Abstract
We recently reported that skeletal muscle fibers of obscurin knockout (KO) mice present altered distribution of ankyrin B (ankB), disorganization of the subsarcolemmal microtubules, and reduced localization of dystrophin at costameres. In addition, these mice have impaired running endurance and increased exercise-induced sarcolemmal damage compared with wild-type animals. Here, we report results from a combined approach of physiological, morphological, and structural studies in which we further characterize the skeletal muscles of obscurin KO mice. A detailed examination of exercise performance, using different running protocols, revealed that the reduced endurance of obscurin KO animals on the treadmill depends on exercise intensity and age. Indeed, a mild running protocol did not evidence significant differences between control and obscurin KO mice, whereas comparison of running abilities of 2-, 6-, and 11-mo-old mice exercised at exhaustion revealed a progressive age-dependent reduction of the exercise tolerance in KO mice. Histological analysis indicated that heavy exercise induced leukocyte infiltration, fibrotic connective tissue deposition, and hypercontractures in the diaphragm of KO mice. On the same line, electron microscopy revealed that, in the diaphragm of exercised obscurin KO mice, but not in the hindlimb muscles, both M-line and H-zone of sarcomeres appeared wavy and less defined. Altogether, these results suggest that obscurin is required for the maintenance of morphological and ultrastructural integrity of skeletal muscle fibers against damage induced by intense mechanical stress and point to the diaphragm as the skeletal muscle most severely affected in obscurin-deficient mice.
Keywords: myopathy, myofibrils, contraction, fibrosis, muscular dystrophy
obscurin is a giant sarcomeric protein expressed in all striated muscles with a localization pattern showing a larger distribution in coincidence of the M-line and a minor distribution over the Z-disk (17, 31, 37). Originally identified as a titin-binding partner, to date four different obscurin isoforms have been characterized. Two high-molecular-weight variants, namely obscurin A and B, are both expressed in adult skeletal muscles and share a modular architecture. Both isoforms present immunoglobulin and fibronectin type III domains, arranged in tandem, and several modules involved in signaling and protein-protein interaction (22, 37). Obscurin A contains two COOH-terminal binding sites able to mediate interactions with muscle-specific isoforms of ankyrin proteins (3, 19). Obscurin B, the higher-size isoform, lacks the COOH-terminal tail present in obscurin A but contains two serine-threonine kinase domains. Finally, two additional variants (tandem obscurin-myosin light chain kinase and single obscurin-myosin light chain kinase), predominantly expressed in cardiac muscle cells, have been also characterized (5, 12, 33). The modular layout allows obscurin to be part of a molecular network that connects the sarcomere with two other separate intracellular districts: the sarcoplasmic reticulum (SR) and the costameres (8, 11, 28). Indeed, in vitro characterization of the functional role of obscurin revealed its contribution, together with titin, myomesin, and myosin-binding protein C, to the organization and maintenance of an intrasarcomeric cytoskeleton that stabilizes the M-line during contraction (13, 14, 29, 37). Also, the ankyrin-binding sites present in the COOH-terminus tail allow the interaction between obscurin and the small muscle-specific ankyrin sAnk1.5, an integral protein of the SR membrane, suggesting a role of obscurin in tethering the SR to the contractile apparatus (1, 3, 24, 32, 38). This was elegantly confirmed by the evidence that mice KO for obscurin and mice KO for sAnk1.5 (16, 25) both present an aberrant architecture of the longitudinal SR in skeletal muscle fibers. Recently, we reported that obscurin is also required for the localization of ankB at the M-line of skeletal muscle fibers and that, in obscurin KO mice, this localization is lost, leading to altered organization of the subsarcolemmal microtubule network and reduced distribution of dystrophin at costameres. Moreover, when exercised on the treadmill using heavy running protocols, these mice show reduced running endurance associated with an increased fragility of the sarcolemma (31).
Altered organization of costameres, usually as a consequence of mutations or loss of dystrophin and/or other components of the dystrophin associated protein complex, results in failure of muscle fibers' sarcolemma to support the mechanical stress associated with repetitive cycles of muscle contraction and relaxation, leading to fiber damage, atrophy, and degeneration. Accordingly, histological evidence of fiber necrosis and regeneration, inflammatory infiltrate, fibrosis, changes in fiber size, and replacement of muscle fibers with adipocytes can be observed in dystrophic muscles, but degree and occurrence of these features are both variable. The presence of one or more of these histological features may vary depending on the specific type of muscular dystrophy and can be either generalized or selective for specific muscles. In dystrophic mice, the most intense damage is usually observed in the diaphragm muscle, likely because of its involvement in respiration, and hence exposed to the injury caused by continuous contraction-relaxation cycles even at rest (4, 10, 36). Interestingly, a similar sequence of events can be observed in almost all models of muscular dystrophy (30, 34, 36). Analysis of both human patients and mouse models of muscular dystrophy indicate that the establishment of histological evidence of muscle damage is usually induced by “regular” muscle activity and does not require strenuous activation of contraction. This contrasts with observation of the characterization of obscurin KO mice that, under resting conditions, do not show significant alterations in skeletal muscle tissue other than reduction in longitudinal SR volume, presence of few fibers with centrally positioned nuclei, and reduced localization of dystrophin (25, 31).
Here, we report a further characterization of obscurin KO skeletal muscles obtained from the combination of physiological, morphological, and ultrastructural approaches. Our results indicate that the reduced endurance ability of the obscurin KO mice is exercise intensity dependent and increases with age. In obscurin KO mice, an intense training determines inflammatory response and fibrosis, both mainly evident in the diaphragm muscle, where these histopathological features are paralleled by alteration of the H-zone and loss of the M-line at the myofibrillar level.
MATERIALS AND METHODS
Mice.
All experiments followed the official guidelines laid down by the European Community Council (directive 86/609/EEC) incorporated into Italian Government Legislation. Experiments have been performed with the approval by the Local Ethical Committee and from Ministero della Salute (Rome, Italy). The development of obscurin global KO mice has been previously described (25). All animals used in this study were Black Swiss male mice. The genotype was verified by duplex PCR performed on genomic DNA purified from tail tissue with the Gentra Puregene (QIAGEN) genotyping kit, according to the manufacturer's instructions, using the following primers: obscurin forward, 5′-TAAGATTCTTTTCTGCAAGCAGTC-3′; obscurin reverse, 5′-AGTGTGTTTTGAGGAAGGAGAGAG-3′; Neo FLP recognition target forward, 5′-AATGGGCTGACCGCTTCCTCGT-3′.
Treadmill exercise.
Running capacity of control and obscurin KO mice was tested with a treadmill (LE 8710M; Panlab) equipped with an electrified grid, using various running protocols. In all uphill running experiments, 2-, 6-, and 11-mo-old mice (n = 4, 20, and 4, respectively) were tested as previously described (31). After the running sessions, a group of 6-mo-old mice (n = 3/genotype) was housed in standard conditions for 14 days and then further exercised with the uphill running protocol. In the low-intensity protocol (mild), 6-mo-old mice (n = 3) were exercised two times a day for 3 days with the following parameters: 20 cm/s, 10 min, 0° incline. In the running exercise performed to exhaustion, 2-, 6-, and 11-mo-old mice (n = 5) were tested one time a day for three consecutive days using the following parameters: 25 cm/s, 5° uphill incline. In all experiments, running was considered concluded when mice paused running for more than 20 s, resisting to gentle or even stronger stimulation.
Neuromuscular junction morphology analysis.
Fibers obtained from diaphragm and hindlimb muscles of 6-mo-old control and of obscurin KO mice (n = 3) were fixed in 1% paraformaldehyde-0.5% Triton X-100 for 1 h at room temperature. The bundles of dissected fibers were further permeabilized with 0.5% Triton X-100 in HEPES buffer (20 mM HEPES, pH 7.4, 300 mM sucrose, 50 mM NaCl, 3 mM MgCl2, and 0.5% Triton X-100) for 3 min and blocked with 10% goat serum in BSA fraction V (A7906; Sigma-Aldrich) for 1 h. Fibers were stained with fluorescently labeled α-bungarotoxin Alexa Fluor 594 (B13423; Molecular Probes, Invitrogen) prepared in 1% BSA for 30 min at room temperature. After two additional washes in 1% BSA, fibers were mounted on glass slides and covered with cover slips using the ProLong Gold antifade reagent (P36934; Molecular Probes) as mounting medium. Z-stack acquisition of neuromuscular junctions (NMJs) was performed using the LSM 510 META confocal microscope (Carl Zeiss) with a Plan-Apochromat ×63, 1.4 numeric aperture (NA) oil immersion objective. After Z-stack acquisition and 3D projection rendering using the LSM Image browser (Carl Zeiss), measurement of NMJ surface areas (n = 23) was performed using the ImageJ software (National Institutes of Health).
In vivo muscle physiology.
Contraction was elicited by electrical stimulation of the sciatic nerve. Teflon-coated 7 multistranded steel wires (AS 632; Cooner Sales, Chatsworth, CA) were implanted with sutures on either side of the sciatic nerve before its branching proximally to the knee. At the distal ends of the two wires the insulation was removed, whereas the proximal ends were connected to a stimulator (Grass S88). To avoid fatigue, 60 contractions were performed with a pause of at least 30 s between stimuli.
Histology.
Skeletal muscles were removed from 6-mo-old sedentary and exercised mice (n = 4/genotype and experimental condition). An additional group of 6 mice/genotype was allowed to recover after completion of the heavy running protocol, and, after 14 days of rest, the diaphragm muscle was isolated for histological analysis (n = 3/genotype). The diaphragm muscle was removed from 2- and 11-mo-old mice immediately after completion of the heavy running protocol (n = 4/genotype and age) and processed for histological analysis. Skeletal muscle specimens were snap-frozen using optimum cutting temperature compound (Tissue-Tek-Sakura) in liquid nitrogen-cooled isopenthane. Cross sections (8 μm) were obtained using a Leica CM1850 cryostat (Leica Microsystem, Vienna, Austria) and mounted on Superfrost Plus slides (Thermo Scientific). Cryosections were stained with Carazzi's modified 0.1% hematoxylin (Panreac) solution for 7 min, washed in double-distilled water for 4 min, stained with 0.1% eosin yellowish (Panreac) solution for 4 min, washed in double-distilled water for 1 min, dehydrated by passages in ascending alcohols (1 min each), and finally clarified with two passages in xylene (2 min each). Cover slips were mounted using Eukitt quick-hardening mounting medium (Fluka). Analysis of contractile apparatus was performed on longitudinal semithin sections (4 μm) obtained using an auto-cut microtome (Supercut 2050; Reichert Jung). Briefly, diaphragm and hindlimb muscles from 6-mo-old sedentary mice (n = 3/genotype); 2-, 6-, and 11-mo-old uphill-exercised mice (n = 2, 3, and 2/genotype, respectively); and 6-mo-old exercised mice after 14 days of rest (n = 3/genotype) were removed and fixed in Karnovsky's buffer overnight at 4°C. The next day, muscles were dehydrated by passages in ascending alcohols and infiltrated with methacrylate resin Technovit 7100 (Bio-Optica) according to the manufacturer's instructions. Sections were stained using a 0.1% toluidine blue solution and then clarified by two passages in ethanol absolute and xylene. Cover slips were mounted using Eukitt quick-hardening mounting medium (Fluka). Samples were imaged using an Eclipse E600 microscope (Nikon) with a Plan-Fluor ×20, 0.50 NA objective, and images were acquired using the Nis-Element 2.3 software (Nikon).
Immunofluorescence and immunohistochemistry.
Cross sections (8 μm) were prepared from muscles of 6-mo-old wild-type and obscurin KO sedentary and exercised mice (n = 4/genotype and experimental condition), using a Leica CM1850 cryostat (Leica Microsystem, Vienna, Austria), mounted on Superfrost Plus slides (Thermo Scientific), and stored at −80°C until use. For immunofluorescence analysis, cryosections were fixed in 3% paraformaldehyde for 7 min at room temperature and further permeabilized with 0.5% Triton X-100 in HEPES buffer (20 mM HEPES, pH 7.4, 300 mM sucrose, 50 mM NaCl, 3 mM MgCl2, and 0.5% Triton X-100) for 3 min. Next, sections were blocked with 5% goat serum in BSA fraction V (A7906; Sigma-Aldrich) for 1 h. Rabbit polyclonal antibodies against CD45 antigen (ab10558) (diluted 1:300) to stain leukocyte cells and against type III collagen (ab7778) (diluted 1:300) used in immunofluorescence experiments were both purchased from Abcam. Primary antibodies were prepared in 0.2% BSA and incubated overnight at 4°C. The next day, after three washes of 10 min each, samples were incubated for 1 h at room temperature with the secondary antibodies. Fluorescent Cy3-conjugated secondary antibodies were obtained from Jackson Immuno-Research Laboratories. After a final washing step (3 times, 10 min each), samples were mounted on glass slides and covered with cover slips using the ProLong Gold antifade reagent (P36934; Molecular Probes) as mounting medium. Samples were imaged using the LSM510 META confocal microscope (Carl Zeiss) with a Plan-Neofluar ×20, 0.50 NA objective. Images were acquired at 23°C with the LSM acquisition software (Carl Zeiss). Inflammation was assessed by calculating the average percentage of CD45+ cells on the total number of nuclei in at least five optical fields per section counterstained with TO-PRO-3 (T3605; Molecular Probes, Invitrogen). For evaluation of interstitial fibrosis, sections from muscles of four different mice per experimental condition were scored. Collagen deposition was quantified as mean of the fluorescence intensity in at least five optical fields per section positive to type III collagen staining, using the ImageJ software (National Institutes of Health). In all sections analyzed, the parameters for image acquisition, threshold, and background subtraction were identical, and the evaluation and quantification of both collagen deposition and CD45+ cells in diaphragm and hindlimb muscles were done blindly to avoid subjective interference.
For immunohistochemistry analysis, cryosections were treated with acetone for 2 min, blocked with 5% BSA fraction V (A7906; Sigma-Aldrich) for 30 min, and incubated with primary antibodies prepared in 1% BSA overnight at 4°C. Mouse monoclonal antibodies against fast skeletal myosin (M4276-clone MY-32) (diluted 1:10) to stain the type II isomyosins and against slow skeletal myosin (M8421-clone NOQ7.5.4D) (diluted 1:10) to stain type I myosin were both purchased from Sigma-Aldrich. The next day, samples were washed three times for 10 min each, treated with 5% hydrogen peroxide (in methanol) for 20 min, and incubated with secondary anti-mouse horseradish peroxidase-linked antibody (NA931; GE Healthcare) for 1 h at room temperature. After three washes of 10 min each, samples were incubated with SigmaFast 3,3′-diaminobenzidine/urea (D4168; Sigma-Aldrich) for 5 min. Reaction was blocked with tap water, and nuclei were counterstained with Carazzi's modified 0.1% hematoxylin (Panreac) solution. Finally, samples were dehydrated by passages in ascending alcohols and clarified in xylene. Cover slips were mounted using Eukitt quick-hardening mounting medium (Fluka). Samples were imaged using an Eclipse E600 microscope (Nikon), and images were acquired using the Nis-Element 2.3 software (Nikon).
Electron microscopy.
Wild-type and obscurin KO mice at 6 mo of age (n = 4/genotype) were euthanized after performing the 3 days uphill running protocol on the treadmill. Muscles were processed as previously described (16, 27). Briefly, small strips of diaphragm muscle were dissected and pinned on a Sylgard dish, fixed at room temperature in 3.5% glutaraldehyde in 0.1 M sodium cacodylate buffer, pH 7.2 for 2 h, and kept in fixative until further use. The small bundles of fixed fibers were then postfixed in 2% OsO4 in the same buffer for 2 h and block-stained in aqueous saturated uranyl acetate. After dehydration, specimens were embedded in an epoxy resin (Epon 812). Ultrathin sections (40 nm) were cut in a Leica Ultracut R microtome (Leica Microsystem, Vienna, Austria) using a Diatome diamond knife (Diatome, Biel, Switzerland). After being stained in 4% uranyl acetate and lead citrate, sections were examined with a Morgagni Series 268D electron microscope (FEI, Brno, Czech Republic) equipped with a Megaview III digital camera. Ultrastructural alterations and organization of the M-line with respect to the sarcomere were analyzed in histological sections and electron microscopy (EM) micrographs, respectively.
Evans blue dye uptake assay after eccentric and concentric contractions.
Contraction stimulation was performed as above described. Immediately after stimulation, Evans blue dye (EBD, E2129; Sigma-Aldrich) was injected intraperitoneally (1 mg·0.1 ml−1·10 g body wt−1), as previously described (31). Mice were euthanized after 24 h, and tibialis anterior, extensor digitorum longus (EDL), soleus, and gastrocnemius muscles were removed and snap-frozen using optimum cutting temperature compound (Tissue-Tek-Sakura) in nitrogen-cooled isopenthane at −165°C. Cross sections (20 μm) throughout the length of the muscles were prepared to evaluate the extent of EBD uptake. Positive fibers were imaged with a Plan-Neofluar ×20, 0.50 NA objective using the LSM 510 META confocal microscope and the LSM image browser (Carl Zeiss).
Statistics.
Statistical analysis was performed using the two-tailed Student's t-test. Data are presented as means ± SE, and P values are shown. P values <0.05 were considered significant.
RESULTS
The reduced running ability of obscurin KO mice depends on exercise intensity and increases with age.
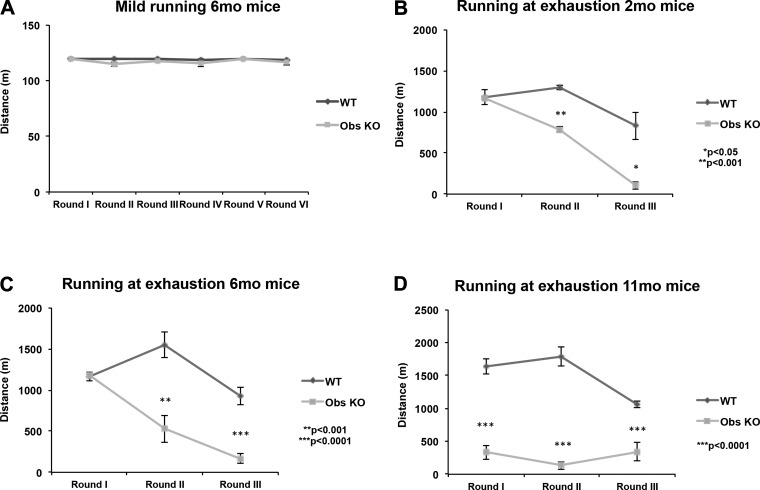
We reported that obscurin KO mice of 6–8 mo of age, when requested to perform uphill running sessions two times a day on the treadmill, showed a marked reduction in their exercise performance compared with wild-type counterparts (31). Given that our previous experiments were based on a heavy running protocol, this approach was unable to discriminate whether the impaired exercise performance showed by obscurin KO mice may depend on the high intensity of the running protocol used and/or on the age of exercised mice. To better characterize the running ability of obscurin KO mice, the animals were again tested, in a slow running protocol based on zero slope, 20 cm/s speed running settings for 10 min. Interestingly, this “mild” exercise did not reveal significant differences, in terms of performance, between control and KO mice, since both groups displayed comparable running endurance during all running sessions (Fig. 1A). These results prompted us to verify how much the intensity of the running protocol might impact the endurance of obscurin KO mice. In addition, we extended the age range of exercised mice. Groups of 2-, 6-, and 11-mo-old animals were therefore forced to run to exhaustion with a heavy protocol (25 cm/s, 5° incline). Determination of the time-to-exhaustion of exercised mice confirmed our previous results and clarified that the gap in terms of exercise tolerance between obscurin KO and control mice progressively augmented with the increasing age of mice (Fig. 1, B–D). Furthermore, the performance of obscurin KO mice worsened in the 2nd and 3rd day of each running protocol, suggesting that the heavy physical activity had an overall negative impact on the endurance of KO mice and that even 24 h of delay between two consecutive running sessions were not sufficient to recover.
Fig. 1.
Analysis of running performance of wild-type (WT) and obscurin (Obs) knockout (KO) mice under different exercise protocols. A low-intensity running protocol revealed that WT and obscurin KO mice have comparable exercise endurance under mild training conditions (A) (see materials and methods for training parameters). Conversely, a markedly reduced performance of obscurin KO mice on the treadmill is induced by higher exercise intensity (running at exhaustion) and progressively worsens with the increasing age of trained mice (B–D). P values are shown.
Synaptic transmission at NMJs is not altered in obscurin KO mice.
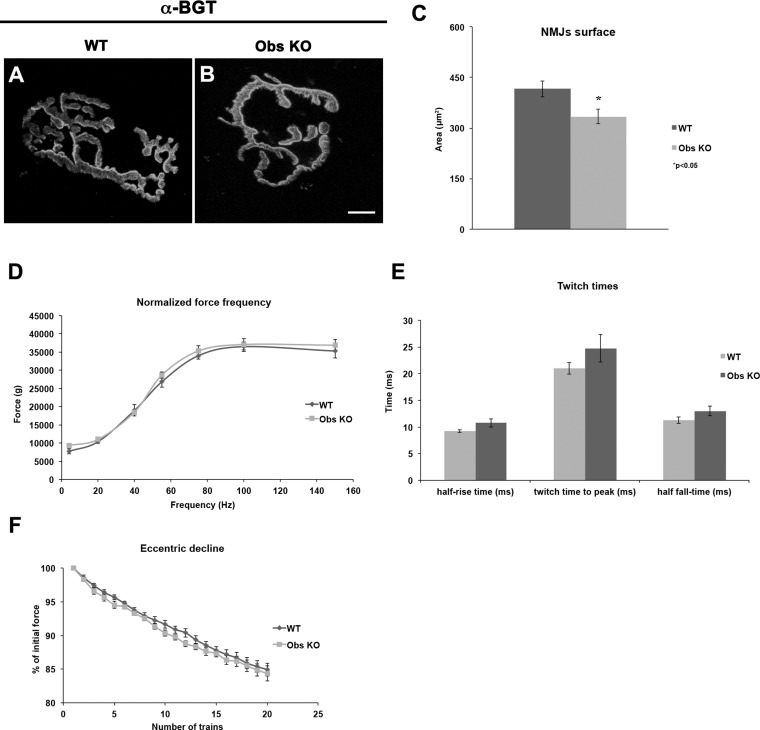
In addition to the sarcomeric and subsarcolemmal localization in skeletal muscle fibers, obscurin has been reported to be present at NMJs (7). To verify whether the lack of obscurin may negatively impact the organization and function of NMJs, therefore affecting their running performance, we analyzed the NMJs of diaphragm and hindlimb muscles from 6-mo-old control and obscurin KO mice. Immunostaining of α-bungarotoxin showed a less branched architecture of NMJs in obscurin-deficient muscle fibers compared with control counterparts (Fig. 2, A and B), revealing also a surface extension reduced by an average 20% (control mice: 416 ± 23.8 μm2; obscurin KO mice: 334 ± 21.1 μm2, P < 0.05, n = 3) (Fig. 2C). We asked whether an impaired synaptic transmission could occur in these mice. Nevertheless, the full efficiency of the in vivo electrical stimulation of gastrocnemius via sciatic nerve, the similar force generated in twitch and tetanus stimulations, and the lack of any delay in twitch time parameters of obscurin KO muscle fibers compared with controls allowed to exclude that the reduced running performance of obscurin KO mice relies on altered synaptic transmission (Fig. 2, D–F).
Fig. 2.
Staining of neuromuscular junctions (NMJs) with fluorescently labeled α-bungarotoxin (α-BGT). Analysis of NMJ morphology by immunostaining of α-BGT to mark the acetylcholine receptors shows that, in obscurin KO mice, NMJs have a reduced surface area compared with control counterparts (A–C). P value is shown. Scale bar, 10 μm. Results obtained from in vivo electrical stimulation of gastrocnemius muscle through repeated high-intensity eccentric contractions did not evidence significant differences on force frequency (D), twitch times (E), and force decline values measured after eccentric contraction (F) between control and obscurin KO mice.
The diaphragm of exercised obscurin KO mice shows evidence of muscle injury, CD45+ cell infiltration, and increased amount of type III collagen.
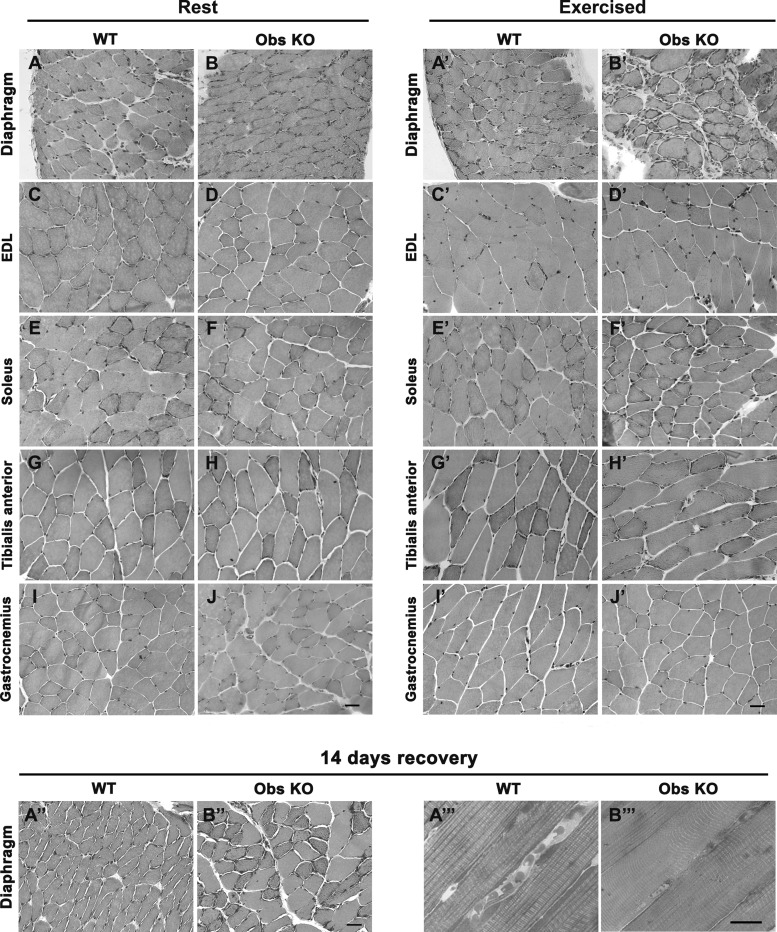
Next, we decided to investigate whether the altered performance of obscurin KO mice on the treadmill may depend on the presence a priori of histological alterations in their skeletal muscles. Accordingly, we performed hematoxylin and eosin staining of cross sections prepared from several hindlimb muscles (EDL, soleus, tibialis anterior, and gastrocnemius) and from the diaphragm of 6-mo-old wild-type and obscurin KO mice, kept either at rest or subjected to a heavy uphill running protocol (total of 16 mice, n = 4/genotype and experimental condition), as previously described (31). Histological preparations showed that, in sedentary mice, no evident morphological alterations were present in skeletal muscles, neither in control nor in obscurin KO (Fig. 3, A–J). Conversely, muscles from exercised mice revealed that, while no evidence of morphological alterations was detected in hindlimb muscles (Fig. 3, C′–J′), four out of four obscurin KO mice had evident signs of damage in the diaphragm, referable to blood cell infiltration and interstitial fibrosis (Fig. 3, A′ and B′). The histological analysis was also extended to diaphragm muscle of 2- and 11-mo-old mice. No significant morphological alterations were observed in obscurin KO mice of 2 mo of age after intense exercise with respect to age-matched WT mice, while, in analogy with results obtained with exercised 6-mo-old mice, evidence of muscle damage, including cell infiltration and increased interstitial fibrosis, was observed in four out of four 11-mo-old obscurin KO mice but not in age-matched wild-type mice (data not shown).
Fig. 3.
Histological analysis of diaphragm and hindlimb muscles from 6-mo-old WT and obscurin KO mice. Hematoxylin and eosin staining of cross sections from diaphragm muscle of mice maintained at rest reveals no significant morphological differences between WT and obscurin KO mice (compare A and B). After a heavy exercise (uphill running), alterations qualitatively referable to blood cell infiltration and interstitial connective tissue deposition become evident in the diaphragm of obscurin KO mice (compare A′ and B′). Cross sections from several other hindlimb muscles do not display evident morphological differences from WT and obscurin KO mice neither at rest (C–J) nor after heavy uphill exercise (C′-J′). Following recovery (14 days), rescue of morphology in the diaphragm of trained obscurin KO mice is evident both in hematoxylin and eosin (A″–B″) and in toluidine-stained sections (A″′–B″′). EDL, extensor digitorum longus. Scale bars, 10 μm.
To verify the ability of diaphragm fibers from obscurin KO mice to recover from uphill running injuries, we performed experiments in which 6-mo-old wild-type and obscurin KO mice (n = 9/genotype) were allowed to rest for 14 days after intense exercise. After this period of rest, these mice were capable of running performances similar to those recorded 14 days earlier (data not shown). In addition, histological analysis revealed a significant recovery of muscle morphology in the diaphragm of obscurin KO mice (although a few damaged muscle fibers were still present), indicating that deletion of the obscurin gene did not impair the ability to repair muscle damage (Fig. 3, A″ and B″′).
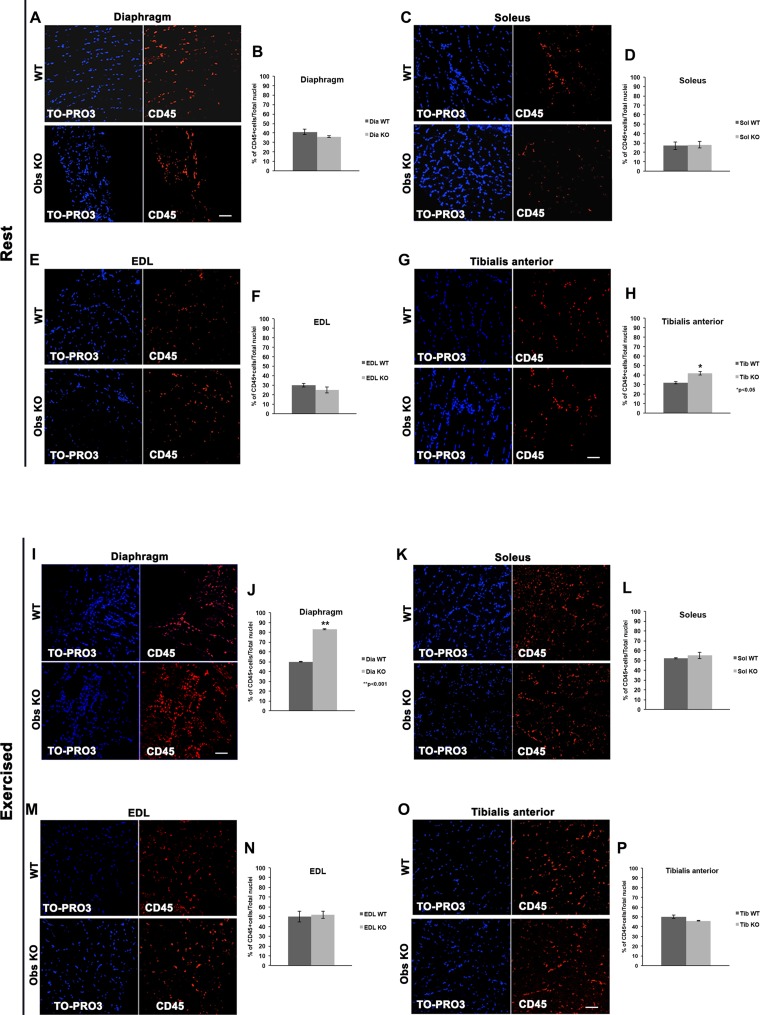
To follow up and confirm the observation that inflammatory cells and fibrosis were present in the diaphragms of obscurin KO mice as a result of intense exercise, we first performed the immunofluorescence staining of diaphragm and hindlimb muscles using an antibody for the CD45 antigen, a pan leukocyte marker (20). Evaluation of the average percentage of CD45+ cells on total nuclei revealed no significant differences between control and obscurin KO muscles at rest in the diaphragm (Fig. 4, A and B) while, among hindlimb muscles (Fig. 4, C–H), the only significant difference detected in the tibialis anterior was the amount of CD45+ cells detected in KO mice that was slightly higher (1.3-fold) than in control animals (control mice: 32 ± 1.2%; obscurin KO mice: 42 ± 1.9%, P < 0.05) (Fig. 4, G and H). As for exercised mice, the number of CD45+ cells was markedly increased (1.7-fold) in the diaphragm of obscurin KO mice (control mice: 50 ± 0.4%; obscurin KO mice: 83 ± 0.5%, P < 0.01) (Fig. 4, I and J), whereas our analysis revealed no significant changes among hindlimb muscles (Fig. 4, K–P).
Fig. 4.
Immunostaining for CD45 of diaphragm and hindlimb muscle fibers from WT and obscurin KO mice. At rest, the amount of CD45+ cells revealed by immunofluorescence in the diaphragm muscle is similar between WT and obscurin KO mice (A and B). As for hindlimb muscles, only a slight increase (1.3-fold) in the amount of CD45+ cells is found in tibialis anterior muscle of obscurin KO mice at rest (C–H). After a heavy uphill exercise, the diaphragm of obscurin KO mice shows a remarkable increase (1.7-fold) of CD45+ cells (I–J) compared with WT. Conversely, no significant differences in the amount of CD45+ cells are detected in hindlimb muscles of WT and obscurin KO mice (K–P). Scale bars, 20 μm. P values are shown.
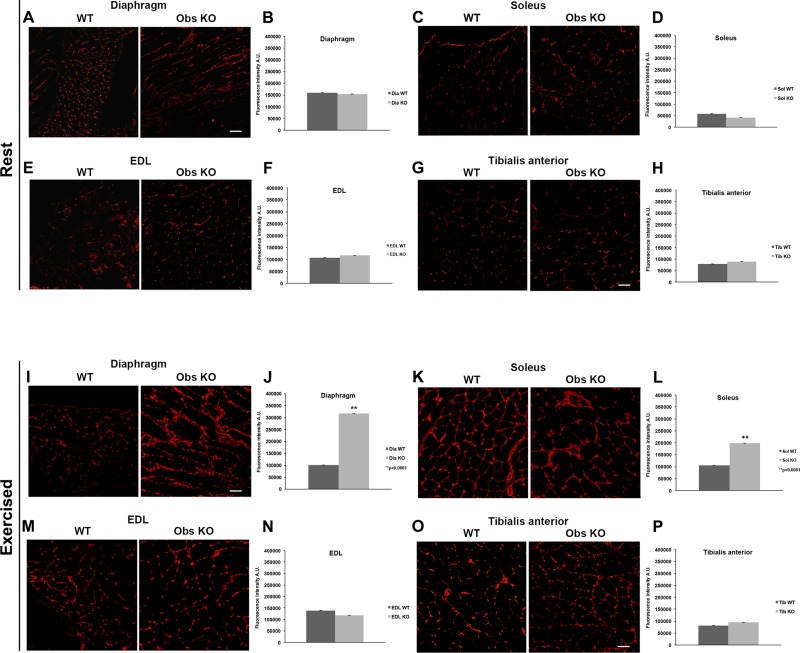
Next, to confirm the observation that interstitial fibrosis developed in parallel to leukocyte infiltration in exercised obscurin KO mice, we performed the immunofluorescence analysis using an antibody for type III collagen, a known marker of fibrosis in muscle and other tissue diseases (20). Analysis of the fluorescence intensity of collagen-positive areas within muscle sections revealed no significant differences between control and obscurin KO muscles of sedentary mice (Fig. 5, A–H). Conversely, in exercised mice, the areas of newly formed collagen III-positive connective tissue in the diaphragm of obscurin-deficient mice were 3.1-fold wider compared with controls [control mice: 100,351 ± 0.6 arbitrary units (AU); obscurin KO mice: 315,857 ± 0.8 AU, P < 0.001] (Fig. 5, I and J) and, among hindlimb muscles (Fig. 5, K–P), the larger increase (1.9-fold) was observed in the soleus of obscurin KO mice (Fig. 5, K and L) (control mice: 105,432 ± 0.4 AU; obscurin KO mice: 197,370 ± 0.7 AU, P < 0.001) while only minimal changes were detected in the other muscles. Altogether, these results point to inflammation and fibrosis as the main alterations of skeletal muscles in obscurin KO mice after heavy exercise, in line with the histological analysis, both severely affecting the diaphragm muscle while selective (i.e., inflammation or fibrosis) and less extended alterations could be detected in some of the hindlimb muscles.
Fig. 5.
Immunostaining for type III collagen of diaphragm and hindlimb muscle fibers from WT and obscurin KO mice. At rest, the amount of type III collagen revealed by immunofluorescence in the diaphragm and hindlimb muscles is comparable between WT and obscurin KO mice (A–H). A heavy uphill exercise induces an augmented deposition (3.1-fold) of collagen-positive fibrotic areas in the diaphragm of obscurin KO mice (I–J). As for the hindlimb muscles, the exercise increased the level of type III collagen in the soleus (2-fold) of obscurin KO mice (K and L), whereas the amount of type III collagen remains comparable between control and obscurin KO for EDL (M and N) and tibialis anterior (O and P) muscles. Scale bars, 20 μm. P values are shown.
The H-zone and the M-line of sarcomeres are altered in diaphragm slow-type fibers of obscurin KO mice after exercise.
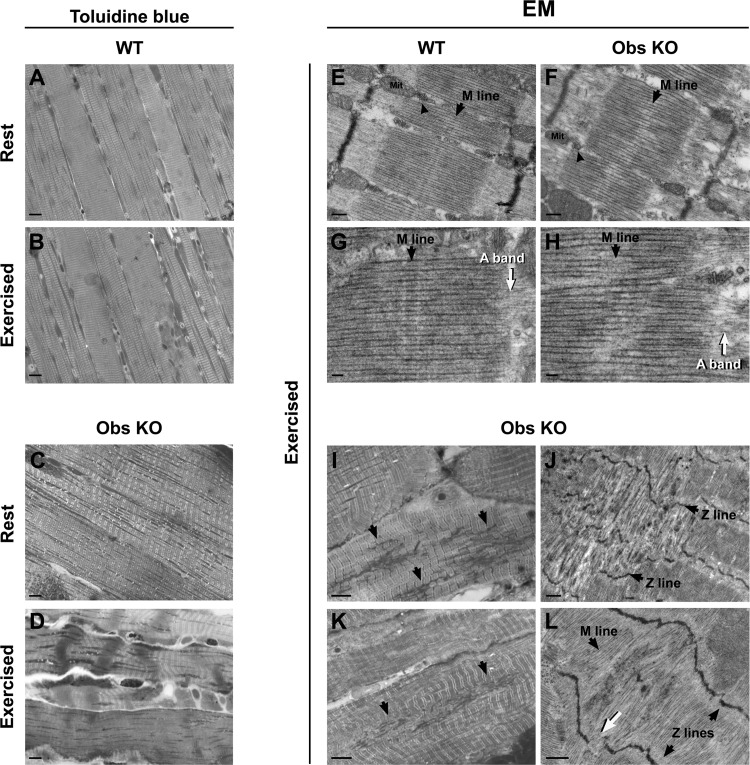
A large amount of data in the literature indicate that obscurin plays an important role in the sarcomerogenesis and in the maintenance of sarcomeric organization in adult life (14, 23, 29). We therefore took a closer look at the contractile apparatus (using histological and EM sections) comparing diaphragm muscles of sedentary and exercised wild-type and obscurin KO mice (6 mo of age). Diaphragm muscles of obscurin KO mice often present fiber areas with structural alterations characterized by loss of striations, unusual sarcomere shortening (contractures), or even hypercontracted sarcomeres following uphill exercise. The count of contracture knots in skeletal muscle fibers of sedentary mice yielded an average 1.7% (n = 450) in wild-type mice (n = 3) and an average 4.8% (n = 500) in obscurin KO mice (n = 3) (Fig. 6, A and C). After heavy exercise, the analysis revealed an average 2.5% (n = 400) of fibers presenting contracture knots in wild-type mice (n = 3), whereas an average 17% (n = 800) was detected in obscurin KO mice (n = 3) (Fig. 6, B and D). No hypercontracted sarcomeres were detected in the hindlimb muscles of either mice exercised or maintained under resting conditions (data not shown). Altogether, these results show, on one side, that a heavy exercise is able to induce structural alterations in obscurin-deficient skeletal muscle fibers but, on the other side, that these alterations are evident only in the diaphragm.
Fig. 6.
Structural and ultrastructural analysis of diaphragm muscle fibers from WT and obscurin KO mice. While diaphragm fibers of WT mice do not present significant structural alterations at rest or after exercise (A and B), in obscurin KO mice some contracture knots are present at rest (C) and become more frequent following exercise (D). Scale bars, 20 μm. Ultrastructural evaluation by electron microscopy (EM) of diaphragm muscle fibers shows that in WT the M-lines are placed at the center of sarcomeres and well aligned with those of adjacent myofibrils (E and G, black arrows), whereas in obscurin KO fibers they appear wavy and blurry (F and H, black arrows). Also, a loss of sharpness of A-bands is visible at the junction with the I-band (G and H, white arrows). In E and F, arrowheads point to a Ca2+ release unit (triad). Mit, mitochondrion. Scale bars: E and F, 0.2 μm; G and H, 0.1 μm. Sporadically, only in diaphragm fibers of exercised obscurin KO mice, Z-line streaming and complete disruption of M-lines are visible in histological (I and K) and EM (J and L) images. In areas presenting streaming, Z-lines are wavy (J and L, black arrows) or missing (L, white arrow) with Z-disk material that appears ripped out (I and K, black arrows) and missing M-lines (L, black arrow). Scale bars: I and K, 5 μm; J and L, 0.5 μm.
The contractile apparatus of sedentary and exercised control and obscurin KO mice was also analyzed by EM. Diaphragm fibers from sedentary mice did not show any significant modification in control and obscurin KO animals (data not shown). Conversely, following the 3-day protocol of uphill treadmill running, EM revealed partial disruption of M-lines in sarcomeres of obscurin KO diaphragm fibers. Indeed, although in control fibers the M-lines are placed at the center of sarcomeres and well aligned with those of adjacent myofibrils (Fig. 6, E and G), in obscurin KO mice (n = 4) an average 25% of fibers (n = 76) showed an M-line that appeared wavy and blurry (Fig. 6, F and H). In turn, the loss of sharpness of M-lines caused also the H-zones and the A-band to be less defined, with the bare zones of thick filaments shifted from the middle of the sarcomere. Occasionally, some fibers presented even more severe disruptions of the myofibrillar structure (Fig. 6, I and L) in form of Z-line streaming (Fig. 6J), areas in which Z-line material appeared ripped out (Fig. 6, I and K) while M-lines and A-bands were missing (Fig. 6L).
Stimulation of eccentric contractions reveals increased sarcolemmal fragility of obscurin-deficient skeletal muscle fibers.
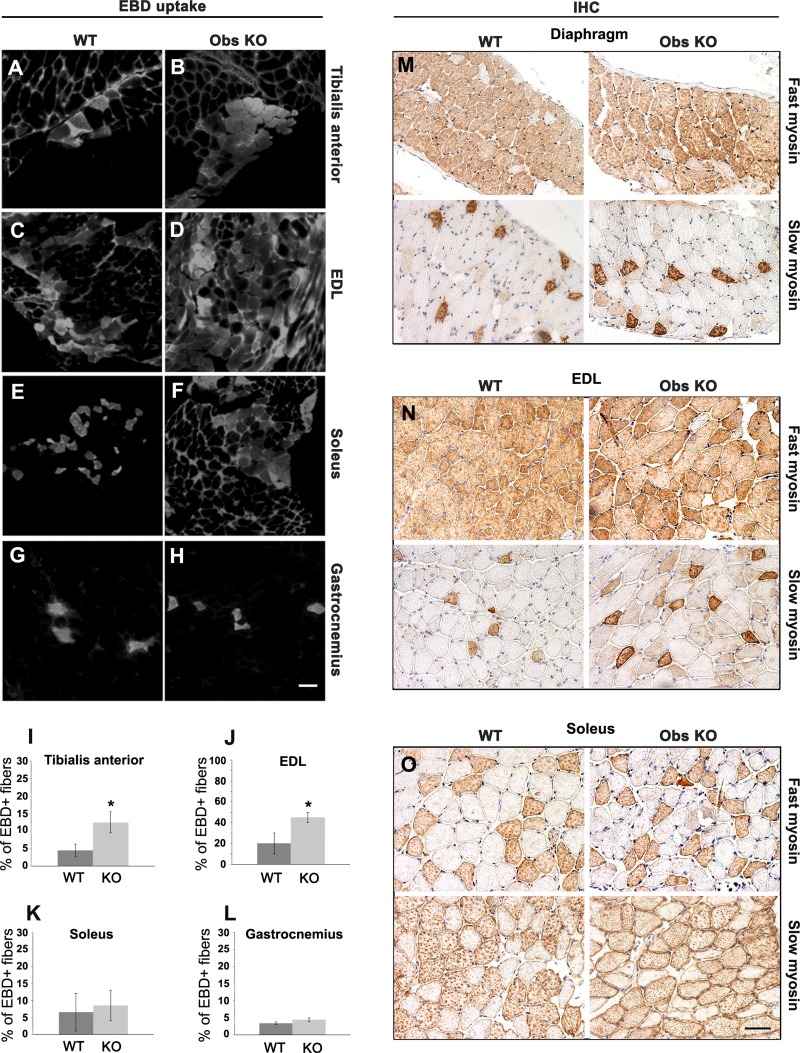
In obscurin KO mice, the reduced localization of dystrophin at costameres is associated with increased sarcolemmal fragility, as previously determined by EBD uptake analysis after completing an intense uphill running protocol (31). To better characterize the sarcolemmal sensitivity to stretch of obscurin-deficient fibers, we performed experiments of in vivo electrical stimulation to induce eccentric and concentric contractions in hindlimb muscles (35). The sciatic nerve was stimulated 60 times for a total duration of 30 min, leading to activation of tibialis anterior and EDL muscles (eccentric stimulation), and of gastrocnemius and soleus muscles (concentric stimulation). Immediately after the end of stimulation, EBD was injected intraperitoneally. The next day, hindlimb muscles were removed, cross sectioned, and analyzed by immunofluorescence. The percentage of EBD-positive fibers was assessed, revealing that the number of damaged fibers detected was increased by 2.8-fold in the tibialis anterior (control mice: 4.5 ± 1.8%; obscurin KO mice: 12.5 ± 3%, P < 0.05) and by 2.25-fold in the EDL (control mice: 20 ± 10%; obscurin KO mice: 45 ± 5%, P < 0.05) of obscurin KO compared with control mice (n = 4) (Fig. 7, A–D, I, and J), whereas no significant differences were observed in soleus and gastrocnemius (Fig. 7, E–H, K, and L), indicating that, under these experimental conditions, eccentric contractions emphasize an increased susceptibility to sarcolemmal damage of obscurin KO skeletal muscle fibers. Because in dystrophin-deficient mice the increased susceptibility to exercise-induced damage is less pronounced in muscles with an increased percentage of slow type 1 fibers (18), we next tested whether a change in the fiber phenotype could occur in obscurin KO mice. We examined the fiber type distribution in various muscles and, as shown in Fig. 7, M–O, no change in fiber type was found in diaphragm and soleus, whereas a shift toward a slower fiber phenotype was observed in the EDL of the obscurin KO animals (see also Table 1).
Fig. 7.
Evans blue dye (EBD) uptake after in vivo electrical stimulation of hindlimb skeletal muscles and fiber type analysis of diaphragm, EDL, and soleus muscles of WT and obscurin KO mice. The analysis shows that obscurin KO tibialis anterior (A, B, and I) and EDL (C, D, and J) subjected to eccentric contractions present a significantly higher number of EBD-positive fibers, compared with WT counterparts, than soleus (E, F, and K) and gastrocnemius (G, H, and L) muscles stimulated by concentric contractions. Scale bar, 20 μm. *P value <0.05; Student's t-test. Cross sections of diaphragm, EDL, and soleus muscles were stained by immunohistochemistry using antibodies against fast and slow myosin isotypes (M–O). A slight increase in the count of slow-type fibers was detected in the EDL (N) of obscurin KO mice (see Table 1 for percentages). Scale bar, 20 μm.
Table 1.
Characterization of fast- and slow-type fibers in diaphragm and hindlimb muscles of WT and obscurin KO mice
| Dia WT | Dia KO | EDL WT | EDL KO | Sol WT | Sol KO | |
|---|---|---|---|---|---|---|
| Fast | 84.5 | 83.5 | 95.5 | 87.5 | 39 | 37.5 |
| Slow | 15.5 | 16.5 | 4.5 | 12.5* | 61 | 62.5 |
Values are calculated from n = 4 independent experiments. Units are %. No significant differences in the count of fast vs. slow fiber type were observed in diaphragm (Dia) and soleus (Sol) muscles between wild-type (WT) and obscurin knockout (KO) mice. A shift toward the slow phenotype was detected in the extensor digitorum longus (EDL) of obscurin KO mice (
P < 0.05).
DISCUSSION
Exercise intensity and age of trained animals both coincide with the impaired running endurance of obscurin KO mice.
Here, we report on the age-dependent decreased ability of obscurin KO mice to perform in different running protocols and on the evidence of pathological features in obscurin KO skeletal muscles following a heavy exercise. Obscurin KO mice are able to accomplish a mild running protocol without remarkable difference compared with controls. Conversely, an exercise based on a more intense protocol revealed a marked reduction in their running capacity, suggesting that the effect of obscurin deletion on exercise performance becomes evident when a “threshold” of mechanical stress is exceeded. Moreover, our analysis shows that the reduced performance of the obscurin KO mice can be already observed in mice at 2 mo of age and is even more pronounced in 6- and 11-mo-old mice.
Obscurin has been reported to localize at the NMJs (7). Nevertheless, the observed reduction in the running ability of obscurin KO mice cannot be causally linked to reduced and/or altered synaptic transmission. In fact, even though the NMJs in obscurin KO mice presented reduced surface distribution and less organized architecture, the efficacy of synaptic transmission is comparable between control and KO mice. It is conceivable that the altered organization of NMJs may depend on the mislocalization of ankB, characteristic of obscurin KO skeletal muscles, as previously reported (2, 31).
Heavy exercise negatively impacts morphology and ultrastructure of diaphragm muscle fibers in obscurin KO mice.
The histological analysis shows that skeletal muscle fibers of untrained obscurin KO mice have no evident pathological features, analogous to a previous report (25). At variance, leukocyte infiltration and increased deposition of connective tissue were observed in KO mice after heavy exercise and were both confirmed by immunoreactivity to antibodies directed against CD45 and type III collagen, respectively. Clearly, the most pronounced alterations were detected in the diaphragm. On the same line, toluidine blue staining revealed hypercontractures in several diaphragm fibers of exercised obscurin KO mice. These data were further supported by results from EM analysis that showed misalignment of thick filaments resulting from disruption of the M-line, causing loss of sharpness of both H- and A-bands. These results indicate that, when the workload applied to the contractile apparatus is higher, as imposed to diaphragm fibers during heavy exercise, the effect of obscurin deletion becomes more evident. Notably, a similar effect has been recently described in Drosophila where knockdown of obscurin expression resulted on the complete loss of the M-line within sarcomeres (21). Why in obscurin KO mice the effect of obscurin deletion on sarcomere structure is evident only in the diaphragm and only in a definite percentage of fibers compared with the higher frequency of M-line alterations observed in flies is not clear. A possible explanation may be represented by a compensatory function contributed by the product of the obscurin-like 1 (Obsl-1) gene, which is present in the mice genome but not in that of Drosophila (13, 15). As it concerns the preferential localization of muscle damage to the diaphragm muscle in the obscurin KO mice, this might reflect the continued and rhythmic activity of this muscle in the context of the respiratory function and the occurrence of eccentric contractions, which are known to be more prone to cause muscle damage, mainly in the so-called postinspiration inspiratory activity phase (9).
Phenotype of obscurin KO mice: analogies and incongruities with other murine models of muscle pathology.
The present study highlights that, in obscurin KO mice, heavy exercise results in pathological alterations of skeletal muscle tissue. Intriguingly, these alterations are common to several murine models of muscle pathology like, for example, the mdx mice, a model of human Duchenne muscular dystrophy (6). Indeed, they both show that skeletal muscles can be differentially affected by pathological alterations and that muscle damage is predominantly observed in the diaphragm (34). Nevertheless, although on one hand the obscurin KO and the mdx mice show similar peculiar signs of muscle histopathology such as leukocyte infiltration and fibrosis, on the other hand a significant difference resides in the “inducible” nature of these pathological features since in obscurin KO mice they arise only after strenuous activation of muscle contraction, whereas in mdx mice chronic muscle damage develops even under resting conditions. Also, at variance with mdx, in obscurin KO muscles no marked change in the fast vs. slow fiber type ratio could be observed with the exception of a slight increase in the amount of slow fibers in the EDL. Last, the absence of evident degenerating and regenerating fibers, centrally nucleated fibers, or variation in fiber size in obscurin KO mice (data not shown) appears in agreement with the muscle damage being induced by an “external” stimulus, i.e., the execution of strenuous running sessions, in contrast to the intrinsic spontaneously occurring degenerative phenomena observed in mdx mice (36). Accordingly, obscurin KO and mdx mice markedly diverge on the ability of skeletal muscles to recover from damage, a feature that is indeed maintained in obscurin-deficient mice while it is severely impaired in mdx animals, where it plays a predominant role on both the onset and progression of the dystrophic pathology.
It is also worth comparing the results from the characterization of obscurin KO mice with those obtained from the sAnk1.5 KO model (16, 25) given the complementary roles played by obscurin and sAnk1.5. In both mouse models the diaphragm is the most severely affected muscle although, as for the mdx mice, the occurrence of pathological alterations in mice lacking sAnk1.5 is spontaneous (16). Intriguingly, the decrease in the running ability of obscurin KO mice is more severe than in sAnk1.5 KO mice. The interpretation of these dissimilarities is not obvious, but it is conceivable that they may reflect the diverse network of interactions participated by obscurin in muscle cells (22). In fact, while sAnk1.5 is known for stabilizing (in association with obscurin) the SR around the myofibrils, obscurin is involved in a wider molecular network through the specific interaction with ankB, connecting obscurin itself with the subsarcolemmal microtubules and dystrophin (31) that, in turn, might justify the observed sarcolemmal fragility.
Obscurin in needed for myofibrils to maintain their integrity in the presence of high-level mechanical workloads.
The results presented in this work and the available knowledge on the molecular mechanisms in which obscurin is involved may help to build a model to explain how its deletion causes the phenotype observed in KO mice. Analysis of muscle contraction induced by stimulation of Ca2+ release in obscurin KO mice did not reveal differences with control mice (25), indicating that the decreased SR volume observed in these mice does not explain their reduced running ability. At the sarcolemmal level, it is plausible that the remaining amount of dystrophin at costameres of obscurin-deficient muscle fibers can still provide the sarcolemma the ability to cope with a low-intensity muscle contraction, but not with the mechanical stress generated by an intense contractile activity. Similarly, at the level of the sarcomere and more specifically of the M-line, obscurin may probably be compensated by the presence of the obsl- 1 protein within the limit of a not too strenuous regime of contractions (13, 29). Obscurin is itself part of a molecular scaffold, together with titin and myomesin, able to “shield” the mechanical stress generated by muscle contraction at the myofibrillar level (8, 13, 14, 22, 29). Hence, the lack of obscurin may negatively affect the “functional quality” of this intrasarcomeric cytoskeleton that, as a consequence, would no longer be capable of tolerating muscle contractions exceeding a definite level of intensity. Accordingly, when animals are required to perform an intense activity, the effect of obscurin deletion on sarcomere solidity becomes evident, resulting in the observed alterations of the M-line and the H-zone and mirrored by the histopathological changes in the obscurin-deficient skeletal muscle tissue.
Finally, it is worth mentioning that obscurin haploinsufficiency, as a result of mutations in the OBSCN gene, has been causally linked to development of the familial dilated cardiomyopathy in humans (26), thus reinforcing the assumption that a diminished amount of obscurin plays a significant role in the impaired ability of muscle cells to deal with a continuous intense mechanical workload.
In summary, the data reported in this work indicate that the obscurin KO mice have a reduced exercise tolerance that becomes evident when the KO animals are subjected to a heavy running protocol. The lack of obscurin, although unable to generate measurable alterations in a poorly stressed muscle fiber (rest), impacts the structural integrity of the muscle fibers when associated with an intense and prolonged workload (exercise). On this basis, it is conceivable that the remarkable differences between normal and obscurin-deficient mice in terms of exercise performance and the effect of exercise on obscurin-deficient muscles support the role played by obscurin in maintaining the functional and structural integrity of two distinct compartments of skeletal muscle cells: the sarcomere and the sarcolemma.
GRANTS
This study was supported by the following grants: 1) Italian Telethon ONLUS Foundation (Rome, Italy): GGP13213 to F. Protasi, C. Reggiani, and V. Sorrentino; 2) National Institutes of Health Grants HL-107744 and P30-AR-061303 to S. Lange; 3) AFM Telethon no. 18822 to V. Sorrentino; 4) WF GR-2011-02350912 to C. Paolini; 5) MIUR-FIR 2013 RBFR13A20K to E. Pierantozzi; and 6) PAR FAS 2007-2013 grant ToRSADE) Regione Toscana to V. Sorrentino.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
D.R., B.B., C.R., and V.S. conception and design of research; D.R., B.B., C.P., E.P., and S.S. performed experiments; D.R., B.B., C.P., E.P., C.R., and V.S. analyzed data; D.R., B.B., C.P., F.P., C.R., and V.S. interpreted results of experiments; D.R. prepared figures; D.R. and V.S. drafted manuscript; D.R., B.B., C.P., E.P., S.L., J.C., F.P., C.R., and V.S. edited and revised manuscript; D.R., B.B., C.P., S.L., J.C., F.P., C.R., and V.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Prof. Elisabetta Weber (University of Siena) for sharing reagents for histological analysis and Dr. Nila Volpi (University of Siena) for the insightful discussions on the histopathology of skeletal muscle diseases.
REFERENCES
- 1.Armani A, Galli S, Giacomello E, Bagnato P, Barone V, Rossi D, Sorrentino V. Molecular interactions with obscurin are involved in the localization of muscle-specific small ankyrin1 isoforms to subcompartments of the sarcoplasmic reticulum. Exp Cell Res 312: 3546–3558, 2006. [DOI] [PubMed] [Google Scholar]
- 2.Ayalon G, Davis JQ, Scotland PB, Bennett V. An ankyrin-based mechanism for functional organization of dystrophin and dystroglycan. Cell 135: 1189–1200, 2008. [DOI] [PubMed] [Google Scholar]
- 3.Bagnato P, Barone V, Giacomello E, Rossi D, Sorrentino V. Binding of an ankyrin-1 isoform to obscurin suggests a molecular link between the sarcoplasmic reticulum and myofibrils in striated muscles. J Cell Biol 160: 245–253, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barresi R. From proteins to genes: immunoanalysis in the diagnosis of muscular dystrophies. Skelet Muscle 1: 24, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bowman AL, Kontrogianni-Konstantopoulos A, Hirsch SS, Geisler SB, Gonzalez-Serratos H, Russell MW, Bloch RJ. Different obscurin isoforms localize to distinct sites at sarcomeres. FEBS Lett 581: 1549–1554, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bulfield G, Siller WG, Wight PA, Moore KJ. X chromosome-linked muscular dystrophy (mdx) in the mouse. Proc Natl Acad Sci USA 81: 1189–1192, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carlsson L, Yu JG, Thornell LE. New aspects of obscurin in human striated muscles. Histochem Cell Biol 130: 91–103, 2008. [DOI] [PubMed] [Google Scholar]
- 8.Clark KA, McElhinny AS, Beckerle MC, Gregorio CC. Striated muscle cytoarchitecture: an intricate web of form and function. Annu Rev Cell Dev Biol 18: 637–706, 2002. [DOI] [PubMed] [Google Scholar]
- 9.Dutschmann M, Jones SE, Subramanian HH, Stanic D, Bautista TG. The physiological significance of postinspiration in respiratory control. Prog Brain Res 212: 113–130, 2014. [DOI] [PubMed] [Google Scholar]
- 10.Engel AG, Franzini-Armstrong C. Myology, Basic and Clinical (3rd ed) New York, NY: McGraw-Hill, 2004. [Google Scholar]
- 11.Ervasti JM. Costameres: the Achilles' heel of Herculean muscle. J Biol Chem 278: 13591–13594, 2003. [DOI] [PubMed] [Google Scholar]
- 12.Fukuzawa A, Idowu S, Gautel M. Complete human gene structure of obscurin: implications for isoform generation by differential splicing. J Muscle Res Cell Motil 26: 427–434, 2005. [DOI] [PubMed] [Google Scholar]
- 13.Fukuzawa A, Lange S, Holt M, Vihola A, Carmignac V, Ferreiro A, Udd B, Gautel M. Interactions with titin and myomesin target obscurin and obscurin-like 1 to the M-band: implications for hereditary myopathies. J Cell Sci 121: 1841–1851, 2008. [DOI] [PubMed] [Google Scholar]
- 14.Gautel M. The sarcomeric cytoskeleton: who picks up the strain? Curr Opin Cell Biol 23: 39–46, 2011. [DOI] [PubMed] [Google Scholar]
- 15.Geisler SB, Robinson D, Hauringa M, Raeker MO, Borisov AB, Westfall MV, Russell MW. Obscurin-like 1, OBSL1, is a novel cytoskeletal protein related to obscurin. Genomics 89: 521–531, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giacomello E, Quarta M, Paolini C, Squecco R, Fusco P, Toniolo L, Blaauw B, Formoso L, Rossi D, Birkenmeier C, Peters L, Francini F, Protasi F, Reggiani C, Sorrentino V. Deletion of small ankyrin 1 (sAnk1) isoforms results in structural and functional alterations in aging skeletal muscles fibers. Am J Physiol Cell Physiol 308: C123–C138, 2015. [DOI] [PubMed] [Google Scholar]
- 17.Giacomello E, Sorrentino V. Localization of ank1.5 in the sarcoplasmic reticulum precedes that of SERCA and RyR: relationship with the organization of obscurin in developing sarcomeres. Histochem Cell Biol 131: 371–382, 2009. [DOI] [PubMed] [Google Scholar]
- 18.Head SI, Williams DA, Stephenson DG. Abnormalities in structure and function of limb skeletal muscle fibres of dystrophic mdx mice. Proc Biol Sci 248: 163–169, 1992. [DOI] [PubMed] [Google Scholar]
- 19.Hopitzan AA, Baines AJ, Ludosky MA, Recouvreur M, Kordeli E. Ankyrin-G in skeletal muscle: tissue-specific alternative splicing contributes to the complexity of the sarcolemmal cytoskeleton. Exp Cell Res 309: 86–98, 2005. [DOI] [PubMed] [Google Scholar]
- 20.Huang P, Zhao XS, Fields M, Ransohoff RM, Zhou L. Imatinib attenuates skeletal muscle dystrophy in mdx mice. FASEB J 23: 2539–2548, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katzemich A, Kreisköther N, Alexandrovich A, Elliott C, Schöck F, Leonard K, Sparrow J, Bullard B. The function of the M-line protein obscurin in controlling the symmetry of the sarcomere in the flight muscle of Drosophila. J Cell Sci 125: 3367–3379, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kontrogianni-Konstantopoulos A, Ackermann MA, Bowman AL, Yap SV, Bloch RJ. Muscle giants: molecular scaffolds in sarcomerogenesis. Physiol Rev 89: 1217–1267, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kontrogianni-Konstantopoulos A, Catino DH, Strong JC, Sutter S, Borisov AB, Pumplin DW, Russell MW, Bloch RJ. Obscurin modulates the assembly and organization of sarcomeres and the sarcoplasmic reticulum. FASEB J 20: 2102–2111, 2006. [DOI] [PubMed] [Google Scholar]
- 24.Kontrogianni-Konstantopoulos A, Jones EM, Van Rossum DB, Bloch RJ. Obscurin is a ligand for small ankyrin 1 in skeletal muscle. Mol Biol Cell 14: 1138–1148, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lange S, Ouyang K, Meyer G, Cui L, Cheng H, Lieber RL, Chen J. Obscurin determines the architecture of the longitudinal sarcoplasmic reticulum. J Cell Sci 122: 2640–2650, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marston S, Montgiraud C, Munster AB, Copeland O, Choi O, Dos Remedios C, Messer AE, Ehler E, Knöll R. OBSCN mutations associated with dilated cardiomyopathy and haploinsufficiency. PLoS One 10: e0138568, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paolini C, Quarta M, D'Onofrio L, Reggiani C, Protasi F. Differential effect of calsequestrin ablation on structure and function of fast and slow skeletal muscle fibers. J Biomed Biotechnol 634075, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pardo JV, Siliciano JD, Craig SW. A vinculin-containing cortical lattice in skeletal muscle: transverse lattice elements (“costameres”) mark sites of attachment between myofibrils and sarcolemma. Proc Natl Acad Sci USA 80: 1008–1012, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pernigo S, Fukuzawa A, Bertz M, Holt M, Rief M, Steiner RA, Gautel M. Structural insight into M-band assembly and mechanics from the titin-obscurin-like-1 complex. Proc Natl Acad Sci USA 107: 2908–2913, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Petrof BJ, Shrager JB, Stedman HH, Kelly AM, Sweeney HL. Dystrophin protects the sarcolemma from stresses developed during muscle contraction. Proc Natl Acad Sci USA 90: 3710–3714, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Randazzo D, Giacomello E, Lorenzini S, Rossi D, Pierantozzi E, Blaauw B, Reggiani C, Lange S, Peter AK, Chen J, Sorrentino V. Obscurin is required for ankyrinB-dependent dystrophin localization and sarcolemma integrity. J Cell Biol 200: 523–536, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rossi D, Bencini C, Maritati M, Benini F, Lorenzini S, Pierantozzi E, Scarcella AM, Paolini C, Protasi F, Sorrentino V. Distinct regions of triadin are required for targeting and retention at the junctional domain of the sarcoplasmic reticulum. Biochem J 458: 407–417, 2016. [DOI] [PubMed] [Google Scholar]
- 33.Russell MW, Raeker MO, Korytkowski KA, Sonneman KJ. Identification, tissue expression and chromosomal localization of human Obscurin-MLCK, a member of the titin and Dbl families of myosin light chain kinases. Gene 282: 237–246, 2002. [DOI] [PubMed] [Google Scholar]
- 34.Stedman HH, Sweeney HL, Shrager JB, Maguire HC, Panettieri RA, Petrof B, Narusawa M, Leferovich JM, Sladky JT, Kelly AM. The mdx mouse diaphragm reproduces the degenerative changes of Duchenne muscular dystrophy. Nature 352: 536–539, 1991. [DOI] [PubMed] [Google Scholar]
- 35.Vilquin JT, Brussee V, Asselin I, Kinoshita I, Gingras M, Tremblay JP. Evidence of mdx mouse skeletal muscle fragility in vivo by eccentric running exercise. Muscle Nerve 21: 567–576, 1998. [DOI] [PubMed] [Google Scholar]
- 36.Whitmore C, Morgan J. What do mouse models of muscular dystrophy tell us about the DAPC and its components? Int J Exp Pathol 95: 365–377, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Young P, Ehler E, Gautel M. Obscurin, a giant sarcomeric Rho guanine nucleotide exchange factor protein involved in sarcomere assembly. J Cell Biol 154: 123–136, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou D, Birkenmeier CS, Williams MW, Sharp JJ, Barker JE, Bloch RJ. Small, membrane-bound, alternatively spliced forms of ankyrin 1 associated with the sarcoplasmic reticulum of mammalian skeletal muscle. J Cell Biol 136: 621–631, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]