Nasal high-flow (NHF) therapy can support ventilation in patients with chronic obstructive pulmonary disease during sleep by decreasing the work of breathing and improving CO2 levels. On the other hand, oxygen supplementation corrects hypoxemia, but it produces only a minimal reduction in work of breathing and is associated with increased CO2 levels. Therefore, NHF can be a useful method to assist ventilation in patients with increased respiratory mechanical loads.
Keywords: COPD, work of breathing, nasal high flow, oxygen, sleep
Abstract
Patients with chronic obstructive pulmonary disease (COPD) endure excessive resistive and elastic loads leading to chronic respiratory failure. Oxygen supplementation corrects hypoxemia but is not expected to reduce mechanical loads. Nasal high-flow (NHF) therapy supports breathing by reducing dead space, but it is unclear how it affects mechanical loads of patients with COPD. The objective of this study was to compare the effects of low-flow oxygen and NHF therapy on ventilation and work of breathing (WOB) in patients with COPD and controls during sleep. Patients with COPD (n = 12) and controls (n = 6) were recruited and submitted to polysomnography to measure sleep parameters and ventilation in response to administration of oxygen and NHF. A subset of six patients also had an esophageal catheter inserted for the purpose of measuring WOB. Patients with COPD had similar minute ventilation (V̇e) but lower tidal volumes than matched controls. With oxygen, was increased and V̇e was reduced in both controls and patients with COPD, but there was an increase in transcutaneous CO2 levels. NHF produced a greater reduction in V̇e and was associated with a reduction in CO2 levels. Although NHF halved WOB, oxygen produced only a minor reduction in this parameter. We conclude that oxygen produced little change in WOB, which was associated with CO2 elevations. On the other hand, NHF produced a large reduction in V̇e and WOB with a concomitant decrease in CO2 levels. Our data indicate that NHF improves alveolar ventilation during sleep compared with oxygen and room air in patients with COPD and therefore can decrease their cost of breathing.
NEW & NOTEWORTHY Nasal high-flow (NHF) therapy can support ventilation in patients with chronic obstructive pulmonary disease during sleep by decreasing the work of breathing and improving CO2 levels. On the other hand, oxygen supplementation corrects hypoxemia, but it produces only a minimal reduction in work of breathing and is associated with increased CO2 levels. Therefore, NHF can be a useful method to assist ventilation in patients with increased respiratory mechanical loads.
in patients with acute hypoxic respiratory failure, an open nasal cannula system for delivering warm and humidified air or oxygen at high flow rates ranging between 15 and 50 l/min has been shown to improve patients' oxygenation and comfort (11) and to decrease mortality (5). In patients with chronic obstructive pulmonary disease (COPD), nasal high-flow (NHF) therapy was also shown to reduce exacerbations, increase exercise capacity, and improve lung function, arterial blood gases, and quality of life (16, 21). Therapeutic advantages for patients with COPD using NHF therapy are derived from reduction of anatomic dead space from the nasal and pharyngeal airways, improvement in mucociliary clearance in large bronchial and conducting airways, as well as passive elevation in end-expiratory pressure (6, 7, 13, 14). Although NHF can provide ventilatory support in both acute and chronic respiratory failure, it remains unclear whether it reduces mechanical respiratory loads in patients with COPD.
Airway collapse and increased respiratory resistance in COPD lead to dynamic hyperinflation that may generate elastic loads. Both elastic and resistive loads on the respiratory system (respiratory loads) increase the effort required for ventilation. An excessive work of breathing (WOB), particularly during periods of high ventilatory demand, places respiratory muscles under distress. Associated with systemic inflammation and oxidative stress, respiratory muscle loading can lead to muscle fiber atrophy of the diaphragm and accessory respiratory muscles. As a consequence, patients develop reduced exercise capacity and respiratory failure with both respiratory and general muscle weakening (10, 18, 22).
Long-term oxygen therapy is standard of care for chronic hypoxemic respiratory failure (12) and can improve hypoxemia, but it has not been shown to affect the WOB. On the other hand, NHF therapy has been demonstrated to reduce inspiratory loads in adults (15) and WOB in infants with bronchiolitis (20). Recently, it has been shown that NHF therapy can be used in exercise trials to improve exercise capacity and muscle fiber function (3). In the present study we show that NHF significantly reduces WOB during sleep. It is possible that extended use of NHF during sleep may slow COPD disease progression and can be used to preserve exercise capacity and increase daily activity in these patients.
We have recently shown that breathing pattern responses to NHF depend on the wake/sleep state in normal healthy adults (15) and vary widely during wakefulness in patients with COPD (16). We hypothesize that NHF would both reduce ventilation and decrease WOB during sleep compared with low-flow oxygen. Thus, in the present study, we examined the effects of NHF and oxygen on ventilation and WOB during sleep in patients with COPD, and in a control group individuals who smoke tobacco.
METHODS
Study Population
Subjects were recruited from the Johns Hopkins Pulmonary Clinic and the surrounding community (Johns Hopkins University, Baltimore, MD). Participants with COPD and FEV1 predicted above 30% were included. We also included a control group of otherwise healthy smoking subjects. Participants were excluded from COPD and control groups if they had an acute respiratory illness in the 8 wk preceding the evaluation; severe chronic cardiac or liver disease; home use of oxygen; chronic use of opioids, benzodiazepines, or other sedatives; or allergy to lidocaine. The study was approved by the Johns Hopkins Medical Institution Human Investigations Review Board, and signed informed consent forms were obtained from all participants.
Study Procedures
Baseline polysomnography.
Signals were recorded on a polysomnography (PSG) recording station (Remlogic Natus, Pleasanton, CA). The recorded signals for the baseline PSG (baseline) included EEGs (Fz-A2, C3-A2, A2-O1), left and right electrooculograms, submental electromyogram (EMG), tibial EMG, ECG, oxyhemoglobin saturation, and body position via infrared video camera. Breathing was assessed by recording nasal pressure from a standard PSG cannula. Thoracic and abdominal belts were used to measure respiratory effort.
Ventilation and WOB during PSG (intervention).
An overnight PSG was performed as described above with the addition of instrumentation required to quantify ventilation and measure esophageal pressure. A lightweight, low-dead space pneumotachograph (9) was attached to a nasal continuous positive airway pressure mask (volume, 50 ml; Respironics, Murraysville, PA) or a full face mask (volume, 150 ml; Hans Rudolf, Shawnee, KS) to continuously record airflow. In the entire group, nine individuals (four controls and five with COPD) wore the full face mask. The remaining nine participants (two controls and seven with COPD) wore a nasal mask. Esophageal pressure was measured using an air-filled catheter (CooperSurgical, Trumbull, CT) positioned 10 cm superior to the gastroesophageal sphincter in a subset of six participants (two controls and four with COPD). In this subgroup, two participants (one control and one with COPD) wore a full face mask and the remaining four participants (one control and three with COPD) wore a nasal mask. Arterial CO2 levels were monitored using a transcutaneous CO2 probe (TCM4; Radiometer, Copenhagen, Denmark). Patients also wore a therapy nasal cannula (TNI Medical, Wuerzburg, Germany) through which no flow (room air condition), low-flow oxygen (oxygen condition), or NHF (NHF condition) was delivered. During the room air condition, no extra flow was delivered through the nasal cannula, but participants could breathe normally through the opening of the mask. On a separate night, a subset (n = 6) of the patients slept an additional night with a high-flow nasal cannula without wearing a mask or having an esophageal catheter. On that night, ventilation was monitored by inductive PSG as previously described (15). Three of those six individuals had worn full face mask, three work a nasal mask, and four had an esophageal catheter during the previous intervention night.
Nasal high flow.
The NHF system comprises an air compressor (TNI Medical) that delivered air at a constant flow rate of up to 20 l/minute at the tip of the nasal cannula. A heater, humidifier, and heated wire running inside the nasal cannula maintained the air temperature between 30 and 33°C, and the relative humidity at ~80% at the nasal outlet.
Low-flow oxygen.
An oxygen compressor was used to deliver 2 l/min oxygen through the same nasal cannula that was used for the NHF.
Study Protocol
Each participant was scheduled for a minimum of two visits. At the first visit demographic information (age, sex, height, and weight) for each participant was collected followed by a medical history and physical examination, pulmonary lung function testing, and baseline PSG study. At the second visit (intervention) subjects were instrumented with PSG, ventilatory measurement equipment, and a therapy nasal cannula. All subjects initiated sleep with NHF at 20 l/min because the warmed, humidified air was most comfortable. Following a stable period (~10 min) of non–rapid eye movement (NREM) sleep in the supine position, therapy was randomly alternated through the nasal cannula for 10-min periods for each of the conditions: 1) room air (no airflow through the therapy cannula), 2) oxygen (delivered at 2 l/min), and 3) NHF (delivered at 20 l/min) (see Fig. 1). Each condition was repeated two times.
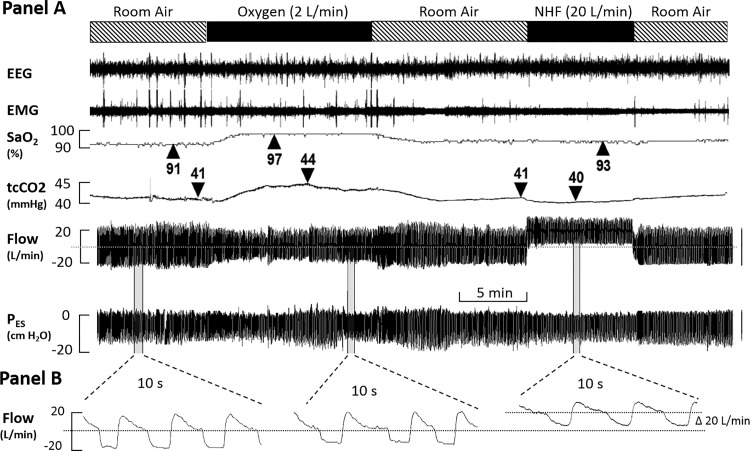
Fig. 1.
Study protocol during the intervention night (see text). Participants underwent polysomnography with EEG, EMG, , and CO2 monitoring. Additionally, individuals wore a mask attached to a pneumotacograph, allowing ventilation monitoring. Six patients also had an esophageal catheter inserted to measure work of breathing (WOB). During stable non–rapid eye movement (NREM) sleep, delivery through a nasal cannula was alternated among room air (no additional flow), oxygen (2 l/min), and nasal high flow (NHF) at 20 l/min. tcCO2, transcutaneous CO2, PES, esophageal pressure.
Analysis
To detect mask-leak free periods, we measured airflow without using any high-pass filter. This allowed us to use the mean airflow or specific points to determine the degree of bias airflow. These points were either end-expiration and swallowing events during which dynamic breathing airflow signal suddenly stopped and returned to baseline. For instance, during room air, the mean airflow was zero (0 l/min), with oxygen it was 2 l/min, and with NHF it was 20 l/min. Any deviation of the mean airflow, the end-expiratory points, or baseline during swallowing events (if present) of greater than 0.5 l/min for the oxygen condition and 2 l/min for the NHF condition were considered as periods with significant mask leaks and were not selected for our analysis. In Fig. 1 we illustrate our approach: when NHF was turned on, there was a significant shift in the bias (baseline) airflow signal of 20 l/min compared with the zero line at room air (dashed horizontal line). One can easily see that this baseline remained stable at zero throughout the entire time (compare baseline at the far left and far right baseline conditions). A shift in the baseline of 2 l/min can also be detected during the administration of 2 l/min oxygen (see expanded time window in lower traces).
Data Processing
Sleep scoring was performed as previously described (8) to determine standard PSG indices from the baseline PSG. Respiratory signals were processed with the custom analytic software IgorPro (WaveMetics, Lake Oswego, OR). Minute ventilation (V̇e), respiratory rate, and timing parameters were derived from flow signal. Inspired and expired volumes were calculated by integrating flow signals over time. Esophageal pressure measurements were used to calculate WOB for each breath by integrating the volume signal over esophageal pressure signal, which was used as a surrogate of intrathoracic pressure changes as previously described (1, 2). WOB per minute was calculated from the per breath average multiplied by the respiratory rate. Additionally, we measured the maximum esophageal pressure swing per breath and the pressure time product per breath (i.e., average esophageal pressure drop during inspiration × inspiratory time). Esophageal pressure swings and pressure time product are measurements of the amount of effort required to generate inspiratory flows. High pressure time product and esophageal swings indicate a need for higher levels of ventilation or suggest the presence of impaired lung function, which would require high degrees of effort to generate regular ventilation. Esophageal pressure, volume, and flow signals were then used in a curve-fitting linear model to obtain respiratory system resistance and compliance.
Statistical Analysis
We used nonpaired t-tests to compare demographics, pulmonary function tests, and baseline PSG characteristics between the COPD and control groups. Repeated-measures ANOVA was used to compare ventilation and WOB during PSG between the treatment conditions (room air, oxygen, or NHF). A post hoc t-test with Holm adjustment was used for between-group comparisons. Values of P < 0.05 were considered statistically significant.
RESULTS
Eighteen individuals (12 with COPD, 6 controls) agreed to participate and underwent both the standard PSG (baseline) for characterizing sleep and the interventional study night (intervention) to assess measures of ventilation and WOB with NHF or oxygen (compared with room air). Demographic, respiratory, and sleep characteristics from the baseline assessment are presented in Tables 1 and 2. In general, individuals in the COPD group were 6 yr older than controls, with no difference in height, weight, or body mass index. By design, the absolute FEV1, FEV1 percent of predicted, and FEV1/FVC ratio were lower in the COPD group compared with measurements in the control group. On average in the COPD group there was lower total sleep time and sleep efficiency compared with controls. Of the seven participants who consented to the use of esophageal catheter, six were able to tolerate the catheter and their data were used for measurement of WOB. The group of patients in whom WOB was performed had demographic and sleep characteristics that were similar to those of the entire group.
Table 1.
Baseline characteristics
| Control Subjects | Subjects with COPD | P | |
|---|---|---|---|
| Demographics | |||
| Number | 6 | 12 | |
| Gender | 2 M/4 F | 5 M/7 F | |
| Age, yr | 50 ± 5 | 56 ± 6 | 0.038 |
| Height, cm | 167 ± 8 | 171 ± 13 | 0.695 |
| Weight, kg | 84 ± 10 | 75 ± 20 | 0.205 |
| BMI, kg/m2 | 31 ± 5 | 26 ± 4 | 0.077 |
| Lung function | |||
| FEV1, liter | 2.6 ± 0.6 | 1.7 ± 0.6 | 0.015 |
| FEV1, % | 90 ± 14 | 59 ± 23 | 0.001 |
| FVC, liter | 3.3 ± 0.8 | 2.8 ± 0.6 | 0.234 |
| FEV1/FVC | 0.8 ± 0.1 | 0.6 ± 0.1 | 0.000 |
| Arterial blood gases | |||
| Po2, mmHg | 81 ± 7 | 87 ± 47 | 0.390 |
| Pco2, mmHg | 41 ± 22 | 42 ± 19 | 0.813 |
| , % | 96.3 ± 49.7 | 95.9 ± 43.4 | 0.722 |
Values are means ± SD. A nonpaired t-test was used for comparisons. BMI, body mass index; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; Pco2, partial pressure of CO2; Po2, partial pressure of O2; , arterial O2 saturation.
Table 2.
Sleep and baseline ventilation data
| Control Subjects | Subjects with COPD | P | |
|---|---|---|---|
| Sleep data | |||
| TST, min | 422 ± 39 | 369 ± 61 | 0.040 |
| SE, % | 86 ± 4 | 77 ± 11 | 0.041 |
| NREM1, % | 12 ± 5 | 20 ± 16 | 0.133 |
| NREM2, % | 44 ± 11 | 49 ± 16 | 0.449 |
| NREM3/4, % | 20 ± 7 | 14 ± 9 | 0.126 |
| REM, % | 24 ± 8 | 18 ± 6 | 0.101 |
| RDI, n/h | 15 ± 17 | 10 ± 10 | 0.507 |
| RDI during NREM, n/h | 13 ± 17 | 9 ± 12 | 0.607 |
| RDI during REM, n/h | 24 ± 23 | 17 ± 14 | 0.501 |
| Baseline , % | 96 ± 1.5 | 94.1 ± 2.2 | 0.050 |
| Lowest , % | 92.5 ± 1.6 | 90.5 ± 2.8 | 0.075 |
| Ventilation during NREM sleep | |||
| V̇e, l/min | 7.9 ± 1.5 | 7.0 ± 2.6 | 0.444 |
| Tidal volume, liter | 0.55 ± 0.12 | 0.42 ± 0.12 | 0.042 |
| Respiratory rate, min−1 | 15 ± 3 | 17 ± 3 | 0.196 |
| Duty cycle | 0.40 ± 0.09 | 0.44 ± 0.04 | 0.188 |
Sleep data on the baseline night and ventilation parameters during NREM sleep under room air condition. Values are means ± SD. NREM1 to 5 and REM, percent of time under each sleep stage; RDI, respiratory disturbance index; , arterial O2 saturation; SE, sleep efficiency; TST, total sleep time; V̇e, minute ventilation. A nonpaired t-test was used for comparisons.
Ventilation and Respiratory Characteristic in COPD and Controls
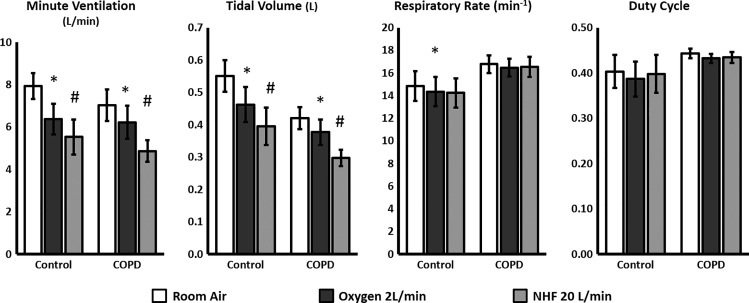
Ventilatory characteristics for the COPD and control subjects for the room air condition during NREM sleep on the intervention night are shown in Table 2. Compared with individuals in the control group, those in the COPD group had lower tidal volumes but there was a trend toward higher respiratory rates. There was no difference in V̇e between individuals in the COPD and control groups.
Effect of NHF and O2 on Ventilation
In both groups of subjects (those with COPD and controls) there was a reduction in V̇e and tidal volume for both NHF and oxygen conditions (Fig. 2, *P < 0.01) compared with room air. There was a greater decrease in V̇e with NHF compared with oxygen (t-test with Holm adjustment, P < 0.01). For both the COPD and control groups there was no difference in the duty cycle for either NHF or oxygen compared with room air. There was also no difference in the respiratory rate in either the COPD or control groups with NHF. With oxygen, there was a statistically significant decrease in respiratory rate in the control group, but with no clinical significance (<0.3 breaths/min). In the six patients that also performed the intervention night study using inductive PSG the baseline V̇e was 5.2 l/min in the room air condition and decreased to 4.5 l/min with the use of NHF (P < 0.01).
Fig. 2.
Ventilation and breathing parameters during stable NREM sleep under room air, oxygen or NHF conditions in subjects with chronic obstructive pulmonary disease (COPD, n = 12) and control subjects (n = 6). Both oxygen and NHF decreased minute ventilation (V̇e) and tidal volume, but the decrease was greater under the NHF condition. With oxygen, there was also a statistically significant but clinically irrelevant decrease in respiratory rate in the control group. Graphs represent averages ± SE. Repeated-measures ANOVA was used for comparing the three groups, with a Holm adjustment for two-group comparisons. *P < 0.05 for comparison with room air condition, #P < 0.05 for comparison with room air and oxygen conditions.
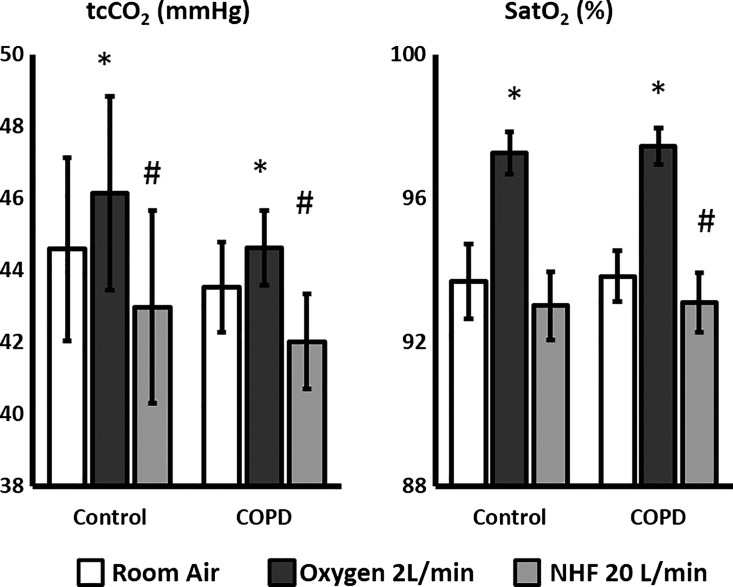
In both the COPD and control groups the reduction in V̇e with NHF was accompanied by a reduction in transcutaneous CO2 (tcCO2) (Fig. 3), which contrasts with the rise in tcCO2 in the oxygen condition (all P < 0.01 repeated-measures ANOVA). In both the COPD and control groups there was an increase in O2 saturation in the oxygen condition despite a decrease in ventilation (P < 0.01). NHF did not change oxygen saturation in the control group; however, there was a small yet clinically insignificant reduction in in the COPD group.
Fig. 3.
and transcutaneous CO2 during stable NREM sleep under room air, oxygen, or NHF conditions in subjects with COPD (n = 12) and control subjects (n = 6). Oxygen significantly increased , but it also led to a rise in CO2 levels. Despite reducing V̇e (see Fig. 2), NHF decreased CO2 levels and, in patients with COPD, slightly decreased . Graphs represent averages ± SE. Repeated-measures ANOVA was used to compare the three groups, with a Holm adjustment for two-group comparisons. *P < 0.05 for comparison with room air condition, #P < 0.05 for comparison with room air and oxygen conditions.
Effect of NHF and O2 on WOB and lung mechanics.
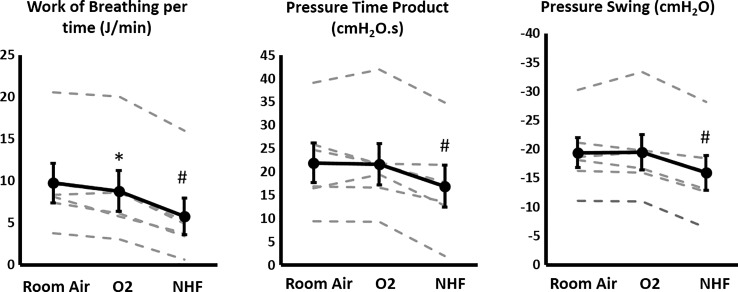
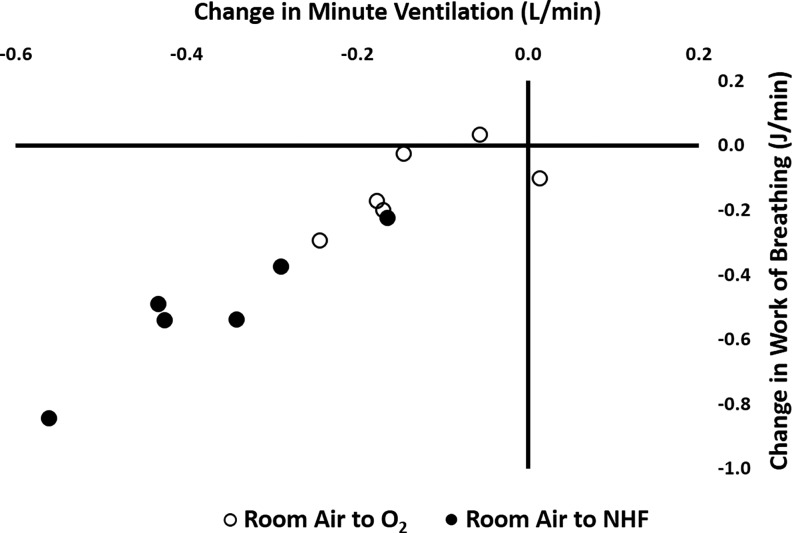
Individual data for WOB measurement are displayed in Fig. 4. There was a reduction in the WOB with the use of either oxygen (8.7 ± 5.9 J/min, means ± SD) or NHF (5.7 ± 5.3 J/min), compared with room air (9.7 ± 5.7 J/min, P < 0.01). There was a greater reduction in the WOB in the NHF condition compared with the oxygen condition (P < 0.01). NHF, but not oxygen, also reduced the pressure time product (17.1 ± 10.9 cmH2O/s, P < 0.01) compared with room air (22.1 ± 10.3 cmH2O/s). Similarly esophageal pressure swings were reduced in the NHF (−15.8 ± 7.3 cmH2O, P < 0.01) but not the oxygen (−19.4 ± 7.5 cmH2O) condition compared with room air (−19.2 ± 6.3 cmH2O). There were no statistically significant changes in lung compliance (room air, 70.9 ± 24.6 ml/cmH2O) with the use of either oxygen (69.1 ± 22.7 ml/cmH2O) or NHF (72.6 ± 36.8 ml/cmH2O). Similarly airway resistance (room air, 30.8 ± 10.2 cmH2O·s−1·l−1) did not significantly change with the use of oxygen (31.4 ± 11.2 cmH2O·s−1·l−1) or NHF (38.3 ± 11.6 cmH2O·s−1·l−1). Therefore, decreases in V̇e are the main drivers for the reduction in WOB observed with the use of NHF or oxygen, which can be appreciated in Fig. 5.
Fig. 4.
WOB, pressure time product, and pressure swings during stable NREM sleep under room air, oxygen, or NHF conditions in a subset of six patients who had an esophageal catheter inserted. Although oxygen only minimally reduced WOB, NHF resulted in a large decrease in WOB. NHF also decreased esophageal pressure swings and pressure time product, but could not be noticed with the use of oxygen. Gray dashed lines represent individual values. Dark lines represent averages with SE bars. Repeated-measures ANOVA was used for comparing the three groups, with a Holm adjustment for two-group comparisons. Error bars depict high intragroup variability. The statistical test (repeated-measures ANOVA) corrects for this variability and evaluates changes on each individual under the different experimental conditions. *P < 0.05 for comparison with room air condition, #P < 0.05 for comparison with room air and oxygen condition.
Fig. 5.
Change in minute ventilation vs. change in WOB in the six subjects who agreed to the use of an esophageal catheter. Black symbols represent changes in WOB and V̇e with the use of NHF compared with room air. White symbols represent changes in WOB and V̇e with the use of oxygen compared with room air. The figure shows the close correlation between the two parameters, suggesting there are few changes in respiratory mechanics. The observed reduction in WOB parallels the reduction in V̇e. We can notice a greater change with the use of NHF compared with oxygen.
DISCUSSION
In the present study we compared ventilation and WOB during NREM sleep in patients with COPD and healthy controls who smoke under three experimental conditions: room air, oxygen at 2 l/min, and NHF at 20 l/min. First, we showed that during sleep patients with COPD had a more rapid shallow breathing pattern compared with that of the control group. Next, in both the control and COPD subjects, oxygen decreased V̇e due to a reduction in tidal volume but it increased arterial CO2. NHF decreased V̇e and tidal volume to greater degrees than oxygen, yet in contrast, arterial CO2 was decreased. Last, oxygen reduced WOB minimally, whereas NHF resulted in WOB being reduced by half. We conclude that NHF offloaded breathing and improved arterial CO2 levels during NREM sleep compared with oxygen and room air in both control and COPD groups.
Effect of NREM Sleep on Breathing Pattern in COPD
Compared with controls, patients with COPD had similar V̇e but lower tidal volumes and slightly higher respiratory rates during sleep. Patients with COPD have increased intrapulmonary dead space and should require higher V̇e for maintaining CO2 homeostasis. However, increased airway resistance and increased respiratory rates can lead to dynamic hyperinflation, driving patients to adopt a shallower breathing pattern (22). During sleep, the decrease in muscle tone further increases airway resistance and can exacerbate air trapping (17). Although we cannot identify the precise mechanism for this, we noticed a small decrease in tidal volume in our group of participants with COPD, without a simultaneous increase in CO2 levels. This change in respiratory pattern during sleep could be an early indicator of increased mechanical loads in our subset of patients with COPD.
Effect of O2 and NHF on Ventilation During Sleep
We observed a marked reduction in ventilation with the use of NHF in both the control and COPD subjects. The reduction in V̇e was greater with the use of NHF than with oxygen. We have recently shown that the use of NHF decreases V̇e on healthy individuals during sleep by ~15% (15) and that this decrease is due to washout of the anatomic dead space of the upper airways (14). With the reduction in anatomic dead space during the use of NHF, a patient's V̇e would decrease while maintaining similar levels of alveolar ventilation and CO2. Nevertheless, we show that tcCO2 decreases with NHF, indicating that either alveolar ventilation has increased, or that metabolic demand (and CO2 production) has decreased, or both. We believe that both mechanisms are likely to occur simultaneously. We and others have shown that NHF increases positive end-expiratory pressure (PEEP) (4, 6, 13, 19), which may change the ventilation/perfusion ratio, improve alveolar ventilation, and alleviate dynamic hyperinflation. Furthermore, we monitored ventilation with a pneumotachograph attached to a nasal/face mask, both of which added dead space volume. Part of the reduction in ventilation may be related to a washout of dead space volume in the mask. To address this limitation we measured ventilation without a mask (with inductive PSG, n = 6) and also observed a 13% reduction in V̇e. Therefore, we demonstrated that NHF during sleep markedly reduced ventilation in individuals with COPD and controls alike to a greater extent than oxygen therapy, and therefore can decrease ventilatory demand.
Oxygen Versus High Flow on WOB, CO2, and O2
The major difference between O2 and NHF was that arterial CO2 rose by ~3% when patients received oxygen, whereas arterial CO2 fell by ~4% during NHF. Additionally, NHF was associated with a fourfold greater reduction in WOB compared with oxygen. The reduction in WOB was primarily due to the reduction in V̇e (see Fig. 5), because we detected no changes in airway resistance or lung compliance. Oxygen possibly reduced the respiratory drive, thereby decreasing V̇e, WOB, and alveolar ventilation, and consequently, impairing CO2 clearance. In contrast, NHF must have increased alveolar ventilation even at a markedly lower WOB. Thus, NHF led to a greater offloading of breathing than oxygen. Alternatively, it is possible that the reduction in WOB decreased CO2 production, thereby reducing arterial CO2. Further studies measuring CO2 production and O2 consumption are required to determine the precise mechanisms. We concluded that NHF led to significantly greater respiratory offloading compared with oxygen, thereby decreasing V̇e and WOB without increasing CO2 levels.
We also noted a small but significant reduction in with the use of NHF in patients with COPD. Those changes were small, in the order of 1%, and were not expected to be of any clinical relevance. We cannot determine the precise mechanism for this reduction, but regional changes in ventilation distribution as V̇e decreases can explain the decrease in . We could expect a greater reduction in ventilation in areas with a low ventilation/perfusion (V/Q) relationship, because those areas might need a longer time to inflate. Alternatively, NHF generates a small PEEP, which could increase pressure particularly in very high V/Q areas. This could move blood away from those areas and toward regions with lower V/Q and, therefore, produce small reductions in . However, despite eliciting interesting mechanistic discussion, changes in O2 were small and of no clinical relevance.
Strengths and Limitations
There are several strengths and limitations in our study. The main strength is the accurate quantification of the ventilatory parameter allowing analysis of the behavior of each patient under the three conditions. The main limitation is the small number of patients who agreed to have the esophageal catheter placed. Therefore, we could measure WOB and effort-related parameters in six patients only. Nevertheless, the patients who submitted to WOB measurement were similar to those of the entire group, and the results on ventilation are also comparable between them. The use of a nasal/facial mask to allow accurate ventilation measurements increases the dead space and thus may have magnified the NHF and oxygen effect on ventilation. To address that, we measured the NHF effects in a subset of patients undergoing PSG without any interface, in whom we measured ventilation with inductance PSG, and we could also detect a similar decrease in ventilation with the use of NHF.
Implications and Conclusion
The importance of this study is to demonstrate that although oxygen and NHF reduced V̇e during sleep, oxygen was associated with increases in CO2 and minimal reduction in WOB. In contrast, NHF was associated with both a significant reduction in WOB and CO2, indicating that NHF improved alveolar ventilation. Therefore NHF may be used as an alternative means to assist ventilation in patients prone to develop respiratory failure due to increased respiratory loads or insufficient alveolar ventilation.
GRANTS
Support for this study was provided by National Heart, Lung, and Blood Institute Grant HL-105546; Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), Brazil, Grant 2012/05190-0; Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Brazil, Grant 200817/2012-4; and Heinen + Loewenstein GmbH & Co KG, Bad Ems, Germany.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
P.J.C.B., J.P.K., A.R.S., P.L.S., and H.S. conceived and designed research; P.J.C.B., J.P.K., and H.S. performed experiments; P.J.C.B., J.P.K., K.F., A.R.S., P.L.S., and H.S. analyzed data; P.J.C.B., J.P.K., A.R.S., P.L.S., and H.S. interpreted results of experiments; P.J.C.B. and H.S. prepared figures; P.J.C.B., K.F., and H.S. drafted manuscript; P.J.C.B., J.P.K., L.G., K.F., A.R.S., P.L.S., and H.S. edited and revised manuscript; P.J.C.B., J.P.K., and H.S. approved final version of manuscript.
REFERENCES
- 1.Akoumianaki E, Maggiore SM, Valenza F, Bellani G, Jubran A, Loring SH, Pelosi P, Talmor D, Grasso S, Chiumello D, Guérin C, Patroniti N, Ranieri VM, Gattinoni L, Nava S, Terragni PP, Pesenti A, Tobin M, Mancebo J, Brochard L; PLUG Working Group (Acute Respiratory Failure Section of the European Society of Intensive Care Medicine) . The application of esophageal pressure measurement in patients with respiratory failure. Am J Respir Crit Care Med 189: 520–531, 2014. doi: 10.1164/rccm.201312-2193CI. [DOI] [PubMed] [Google Scholar]
- 2.Benditt JO. Esophageal and gastric pressure measurements. Respir Care 50: 68–75, 2005. [PubMed] [Google Scholar]
- 3.Chatila W, Nugent T, Vance G, Gaughan J, Criner GJ. The effects of high-flow vs low-flow oxygen on exercise in advanced obstructive airways disease. Chest 126: 1108–1115, 2004. doi: 10.1378/chest.126.4.1108. [DOI] [PubMed] [Google Scholar]
- 4.Corley A, Caruana LR, Barnett AG, Tronstad O, Fraser JF. Oxygen delivery through high-flow nasal cannulae increase end-expiratory lung volume and reduce respiratory rate in post-cardiac surgical patients. Br J Anaesth 107: 998–1004, 2011. doi: 10.1093/bja/aer265. [DOI] [PubMed] [Google Scholar]
- 5.Frat J-P, Thille AW, Mercat A, Girault C, Ragot S, Perbet S, Prat G, Boulain T, Morawiec E, Cottereau A, Devaquet J, Nseir S, Razazi K, Mira JP, Argaud L, Chakarian J-C, Ricard JD, Wittebole X, Chevalier S, Herbland A, Fartoukh M, Constantin JM, Tonnelier JM, Pierrot M, Mathonnet A, Béduneau G, Delétage-Métreau C, Richard JC, Brochard L, Robert R; FLORALI Study Group; REVA Network . High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N Engl J Med 372: 2185–2196, 2015. doi: 10.1056/NEJMoa1503326. [DOI] [PubMed] [Google Scholar]
- 6.Groves N, Tobin A. High flow nasal oxygen generates positive airway pressure in adult volunteers. Aust Crit Care 20: 126–131, 2007. doi: 10.1016/j.aucc.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 7.Hasani A, Chapman TH, McCool D, Smith RE, Dilworth JP, Agnew JE. Domiciliary humidification improves lung mucociliary clearance in patients with bronchiectasis. Chron Respir Dis 5: 81–86, 2008. doi: 10.1177/1479972307087190. [DOI] [PubMed] [Google Scholar]
- 8.Iber C, Ancoli-Israel S, Chesson AL Jr, Quan SF. The AASM Manual for the Scoring of Sleep and Associated Events: Rules Terminology and Technical Specifications (1st ed.), Darien, IL: American Academy of Sleep Medicine, 2007, p. 57. [Google Scholar]
- 9.Kirkness JP, Verma M, McGinley BM, Erlacher M, Schwartz AR, Smith PL, Wheatley JR, Patil SP, Amis TC, Schneider H. Pitot-tube flowmeter for quantification of airflow during sleep. Physiol Meas 32: 223–237, 2011. doi: 10.1088/0967-3334/32/2/006. [DOI] [PubMed] [Google Scholar]
- 10.MacIntyre NR. Muscle dysfunction associated with chronic obstructive pulmonary disease. Respir Care 51: 840–847, 2006. [PubMed] [Google Scholar]
- 11.Maggiore SM, Idone FA, Vaschetto R, Festa R, Cataldo A, Antonicelli F, Montini L, De Gaetano A, Navalesi P, Antonelli M. Nasal high-flow versus Venturi mask oxygen therapy after extubation. Effects on oxygenation, comfort, and clinical outcome. Am J Respir Crit Care Med 190: 282–288, 2014. doi: 10.1164/rccm.201402-0364OC. [DOI] [PubMed] [Google Scholar]
- 12.McDonald CF. Oxygen therapy for COPD. J Thorac Dis 6: 1632–1639, 2014. doi: 10.3978/j.issn.2072-1439.2014.10.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McGinley BM, Patil SP, Kirkness JP, Smith PL, Schwartz AR, Schneider H. A nasal cannula can be used to treat obstructive sleep apnea. Am J Respir Crit Care Med 176: 194–200, 2007. doi: 10.1164/rccm.200609-1336OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Möller W, Celik G, Feng S, Bartenstein P, Meyer G, Oliver E, Schmid O, Tatkov S. Nasal high flow clears anatomical dead space in upper airway models. J Appl Physiol 118: 1525–1532, 2015. doi: 10.1152/japplphysiol.00934.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mündel T, Feng S, Tatkov S, Schneider H. Mechanisms of nasal high flow on ventilation during wakefulness and sleep. J Appl Physiol 114: 1058–1065, 2013. doi: 10.1152/japplphysiol.01308.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nilius G, Franke KJ, Domanski U, Rühle KH, Kirkness JP, Schneider H. Effects of nasal insufflation on arterial gas exchange and breathing pattern in patients with chronic obstructive pulmonary disease and hypercapnic respiratory failure. Adv Exp Med Biol 755: 27–34, 2013. doi: 10.1007/978-94-007-4546-9_4. [DOI] [PubMed] [Google Scholar]
- 17.O’Donoghue FJ, Catcheside PG, Eckert DJ, McEvoy RD. Changes in respiration in NREM sleep in hypercapnic chronic obstructive pulmonary disease. J Physiol 559: 663–673, 2004. doi: 10.1113/jphysiol.2004.066084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ottenheijm CA, Heunks LM, Dekhuijzen PN. Diaphragm muscle fiber dysfunction in chronic obstructive pulmonary disease: toward a pathophysiological concept. Am J Respir Crit Care Med 175: 1233–1240, 2007. doi: 10.1164/rccm.200701-020PP. [DOI] [PubMed] [Google Scholar]
- 19.Parke R, McGuinness S, Eccleston M. Nasal high-flow therapy delivers low level positive airway pressure. Br J Anaesth 103: 886–890, 2009. doi: 10.1093/bja/aep280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pham TM, O’Malley L, Mayfield S, Martin S, Schibler A. The effect of high flow nasal cannula therapy on the work of breathing in infants with bronchiolitis. Pediatr Pulmonol 50: 713–720, 2015. doi: 10.1002/ppul.23060. [DOI] [PubMed] [Google Scholar]
- 21.Rea H, McAuley S, Jayaram L, Garrett J, Hockey H, Storey L, O’Donnell G, Haru L, Payton M, O’Donnell K. The clinical utility of long-term humidification therapy in chronic airway disease. Respir Med 104: 525–533, 2010. doi: 10.1016/j.rmed.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 22.Vitacca M, Lanini B, Nava S, Barbano L, Portal R, Clini E, Ambrosino N. Inspiratory muscle workload due to dynamic intrinsic PEEP in stable COPD patients: effects of two different settings of non-invasive pressure-support ventilation. Monaldi Arch Chest Dis 61: 81–85, 2004. doi: 10.4081/monaldi.2004.704. [DOI] [PubMed] [Google Scholar]