microvascular assessment and not a measure of conduit function, which may also be more clinically relevant. However, as with all burgeoning techniques, PLM needs to be refined and questions answered. Specifically, and from a practical standpoint, the need to perform PLM for, originally, 3 min, although this study has only focused on the first 60 s, which seems reasonable, and concerns regarding the potential role of central hemodynamics superimposed to differing degrees in various populations have been aired (10, 24, 34, 35, 38). Indeed, the findings of this investigation build on our laboratory's previous work, in a clinical population (37), now revealing that in young healthy subjects sPLM, a variant of PLM, addresses both of these methodological concerns by simplifying the peripheral vascular assessment, down to a single movement, and avoiding the potentially confounding, or at least distracting, central hemodynamic responses. However, the utility of this new method for the vascular function assessment is not only based on the simplicity of the protocol. Indeed, it is also important to note that the lack of discomfort, associated with the 5-min cuff occlusion employed during FMD testing, will also, likely, facilitate the use of sPLM on a greater range of clinical and preclinical populations. Furthermore, in the relatively common case of a proximal split in the brachial artery, where FMD cannot be validly performed, sPLM would be unaffected by this issue. Therefore, sPLM may be a clinically useful approach with which to routinely assess vascular function in health and disease.
The role of central hemodynamics in movement-induced hyperemia.
The comprehensive analysis of central and peripheral hemodynamics, during PLM and sPLM, afforded the opportunity to evaluate which factors contribute to leg movement-induced hyperemia. In addition to the robust increase in both LBF and LVC (Fig. 3), PLM also evoked a rapid rise in both HR and CO, likely a result of afferent feedback from joint and muscle mechanoreceptors (Fig. 2) (1, 2, 15, 31). However, concomitant with these responses to PLM, as is commonly observed in young healthy subjects (18), despite the increase in CO, there was a small, but significant, decrease in MAP of ~5 mmHg (Fig. 2). This fall in MAP was likely the consequence of the acute vasodilation in the passively moved leg, but with PLM it cannot be discerned if the baroreflex response to this pressure change also contributed to the proposed afferent driven increase in HR and CO (Fig. 2). Nevertheless, although it is pressure and resistance across the leg that determine LBF (Ohm’s law), this increase in CO could be an essential component of the PLM response as CO is, systemically, linked to MAP. Specifically, it appears that, at the onset of PLM, LVC increases, driven by mechanically induced vasodilation (29) and shear stress-induced FMD (16, 26), to such an extent that, not only does MAP fall, but, in the face of this fall, the greatly increased LVC facilitates an increase in LBF. The PLM-induced increase in CO likely plays an important role in limiting this fall in MAP, which could soon outstrip the rise in LVC and not elicit an increase in LBF. However, by only examining the PLM-induced responses, which include an increase in HR and CO, this is difficult to discern.
By utilizing sPLM, employed to reduce the amount of afferent feedback from joint and muscle mechanoreceptors (1/60 compared with PLM), the central hemodynamic responses, HR and CO, were, as anticipated, ablated (Fig. 2). Interestingly, however, despite the absence of cardioacceleration and therefore no increase in CO, peak hyperemia was similar to the peak PLM LBF response. Additionally, it should be noted that, although of undetermined origin, this increase in LBF with PLM, without a central hemodynamic response, suggests a simultaneous vasoconstriction somewhere in the circulatory system.
Furthermore, the sPLM-induced fall in MAP (~5 mmHg) was of a similar magnitude to the PLM response (Fig. 2). These findings afford the unique opportunity to better understand the role of central hemodynamics in movement-induced hyperemia. Specifically, the lack of a HR and CO response with sPLM, but a similar fall in MAP compared with PLM, implies that the main drive to increase central hemodynamic responses is, indeed, afferent feedback from joint and muscle mechanoreceptors, and not the baroreflex, which, if a key player, would have been evoked by the similar drop in MAP in both sPLM and PLM. It is also clear from this comparison of the sPLM and PLM responses that, with these passive movement interventions, there is likely a minimal role for CO in protecting MAP and, therefore, facilitating the movement-induced increase in LBF. This is based on the observation that, despite an unchanged CO with sPLM, both the movement-induced increase in LBF and the subsequent fall in MAP were not different from PLM in which there was a significant central hemodynamic response (Figs. 1 and 2). It is also important to note that these impressively similar initial peak LBF and LVC responses to sPLM and PLM, occurring in the first 10–15 s (Fig. 3), were the consequence of just one knee flexion and extension in the case of sPLM, which occurred over just 1 s. Indeed, the PLM and sPLM peripheral hemodynamic responses were reasonably well correlated, with the relationship between the three indexes of LBF illustrated in Fig. 4. It is important to note, as documented by the Bland-Altman plots, that the congruence of peak LBF evoked during PLM and sPLM was high (Fig. 4B), while the agreement between LBF Δpeak and AUC was less strong (Fig. 4, D and F). Nevertheless, over time there were some clear differences between the sPLM and PLM responses. For instance, the duration of the sPLM-induced hyperemia was shorter, returning to baseline in ~20 s, whereas in PLM, LBF and LVC were still elevated at 60 s (Fig. 3). However, unfortunately, in this particular case, the role of central hemodynamics and the sustained passive movement cannot be parsed out so simply, although the timing of responses and the information gleaned from the mechanical influences of the passive movement (below) again support a negligible role for both HR and CO.
Taken together, these findings suggest that the central hemodynamic responses to PLM (HR and CO) are evoked by afferent feedback and not the baroreflex. However, at least in terms of the initial response to the passive movement, HR and CO appear to play a minimal role in the passive movement-induced hyperemia, as evidenced by a similar fall in MAP and increase in LBF without these responses. Regardless, in addition to the relative simplicity of performance, sPLM minimizes the potentially complicating, or at least distracting, central hemodynamic responses, while still producing a related and robust peripheral hemodynamic response that can be used to assess vascular function.
Mechanical determinants of movement-induced hyperemia.
During PLM, net LBF was significantly elevated within 4 s of the passive movement beginning and remained elevated for at least 60 s. This net PLM LBF response comprised an increase in both anterograde and retrograde blood flow, with the magnitude of change in anterograde flow being greater than the magnitude of change in retrograde flow (Fig. 5). Interestingly, after the initial rapid peak response, the anterograde blood flow fell, but remained elevated, while the retrograde blood flow rose to become different from baseline and remained elevated. These responses are likely attributable to the continuous repeated muscle length-dependent changes in vessel tortuosity that produced intermittent periods of oscillating vascular resistance. Indeed, in a previous investigation from our group (19), it was revealed that femoral artery blood flow is clearly influenced by knee joint angle. Specifically, femoral artery blood flow was documented to increase as the knee was extended from the lower (90°) to the middle and upper (180°) range of knee joint angle. It was concluded that the factors likely resulting in this response were muscle length-dependent changes in capillary tortuosity and vessel diameter (17, 27). Although in the present study both the central hemodynamic responses (HR and CO) were evoked beyond the initial response to PLM, the timing of the HR and CO responses (Fig. 2) and the information gleaned from the anterograde and retrograde LBF responses over time (Fig. 5) suggest a negligible role for both HR and CO and a more likely important contribution from the repeated passive movements in terms of the sustained PLM-induced blood flow. The effect of the continued movement is likely both mechanical and physiological in that the repetitive movements probably continue to stimulate NO release, but perhaps in a more complex fashion than with just a single movement. However, it should be recognized that a limitation of this study was the lack of an attempt to block NO synthase during sPLM, which warrants further investigation to better elucidate the role of NO in these two paradigms.
As a consequence of sPLM, net LBF was also significantly elevated within 3 s of commencing the single and only passive movement, but, in contrast to the PLM, LBF returned to baseline levels in ~20 s. Interestingly, this increase in net LBF was only composed of an increase in anterograde blood flow (Fig. 5), with retrograde blood flow only being significantly increased during the single knee flexion of the sPLM, following which retrograde blood flow was clearly reduced for 20 s (Fig. 5, B and D). These data are highly supportive of the contention that LBF during passive movement is influenced by knee joint angle subsequent to changes in muscle length-dependent vessel tortuosity, vessel diameter, and, consequently, resistance. Thus the rapid and very short-lived increase in retrograde blood flow associated with the knee flexion in sPLM (Fig. 5, B and D) was likely induced by an acute, joint angle-related increase in vascular resistance. During the subsequent knee extension, and then the maintenance of muscle length for the following 59 s, vascular resistance was clearly decreased, leading to a transient period of diminished retrograde blood flow.
Taken together, these findings suggest that there are, indeed, significant mechanical determinants of passive movement-induced hyperemia. Specifically, knee-joint angle indirectly alters muscle and vessel geometry, both of which can facilitate or impede LBF. However, whether the leg was exposed to either PLM or sPLM, the initial peripheral hemodynamic responses were very similar. In terms of the sustained increase in LBF with PLM, although a role for the central hemodynamic responses cannot be ruled out, there is likely an important contribution from the repeated passive movements, both mechanically and physiologically, due to the continued vasodilatory stimulus.
In conclusion, this study sought to characterize sPLM, a variant of the vascular function assessment PLM, which, due to simplicity, may be more clinically useful than conventional PLM. Somewhat unexpectedly, despite a lack of central hemodynamic responses and a smaller peripheral stimulus, the initial magnitude of the peripheral hemodynamic response, as assessed by peak LBF and Δpeak LBF, was not significantly different between sPLM and PLM. These data, in combination with the similar drop in MAP with both PLM and sPLM, imply a somewhat minimal impact of the central hemodynamic response on the peripheral hemodynamic response to passive movement. These findings document that the more easily performed sPLM does not evoke a potentially confounding central hemodynamic response, but does afford a similar assessment of peripheral vascular function, potentially making sPLM more useful clinically.
GRANTS
This work was funded, in part, by the National Heart, Lung, and Blood Institute at the National Institutes of Health (PO1 HL1091830 and K99HL125756), the Veteran’s Administration Rehabilitation Research and Development Service (E6910-R, E1697-R, E1433-P, and E9275-L), and by grants from the Flight Attendant Medical Research Institute (FAMRI).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
M.V., G.L., J.D.T., C.R.H., and R.M.B. performed experiments; M.V., G.L., J.D.T., and C.R.H. interpreted results of experiments; M.V. prepared figures; M.V., G.L., J.D.T., and C.R.H. drafted manuscript; M.V., J.D.T., C.R.H., and R.S.R. edited and revised manuscript; M.V., G.L., J.D.T., C.R.H., and R.S.R. approved final version of manuscript; G.L., C.R.H., and R.M.B. analyzed data.
REFERENCES
- 1.Adreani CM, Hill JM, Kaufman MP. Responses of group III and IV muscle afferents to dynamic exercise. J Appl Physiol (1985) 82: 1811–1817, 1997. [DOI] [PubMed] [Google Scholar]
- 2.Adreani CM, Kaufman MP. Effect of arterial occlusion on responses of group III and IV afferents to dynamic exercise. J Appl Physiol (1985) 84: 1827–1833, 1998. [DOI] [PubMed] [Google Scholar]
- 3.Anderson TJ, Uehata A, Gerhard MD, Meredith IT, Knab S, Delagrange D, Lieberman EH, Ganz P, Creager MA, Yeung AC, Selwyn AP. Close relation of endothelial function in the human coronary and peripheral circulations. J Am Coll Cardiol 26: 1235–1241, 1995. doi: 10.1016/0735-1097(95)00327-4. [DOI] [PubMed] [Google Scholar]
- 4.Azabji Kenfack M, Lador F, Licker M, Moia C, Tam E, Capelli C, Morel D, Ferretti G. Cardiac output by Modelflow method from intra-arterial and fingertip pulse pressure profiles. Clin Sci (Lond) 106: 365–369, 2004. doi: 10.1042/CS20030303. [DOI] [PubMed] [Google Scholar]
- 5.Becker L, Prado K, Foppa M, Martinelli N, Aguiar C, Furian T, Clausell N, Rohde LE. Endothelial dysfunction assessed by brachial artery ultrasound in severe sepsis and septic shock. J Crit Care 27: 316.e319–e314, 2012. doi: 10.1016/j.jcrc.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 6.Bogert LW, van Lieshout JJ. Non-invasive pulsatile arterial pressure and stroke volume changes from the human finger. Exp Physiol 90: 437–446, 2005. doi: 10.1113/expphysiol.2005.030262. [DOI] [PubMed] [Google Scholar]
- 7.Cameron JD, Asmar R, Struijker-Boudier H, Shirai K, Sirenko Y, Kotovskaya Y, Topouchian J. Current and future initiatives for vascular health management in clinical practice. Vasc Health Risk Manag 9: 255–264, 2013. doi: 10.2147/VHRM.S42947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Celermajer DS, Sorensen KE, Gooch VM, Spiegelhalter DJ, Miller OI, Sullivan ID, Lloyd JK, Deanfield JE. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet 340: 1111–1115, 1992. doi: 10.1016/0140-6736(92)93147-F. [DOI] [PubMed] [Google Scholar]
- 9.Clarenbach CF, Senn O, Sievi NA, Camen G, van Gestel AJ, Rossi VA, Puhan MA, Thurnheer R, Russi EW, Kohler M. Determinants of endothelial function in patients with COPD. Eur Respir J 42: 1194–1204, 2013. doi: 10.1183/09031936.00144612. [DOI] [PubMed] [Google Scholar]
- 10.Crow MT, Kushmerick MJ. Chemical energetics of slow- and fast-twitch muscles of the mouse. J Gen Physiol 79: 147–166, 1982. doi: 10.1085/jgp.79.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deanfield JE, Halcox JP, Rabelink TJ. Endothelial function and dysfunction: testing and clinical relevance. Circulation 115: 1285–1295, 2007. doi: 10.1161/CIRCULATIONAHA.106.652859. [DOI] [PubMed] [Google Scholar]
- 12.Dede DS, Yavuz B, Yavuz BB, Cankurtaran M, Halil M, Ulger Z, Cankurtaran ES, Aytemir K, Kabakci G, Ariogul S. Assessment of endothelial function in Alzheimer’s disease: is Alzheimer’s disease a vascular disease? J Am Geriatr Soc 55: 1613–1617, 2007. doi: 10.1111/j.1532-5415.2007.01378.x. [DOI] [PubMed] [Google Scholar]
- 13.Gori T, Dragoni S, Lisi M, Di Stolfo G, Sonnati S, Fineschi M, Parker JD. Conduit artery constriction mediated by low flow a novel noninvasive method for the assessment of vascular function. J Am Coll Cardiol 51: 1953–1958, 2008. doi: 10.1016/j.jacc.2008.01.049. [DOI] [PubMed] [Google Scholar]
- 14.Groot HJ, Trinity JD, Layec G, Rossman MJ, Ives SJ, Morgan DE, Bledsoe A, Richardson RS. The role of nitric oxide in passive leg movement-induced vasodilatation with age: insight from alterations in femoral perfusion pressure. J Physiol 593: 3917–3928, 2015. doi: 10.1113/JP270195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herr MD, Imadojemu V, Kunselman AR, Sinoway LI. Characteristics of the muscle mechanoreflex during quadriceps contractions in humans. J Appl Physiol (1985) 86: 767–772, 1999. [DOI] [PubMed] [Google Scholar]
- 16.Koller A, Kaley G. Endothelium regulates skeletal muscle microcirculation by a blood flow velocity-sensing mechanism. Am J Physiol Heart Circ Physiol 258: H916–H920, 1990. [DOI] [PubMed] [Google Scholar]
- 17.Mathieu-Costello O. Muscle capillary tortuosity in high altitude mice depends on sarcomere length. Respir Physiol 76: 289–302, 1989. doi: 10.1016/0034-5687(89)90070-4. [DOI] [PubMed] [Google Scholar]
- 18.McDaniel J, Fjeldstad AS, Ives S, Hayman M, Kithas P, Richardson RS. Central and peripheral contributors to skeletal muscle hyperemia: response to passive limb movement. J Appl Physiol (1985) 108: 76–84, 2010. doi: 10.1152/japplphysiol.00895.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McDaniel J, Ives SJ, Richardson RS. Human muscle length-dependent changes in blood flow. J Appl Physiol (1985) 112: 560–565, 2012. doi: 10.1152/japplphysiol.01223.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mohty D, Pibarot P, Echahidi N, Poirier P, Dagenais GR, Dumesnil JG. Reduced systemic arterial compliance measured by routine Doppler echocardiography: a new and independent predictor of mortality in patients with type 2 diabetes mellitus. Atherosclerosis 225: 353–358, 2012. doi: 10.1016/j.atherosclerosis.2012.09.025. [DOI] [PubMed] [Google Scholar]
- 21.Mortensen SP, Askew CD, Walker M, Nyberg M, Hellsten Y. The hyperaemic response to passive leg movement is dependent on nitric oxide: a new tool to evaluate endothelial nitric oxide function. J Physiol 590: 4391–4400, 2012. doi: 10.1113/jphysiol.2012.235952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Naka KK, Papathanassiou K, Bechlioulis A, Kazakos N, Pappas K, Tigas S, Makriyiannis D, Tsatsoulis A, Michalis LK. Determinants of vascular function in patients with type 2 diabetes. Cardiovasc Diabetol 11: 127, 2012. doi: 10.1186/1475-2840-11-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nelson AD, Rossman MJ, Witman MA, Barrett-O’Keefe Z, Groot HJ, Garten RS, Richardson RS. Nitric oxide-mediated vascular function in sepsis using passive leg movement as a novel assessment: a cross-sectional study. J Appl Physiol (1985) 120: 991–999, 2016. doi: 10.1152/japplphysiol.00961.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nóbrega AC, Araújo CG. Heart rate transient at the onset of active and passive dynamic exercise. Med Sci Sports Exerc 25: 37–41, 1993. doi: 10.1249/00005768-199301000-00007. [DOI] [PubMed] [Google Scholar]
- 25.Parker BA, Tschakovsky ME, Augeri AL, Polk DM, Thompson PD, Kiernan FJ. Heterogenous vasodilator pathways underlie flow-mediated dilation in men and women. Am J Physiol Heart Circ Physiol 301: H1118–H1126, 2011. doi: 10.1152/ajpheart.00400.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pohl U, Holtz J, Busse R, Bassenge E. Crucial role of endothelium in the vasodilator response to increased flow in vivo. Hypertension 8: 37–44, 1986. doi: 10.1161/01.HYP.8.1.37. [DOI] [PubMed] [Google Scholar]
- 27.Poole DC, Musch TI, Kindig CA. In vivo microvascular structural and functional consequences of muscle length changes. Am J Physiol Heart Circ Physiol 272: H2107–H2114, 1997. [DOI] [PubMed] [Google Scholar]
- 28.Pyke KE, Tschakovsky ME. The relationship between shear stress and flow-mediated dilatation: implications for the assessment of endothelial function. J Physiol 568: 357–369, 2005. doi: 10.1113/jphysiol.2005.089755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Segal SS. Integration of blood flow control to skeletal muscle: key role of feed arteries. Acta Physiol Scand 168: 511–518, 2000. doi: 10.1046/j.1365-201x.2000.00703.x. [DOI] [PubMed] [Google Scholar]
- 30.Tam E, Azabji Kenfack M, Cautero M, Lador F, Antonutto G, di Prampero PE, Ferretti G, Capelli C. Correction of cardiac output obtained by Modelflow from finger pulse pressure profiles with a respiratory method in humans. Clin Sci (Lond) 106: 371–376, 2004. doi: 10.1042/CS20030302. [DOI] [PubMed] [Google Scholar]
- 31.Trinity JD, Amann M, McDaniel J, Fjeldstad AS, Barrett-O’Keefe Z, Runnels S, Morgan DE, Wray DW, Richardson RS. Limb movement-induced hyperemia has a central hemodynamic component: evidence from a neural blockade study. Am J Physiol Heart Circ Physiol 299: H1693–H1700, 2010. doi: 10.1152/ajpheart.00482.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trinity JD, Groot HJ, Layec G, Rossman MJ, Ives SJ, Runnels S, Gmelch B, Bledsoe A, Richardson RS. Nitric oxide and passive limb movement: a new approach to assess vascular function. J Physiol 590: 1413–1425, 2012. doi: 10.1113/jphysiol.2011.224741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tschakovsky ME, Pyke KE. Counterpoint: Flow-mediated dilation does not reflect nitric oxide-mediated endothelial function. J Appl Physiol (1985) 99: 1235–1237, 2005. doi: 10.1152/japplphysiol.00607.2005. [DOI] [PubMed] [Google Scholar]
- 34.Venturelli M, Amann M, Layec G, McDaniel J, Trinity JD, Fjeldstad AS, Ives SJ, Yonnet G, Richardson RS. Passive leg movement-induced hyperaemia with a spinal cord lesion: evidence of preserved vascular function. Acta Physiol (Oxf) 210: 429–439, 2014. doi: 10.1111/apha.12173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Venturelli M, Amann M, McDaniel J, Trinity JD, Fjeldstad AS, Richardson RS. Central and peripheral hemodynamic responses to passive limb movement: the role of arousal. Am J Physiol Heart Circ Physiol 302: H333–H339, 2012. doi: 10.1152/ajpheart.00851.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vita JA, Keaney JF Jr. Endothelial function: a barometer for cardiovascular risk? Circulation 106: 640–642, 2002. doi: 10.1161/01.CIR.0000028581.07992.56. [DOI] [PubMed] [Google Scholar]
- 37.Witman MA, Ives SJ, Trinity JD, Groot HJ, Stehlik J, Richardson RS. Heart failure and movement-induced hemodynamics: partitioning the impact of central and peripheral dysfunction. Int J Cardiol 178: 232–238, 2015. doi: 10.1016/j.ijcard.2014.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wray DW, Donato AJ, Uberoi A, Merlone JP, Richardson RS. Onset exercise hyperaemia in humans: partitioning the contributors. J Physiol 565: 1053–1060, 2005. doi: 10.1113/jphysiol.2005.084327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wray DW, Witman MA, Ives SJ, McDaniel J, Trinity JD, Conklin JD, Supiano MA, Richardson RS. Does brachial artery flow-mediated vasodilation provide a bioassay for NO? Hypertension 62: 345–351, 2013. doi: 10.1161/HYPERTENSIONAHA.113.01578. [DOI] [PMC free article] [PubMed] [Google Scholar]