Subcortical disconnection of cognitive neural networks is a key mechanism of cognitive impairment in patients with probable vascular cognitive disorder.
Abstract
Purpose
To investigate associations between neuroimaging markers of cerebrovascular disease, including lesion topography and extent and severity of strategic and global cerebral tissue injury, and cognition in carotid artery disease (CAD).
Materials and Methods
All participants gave written informed consent to undergo brain magnetic resonance imaging and the Addenbrooke’s Cognitive Examination–Revised. One hundred eight patients with symptomatic CAD but no dementia were included, and a score less than 82 represented cognitive impairment. Group comparison and interrelations between global cognitive and fluency performance, lesion topography, and ultrastructural damage were assessed with voxel-based statistics. Associations between cognition, medial temporal lobe atrophy (MTA), lesion volumes, and global white matter ultrastructural damage indexed as increased mean diffusivity were tested with regression analysis by controlling for age. Diagnostic accuracy of imaging markers selected from a multivariate prediction model was tested with receiver operating characteristic analysis.
Results
Cognitively impaired patients (n = 53 [49.1%], classified as having probable vascular cognitive disorder) were older than nonimpaired patients (P = .027) and had more frequent MTA (P < .001), more cortical infarctions (P = .016), and larger volumes of acute (P = .028) and chronic (P = .009) subcortical ischemic lesions. Lesion volumes did not correlate with global cognitive performance (lacunar infarctions, P = .060; acute lesions, P = .088; chronic subcortical ischemic lesions, P = .085). In contrast, cognitive performance correlated with presence of chronic ischemic lesions within the interhemispheric tracts and thalamic radiation (P < .05, false discovery rate corrected). Skeleton mean diffusivity showed the closest correlation with cognition (R2 = 0.311, P < .001) and promising diagnostic accuracy for vascular cognitive disorder (area under the curve, 0.82 [95% confidence interval: 0.75, 0.90]). Findings were confirmed in subjects with a low risk of preclinical Alzheimer disease indexed by the absence of MTA (n = 85).
Conclusion
Subcortical white matter ischemic lesion locations and severity of ultrastructural tract damage contribute to cognitive impairment in symptomatic CAD, which suggests that subcortical disconnection within large-scale cognitive neural networks is a key mechanism of vascular cognitive disorder.
Introduction
The prevalence of vascular cognitive impairment and vascular dementia, which can be summarized as vascular cognitive disorder (1), is increasing in the elderly (2). Diagnosing vascular cognitive disorder remains challenging, and up to 44% of patients have coincident vascular and Alzheimer disease pathologic processes (3). New diagnostic criteria have been proposed to classify the disease as being probable or possible vascular cognitive disorder (4). To date, no useful imaging biomarkers have been identified for vascular cognitive disorder, and exclusion of amyloid pathologic abnormalities by using positron emission tomographic (PET) imaging (5) or cerebrospinal fluid biomarkers (6) cannot be used in clinical practice, as these techniques are prohibitively expensive and moderately invasive. There is a need for imaging biomarkers that can be used to predict and track vascular cognitive disorder. Moreover, neuroimaging markers may afford mechanistic insights into the key processes that underlie vascular cognitive disorder.
Several imaging studies yielded only a modest association between visible ischemic lesion burden and cognitive impairment, which is thought to be an underestimation of the effect of ischemia on cognition, as measurement of lesion burden fails to take ultrastructural tissue damage into account (7,8). Although the initial ischemic damage is focal, there is recognition of structural and functional alteration of distant brain regions due to the remote effects of lesions mainly reflecting Wallerian and transsynaptic degeneration (9).
White matter ultrastructural damage can be quantified with diffusion-tensor imaging (DTI), which allows the study of interrelations between the full extent of vascular tissue damage beyond visible lesions and cognitive impairment. Two DTI metrics, fractional anisotropy (FA) and mean diffusivity, were shown to be correlated with myelin content and axonal loss (10). Furthermore, FA and mean diffusivity maps can be subjected to whole-brain voxel-based or tract-based analysis to overcome the recognized limitations of region of interest analysis (11).
Patients with symptomatic carotid artery disease (CAD) have an increased risk of developing vascular cognitive disorder (12) as a result of several mechanisms, including direct risks of emboli and hypoperfusion via shared risk factors, brain tissue injury resulting from small-vessel disease, and arterial stiffness (4), in addition to being prone to age-related neurodegeneration. Elderly patients with symptomatic CAD are thus a particularly interesting group in which to assess the value of neuroimaging markers because of their heightened risk for multiple mechanisms.
We aimed to investigate the role of vascular tissue damage for global cognition and fluency, as this domain is believed to be impaired early in vascular cognitive disorder (13). We hypothesized that strategic lesion location and tissue damage severity are better indexes of vascular cognitive disorder than is lesion volume. To explore the role of putative commixed pathologic processes in our elderly cohort, we assessed whether imaging and cognitive associations were similar in the subgroup of patients at low risk for preclinical Alzheimer disease—namely, those without medial temporal lobe atrophy (MTA). Second, we examined whether presence of MTA affected the vascular lesion load and nonlesional tissue damage and used a multivariate prediction model of cognitive disorder to assess the independent contributions of vascular damage and MTA.
Materials and Methods
Subjects
The study protocol was approved by the ethics committee, and all participants gave written informed consent to undergo brain magnetic resonance (MR) imaging and the Addenbrooke’s Cognitive Examination–Revised (ACE-R). Participants were recruited from a CAD imaging study that will be reported elsewhere. From 2010 to 2015, subjects were recruited for the CAD imaging study (by A.A.H. and R.J.S.) if they experienced a recent nondisabling cerebrovascular event (stroke, transient ischemic attack, or amaurosis fugax) and were found to have ipsilateral carotid arterial stenosis of more than 30%. Exclusion criteria were contraindications to MR imaging, language or sensory difficulties that would interfere with cognitive assessment, and pre-existing cognitive impairment with possible causes other than vascular events. Research investigators (A.A.H. and R.J.S.) reviewed the clinical notes, spoke to all participants, and excluded (a) patients without the capacity to consent and (b) those with clinically apparent or documented dementia before enrollment.
Cognitive Assessment and Criteria of Possible and Probable Vascular Cognitive Disorder
One hundred forty-seven CAD imaging study participants agreed to undergo cognitive assessments by using the ACE-R (14), which was conducted by trained researchers (A.A.H., R.J.S.) on the day of MR imaging. Participants were categorized as being cognitively intact or impaired on the basis of a cutoff value of 82, according to proven good sensitivity and specificity for defining cognitive impairment (14). Normal daily functioning could be normal or mildly impaired, independent of the presence of motor and/or sensory symptoms.
In the cognitively impaired subgroup, we further excluded subjects in whom a nonvascular cause of cognitive disorder was considered clinically. According to published diagnostic criteria (4), the following criteria for probable vascular cognitive disorder had to be met: (a) cognitive impairment (defined in our study as ACE-R score < 82), (b) imaging evidence of cerebrovascular disease (defined in our study as a chronic or acute ischemic lesion at fluid-attenuated inversion-recovery [FLAIR] imaging or diffusion-weighted imaging), (c) clear temporal relationship between cerebrovascular disease and cognitive impairment, (d) no evidence of another neurodegenerative disorder that may affect cognition before the onset of cerebrovascular disease, and (e) no history of gradually progressive cognitive impairment before or after manifestation of the cerebrovascular disease. Criteria b–e were assessed clinically (by A.A.H., with 9 years of clinical experience).
Image Acquisition
All MR images were acquired with a 3.0-T imaging unit (Achieva; Philips Medical Systems, Best, the Netherlands). The imaging protocol included axial DTI (spin-echo single-shot diffusion-weighted echo-planar imaging with a b value [gradient factor] of 1000 sec/mm2, 15 directions, one volume with a b value of 0 sec/mm2 acquired, repetition time [sec]/echo time [msec] of 4.2/57, field of view of 224 × 224, matrix of 112 × 112, and 45 sections of 3-mm thickness acquired), axial FLAIR imaging (repetition time [sec]/echo time [sec] of 10.5/0.14; inversion time, 2.75 seconds; field of view, 224 × 192; matrix, 224 × 181; and 45 sections of 3-mm thickness acquired), and a plaque hemorrhage–sensitive, blood-nulled, water-selective T1-weighted three-dimensional gradient-echo sequence (repetition time msec/echo time msec, 8.8/4.11; inversion time, 570 msec; field of view, 92 × 162; matrix, 384 × 384; and 102 sections of 0.9-mm thickness acquired, covering the hippocampal formation fully in 50 cases).
Preprocessing of DTI data sets and computation of FA and mean diffusivity maps were performed by using the diffusion toolbox of FSL (Oxford Centre for Functional Magnetic Resonance Imaging of the Brain [FMRIB] Software Library, Oxford, England) (15). Isotropic maximum diffusion images with a b value of 1000 sec/mm2 extracted from DTI images and mean diffusivity maps were used to outline acute ischemic lesions.
MTA Assessment
MTA was rated per subject on reconstructed coronal FLAIR images by using the Scheltens scale (16) (D.M., with 6 years of neuroimaging training; and Q.S., a neurology trainee with a master of science in translational neuroimaging; supervised by D.P.A., with more than 25 years of quantitative neuroimaging research experience). Visual rating for all subjects was performed twice to study intrarater reliability. To validate the reliability of using reconstructed FLAIR images to assess MTA, the Scheltens scale was also applied to all subjects who had coronal T1-weighted images sufficiently covering the hippocampal formation (n = 50, D.M.). Assessments were performed while blinded to the patients’ age and clinical information. Presence of MTA was defined by using the following age range–specific cutoffs for the mean MTA scores (the averaged value of both hemispheres): MTA of more than 1.5 in patients younger than 75 years of age, MTA of more than 2 in patients at least 75 years of age, and MTA of more than 2.5 in patients at least 85 years of age (17). To validate the reliability of MTA assessment, intermodality agreement (n = 50), interrater agreement (n = 108), and intrarater agreement (n = 108) were analyzed. Patients were further classified into two subgroups according to their MTA score: those with MTA and those without. Patients without MTA who have clinically probable vascular cognitive disorder can be considered to have a low risk of having comorbid Alzheimer disease (18).
Lesion Mapping
FLAIR and diffusion images were registered into Montreal Neurological Institute (MNI) template space by using FSL tools (19). Lacunar infarctions, acute ischemic infarctions, chronic subcortical ischemic lesions, and cortical infarctions were separately and manually outlined (by D.M., a trained investigator) on coregistered images by using locally developed NeuRoi software (http://www.nottingham.ac.uk/research/groups/clinicalneurology/neuroi.aspx). Lacunar infarctions were defined as small (<20-mm) hypointense subcortical areas on FLAIR images. Hyperintense lesions on images acquired with a b value of 1000 sec/mm2 that were hypointense on mean diffusivity maps were considered acute ischemic infarctions. Subcortical FLAIR hyperintense lesions were considered to reflect chronic subcortical ischemic lesions, and cortical FLAIR hypointensities were considered to reflect chronic cortical infarctions. Then, three kinds of lesion maps were generated: (a) an “infarction frequency map” included acute ischemic infarctions and chronic cortical and lacunar infarctions, (b) a “chronic subcortical ischemia frequency map” was generated by adding chronic subcortical ischemic lesions, and (c) a “total ischemic lesion frequency map” was created by combining both maps.
Lesion Burden
Lesion type–specific volumes were calculated by using NeuRoi software. FLAIR brain images without skull, extracted by using the “Brain Extraction Tool” of the Oxford Centre for Functional Magnetic Resonance Imaging of the Brain (20), were loaded into NeuRoi for calculating total brain volumes. Lesion volumes were divided by total brain volumes for normalization. The total ischemic lesion load was calculated by adding the normalized volumes of nonoverlapping acute and chronic lesions and was logarithmically expressed to account for nonnormal distribution.
Lesion Topography, White Matter Tract Topography, and Tissue Damage Severity
Since results of a previous study demonstrated the strategic role of lacunar infarctions located at the hippocampus, thalamus, mesial temporal areas, basal forebrain, and frontocingular cortex in vascular dementia (21), the frequency of infarctions at these locations was recorded.
Voxel-based lesion-symptom mapping (VLSM) (http://www.neuroling.arizona.edu/resources.html) was used to determine lesion locations associated with cognition and fluency (22). All analyses were controlled for age and total ischemic lesion load (logarithmically expressed). Additionally, performance of memory, language, attention, and visuospatial ability derived from ACE-R was controlled for when assessing the association between lesion locations and fluency. Significant voxels were projected onto the 1 × 1 × 1-mm MNI template, and major white matter tracts (WMTs) that contained significant clusters were identified from the WMT atlas within FSL (23).
Whole white matter ultrastructural damage and its association with global cognition and fluency were investigated by means of tract-based spatial statistics (TBSS) on FA and mean diffusivity maps. The standard TBSS procedure was applied to this study (24).
Mean diffusivity values were derived from each individual’s maps of chronic subcortical ischemic lesions, normal-appearing white matter (NAWM), and WMT skeleton to investigate tissue-specific injury. Infarction severity was not studied owing to the mixed type of infarctions, which may confound the assessment of severity of tissue damage.
To confirm whether the imaging-cognitive associations were independent of potentially comorbid but preclinical Alzheimer disease pathologic processes, as indexed by the presence of hippocampal atrophy, we repeated all imaging analysis in the subgroup of patients without MTA.
Statistical Analysis
The spatial distribution of two kinds of lesion maps was compared on a voxel-by-voxel basis by using a Fisher test (with the false discovery rate controlled at 0.05) as implemented in the NeuRoi software package (http://www.nottingham.ac.uk/research/groups/clinicalneurology/neuroi.aspx).
Reliability and agreement analysis.—The inter- and intrarater reliability and the agreement of MTA measurement on reconstructed FLAIR images with that on coronal three-dimensional T1-weighted gradient-echo images were assessed by using the Cohen κ coefficient.
VLSM analysis.—Nonparametric mapping was used to relate lesion location and cognitive performance (t test). Voxels affected in fewer than four subjects (the default setting in VLSM) were not considered for analysis. False discovery rate was used to correct for multiple comparisons.
TBSS analysis.—The voxelwise statistical analysis of the DTI data was based on a nonparametric method by using a permutation test (n = 5000) with a general linear-model design matrix (25).
Tissue damage severity.—The age-independent interrelations between severity of tissue damage and cognition were assessed by using SPSS version 21 software (SPSS, Chicago, Ill). The correlation coefficients were compared by using VassarStats software (http://www.vassarstats.net/index.html).
Prediction models.—Univariate regression analysis was first performed to initially explore the predictive value of age and various imaging markers, including normalized volumes of lacunar infarctions, acute and chronic ischemic lesions, mean diffusivity values of infarctions, chronic subcortical ischemic lesions, NAWM and WMT skeleton, and MTA for cognitive status. Given the number of cognitively impaired subjects (n = 53), five factors were selected for creating the multivariate prediction model.
The area under the receiver operating characteristic curve for the diagnosis of probable vascular cognitive disorder was constructed by using SPSS software for the imaging metrics selected from the prediction model.
A P value less than .05 was considered to indicate a significant difference for all analyses.
Results
Of the 147 participants, 38 had to be excluded owing to incomplete MR examination or poor quality of MR images as assessed visually, and one subject was excluded because of comorbid Parkinson disease that likely contributed to cognitive impairment. This resulted in a cohort of 108 subjects. Fifty-three patients (49.1%) were cognitively impaired and were classified as having probable vascular cognitive disorder. Forty-eight of these had experienced first-ever stroke without any known cognitive impairment or other relevant background. Four subjects had a previous history of poststroke cognitive impairment with subsequent partial improvement, and one subject later developed cerebral hemorrhage, which was considered to be amyloid angiopathy. Patients with abnormal cognition were older (P = .027), had a higher percentage of smokers (P = .015), and had more peripheral vascular disease (P = .017) than those with normal cognition (n = 55, 50.9%). Of the 108 subjects, 98 subjects (90.7%) were not at risk for cerebral hemodynamic impairment due to carotid stenosis of less than 80% on either side or both sides (Table 1). Among 10 subjects who had carotid stenosis of more than 80% on either side, three subjects had bilaterial carotid stenosis of more than 80%.
Table 1.
Demographics, MR Imaging Characteristics, and Cognitive Profile of the Study Cohort and Comparison between Cognitive Subgroups
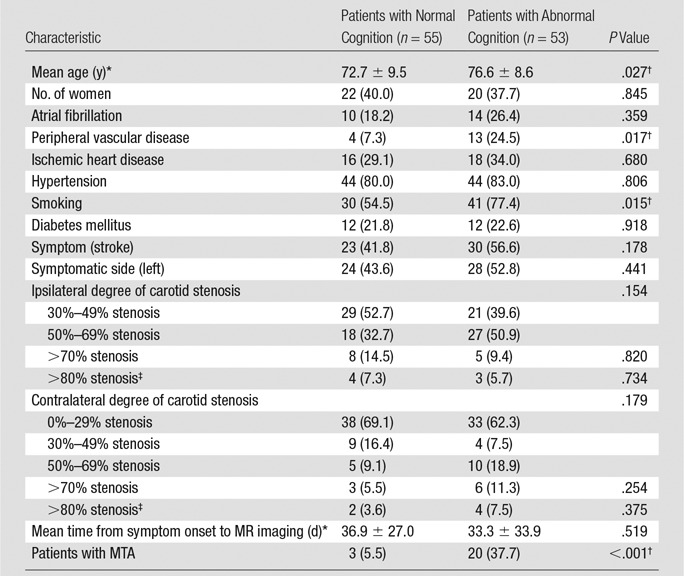
Note.—Numbers in parentheses are percentages.
*Data are means ± standard deviations.
†Statistically significant (P < .05).
‡Threshold for possible hemodynamic impairment > 80%—that is, subgroup of >70%. P value refers to >80% vs rest.
MTA scores showed high inter- and intrarater reliability and similarly good agreement between assessment on FLAIR images and coronal T1-weighted images (Appendix E1 [online]). Twenty-three subjects (21.3%) had MTA, while 85 subjects (78.7%) did not. Most subjects with MTA were cognitively impaired (n = 20, 87.0%), compared with 33 (38.8%) of those without MTA (P < .001).
Neuroimaging Differences between Cognitively Impaired Patients and Unimpaired Patients
Cognitively impaired patients had larger normalized acute lesion volumes (mean ± standard deviation, [5.9 ± 11.3] × 10−4 for cognitively impaired patients vs [2.7 ± 5.1] × 10−4 for cognitively intact patients; P = .028), higher chronic subcortical ischemic volumes ([15.5 ± 15.5] × 10−3 for cognitively impaired patients vs [8.0 ± 10.5] × 10−3 for cognitively intact patients, P = .009), and more cortical infarctions (n = 4 [3.7%] cognitively impaired patients; n = 0 [0%] cognitively intact patients; P = .016) after controlling for age (Table 2). Similar group differences were found in the subgroup without MTA (Table E1 [online]).
Table 2.
Lesion Volumes, MTA, and Cognition
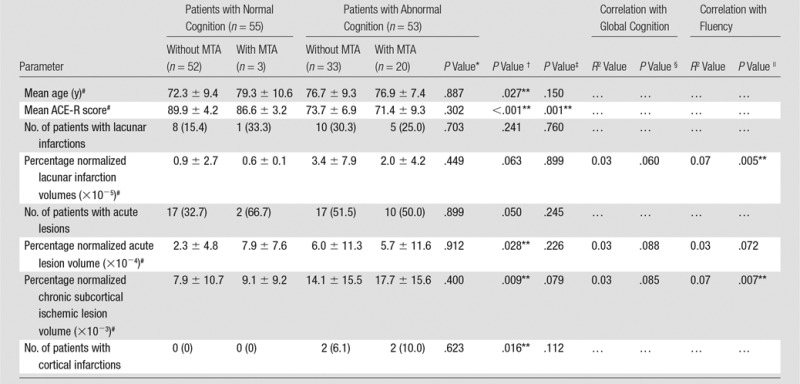
Note.—Numbers in parentheses are percentages.
*P value for the comparison between cognitively impaired patients with MTA and those without MTA, controlled for age.
†P value for the comparison between patients with normal cognition and those with abnormal cognition, controlled for age.
‡P value for the comparison between patients with MTA and patients without MTA, controlled for age.
§P value for the correlation between lesion volumes and global cognitive performance, controlled for age.
||P value for the correlation between lesion volumes and fluency, controlled for age.
#Data are means ± standard deviations.
**Statistically significant (P < .05).
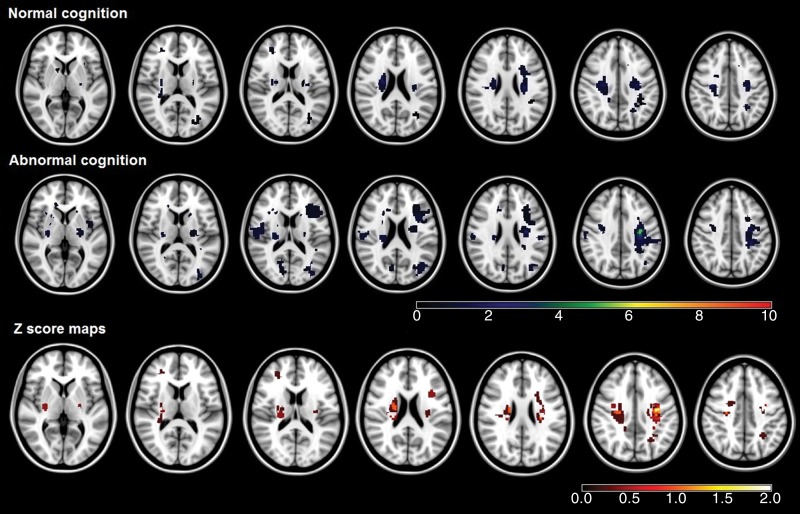
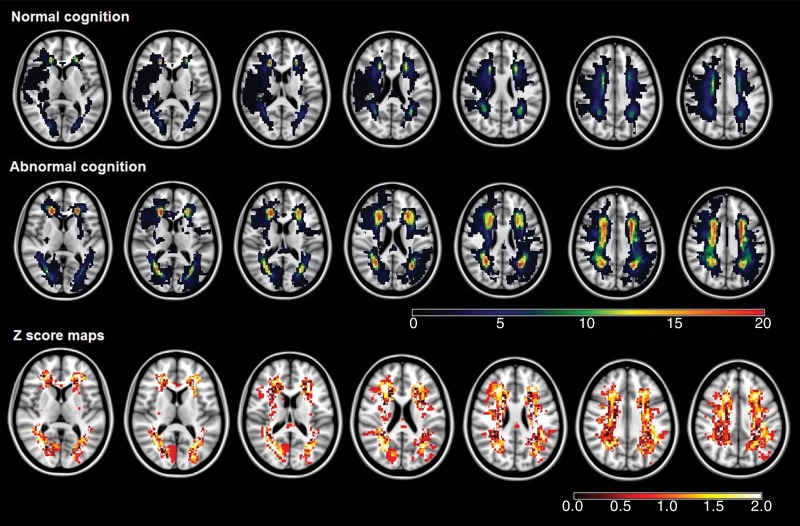
Three strategic thalamic lacunar infarctions were seen in cognitively impaired patients (5.6%), but none were seen in cognitively intact subjects. Cognitively impaired subjects (n = 53, 49.1%) also showed qualitatively more extended patterns of the infarctions (Fig 1a) and chronic subcortical ischemia frequencies (Fig 1b), but the difference was not significant by using voxel-based analysis (false discovery rate corrected, P > .05). Additionally, according to visual inspection, no infarctions or chronic subcortical ischemic lesions were located in the known language brain areas, such as superior and middle temporal gyri, inferior frontal gyri, and insula, which may affect language performance (26).
Figure 1a:
(a) Infarction maps in patients with normal cognition and those with abnormal cognition. Maps were superimposed onto the 1 × 1 × 1-mm MNI152 standard-space T1-weighted average structural template. The color bars show the variation between the minimum and maximum number of lesions. Z score maps represent the result of group comparison between patients with normal cognition and those with abnormal cognition. The significant voxels (according to results of the Fisher test) did not survive the false discovery rate correction. The color bar of the Z score maps shows the variation in Z score between the minimum and maximum values. (b) Chronic subcortical ischemic lesion frequency maps in patients with normal cognition and those with abnormal cognition. Details are the same as for a.
Figure 1b:
(a) Infarction maps in patients with normal cognition and those with abnormal cognition. Maps were superimposed onto the 1 × 1 × 1-mm MNI152 standard-space T1-weighted average structural template. The color bars show the variation between the minimum and maximum number of lesions. Z score maps represent the result of group comparison between patients with normal cognition and those with abnormal cognition. The significant voxels (according to results of the Fisher test) did not survive the false discovery rate correction. The color bar of the Z score maps shows the variation in Z score between the minimum and maximum values. (b) Chronic subcortical ischemic lesion frequency maps in patients with normal cognition and those with abnormal cognition. Details are the same as for a.
Voxelwise group comparisons of mean diffusivity and/or FA maps demonstrated widespread mean diffusivity increases and FA decreases between patients with abnormal cognition and those with normal cognition (Fig E1 [online]).
In cognitively impaired patients versus normal subjects, mean diffusivity (Table 3) was increased in chronic subcortical ischemic lesions ([1.05 ± 0.09] × 10−9 m2/sec in cognitively impaired patients vs [0.99 ± 0.11] × 10−9 m2/sec in normal patients [P = .007]; partial η2 = 0.068 [95% confidence interval {CI}: 0.016, 0.095), NAWM ([0.78 ± 0.04] × 10−9 m2/sec for cognitively impaired patients and [0.75 ± 0.03] × 10−9 m2/sec for normal patients [P = .002]; η2 = 0.086 [95% CI: 0.008, 0.037]), and WMT skeleton ([0.83 ± 0.05] × 10−9 m2/sec for cognitively impaired patients vs [0.77 ± 0.03] × 10−9 m2/sec for normal patients [P < .001]; η2 = 0.271 [95% CI: 0.035, 0.067]).
Table 3.
Severity of Tissue Damage and Cognitive Performance
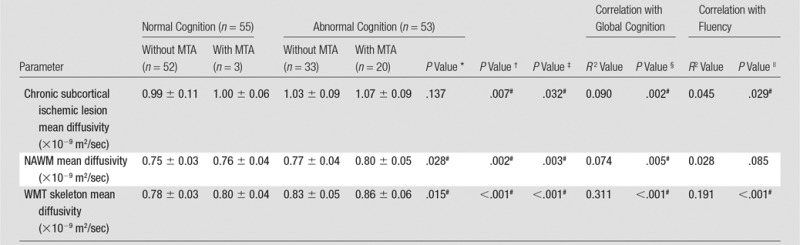
Note.—Data are means ± standard deviations, unless indicated otherwise.
*P value for the comparison between cognitively impaired patients with MTA and those without MTA, controlled for age.
†P value for the comparison between patients with normal cognition and those with abnormal cognition, controlled for age.
‡P value for the comparison between patients with MTA and patients without MTA, controlled for age.
§P value for the age-independent correlation between mean diffusivity of chronic subcortical ischemic lesions, NAWM, WMT skeleton, and global cognitive performance.
||P value for the age-independent correlation between mean diffusivity of chronic subcortical ischemic lesions, NAWM, WMT skeleton, and global cognitive performance in subjects without MTA.
#Statistically significant (P < .05).
In patients without MTA (n = 85), both NAWM and WMT skeleton mean diffusivity were also significantly higher in cognitively impaired patients versus normal patients (NAWM, P = .047 [η2 = 0.047; 95% CI: 0.001, 0.028]; WMT skeleton, P < .001 [η2 = 0.230; 95% CI: 0.024, 0.057]) (Table E2 [online]).
Neuroimaging Findings and Cognitive Impairment: Correlation Analyses
None of the normalized lesion type volumes were significantly correlated with global cognition (lacunar infarctions, P = .060; acute lesions, P = .088; chronic subcortical ischemic lesions, P = .085). In contrast, normalized lacunar infarction volumes (R2 = 0.07, P = .005) and chronic subcortical ischemic lesion volumes (R2 = 0.07, P = .007) were negatively correlated with fluency performance (Table 2). Interestingly, in patients without MTA, normalized lacunar infarction volumes were correlated with both global cognition (R2 = 0.06, P = .030) and fluency (R2 = 0.08, P = .007), while normalized acute lesion volumes were correlated with global cognition (R2 = 0.06, P = .020) but not fluency (Table E1 [online]).
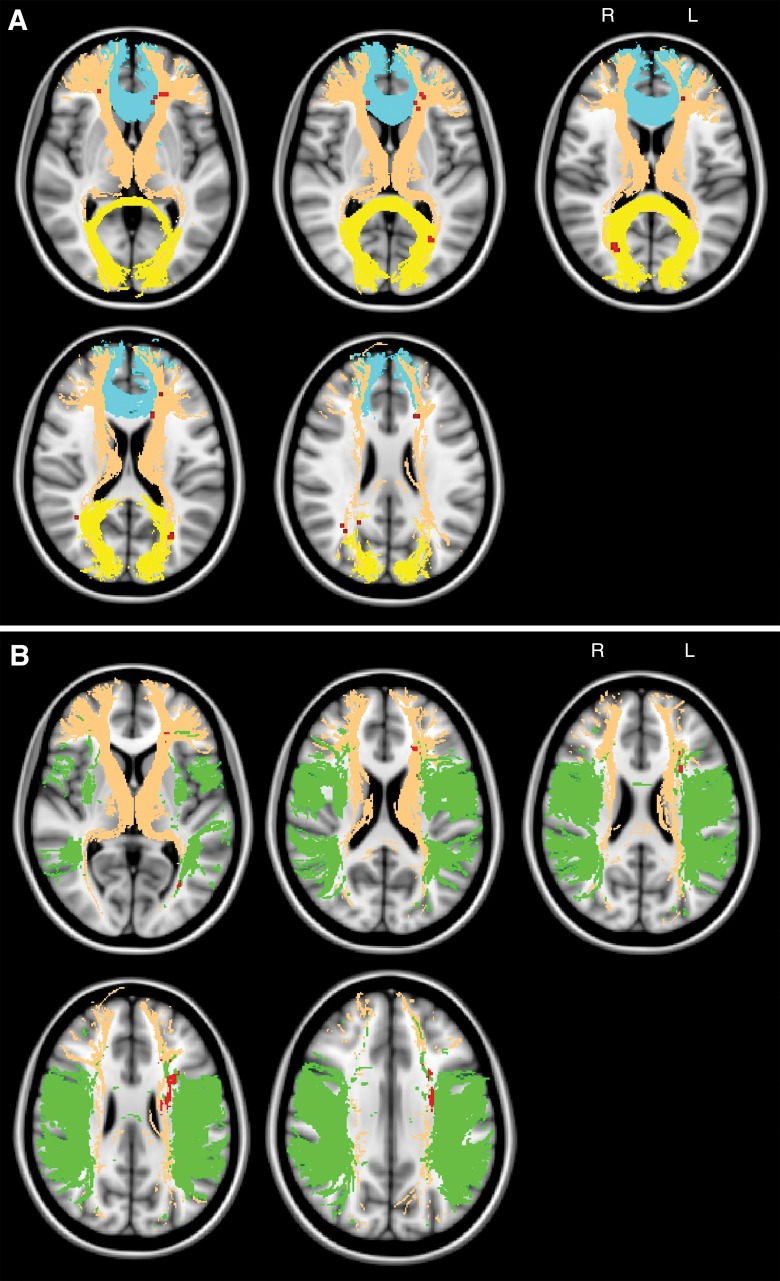
Significant correlations were identified between clusters of chronic subcortical ischemia presence and global cognition and fluency (false discovery rate, q = 0.05; threshold, t = 2.4) by using VLSM. A total of 159 and 1351 voxels were identified in which presence of chronic subcortical ischemia was significantly related to impaired global cognition and fluency, respectively. Clusters for global cognition were mainly projected onto the anterior thalamic radiation, forceps minor, and forceps major (Table 4; Fig 2, A). Clusters for fluency were mainly projected onto the anterior thalamic radiation and superior longitudinal fasciculus (Table 4; Fig 2, B). No significant clusters were identified for the infarction frequency maps and global cognition or fluency. Similar results were identified in patients without MTA (n = 85, Fig E2 [online]).
Table 4.
MNI Coordinates for the Local Maxima of Clusters That Correlate with Cognition
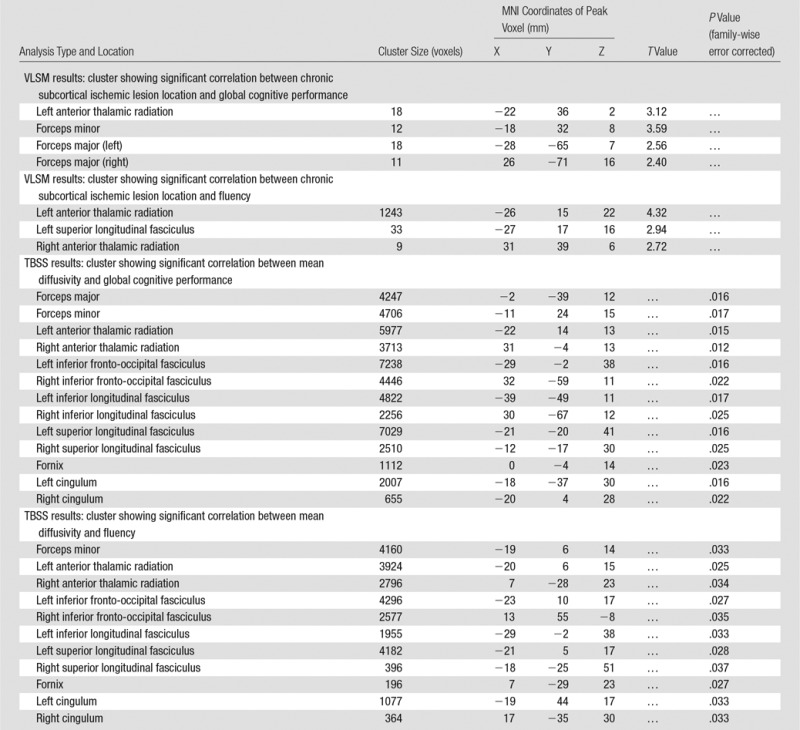
Figure 2:
A, Maps show the topography of chronic ischemic lesions associated with global cognitive impairment. Significant clusters (red) according to VLSM analysis (P < .05, false discovery rate corrected, controlled for age and normalized total ischemic lesion volumes after logarithmic transformation) are projected onto the MNI152 standard-space T1-weighted average structural template image with overlain selected WMTs from the Johns Hopkins University DTI-based white matter atlas (yellow, forceps major; brown, anterior thalamic radiation; blue, forceps minor). B, Maps show the topography of chronic ischemic lesions associated with impaired fluency. Details are the same as for A (green, superior longitudinal fasciculus). L = left, R = right.
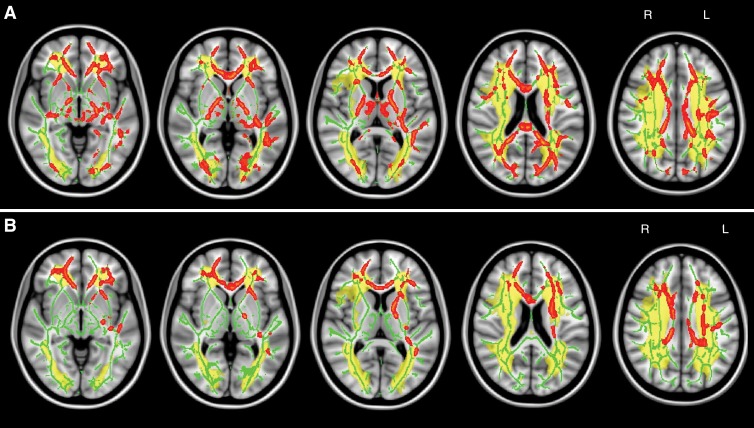
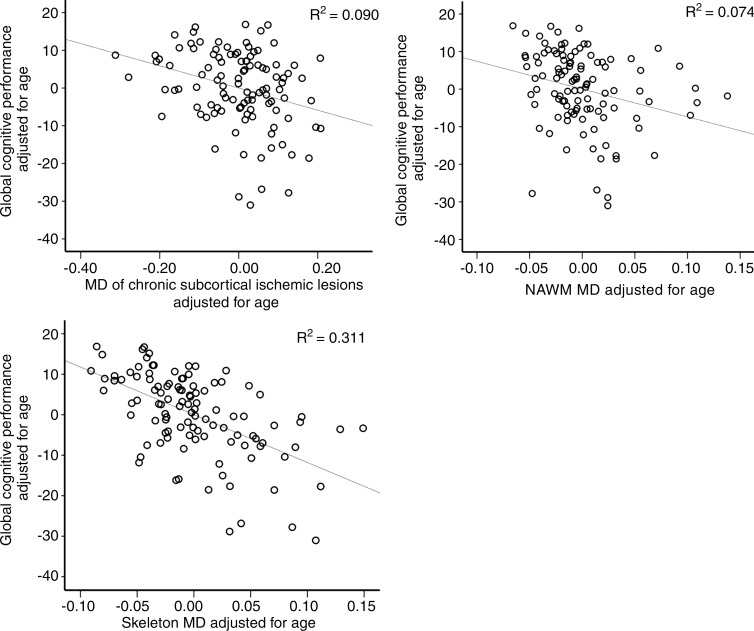
Age-independent correlation between WMT mean diffusivity and global cognition was widespread on the basis of TBSS (Table 4; Fig 3, A). A similar correlation pattern was identified between fluency and mean diffusivity of widespread white matter except forceps major and right inferior longitudinal fasciculus (Table 4; Fig 3, B). The analyses of correlation between FA and global cognition yielded similar results (Fig E3 [online]). No significant correlation was identified between FA and fluency. The pattern of widespread WMT damage associated with impaired cognition in the subgroup of patients without MTA was similar to that in the whole study cohort (Fig E4 [online]). We found a consistent, indirect interrelation between tissue-specific mean diffusivity and cognitive performance after controlling for age (chronic subcortical ischemic lesions, R2 = 0.090 [P = .002]; NAWM, R2 = 0.074 [P = .005]; WMT skeleton, R2 = 0.311 [P < .001]) (Table 3, Fig 4). Similar interrelations were also identified in patients without MTA (n = 85) (Table E2 [online]). Also, the partial correlation between global cognition and WMT skeleton mean diffusivity was significantly stronger than that between global cognition and chronic subcortical ischemic lesion mean diffusivity (P = .018) or NAWM mean diffusivity (P = .004).
Figure 3:
A, Maps show WMT mean diffusivity and global cognition. Significant TBSS results show skeletal voxels (red) where increased mean diffusivity correlated with global cognitive decline (controlled for age and normalized total ischemic lesion volumes after logarithmic transformation, P < .05, corrected). Mean TBSS tract skeleton (green) and total ischemic lesion distribution map (yellow) were overlaid on the MNI152 standard-space T1-weighted average structural template image. B, Maps show WMT mean diffusivity and fluency. Details are the same as for A. L = left, R = right.
Figure 4:
Scatterplots show the correlation between mean diffusivity (MD) within chronic subcortical ischemic lesions, NAWM, WMT skeleton, and global cognitive performance, corrected for age.
Predictive Value and Diagnostic Accuracy of Imaging Measures
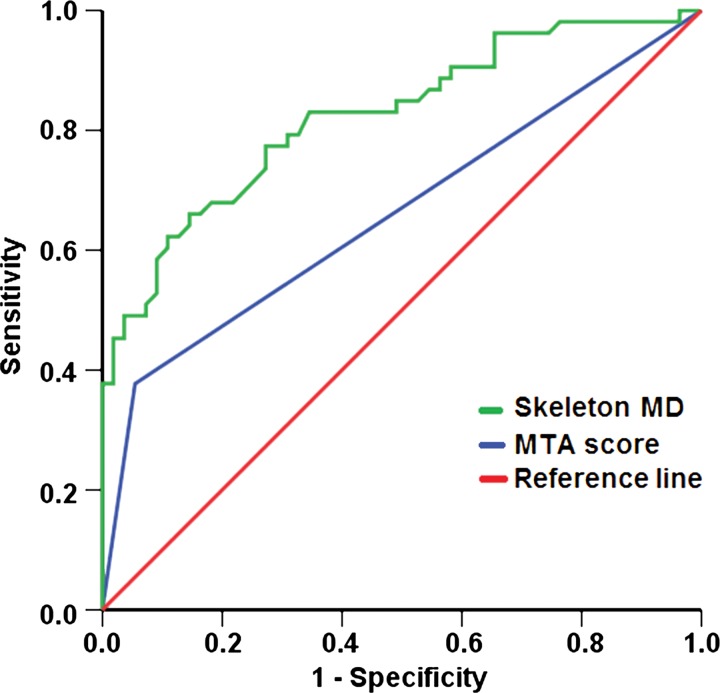
On the basis of univariate regression analyses, normalized volume of chronic subcortical ischemic lesions (P = .007), mean diffusivity of chronic subcortical ischemic lesions (P = .004), NAWM (P = .001), WMT skeleton (P < .001), and MTA (P < .001) were found to be predictors of cognitive status. After entering these factors into the multivariate prediction model, WMT skeleton mean diffusivity (P = .001, exp[β] = 38.880 [95% CI: 4.207, 359.336]) and MTA score (P = .015, exp[β] = 6.074 [95% CI: 1.426, 25.867]) remained as the only independent significant factors (Table E3 [online]).
The receiver operating characteristic curves of WMT skeleton mean diffusivity and MTA score for the diagnosis of cognitive deficits were constructed separately (Fig 5), with an area under the receiver operating characteristic curve of 0.82 (95% CI: 0.75, 0.90) for WMT skeleton mean diffusivity and an area under the curve of 0.66 (95% CI: 0.56, 0.77) for MTA score. At a mean diffusivity cutoff value of 0.797 × 10−9 m2/sec, sensitivity and specificity were 77.4% and 72.7%, respectively. At a cutoff value of 0.75 for mean MTA score, sensitivity was 72.2%, and specificity was 55.5%.
Figure 5:
Receiver operating characteristic curves with a diagonal reference line (red) for prediction of global cognitive performance according to WMT skeleton mean diffusivity (MD, green) and MTA score (blue).
Discussion
In this multimodal MR imaging study in 108 patients with advanced arteriosclerosis and a recent cerebrovascular event, we found that the location of chronic subcortical ischemic lesions in the anterior thalamic radiation and interhemispheric fiber tracts and the severity of WMT damage, indexed according to mean diffusivity increase, were associated with cognitive impairment. These results suggest that subcortical disconnection of large-scale cognitive neural networks that result from strategic and cumulative lesional and nonlesional tract damage play a key role in cognitive impairment in advanced arteriosclerosis.
Our findings confirm and extend the strategic role of anterior thalamic radiation and interhemispheric fiber damages as a mechanism of cognitive impairment, as previously shown in small-vessel disease (27) and in manifest vascular disease due to arteriosclerosis anywhere (28). The identification of anterior thalamic radiation and superior longitudinal fasciculus as strategic WMTs for fluency in our study also closely replicates results in manifest vascular disease (28). The consistency of findings across different patient cohorts highlights that lesion location rather than disease origin determines cognitive outcomes. This in turn is concordant with our current understanding that disconnection of large-scale cognitive networks through the anterior thalamic radiation and the forceps minor underlies vascular cognitive disorder in both small- and large-artery disease. In contrast to previous studies in vascular disease, we found a key role of the forceps major in global cognition (27,28). This partial discrepancy could be due to the differences in the underlying vasculopathy but is more likely due to variation in cognitive assessments. In previous studies, investigators focused on processing speed or executive function, which mainly reflect frontal lobe dysfunctions (27,28). Forceps major integrity, on the other hand, has been related to memory, attention, and executive functions (29). Interestingly, the global cognitive role of the forceps major in nonvascular brain diseases, such as multiple sclerosis (30,31), suggests that consistent structural-cognitive interrelations exist in disconnection disorders across different diseases.
The observed widespread age-independent associations between ultrastructural white matter damage and global cognition, as well as fluency, agree well with the results of previous studies in patients with subcortical vascular dementia (32), cerebral autosomal-dominant arteriopathy with subcortical infarcts and leukoencephalopathy (33), and mild cognitive impairment (34). Diffusional changes are thought to reflect primary or secondary tissue damage, including Wallerian degeneration with myelin damage, axonal loss, gliosis, and incomplete ischemia (35,36). We thus consider that the observed WMT ultrastructural damage reflects cumulative focal and remote effects of vascular tissue damage, thereby providing a more reliable index of cerebrovascular lesion burden than lesions visible on MR images do. This is corroborated by the finding that lesion volumes played no role or only a minor role in explaining global cognitive and fluency performance, which is well in line with previous reports (7,36). Taken together, topographic, volumetric, and diffusivity findings converge and point to a key role of subcortical disconnection within large-scale cognitive neural networks that may underpin vascular cognitive disorder.
It is noteworthy that cognitively impaired subjects with MTA showed more extensive ultrastructural white matter damage than those without MTA, even after age correction (Table 3). While speculative, this association would be consistent with the notion of supra-additive cognitive effects of vascular and neurodegenerative pathologic processes (37,38) that may further contribute to the recognized increasing prevalence of mixed pathologic findings in the elderly (3). Future research is needed to confirm and further characterize such interactions and putative responsiveness to therapeutic interventions. This will be facilitated by the combined use of two simple imaging measures from standard brain MR imaging examinations: MTA as an established marker of hippocampal pathologic abnormalities and WMT skeleton mean diffusivity, which we propose as a new, promising marker of subcortical disconnection. WMT skeleton mean diffusivity also has potential as an imaging biomarker of vascular cognitive disorder (area under the receiver operating characteristic curve, 0.82; sensitivity, 77.4%; specificity, 72.7%). The lower specificity compared with cerebrospinal fluid biomarkers (sensitivity, 68%; specificity, 87%) (39) could be explained by the milder end of the cognitive deficit profile in our study.
The main limitations of our study are a lack of neuropsychometric information on premorbid cognition due to the study design and a lack of direct diagnostic tools for amyloid pathologic abnormalities. We are, however, confident that we excluded patients with premorbid dementia, as a research investigator with up to 9 years of clinical experiences (A.A.H.) spoke to all participants and carefully reviewed clinical notes to exclude patients without the capacity to consent and those with clinically apparent dementia before MR imaging examination. Also, on the basis of the clinical information and the published diagnostic criteria, all 53 cognitively impaired subjects in our cohort were considered to have probable vascular cognitive disorder. Nevertheless, we cannot exclude premorbid Alzheimer disease, with an expected prevalence of 11%–20% in those aged 65 and older (40,41). In the absence of amyloid PET data, we used the indirect marker of MTA with proven high positive and negative predictive value for Alzheimer disease (17,18). We found that just over 21% of our cohort showed MTA. Importantly, all main findings reported in the whole study group were confirmed in the subgroup of subjects without MTA, considered to be at low risk of Alzheimer disease. This allows us to largely exclude a confounding effect from undetected Alzheimer disease. Second, we have a variable time interval from the cerebrovascular event to MR imaging. This is, however, unlikely to have biased our results, as we did not observe cognitive associations with acute infarction metrics. Last, in this study, we did not investigate the presence of hemodynamic impairment and hence cannot exclude this as a contributory factor in individual subjects. Nevertheless, only 10 of 108 subjects (9.3%) had more than 80% carotid arterial stenosis, and only a subgroup of these can be expected to show hemodynamic impairment.
In conclusion, we demonstrated that lesional and ultrastructural affection of the WMT skeleton contributes to cognitive impairment in patients with recently symptomatic CAD, independent of the effect of age, and also in patients without MTA. This supports the notion that subcortical disconnection of cognitive neural networks is a key mechanism of cognitive impairment in patients with probable vascular cognitive disorder.
Advances in Knowledge
■ In patients with carotid artery disease, cerebrovascular lesion location in the thalamic radiation and interhemispheric fiber tracts contributes to global cognitive deficits, and lesions in the thalamic radiation and long association fibers affect fluency performance.
■ Severity of subcortical tissue damage, preferentially in major white matter tracts, contributes to global cognitive impairment (R2 = 0.311, P < .001), with skeleton mean diffusivity as the best-performing imaging marker for the prediction of probable vascular cognitive disorder (area under the receiver operating characteristic curve, 0.82 [95% confidence interval: 0.75, 0.90]).
■ Findings were confirmed in patients with low risk of comorbid Alzheimer disease (absence of medial temporal lobe atrophy [MTA]), while presence of MTA was associated with more severe cognitive impairment, increased vascular lesion load, and more severe tissue damage.
■ Disconnection of large-scale cognitive neural networks from cumulative lesional and nonlesional tract injuries is proposed as a mechanism of cognitive impairment in advanced arteriosclerosis.
Implication for Patient Care
■ The findings provide insights into how cerebrovascular disease contributes to cognitive impairment and/or dementia and highlight the need to combat progressive subcortical brain tissue damage; average white matter skeleton mean diffusivity shows potential as a simple diagnostic marker of subcortical disconnection underlying vascular cognitive disorder.
APPENDIX
SUPPLEMENTAL FIGURES
Acknowledgments
Acknowledgments
The authors are grateful to the CAD imaging study participants and the MR imaging technologists at the Queen’s Medical Centre, Nottingham, England, for imaging our participants.
Received December 7, 2015; revision requested February 15, 2016; revision received May 25; accepted May 27; final version accepted June 3.
Study supported by the National Institute for Health Research (NIHR) under its Research for Patient Benefit (RfPB) Program (PB-PG-0107-11438). D.M. funded by the University of Nottingham. R.J.S. funded by the NIHR, Stroke Association UK, and Nottingham Vascular Surgery Research Fund.
The views expressed are those of the authors and are not necessarily those of the National Health Service, the NIHR, or the Department of Health. None of the sponsors had any role in the study design, data collection, data analysis, data interpretation, writing of the report, or decision to submit the article for publication.
See also the editorial by Haller and Barkhof in this issue.
Disclosures of Conflicts of Interest: D.M. disclosed no relevant relationships. A.A.H. disclosed no relevant relationships. R.J.S. disclosed no relevant relationships. Q.S. disclosed no relevant relationships. C.R.T. disclosed no relevant relationships. R.A.D. disclosed no relevant relationships. D.P.A. disclosed no relevant relationships.
Abbreviations:
- ACE-R
- Addenbrooke’s Cognitive Examination–Revised
- CAD
- carotid artery disease
- CI
- confidence interval
- DTI
- diffusion-tensor imaging
- FA
- fractional anisotropy
- FLAIR
- fluid-attenuated inversion recovery
- MNI
- Montreal Neurological Institute
- MTA
- medial temporal lobe atrophy
- NAWM
- normal-appearing white matter
- TBSS
- tract-based spatial statistics
- VLSM
- voxel-based lesion-symptom mapping
- WMT
- white matter tract
References
- 1.Sachdev P, Kalaria R, O’Brien J, et al. Diagnostic criteria for vascular cognitive disorders: a VASCOG statement. Alzheimer Dis Assoc Disord 2014;28(3):206–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Plassman BL, Langa KM, Fisher GG, et al. Prevalence of dementia in the United States: the aging, demographics, and memory study. Neuroepidemiology 2007;29(1-2):125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chui HC, Ramirez-Gomez L. Clinical and imaging features of mixed Alzheimer and vascular pathologies. Alzheimers Res Ther 2015;7(1):21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gorelick PB, Scuteri A, Black SE, et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2011;42(9):2672–2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Quigley H, Colloby SJ, O’Brien JT. PET imaging of brain amyloid in dementia: a review. Int J Geriatr Psychiatry 2011;26(10):991–999. [DOI] [PubMed] [Google Scholar]
- 6.Tapiola T, Alafuzoff I, Herukka SK, et al. Cerebrospinal fluid beta-amyloid 42 and tau proteins as biomarkers of Alzheimer-type pathologic changes in the brain. Arch Neurol 2009;66(3):382–389. [DOI] [PubMed] [Google Scholar]
- 7.Schiemanck SK, Kwakkel G, Post MW, Prevo AJ. Predictive value of ischemic lesion volume assessed with magnetic resonance imaging for neurological deficits and functional outcome poststroke: a critical review of the literature. Neurorehabil Neural Repair 2006;20(4):492–502. [DOI] [PubMed] [Google Scholar]
- 8.Vernooij MW, Ikram MA, Vrooman HA, et al. White matter microstructural integrity and cognitive function in a general elderly population. Arch Gen Psychiatry 2009;66(5):545–553. [DOI] [PubMed] [Google Scholar]
- 9.Ovadia-Caro S, Villringer K, Fiebach J, et al. Longitudinal effects of lesions on functional networks after stroke. J Cereb Blood Flow Metab 2013;33(8):1279–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmierer K, Wheeler-Kingshott CA, Boulby PA, et al. Diffusion tensor imaging of post mortem multiple sclerosis brain. Neuroimage 2007;35(2):467–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alves GS, Oertel Knöchel V, Knöchel C, et al. Integrating retrogenesis theory to Alzheimer’s disease pathology: insight from DTI-TBSS investigation of the white matter microstructural integrity. BioMed Res Int 2015;2015:291658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bakker FC, Klijn CJ, Jennekens-Schinkel A, Kappelle LJ. Cognitive disorders in patients with occlusive disease of the carotid artery: a systematic review of the literature. J Neurol 2000;247(9):669–676. [DOI] [PubMed] [Google Scholar]
- 13.O’Brien JT, Erkinjuntti T, Reisberg B, et al. Vascular cognitive impairment. Lancet Neurol 2003;2(2):89–98. [DOI] [PubMed] [Google Scholar]
- 14.Mioshi E, Dawson K, Mitchell J, Arnold R, Hodges JR. The Addenbrooke’s Cognitive Examination Revised (ACE-R): a brief cognitive test battery for dementia screening. Int J Geriatr Psychiatry 2006;21(11):1078–1085. [DOI] [PubMed] [Google Scholar]
- 15.Behrens TEJ, Johansen-Berg H, Woolrich MW, et al. Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat Neurosci 2003;6(7):750–757. [DOI] [PubMed] [Google Scholar]
- 16.Scheltens P, Leys D, Barkhof F, et al. Atrophy of medial temporal lobes on MRI in “probable” Alzheimer’s disease and normal ageing: diagnostic value and neuropsychological correlates. J Neurol Neurosurg Psychiatry 1992;55(10):967–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferreira D, Cavallin L, Larsson EM, et al. Practical cut-offs for visual rating scales of medial temporal, frontal and posterior atrophy in Alzheimer’s disease and mild cognitive impairment. J Intern Med 2015;278(3):277–290. [DOI] [PubMed] [Google Scholar]
- 18.Burton EJ, Barber R, Mukaetova-Ladinska EB, et al. Medial temporal lobe atrophy on MRI differentiates Alzheimer’s disease from dementia with Lewy bodies and vascular cognitive impairment: a prospective study with pathological verification of diagnosis. Brain 2009;132(Pt 1):195–203. [DOI] [PubMed] [Google Scholar]
- 19.Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 2002;17(2):825–841. [DOI] [PubMed] [Google Scholar]
- 20.Smith SM. Fast robust automated brain extraction. Hum Brain Mapp 2002;17(3):143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jellinger KA. Pathology and pathogenesis of vascular cognitive impairment—a critical update. Front Aging Neurosci 2013;5:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bates E, Wilson SM, Saygin AP, et al. Voxel-based lesion-symptom mapping. Nat Neurosci 2003;6(5):448–450. [DOI] [PubMed] [Google Scholar]
- 23.Hua K, Zhang J, Wakana S, et al. Tract probability maps in stereotaxic spaces: analyses of white matter anatomy and tract-specific quantification. Neuroimage 2008;39(1):336–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith SM, Jenkinson M, Johansen-Berg H, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage 2006;31(4):1487–1505. [DOI] [PubMed] [Google Scholar]
- 25.Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp 2002;15(1):1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Geva S, Baron JC, Jones PS, Price CJ, Warburton EA. A comparison of VLSM and VBM in a cohort of patients with post-stroke aphasia. Neuroimage Clin 2012;1(1):37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duering M, Zieren N, Hervé D, et al. Strategic role of frontal white matter tracts in vascular cognitive impairment: a voxel-based lesion-symptom mapping study in CADASIL. Brain 2011;134(Pt 8):2366–2375. [DOI] [PubMed] [Google Scholar]
- 28.Biesbroek JM, Kuijf HJ, van der Graaf Y, et al. Association between subcortical vascular lesion location and cognition: a voxel-based and tract-based lesion-symptom mapping study. The SMART-MR study. PLoS One 2013;8(4):e60541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kraus MF, Susmaras T, Caughlin BP, Walker CJ, Sweeney JA, Little DM. White matter integrity and cognition in chronic traumatic brain injury: a diffusion tensor imaging study. Brain 2007;130(Pt 10):2508–2519. [DOI] [PubMed] [Google Scholar]
- 30.Dineen RA, Vilisaar J, Hlinka J, et al. Disconnection as a mechanism for cognitive dysfunction in multiple sclerosis. Brain 2009;132(Pt 1):239–249. [DOI] [PubMed] [Google Scholar]
- 31.Welton T, Kent D, Constantinescu CS, Auer DP, Dineen RA. Functionally relevant white matter degradation in multiple sclerosis: a tract-based spatial meta-analysis. Radiology 2015;275(1):89–96. [DOI] [PubMed] [Google Scholar]
- 32.Kim YJ, Kwon HK, Lee JM, et al. White matter microstructural changes in pure Alzheimer’s disease and subcortical vascular dementia. Eur J Neurol 2015;22(4):709–716. [DOI] [PubMed] [Google Scholar]
- 33.O’Sullivan M, Barrick TR, Morris RG, Clark CA, Markus HS. Damage within a network of white matter regions underlies executive dysfunction in CADASIL. Neurology 2005;65(10):1584–1590. [DOI] [PubMed] [Google Scholar]
- 34.Grambaite R, Selnes P, Reinvang I, et al. Executive dysfunction in mild cognitive impairment is associated with changes in frontal and cingulate white matter tracts. J Alzheimers Dis 2011;27(2):453–462. [DOI] [PubMed] [Google Scholar]
- 35.Huang J, Auchus AP. Diffusion tensor imaging of normal appearing white matter and its correlation with cognitive functioning in mild cognitive impairment and Alzheimer’s disease. Ann N Y Acad Sci 2007;1097:259–264. [DOI] [PubMed] [Google Scholar]
- 36.Duering M, Righart R, Wollenweber FA, Zietemann V, Gesierich B, Dichgans M. Acute infarcts cause focal thinning in remote cortex via degeneration of connecting fiber tracts. Neurology 2015;84(16):1685–1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang R, Fratiglioni L, Laukka EJ, et al. Effects of vascular risk factors and APOE ε4 on white matter integrity and cognitive decline. Neurology 2015;84(11):1128–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vemuri P, Lesnick TG, Przybelski SA, et al. Vascular and amyloid pathologies are independent predictors of cognitive decline in normal elderly. Brain 2015;138(Pt 3):761–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de Jong D, Jansen RW, Kremer BP, Verbeek MM. Cerebrospinal fluid amyloid beta42/phosphorylated tau ratio discriminates between Alzheimer’s disease and vascular dementia. J Gerontol A Biol Sci Med Sci 2006;61(7):755–758. [DOI] [PubMed] [Google Scholar]
- 40.Alzheimer’s Association . 2015 Alzheimer’s disease facts and figures. Alzheimers Dement 2015;11(3):332–384. [DOI] [PubMed] [Google Scholar]
- 41.Jansen WJ, Ossenkoppele R, Knol DL, et al. Prevalence of cerebral amyloid pathology in persons without dementia: a meta-analysis. JAMA 2015;313(19):1924–1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.