Abstract
Within the CCD of the distal nephron of the rabbit, the BK (maxi K) channel mediates Ca2+- and/or stretch-dependent flow-induced K+ secretion (FIKS) and contributes to K+ adaptation in response to dietary K+ loading. An unresolved question is whether BK channels in intercalated cells (ICs) and/or principal cells (PCs) in the CCD mediate these K+ secretory processes. In support of a role for ICs in FIKS is the higher density of immunoreactive apical BKα (pore-forming subunit) and functional BK channel activity than detected in PCs, and an increase in IC BKα expression in response to a high-K+ diet. PCs possess a single apical cilium which has been proposed to serve as a mechanosensor; direct manipulation of cilia leads to increases in cell Ca2+ concentration, albeit of nonciliary origin. Immunoperfusion of isolated and fixed CCDs isolated from control K+-fed rabbits with channel subunit-specific antibodies revealed colocalization of immunodetectable BKα- and β1-subunits in cilia as well as on the apical membrane of cilia-expressing PCs. Ciliary BK channels were more easily detected in rabbits fed a low-K+ vs. high-K+ diet. Single-channel recordings of cilia revealed K+ channels with conductance and kinetics typical of the BK channel. The observations that 1) FIKS was preserved but 2) the high-amplitude Ca2+ peak elicited by flow was reduced in microperfused CCDs subject to pharmacological deciliation suggest that cilia BK channels do not contribute to K+ secretion in this segment, but that cilia serve as modulators of cell signaling.
Keywords: maxi K channel, Ca2+ signaling, intercalated cell, flow-induced K+ secretion, dietary K+ adaptation, Ca2+ channel
it is now well established that within the cortical collecting duct (CCD) of the distal nephron, a heterogeneous segment comprised of both principal and intercalated cells, the BK (maxi K) channel mediates 1) Ca2+- and/or stretch-dependent flow-induced K+ secretion (FIKS) and thus likely mediates, at least in part, the kaliuresis associated with diuretic use or volume expansion; and 2) contributes to the renal adaptation to a high-K+ (HK) diet (2, 25, 29, 37, 84, 86). However, a major unresolved question is the identity of the specific cell type in the CCD that mediates FIKS and K+ adaptation.
The principal cell, possessing robust basolateral Na+-K+-ATPase activity, has traditionally been considered to mediate transepithelial Na+ absorption and K+ secretion (20, 21, 71, 83). However, the density of apical conducting BK channels is low in these cells (35, 54, 55, 72) as is immunoreactivity to antibodies directed against the pore-forming BKα-subunit (18, 24, 48, 59, 86). We initially attributed our inability to visualize immunoreactive BKα in rabbit principal cells to cell-specific expression of BKα splice variants that were not recognized by the polyclonal anti-BKα antibody we had previously raised in chicken against an epitope at the extreme C terminus of the protein (86). However, similar results have been reported by others in rabbit and mouse CCD principal cells (24, 59, 81) using a commercially available mouse anti-Slo1 antibody raised against a highly conserved upstream (a.a. 690–715) epitope in the pore-forming subunit (45), and thus expected to identify all splice variants.
In contrast to the situation prevailing in principal cells, the density of apical immunodetectable BKα (18, 24, 48, 59, 86) and functional (35, 54, 55, 72) BK channels is high in intercalated cells, cells that are primarily responsible for acid-base transport. Immunocytochemical and functional analyses suggest that intercalated cells may have insufficient Na+-K+-ATPase activity to sustain high rates of luminal K+ secretion (3, 55, 69, 74) but can sustain FIKS via basolateral NKCC1-mediated K+ uptake (39).
While the observations summarized above support a role for intercalated cells in mediating FIKS and K+ adaptation, the BK channel in mouse intercalated cells assembles with the BKβ4-subunit (23), a protein that renders BKα resistant to low nanomolar concentrations of iberiotoxin (IbTX) (44, 51), a selective inhibitor of BK channels (2, 9, 22, 79). Coassembly of BKα with β1-subunits, the latter identified in principal cells in rabbit initial CCD (57, 59), generates a channel sensitive to low concentrations of IbTX. Given that FIKS is inhibited by IbTX in rabbit CCD (39, 86), the latter observations support the notion that FIKS is mediated by a BKα/β1 channel in principal cells.
Principal but not intercalated cells possess a single apical cilium, which extends from the basal body into the lumen and has been implicated as a sensor that transduces mechanical signals, including fluid shear and direct mechanical perturbation, into global increases in cell Ca2+ concentration ([Ca2+]i) (49, 61, 64–66, 75), due to both luminal Ca2+ entry and internal store release (37, 41). Mechanosensitive Ca2+ channels have been localized to cilia, which maintain a resting Ca2+ concentration ([Ca2+]cilium) significantly higher (>300 nM) than that measured in the cytoplasm of the same cell (~100 nM) (14, 15, 77). Based on the above, we hypothesized that BKα localizes to the cilia of principal cells and that cilia BK channels play a role in FIKS. Using an in vitro immunoperfusion approach with three-dimensional (3D) reconstructions of confocal images, we now localize immunoreactive BKα to the principal cell central cilium, a structure that has been difficult to detect by conventional analysis of kidney sections. Electrophysiological analyses of cilia reveal conducting K+ channels with the fingerprint of BK channels. However, these cilia BK channels appear not to mediate FIKS, as evidenced by our findings that 1) cilia BKα labeling is robust in CCDs isolated from low K+ (LK)-fed rabbits, which do not exhibit FIKS (48); and 2) microperfused CCDs subject to pharmacological deciliation retain their capacity for FIKS.
METHODS
Animals and isolation of single tubules.
Adult (>6 wk) female New Zealand White rabbits (Covance, Denver, PA) were housed in the Center for Comparative Medicine at the Icahn School of Medicine (ISMMS) at Mount Sinai. All animals were allowed free access to tap water and chow. Some rabbits were randomized to receive a LK (0.13%, or 34 meq K+/kg) or HK (1.56%, or 398 meq K+/kg) diet, both containing 0.29% Na+, or 7.5 g/kg, for 10 days (48). Special diets were obtained from Harlan Teklad (Madison, WI). Animal weights and the amount of food and water consumed were monitored. Animals were euthanized in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Animal protocols were approved by the Institutional Animal Care and Use Committee at the ISMMS.
Rabbit kidneys were removed via a midline incision, and single tubules were dissected freehand in cold (4°C) Na+-Ringer solution containing (in mM) 135 NaCl, 2.5 K2HPO4, 2.0 CaCl2, 1.2 MgSO4, 4.0 lactate, 6.0 l-alanine, 5.0 HEPES, and 5.5 d-glucose, pH 7.4, 290 ± 2 mosmol/kgH2O, as previously described (41). A single tubule was studied from each animal, unless otherwise indicated.
Microperfusion of single tubules.
Each isolated tubule was immediately transferred to a temperature- and O2/CO2-controlled specimen chamber set on the stage of a Nikon inverted epifluorescence microscope (Eclipse TE300), mounted on concentric glass pipettes, and perfused and bathed at 37°C with a -containing solution [Burg’s perfusate (in mM): 120 NaCl, 25 NaHCO3, 2.5 K2HPO4, 2.0 CaCl2, 1.2 MgSO4, 4.0 Na+ acetate, 1.0 Na3 citrate, 6.0 l-alanine, and 5.5 d-glucose, pH 7.4, 290 ± 2 mosmol/kgH2O] (41). During the 45-min equilibration period and thereafter, the perfusion chamber was continuously suffused with a gas mixture of 95% O2-5% CO2 to maintain pH of the Burg’s solution at 7.4 at 37°C. The bathing solution was continuously exchanged at a rate of 10 ml/h using a syringe pump (Razel, Stamford, CT).
Some CCDs, as indicated, were subject to pharmacological deciliation accomplished by luminal perfusion with 1 mM dibucaine × 30 min, a treatment that leads to rapid shedding of cilia in other cells (11, 53), followed by a 1-h recovery period. Treatment of polarized Madin-Darby canine kidney (MDCK) cells with dibucaine at a dose of 0.75 mM × 30 min leads to rapid shedding of cilia, accompanied by tight junctional remodeling, leading to an increase in transepithelial resistance, without epithelial monolayer disruption or changes in gross morphology (53).
Immunoperfusion of single tubules.
After equilibration, tubules were fixed while cannulated on the perfusion rig with 2.5% paraformaldehyde in PBS added to the bathing solution for 1 h at room temperature. Fixed CCDs were then permeabilized with 0.3% Triton X-100 in PBS, blocked with PBS containing 0.1% glycine and 1% BSA and subsequently with PBS supplemented with 1% BSA and 10% normal goat serum (NGS). Tubules were then sequentially perfused at 37°C with a chicken IgY anti-BKα (1:1,000; Aves Laboratory) for 2 h visualized by subsequent perfusion for 1 h, after rinsing, with a DL594-rabbit IgG anti-chicken IgY or A594-goat IgG anti-chicken IgY (1:1,000; Abcam: ab96957 or ab150176, respectively). A subset of CCDs was then colabeled by sequential luminal perfusion with another 1° and 2° antibody (Ab) pair, as indicated, using reagents listed in Table 1. After immunolabeling, tubules were collected, deposited on a coverslip with a drop of Vectashield mounting medium with 4′-6-diamidino-2-phenylindole (DAPI; to label nuclei) for visualization by confocal microscopy using a ×63 oil-immersion plan-Apochromat objective (NA 1.4) and a laser-scanning Leica SP5 DM. Stacks of confocal sections were collected at a pinhole = 80.0 µm and step size = 0.21 µm to capture the cilia in a projection of 10 (for tubule sections) to 200 (for a full thickness tubule image) confocal slices. 3D reconstructions were generated using Leica Application Suite (LAS AF) software.
Table 1.
Antibodies (Abs) used for immunoperfusion
| Primary Abs | Dilution | Provider | Secondary Abs | Dilution | Provider |
|---|---|---|---|---|---|
| Goat IgG anti-maxi-Kβ1 (N-15) | 1:100 | Santa Cruz Biotechnology (sc-14749) | A488-rabbit IgG anti-goat IgG | 1:500 | Molecular Probes (A11078) |
| Mouse IgG2a anti-maxi-Kβ4 | 1:100 | Stress Marq Biosciences (SMC-38D) | A488-goat IgG anti-mouse IgG2a | 1:500 | Molecular Probes (A21131) |
| Mouse IgG2b anti-acetylated α-tubulin (6-11B-1) | 1:1,000 | Abcam (ab24610) | A488-goat IgG anti-mouse IgG | 1:1,000 | Molecular Probes (A11029) |
| Goat IgG anti-aquaporin-2 (C-17) | 1:100 | Santa Cruz Biotechnology (sc-9882) | A488-rabbit IgG anti-goat IgG | 1:500 | Molecular Probes (A11078) |
| Mouse IgG2a anti-polycystin-2 (YCE2) | 1:100 | Santa Cruz Biotechnology (sc-47734) | A647-goat IgG anti-mouse IgG | 1:500 | Molecular Probes (A21236) |
| Rabbit anti-pendrin | 1:2,000 | Gift from Dr. S. Wall, Emory University School of Medicine | A488-goat IgG anti-rabbit IgG | 1:500 | Molecular Probes (A11008) |
The effect of K+ intake on relative BKα expression in the cilia was determined by analysis of CCDs isolated from HK (n = 4)- or LK (n = 4)-fed rabbits and immunoperfused simultaneously with Abs directed against BKα and acetylated α-tubulin (Ac α-tubulin), followed by appropriate secondary Abs (Table 1). Stacks of confocal sections were collected as described above and cilia, whose full length was visualized in the x–y plane, were outlined for analysis using the freehand tool. For each outlined cilium, the sum of grayscale pixels corresponding to the BKα or Ac α-tubulin fluorescence intensity signals were acquired in five consecutive stacks. The ratios of BKα/Ac α-tubulin fluorescence intensity signals were calculated for 22 cilia in LK and 14 cilia in HK CCDs and averaged for each diet.
The effect of K+ intake on relative BKα distribution between the apical+subapical region and whole cells in the CCD was studied using LAS AF Lite software from Leica Microsystems. Stacks of confocal sections (pinhole = 90.0 µm; step size = 0.5 µm) were collected from single CCDs isolated from HK (n = 4)- and LK (n = 4)-fed animals and immunoperfused with Abs directed against BKα and pendrin (Table 1), the latter to identify β-type intercalated cells. Five to 10 ciliated principal cells and an equivalent number of pendrin (+) intercalated cells in the wall of each tubule were selected for analysis by outlining the whole cell as well as the apical+subapical regions using the freehand tool of the software. For each outlined region, the sum of grayscale pixels corresponding to the BKα fluorescence intensity signal in three sequential confocal sections was acquired. For each individual cell, the value obtained for the apical+subapical region was divided by that of the whole cell to generate a relative fluorescence intensity of BKα in the apical+subapical region. Data for specific cell types were averaged for each diet and then normalized to the value for LK-fed animals.
Measurement of transepithelial transport.
Transport measurements were performed in the absence of transepithelial osmotic gradients, and thus water transport was assumed to be zero. Three to four samples of tubular fluid were collected under water-saturated light mineral oil by timed filling of a calibrated ~20-nl volumetric constriction pipette at slow (~1) and fast (~5 nl·min–1·mm–1) flow rates, as indicated (41, 86). To determine the concentrations of K+ and Na+ delivered to the tubular lumen, ouabain (200 μM) was added to the bath at the conclusion of each experiment to inhibit all active transport, and an additional three to four samples of tubular fluid were obtained for analysis. Thereafter, each tubule was fixed and immunoperfused, as described above, with Abs directed against BKα to demonstrate successful deciliation. Data from CCDs subject to the deciliation protocol but found to have residual cilia were not included in subsequent analyses. To examine the effect of dibucaine on structural integrity of the preparation, some fixed CCDs were labeled with rhodamine-phalloidin (1:40, Molecular Probes) to identify F-actin and fluorescein-conjugated Dolichos biflorus agglutinin (FITC-DBA; 1:100, Vector Laboratories) to label apical membranes of principal cells.
The cation concentrations of perfusate and collected tubular fluid samples were determined by helium glow photometry, and the rates of net transport (Jx; in pmol·min–1·mm–1 tubular length) were calculated using standard flux equations, as previously described (18). The calculated ion fluxes were averaged to obtain a single mean rate of ion transport for the CCD at each flow rate under each condition. The flow rate was varied by adjusting the height of the perfusate reservoir. The sequence of flow rates was randomized within each group of tubules to minimize any bias induced by time-dependent changes in ion transport.
Measurement of [Ca2+]i.
Isolated CCDs were transferred to the specimen chamber assembled with a No. 1 coverslip (Corning) painted with a 1-µl drop of poly-d-lysine hydrobromide 0.01% (BD Biosciences, Bedford, MA), set on the stage of the Nikon inverted epifluorescence microscope linked to a Cascade 512F camera (Photometrics) or a cooled Pentamax CCD camera (Princeton Instruments), interfaced with a digital imaging system (MetaFluor, Universal Imaging, Westchester, PA). Each microperfused CCD was positioned directly on the poly-d-lysine to immobilize the segment for the duration of the experiment. Following a 1-h equilibration, CCDs were loaded with 20 μM acetoxymethyl ester of fura 2 (Calbiochem, La Jolla, CA) added to the bath for 20 min. The tubule was then rinsed with perfusate for 30 min. Fura 2-loaded CCDs were alternately excited at 340 and 380 nm, and images, acquired every 3 s, were digitized for subsequent analysis. After stable baseline 340 nm/380 nm fluorescence intensity ratios (FIRs) were obtained at a slow flow rate, the luminal flow rate was increased acutely and FIRs were monitored, using our commercially available digital image-analysis system (MetaFluor).
At the conclusion of each experiment in this series, each tubule was immunoperfused, as described above, with Abs directed against acetylated α-tubulin to demonstrate successful deciliation; data from CCDs subject to the deciliation protocol but found to have residual cilia were not included in subsequent analyses. The mean FIRs for dull (presumably principal) and bright (presumably intercalated) cells were calculated. We have previously reported that intercalated cells, characterized by high levels of carbonic anhydrase activity, generally appear brighter under epifluorescence illumination than do principal cells secondary to their selective accumulation of functional fluorescent dyes delivered as acetoxymethyl (AM) esters, such as fura 2-AM (38, 41).
Patch-clamp analysis of cilia channels.
Patch-clamp pipettes, made with borosilicate glass (1.7-mm OD) with a Narishege electrode puller, had resistances of 2–4 MΩ when filled with 140 mM KCl. An Axon200B patch-clamp amplifier was used to record single -channel currents which were low-pass filtered at 1 KHz, and digitized by an Axon interface (Digidata 1322). The channel conductance was calculated by measuring currents at several holding potentials. Data were analyzed using the pClamp software system 9 (Axon).
Cilia were visualized directly under epifluorescence illumination in living mpkCCD cells stably expressing somatostatin receptor 3 (localized to cilia) fused to green fluorescent protein (GFP) (52) (gift from B. Yoder, U. Alabama Hepato/Renal Fibrocystic Disease Core Center). Cells were grown in DMEM:Ham's F12 (with 60 nM sodium selenate, 5 μg/ml transferrin, 2 mM glutamine, 50 nM dexamethasone, 1 nM tri-iodothyronine, 10 ng/ml epidermal growth factor, 5 μg/ml insulin, 20 mM d-glucose, 2% fetal calf serum, and 20 mM HEPES), as previously described (10). To patch cilia, we placed the pipette on the side of the cilium and then applied gentle negative suction. Recordings were performed with a pipette solution containing 140 mM KCl, 1.8 mM MgCl2, 1.8 mM CaCl2, and 10 mM HEPES (pH 7.4) and a bath solution with 140 mM NaCl, 5 mM KCl, 1.8 mM MgCl2, 1.8 mM CaCl2, and 10 HEPES (pH = 7.4).
Statistics.
All results are expressed as means ± SE; n equals the number of animals unless otherwise indicated. For functional studies, significant differences were determined by paired or unpaired t-tests, as appropriate, using the statistical software program SigmaStat (SPSS, Chicago, IL). Unpaired data that did not fit a normal distribution were analyzed using the nonparametric Mann-Whitney rank test. Significance was asserted if P < 0.05.
RESULTS
Immunoreactive BKα/β1 channels are localized to cilia in rabbit CCD.
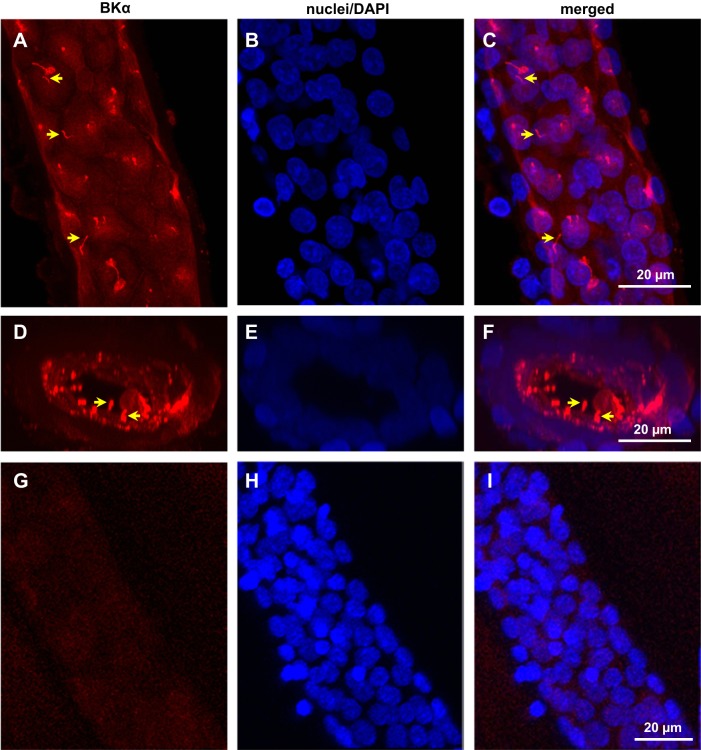
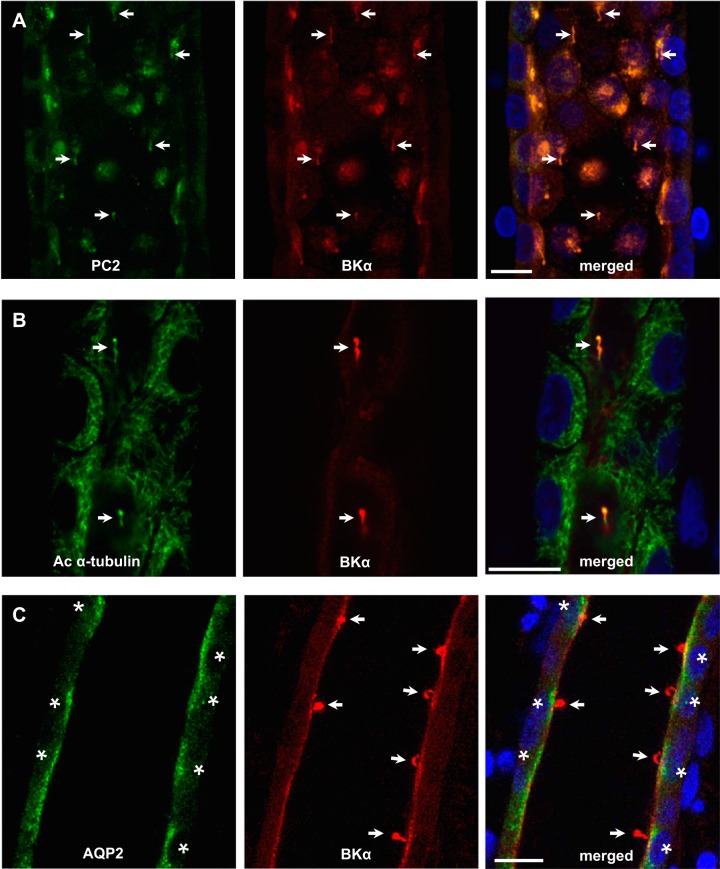
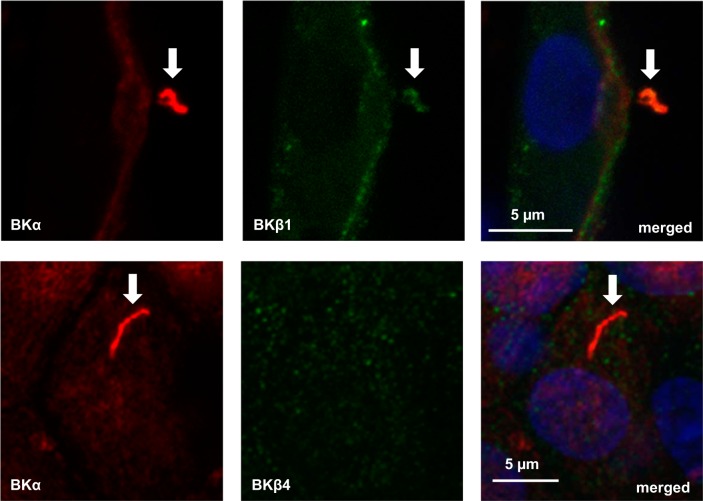
BKα labeling was readily detected by confocal microscopy in fixed and immunoperfused CCDs isolated from control K+-fed rabbits (Fig. 1, A and D) using a 3D reconstruction approach. BKα labeling was not detected in rabbit CCDs perfused with the chicken anti-BKα Ab preadsorbed with its antigen (Fig. 1, G and I) or 2° Ab alone (data not shown). Modest immunoreactivity for BKα was detected along the apical membranes of ciliated as well as nonciliated cells in rabbit CCDs (Fig. 1, A and D), with the most intense labeling localized to organelles projecting into the tubular lumen (Fig. 1D) that labeled with Abs directed against the Ca2+ channel polycystin 2 (PC2; Fig. 2A) and Ac α-tubulin (Fig. 2B), a marker specific for cilia. Notably, luminal immunoperfusion with an anti-AQP2 Ab labeled apical membrane but not cilia of principal cells (Fig. 2C). Immunodetectable BKα in the cilia colocalized with immunoreactivity for BKβ1- (Fig. 3, top) but not β4-subunits (Fig. 3, bottom), using subunit-specific Abs. BKβ1 labeling was also present along the apical membrane of principal cells (Fig. 3, top), whereas β4 was distributed diffusely throughout the cytoplasm of all CCD cells (Fig. 3, bottom). In sum, these data suggest that BKα/β1 channels are abundant in cilia of principal cells.
Fig. 1.
Immunodetectable maxi K channel subunit (BKα) is expressed in cilia in fixed microperfused rabbit cortical collecting duct (CCD): 3D reconstructions of confocal images. Top: CCD immunolabeled with anti-BKα antibody (Ab) and appropriate 2° Ab (A) and stained with 4′-6-diamidino-2-phenylindole (DAPI; B), viewed en face, with merged image shown in C. Middle: same CCD as in A–C, immunolabeled for BKα (D) and stained with DAPI (E), viewed down the internal axis of the tubule, with merged image in F. Bottom, negative control: CCD immunolabeled with anti-BKα Ab preadsorbed with the control peptide (G) and stained with DAPI (H), viewed en face, with merged image shown in I. Cilia are identified by arrows in A, C, D, and F. Similar patterns of expression were observed in at least 10 rabbit CCDs.
Fig. 2.
Immunodetectable BKα colocalizes with polycystin 2 (PC2) in cilia in principal cells in microperfused rabbit CCD. Anti-BKα Ab (arrows; red, middle column) colocalizes with anti-PC2 Ab (arrows; artificial green in A) in organelles that label with acetylated (Ac) α-tubulin (arrows; green in B) in aquaporin-2 (AQP2)-positive (*; green in C) cells, as demonstrated by merged images in right column. Note apical membrane colocalization of anti-BKα Ab with immunoreactive PC2 and AQP2 (A and C, merged images). Bar = 10 µm. Similar patterns of expression were observed in at least 4 CCDs labeled with each Ab set.
Fig. 3.
Immunodetectable BKα colocalizes with β1-subunit in cilia in principal cells in microperfused rabbit CCD. Anti-BKα Ab (red, left) in cilia (arrows) colocalizes with the immunoreactive β1-subunit in cilia as well as in the apical membrane of principal cells (top, middle, and right) but not with the β4-subunit, which appears diffusely distributed throughout the cytoplasm of ciliated principal cells (bottom, middle, and right). Similar patterns of expression were observed in 4 CCDs labeled with each Ab set.
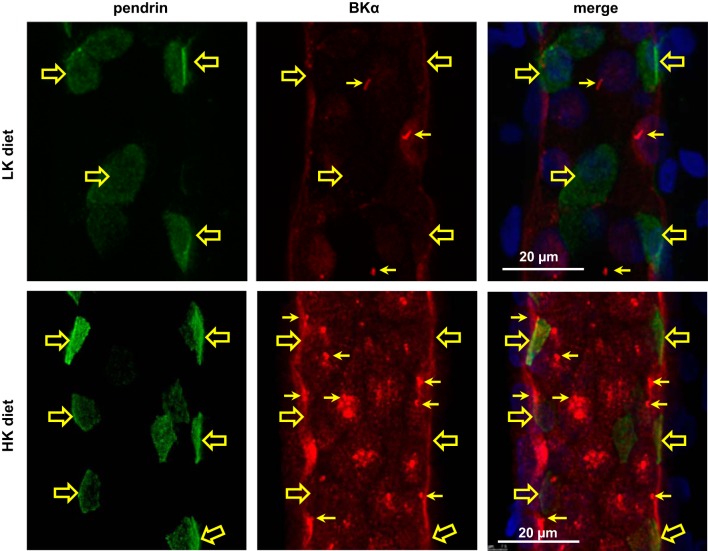
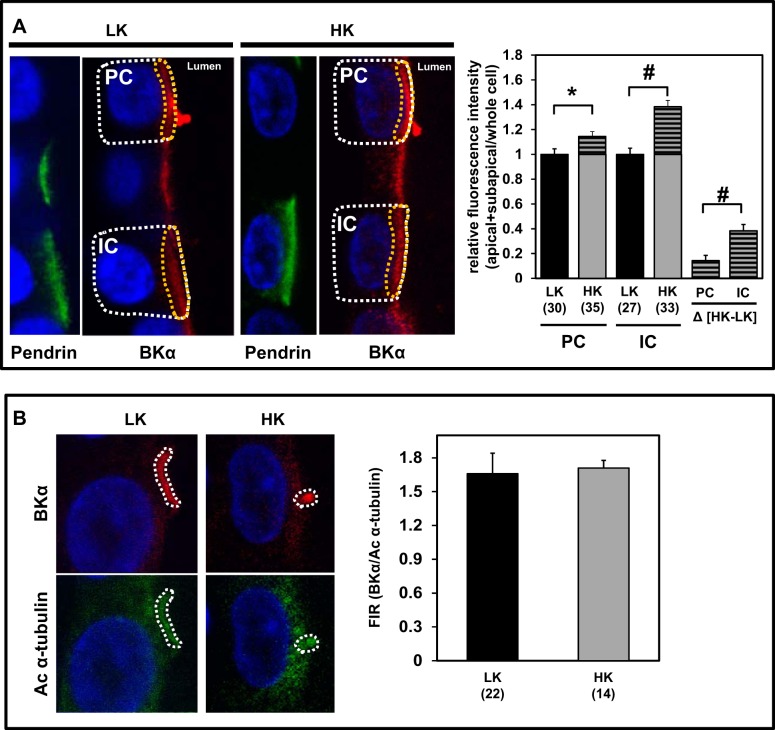
In immunoperfused CCDs from rabbits fed a LK diet for 10 days, BKα labeling was clearly evident in cilia, and minimally present along the apical membranes of ciliated principal cells, but was poorly expressed in pendrin (+) intercalated cells (Fig. 4, top). Note that mice fed a LK diet for 14 days exhibit a metabolic alkalosis, reduction in pendrin protein levels, and shift in pendrin expression to an intracellular pool, with a downregulation in the number of pendrin-expressing cells (67, 82). In response to dietary K+ loading, BKα labeling in apical membranes of nonciliated (including pendrin (+)) cells and thus presumably intercalated cells, was markedly enhanced (Fig. 4, bottom). BKα expression was quantified in the apical+subapical region, relative to whole cell BKα expression, in individual cells. We observed an increase in the BKα apical+subapical expression, relative to its whole cell expression, in response to dietary K+ loading in both principal and intercalated cells. Interestingly, the percent increase BKα apical+subapical distribution in intercalated cells (38.4 ± 5.1%) was greater than that detected in principal cells (14.4 ± 4.2%; P < 0.05) (Fig. 5A).
Fig. 4.
Effect of dietary K+ on BKα expression in immunoperfused rabbit CCDs. Representative 3D reconstructions of CCDs, isolated from low-K+ (LK; top)- and high-K+ (HK; bottom)-fed animals, fixed and immunoperfused with Abs directed against pendrin (green, left) and BKα (red, middle). In CCDs from LK-fed animals, immunoreactive BKα was observed in principal cell cilia (arrows) but barely detected in pendrin (+) and thus β-type intercalated cells (open arrowheads). In HK-fed rabbits, apical BKα expression was clearly apparent in both pendrin (+) intercalated and ciliated principal cells. Similar observations were made in 3 additional HK- and LK-fed animals.
Fig. 5.
Effect of dietary K+ on subcellular localization of BKα in rabbit CCD. Representative confocal images of principal (PC) and intercalated (IC) cells from CCDs isolated from LK (n = 4)- and HK (n = 4)-fed rabbits and immunoperfused with Abs directed against BKα (red in A and B) and pendrin (green in A) or acetylated α-tubulin (Ac α-tubulin; green in B) are shown. A: fluorescence intensity of BKα in the apical+subapical region (delineated by yellow dots, left) relative to that in the same whole cell (white dots) for individually identified cells in the tubular wall was calculated and averaged for cell types (ciliated PC, pendrin (+) IC) and diet; averages were normalized to cell-specific values obtained in LK-fed animals. This analysis (right) revealed a relative increase in apical+subapical BKα distribution in response to dietary K+ loading for both PC and IC, but the increase [(Δ(HK − LK)] in IC was greater than that detected in PC. B: cilia labeled with Abs directed against BKα and Ac α-tubulin were outlined for analysis. The ratios of BKα/Ac α-tubulin fluorescence intensity signals were calculated for each cilium, and ratios were averaged for LK and HK diets. There was no significant difference in the relative cilia BKα expression between the 2 diets (right). Values are means ± SE; n = number of cells or cilia. *P = 0.017. #P ≤ 0.002.
The number of immunolabeled cilia in CCDs isolated from HK (257 ± 23/mm tubular length; n = 162 cilia in 4 CCDs)- and LK (273 ± 32/mm tubular length; n = 139 cilia in 4 CCDs)-fed animals did not differ (P = 0.43). Nor did BKα expression in cilia, relative to that of Ac α-tubulin, show any change with enhanced dietary K+ intake (Fig. 5B). However, cilia were significantly shorter in HK- compared with LK-fed animals (Fig. 6). These observations suggest that the renal adaptation to a HK diet includes a greater upregulation of BKα expression in the apical+subapical regions of intercalated than principal cells in the rabbit CCD.
Fig. 6.
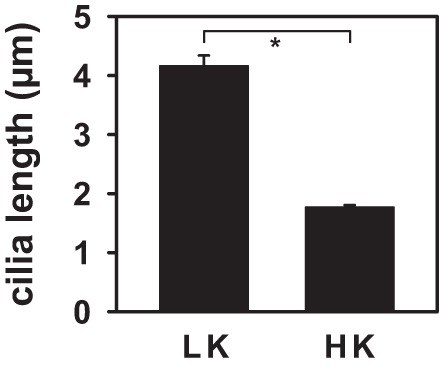
Effect of dietary K+ on cilia length in rabbit CCDs. Cilia length, measured as described in methods, was ~2.5 times longer in LK-fed rabbits (4.2 ± 1.4 µm in 57 cilia in 4 CCDs) than in HK-fed animals (1.8 ± 0.5 µm in 116 cilia in 4 CCDs). Values are means ± SE. *P ≤ 0.001.
BKα channels are functional in cilia.
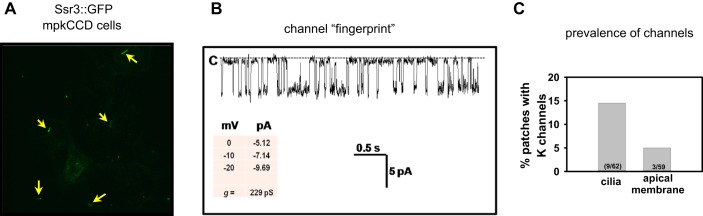
To test whether immunoreactive BKα in cilia comprised functional channels in living cells, we performed single-channel recordings of cilia- and apical membrane-attached patches in mpkCCD cells stably expressing the cilia-localized somatostatin receptor 3 protein fused with GFP (Sstr3::GFP; Fig. 7A) (52). A large-conductance (>200 pS) K+-conducting channel with voltage-dependent kinetics expected for the BK channel (Fig. 7B), as previously described (35, 54), was detected in both cilia- and apical membrane-attached patches of these cells. However, the prevalence of conducting channels (number of patches with high-conductance channels/total number of patches × 100%) was greater in cilia (9/62; 14.5%) than in the associated apical membrane (3/59; 5.1%) of these cells (Fig. 7C). These data suggest that BK channels present in cilia of CCD cells are functional.
Fig. 7.
Cilia possess functional high-conductance K+ channels. Single-channel recordings were performed in cilia, identified by their green fluorescence (A, arrows), in Ssr3::GFP mpkCCD cells (gift from B. Yoder, U. of Alabama Hepato/Renal Fibrocystic Disease Core Center). Channel activity was recorded with 140 mM KCl in the pipette and 140 mM NaCl/5 mM KCl in bath. The channel closed state (C) is indicated by a dashed line in B, a representative recording of a cilia-attached patch at 0 mV. Channel openings are shown as downward deflections. The conductance of this channel was >200 pS. C: prevalence of conducting channels (number of patches with high-conductance K+ channels/total number of patches × 100%) was greater in cilia (9/62; 14.5%) than in the associated apical membrane (3/59; 5.1%) of these cells.
Cilia BKα channels do not mediate FIKS.
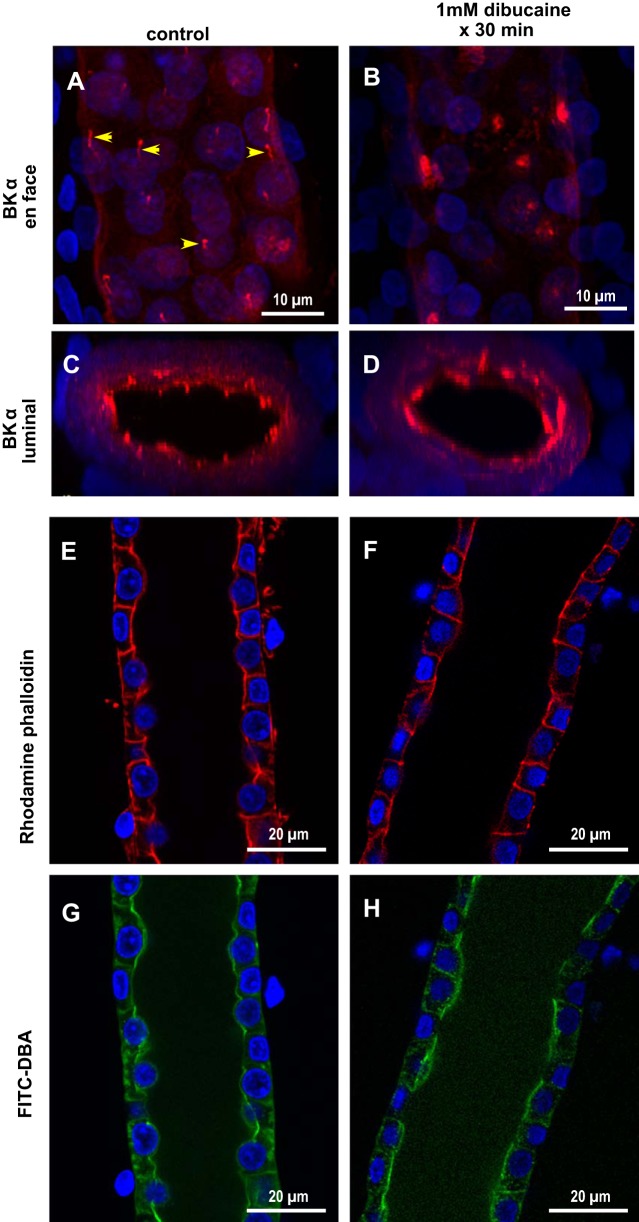
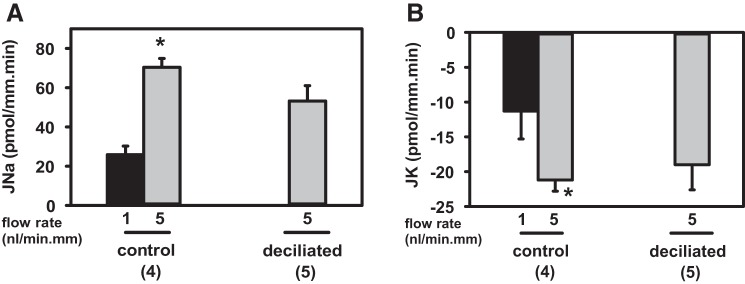
To test whether cilia BK channels mediate FIKS, Jx of Na+ and K+ was measured in microperfused control (n = 4) and “deciliated” (n = 5) rabbit CCDs. Luminal perfusion with 1 mM dibucaine × 30 min led to loss of cilia, although apical immunoreactivity for BKα was retained in deciliated tubules (Fig. 8, A–D). Pharmacological deciliation of CCDs did not lead to a loss in gross structural integrity, as evidenced by similar F-actin (visualized with rhodamine-phalloidin) and FITC-DBA labeling in control and deciliated CCDs (Fig. 8, E–H), and was not accompanied by cell death, as evidenced by trypan blue exclusion (data not shown). Nor did pharmacological deciliation significantly reduce flow-stimulated JNa (70.4 ± 4.5 to 53.2 ± 7.8 pmol·min−1·mm−1; P = 0.12) or FIKS (−21.2 ± 1.6 to −19.0 ± 3.6 pmol·min−1·mm−1; P = 0.63) (Fig. 9). Transport data for deciliated CCDs were included in this analysis only if deciliation was confirmed. Given the lengthy duration of the microperfusion experiments (equilibration, perfusion with dibucaine or vehicle, measurement of net cation transport before and after ouabain, and then immunoperfusion), we did not examine whether deciliation alone activated FIKS or confirm that FIKS was sensitive to IbTx. In sum, these data indicate that BK channels in principal cell cilia do not mediate FIKS.
Fig. 8.
Effect of deciliation on CCD BK channel expression and structural integrity in microperfused rabbit CCDs. Confocal images of perfused CCDs fixed and labeled with anti-BKα Ab (red) and DAPI (blue) after treatment with luminal vehicle (A and C) or 1 mM dibucaine × 30 min (B and D) are shown. Dibucaine led to complete loss of cilia, although apical immunoreactivity for BKα was retained (B). Dibucaine did not disrupt F-actin cytoskeletal integrity, as evidenced by similar phalloidin (E and F) and FITC-DBA (G and H) staining in control and experimental CCDs.
Fig. 9.
Effect of deciliation on CCD BK channel function in microperfused rabbit CCDs. A 5-fold increase in luminal flow rate in microperfused rabbit CCDs from ~1 (slow; black bars) to 5 (fast; grey bars) nl·min–1·mm–1 led to a significant increase in JNa (left) and JK (right) in control (n = 4) CCDs. Flow-stimulated JNa and JK (FIKS) were similar in control and deciliated (n = 5) CCDs (P > 0.1). Values are means ± SE. *P < 0.05 compared with Jx at 1 nl·min–1·mm–1 in same tubules.
Deciliation dampens the flow-induced high amplitude [Ca2+]i transient in CCD.
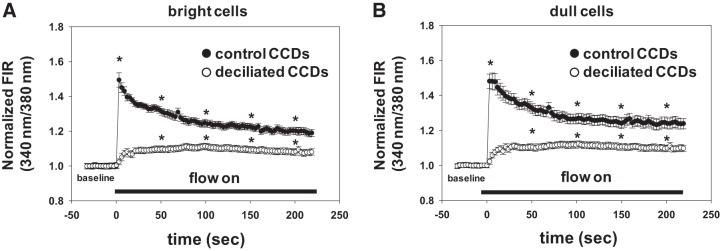
The observation that FIKS, mediated by the flow/stretch- and Ca2+-activated BK channel, is preserved in deciliated CCDs suggested to us that principal cell cilia were not required for the flow-induced Ca2+ transient necessary for BK channel activation. We thus sought to test the effect of pharmacological deciliation on [Ca2+]i transients in cells comprising a native, non-genetically modified CCD, comprised of both principal and intercalated cells. As it was necessary to confirm deciliation of CCDs, as appropriate, in this series of experiments, fura 2 calibrations were not performed. Data are thus presented as fluorescence intensity ratios (FIRs), a measure of [Ca2+]i, in bright (presumably intercalated) and dull (presumably principal) cells.
In intact control CCDs (n = 3), a rapid increase in tubular fluid flow rate, sufficient to increase diameter by 16.1 ± 0.7% within 1 s, led to a typical high-amplitude increase in FIR in both dull and bright cells (Fig. 10), followed by a gradual decay to a plateau value. As previously reported, the FIR remained significantly elevated above baseline for at least 20 min during a period of sustained high flow. Deciliation led to the loss of the initial high-amplitude Ca2+ peak in response to an increase in flow sufficient to increase diameter by 14.1 ± 1.9% (n = 3) within 1 s, but the plateau elevation in FIR (and thus Ca2+) was retained (Fig. 10). Note that these changes in FIR, and thus global cytoplasmic [Ca2+]i, may not accurately reflect changes in membrane [Ca2+] in the immediate vicinity of the BK channel.
Fig. 10.
Effect of deciliation on the flow-induced increase in fura 2 fluorescence intensity ratio (FIR), a measure of intracellular Ca2+ concentration ([Ca2+]i), in microperfused CCDs. Summary tracings of the effect of an acute increase in flow rate on the normalized fura 2 FIR in bright (presumably intercalated) and dull (principal) cells in control (vehicle-treated) and deciliated (dibucaine-treated) CCDs loaded with the Ca2+ indicator are shown. In control CCDs, an acute increase in luminal flow rate led to a typical biphasic response including an immediate rapid increase in FIR to a peak value within ~10 s, presumably secondary to release of Ca2+ from internal stores, followed by a gradual decay to a plateau [Ca2+]i value that significantly exceeds baseline for at least 10 min of sustained high flow. The latter plateau elevation in FIR is believed to represent mechano-induced Ca2+ influx into cells. Treatment of CCDs with dibucaine eliminated the immediate peak response but not the plateau elevation in FIR. Values are means ± SE; n = 3 CCDs/group. *P ≤ 0.001 compared with baseline FIR in that subset of cells.
DISCUSSION
The results of the current investigation demonstrate that immunoreactive BKα is abundant in principal cells of the CCD isolated from rabbits fed a standard K+ diet, but its expression is concentrated in the apical central cilium (Figs. 1–3). We speculate that this subcellular localization has been overlooked because investigators, including ourselves, have long focused their efforts on studying apical membrane expression of ion channels, whether by immunolabeling or patch-clamp analysis, and ignoring the cilia, long considered to be a vestigial organelle. Immunoperfusion of isolated CCDs, while laborious, allows for subsequent visualization of labeling in 3D reconstructions of tubules fixed in their native geometry (e.g., Fig. 1), and thus examination of both apical membrane and cilia in a single tubule, with little background artifact. We propose that the immunodetectable BKα, which colocalizes in the cilia with the β1-subunit, represents the functional high-conductance K+ channel detected by patch-clamp analysis of these organelles (Fig. 7). The observations that deciliation of CCDs does not affect FIKS (Fig. 9), and that dietary K+ loading results in a marked upregulation of BKα expression predominantly in the apical membrane of pendrin (+) (and thus β-type intercalated) cells in the CCD (Figs. 4 and 5) leads us to conclude that the cilia BK channel does not participate in K+ secretion. However, the impact of deciliation on mechano-induced [Ca2+]i transients in both principal and adjacent nonciliated intercalated cells in the CCD (Fig. 10) underscores the importance of this organelle as a modulator of Ca2+ and autocrine/paracrine signaling in the distal nephron.
The identification of the BK channel in cilia of principal cells is compatible with the subcellular localization of this channel reported by others in simple organisms (e.g., the Flagellar Apparatus Basal Body Proteome) (36) and other mammalian cells, such as ciliated olfactory sensory neurons (13). In the paramecium (80), the repertoire of ion channels, including KCa channels, receptors, and other transmembrane proteins in the thousands of cilia decorating the surface of the cell controls the beat frequency and wave form of the cilia, which determine swimming behavior (17, 42). Transient backward swimming, caused by reversal of the cilia power stroke, correlates with an increase in intraciliary Ca2+, generated by depolarization-induced opening of voltage-gated Ca2+ channels (Cav) that are concentrated in the cilia (16, 17, 42). Membrane potential is then returned to resting levels by the rapid activation of a voltage-gated K+ conductance and slower Ca2+-activated K+ conductance (IKCa), also both localized to the cilium (6). The Ca2+ that activates the latter KCa is local in origin, entering cilia through the ciliary Cav channels (6, 73). In cilia of olfactory sensory neurons, Ca2+-dependent K+ (13) and Cl− (33) channels contribute to olfactory sensory function. The KCa current generates a hyperpolarizing receptor potential, causing the spiking rate to decrease, leading to odor inhibition (46, 47, 70).
The patch-clamp technique has been used to examine single-channel activity in primary cilia isolated from the kidney LLC-PK1 cell line (68). Three cation-selective channels were observed, but the channels were not characterized in terms of ion selectivity and inhibitor sensitivity. Among the channels immunolocalized to the cilia of LLC-PK1 cells were the nonselective Ca2+-permeable channel PKD2 (TRPP2, or polycystin 2), TRPC1, and α-ENaC (68). Indeed, it is well established that the PKD1 gene product polycystin-1 and TRPP2 are present in primary cilia of mammalian cells (49, 56, 89) where they form heteromeric Ca2+-permeant channels that can be indirectly activated by purines, such as ATP (12). Based on the findings that TRPV4 and TRPP2 physically and functionally interact, and colocalize to the cilia of polarized collecting duct cells, a TRPP2/TRPV4 heteromer has been proposed to comprise the mechano- and thermosensitive molecular sensor in the renal epithelial cell cilium (34). The colocalization of BKα and TRPP2/TRPV4 in the cilium would be expected to provide for the temporal and spatial restriction of a mechano-induced Ca2+ signal of sufficient magnitude (μM) to activate the BK channel without deleterious effects on the cell (1, 4, 7, 50). To the extent that sustained mechano-induced Ca2+ entry into cilia will depolarize the membrane and would be expected to dampen further Ca2+ influx, the cilia BKα/β1 channel in the CCD principal cell may be necessary for repolarizing the membrane potential in response to inward depolarizing currents through cilia Ca2+ channels, as has been proposed in cell lines and lower organisms (6, 61, 68, 80).
It has been a commonly held belief that the apical central cilium, which extends from the basal body into the lumen, plays a major role in Ca2+ signaling in the CCD (49, 61, 64–66, 75). Studies in principal cell models (MDCK cells), cultured in the absence of intercalated cells, have demonstrated that direct or indirect manipulation of the cilium leads to an increase in global [Ca2+]i and that removal of the cilium in these cells abolishes flow sensing (64–66). We have shown that fluid shear acting on the apical membrane or cilium of the microperfused rabbit CCD stimulates release of Ca2+ from inositol 1,4,5-trisphosphate (IP3)-sensitive internal stores and influx of Ca2+ from the extracellular space in both principal and nonciliated intercalated cells comprising this segment (41). Furthermore, we have shown that the flow-induced plateau elevation in [Ca2+]i in perfused CCDs is mediated by luminal Ca2+ entry (37, 38, 41).
The relatively recent development of genetically encoded Ca2+ indicators targeted to the cilia of epithelial cells have allowed for the analysis of the effect of mechanical forces on changes in cilia-specific Ca2+ concentration, or [Ca2+]cilia (14, 32, 77). In mIMCD3 cells, initiation of superfusate fluid flow resulting in mechanical deformation of the cilia increased [Ca2+]cilia within 15 s of a FSS of 1 dyne/cm2 (77). In human retinal epithelial cells, changes in [Ca2+]cilia were shown to occur without substantially altering global [Ca2+]i and were proposed to be mediated by PC2 (14). Studies in LLC-PK cells revealed that the PC2-dependent flow-induced increase in [Ca2+]cilia is propogated to the cytosol and amplified through the ryanodine receptor via Ca2+-induced Ca2+ release (CICR) (32, 49). However, a very recent report, performed using mIMCD cells isolated from postpartum day P14–P21 mice expressing a ratiometric genetically encoded Ca2+ indicator and confocal microscopy with rapid image acquisition rates (1,000 frames/s), did not detect cilia-initiated Ca2+ signaling in response to physiological and supraphysiological increases in fluid flow (15). Based on their data, these authors concluded that cilia-associated mechanosensation does not originate from activation of Ca2+ channels and signaling in this organelle. Instead, the increase in [Ca2+]cilium was proposed to be initiated at other sites in the cell, with Ca2+ diffusing from the cytoplasm into the cilia (15). In fact, studies by others in MDCK cells had suggested that flow-induced [Ca2+]i signals are likely not due exclusively to direct mechano-induced Ca2+ entry through TRPP2 and other TRP channels into cilia, but likely involve release of autocrine/paracrine factors (30, 60, 62, 63, 87). The Ca2+ response is also thought to involve [Ca2+]i-dependent priming of “releasable” pools of ATP at the base of the cilium which, in turn, activates autocrine/paracrine purinergic signaling, transduced by P2 receptors on or near the cilium (30). A well-formed monocilium is essential for mechano-induced ATP secretion to occur via this mechanism.
Whereas we observed flow-induced [Ca2+]i transients in both principal and intercalated cells in deciliated CCDs, the response lacked the typical immediate (within 10 s) high-amplitude initial increase presumed to be secondary to IP3-mediated release (38, 41). In fact, the response was similar to that expected for luminal Ca2+ influx through mechanosensitiv e channels in the apical membrane (38, 41, 64). We propose that the flow-induced elevations in [Ca2+]i in the deciliated CCDs were mediated by mechano-activated cilium-independent autocrine/paracrine signaling between cells in the distal nephron. Direct mechanical, circumferential stretch, and osmotic cell swelling lead to ATP release, presumably across apical and basolateral membranes, from many epithelia, including that of the distal nephron, with subsequent P2Y2 receptor activation to increase [Ca2+]i (5, 28, 31, 60, 76, 78, 85). Although loss of apical monocilia from monolayers of collecting duct principal cells impairs apical ATP secretion (30), the isolated perfused CCD, unlike monolayers of renal epithelial cell lines, includes β intercalated cells, known to express the apical ATP-permeable mechanosensitive hemichannel connexin 30 (Cx30) in mouse, rat and rabbit kidney (26, 43, 76). Pannexin-1 (Panx1) channels, also expressed along the apical membranes of intercalated cells, may also participate in ATP secretion, as Panx1-deficient mice excrete less ATP than do wild-type animals (27). Finally, flow stimulates release into the urinary fluid of other autocrine/paracrine factors that act on their cognate receptors to cause an increase in Ca2+, such as PGE2 that activates EP receptor-associated signaling cascades (19).
The data presented in the current investigation provide additional insight into the unresolved question as to whether BK channels in intercalated and/or principal cells mediate FIKS and K+ adaptation. Support for a role for intercalated cells in these physiological processes includes the findings that 1) the density of immunodetectable apical BKα in the rabbit (18, 48) and mouse (23, 24, 59), and functional (35, 54, 55, 72) BK channel activity are greater in intercalated than in principal cells; and 2) the apical membrane voltage of intercalated cells is depolarized relative to that of principal cells (55), predicting that BK channel open probability (Po) would be high (due to the voltage sensitivity of Po), reducing the requirement for elevation of [Ca2+]i to open the channel (8, 55). However, the pathways allowing for robust basolateral K+ uptake sufficient to sustain luminal K+ secretion by intercalated cells remain to be fully defined, given that immunocytochemical (69) and functional (3, 55, 74) studies suggest that intercalated cells have low levels of Na+-K+ pump activity. Whereas BK channel-mediated FIKS in rabbit CCD requires basolateral NKCC1 (39), it remains uncertain as to how Na+, taken up at the basolateral membrane by this cotransporter, is extruded back out of the renal epithelial cells. Our current observations that FIKS is preserved in deciliated CCDs and a HK diet leads to a dramatic increase in immunodetectable BKα predominantly in pendrin (+) (β-type) intercalated cells provide additional support for the notion that this population of intercalated cells is responsible for BK channel-mediated FIKS and K+ adaptation. This hypothesis awaits testing in mouse models with cell-specific targeted genetic deletion of BKα. The predominance of cilia BKα labeling in CCDs isolated from LK-fed rabbits, which do not exhibit FIKS (48), again underscores the notion that cilia BKα/β1 channels play a role other than in FIKS.
The results of the current investigation, however, do not permit us to definitively conclude that principal cells do not mediate FIKS. BK channel activity in principal cells increases in CCDs from HK-fed rats (35), and in rabbits and rats fed a normal K+ diet, following inhibition of PKA (40) or p38/ERK (35). These observations suggest that a pool of constitutively suppressed BK channels can be activated in this cell population and recruited to secrete K+ under high flow conditions or in response to dietary K+. In fact, while immunolocalization of the BKα and β1-subunits in rabbit CCD revealed dense expression in cilia, these subunits were also detected along the apical membrane of ciliated and thus principal cells (Fig. 3). As 1) BKβ1 knockout mice subject to volume expansion reveal an attenuated kaliuretic response (58, 59), 2) coassembly of BKα- with β1-subunits generates a channel sensitive to low concentrations (50 nM) of IbTX, and 3) FIKS in mouse kidney and rabbit CCDs is inhibited by IbTX, it would seem logical to conclude that FIKS is mediated, at least in part, by a BKα/β1 channel in mice.
The BKβ1-subunit has not been detected in intercalated cells, a cell population reported to express the β4-subunit in mouse distal CNT and CCD (23). In the present study, immunolabeling of rabbit CCDs revealed diffuse β4 labeling throughout all cells (Fig. 3). Notably, β4 renders BKα resistant to low nanomolar concentrations of IbTx (44, 51). While mice lacking BKβ4 have no detectable phenotype under baseline conditions when fed a normal diet, they do show an impaired kaliuresis in response to dietary K+ loading (29). As the focus of this investigation was on the cilia BK channel, we did not define whether β-subunits are upregulated in CCDs of HK-fed animals. The composition of the BK channel mediating FIKS and K+ adaptation in the CCD, whether comprised of an α-subunit alone, or a channel assembled with a β1- or with an as yet undescribed β-subunit, remains to be clarified. Also possible, but as yet unexplored, is that the functional BK channel is assembled with a member of the recently identified leucine-rich repeat-containing subunits, also referred to as γ-subunits, which allow BK channels to open at near-physiological Ca2+ concentration and membrane voltage in nonexcitable cells (88, 90).
In sum, the results of the present study identify a high density of immunoreactive BKα and functional high-conductance K+ channels in the primary cilia of principal cells. Pharmacological deciliation of CCDs does not affect FIKS, and a HK diet leads to a greater increase in apical immunoreactive BKα in intercalated than in principal cells, consistent with the notion that intercalated, and not principal cells, mediate FIKS and contribute to the renal adaptation to dietary K+ loading. Left unanswered is the question: what is the role of the BK channel in principal cell cilia? We hypothesize that cilia BK channels are responsible for localized hyperpolarization of membrane potential in this organelle. How this affects ciliary-dependent signaling events requires further study.
GRANTS
This work was supported by National Institutes of Health (NIH) Grants DK038470 (to L. M. Satlin), DK051391 (to T. R. Kleyman), P30 DK079307 (The Pittsburgh Center for Kidney Research), and DK54983 (to W.H. Wang) and an American Heart Association Fellowship Grant (to C. Schreck).
DISCLOSURES
Conflict of interest statement: No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.C.-G., T.R.K., W.-H.W., and L.M.S. provided conception and design of research; R.C.-G., L.W., C.M.N.S., and L.M.S. performed experiments; R.C.-G., L.W., W.-H.W., and L.M.S. analyzed data; R.C.-G., T.R.K., W.-H.W., and L.M.S. interpreted results of experiments; R.C.-G., L.W., W.-H.W., and L.M.S. prepared figures; R.C.-G. and L.M.S. drafted manuscript; R.C.-G., C.M.N.S., T.R.K., W.-H.W., and L.M.S. edited and revised manuscript; R.C.-G., L.W., C.M.N.S., T.R.K., W.-H.W., and L.M.S. approved final version of manuscript.
REFERENCES
- 1.Augustine GJ, Santamaria F, Tanaka K. Local calcium signaling in neurons. Neuron 40: 331–346, 2003. doi: 10.1016/S0896-6273(03)00639-1. [DOI] [PubMed] [Google Scholar]
- 2.Bailey MA, Cantone A, Yan Q, MacGregor GG, Leng Q, Amorim JB, Wang T, Hebert SC, Giebisch G, Malnic G. Maxi-K channels contribute to urinary potassium excretion in the ROMK-deficient mouse model of Type II Bartter’s syndrome and in adaptation to a high-K diet. Kidney Int 70: 51–59, 2006. doi: 10.1038/sj.ki.5000388. [DOI] [PubMed] [Google Scholar]
- 3.Beck FX, Dörge A, Blümner E, Giebisch G, Thurau K. Cell rubidium uptake: a method for studying functional heterogeneity in the nephron. Kidney Int 33: 642–651, 1988. doi: 10.1038/ki.1988.47. [DOI] [PubMed] [Google Scholar]
- 4.Berkefeld H, Sailer CA, Bildl W, Rohde V, Thumfart JO, Eble S, Klugbauer N, Reisinger E, Bischofberger J, Oliver D, Knaus HG, Schulte U, Fakler B. BKCa-Cav channel complexes mediate rapid and localized Ca2+-activated K+ signaling. Science 314: 615–620, 2006. doi: 10.1126/science.1132915. [DOI] [PubMed] [Google Scholar]
- 5.Bjaelde RG, Arnadottir SS, Overgaard MT, Leipziger J, Praetorius HA. Renal epithelial cells can release ATP by vesicular fusion. Front Physiol 4: 238, 2013. doi: 10.3389/fphys.2013.00238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brehm P, Dunlap K, Eckert R. Calcium-dependent repolarization in Paramecium. J Physiol 274: 639–654, 1978. doi: 10.1113/jphysiol.1978.sp012171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brenner R, Jegla TJ, Wickenden A, Liu Y, Aldrich RW. Cloning and functional characterization of novel large conductance calcium-activated potassium channel beta subunits, hKCNMB3 and hKCNMB4. J Biol Chem 275: 6453–6461, 2000. doi: 10.1074/jbc.275.9.6453. [DOI] [PubMed] [Google Scholar]
- 8.Butler A, Tsunoda S, McCobb DP, Wei A, Salkoff L. mSlo, a complex mouse gene encoding “maxi” calcium-activated potassium channels. Science 261: 221–224, 1993. doi: 10.1126/science.7687074. [DOI] [PubMed] [Google Scholar]
- 9.Candia S, Garcia ML, Latorre R. Mode of action of iberiotoxin, a potent blocker of the large conductance Ca(2+)-activated K+ channel. Biophys J 63: 583–590, 1992. doi: 10.1016/S0006-3495(92)81630-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carrisoza-Gaytan R, Liu Y, Flores D, Else C, Lee HG, Rhodes G, Sandoval RM, Kleyman TR, Lee FY, Molitoris B, Satlin LM, Rohatgi R. Effects of biomechanical forces on signaling in the cortical collecting duct (CCD). Am J Physiol Renal Physiol 307: F195–F204, 2014. doi: 10.1152/ajprenal.00634.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choi YH, Suzuki A, Hajarnis S, Ma Z, Chapin HC, Caplan MJ, Pontoglio M, Somlo S, Igarashi P. Polycystin-2 and phosphodiesterase 4C are components of a ciliary A-kinase anchoring protein complex that is disrupted in cystic kidney diseases. Proc Natl Acad Sci USA 108: 10679–10684, 2011. doi: 10.1073/pnas.1016214108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeCaen PG, Delling M, Vien TN, Clapham DE. Direct recording and molecular identification of the calcium channel of primary cilia. Nature 504: 315–318, 2013. doi: 10.1038/nature12832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Delgado R, Saavedra MV, Schmachtenberg O, Sierralta J, Bacigalupo J. Presence of Ca2+-dependent K+ channels in chemosensory cilia support a role in odor transduction. J Neurophysiol 90: 2022–2028, 2003. doi: 10.1152/jn.01167.2002. [DOI] [PubMed] [Google Scholar]
- 14.Delling M, DeCaen PG, Doerner JF, Febvay S, Clapham DE. Primary cilia are specialized calcium signalling organelles. Nature 504: 311–314, 2013. doi: 10.1038/nature12833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Delling M, Indzhykulian AA, Liu X, Li Y, Xie T, Corey DP, Clapham DE. Primary cilia are not calcium-responsive mechanosensors. Nature 531: 656–660, 2016. doi: 10.1038/nature17426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dunlap K. Localization of calcium channels in Paramecium caudatum. J Physiol 271: 119–133, 1977. doi: 10.1113/jphysiol.1977.sp011993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eckert R. Bioelectric control of ciliary activity. Science 176: 473–481, 1972. doi: 10.1126/science.176.4034.473. [DOI] [PubMed] [Google Scholar]
- 18.Estilo G, Liu W, Pastor-Soler N, Mitchell P, Carattino MD, Kleyman TR, Satlin LM. Effect of aldosterone on BK channel expression in mammalian cortical collecting duct. Am J Physiol Renal Physiol 295: F780–F788, 2008. doi: 10.1152/ajprenal.00002.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flores D, Liu Y, Liu W, Satlin LM, Rohatgi R. Flow-induced prostaglandin E2 release regulates Na and K transport in the collecting duct. Am J Physiol Renal Physiol 303: F632–F638, 2012. doi: 10.1152/ajprenal.00169.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frindt G, Palmer LG. Apical potassium channels in the rat connecting tubule. Am J Physiol Renal Physiol 287: F1030–F1037, 2004. doi: 10.1152/ajprenal.00169.2004. [DOI] [PubMed] [Google Scholar]
- 21.Frindt G, Palmer LG. Low-conductance K channels in apical membrane of rat cortical collecting tubule. Am J Physiol Renal Physiol 256: F143–F151, 1989. [DOI] [PubMed] [Google Scholar]
- 22.Galvez A, Gimenez-Gallego G, Reuben JP, Roy-Contancin L, Feigenbaum P, Kaczorowski GJ, Garcia ML. Purification and characterization of a unique, potent, peptidyl probe for the high conductance calcium-activated potassium channel from venom of the scorpion Buthus tamulus. J Biol Chem 265: 11083–11090, 1990. [PubMed] [Google Scholar]
- 23.Grimm PR, Foutz RM, Brenner R, Sansom SC. Identification and localization of BK-beta subunits in the distal nephron of the mouse kidney. Am J Physiol Renal Physiol 293: F350–F359, 2007. doi: 10.1152/ajprenal.00018.2007. [DOI] [PubMed] [Google Scholar]
- 24.Grimm PR, Irsik DL, Liu L, Holtzclaw JD, Sansom SC. Role of BKβ1 in Na+ reabsorption by cortical collecting ducts of Na+-deprived mice. Am J Physiol Renal Physiol 297: F420–F428, 2009. doi: 10.1152/ajprenal.00191.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grimm PR, Irsik DL, Settles DC, Holtzclaw JD, Sansom SC. Hypertension of Kcnmb1−/− is linked to deficient K secretion and aldosteronism. Proc Natl Acad Sci USA 106: 11800–11805, 2009. doi: 10.1073/pnas.0904635106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gueutin V, Vallet M, Jayat M, Peti-Peterdi J, Cornière N, Leviel F, Sohet F, Wagner CA, Eladari D, Chambrey R. Renal β-intercalated cells maintain body fluid and electrolyte balance. J Clin Invest 123: 4219–4231, 2013. doi: 10.1172/JCI63492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hanner F, Lam L, Nguyen MT, Yu A, Peti-Peterdi J. Intrarenal localization of the plasma membrane ATP channel pannexin1. Am J Physiol Renal Physiol 303: F1454–F1459, 2012. doi: 10.1152/ajprenal.00206.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holtzclaw JD, Cornelius RJ, Hatcher LI, Sansom SC. Coupled ATP and potassium efflux from intercalated cells. Am J Physiol Renal Physiol 300: F1319–F1326, 2011. doi: 10.1152/ajprenal.00112.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holtzclaw JD, Grimm PR, Sansom SC. Intercalated cell BK-α/β4 channels modulate sodium and potassium handling during potassium adaptation. J Am Soc Nephrol 21: 634–645, 2010. doi: 10.1681/ASN.2009080817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hovater MB, Olteanu D, Hanson EL, Cheng NL, Siroky B, Fintha A, Komlosi P, Liu W, Satlin LM, Bell PD, Yoder BK, Schwiebert EM. Loss of apical monocilia on collecting duct principal cells impairs ATP secretion across the apical cell surface and ATP-dependent and flow-induced calcium signals. Purinergic Signal 4: 155–170, 2008. doi: 10.1007/s11302-007-9072-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jensen ME, Odgaard E, Christensen MH, Praetorius HA, Leipziger J. Flow-induced [Ca2+]i increase depends on nucleotide release and subsequent purinergic signaling in the intact nephron. J Am Soc Nephrol 18: 2062–2070, 2007. doi: 10.1681/ASN.2006070700. [DOI] [PubMed] [Google Scholar]
- 32.Jin X, Mohieldin AM, Muntean BS, Green JA, Shah JV, Mykytyn K, Nauli SM. Cilioplasm is a cellular compartment for calcium signaling in response to mechanical and chemical stimuli. Cell Mol Life Sci 71: 2165–2178, 2014. doi: 10.1007/s00018-013-1483-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kleene SJ. Origin of the chloride current in olfactory transduction. Neuron 11: 123–132, 1993. doi: 10.1016/0896-6273(93)90276-W. [DOI] [PubMed] [Google Scholar]
- 34.Köttgen M, Buchholz B, Garcia-Gonzalez MA, Kotsis F, Fu X, Doerken M, Boehlke C, Steffl D, Tauber R, Wegierski T, Nitschke R, Suzuki M, Kramer-Zucker A, Germino GG, Watnick T, Prenen J, Nilius B, Kuehn EW, Walz G. TRPP2 and TRPV4 form a polymodal sensory channel complex. J Cell Biol 182: 437–447, 2008. doi: 10.1083/jcb.200805124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li D, Wang Z, Sun P, Jin Y, Lin DH, Hebert SC, Giebisch G, Wang WH. Inhibition of MAPK stimulates the Ca2+ -dependent big-conductance K channels in cortical collecting duct. Proc Natl Acad Sci USA 103: 19569–19574, 2006. doi: 10.1073/pnas.0609555104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li JB, Gerdes JM, Haycraft CJ, Fan Y, Teslovich TM, May-Simera H, Li H, Blacque OE, Li L, Leitch CC, Lewis RA, Green JS, Parfrey PS, Leroux MR, Davidson WS, Beales PL, Guay-Woodford LM, Yoder BK, Stormo GD, Katsanis N, Dutcher SK. Comparative genomics identifies a flagellar and basal body proteome that includes the BBS5 human disease gene. Cell 117: 541–552, 2004. doi: 10.1016/S0092-8674(04)00450-7. [DOI] [PubMed] [Google Scholar]
- 37.Liu W, Morimoto T, Woda C, Kleyman TR, Satlin LM. Ca2+ dependence of flow-stimulated K secretion in the mammalian cortical collecting duct. Am J Physiol Renal Physiol 293: F227–F235, 2007. doi: 10.1152/ajprenal.00057.2007. [DOI] [PubMed] [Google Scholar]
- 38.Liu W, Murcia NS, Duan Y, Weinbaum S, Yoder BK, Schwiebert E, Satlin LM. Mechanoregulation of intracellular Ca2+ concentration is attenuated in collecting duct of monocilium-impaired orpk mice. Am J Physiol Renal Physiol 289: F978–F988, 2005. doi: 10.1152/ajprenal.00260.2004. [DOI] [PubMed] [Google Scholar]
- 39.Liu W, Schreck C, Coleman RA, Wade JB, Hernandez Y, Zavilowitz B, Warth R, Kleyman TR, Satlin LM. Role of NKCC in BK channel-mediated net K+ secretion in the CCD. Am J Physiol Renal Physiol 301: F1088–F1097, 2011. doi: 10.1152/ajprenal.00347.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu W, Wei Y, Sun P, Wang WH, Kleyman TR, Satlin LM. Mechanoregulation of BK channel activity in the mammalian cortical collecting duct: role of protein kinases A and C. Am J Physiol Renal Physiol 297: F904–F915, 2009. doi: 10.1152/ajprenal.90685.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu W, Xu S, Woda C, Kim P, Weinbaum S, Satlin LM. Effect of flow and stretch on the [Ca2+]i response of principal and intercalated cells in cortical collecting duct. Am J Physiol Renal Physiol 285: F998–F1012, 2003. doi: 10.1152/ajprenal.00067.2003. [DOI] [PubMed] [Google Scholar]
- 42.Machemer H, Ogura A. Ionic conductances of membranes in ciliated and deciliated Paramecium. J Physiol 296: 49–60, 1979. doi: 10.1113/jphysiol.1979.sp012990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McCulloch F, Chambrey R, Eladari D, Peti-Peterdi J. Localization of connexin 30 in the luminal membrane of cells in the distal nephron. Am J Physiol Renal Physiol 289: F1304–F1312, 2005. doi: 10.1152/ajprenal.00203.2005. [DOI] [PubMed] [Google Scholar]
- 44.Meera P, Wallner M, Toro L. A neuronal beta subunit (KCNMB4) makes the large conductance, voltage- and Ca2+-activated K+ channel resistant to charybdotoxin and iberiotoxin. Proc Natl Acad Sci USA 97: 5562–5567, 2000. doi: 10.1073/pnas.100118597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Misonou H, Menegola M, Buchwalder L, Park EW, Meredith A, Rhodes KJ, Aldrich RW, Trimmer JS. Immunolocalization of the Ca2+-activated K+ channel Slo1 in axons and nerve terminals of mammalian brain and cultured neurons. J Comp Neurol 496: 289–302, 2006. doi: 10.1002/cne.20931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morales B, Madrid R, Bacigalupo J. Calcium mediates the activation of the inhibitory current induced by odorants in toad olfactory receptor neurons. FEBS Lett 402: 259–264, 1997. doi: 10.1016/S0014-5793(97)00005-7. [DOI] [PubMed] [Google Scholar]
- 47.Morales B, Ugarte G, Labarca P, Bacigalupo J. Inhibitory K+ current activated by odorants in toad olfactory neurons. Proc Biol Sci 257: 235–242, 1994. doi: 10.1098/rspb.1994.0120. [DOI] [PubMed] [Google Scholar]
- 48.Najjar F, Zhou H, Morimoto T, Bruns JB, Li HS, Liu W, Kleyman TR, Satlin LM. Dietary K+ regulates apical membrane expression of maxi-K channels in rabbit cortical collecting duct. Am J Physiol Renal Physiol 289: F922–F932, 2005. doi: 10.1152/ajprenal.00057.2005. [DOI] [PubMed] [Google Scholar]
- 49.Nauli SM, Alenghat FJ, Luo Y, Williams E, Vassilev P, Li X, Elia AE, Lu W, Brown EM, Quinn SJ, Ingber DE, Zhou J. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet 33: 129–137, 2003. doi: 10.1038/ng1076. [DOI] [PubMed] [Google Scholar]
- 50.Neher E. Vesicle pools and Ca2+ microdomains: new tools for understanding their roles in neurotransmitter release. Neuron 20: 389–399, 1998. doi: 10.1016/S0896-6273(00)80983-6. [DOI] [PubMed] [Google Scholar]
- 51.Nehrke K, Quinn CC, Begenisich T. Molecular identification of Ca2+-activated K+ channels in parotid acinar cells. Am J Physiol Cell Physiol 284: C535–C546, 2003. doi: 10.1152/ajpcell.00044.2002. [DOI] [PubMed] [Google Scholar]
- 52.O’Connor AK, Malarkey EB, Berbari NF, Croyle MJ, Haycraft CJ, Bell PD, Hohenstein P, Kesterson RA, Yoder BK. An inducible CiliaGFP mouse model for in vivo visualization and analysis of cilia in live tissue. Cilia 2: 8, 2013. doi: 10.1186/2046-2530-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Overgaard CE, Sanzone KM, Spiczka KS, Sheff DR, Sandra A, Yeaman C. Deciliation is associated with dramatic remodeling of epithelial cell junctions and surface domains. Mol Biol Cell 20: 102–113, 2009. doi: 10.1091/mbc.E08-07-0741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pácha J, Frindt G, Sackin H, Palmer LG. Apical maxi K channels in intercalated cells of CCT. Am J Physiol Renal Physiol 261: F696–F705, 1991. [DOI] [PubMed] [Google Scholar]
- 55.Palmer LG, Frindt G. High-conductance K channels in intercalated cells of the rat distal nephron. Am J Physiol Renal Physiol 292: F966–F973, 2006. doi: 10.1152/ajprenal.00191.2006. [DOI] [PubMed] [Google Scholar]
- 56.Pazour GJ, San Agustin JT, Follit JA, Rosenbaum JL, Witman GB. Polycystin-2 localizes to kidney cilia and the ciliary level is elevated in orpk mice with polycystic kidney disease. Curr Biol 12: R378–R380, 2002. doi: 10.1016/S0960-9822(02)00877-1. [DOI] [PubMed] [Google Scholar]
- 57.Pluznick JL, Sansom SC. BK channels in the kidney: role in K(+) secretion and localization of molecular components. Am J Physiol Renal Physiol 291: F517–F529, 2006. doi: 10.1152/ajprenal.00118.2006. [DOI] [PubMed] [Google Scholar]
- 58.Pluznick JL, Wei P, Carmines PK, Sansom SC. Renal fluid and electrolyte handling in BKCa-β1−/− mice. Am J Physiol Renal Physiol 284: F1274–F1279, 2003. doi: 10.1152/ajprenal.00010.2003. [DOI] [PubMed] [Google Scholar]
- 59.Pluznick JL, Wei P, Grimm PR, Sansom SC. BK-β1 subunit: immunolocalization in the mammalian connecting tubule and its role in the kaliuretic response to volume expansion. Am J Physiol Renal Physiol 288: F846–F854, 2004. doi: 10.1152/ajprenal.00340.2004. [DOI] [PubMed] [Google Scholar]
- 60.Praetorius HA, Frøkiaer J, Leipziger J. Transepithelial pressure pulses induce nucleotide release in polarized MDCK cells. Am J Physiol Renal Physiol 288: F133–F141, 2005. doi: 10.1152/ajprenal.00238.2004. [DOI] [PubMed] [Google Scholar]
- 61.Praetorius HA, Frokiaer J, Nielsen S, Spring KR. Bending the primary cilium opens Ca2+-sensitive intermediate-conductance K+ channels in MDCK cells. J Membr Biol 191: 193–200, 2003. doi: 10.1007/s00232-002-1055-z. [DOI] [PubMed] [Google Scholar]
- 62.Praetorius HA, Leipziger J. Fluid flow sensing and triggered nucleotide release in epithelia. J Physiol 586: 2669, 2008. doi: 10.1113/jphysiol.2008.155085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Praetorius HA, Leipziger J. Primary cilium-dependent sensing of urinary flow and paracrine purinergic signaling. Semin Cell Dev Biol 24: 3–10, 2013. doi: 10.1016/j.semcdb.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 64.Praetorius HA, Spring KR. Bending the MDCK cell primary cilium increases intracellular calcium. J Membr Biol 184: 71–79, 2001. doi: 10.1007/s00232-001-0075-4. [DOI] [PubMed] [Google Scholar]
- 65.Praetorius HA, Spring KR. Removal of the MDCK cell primary cilium abolishes flow sensing. J Membr Biol 191: 69–76, 2003. doi: 10.1007/s00232-002-1042-4. [DOI] [PubMed] [Google Scholar]
- 66.Praetorius HA, Spring KR. The renal cell primary cilium functions as a flow sensor. Curr Opin Nephrol Hypertens 12: 517–520, 2003. doi: 10.1097/00041552-200309000-00006. [DOI] [PubMed] [Google Scholar]
- 67.Procino G, Milano S, Tamma G, Dossena S, Barbieri C, Nicoletti MC, Ranieri M, Di Mise A, Nofziger C, Svelto M, Paulmichl M, Valenti G. Co-regulated pendrin and aquaporin 5 expression and trafficking in Type-B intercalated cells under potassium depletion. Cell Physiol Biochem 32: 184–199, 2013. doi: 10.1159/000356638. [DOI] [PubMed] [Google Scholar]
- 68.Raychowdhury MK, McLaughlin M, Ramos AJ, Montalbetti N, Bouley R, Ausiello DA, Cantiello HF. Characterization of single channel currents from primary cilia of renal epithelial cells. J Biol Chem 280: 34718–34722, 2005. doi: 10.1074/jbc.M507793200. [DOI] [PubMed] [Google Scholar]
- 69.Sabolić I, Herak-Kramberger CM, Breton S, Brown D. Na/K-ATPase in intercalated cells along the rat nephron revealed by antigen retrieval. J Am Soc Nephrol 10: 913–922, 1999. [DOI] [PubMed] [Google Scholar]
- 70.Sanhueza M, Schmachtenberg O, Bacigalupo J. Excitation, inhibition, and suppression by odors in isolated toad and rat olfactory receptor neurons. Am J Physiol Cell Physiol 279: C31–C39, 2000. [DOI] [PubMed] [Google Scholar]
- 71.Satlin LM, Palmer LG. Apical K+ conductance in maturing rabbit principal cell. Am J Physiol 272: F397–F404, 1997. [DOI] [PubMed] [Google Scholar]
- 72.Satlin LM, Palmer LG. Apical Na+ conductance in maturing rabbit principal cell. Am J Physiol Renal Physiol 270: F391–F397, 1996. [DOI] [PubMed] [Google Scholar]
- 73.Satow Y, Kung C. Ca-induced K+-outward current in Paramecium tetraurelia. J Exp Biol 88: 293–303, 1980. [DOI] [PubMed] [Google Scholar]
- 74.Sauer M, Flemmer A, Thurau K, Beck FX. Sodium entry routes in principal and intercalated cells of the isolated perfused cortical collecting duct. Pflugers Arch 416: 88–93, 1990. doi: 10.1007/BF00370227. [DOI] [PubMed] [Google Scholar]
- 75.Schwartz EA, Leonard ML, Bizios R, Bowser SS. Analysis and modeling of the primary cilium bending response to fluid shear. Am J Physiol Renal Physiol 272: F132–F138, 1997. [DOI] [PubMed] [Google Scholar]
- 76.Sipos A, Vargas SL, Toma I, Hanner F, Willecke K, Peti-Peterdi J. Connexin 30 deficiency impairs renal tubular ATP release and pressure natriuresis. J Am Soc Nephrol 20: 1724–1732, 2009. doi: 10.1681/ASN.2008101099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Su S, Phua SC, DeRose R, Chiba S, Narita K, Kalugin PN, Katada T, Kontani K, Takeda S, Inoue T. Genetically encoded calcium indicator illuminates calcium dynamics in primary cilia. Nat Methods 10: 1105–1107, 2013. doi: 10.1038/nmeth.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Svenningsen P, Burford JL, Peti-Peterdi J. ATP releasing connexin 30 hemichannels mediate flow-induced calcium signaling in the collecting duct. Front Physiol 4: 292, 2013. doi: 10.3389/fphys.2013.00292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Taniguchi J, Imai M. Flow-dependent activation of maxi K+ channels in apical membrane of rabbit connecting tubule. J Membr Biol 164: 35–45, 1998. doi: 10.1007/s002329900391. [DOI] [PubMed] [Google Scholar]
- 80.Valentine MS, Rajendran A, Yano J, Weeraratne SD, Beisson J, Cohen J, Koll F, Van Houten J. Paramecium BBS genes are key to presence of channels in Cilia. Cilia 1: 16, 2012. doi: 10.1186/2046-2530-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.van der Lubbe N, Moes AD, Rosenbaek LL, Schoep S, Meima ME, Danser AH, Fenton RA, Zietse R, Hoorn EJK. K+-induced natriuresis is preserved during Na+ depletion and accompanied by inhibition of the Na+-Cl− cotransporter. Am J Physiol Renal Physiol 305: F1177–F1188, 2013. doi: 10.1152/ajprenal.00201.2013. [DOI] [PubMed] [Google Scholar]
- 82.Wagner CA, Finberg KE, Stehberger PA, Lifton RP, Giebisch GH, Aronson PS, Geibel JP. Regulation of the expression of the Cl-/anion exchanger pendrin in mouse kidney by acid-base status. Kidney Int 62: 2109–2117, 2002. doi: 10.1046/j.1523-1755.2002.00671.x. [DOI] [PubMed] [Google Scholar]
- 83.Wang WH, Schwab A, Giebisch G. Regulation of small-conductance K+ channel in apical membrane of rat cortical collecting tubule. Am J Physiol Renal Physiol 259: F494–F502, 1990. [DOI] [PubMed] [Google Scholar]
- 84.Woda CB, Bragin A, Kleyman TR, Satlin LM. Flow-dependent K+ secretion in the cortical collecting duct is mediated by a maxi-K channel. Am J Physiol Renal Physiol 280: F786–F793, 2001. [DOI] [PubMed] [Google Scholar]
- 85.Woda CB, Leite M Jr, Rohatgi R, Satlin LM. Effects of luminal flow and nucleotides on [Ca(2+)](i) in rabbit cortical collecting duct. Am J Physiol Renal Physiol 283: F437–F446, 2002. doi: 10.1152/ajprenal.00316.2001. [DOI] [PubMed] [Google Scholar]
- 86.Woda CB, Miyawaki N, Ramalakshmi S, Ramkumar M, Rojas R, Zavilowitz B, Kleyman TR, Satlin LM. Ontogeny of flow-stimulated potassium secretion in rabbit cortical collecting duct: functional and molecular aspects. Am J Physiol Renal Physiol 285: F629–F639, 2003. doi: 10.1152/ajprenal.00191.2003. [DOI] [PubMed] [Google Scholar]
- 87.Xu C, Shmukler BE, Nishimura K, Kaczmarek E, Rossetti S, Harris PC, Wandinger-Ness A, Bacallao RL, Alper SL. Attenuated, flow-induced ATP release contributes to absence of flow-sensitive, purinergic Cai2+ signaling in human ADPKD cyst epithelial cells. Am J Physiol Renal Physiol 296: F1464–F1476, 2009. doi: 10.1152/ajprenal.90542.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yan J, Aldrich RW. LRRC26 auxiliary protein allows BK channel activation at resting voltage without calcium. Nature 466: 513–516, 2010. doi: 10.1038/nature09162. [DOI] [PubMed] [Google Scholar]
- 89.Yoder BK, Hou X, Guay-Woodford LM. The polycystic kidney disease proteins, polycystin-1, polycystin-2, polaris, and cystin, are co-localized in renal cilia. J Am Soc Nephrol 13: 2508–2516, 2002. doi: 10.1097/01.ASN.0000029587.47950.25. [DOI] [PubMed] [Google Scholar]
- 90.Zhang J, Yan J. Regulation of BK channels by auxiliary γ subunits. Front Physiol 5: 401, 2014. doi: 10.3389/fphys.2014.00401. [DOI] [PMC free article] [PubMed] [Google Scholar]