Abstract
Autosomal dominant polycystic kidney disease (ADPKD) is the most common life-threatening monogenic renal disease. ADPKD results from mutations in either of two proteins: polycystin-1 (also known as PC1 or PKD1) or transient receptor potential cation channel, subfamily P, member 2 (TRPP2, also known as polycystin-2, PC2, or PKD2). Each of these proteins is expressed in the primary cilium that extends from many renal epithelial cells. Existing evidence suggests that the cilium can promote renal cystogenesis, while PC1 and TRPP2 counter this cystogenic effect. To better understand the function of TRPP2, we investigated its electrophysiological properties in the native ciliary membrane. We recorded directly from the cilia of mIMCD-3 cells, a murine cell line of renal epithelial origin. In one-third of cilia examined, a large-conductance channel was observed. The channel was not permeable to Cl¯ but conducted cations with permeability ratios PK:PCa:PNa of 1:0.55:0.14. The single-channel conductance ranged from 97 pS in typical physiological solutions to 189 pS in symmetrical 145 mM KCl. Open probability of the channel was very sensitive to membrane depolarization or increasing cytoplasmic free Ca2+ in the low micromolar range, with the open probability increasing in either case. Knocking out TRPP2 by CRISPR/Cas9 genome editing eliminated the channel current, establishing it as TRPP2 dependent. Possible mechanisms for activating the TRPP2-dependent channel in the renal primary cilium are discussed.
Keywords: primary cilium, polycystic kidney disease, TRPP2, PC2, polycystin
autosomal dominant polycystic kidney disease (ADPKD) is one of the most common monogenic diseases, with an incidence between 1:400 and 1:1,000 (22). More than half of patients progress to end-stage renal disease by the age of 60 (56). ADPKD is caused by mutations in either of two membrane proteins: polycystin-1 (also known as PC1 or PKD1) or TRPP2 (also known as polycystin-2, PC2, or PKD2) (14, 42). TRPP2 is a member of subfamily P of the transient receptor potential (TRP) family of cation channels (57).
It has long been proposed that ADPKD is a ciliopathy—a disease due to a defect in the primary cilia of the renal epithelial cells (63). Early evidence for this was circumstantial. PC1 and TRPP2, which when mutated lead to ADPKD, reside in the cilium (47, 66), but not exclusively (35). It has also been noted that animals lacking renal cilia develop renal cysts (38, 43, 46). However, these are imperfect models of human disease, since human ADPKD patients have renal cilia that appear to be grossly normal (63). A recent study provides strong evidence that the cilium contributes to cystogenesis. In mice that lack PC1 or TRPP2, cystogenesis is more severe when the cilium is present than when it is removed (40). This indicates that the cilium itself has a pro-cystogenic function; the mechanism of this is unknown. In a normal renal cell, PC1 and TRPP2 must oppose the pro-cystogenic function of the cilium so that no cysts result (40). Since TRPP2 channels conduct Ca2+, it has been widely proposed that an influx of Ca2+ into the primary cilium suppresses cystogenesis (1, 27, 40). Identifying the function of TRPP2, particularly in the primary cilium, has been identified as a key problem in trying to understand cystic kidney disease (20).
To date, the small size of the cilium has largely precluded direct measurement of the electrophysiological properties of ciliary TRPP2 channels. Nonetheless, there has been extensive study of TRPP2 and TRPP2-like channels: in channel subunits expressed exogenously (2, 9, 12, 19, 21, 39, 41, 62, 67, 68) or in artificial bilayers (9, 18, 19, 36, 52, 53, 67); in detached, previously frozen cilia (52); or in non-ciliary cellular compartments (3, 18, 19, 28, 39, 48, 67, 68). As noted elsewhere (3, 7, 61, 64), the conclusions have sometimes been contradictory. In different preparations, for example, channels that incorporate the TRPP2 channel subunit opened almost exclusively at negative potentials (18, 19, 36) or only at positive potentials (3). It remained unclear how the channels function in the unique microenvironment of the cilium.
It has recently become possible to directly measure channel activities in the membrane of an attached or freshly detached primary cilium (8, 10, 30). In developing such a method, we noted a large-conductance ciliary channel in a cell line derived from murine renal epithelium (30). We now identify this channel as a TRPP2-dependent channel and describe its gating and conductance properties.
MATERIALS AND METHODS
Electrical recording.
Electrical recordings were made from primary cilia of mIMCD-3 cells as described previously (30). In short, mIMCD-3 cells [murine epithelial cells from the renal inner medullary collecting duct, CRL-2123, American Type Culture Collection, Manassas, VA (51)] were cultured on beads that were free to move in the recording chamber. Suction was applied to a recording pipette so that a single primary cilium entered the pipette. If a resistance of at least 1 GΩ formed between the membrane and the pipette, the cilium was further studied. Recordings were made with the cilium still attached to the cell or after the cilium was excised from the cell. Excision left the cilium inside the recording pipette in the inside-out configuration. The pipette containing the cilium could then be transferred among different solutions that bathed the cytoplasmic face of the membrane. When comparing the wild-type (WT) and TRPP2 knockout (KO) lines, the electrophysiologist was not told from which line he was recording until analysis of all of the recordings was completed.
During recording, the beads coated with cells were stored in a standard external solution (Table 1). The recording pipettes also contained this solution except as noted. Leak currents were not subtracted except as indicated. Cytoplasmic solutions contained from 1 µM to 300 µM free Ca2+ as noted. At 300 µM Ca2+ only, another ciliary channel (TRPM4) may be activated (15). When 300 µM Ca2+ was used in single-channel studies, cilia were rejected if TRPM4-dependent current precluded analysis of the TRPP2 channels (as judged by the amplitude histogram). For some recordings as noted, inside-out excised patches of apical membrane were used instead of cilia. The presence or absence of active TRPP2 channels was assessed with the cilium or patch exposed to 3 µM cytoplasmic Ca2+ and the voltage clamped to +40 mV. If no large-conductance channels were seen to open within 2 min, active TRPP2 channels were judged to be absent.
Table 1.
Compositions of solutions (mM)
| KCl | NaCl | KMeSO3 | CaCl2 | CaGluc | MgCl2 | MgGluc | HEPES | BAPTA | DibromoBAPTA | HEDTA | d-Glucose | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytoplasmic | ||||||||||||
| 0.1 µM free Ca2+ (standard) | 140 | 5 | 0.7 | 2 | 5 | 2 | 5 | |||||
| 1–3 µM free Ca2+ | 140 | 5 | 0.9–1.4 | 2 | 5 | 2 | 5 | |||||
| 10–30 µM free Ca2+ | 140 | 5 | 0.8–1.4 | 2 | 5 | 2 | 5 | |||||
| 300 µM free Ca2+ | 140 | 5 | 2.3 | 2 | 5 | 2 | 5 | |||||
| 1 mM free Ca2+ | 140 | 5 | 1 | 2 | 5 | 5 | ||||||
| High-K+, 0.1–300 µM free Ca2+ | 145 | * | 2 | 5 | * | * | * | 5 | ||||
| High-Na+, 3 µM free Ca2+ | 145 | 1.4 | 2 | 5 | 2 | 5 | ||||||
| High-Na+, 300 µM free Ca2+ | 145 | 2.3 | 2 | 5 | 2 | 5 | ||||||
| Cl¯-free, 3 µM free Ca2+ | 145 | 1.4 | 2 | 5 | 2 | 5 | ||||||
| Cl¯-free, 300 µM free Ca2+ | 145 | 2.3 | 2 | 5 | 2 | 5 |
| NaCl | KCl | CaCl2 | MgCl2 | HEPES | NaPyr | d-Glucose | |
|---|---|---|---|---|---|---|---|
| External | |||||||
| Standard | 140 | 5 | 2 | 2 | 5 | 2 | 9.4 |
| High-K+ | 145 | 2 | 2 | 5 | 2 | 9.4 | |
| High-Ca2+ | 100 | 2 | 5 | 9.4 |
BAPTA and dibromoBAPTA were added as the tetrapotassium salts and HEDTA as the tripotassium salt. In solutions containing 300 µM free Ca2+, the Ca2+ buffer (BAPTA) was saturated. Solutions were adjusted to pH 7.4 with the hydroxide of the principal cation, or with Tris base (high-Ca2+ external solution). KMeSO3, potassium methanesulfonate; CaGluc, calcium gluconate; MgGluc, magnesium gluconate; NaPyr, sodium pyruvate.
Concentrations of CaCl2 and Ca2+ buffer (BAPTA, dibromoBAPTA, or HEDTA) are as shown for the first set of four solutions.
All recordings were done under voltage clamp at room temperature (24°C). The recording pipette and chamber were coupled by Ag/AgCl electrodes to an Axopatch 200B patch-clamp amplifier with a CV203BU headstage and Digidata 1200A BNC data-acquisition system, controlled by pCLAMP 5.7.1 software (all from Axon Instruments/Molecular Devices, Sunnyvale, CA). The voltage ramps were of 1-s duration. The pipette was positioned with a Narishige MO-103M hydraulic micromanipulator (Narishige, Tokyo, Japan). During acquisition, currents were low-pass filtered at 2 kHz and digitized at 5 kHz. As described elsewhere (31), corrections were applied for each of two liquid junction potentials. The first, which was <5 mV, existed between each cytoplasmic bath and its salt bridge. The second potential existed during the patch procedure when the pipette solution was different from the solution bathing the cells. The largest such correction was 7 mV between high-Ca2+ (pipette) and standard (bath) external solutions. For excised cilia, potentials are given as membrane potential (i.e., cytoplasmic relative to external). Software for analysis included Origin 7.0 (OriginLab, Northampton, MA) and QuB 1.5 (www.qub.buffalo.edu, State University of New York, Buffalo, NY). Relative cationic permeabilities were calculated with a Goldman-Hodgkin-Katz equation extended to include Ca2+ (24). For this purpose, ionic concentrations were converted to activities using published activity coefficients (appendix 8.10 of Ref. 54).
Macroscopic currents were simulated by averaging currents from 10 to 40 voltage ramps. Open probability at each voltage on the ramp was estimated as the mean macroscopic current divided by the number of channels present and the single-channel current at that voltage. The latter was taken from the current-voltage relation shown in Fig. 4A. Data near 0 mV are not shown. The single-channel current there was near 0 pA, so the open probability could not be accurately estimated. A leak current was subtracted. The leak was estimated as follows. The linear portion of the current-voltage relation in 0.1 µM cytoplasmic Ca2+ (−100 to +40 mV) was fit to a straight line and extrapolated to +100 mV. The relation between estimated open probability and voltage was fit to a Boltzmann function:
where Vm is the membrane potential; V1/2 is the potential at which open probability is 0.5; and k is a slope factor.
Fig. 4.
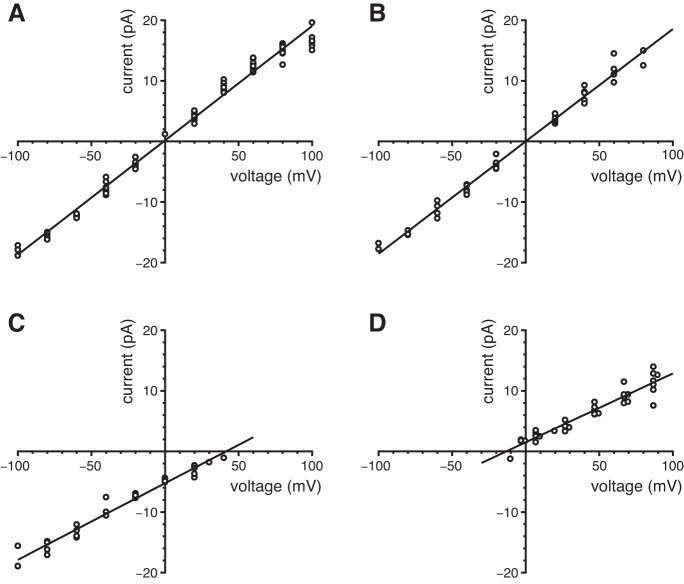
Ionic selectivity of the ciliary large-conductance channel. Mean single-channel current-voltage relations were determined in solutions as described (Table 1). A: symmetrical high-K+ solutions. The pipette contained the high-K+ external solution, and high-K+ cytoplasmic solutions were used (0.1 to 300 µM free Ca2+). Single-channel currents were measured in 11 cilia as described for Fig. 3C. Single-channel conductance 189 pS; reversal potential −1.0 mV. B: test of relative Cl¯ permeability. The pipette contained the high-K+ external solution, and Cl¯-free cytoplasmic solutions (which also have high K+) were used (3 µM or 300 µM free Ca2+). Single-channel currents were measured in 5 cilia. Single-channel conductance 185 pS; reversal potential 0.0 mV. C: test of relative Na+ permeability. The pipette contained the high-K+ external solution, and high-Na+ cytoplasmic solutions were used (3 µM or 300 µM free Ca2+). Single-channel currents were measured in 4 cilia. Single-channel conductance 126 pS; reversal potential +42 mV. D: test of Ca2+ permeability. The pipette contained the high-Ca+ external solution, and high-K+ cytoplasmic solutions were used (0.1, 3, or 300 µM free Ca2+). Single-channel currents were measured in 7 cilia. Single-channel conductance 114 pS; reversal potential −13 mV.
BAPTA, dibromoBAPTA, or HEDTA was used to buffer the concentration of free Ca2+ in the range 0.1 to 30 µM (Table 1). In buffered solutions, concentrations of free Ca2+ were estimated by the method of Bers (5) as described previously (33). BAPTA, HEDTA, amiloride hydrochloride, and charybdotoxin were purchased from Sigma-Aldrich (St. Louis, MO). DibromoBAPTA was purchased from Molecular Probes /Thermo Fisher Scientific (Waltham, MA). Apamin and iberiotoxin were from EMD Chemicals (Gibbstown, NJ).
CRISPR/Cas9 knockout of TRPP2.
To confirm that we were recording from a TRPP2-dependent channel, we generated two clonal cell lines in which TRPP2 protein expression was knocked out by CRISPR/Cas9 genome editing of the Pkd2 gene. A CRISPR plasmid with constitutive GFP expression and containing the guide RNA sequence 5′-CGGCTGCGGCTGCACGCGTCTGG-3′ was made by the Cincinnati Children’s Hospital Medical Center (CCHMC) Transgenic Animal Genome Editing Core Facility. The Core selected the guide RNA sequence using an algorithm provided by the Zhang laboratory (http://crispr.mit.edu/, Ref. 23). The Core validated the mutation efficiency in a murine cell line with a T7 endonuclease I test. We transfected mIMCD-3 cells with this plasmid using Lipofectamine 3000 (Thermo Fisher Scientific). Two days after transfection, single GFP-positive cells were sorted into separate wells for expansion (MoFlo XDP, Beckman Coulter, CCHMC Research Flow Cytometry Core). After expansion, genomic DNA extracted from the clones (GeneJET Genomic DNA Purification Kit, K0721, Thermo Fisher Scientific) was used in a polymerase chain reaction (PCR, Phusion Hot Start II DNA polymerase, F549, Thermo Fisher Scientific) to amplify the targeted region. These PCR products were screened for loss of the AflIII restriction site (New England Biolabs, Ipswich, MA). PCR products of restriction-site mutants were purified (GeneJet PCR Purification Kit, Thermo Fisher Scientific) and sequenced (CCHMC DNA Sequencing and Genotyping Core). We selected clonal lines that had, in both alleles, a frameshifting mutation that resulted in an early (first exon) stop codon. Potential off-target sites were identified and scored using an algorithm provided by the Zhang laboratory (http://crispr.mit.edu/, Ref. 23). All of the off-target sites had scores ≤ 0.7, which indicates a low chance of being mutated by the guide RNA. Four of the top five sites were amplified via PCR with genomic DNA and were sequenced to confirm that they were unchanged in the TRPP2 KO cell lines. We were unable to amplify one of the top five potential sites, perhaps because of its high guanine-cytosine content. Failure to amplify does not indicate there was a mutation; the primers do not bind on or near the guide RNA binding site.
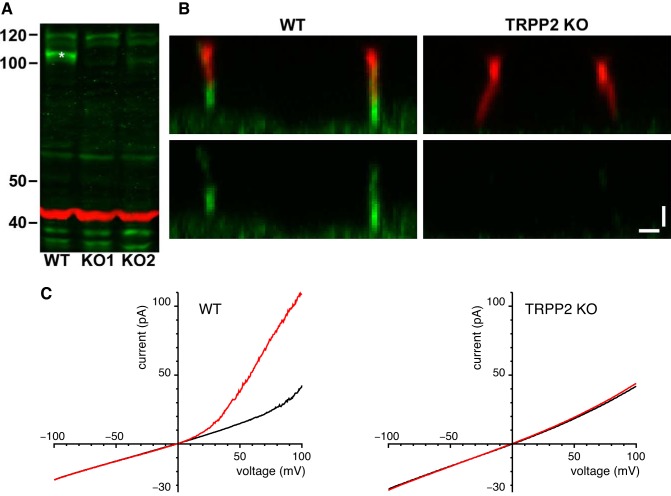
We used Western blotting and immunocytochemistry to confirm the absence of TRPP2 protein in the TRPP2 KO cell lines. Wild-type and TRPP2 KO mIMCD-3 cells were grown for several days after confluence on plastic, tissue-culture-treated, petri dishes with the same medium used to culture the cells for recording (30). Cells were rinsed with cold PBS three times. PBS contained (in mM) KCl 2.7, KH2PO4 1.5, Na2HPO4 8.1, NaCl 140, pH 7.4. Then the cells were scraped from the dish with lysis buffer (50 mM Tris pH 7.5, 150 mM NaCl, 1 mM EDTA, 0.05% sodium deoxycholate, 0.1% sodium dodecyl sulfate, 1% Triton X-100). Lysate buffer contained Halt protease inhibitor cocktail (87785, Thermo Fisher Scientific) at 1 µl per 100 µl lysate. The samples were vortexed and then set on ice for 10 min. Then the samples were sonicated for 15 s with a Cell Disruptor Sonicator (setting 4 on model W-220F, Qsonica, Newtown, CT). Lysates were spun at 15,000 rpm for 10 min and the supernatant was collected. Protein concentration was determined with a BCA assay (Thermo Fisher Scientific). Equal amounts (40 µg) of reduced, denatured protein were loaded into wells of a NuPAGE Novex 3–8% Tris-acetate gel (Invitrogen/Thermo Fisher Scientific) and subjected to electrophoresis with sodium dodecyl sulfate at 150 V for 90 min per Invitrogen’s protocol. Proteins were transferred to a PVDF membrane (Immobilon-P, EMD Millipore, Billerica, MA) for 1 h at 30 V. The membrane was blocked in 50% PBS-50% Odyssey blocking buffer (LI-COR Biotechnology, Lincoln, NE). The membrane was incubated with an antibody to TRPP2 (1/750 dilution, sc-25749, Santa Cruz Biotechnology, Dallas, TX) overnight at 4°C per LI-COR instructions. The membrane was incubated with donkey anti-rabbit IgG conjugated to Alexa Fluor 790 (1/15,000, A11369, Molecular Probes/Thermo Fisher Scientific). Equal loading was confirmed by staining with a mouse monoclonal antibody to β-actin (1/20,000, A5441, Sigma-Aldrich) and a goat anti-mouse IgG conjugated to Alexa Fluor 680 (1/10,000, A21057 Molecular Probes/Thermo Fisher Scientific). The membrane was scanned on an Odyssey CLx Infrared Imaging System (LI-COR Biotechnology). Contrast was enhanced, gamma was kept at 1, and labels were added using Image Studio (LI-COR Biotechnology) and Photoshop (Adobe Systems, San Jose, CA). The membrane shown is representative of two independent cell culture experiments.
For immunocytochemistry, mIMCD-3 cells were grown on glass coverslips for several days after confluence and then were fixed with 3% paraformaldehyde in PBS (pH 7.4) for 10 min followed by 4% paraformaldehyde in 38 mM sodium tetraborate (pH 11) for 15 min (17). Cells were treated with 1% sodium dodecyl sulfate (17) in a sodium phosphate buffered saline [0.9% NaCl in 10 mM Na2HPO4, pH 7.4 (6)] for 5 min and rinsed thoroughly with PBS. The cells were blocked in 5% bovine serum albumin (BSA), 10% normal donkey serum, and 0.02% sodium azide in PBS for 1 to 2 h at room temperature. The anti-TRPP2 antibody was applied overnight at 4°C (1/250, sc-25749, Santa Cruz Biotechnology). Antibodies were diluted in 1% BSA and 0.02% sodium azide in PBS. Anti-acetylated α-tubulin (1/15,000, T6793, Sigma-Aldrich) was applied for 1 to 2 h at room temperature. Secondary antibodies (1/1000, Cy5-conjugated donkey anti-mouse IgG (715–175–150, Jackson ImmunoResearch, West Grove, PA) and donkey anti-rabbit IgG conjugated with Alexa Fluor 488 (A21206, Molecular Probes/Thermo Fisher Scientific)) were applied together for 1 h at room temperature. For Fig. 9B, stacks of optically sectioned XY images were acquired every 0.17 µm in Z with a ×100 oil objective (numerical aperture = 1.45, Nikon, Melville, NY) and a Nikon A1R confocal microscope (CCHMC Confocal Imaging Core). The acquisition parameters were the same for both wild-type and KO samples. A maximum XZ projection of each image stack was made using NIS Elements software (Nikon). Contrast was enhanced equally for wild-type and KO images, gamma was kept at 1, and labels were added using Photoshop. Images are representative of three independent cell culture/immunocytochemistry experiments, each with both the wild-type and TRPP2 KO line.
Fig. 9.
TRPP2 knockout (KO) mIMCD-3 cell lines. A: Western blot for TRPP2 protein with 40 µg of whole cell lysate from mIMCD‐3 wild-type (WT) and two TRPP2 KO cell lines. The band of TRPP2 protein (green, *) is present in the WT but not in the KO cells. β-Actin (red) was immunostained as a control for loading. Molecular mass markers are in kDa. B: immunostaining of mIMCD‐3 cells with the ciliary marker (37) anti-acetylated α-tubulin (red, top) and with anti‐TRPP2 (green, all images) shows that the TRPP2 KO cells have lost ciliary labeling for TRPP2. Each image is an XZ maximum intensity projection of a stack of XY images acquired at a range of Z depths on a confocal microscope. Bars, 1 μm. C: absence of current activated by 3 µM cytoplasmic Ca2+ in cells lacking TRPP2. For each cilium, macroscopic currents were determined by averaging currents from 10 to 40 voltage ramps. High-K+ external and cytoplasmic solutions were used; the cytoplasmic solutions contained 0.1 µM free Ca2+ (black) or 3 µM free Ca2+ (red). Results shown are averages from 9 cilia (wild-type with detectable large-conductance channels, at left) or 10 cilia (TRPP2 knockout, at right).
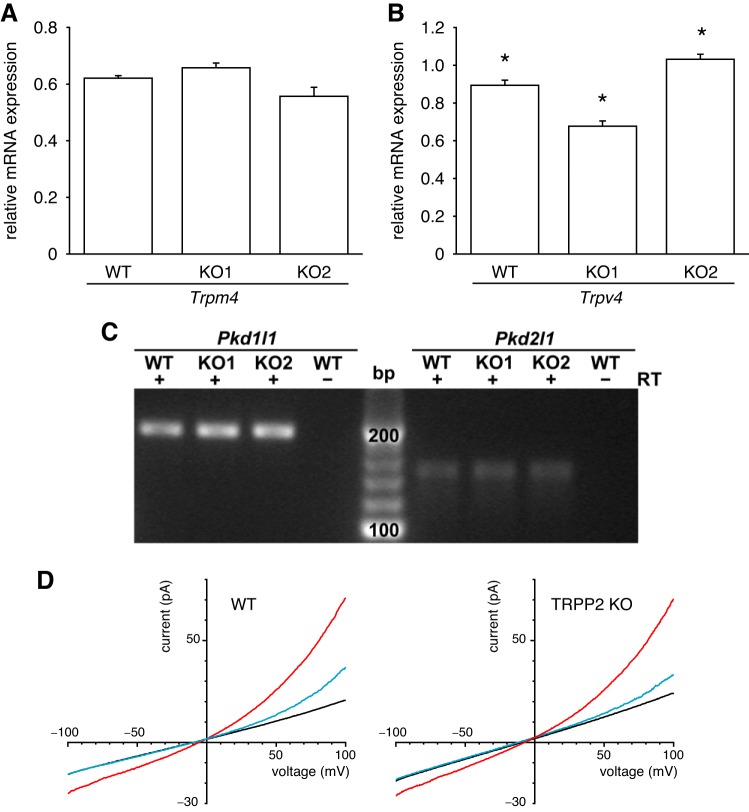
We used reverse transcription–quantitative polymerase chain reaction (RT-qPCR) to monitor the expression of mRNA in the wild-type and TRPP2 KO lines. Cells were cultured for several days after confluence in tissue-culture-treated petri dishes with the same medium used to culture the cells for recording (30). Total RNA was extracted (RNeasy 74104, Qiagen, Valencia, CA), treated with DNase (EN0525, Thermo Fisher Scientific), and reverse-transcribed to cDNA with random hexamers (Maxima reverse transcriptase, EP0742, Thermo Fisher Scientific). For reverse transcription, DNase-treated RNA was added to a final concentration of 0.03 μg/μl. The amount of RNA in the RT reaction was within the linear range of the efficiency of the RT. For Pkd1l1 and Pkd2l1, each 15-μl qPCR reaction contained 0.66 μl of undiluted RT reaction product. For Trpm4, Trpv4, and Sdha, the RT reaction product was diluted 25-fold in water, and each 15-μl qPCR reaction contained 0.66 μl of diluted RT reaction product. qPCR was done with HotStart-IT SYBR Green qPCR Master Mix (75762, Affymetrix, Santa Clara, CA) and was followed on a OneStepPlus thermal cycler (Thermo Fisher Scientific) with an annealing temperature of 64°C. Primer pairs were selected using Primer-BLAST (National Center for Biotechnology Information, Database: Mus musculus refseq_rna) and were designed to cross at least one intron (Table 2). We did not compare the amounts of Pkd1l1 and Pkd2l1 mRNA across cell lines. Little product was detected (amplification after 33 of the 40 cycles), and in the case of Pkd2l1, some reactions/wells showed no product. For Pkd1l1 and Pkd2l1, we subjected the qPCR products to electrophoresis on an ethidium bromide-stained, 4% agarose gel. We quantified the expression of Trpm4 and Trpv4. Using LinRegPCR software (55), we determined the starting amount of mRNA transcript (N0, in arbitrary fluorescence units) for each qPCR reaction/well in a multiwell plate. The efficiency of the qPCR amplification was calculated for each reaction, and an average efficiency for a given primer pair was calculated for each plate. (Occasional efficiencies that varied from the median by >2.5% were excluded from the calculation of the mean efficiency.) This mean efficiency was taken into account when calculating the starting amount of transcript (N0). Relative expression of mRNA (the vertical axis in Fig. 10, A and B) was calculated by dividing N0 for the target transcript by N0 for the reference transcript (Sdha). The reference gene (Sdha) was selected because we found that equal amounts of RNA from several samples yielded a similar starting amount of transcript (N0) for this gene. As negative controls, we used samples processed without RT or reactions with water in place of RNA. The Trpm4 data were examined with a Kruskal-Wallis one-way analysis of variance on ranks. The Trpv4 data passed normality (Shapiro-Wilk) and equal variance (Brown-Forsythe) tests and were examined with a one-way analysis of variance followed by the all-pairwise multiple comparison procedure: Holm-Sidak (SigmaPlot, Systat Software, San Jose, CA). Two independent cell culture experiments were analyzed for each of the cell lines. Three to four wells per condition were used as technical replicates. Melt curves were used to confirm that only the targeted product was made. Sequencing confirmed the identities of the qPCR products.
Table 2.
qPCR primers
| Gene | Product Size, bp | Primer Sequence (5′–3′) | NCBI No. |
|---|---|---|---|
| Pkd1l1 | 184 | ATGCCACTCTTGAAGTGAGCA | XM_017314870.1 |
| CCAGGCAGTGTATCTTCTTCCA | |||
| Pkd2l1 | 141 | GAAAGAGCGGGTTTCTGATGTG | NM_181422.3 |
| TCCTGATCAAACCTGGTGAAGG | |||
| Sdha | 75 | AAGAAGGCATCAGCTAAAGTTTCA | NM_023281.1 |
| CACAGCATCAAATTCATGATCCAC | |||
| Trpm4 | 146 | ACGGCTCCGAGGAGTTTGAGACTA | NM_175130.4 |
| CCCACGGAAAAGTTCACTTTGGGC | |||
| Trpv4 | 143 | CGTTCCTTCCCTGTGTTCC | NM_022017.3 |
| CCCAAGTTCTGGTTCCAGTG |
bp, Base pairs. NCBI no., National Center for Biotechnology Information accession number.
Fig. 10.
Checking for off-target effects in the TRPP2 KO, mIMCD-3 cell lines. A: survival of Trpm4 mRNA expression following knockout of TRPP2. RT-qPCR was performed to determine Trpm4 mRNA expression relative to the reference gene Sdha. There was no significant difference between the WT and two TRPP2 KO cell lines (Kruskal-Wallis one-way analysis of variance on ranks, n = 8 wells, 4 wells/cell culture passage). B: survival of Trpv4 mRNA expression following knockout of TRPP2. RT-qPCR was performed to determine Trpv4 mRNA expression relative to the reference gene Sdha. *All lines were significantly different from the other two lines (P < 0.004, Holm-Sidak, n = 8 wells, 4 wells/passage). C: survival of Pkd1l1 and Pkd2l1 mRNA expression following knockout of TRPP2. Pkd1l1 and Pkd2l1 mRNA was detected by RT-qPCR in mIMCD-3 RNA samples treated with reverse transcriptase (RT+) but not in untreated samples (RT−). The qPCR products were subjected to electrophoresis on a 4% agarose gel and stained with ethidium bromide. The expected size of the qPCR products is 184 base pairs (bp) for Pkd1l1 and 141 bp for Pkd2l1. Sequencing confirmed gene identity. D: survival of TRPM4-dependent current following knockout of TRPP2. For each cilium, macroscopic currents were determined by averaging currents from 10 voltage ramps. Standard external and cytoplasmic solutions were used; the cytoplasmic solutions contained 0.1 µM free Ca2+ (black), 1 mM free Ca2+ (red), or 1 mM free Ca2+ + 2 mM MgATP (blue). Results shown are averages from 13 cilia (wild-type, at left) or 8 cilia (TRPP2 knockout, at right).
Statistical analyses.
Results of repeated experiments are reported as mean ± SE. The Shapiro-Wilk test was used to verify normal distribution. Student’s t-tests reported are two-tailed. Channel open probabilities are reported only when the number of channels in the membrane was unambiguous.
RESULTS
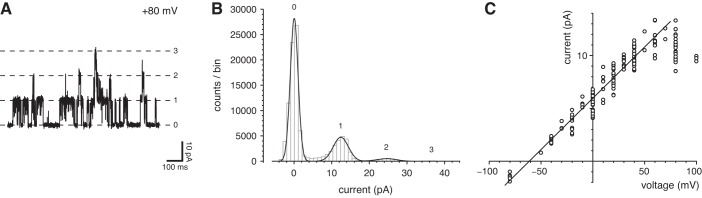
The entire primary cilium of an mIMCD-3 cell can be pulled into a recording pipette while the cilium remains attached to the cell. This allows recording of currents conducted through the ciliary membrane (Fig. 1A). In this configuration, recordings from 37 of 170 cilia tested showed clear single-channel fluctuations at depolarizing potentials (Fig. 2A). In the example shown, the single-channel conductance was linear over most of the range of voltages tested and measured 97 pS (Fig. 2B). At the most depolarizing potentials, there was no further increase in single-channel current (Fig. 2B).
Fig. 1.
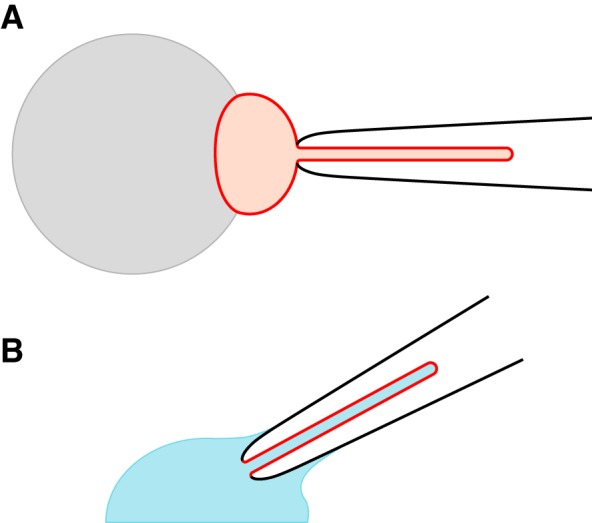
Configurations for recording ciliary transmembrane currents (30). A: recording from a cilium that remains attached to its cell. On a glass-coated bead (gray), renal epithelial cells are grown to confluence. Just one cell is shown (red). Suction from the recording pipette (black) pulls the cell’s primary cilium into the pipette. The pipette contains an external solution, as does the bath surrounding the cell. B: recording from a cilium detached from the cell. From the attached state (A), the pipette is lifted briefly into the air, which breaks off the cell body and leaves the excised cilium in the pipette. The pipette is quickly immersed in a cytoplasmic solution (blue), which diffuses into the cilium. In both configurations, currents seen principally reflect ciliary channels. The attached configuration (A) was used only for the work shown in Fig. 2; all other experiments used excised cilia (B). The drawings are not to scale.
Fig. 2.
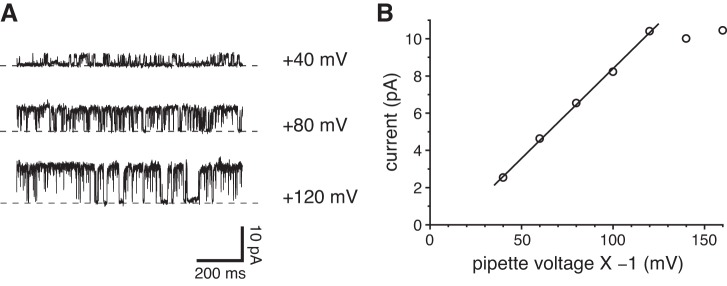
Single ciliary channel observed while cell attached. A: current fluctuations from opening and closing of a single channel at the potentials shown. The recording pipette contained a single cilium and was attached to the intact cell near the base of the cilium. The dashed lines indicate the current level when the channel was closed. The recordings were low-pass filtered at 1 kHz following acquisition. The standard external solution was used to bathe the cell and to fill the pipette. B: current-voltage relation for the single channel shown in A. The relation between +40 and +120 mV was fit to a straight line; the slope of that line (single-channel conductance) is 97 pS. Voltages in this figure only are reported as pipette potential × −1. This represents the contribution of the pipette potential to the membrane potential of the patched cilium. That membrane potential also includes the resting cell potential, which was not measured.
The primary cilium can subsequently be excised from the cell. The resulting configuration is equivalent to an inside-out patch, with the patch consisting of one whole cilium (Fig. 1B). In some excised cilia, one or more large-conductance channels opened and closed spontaneously at a depolarizing potential (Fig. 3, A and B). Figure 3C shows the current-voltage relation measured for this channel using physiological solutions containing primarily external NaCl and cytoplasmic KCl. From −80 to +70 mV, the relation was linear. The slope of the relation (single-channel conductance) was 97 pS, which matches the conductance seen when cell-attached (Fig. 2B). As in the cell-attached cilia, the conductance failed to increase at the most depolarizing voltages tested (Fig. 3C). The reversal potential of the single-channel current was −61 mV, suggesting that K+ is more permeant than Na+. Channel openings at potentials more negative than −61 mV were rare, for reasons discussed below. These large-conductance channels were observed in 103 of 304 cilia tested. Many cilia showed just one active channel, and it was uncommon to see more than six channels. Of 22 patches excised from the apical non-ciliary membrane, only one showed the large-conductance channel.
Fig. 3.
Large-conductance channels in excised single cilia. A: current fluctuations from opening and closing of single channels at +80 mV. Numbers at the right of each recording indicate the number of channels open at each current level; the levels are indicated by dashed lines. The standard external solution was used to bathe the cell and to fill the pipette; the cytoplasmic solution was the standard solution, which contained 0.1 µM free Ca2+. B: an amplitude histogram from the full 20 s of recorded current. At least 3 channels were present in this cilium. (A peak corresponding to 3 channels was present but too small to be seen at the scale shown.) The average single-channel current was 12.2 pA. C: single-channel current-voltage relation. In each of 66 cilia, single-channel current amplitudes were determined from amplitude histograms of 20-s recordings as in A and B. In many cilia, single-channel currents were measured at each of several voltages. In all cases, the standard external solution was used. For each measurement, single-channel current was determined as shown in A and B. Solutions were as in A, except that cytoplasmic free Ca2+ ranged from 0.1 to 300 µM. The mean single-channel conductance (slope) is 97 pS, and the reversal potential (x-intercept) is −61 mV.
To determine the ionic selectivity of the large-conductance channel, excised cilia were tested in solutions of various compositions (Fig. 4). In nearly symmetrical KCl solutions, the mean reversal potential was −1.0 mV (near 0 mV as expected; Fig. 4A). The single-channel conductance was 189 pS, which is almost twice the value measured in the typical physiological solutions (97 pS; Fig. 3C). When cytoplasmic KCl was replaced with potassium methanesulfonate, the conductance (185 pS) and reversal potential (0.0 mV) were not changed (Fig. 4B). Thus the channels do not select between Cl¯ and methanesulfonate¯; they probably do not conduct anions. With external KCl and cytoplasmic NaCl, the reversal potential shifted to +42 mV; the single-channel conductance was 126 pS (Fig. 4C). We also recorded from excised cilia using a pipette (external) solution containing isotonic (100 mM) CaCl2 and a cytoplasmic solution with K+ as the principal cation. In this case the average current-voltage relation reversed at −13 mV, and the channel conductance was 114 pS (Fig. 4D). From the reversal potentials, we derived relative ionic permeability ratios of 1:0.55:0.14 (PK:PCa:PNa).
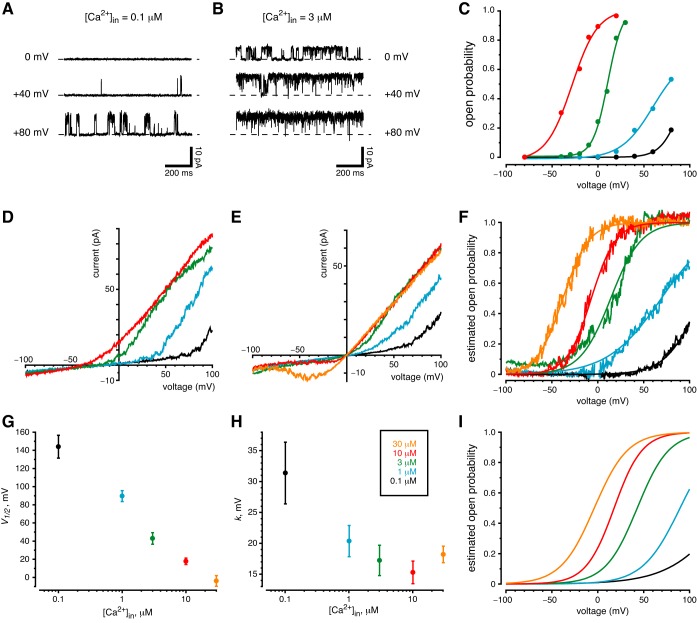
As indicated in a preliminary report (30), the large-conductance ciliary channel is activated by both depolarization and cytoplasmic Ca2+. With cytoplasmic Ca2+ buffered to 0.1 µM, an excised cilium showed few channel openings at 0 mV or +40 mV (Fig. 5A). At +80 mV, the single channel present opened more often (Fig. 5A). When cytoplasmic free Ca2+ was elevated to 3 µM, the channel opened frequently at each of the three potentials (Fig. 5B). At each potential, the open probability increased when cytoplasmic Ca2+ was elevated (Fig. 5, A and B). From one cilium that survived many changes of solution and voltage, we obtained the relations between open probability and voltage at each of four cytoplasmic Ca2+ levels (Fig. 5C). At any given level of cytoplasmic Ca2+, the open probability increased substantially with depolarization. At any given voltage, the open probability was very sensitive to increases in cytoplasmic Ca2+. Recordings from a second cilium (not shown) gave a very similar result.
Fig. 5.
Channel sensitivity to membrane potential and cytoplasmic Ca2+. The color key shown in H applies to all of parts C–I. A–C: measurements from observing single channels. A: the activity of a single ciliary channel increased with membrane depolarization. The standard external solution was used to bathe the cell and to fill the pipette; the cytoplasmic solution was the standard solution, which contained 0.1 µM free Ca2+. B: channel activity at each voltage was increased after cytoplasmic free Ca2+ was increased to 3 µM. The same cilium was used for parts A and B. C: open probability as a function of membrane potential and the concentration of cytoplasmic Ca2+. Cytoplasmic Ca2+ was varied from 0.1 to 10 µM as indicated; the standard external solution was used. Open probabilities were determined from amplitude histograms made from 20-s recordings at each voltage and the Ca2+ concentration shown. All measurements in C are from a single cilium having 4 of the large-conductance channels. D–I: measurements from macroscopic currents. D: current-voltage relation in a cilium with several large-conductance channels. Solutions were as in C. Each record shown is the average of 20 ramps from −100 to +100 mV. E: the same protocol as D, but with high-K+ external and cytoplasmic solutions. Each record shown is the average of 40 ramps. This cilium had 4 large-conductance channels. F: estimated channel open probability as a function of membrane potential and the concentration of cytoplasmic Ca2+ from the recordings in E. Open probability was estimated and fit to a Boltzmann function as described in materials and methods. The Boltzmann functions are shown as smooth curves in F. G and H: mean values of the Boltzmann constants V1/2 and k, respectively, measured in 10 cilia as functions of cytoplasmic Ca2+ concentration. I: the Boltzmann functions resulting from the mean constants shown in G and H.
The macroscopic current-voltage relation for a population of channels reflects the effects of voltage on both single-channel current and open probability. Because most cilia had few large-conductance channels, we simulated macroscopic current-voltage relations in excised cilia by averaging a large number of individual current-voltage relations, each measured with a voltage ramp. In typical physiological solutions, the relation showed very strong outward rectification (Fig. 5D, black). As cytoplasmic Ca2+ was increased, outward current also increased (Fig. 5D), but the rectification was maintained. Even at very negative potentials, there was almost no inward current (as also noted for Fig. 3C). There are two reasons for this. First, the channel rarely opens at such negative potentials (Fig. 5C). Second, in these solutions, an inward current would be primarily carried by Na+. As noted above, the permeability of the channel to Na+ is relatively low. For any given concentration of cytoplasmic Ca2+, outward currents increased with depolarization (Fig. 5D). This arose from two consequences of depolarization: an increased outward driving force on K+ and the increase in open probability (Fig. 5C).
The ciliary TRPP2-dependent channel was present at surprisingly low density. The largest TRPP2-dependent macroscopic current observed was 360 pA at +60 mV with the standard external (pipette) solution and 300 µM cytoplasmic free Ca2+. Given the mean single-channel current at +60 mV (11.4 pA; Fig. 3C) and a likely open probability near 1, we infer that this cilium had ~30 active TRPP2-dependent channels. This macroscopic current (not shown) exhibited the properties associated with the TRPP2-dependent channels: very strong outward rectification, a negative reversal potential (−54 mV), and a decreasing conductance at potentials more positive than +70 mV.
When solutions on both sides of the membrane contained primarily KCl, the macroscopic current-voltage relations were similar but, as expected, reversed near 0 mV (Fig. 5E). In these solutions, elevating cytoplasmic Ca2+ to 30 µM allowed an inward current near the presumed resting potential of a renal epithelial cell [−40 to −80 mV (13, 58, 65)]. Given the macroscopic currents (Fig. 5E), the number of channels in the cilium, and the single-channel current-voltage relation in these solutions (Fig. 4A), it was possible to estimate the channel’s open probability as a function of voltage at each concentration of cytoplasmic Ca2+ (Fig. 5F). These functions were well fit by Boltzmann functions (Fig. 5F, smooth curves). Each of 10 cilia tested showed a similar dependence of estimated open probability on voltage and cytoplasmic Ca2+ (Fig. 5, G–I).
In excised cilia, the large-conductance ciliary channel spontaneously inactivated in many but not all cases (Fig. 6). To measure inactivation, a cilium was left undisturbed as channel open probability was measured. The standard external (pipette) solution was used, together with a cytoplasmic solution containing 3 µM free Ca2+. Initially, this activated the channel, but over 40 min, the activity often decreased. The rate of inactivation did not correlate with the holding potential (listed for each cilium in the legend to Fig. 6).
Fig. 6.
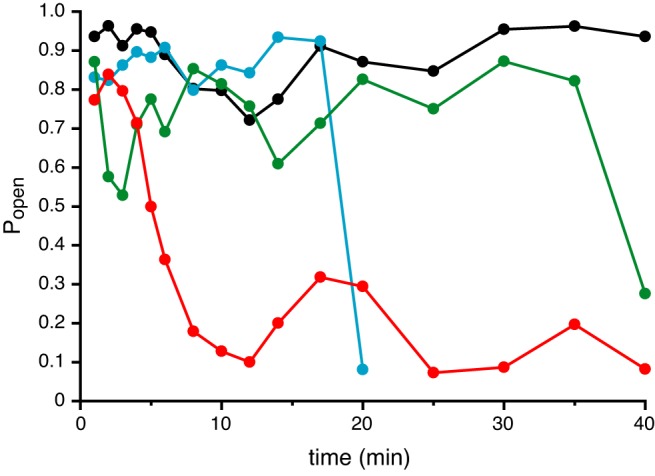
Spontaneous inactivation of the large-conductance channel. Channel open probability (Popen) was determined in each of 4 cilia left undisturbed for up to 40 min. The standard external solution was used to bathe the cell and to fill the pipette; the cytoplasmic solution contained 3 µM free Ca2+. The holding potentials and numbers of channels were: +10 mV, 1 channel (black); +30 mV, 1 channel (blue); +80 mV, 1 channel (green); +10 mV, 2 channels (red). Each open probability was determined from an amplitude histogram from a recording of duration 20 s.
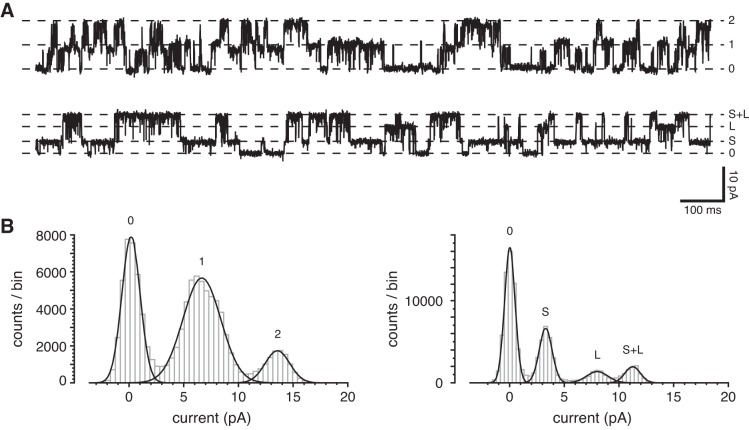
On a few occasions, the single-channel conductance changed over time. Figure 7 shows a cilium that initially had two typical large-conductance channels. With time, these were replaced by two other channels, one with a smaller current than the typical channels, and one larger. Channels of these sorts were only seen following the observation of typical large-conductance channels. Owing to their infrequency, we have not studied them further. Conductance substates of apparent TRPP2-dependent channels have been described elsewhere (9, 18, 19, 67).
Fig. 7.
Change in channel conductance with time. A: the upper recording shows current fluctuations from opening and closing of two large-conductance channels in a cilium 6 min following excision from the cell. The lower recording shows the fluctuations in the same cilium 35 min following excision. The standard external solution was used to bathe the cell and to fill the pipette; the cytoplasmic solution contained 3 µM free Ca2+. Holding potential was +10 mV. B: amplitude histograms from the full 20-s recordings parts of which are shown in A. The left histogram (from the upper recording in A) shows peaks corresponding to two equivalent channels, each conducting 6.8 pA. The right histogram (lower recording), shows a smaller channel conducting 3.3 pA (S) and a larger channel conducting 8.0 pA (L). The third peak (S+L, 11.3 pA) represents simultaneous openings of each of the two channels.
Cytoplasmic amiloride did not block the large-conductance ciliary channel (Fig. 8, left). The ratio of current following treatment to current before treatment averaged 1.01 ± 0.16 (n = 7). Amiloride was ineffective regardless of whether cytoplasmic free Ca2+ was 0.1 µM (n = 3) or 3 µM (n = 4). Since the large-conductance channel is activated by Ca2+ and conducts K+ well, we also tested a mixture of reagents that block Ca2+-activated K+ channels: 100 nM apamin + 10 nM iberiotoxin + 10 nM charybdotoxin, applied to the cytoplasmic face of the cilium (Fig. 8, right). These were also ineffective; the ratio of current after and before treatment averaged 0.94 ± 0.13 (n = 8). Both 0.1 µM (n = 4) and 3 µM (n = 4) cytoplasmic free Ca2+ were tested.
Fig. 8.
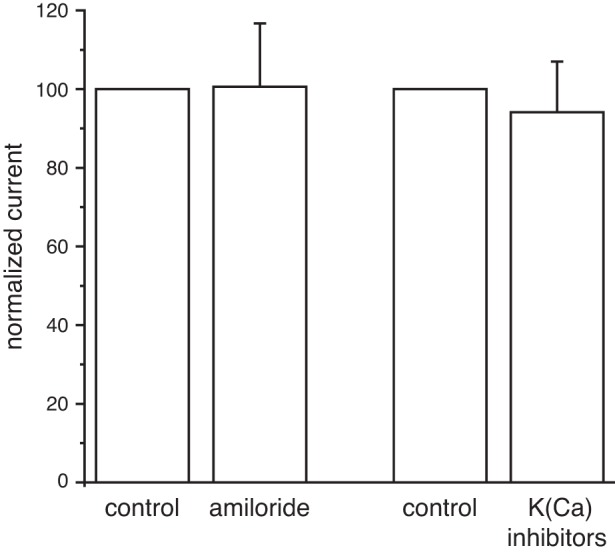
Lack of channel block by amiloride and by antagonists of Ca2+-activated K+ channels. Left: in 7 cilia with the large-conductance channel, mean channel current was measured in the absence (control) and presence of 1 mM cytoplasmic amiloride. Right: in 8 cilia with the large-conductance channel, the effect of a mixture of blockers of Ca2+-activated K+ channels was determined. For both parts of this figure, mean channel current was measured as the mean current minus the current attributed to leak channels. The latter was determined from an amplitude histogram. The control current was arbitrarily assigned a value of 100. The standard external solution was used to bathe the cell and to fill the pipette; the cytoplasmic solution contained 0.1 µM or 3 µM free Ca2+. For both antagonists, holding potentials ranging from −20 to +70 mV were tested.
It has been established by immunocytochemistry that the primary cilia of mIMCD-3 cells express the channel protein TRPP2 (2, 39). To determine whether the large-conductance channels we observe depend on expression of TRPP2, we knocked out TRPP2 protein expression in mIMCD-3 cells using CRISPR/Cas9 genome editing of the Pkd2 gene. TRPP2 protein was not detected by Western blotting in whole cell lysate from two knockout (KO) cell lines (Fig. 9A). Immunostaining for TRPP2 in primary cilia was nearly absent in the KO cell line examined (Fig. 9B). We suspect the faint, residual ciliary staining is nonspecific labeling by the non-affinity- purified, polyclonal TRPP2 antibody. In electrical recordings from the primary cilia of 38 cells lacking TRPP2, the large-conductance channel was never seen. This is significantly different from the channel’s incidence in cilia from wild-type cells (103 of 304 cilia tested, P < 0.000001, Fishers exact test). To check for the chance that the reduced current was an artifact of clonal selection, cilia of cells from a second knockout clonal line were tested. These also lacked the channels (n = 36, P < 0.000002). In simulated macroscopic ciliary currents, 3 µM cytoplasmic free Ca2+ activated an outwardly rectifying current in wild-type cilia with detectable large-conductance channels (Fig. 9C, left) but not in cilia lacking TRPP2 (Fig. 9C, right).
We found no evidence that knocking out TRPP2 had off-target effects on ciliary channels related to TRPP2. RT-qPCR indicated no differences in quantities of Trpm4 mRNA between the wild-t ype (WT) and the two TRPP2 KO lines (Fig. 10A). For Trpv4, the two KO lines had slightly more or less Trpv4 mRNA than the WT did (Fig. 10B). We detected Pkd1l1 and Pkd2l1 mRNA in the WT and both TRPP2 KO lines (Fig. 10C) but only by using more cDNA than for Trpm4 and Trpv4. Detection was possible only after 33 cycles of amplification, and not even with 40 cycles in some of the Pkd2l1 reactions. The identities of the qPCR products were confirmed with sequencing. The WT and both TRPP2 KO lines showed normal polarization as judged by the presence of primary cilia. We also tested for off-target effects on ciliary channels with an electrophysiological assay. The cilia of mIMCD-3 cells contain a TRPM4-dependent current that is activated by high cytoplasmic Ca2+ and inhibited by cytoplasmic MgATP (15). The TRPM4-dependent current was not detectably different in cilia from wild-type cells (Fig. 10D, left) and cells lacking TRPP2 (Fig. 10D, right). We compared the current activated by 1 mM cytoplasmic Ca2+ (difference between the red and black curves in Fig. 10D) at +100 mV. The current averaged 50 ± 10 pA (n = 13) in cilia from wild-type cells and 48 ± 8 pA (n = 8) in cilia from cells lacking TRPP2. These two values are not significantly different (t-test for independent measures). The fraction of this current remaining after inhibition by 2 mM cytoplasmic MgATP was also unaffected by knocking out TRPP2 (0.28 ± 0.06 for wild-type; 0.22 ± 0.04 for the TRPP2 knockout).
DISCUSSION
We describe the native TRPP2-dependent channel of primary cilia of mIMCD-3 cells, which are derived from the inner medullary collecting duct of murine kidney (51). The channel has a large conductance. It has a high permeability to K+ and Ca2+ and conducts Na+ less well. Under typical resting conditions, the channel rarely opens. If the membrane is depolarized, or if cytoplasmic Ca2+ increases to micromolar levels, there is a marked increase in the channel’s open probability. This channel was only seen once in 22 patches excised from the apical non-ciliary cytoplasmic membrane.
The dependence of this channel on expression of TRPP2 could not be established on the basis of its conductive properties. Many TRPP2 and TRPP2-like channels have been described (Table 3). Some were native channels believed to be TRPP2-dependent, and others resulted from exogenous expression of TRPP2. The properties of these channels range widely and do not distinguish them sufficiently from other cation-conducting channels. Some of the properties (e.g., single-channel conductance and rectification) depend strongly on the choice of solutions and range of voltages examined, which further complicates comparison. In general, most of the channels reported have a large conductance (>100 pS). All tested showed some permeability to Ca2+, and about half showed a smaller permeability to Na+. These properties match those of the channel we describe. Like the ciliary channel, exogenously expressed TRPP2 has been shown to be activated by cytoplasmic Ca2+ (36, 62). The voltage dependence of the many apparent TRPP2 channels has not been consistent (Table 3; see Ref. 3 for discussion). Ultimately, we found it necessary to knock out TRPP2 to demonstrate that the ciliary channel is TRPP2-dependent. A similar approach was used by Pelucchi et al. (48), who decreased an endogenous cationic current in HEK-293 cells by siRNA knockdown of TRPP2.
Table 3.
Conductance properties of reported TRPP2-like channels
| Reference | System | g, pS | PCa/PK | PNa/PK | V-dep |
|---|---|---|---|---|---|
| This report | Native | 97–189 | 0.55 | 0.14 | OR |
| Hanaoka et al. (21) | Exogenous† | * | Mild OR | ||
| González-Perrett et al. (19) | Native | 134–177 | 1.3 | 1.1 | IR |
| Vassilev et al. (62) | Exogenous | 23,124 | 0.21 | 0.14 | |
| Koulen et al. (36) | Exogenous | 85–114 | IR | ||
| González-Perrett et al. (18) | Native | 135–172 | IR | ||
| Luo et al. (39) | Native | 23,116 | >0 | 0.19 | OR |
| Delmas et al. (12) | Exogenous† | 128 | 0.58 | 1.02 | NR |
| Raychowdhury et al. (52) | Native | 73–173 | ~1 | ||
| Ma et al. (41) | Exogenous | 71 | 0.28 | 0.2 | NR |
| Pelucchi et al. (48) | Native | 3 | NR | ||
| Kim et al. (28) | Native | OR | |||
| Bai et al. (2) | Exogenous† | 142 | NR | ||
| Bai et al. (3) | Native | OR | |||
| Raychowdhury et al. (53) | Native | 158 | |||
| Zhang et al. (67) | Native | 157 | |||
| Cantero et al. (9) | Exogenous | 127 | |||
| Zhang et al. (68) | Native | 87 |
g, Single-channel conductance; PX, permeability to ion X; V-dep, voltage dependence of channel open probability (in some cases inferred from rectification of the macroscopic current in nearly symmetrical solutions); NR, nonrectifying; OR, outwardly rectifying; IR, inwardly rectifying; native, channels originating in a native membrane, in some cases transferred to an artificial bilayer; exogenous, channels made by exogenous expression.
PCa/PNa ~6;
coexpressed with PC1.
The number of active TRPP2-dependent channels was surprisingly small. A majority of cilia (66%) showed no detectable TRPP2-dependent channel activity. Of the remaining 34% that did, most showed just one to six active channels. The incidence of active channels was not decreased by excision of the cilium from the cell. Our observations contrast with immunocytochemical results reported elsewhere. In the mIMCD-3 cell line we used, ~75% of cilia could be labeled with an antibody to TRPP2 (16). In other renal cells, the rate of labeling was 87% to 99% (47). This suggests that many TRPP2-dependent channels may be present but inactive, given our conditions for cell growth and recording. In our experience, almost all mIMCD-3 primary cilia show no discernible single-channel fluctuations in standard physiological solutions at a typical resting membrane potential. One-third show TRPP2-dependent channels if the membrane is strongly depolarized or if cytoplasmic free Ca2+ is elevated to micromolar levels. In 92% of mIMCD-3 primary cilia, a TRPM4-dependent channel current is seen at higher concentrations of cytoplasmic free Ca2+ (15).
Another large, cation-conducting channel was recently identified in primary cilia of the same cell line we studied, mIMCD-3 (8, 10). This channel has a conductance of 96 pS and conducts Na+ and K+ equally well. A very similar channel in a cell line derived from human retinal pigment epithelium was shown to depend on expression of the proteins PKD1L1 and PKD2L1 but not on expression of TRPP2 (8). The PKD1L1-PKD2L1 channel is blocked by cytoplasmic Ca2+, which has its half-maximal effect at 445 nM (10). We have been unable to detect a channel with those properties by electrophysiological methods, although we do detect Pkd1l1 and Pkd2l1 mRNA. The ciliary channel we describe is seven times more conductive to K+ than Na+, and it is activated by cytoplasmic Ca2+ as high as 300 µM. We do not know why the ciliary PKD1L1-PKD2L1 channel was not detected with our protocol. It should also be noted that the study describing the PKD1L1-PKD2L1 channel did not report TRPP2-dependent channels of the sort we observe. In that study, recordings were made by sampling the distal portion of the cilium (8). We speculate that this could miss channels confined to the proximal portion of the cilium or its base. We recorded from the entire cilium and have not determined the localization of the channels along its length. Since the density of active TRPP2-dependent channels is low, most patches of ciliary membrane probably have none of these channels.
The large-conductance ciliary channel depends on expression of TRPP2. However, we cannot conclude that TRPP2 is a subunit of the channel. It may be that TRPP2 fulfills some other requirement for expression or trafficking of the large-conductance channel. TRPP2 is homologous to other channels, so the channel we report may include TRPP2 as a subunit. TRPP2 is known to form functional channels in association with PC1 (2, 12, 21), which does not form channels by itself. TRPP2 also forms heteromultimeric channels with other TRP channel subunits. With TRPC1, a channel of 40 pS has been described (2), which is much smaller than the channel we observe. TRPP2 associates with TRPV4 to form channels of 23 to 57 pS (34, 68). The primary cilia of mIMCD-3 cells have now been shown to express four types of TRP channel, including TRPP2 (Refs. 2, 39 and this report), TRPC1 (2), PKD1L1-PKD2L1 (8, 10), and TRPM4 (15).
An important question remains: How is the ciliary TRPP2-dependent channel activated under normal circumstances? In primary cilia of cells derived from human retinal pigment epithelium, the resting membrane potential is −18 mV, and the cilium contains 580 nM free Ca2+ (10). Under those conditions, the ciliary TRPP2-dependent channel would be closed. At −18 mV, the channel would only begin to open were free Ca2+ in the cilium to reach ~3 µM. The mechanism for such an initial increase in intraciliary Ca2+ is not yet known. One popular hypothesis is that ciliary TRPP2 is activated by mechanical displacement of the cilium. In the renal epithelium, the cilia are displaced by the flow of renal filtrate. In cell lines derived from renal epithelial cells, flow that deflects the cilia causes increases in cytoplasmic Ca2+ in both the cilium (26, 60) and the soma (26, 44, 49). The response in the cell body requires the cilium, external Ca2+, TRPP2, PC1, and TRPV4 (26, 34, 44, 50). However, a more recent study found no evidence that flow produces a Ca2+ influx through the ciliary membrane in primary renal epithelial cells (11). While it is clear that ciliary deflection elevates Ca2+ in the cell body, and that this response is TRPP2-dependent, the intervening mechanisms are not yet understood (45). The ciliary TRPP2-dependent channel could also be activated by an extracellular ligand. In native mouse embryonic fibroblasts and in an exogenous expression system, some Wnt ligands activate a current that depends on both TRPP2 and polycystin-1 (29).
One can also speculate that TRPP2 may be activated, directly or indirectly, by a chemical stimulus. No direct agonist for TRPP2 (other than cytoplasmic Ca2+) has been reported. However, activation of any of several ciliary TRP channels could depolarize the cilium, which would promote opening of TRPP2 channels. Ca2+ that enters the cilium through another TRP channel could also activate TRPP2. Activation of TRPP2 is expected to lead to positive feedback. Open TRPP2 channels will depolarize the membrane and conduct Ca2+ into the cilium. Depolarization and increasing intraciliary Ca2+ should in turn activate additional TRPP2 channels, and so on. The effectiveness of such an amplifying system will depend on the channels involved, their proximities to one another, and the speed, capacity, and affinity of any intraciliary Ca2+ buffers.
In contemplating the effects of open TRPP2 channels, it is worthwhile to consider the native environment of the renal primary cilium. In the collecting duct, the cilium is bathed in a filtrate that resembles urine. In the mouse, the urine typically contains 257 mM K+ (25). Given the cationic concentrations reported for murine urine (25), the cytoplasmic cationic concentrations in collecting duct cells (4, 59), and the permeability ratios reported here, the channel current should reverse near +19 mV. This, plus the ready availability of the most permeant cation (K+) in the external space, should favor a substantial depolarizing current as the ciliary TRPP2-dependent channels open. In olfactory neurons with high resting membrane resistance, ciliary currents are believed to influence the cell potential (32). In renal epithelial cells, though, the lower resistance should leave the cell potential less sensitive to ciliary currents. In addition, even a large influx of Ca2+ at the cilium has at best a slight influence on the concentration of Ca2+ in the cytoplasm via bulk diffusion (10). Thus the consequences of a ciliary Ca2+ influx for the cell body remain unclear.
GRANTS
S. J. Kleene received support from National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases Grants R21 DK-091917 and P30 DK090868 (Baltimore Polycystic Kidney Disease Research and Clinical Core Center) and the University Research Council of the University of Cincinnati.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
S.J.K. and N.K.K. conceived and designed the research; S.J.K. and N.K.K. performed experiments; S.J.K. and N.K.K. analyzed data; S.J.K. and N.K.K. interpreted results of experiments; S.J.K. and N.K.K. prepared figures; S.J.K. and N.K.K. drafted manuscript; S.J.K. and N.K.K. edited and revised manuscript; S.J.K. and N.K.K. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Priscilla Amofa for excellent technical assistance and Drs. Brian Siroky and Bradley Dixon for helpful discussions. The authors thank the Cincinnati Children’s Hospital Medical Center Transgenic Animal and Genome Editing Core Facility and Dr. Yueh-Chiang Hu for manufacture and testing of the TRPP2 CRISPR plasmid and advice in developing and screening the clones.
REFERENCES
- 1.Abdul-Majeed S, Nauli SM. Calcium-mediated mechanisms of cystic expansion. Biochim Biophys Acta 1812: 1281–1290, 2011. doi: 10.1016/j.bbadis.2010.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bai C-X, Giamarchi A, Rodat-Despoix L, Padilla F, Downs T, Tsiokas L, Delmas P. Formation of a new receptor-operated channel by heteromeric assembly of TRPP2 and TRPC1 subunits. EMBO Rep 9: 472–479, 2008. doi: 10.1038/embor.2008.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bai C-X, Kim S, Li W-P, Streets AJ, Ong ACM, Tsiokas L. Activation of TRPP2 through mDia1-dependent voltage gating. EMBO J 27: 1345–1356, 2008. doi: 10.1038/emboj.2008.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beck F, Dörge A, Rick R, Thurau K. Intra- and extracellular element concentrations of rat renal papilla in antidiuresis. Kidney Int 25: 397–403, 1984. doi: 10.1038/ki.1984.30. [DOI] [PubMed] [Google Scholar]
- 5.Bers DM. A simple method for the accurate determination of free [Ca] in Ca-EGTA solutions. Am J Physiol Cell Physiol 242: C404–C408, 1982. [DOI] [PubMed] [Google Scholar]
- 6.Brown D, Lydon J, McLaughlin M, Stuart-Tilley A, Tyszkowski R, Alper S. Antigen retrieval in cryostat tissue sections and cultured cells by treatment with sodium dodecyl sulfate (SDS). Histochem Cell Biol 105: 261–267, 1996. doi: 10.1007/BF01463929. [DOI] [PubMed] [Google Scholar]
- 7.Cantiello HF. Regulation of calcium signaling by polycystin-2. Am J Physiol Renal Physiol 286: F1012–F1029, 2004. doi: 10.1152/ajprenal.00181.2003. [DOI] [PubMed] [Google Scholar]
- 8.DeCaen PG, Delling M, Vien TN, Clapham DE. Direct recording and molecular identification of the calcium channel of primary cilia. Nature 504: 315–318, 2013. doi: 10.1038/nature12832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cantero MR, Cantiello HF. Effect of lithium on the electrical properties of polycystin-2 (TRPP2). Eur Biophys J 40: 1029–1042, 2011. doi: 10.1007/s00249-011-0715-2. [DOI] [PubMed] [Google Scholar]
- 10.Delling M, DeCaen PG, Doerner JF, Febvay S, Clapham DE. Primary cilia are specialized calcium signalling organelles. Nature 504: 311–314, 2013. doi: 10.1038/nature12833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Delling M, Indzhykulian AA, Liu X, Li Y, Xie T, Corey DP, Clapham DE. Primary cilia are not calcium-responsive mechanosensors. Nature 531: 656–660, 2016. doi: 10.1038/nature17426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Delmas P, Nauli SM, Li X, Coste B, Osorio N, Crest M, Brown DA, Zhou J. Gating of the polycystin ion channel signaling complex in neurons and kidney cells. FASEB J 18: 740–742, 2004. doi: 10.1096/fj.03-0319fje. [DOI] [PubMed] [Google Scholar]
- 13.Ducoudret O, Barbier O, Tauc M, Fuchs M, Poujeol P. Characterization of Zn2+ transport in Madin-Darby canine kidney cells. Biochim Biophys Acta 1611: 171–179, 2003. doi: 10.1016/S0005-2736(03)00052-X. [DOI] [PubMed] [Google Scholar]
- 14.The European Polycystic Kidney Disease Consortium . The polycystic kidney disease 1 gene encodes a 14 kb transcript and lies within a duplicated region on chromosome 16. Cell 77: 881–894, 1994. doi: 10.1016/0092-8674(94)90137-6. [DOI] [PubMed] [Google Scholar]
- 15.Flannery RJ, Kleene NK, Kleene SJ. A TRPM4-dependent current in murine renal primary cilia. Am J Physiol Renal Physiol 309: F697–F707, 2015. doi: 10.1152/ajprenal.00294.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Freedman BS, Lam AQ, Sundsbak JL, Iatrino R, Su X, Koon SJ, Wu M, Daheron L, Harris PC, Zhou J, Bonventre JV. Reduced ciliary polycystin-2 in induced pluripotent stem cells from polycystic kidney disease patients with PKD1 mutations. J Am Soc Nephrol 24: 1571–1586, 2013. doi: 10.1681/ASN.2012111089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gainullin VG, Hopp K, Ward CJ, Hommerding CJ, Harris PC. Polycystin-1 maturation requires polycystin-2 in a dose-dependent manner. J Clin Invest 125: 607–620, 2015. doi: 10.1172/JCI76972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.González-Perrett S, Batelli M, Kim K, Essafi M, Timpanaro G, Moltabetti N, Reisin IL, Arnaout MA, Cantiello HF. Voltage dependence and pH regulation of human polycystin-2-mediated cation channel activity. J Biol Chem 277: 24959–24966, 2002. doi: 10.1074/jbc.M105084200. [DOI] [PubMed] [Google Scholar]
- 19.González-Perrett S, Kim K, Ibarra C, Damiano AE, Zotta E, Batelli M, Harris PC, Reisin IL, Arnaout MA, Cantiello HF. Polycystin-2, the protein mutated in autosomal dominant polycystic kidney disease (ADPKD), is a Ca2+-permeable nonselective cation channel. Proc Natl Acad Sci USA 98: 1182–1187, 2001. doi: 10.1073/pnas.98.3.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guay-Woodford LM, Henske E, Igarashi P, Perrone RD, Reed-Gitomer B, Somlo S, Torres VE, Ketchum CJ, Star RA, Flessner MF, Rasooly RS. Filling the holes in cystic kidney disease research. Clin J Am Soc Nephrol 9: 1799–1801, 2014. doi: 10.2215/CJN.03410414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hanaoka K, Qian F, Boletta A, Bhunia AK, Piontek K, Tsiokas L, Sukhatme VP, Guggino WB, Germino GG. Co-assembly of polycystin-1 and -2 produces unique cation-permeable currents. Nature 408: 990–994, 2000. doi: 10.1038/35050128. [DOI] [PubMed] [Google Scholar]
- 22.Harris PC, Torres VE. Genetic mechanisms and signaling pathways in autosomal dominant polycystic kidney disease. J Clin Invest 124: 2315–2324, 2014. doi: 10.1172/JCI72272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hsu PD, Scott DA, Weinstein JA, Ran FA, Konermann S, Agarwala V, Li Y, Fine EJ, Wu X, Shalem O, Cradick TJ, Marraffini LA, Bao G, Zhang F. DNA targeting specificity of RNA-guided Cas9 nucleases. Nat Biotechnol 31: 827–832, 2013. doi: 10.1038/nbt.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jan LY, Jan YN. L-glutamate as an excitatory transmitter at the Drosophila larval neuromuscular junction. J Physiol 262: 215–236, 1976. doi: 10.1113/jphysiol.1976.sp011593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang Z, Asplin JR, Evan AP, Rajendran VM, Velazquez H, Nottoli TP, Binder HJ, Aronson PS. Calcium oxalate urolithiasis in mice lacking anion transporter Slc26a6. Nat Genet 38: 474–478, 2006. doi: 10.1038/ng1762. [DOI] [PubMed] [Google Scholar]
- 26.Jin X, Mohieldin AM, Muntean BS, Green JA, Shah JV, Mykytyn K, Nauli SM. Cilioplasm is a cellular compartment for calcium signaling in response to mechanical and chemical stimuli. Cell Mol Life Sci 71: 2165–2178, 2014. doi: 10.1007/s00018-013-1483-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jin X, Muntean BS, Aal-Aaboda MS, Duan Q, Zhou J, Nauli SM. L-type calcium channel modulates cystic kidney phenotype. Biochim Biophys Acta 1842: 1518–1526, 2014. doi: 10.1016/j.bbadis.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim I, Li C, Liang D, Chen XZ, Coffy RJ, Ma J, Zhao P, Wu G. Polycystin-2 expression is regulated by a PC2-binding domain in the intracellular portion of fibrocystin. J Biol Chem 283: 31559–31566, 2008. doi: 10.1074/jbc.M805452200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim S, Nie H, Nesin V, Tran U, Outeda P, Bai CX, Keeling J, Maskey D, Watnick T, Wessely O, Tsiokas L. The polycystin complex mediates Wnt/Ca2+ signalling. Nat Cell Biol 18: 752-764, 2016. doi: 10.1038/ncb3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kleene NK, Kleene SJ. A method for measuring electrical signals in a primary cilium. Cilia 1: 17, 2012. doi: 10.1186/2046-2530-1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kleene SJ. A simple intrapipette salt bridge. J Neurosci Methods 46: 11–16, 1993. doi: 10.1016/0165-0270(93)90136-F. [DOI] [PubMed] [Google Scholar]
- 32.Kleene SJ. The electrochemical basis of odor transduction in vertebrate olfactory cilia. Chem Senses 33: 839–859, 2008. doi: 10.1093/chemse/bjn048. [DOI] [PubMed] [Google Scholar]
- 33.Kleene SJ, Gesteland RC. Calcium-activated chloride conductance in frog olfactory cilia. J Neurosci 11: 3624–3629, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Köttgen M, Buchholz B, Garcia-Gonzalez MA, Kotsis F, Fu X, Doerken M, Boehlke C, Steffl D, Tauber R, Wegierski T, Nitschke R, Suzuki M, Kramer-Zucker A, Germino GG, Watnick T, Prenen J, Nilius B, Kuehn EW, Walz G. TRPP2 and TRPV4 form a polymodal sensory channel complex. J Cell Biol 182: 437–447, 2008. doi: 10.1083/jcb.200805124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Köttgen M, Walz G. Subcellular localization and trafficking of polycystins. Pflugers Arch 451: 286–293, 2005. doi: 10.1007/s00424-005-1417-3. [DOI] [PubMed] [Google Scholar]
- 36.Koulen P, Cai Y, Geng L, Maeda Y, Nishimura S, Witzgall R, Ehrlich BE, Somlo S. Polycystin-2 is an intracellular calcium release channel. Nat Cell Biol 4: 191–197, 2002. doi: 10.1038/ncb754. [DOI] [PubMed] [Google Scholar]
- 37.LeDizet M, Piperno G. Detection of acetylated α-tubulin by specific antibodies. Methods Enzymol 196: 264–274, 1991. doi: 10.1016/0076-6879(91)96025-M. [DOI] [PubMed] [Google Scholar]
- 38.Lin F, Hiesberger T, Cordes K, Sinclair AM, Goldstein LS, Somlo S, Igarashi P. Kidney-specific inactivation of the KIF3A subunit of kinesin-II inhibits renal ciliogenesis and produces polycystic kidney disease. Proc Natl Acad Sci USA 100: 5286–5291, 2003. doi: 10.1073/pnas.0836980100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Luo Y, Vassilev PM, Li X, Kawanabe Y, Zhou J. Native polycystin 2 functions as a plasma membrane Ca2+-permeable cation channel in renal epithelia. Mol Cell Biol 23: 2600–2607, 2003. doi: 10.1128/MCB.23.7.2600-2607.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ma M, Tian X, Igarashi P, Pazour GJ, Somlo S. Loss of cilia suppresses cyst growth in genetic models of autosomal dominant polycystic kidney disease. Nat Genet 45: 1004–1012, 2013. doi: 10.1038/ng.2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ma R, Li W-P, Rundle D, Kong J, Akbarali HI, Tsiokas L. PKD2 functions as an epidermal growth factor-activated plasma membrane channel. Mol Cell Biol 25: 8285–8298, 2005. doi: 10.1128/MCB.25.18.8285-8298.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mochizuki T, Wu G, Hayashi T, Xenophontos SL, Veldhuisen B, Saris JJ, Reynolds DM, Cai Y, Gabow PA, Pierides A, Kimberling WJ, Breuning MH, Deltas CC, Peters DJM, Somlo S. PKD2, a gene for polycystic kidney disease that encodes an integral membrane protein. Science 272: 1339–1342, 1996. doi: 10.1126/science.272.5266.1339. [DOI] [PubMed] [Google Scholar]
- 43.Murcia NS, Richards WG, Yoder BK, Mucenski ML, Dunlap JR, Woychik RP. The Oak Ridge Polycystic Kidney (orpk) disease gene is required for left-right axis determination. Development 127: 2347 –2355, 2000. [DOI] [PubMed] [Google Scholar]
- 44.Nauli SM, Alenghat FJ, Luo Y, Williams E, Vassilev P, Li X, Elia AEH, Lu W, Brown EM, Quinn SJ, Ingber DE, Zhou J. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet 33: 129–137, 2003. doi: 10.1038/ng1076. [DOI] [PubMed] [Google Scholar]
- 45.Nauli SM, Pala R, Kleene SJ. Calcium channels in primary cilia. Curr Opin Nephrol Hypertens 25: 452–458, 2016. doi: 10.1097/MNH.0000000000000251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pazour GJ, Dickert BL, Vucica Y, Seeley ES, Rosenbaum JL, Witman GB, Cole DG. Chlamydomonas IFT88 and its mouse homologue, polycystic kidney disease gene Tg737, are required for assembly of cilia and flagella. J Cell Biol 151: 709–718, 2000. doi: 10.1083/jcb.151.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pazour GJ, San Agustin JT, Follit JA, Rosenbaum JL, Witman GB. Polycystin-2 localizes to kidney cilia and the ciliary level is elevated in orpk mice with polycystic kidney disease. Curr Biol 12: R378–R380, 2002. doi: 10.1016/S0960-9822(02)00877-1. [DOI] [PubMed] [Google Scholar]
- 48.Pelucchi B, Aguiari G, Pignatelli A, Manzati E, Witzgall R, Del Senno L, Belluzzi O. Nonspecific cation current associated with native polycystin-2 in HEK-293 cells. J Am Soc Nephrol 17: 388–397, 2006. doi: 10.1681/ASN.2004121146. [DOI] [PubMed] [Google Scholar]
- 49.Praetorius HA, Spring KR. Bending the MDCK cell primary cilium increases intracellular calcium. J Membr Biol 184: 71–79, 2001. doi: 10.1007/s00232-001-0075-4. [DOI] [PubMed] [Google Scholar]
- 50.Praetorius HA, Spring KR. Removal of the MDCK cell primary cilium abolishes flow sensing. J Membr Biol 191: 69–76, 2003. doi: 10.1007/s00232-002-1042-4. [DOI] [PubMed] [Google Scholar]
- 51.Rauchman MI, Nigam SK, Delpire E, Gullans SR. An osmotically tolerant inner medullary collecting duct cell line from an SV40 transgenic mouse. Am J Physiol Renal Physiol 265: F416–F424, 1993. [DOI] [PubMed] [Google Scholar]
- 52.Raychowdhury MK, McLaughlin M, Ramos AJ, Montalbetti N, Bouley R, Ausiello DA, Cantiello HF. Characterization of single channel currents from primary cilia of renal epithelial cells. J Biol Chem 280: 34718–34722, 2005. doi: 10.1074/jbc.M507793200. [DOI] [PubMed] [Google Scholar]
- 53.Raychowdhury MK, Ramos AJ, Zhang P, McLaughin M, Dai X-Q, Chen X-Z, Montalbetti N, Del Rocío Cantero M, Ausiello DA, Cantiello HF. Vasopressin receptor-mediated functional signaling pathway in primary cilia of renal epithelial cells. Am J Physiol Renal Physiol 296: F87–F97, 2009. doi: 10.1152/ajprenal.90509.2008. [DOI] [PubMed] [Google Scholar]
- 54.Robinson RA, Stokes RH. Electrolyte Solutions, the Measurement and Interpretation of Conductance, Chemical Potential, and Diffusion in Solutions of Simple Electrolytes. London: Butterworths, 1970, p. 571. [Google Scholar]
- 55.Ruijter JM, Ramakers C, Hoogaars WMH, Karlen Y, Bakker O, van den Hoff MJB, Moorman AFM. Amplification efficiency: linking baseline and bias in the analysis of quantitative PCR data. Nucleic Acids Res 37: e45, 2009. doi: 10.1093/nar/gkp045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Saigusa T, Bell PD. Molecular pathways and therapies in autosomal-dominant polycystic kidney disease. Physiology (Bethesda) 30: 195–207, 2015. doi: 10.1152/physiol.00032.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Semmo M, Köttgen M, Hofherr A. The TRPP subfamily and polycystin-1 proteins. Handb Exp Pharmacol 222: 675–711, 2014. doi: 10.1007/978-3-642-54215-2_27. [DOI] [PubMed] [Google Scholar]
- 58.Stefani E, Cereijido M. Electrical properties of cultured epithelioid cells (MDCK). J Membr Biol 73: 177–184, 1983. doi: 10.1007/BF01870440. [DOI] [PubMed] [Google Scholar]
- 59.Stokes JB, Grupp C, Kinne RK. Purification of rat papillary collecting duct cells: functional and metabolic assessment. Am J Physiol Renal Physiol 253: F251–F262, 1987. [DOI] [PubMed] [Google Scholar]
- 60.Su S, Phua SC, DeRose R, Chiba S, Narita K, Kalugin PN, Katada T, Kontani K, Takeda S, Inoue T. Genetically encoded calcium indicator illuminates calcium dynamics in primary cilia. Nat Methods 10: 1105–1107, 2013. doi: 10.1038/nmeth.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tsiokas L, Kim S, Ong EC. Cell biology of polycystin-2. Cell Signal 19: 444–453, 2007. doi: 10.1016/j.cellsig.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vassilev PM, Guo L, Chen X-Z, Segal Y, Peng J-B, Basora N, Babakhanlou H, Cruger G, Kanazirska M, Ye C-P, Brown EM, Hediger MA, Zhou J. Polycystin-2 is a novel cation channel implicated in defective intracellular Ca2+ homeostasis in polycystic kidney disease. Biochem Biophys Res Commun 282: 341–350, 2001. doi: 10.1006/bbrc.2001.4554. [DOI] [PubMed] [Google Scholar]
- 63.Winyard P, Jenkins D. Putative roles of cilia in polycystic kidney disease. Biochim Biophys Acta 1812: 1256–1262, 2011. doi: 10.1016/j.bbadis.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 64.Witzgall R. TRPP2 channel regulation. Handb Exp Pharmacol 179: 363–375, 2007. doi: 10.1007/978-3-540-34891-7_22. [DOI] [PubMed] [Google Scholar]
- 65.Wu S, Chen H, Chou Y, Huang Y, Lo Y. Inhibitory actions by ibandronate sodium, a nitrogen-containing bisphosphonate, on calcium-activated potassium channels in Madin-Darby canine kidney cells. Toxicol Rep 2: 1182–1193, 2015. doi: 10.1016/j.toxrep.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yoder BK, Hou X, Guay-Woodford LM. The polycystic kidney disease proteins, polycystin-1, polycystin-2, polaris, and cystin, are co-localized in renal cilia. J Am Soc Nephrol 13: 2508–2516, 2002. doi: 10.1097/01.ASN.0000029587.47950.25. [DOI] [PubMed] [Google Scholar]
- 67.Zhang P, Luo Y, Chasan B, González-Perrett S, Montalbetti N, Timpanaro GA, Cantero MR, Ramos AJ, Goldmann WH, Zhou J, Cantiello HF. The multimeric structure of polycystin-2 (TRPP2): structural-functional correlates of homo- and hetero-multimers with TRPC1. Hum Mol Genet 18: 1238–1251, 2009. doi: 10.1093/hmg/ddp024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang ZR, Chu WF, Song B, Gooz M, Zhang JN, Yu CJ, Jiang S, Baldys A, Gooz P, Steele S, Owsianik G, Nilius B, Komlosi P, Bell PD. TRPP2 and TRPV4 form an EGF-activated calcium permeable channel at the apical membrane of renal collecting duct cells. PLoS One 8: e73424, 2013. doi: 10.1371/journal.pone.0073424. [DOI] [PMC free article] [PubMed] [Google Scholar]