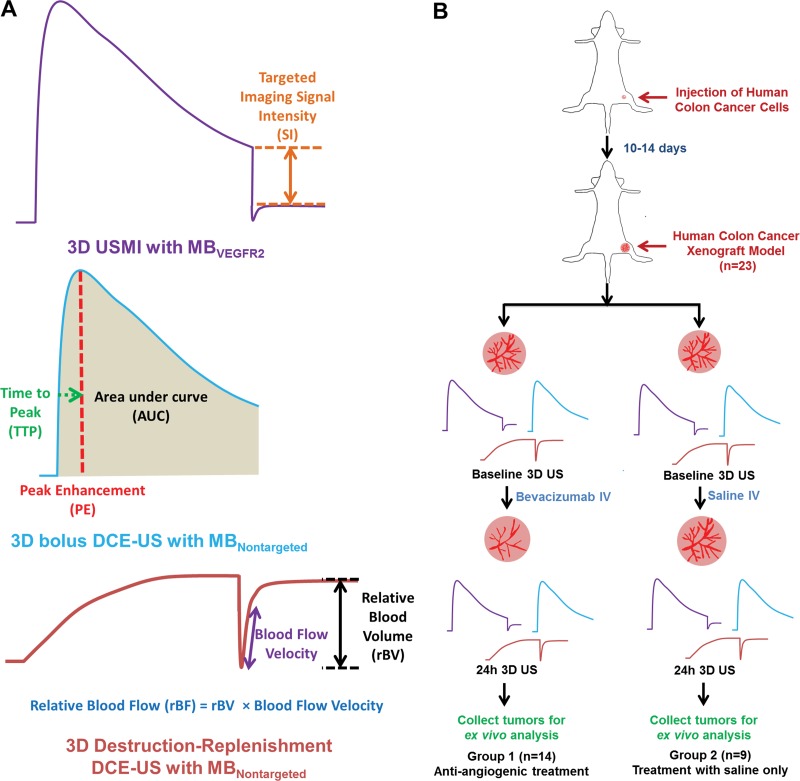
Both three-dimensional (3D) molecularly targeted US and 3D dynamic contrast material–enhanced US provide complementary molecular and functional in vivo information on antiangiogenic treatment effects in a human colon cancer xenograft model in mice.
Abstract
Purpose
To perform an intra-animal comparison between (a) three-dimensional (3D) molecularly targeted ultrasonography (US) by using clinical-grade vascular endothelial growth factor receptor 2 (VEGFR2)–targeted microbubbles and (b) 3D dynamic contrast material–enhanced (DCE) US by using nontargeted microbubbles for assessment of antiangiogenic treatment effects in a murine model of human colon cancer.
Materials and Methods
Twenty-three mice with human colon cancer xenografts were randomized to receive either single-dose antiangiogenic treatment (bevacizumab, n = 14) or control treatment (saline, n = 9). At baseline and 24 hours after treatment, animals were imaged with a clinical US system equipped with a clinical matrix array transducer by using the following techniques: (a) molecularly targeted US with VEGFR2-targeted microbubbles, (b) bolus DCE US with nontargeted microbubbles, and (c) destruction-replenishment DCE US with nontargeted microbubbles. VEGFR2-targeted US signal, peak enhancement, area under the time-intensity curve, time to peak, relative blood volume (rBV), relative blood flow, and blood flow velocity were quantified. VEGFR2 expression and percentage area of blood vessels were assessed ex vivo with quantitative immunofluorescence and correlated with corresponding in vivo US parameters. Statistical analysis was performed with Wilcoxon signed rank tests and rank sum tests, as well as Pearson correlation analysis.
Results
Molecularly targeted US signal with VEGFR2-targeted microbubbles, peak enhancement, and rBV significantly decreased (P ≤ .03) after a single antiangiogenic treatment compared with those in the control group; similarly, ex vivo VEGFR2 expression (P = .03) and percentage area of blood vessels (P = .03) significantly decreased after antiangiogenic treatment. Three-dimensional molecularly targeted US signal correlated well with VEGFR2 expression (r = 0.86, P = .001), and rBV (r = 0.71, P = .01) and relative blood flow (r = 0.78, P = .005) correlated well with percentage area of blood vessels, while other US perfusion parameters did not.
Conclusion
Three-dimensional molecularly targeted US and destruction-replenishment 3D DCE US provide complementary molecular and functional in vivo imaging information on antiangiogenic treatment effects in human colon cancer xenografts compared with ex vivo reference standards.
© RSNA, 2016
Introduction
Colon cancer is the second leading cause of cancer-related death, and it is estimated that there will be 132 700 new cases and more than 50 960 deaths from colorectal cancer in 2015 in the United States (1). The mainstay of treatment for colon cancer is curative surgery for patients at an early stage. Patients with colon cancer metastases, mostly to the liver, are treated with neoadjuvant chemotherapy, which often includes antiangiogenic drugs such as bevacizumab, a vascular endothelial growth factor–targeted monoclonal antibody that was the first antiangiogenic agent approved by the U.S. Food and Drug Administration (2). However, not all patients respond to neoadjuvant therapies, which expose many patients to unnecessary side effects with associated health care costs. Therefore, accurate noninvasive imaging biomarkers are critically needed to allow assessment of treatment success and better patient management.
Owing to its wide availability, relatively low cost, portability, and lack of radiation exposure, ultrasonography (US) is one of the most commonly used imaging techniques in medicine. With the introduction of intravenous microbubble US contrast agents, functional imaging of tumor perfusion, also called dynamic contrast material–enhanced (DCE) US, has become clinically available (3). However, DCE US with current two-dimensional imaging capabilities is suboptimal because it fails to demonstrate the spatial heterogeneity of tumor perfusion, and it is challenging to position the two-dimensional US transducer at the exact same location for longitudinal assessment of tumor response (4–8). This limitation has been addressed by the introduction of three-dimensional (3D) DCE US techniques (9–11). Three-dimensional DCE US enables the assessment of tumor perfusion in a volumetric manner, thereby making this technique more reproducible than two-dimensional DCE US imaging techniques, in particular for the longitudinal assessment of tumor perfusion (11).
In parallel, US performed with molecularly targeted microbubble contrast agent has become a promising technique to visualize, characterize, and quantify biological processes at the molecular level in vivo (12–14), including tumor angiogenesis–associated molecular markers such as vascular endothelial growth factor receptor 2 (VEGFR2) (15–18). A clinical-grade VEGFR2-targeted US microbubble agent has been designed and translated into clinical trials (19). In a recent study, 3D molecularly targeted US was shown to be highly reliable in the assessment of tumor angiogenesis in mice (7). However, to the best of our knowledge, there is no study in which both emerging techniques, 3D molecularly targeted US and 3D DCE US, were compared for the assessment of treatment response after antiangiogenic therapy in an intra-animal comparison study by using a clinical US system and clinical matrix array transducer that supports 3D imaging.
Therefore, the purpose of this study was to perform an intra-animal comparison between 3D molecularly targeted US with clinical-grade VEGFR2-targeted microbubbles and 3D DCE US with nontargeted microbubbles for the assessment of antiangiogenic treatment effects in a murine model of human colon cancer.
Materials and Methods
Mouse Tumor Model
This study was approved by the Institutional Administrative Panel on Laboratory Animal Care. Human colon cancer xenografts were established in 23 female nude mice (6–8 weeks old, weighing 20–25 g; Charles River Laboratories, Hollister, Calif). Human LS174T colon adenocarcinoma cells (ATCC, Manassas, Va) were cultured in Minimum Essential Medium supplemented with 10% fetal bovine serum. The tumor cells were trypsinized at 70%–80% confluence, and then 3 × 106 cells were suspended in 50 μL of Matrigel (BD Biosciences, San Jose, Calif) and injected subcutaneously on the lower hind limb of mice. Tumor growth was assessed by using an electronic caliper available on the US system. When the tumors reached 1–2 cm in maximum diameter (mean size, 1.5 cm), the tumors were imaged.
In Vivo 3D Molecularly Targeted US and 3D DCE US
During scanning, all mice were kept under gas anesthesia (2% isoflurane in room air, administered at 2 L/min). Mice were placed prone on a heated scanning stage. The tail vein was cannulated with a 27-gauge needle (Vevo Micromarker; VisualSonics, Toronto, Ontario, Canada) and attached to an injection pump (Kent Scientific, Torrington, Conn). Three-dimensional US was performed by using a clinical US system (iU22 xMatrix; Philips Healthcare, Andover, Mass) equipped with a clinical matrix array transducer (X6-1, with a center frequency of 3.2 MHz; Philips Healthcare). To minimize motion artifacts, the transducer was held in a fixed position with a clamp. To reduce near-field artifacts, the transducer was coupled to the tumor with a prewarmed custom standoff of US gel to bring the tumor beyond the near field zone of the transducer. The distance between the transducer and the center of the tumor was set at 4 cm.
Each imaging acquisition (performed by H.W., a radiologist with 5 years of experience) was started with B-mode images to outline the tumor before switching to power modulation contrast imaging mode. The following parameters were kept constant for all imaging acquisitions in all mice: voxel dimensions, 320 × 110 × 210 μm3; focal length, 40 mm; mechanical index, 0.05; dynamic range, 40 dB; and volume rate, 1 Hz. Three-dimensional US imaging volumes were streamed in real time by using a built-in digital navigation link on the US machine, with custom in-house MeVisLab modules (Mevis Medical Solutions, Bremen, Germany) written in C++ (20).
Three-dimensional molecularly targeted US protocol.— Three-dimensional molecularly targeted US was performed by using clinical-grade VEGFR2-targeted microbubbles (BR55; Bracco Suisse, Geneva, Switzerland) (21,22). VEGFR2-targeted microbubbles are composed of a mixture of nitrogen and perfluorobutane, functionalized with a heterodimeric peptide binding to VEGFR2. The mean number of heterodimeric peptides per square micrometer on the VEGFR2-targeted microbubble shell ± standard deviation was 34 200 ± 1 300 (range, 31 800–36 600) (7). The mean diameter of the VEGFR2-targeted microbubbles was 1.5 µm ± 0.1 (range, 1–3 µm; Multisizer III Coulter Counter, Beckman Coulter, Fullerton, Calif). The stock solution contained 5 × 108 microbubbles per milliliter. The VEGFR2-targeted microbubbles were injected at a dose of 5 × 107 (in 100 µL) at a constant injection rate of 20 µL/sec. After 4 minutes, which allowed the VEGFR2-targeted microbubbles to circulate through the tumor volume and attach to VEGFR2 (22), imaging acquisition was performed for 15 seconds. A sequence of five high-power destructive pulses (mechanic index = 0.77) was applied over a 5-second period to destroy all VEGFR2-targeted microbubbles within the field of view. After the destructive pulses were administered, imaging data sets were acquired for 60 seconds to allow microbubbles to recirculate into the tumor (Fig 1).
Figure 1:
A, Summary of the time-intensity curve diagrams of 3D VEGFR2-targeted US molecular imaging (USMI) and the two DCE US techniques, along with the imaging parameters measured by using the three different techniques. B, Overall experimental design of 3D molecularly targeted US, bolus DCE US, and destruction-replenishment DCE US imaging experiments. Subcutaneous human colon cancer xenografts were established in 23 nude mice and then randomized into two treatment groups. MBNontargeted = nontargeted microbubbles, MBVEGFR2 = VEGFR2-targeted microbubbles.
Three-dimensional DCE US protocol.—In each mouse, two well-established techniques were used to acquire 3D DCE US data sets by using nontargeted microbubbles (Fig 1). The first method is based on the wash-in washout kinetics of microbubbles after bolus injection of nontargeted microbubbles (henceforth called bolus DCE US) (23). Nontargeted microbubbles (Vevo Micromarker; VisualSonics) consist of a gas core of perfluorobutane and a phospholipid shell with a mean size of 2.6 µm (range, 1–4 µm). Nontargeted microbubbles (5 × 107 microbubbles in 100 µL) were injected over 5 seconds (20 µL/sec). Imaging data sets were acquired continuously for 4 minutes.
With the second method, the unique ability of DCE US to manipulate microbubbles (henceforth called destruction-replenishment DCE US) was used (11,23). Nontargeted microbubbles were continuously infused at a constant infusion rate of 2 × 107 microbubbles per minute (40 µL/min). After steady-state enhancement was reached at 4 minutes, five high-power destructive pulses (mechanic index = 0.77) were applied over a 5-second period to clear nontargeted microbubbles from the entire tumor volume. After microbubble destruction, data were acquired continuously over another 3-minute interval, which allowed the microbubbles to replenish to predestructive values (Fig 1).
In all mice, 3D molecularly targeted US with VEGFR2-targeted microbubbles, bolus DCE US with nontargeted microbubbles, and destruction-replenishment DCE US with nontargeted microbubbles were performed in the same imaging session, and the order of acquisition was randomized. To allow clearance of microbubbles from previous injections, a waiting time of at least 30 minutes was applied between imaging acquisitions (24–26). All mice tolerated the repetitive injections of microbubbles well.
Evaluating antiangiogenic treatment effect with 3D molecularly targeted US and 3D DCE US.—Twenty-three tumor-bearing mice were used to assess the effect of antiangiogenic treatment versus control saline-only treatment on 3D molecularly targeted US and DCE US data sets. Fourteen mice (group 1) were intravenously treated with a single dose of the antiangiogenic agent (bevacizumab, 10 mg per kilogram of body weight; Avastin, Genentech, South San Francisco, Calif), and nine control mice (group 2) were treated with saline only. Three-dimensional US was performed at baseline (day 0) and 24 hours after treatment (Fig 1). After imaging at 24 hours, all animals were sacrificed, and tumor tissues were harvested for ex vivo analysis.
Analysis of 3D US Data Sets
Imaging analysis was performed by one reader who was blinded to the treatment type (antiangiogenic vs saline only) by using in-house custom software developed with MeVisLab (Mevis Medical Solutions) (27). The entire tumor was delineated with a volume of interest on the 3D B-mode images obtained in the axial, sagittal, and coronal imaging planes. This volume of interest was subsequently used to generate time-intensity curves on the images obtained with power modulation contrast imaging mode for the quantification of 3D molecularly targeted US and DCE US data sets.
For 3D molecularly targeted US data sets, the VEGFR2-targeted imaging signal of attached VEGFR2-targeted microbubbles was calculated as the difference in the targeted imaging signal intensity (in arbitrary units) between pre- and postdestructive imaging signals (17,22,25,28). For the quantification of bolus DCE US with nontargeted microbubbles, time-intensity curves were fitted with a log-normal function model (29), and fitted curves were used to calculate the following perfusion parameters (6,23,30): (a) peak enhancement (related to relative blood volume [in arbitrary units]), (b) area under the time-intensity curve (AUC, related to relative blood volume [in arbitrary units]), and (c) time to peak (TTP [in seconds]). For the quantification of destruction-replenishment DCE US data sets, time-intensity curves from continuous infusion of nontargeted microbubbles were fitted with a destruction-replenishment model (31), and the fitted curves were used to calculate relative blood volume (rBV [in arbitrary units]), relative blood flow (rBF [in arbitrary units]), and blood flow velocity (in sec−1) (5,31,32).
Ex vivo analysis of tumors is detailed in Appendix E1 (online).
Statistical analysis was performed with Wilcoxon signed rank tests and rank sum tests, as well as Pearson correlation analysis. Details of statistical analysis are given in Appendix E1 (online).
Results
Three-dimensional Molecularly Targeted US and DCE US for Assessment of Antiangiogenic Treatment
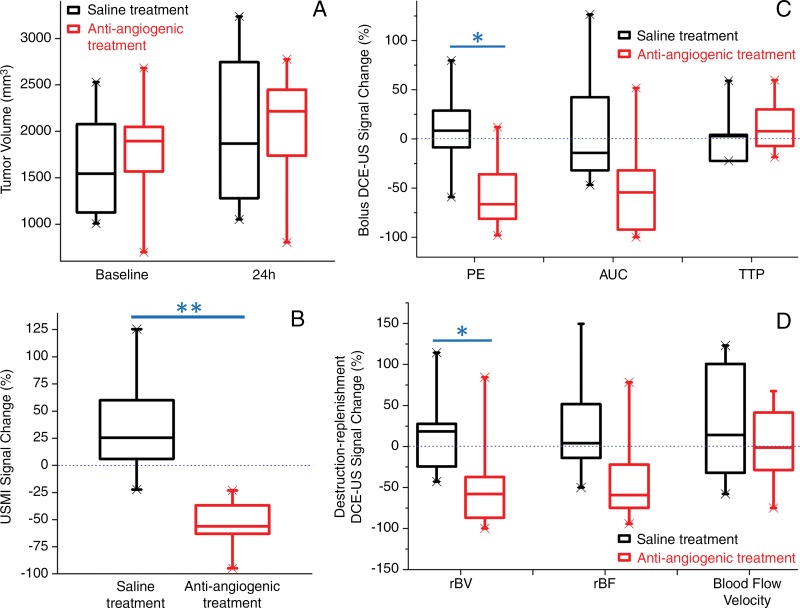
At baseline, tumor volumes in the control group (1657 mm3 ± 644) were not significantly different (P = .54) than those in the antiangiogenic treatment group (1806 mm3 ± 569). At 24 hours, tumor volumes remained not significantly different (P = .90) between the two groups, with a mean increase in volume of 20.7% ± 20.6 (P = .13) in the control group and 15.0% ± 10.8 (P = .21) in the antiangiogenic treatment group (Figs 2, 3).
Figure 2:
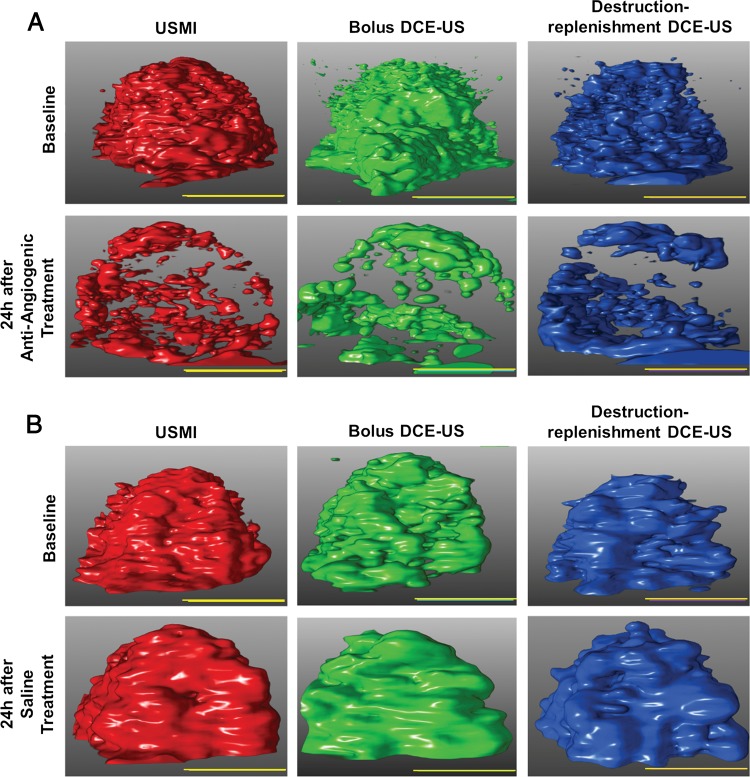
A, US images show the antiangiogenic treatment effect at 3D molecularly targeted US (USMI), bolus DCE US, and destruction-replenishment DCE US in two representative subcutaneous human colon cancer xenografts. After administration of a single dose of bevacizumab, imaging signal (demonstrated at identical volume rendering settings of molecularly targeted US signal [left column], bolus DCE US at peak enhancement [middle column], and destruction-replenishment DCE US at complete replenishment [right column]) substantially decreased 24 hours after antiangiogenic treatment compared with baseline images by using the three imaging techniques. B, In saline-treated tumors, the imaging signal did not change substantially before and after treatment. Scale bar = 10 mm.
Figure 3:
Box and whisker plots of the antiangiogenic treatment effects on A, tumor volume, B, VEGFR2-targeted 3D molecularly targeted US (USMI) signal, and perfusion values obtained with, C, bolus DCE US and, D, destruction-replenishment DCE US. The percentage change from baseline to 24 hours after treatment is plotted in treatment and control groups. Each box in the plot represents the 25th and 75th quartiles; the line inside each box indicates the median, and the whiskers indicate the 5th and 95th percentile of measurements, excluding the outliers (indicated by the X at the end of each line). PE = peak enhancement. * = P < .05, ** = P < .001.
By using 3D molecularly targeted US and 3D DCE US techniques, a significant decrease of 3D molecularly targeted US signal, peak enhancement, and rBV (all P ≤ .03) was observed when compared with baseline after antiangiogenic treatment at 24 hours (Table; Figs 2, 3), while AUC, TTP, rBF, and blood flow velocity did not change significantly (P ≥ .21). In control animals with saline-only treatment, 3D molecularly targeted US signal, peak enhancement, AUC, TTP, rBV, rBF, and blood flow velocity did not change significantly (P > .99) at 24 hours (Table; Figs 2, 3). A significant decrease in molecularly targeted US signal, peak enhancement, and rBV (all P ≤ .03) was observed in the treatment group after antiangiogenic treatment at 24 hours when compared with the control group (Table; Figs 2, 3).
Percentage Changes of in Vivo Imaging Parameters Obtained with 3D Molecularly Targeted US and 3D DCE US after Antiangiogenic and Saline-Only Treatment in Human Colon Cancer Xenografts

Note.—Values are percentage changes (means ± standard deviations) from baseline to 24 hours after treatment. Numbers in parentheses indicate the P values calculated between baseline and 24 hours after treatment in each group. All P values are corrected for multiple comparisons by using the Benjamini-Hochberg procedure.
Ex Vivo Analysis of Tumors
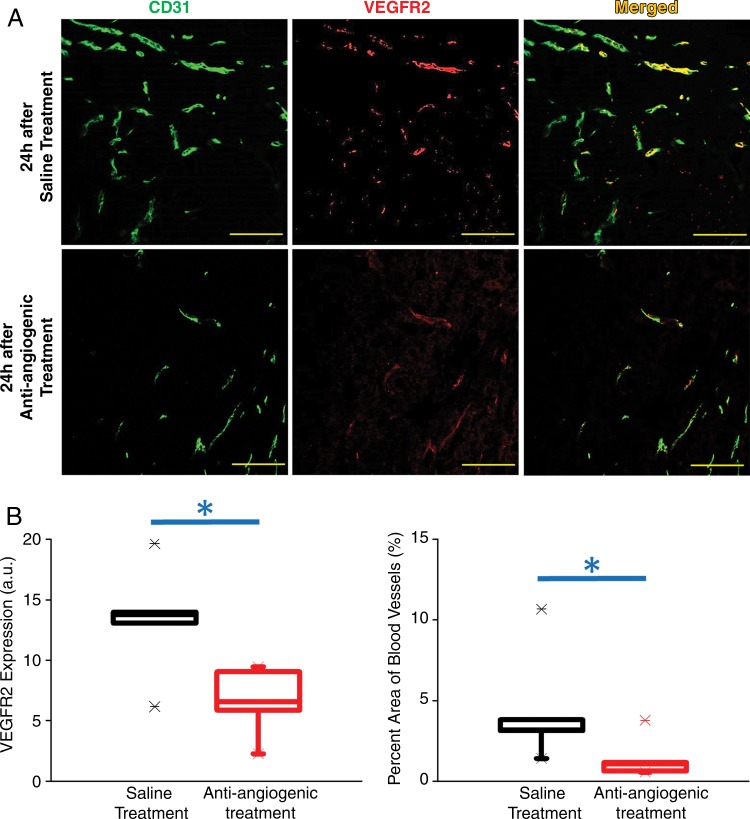
After antiangiogenic treatment, VEGFR2 expression levels at quantitative immunofluorescence of treated tumors (6.6 arbitrary units [a.u.] ± 2.6) were significantly lower (P = .03) when compared with saline-treated tumors (13.3 a.u. ± 4.8). Similarly, percentage area of blood vessels at quantitative immunofluorescence was significantly smaller (P = .03) in treated tumors (1.4% ± 1.2) when compared with saline-treated tumors (4.4% ± 3.6, Fig 4).
Figure 4:
A, Representative photomicrographs of VEGFR2 (red), CD31 (green), and merged (yellow) staining of human colon cancer tissue slices obtained from the tumor center show decreased VEGFR2 expression levels and percentage area of blood vessels in a tumor treated with antiangiogenic therapy (lower row) compared with tumor treated with saline only (upper row) (scale bar = 100 µm). B, Box plots summarize quantitative ex vivo results of immunofluorescence and percentage area of blood vessels in treated and nontreated tumors. Each box in the plot represents the 25th and 75th quartiles; the line inside each box indicates the median, and the whiskers indicate the 5th and 95th percentile of measurements, excluding the outliers (indicated by the X at the end of each line). * = P < .05.
Comparison of 3D Molecularly Targeted US versus 3D DCE US for Assessment of Antiangiogenic Treatment
Three-dimensional molecularly targeted US and 3D destruction-replenishment DCE US were comparably accurate in the assessment of antiangiogenic treatment effects as early as 24 hours after treatment by using ex vivo immunofluorescence findings as the reference standard. There was excellent correlation between in vivo 3D molecularly targeted US signal and ex vivo VEGFR2 expression levels (r = 0.86, P = .001). rBV (r = 0.71, P = .01) and rBF (r = 0.78, P = .005) obtained with 3D destruction-replenishment DCE US correlated well with ex vivo percentage area of blood vessels. The correlation of molecularly targeted US signal with ex vivo VEGFR2 expression levels was not significantly different from the correlation between rBV (P = .44) and rBF (P = .65) with ex vivo percentage area of blood vessels.
In contrast, blood flow velocity obtained with 3D destruction-replenishment DCE US did not correlate with ex vivo percentage area of blood vessels (r = 0.19, P = .57). Also, peak enhancement (r = 0.39, P = .23) and AUC (r = −0.13, P = .71), as well as TTP (r = −0.20, P = .55) obtained with the 3D bolus DCE US technique, did not correlate with ex vivo percentage area of blood vessels.
Discussion
The results of this study demonstrated that 3D molecularly targeted US and 3D DCE US by using a clinical US machine and a clinical matrix array transducer allowed assessment of antiangiogenic treatment effects in a murine xenograft model of human colon cancer. However, only 3D molecularly targeted US and replenishment-destruction 3D DCE US (rBV and rBF) correlated well with the ex vivo reference standards of VEGFR2 expression and percentage vessel area, respectively. Perfusion parameters obtained with bolus 3D DCE US (peak enhancement, AUC, and TTP) did not correlate with the ex vivo reference standard of percentage vessel area.
Early discrimination between chemotherapy responders and nonresponders has major implications for patient care, including the potential to spare nonresponding patients the high morbidity and cost associated with these treatments and the potential for oncologists to change chemotherapy regimens sooner to more effective alternative therapies. Also, several newly introduced therapeutic agents, including antiangiogenic and other molecularly targeted drugs, impose more challenges, because these therapies often induce cytostatic rather than cytotoxic effects, with little or no change in tumor size detectable on anatomic images. Therefore, complementary next-generation imaging approaches are critically needed to allow early evaluation and/or prediction of treatment response before overt anatomic changes occur.
Molecular US and functional US are particularly well suited for the assessment of early postchemotherapy treatment response of both primary tumors and metastases in organs that are easily accessible to US, such as the liver (33–36). US does not expose patients to ionizing radiation, is widespread and available at relatively low cost, and, owing to its portability, can be performed repetitively in sick patients who are not able to be transported to undergo imaging with dedicated scanners. In particular, during early induction chemotherapy, molecular and/or functional US could be performed in the clinic to evaluate whether patients respond well to a given treatment or whether it is necessary to switch to an alternative treatment early on. Furthermore, the introduction of 3D imaging capabilities by using a clinical 3D matrix array transducer can overcome the previous limitations of two-dimensional US—that is, only covering a small fraction of the tumor volume, thereby assessing only an arbitrary plane within the tumor volume, which results in sampling errors and substantial errors in the assessment of treatment response.
In our study, we compared three different molecular and functional US techniques in the same animals to evaluate the capabilities of these techniques in the assessment of antiangiogenic treatment response compared with quantitative immunofluorescence as the ex vivo reference standard. Our findings show that US parameters obtained with both 3D molecularly targeted US and destruction-replenishment DCE US correlated well with the respective ex vivo reference standards, while bolus DCE US did not. Molecularly targeted US is a quantitative technique to monitor molecular markers in vivo (12–14,19). The imaging signal linearly correlates with the number of molecularly attached microbubbles (37), which depends on the number of tumor vessels that express the angiogenic marker, such as VEGFR2, and the density of the marker expression per tumor vessel area. With the destruction-replenishment DCE US method, a constant infusion rate of microbubbles (38) is used, and the method is used to exploit the unique ability of an ultrasound beam to disrupt microbubbles, thus introducing a “negative bolus” into the anatomic area selected (38). The rate of replenishment of microbubbles into the field of view is then captured and modeled by using standard indicator dilution theory. Current models take advantage of the precise knowledge of the indicator input function this method provides, as well as the effect of the ultrasound beam characteristics and the fractal geometry of the vasculature on the resulting distribution of replenishment transit times (39). The destruction-replenishment method is therefore truly quantitative and can be used to extract several perfusion-related parameters, including rBV and rBF (31,40). The advantages of this method over the bolus DCE US method include obviating the need to estimate the indicator input function at the tumor (41). Measuring an input function for bolus DCE US is challenging because of the inability to visualize large vessels (such as the aorta) or a defined feeding vessel of the imaged tumor, owing to the limited field of view (24). Also, bolus DCE US–derived parameters depend on the cardiac output of the animal and the speed of contrast agent injection (24,42,43). These limitations may explain why the in vivo US parameters obtained with bolus DCE US did not correlate with the ex vivo reference standard in our study.
There is limited experience on 3D molecularly targeted US and 3D DCE US techniques tested in intra-animal comparison imaging experiments. In a patient-derived xenograft of pancreatic cancer, αvβ3 integrin–targeted 3D molecularly targeted US, and 3D replenishment-destruction DCE US (using the time it takes to reach 20% of the predestructive value as a perfusion parameter) were performed before and after administration of the orally administered aurora-A kinase inhibitor MLN8237 (44). To obtain 3D data sets, a linear-array transducer was used to scan along the elevational axis with interplane step sizes of 400 μm by using a linear motion stage. Both molecularly targeted US and DCE US showed treatment response earlier than anatomic volumetric imaging in that study, with molecularly targeted US indicating treatment-related changes in imaging signal even earlier than DCE US (44). However, differences in techniques, animal models, and tested drugs make comparison with our results challenging. To the best of our knowledge, our study is the only one so far to involve comparison of the three novel 3D molecular and functional US techniques in a cancer model treated with antiangiogenic therapy and comparison of the imaging findings with histologic end points. Additional studies are warranted to confirm our results in more animal models and in various types of anticancer treatments.
We acknowledge several limitations of our study. First, in our proof-of-principle intra-animal comparison study, we assessed antiangiogenic treatment effects only 24 hours after drug administration. A prospective study design with treatment monitoring over a longer time period and assessment of the value of molecular and functional imaging biomarkers for prediction of treatment response is warranted. Second, the magnitude of a measured treatment response depends on the dose of the therapeutic agent, with higher doses making treatment effects more measurable. While we did not evaluate the effects of varying antiangiogenic doses in our study, we used a body weight–adjusted dose of bevacizumab to be as close as possible to the clinical setting. Third, we only assessed effects of an antiangiogenic monotherapy, while in clinics, antiangiogenic agents are often combined with other chemotherapeutic agents. Future studies are needed to assess the value of 3D molecularly targeted US and DCE US for the assessment of treatment effects of various combination therapies with and without angiogenic agents. Since many conventional chemotherapeutic agents, as well as radiation therapy, have a strong effect on the tumor vasculature, we expect that imaging techniques used to quantify biomarkers of neovasculature will allow sensitive evaluation of treatment responses of those types of therapies, as well. Additional studies are warranted to confirm this hypothesis. Finally, we used a subcutaneous colon cancer model in our study, and further studies in which orthotopically implanted tumors in the liver are used to better represent the tumor microenvironment of metastases in the liver are needed.
In conclusion, our results suggest that both 3D molecularly targeted US and 3D DCE US by using the destruction-replenishment technique provide complementary molecular and functional in vivo information on antiangiogenic treatment effects in a human colon cancer xenograft model in mice. Between the two 3D DCE US imaging techniques, destruction-replenishment DCE US correlates substantially better with the ex vivo reference standard than does bolus DCE US.
Advances in Knowledge
■ Three-dimensional (3D) molecularly targeted US and dynamic contrast material–enhanced (DCE) US are feasible for assessment of antiangiogenic treatment effects in a mouse model of human colon cancer.
■ Vascular endothelial growth factor receptor 2 (VEGFR2)–targeted 3D molecularly targeted US signal correlates well with expression levels of VEGFR2 (r = 0.86, P = .001) as assessed with ex vivo quantitative immunofluorescence.
■ Relative blood volume (r = 0.71, P = .01) and relative blood flow (r = 0.78, P = .005) obtained with 3D destruction-replenishment DCE US correlate well with the percentage area of blood vessels as assessed with ex vivo quantitative immunofluorescence.
■ Blood flow velocity (r = 0.19, P = .57) obtained with the 3D destruction-replenishment DCE US technique, as well as peak enhancement (r = 0.39, P = .23), area under the time-intensity curve (r = −0.13, P = .71), and time to peak (r = −0.20, P = .55) obtained with the 3D bolus DCE US technique, did not correlate with ex vivo percentage area of blood vessels.
Implication for Patient Care
■ Three-dimensional molecularly targeted US and 3D DCE US may further expand the role of US in cancer imaging by providing accurate molecular and functional information on the effects of treatment on the tumor neovasculatures.
APPENDIX
Acknowledgments
Acknowledgments
We acknowledge Vijay Shamdasani, PhD (Philips Healthcare, Bothell, Wash), for providing the clinical US system and transducer and Thierry Bettinger, PhD (Bracco Suisse, Geneva, Switzerland), for providing the VEGFR2-targeted contrast agent BR55. We also acknowledge Philips Healthcare for providing a seed grant that funded our work in parts.
Received January 14, 2016; revision requested March 21; revision received April 30; accepted May 27; final version accepted June 3.
J.K.W. supported by the National Institute of Diabetes and Digestive and Kidney Diseases (R01 DK092509-01A1) and the National Cancer Institute (NIH R01CA195443-01A1, R01 CA155289-01A1).
See also Science to Practice in this issue.
Disclosures of Conflicts of Interest: H.W. disclosed no relevant relationships. A.M.L. disclosed no relevant relationships. D.H. disclosed no relevant relationships. L.T. disclosed no relevant relationships. J.K.W. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: author received payment from Bracco for consulting; author received a grant from Philips. Other relationships: disclosed no relevant relationships.
Abbreviations:
- AUC
- area under the time-intensity curve
- DCE
- dynamic contrast material–enhanced
- rBF
- relative blood flow
- rBV
- relative blood volume
- TTP
- time to peak
- VEGFR2
- vascular endothelial growth factor receptor 2
- 3D
- three-dimensional
References
- 1.American Cancer Society . Cancer facts & figures. Atlanta, Ga: American Cancer Society. 2015. [Google Scholar]
- 2.Bennouna J, Sastre J, Arnold D, et al. Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): a randomised phase 3 trial. Lancet Oncol 2013;14(1):29–37. [DOI] [PubMed] [Google Scholar]
- 3.Wilson SR, Burns PN. Microbubble-enhanced US in body imaging: what role? Radiology 2010;257(1):24–39. [DOI] [PubMed] [Google Scholar]
- 4.Feingold S, Gessner R, Guracar IM, Dayton PA. Quantitative volumetric perfusion mapping of the microvasculature using contrast ultrasound. Invest Radiol 2010;45(10):669–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Williams R, Hudson JM, Lloyd BA, et al. Dynamic microbubble contrast-enhanced US to measure tumor response to targeted therapy: a proposed clinical protocol with results from renal cell carcinoma patients receiving antiangiogenic therapy. Radiology 2011;260(2):581–590. [DOI] [PubMed] [Google Scholar]
- 6.Hoyt K, Sorace A, Saini R. Quantitative mapping of tumor vascularity using volumetric contrast-enhanced ultrasound. Invest Radiol 2012;47(3):167–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang H, Kaneko OF, Tian L, Hristov D, Willmann JK. Three-dimensional ultrasound molecular imaging of angiogenesis in colon cancer using a clinical matrix array ultrasound transducer. Invest Radiol 2015;50(5):322–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baetke SC, Rix A, Tranquart F, et al. Squamous cell carcinoma xenografts: use of VEGFR2-targeted microbubbles for combined functional and molecular US to monitor antiangiogenic therapy effects. Radiology 2016;278(2):430–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li QY, Tang J, He EH, et al. Clinical utility of three-dimensional contrast-enhanced ultrasound in the differentiation between noninvasive and invasive neoplasms of urinary bladder. Eur J Radiol 2012;81(11):2936–2942. [DOI] [PubMed] [Google Scholar]
- 10.Xu HX, Lu MD, Xie XH, et al. Treatment response evaluation with three-dimensional contrast-enhanced ultrasound for liver cancer after local therapies. Eur J Radiol 2010;76(1):81–88. [DOI] [PubMed] [Google Scholar]
- 11.Wang H, Hristov D, Qin J, Tian L, Willmann JK. Three-dimensional dynamic contrast-enhanced US imaging for early antiangiogenic treatment assessment in a mouse colon cancer model. Radiology 2015;277(2):424–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaneko OF, Willmann JK. Ultrasound for molecular imaging and therapy in cancer. Quant Imaging Med Surg 2012;2(2):87–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kiessling F, Huppert J, Palmowski M. Functional and molecular ultrasound imaging: concepts and contrast agents. Curr Med Chem 2009;16(5):627–642. [DOI] [PubMed] [Google Scholar]
- 14.Klibanov AL, Hossack JA. Ultrasound in radiology: from anatomic, functional, molecular imaging to drug delivery and image-guided therapy. Invest Radiol 2015;50(9):657–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fujii H, Matkar P, Liao C, et al. Optimization of ultrasound-mediated anti-angiogenic cancer gene therapy. Mol Ther Nucleic Acids 2013;2:e94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bzyl J, Lederle W, Rix A, et al. Molecular and functional ultrasound imaging in differently aggressive breast cancer xenografts using two novel ultrasound contrast agents (BR55 and BR38). Eur Radiol 2011;21(9):1988–1995. [DOI] [PubMed] [Google Scholar]
- 17.Wei S, Fu N, Sun Y, et al. Targeted contrast-enhanced ultrasound imaging of angiogenesis in an orthotopic mouse tumor model of renal carcinoma. Ultrasound Med Biol 2014;40(6):1250–1259. [DOI] [PubMed] [Google Scholar]
- 18.Pysz MA, Machtaler SB, Seeley ES, et al. Vascular endothelial growth factor receptor type 2–targeted contrast-enhanced US of pancreatic cancer neovasculature in a genetically engineered mouse model: potential for earlier detection. Radiology 2015;274(3):790–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abou-Elkacem L, Bachawal SV, Willmann JK. Ultrasound molecular imaging: moving toward clinical translation. Eur J Radiol 2015;84(9):1685–1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schlosser J, Kirmizibayrak C, Shamdasani V, Metz S, Hristov D. Automatic 3D ultrasound calibration for image guided therapy using intramodality image registration. Phys Med Biol 2013;58(21):7481–7496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pochon S, Tardy I, Bussat P, et al. BR55: a lipopeptide-based VEGFR2-targeted ultrasound contrast agent for molecular imaging of angiogenesis. Invest Radiol 2010;45(2):89–95. [DOI] [PubMed] [Google Scholar]
- 22.Pysz MA, Foygel K, Rosenberg J, Gambhir SS, Schneider M, Willmann JK. Antiangiogenic cancer therapy: monitoring with molecular US and a clinically translatable contrast agent (BR55). Radiology 2010;256(2):519–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greis C. Quantitative evaluation of microvascular blood flow by contrast-enhanced ultrasound (CEUS). Clin Hemorheol Microcirc 2011;49(1-4):137–149. [DOI] [PubMed] [Google Scholar]
- 24.Pysz MA, Foygel K, Panje CM, Needles A, Tian L, Willmann JK. Assessment and monitoring tumor vascularity with contrast-enhanced ultrasound maximum intensity persistence imaging. Invest Radiol 2011;46(3):187–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Willmann JK, Cheng Z, Davis C, et al. Targeted microbubbles for imaging tumor angiogenesis: assessment of whole-body biodistribution with dynamic micro-PET in mice. Radiology 2008;249(1):212–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Palmowski M, Morgenstern B, Hauff P, et al. Pharmacodynamics of streptavidin-coated cyanoacrylate microbubbles designed for molecular ultrasound imaging. Invest Radiol 2008;43(3):162–169. [DOI] [PubMed] [Google Scholar]
- 27.Heckel F, Schwier M, Peitgen HO. Object-oriented application development with MeVisLab and Python. Lecture Notes in Informatics (Informatik 2009: Im Focus das Leben) 2009;154:1338–1351. [Google Scholar]
- 28.Sorace AG, Saini R, Mahoney M, Hoyt K. Molecular ultrasound imaging using a targeted contrast agent for assessing early tumor response to antiangiogenic therapy. J Ultrasound Med 2012;31(10):1543–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Strouthos C, Lampaskis M, Sboros V, McNeilly A, Averkiou M. Indicator dilution models for the quantification of microvascular blood flow with bolus administration of ultrasound contrast agents. IEEE Trans Ultrason Ferroelectr Freq Control 2010;57(6):1296–1310. [DOI] [PubMed] [Google Scholar]
- 30.Quaia E. Assessment of tissue perfusion by contrast-enhanced ultrasound. Eur Radiol 2011;21(3):604–615. [DOI] [PubMed] [Google Scholar]
- 31.Wei K, Jayaweera AR, Firoozan S, Linka A, Skyba DM, Kaul S. Quantification of myocardial blood flow with ultrasound-induced destruction of microbubbles administered as a constant venous infusion. Circulation 1998;97(5):473–483. [DOI] [PubMed] [Google Scholar]
- 32.Wang JW, Zheng W, Chen Y, et al. Quantitative assessment of tumor blood flow changes in a murine breast cancer model after adriamycin chemotherapy using contrast-enhanced destruction-replenishment sonography. J Ultrasound Med 2013;32(4):683–690. [DOI] [PubMed] [Google Scholar]
- 33.Wilson SR, Jang HJ, Kim TK, Burns PN. Diagnosis of focal liver masses on ultrasonography: comparison of unenhanced and contrast-enhanced scans. J Ultrasound Med 2007;26(6):775–787; quiz 788–790. [DOI] [PubMed] [Google Scholar]
- 34.Zocco MA, Garcovich M, Lupascu A, et al. Early prediction of response to sorafenib in patients with advanced hepatocellular carcinoma: the role of dynamic contrast enhanced ultrasound. J Hepatol 2013;59(5):1014–1021. [DOI] [PubMed] [Google Scholar]
- 35.Lassau N, Bonastre J, Kind M, et al. Validation of dynamic contrast-enhanced ultrasound in predicting outcomes of antiangiogenic therapy for solid tumors: the French multicenter support for innovative and expensive techniques study. Invest Radiol 2014;49(12):794–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hudson JM, Williams R, Tremblay-Darveau C, et al. Dynamic contrast enhanced ultrasound for therapy monitoring. Eur J Radiol 2015;84(9):1650–1657. [DOI] [PubMed] [Google Scholar]
- 37.de Jong N, Hoff L. Ultrasound scattering properties of Albunex microspheres. Ultrasonics 1993;31(3):175–181. [DOI] [PubMed] [Google Scholar]
- 38.Dietrich CF, Averkiou MA, Correas JM, Lassau N, Leen E, Piscaglia F. An EFSUMB introduction into dynamic contrast-enhanced ultrasound (DCE-US) for quantification of tumour perfusion. Ultraschall Med 2012;33(4):344–351. [DOI] [PubMed] [Google Scholar]
- 39.Hudson JM, Williams R, Karshafian R, et al. Quantifying vascular heterogeneity using microbubble disruption-replenishment kinetics in patients with renal cell cancer. Invest Radiol 2014;49(2):116–123. [DOI] [PubMed] [Google Scholar]
- 40.Hudson JM, Karshafian R, Burns PN. Quantification of flow using ultrasound and microbubbles: a disruption replenishment model based on physical principles. Ultrasound Med Biol 2009;35(12):2007–2020. [DOI] [PubMed] [Google Scholar]
- 41.Hudson JM, Leung K, Burns PN. The lognormal perfusion model for disruption replenishment measurements of blood flow: in vivo validation. Ultrasound Med Biol 2011;37(10):1571–1578. [DOI] [PubMed] [Google Scholar]
- 42.Derchi LE, Martinoli C, Pretolesi F, Crespi G, Buccicardi D. Quantitative analysis of contrast enhancement. Eur Radiol 1999;9(Suppl 3):S372–S376. [DOI] [PubMed] [Google Scholar]
- 43.Palmowski M, Lederle W, Gaetjens J, et al. Comparison of conventional time-intensity curves vs. maximum intensity over time for post-processing of dynamic contrast-enhanced ultrasound. Eur J Radiol 2010;75(1):e149–e153. [DOI] [PubMed] [Google Scholar]
- 44.Streeter JE, Herrera-Loeza SG, Neel NF, Yeh JJ, Dayton PA. A comparative evaluation of ultrasound molecular imaging, perfusion imaging, and volume measurements in evaluating response to therapy in patient-derived xenografts. Technol Cancer Res Treat 2013;12(4):311–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.