Previous investigations have indicated that spasmolytic polypeptide-expressing metaplasia (SPEM) in the stomach arises from transdifferentiation of chief cells. Nevertheless, the intrinsic properties of chief cells that influence transdifferentiation have been largely unknown. We now report that the ability to transdifferentiate into SPEM is impaired in chief cells that lack full functional maturation, and as chief cells age, they lose their ability to transdifferentiate. Thus chief cell plasticity is dependent on both cell age and maturation.
Keywords: SPEM, spasmolytic polypeptide-expressing metaplasia, lineage mapping, Mist1, transdifferentiation, progenitor cell
Abstract
The plasticity of gastric chief cells is exemplified by their ability to transdifferentiate into spasmolytic polypeptide-expressing metaplasia (SPEM) after parietal cell loss. We sought to determine if chief cell maturity is a limiting factor in the capacity to transdifferentiate. Mist1−/− mice, previously shown to form only immature chief cells, were treated with DMP-777 or L635 to study the capability of these immature chief cells to transdifferentiate into a proliferative metaplastic lineage after acute parietal cell loss. Mist1−/− mice treated with DMP-777 showed fewer chief cell to SPEM transitions. Mist1−/− mice treated with L635 demonstrated significantly fewer proliferative SPEM cells compared with control mice. Thus immature chief cells were unable to transdifferentiate efficiently into SPEM after acute parietal cell loss. To determine whether chief cell age affects transdifferentiation into SPEM, we used tamoxifen to induce YFP expression in chief cells of Mist1CreER/+;RosaYFP mice and subsequently treated the cells with L635 to induce SPEM at 1 to 3.5 mo after tamoxifen treatment. After L635 treatment to induce acute parietal cell loss, 43% of all YFP-positive cells at 1 mo posttamoxifen were SPEM cells, of which 44% of these YFP-positive SPEM cells were proliferative. By 2 mo after tamoxifen induction, only 24% of marked SPEM cells were proliferating. However, by 3.5 mo after tamoxifen induction, only 12% of marked chief cells transdifferentiated into SPEM and none were proliferative. Thus, as chief cells age, they lose their ability to transdifferentiate into SPEM and proliferate. Therefore, both functional maturation and age limit chief cell plasticity.
NEW & NOTEWORTHY Previous investigations have indicated that spasmolytic polypeptide-expressing metaplasia (SPEM) in the stomach arises from transdifferentiation of chief cells. Nevertheless, the intrinsic properties of chief cells that influence transdifferentiation have been largely unknown. We now report that the ability to transdifferentiate into SPEM is impaired in chief cells that lack full functional maturation, and as chief cells age, they lose their ability to transdifferentiate. Thus chief cell plasticity is dependent on both cell age and maturation.
progenitor cells located within the isthmus region of gastric glands in the stomach corpus give rise to multiple cell lineages (10–12). Preparietal cells give rise to acid-secreting parietal cells that migrate downward toward the base of the gland (9). Mucous neck cells arise from premucous neck cells and also migrate toward the gland base. As they exit the neck region of the gland, mucous neck cells reprogram their secretory machinery and transition into zymogen-secreting chief cells (12). Chief cells continue to mature until they reach the base of the gland with a reported lifetime of up to 100–200 days (12, 22). During the “neck to chief cell” transition, the cells begin to expand their rough endoplasmic reticulum and extend their apical membranes to establish a more pyramidal shape (2, 12, 23). This transition also entails the replacement of mucous filled vesicles with zymogen-containing vesicles (12, 23). Upon this transition, chief cells express the chief cell-specific transcription factor Mist1 to obtain functional maturity as they continue migration to the gland base (23). Without Mist1 expression, chief cells retain fewer and smaller zymogen vesicles as well as have defects in apical expansion (23). Thus Mist1−/− chief cells are considered “functionally immature” as broadly define as having undifferentiated or defective mechanisms resulting in an altered cell physiology.
Disruptions in the homeostasis of these lineages such as oxyntic atrophy (parietal cell loss) are associated with the initiation of the metaplastic process in the stomach. In humans, two types of metaplasias arise: spasmolytic polypeptide-expressing metaplasia (SPEM) and intestinal metaplasia (4, 24). Recent studies suggest that SPEM is the preneoplastic lesion that gives rise to intestinal metaplasia and dysplasia (7, 28). Other studies have also indicated that SPEM is a critical participant in the response to acute ulceration (1, 6). Thus metaplastic lineages may provide reparative lineages in the setting of an acute local wound healing response, while functioning as a more deleterious preneoplastic lesion with chronic injury and inflammation. Through lineage tracing studies, we have shown that SPEM develops from transdifferentiation of chief cells following parietal cell loss (15). Our laboratory has developed two acute drug-induced SPEM models in mice: DMP-777 and L635. Administration of the protonophore DMP-777 causes oxyntic atrophy with a blunted inflammatory response that induces SPEM after 14 days (17). L635, a DMP-777 analog, induces oxyntic atrophy accompanied by prominent inflammation. After only 3 days, L635 induces a proliferative and “intestinalized” SPEM (15, 27). It is the presence of inflammation, specifically M2 macrophages, that promotes the advancement of SPEM to a more intestinalized and proliferative metaplastic phenotype (20). While numerous studies focus on the extrinsic influences involved in the progression of metaplasia, far less is understood about the intrinsic properties of chief cell plasticity involved in transdifferentiation into SPEM.
We have now evaluated two critical questions related to the plasticity of chief cells to transdifferentiate into SPEM following acute parietal cell loss. First, we have asked whether completion of functional chief cell maturation is required for efficient transdifferentiation. Second, since chief cells are a long-lived lineage, we have asked whether the ability of chief cells to transdifferentiate changes as they age. Our results suggest that both functional maturation and age affect the ability of chief cells to undergo transdifferentiation into SPEM.
METHODS
Mice.
Germline Mist1−/− mice and Mist1CreER/+ mice have been described previously (15, 23). RosaYFP mice (containing a LoxStopLox-YFP allele) were obtained from Jackson Laboratory (Stock:006148). Mist1CreER/+ and RosaYFP mice were mated and maintained on a C57BL/6J background. Mist1CreER/+;RosaYFP mice were tamoxifen treated between 6 and 8 wk of age. Intraperitoneal injection of tamoxifen (1 mg/0.1 ml corn oil) was administered three times every other day, as previously described (15). All mice were maintained in a temperature-controlled room with 12-h light-dark cycle and given regular mouse chow and water ad libitum. The care, maintenance, and treatment of animals in these studies adhere to the protocols approved by the Institutional Animal Care and Use Committee of Vanderbilt University.
Drug treatment.
Experimental groups contained three male or female mice. Preparation and treatment of DMP-777 have been described previously (17). L635 (synthesized by the Chemical Synthesis Core of the Vanderbilt Institute of Chemical Biology) preparation and treatment has also been previously described (20). For experiments in Mist1−/− mice, DMP-777 and L635 were given by oral gavage to wild-type and Mist1−/−, in parallel, for either 14 or 3 consecutive days, respectively. For Mist1CreER/+;RosaYFP mouse experiments, mice were L635 treated by oral gavage for 3 days either 1, 2, or 3.5 mo after tamoxifen treatment.
Immunohistochemistry.
Stomach tissue preparation, fixation, and sectioning were performed as previously described (20). Periodic acid-Schiff (PAS) staining was performed by the Vanderbilt Translational Pathology Shared Resource. For immunofluorescence, tissue sections were deparaffinized and rehydrated, and Target Retrieval Solution (DakoCyomation, Glostrup, Denmark) was used for antigen retrieval in a pressure cooker for 15 min at high pressure. Tissue sections were blocked overnight using Protein Block Serum-Free (DakoCytomation). Primary antibodies were incubated overnight at 4°C in Antibody Diluent with Background Reducing Components (DakoCytomation). The following primary antibodies were used: rat IgG anti-Ki67 (1:50; Dako), rabbit IgG anti-Ki67 (1:200; Cell Signaling, Danvers, MA), goat anti-gastric intrinsic factor (anti-GIF; 1:2,000; a gift from Dr. David Alpers, Washington University, St. Louis, MO), and rabbit anti-GFP (1:500; Novus Biologicals, Littleton, CO). Fluorescent secondary antibodies and Alexa 488 or 647-conjugated Griffonia simplicifolia lectin II (GSII lectin; 1:1,000; Molecular Probes, Eugene, OR) diluted in Antibody Diluent with Background Reducing Components (DakoCytomation) were incubated at room temperature for 1 h. All tissue sections were analyzed using an Ariol SL-50 automated slide scanner (Leica Biosystems, Buffalo Grove, IL) or a Zeiss Axiophot microscope with an Axiovision digital imaging system (Zeiss, Jena, Germany) in the Vanderbilt Digital Histology Shared Resource.
Quantitation and localization of cell lineages in Mist1−/− and wild-type mice.
From Ariol SL-50-scanned GSII lectin, GIF, and Ki67 triple-immunolabeled tissue sections, regions of well-oriented fundic glands were selected for quantification. For drug-treated mice, only regions with significant parietal cell loss were chosen. A total of ~200 glands (from 3 mice) was quantified for each of the six mouse groups (wild type: untreated, DMP-777, or L635; Mist1−/−: untreated, DMP-777, or L635). With the use of Photoshop (Adobe Systems, San Jose, CA) and adding “layers” named for the markers (GSII, GIF, or Ki67), cells immunolabeled for each marker were manually identified by marking the nuclei of those cells on that specific Photoshop layer (i.e., GSII cells marked in GSII layer). Contour lines representing the top and base of the glands were also designated in individual layers using Photoshop. These five marked layers (GSII, GIF, Ki67, Top, and Base) were then extracted into individual images for subsequent analysis of the total cell numbers and quantitative spatial localization. A custom-built software was developed using MATLAB (Mathworks, Natick, MA) to identify the expression characteristics and localization of each designated cell within the gland (i.e., single-, double-, and triple-labeled expression of Ki67, GSII, and GIF). Double- and triple-labeled cells were identified as cells with overlap of individually-labeled nuclei. Quantitation of the total number of each expression profile is reported in results (see bar graphs in Fig. 4A). For each labeled cell, the height location within the gland was determined by calculating the closest-point distance of the labeled nuclei to a point within both the denoted top and base of the gland. These distances for each cell were used to determine both the overall gland height and the height localization of each cell within the gland. Cell heights within the gland were normalized to the average gland height for each treatment group. Histograms with bin widths equal to 10% of the total normalized gland height were generated for each marker expression profile (i.e., single-, double-, and triple-labeled expression) for each mouse. Cell counts in individual histogram bins for given expression profiles were normalized to the total number of glands manually counted in each mouse to reflect the average number of cells per gland that are localized within a particular 10% height region of the gland. Histograms for each mouse within treatment groups were averaged and are reported as a mean histogram line with standard deviation designated as a shaded region (see Fig. 4B). This software is available for download through the Vanderbilt Digital Histology Shared Resource. For statistical comparison, Kruskal-Wallis test followed by Mann-Whitney U-test for specific comparisons was used.
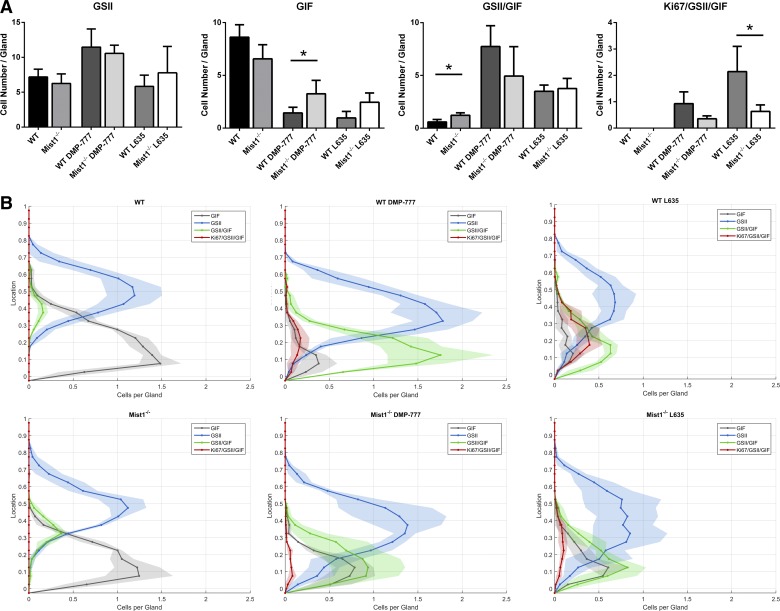
Fig. 4.
Quantitation and distribution of cell lineages in untreated and drug-treated wild-type mice and Mist1−/− mice. The total number (A) and localization (B) of specific immunolabeled cells were analyzed from immunolabeled glands (Ki67, GSII lectin, and GIF) from 3 mice in each group. A: the immunolabeling cell patterns was identified as follows: GSII lectin only (GSII), GIF only (GIF), GSII lectin, and GIF dual-labeled only (GSII+/GIF+), and triple-labeled cells (Ki67+/GSII+/GIF+). Upon DMP-777 treatment, more GIF only (chief cells) remained in Mist1−/− mice than in wild-type mice. No statistically significant difference in GSII+/GIF+ cells was found within each drug treatments. In contrast, while L635 treatment leads to an obvious increase in Ki67+/GSII+/GIF+ cells (proliferating SPEM cells) in wild-type mice; L635-treated Mist1−/− mice have significantly less proliferating SPEM cells (*P value = 0.05). B: the localization of each immunolabeling group from A is shown in distribution histograms with the y-axis representing the gastric gland divided into 10% increments (1 = lumen and 0 = base of gland) and x-axis depicting the number of cells per gland. This novel depiction of cell localization allows for visualization of the different cell labeling patterns observed in wild-type (top row) and Mist1−/− (bottom row) mice as well as cellular changes that occur upon drug treatment (untreated: left column; DMP-777: middle column; L635: right column). The histograms of Mist1−/− mice show the chief cells that resist transdifferentiation into SPEM are predominately localized to the bottom of the glands. GSII (blue), GIF (gray), GSII+/GIF+ (green), and Ki67+/GSII+/GIF+ (red).
Quantitation of YFP marked chief cells and SPEM.
Tissue sections immunolabeled for YFP, Ki67, and GSII lectin were scanned using the Ariol SL-50. For total quantifications, each mouse had 4.5 mm of fundic tissue assessed for lineage-traced cells (YFP positive) and for metaplasia markers (GSII lectin) and proliferation (Ki67). Tissue identification was blinded and the total number of YFP-only positive cells, YFP and GSII lectin copositive cells and YFP, GSII, and Ki67 triple-positive cells was manually counted. The percentage of YFP-positive SPEM cells was calculated by dividing the number of YFP and GSII copositive cells into the total number of YFP-positive cells. The percentage of YFP-positive proliferating SPEM cells was calculated by dividing the number of YFP-, GSII lectin-, and Ki67-positive cells by the total number of YFP and GSII copositive cells (considered marked SPEM cells). Statistical significance was determined using Kruskal-Wallis test followed by Mann-Whitney U-test for specific comparisons.
RESULTS
Functional immaturity of Mist1−/− chief cells impairs the progression to proliferative SPEM.
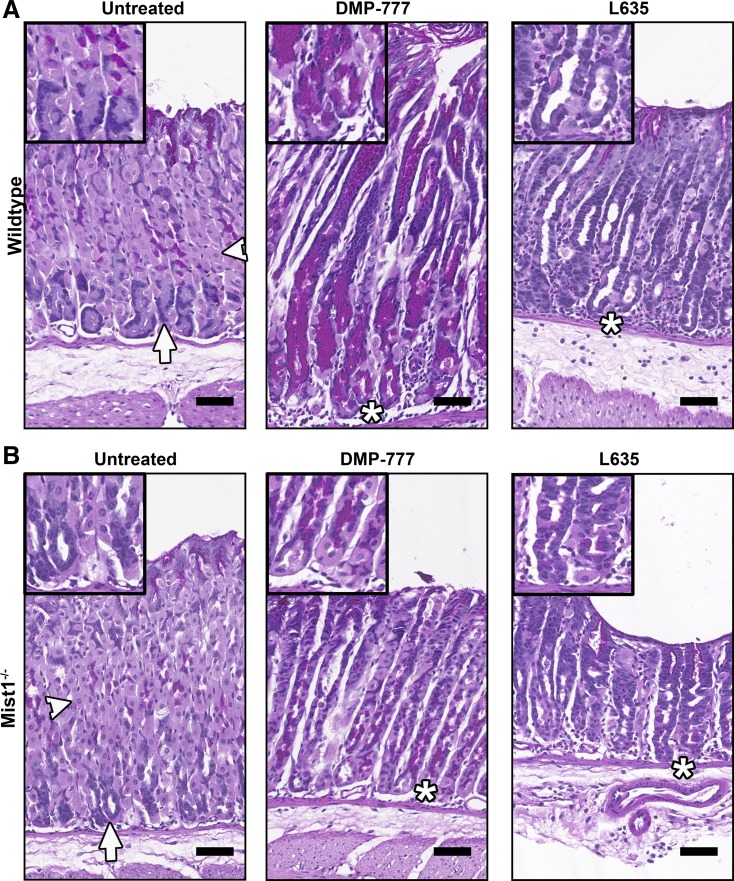
Previous investigations have noted that Mist1−/− mice demonstrate incomplete maturation, as exemplified by fewer and smaller zymogen granules (23). To study the emergence and progression of SPEM from functionally immature chief cells, wild-type mice and Mist1−/− mice were given DMP-777 or L635 by oral gavage for 14 or 3 days, respectively. The presence or absence of the various gastric cell lineages was analyzed by PAS staining. In PAS-stained sections of untreated control mice, parietal cells are light pink, mucous neck cells are dark magenta (located between parietal cells), and chief cells are blue (Fig. 1A). As previously reported (2, 23), the patterning of cells in untreated Mist1−/− mice differs slightly from untreated wild-type mice. Most notably, Mist1−/− gastric glands showed an increase of parietal cells localized near the base and aberrant chief cell structure (Fig. 1B). Wild-type mice treated with DMP-777 or L635 demonstrated significant parietal cell and chief cell loss and the emergence of magenta PAS-positive-stained cells at the base of the glands (Fig. 1A). Although similar in color to mucous neck cells, the localization of these magenta-stained cells at the base of the glands distinguishes them as SPEM cells and not the mucous neck cells located in the neck region (Fig. 1A). In Mist1−/− mice, both DMP-777 treatment and L635 treatment resulted in a reduction in parietal cell numbers as compared with untreated wild-type and Mist1−/− mice (Fig. 1B). However, some residual parietal cells remained in DMP-777-treated Mist1−/− mice as compared with treated wild-type mice. Unlike in wild-type mice, the amount of parietal cell loss varied throughout the fundus of Mist1−/− mice and appeared in patches, suggesting a more focal effect of DMP-777 in Mist1−/− mice (data not shown). This focal parietal cell loss was not observed in L635-treated Mist1−/− mice. Only regions with significant parietal cell loss were selected for further analysis of treated Mist1−/− mice.
Fig. 1.
Periodic acid-Schiff (PAS) staining of untreated, DMP-777-treated, and L635-treated wild-type and Mist1−/− mice. DMP-777 or L635 was administered to wild-type mice (A) and Mist1−/− mice (B) for 14 or 3 days, respectively. DMP-777 and L635 treatment caused significant parietal cell loss (light pink, arrowheads) in both wild-type mice (A) and Mist1−/− mice (B). SPEM cells were identified as magenta-PAS-positive cells at the base of the glands (*), replacing the chief cells (blue, arrows) in drug-treated wild-type mice (A) and Mist1−/− mice (B). Note that L635-induced spasmolytic polypeptide-expressing metaplasia (SPEM) cells do not stain as bright magenta as DMP-777-induced SPEM cells. Scale bar = 50 µm.
To quantitate the development of SPEM in DMP-777 and L635-treated Mist1−/− mice, gastric tissue sections were stained for GSII lectin, GIF, and Ki67 (Figs. 2 and 3). Mucous neck cells were identified as cells labeling solely with GSII lectin. Similarly, exclusive GIF expression (GSII lectin and Ki67 negative) defined chief cells. GSII lectin and GIF colabeling was predominantly observed in SPEM cells at the base of glands. However, a small number of “mucous neck to chief” transition cells in untreated glands also displayed this dual label profile, which increased slightly in Mist1−/− mice, as previously reported (23). Furthermore, previous studies have shown that the more “intestinalized” L635-induced SPEM cells prominently regain the ability to proliferate (15, 20, 27). Thus GSII lectin and GIF copositive cells were further delineated via Ki67 expression into two separate groups, nonproliferating and proliferating. In all analyses, GSII+/GIF+/ Ki67− signified nonproliferating SPEM cells as well as transition cells, while proliferative SPEM cells were GSII+/GIF+/Ki67+. The total number of each cell type was quantitated in untreated, DMP-777-treated, and L635-treated wild-type and Mist1−/− mice (Fig. 4A). By utilizing the ordered structure of the gastric gland, the localization of each cell type was compared between wild-type and Mist1−/− mice to demonstrate more comprehensively and visibly the effects of DMP-777 and L635 treatment (Fig. 4B).
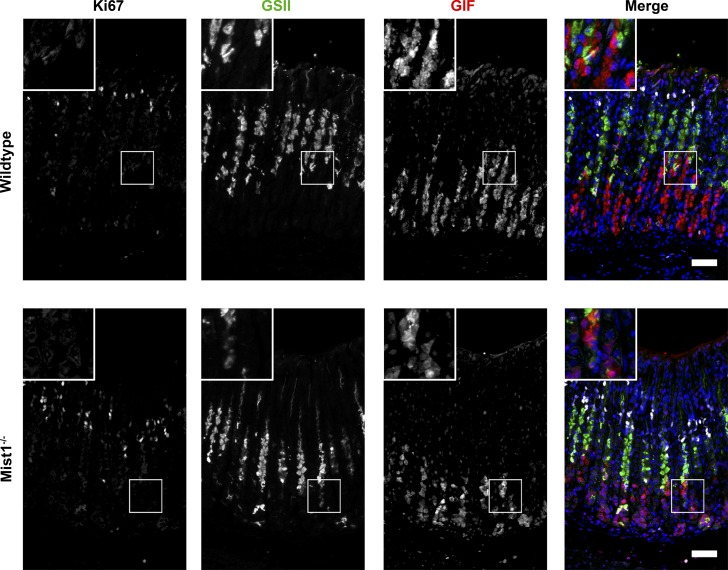
Fig. 2.
Proliferation in untreated wild-type and Mist1−/− mice. Sections were immunostained for Ki67 (white), GSII lectin (green), and gastric intrinsic factor (GIF; red). Untreated wild-type and Mist1−/− mice have low levels of proliferation localized only to the progenitor region. Additionally, untreated mice had distinctly separate regions of either GSII lectin or GIF labeling with only occasional “neck to chief” transition cells GSII lectin and GIF-dual labeled DAPI (blue). Scale bar = 50 µm.
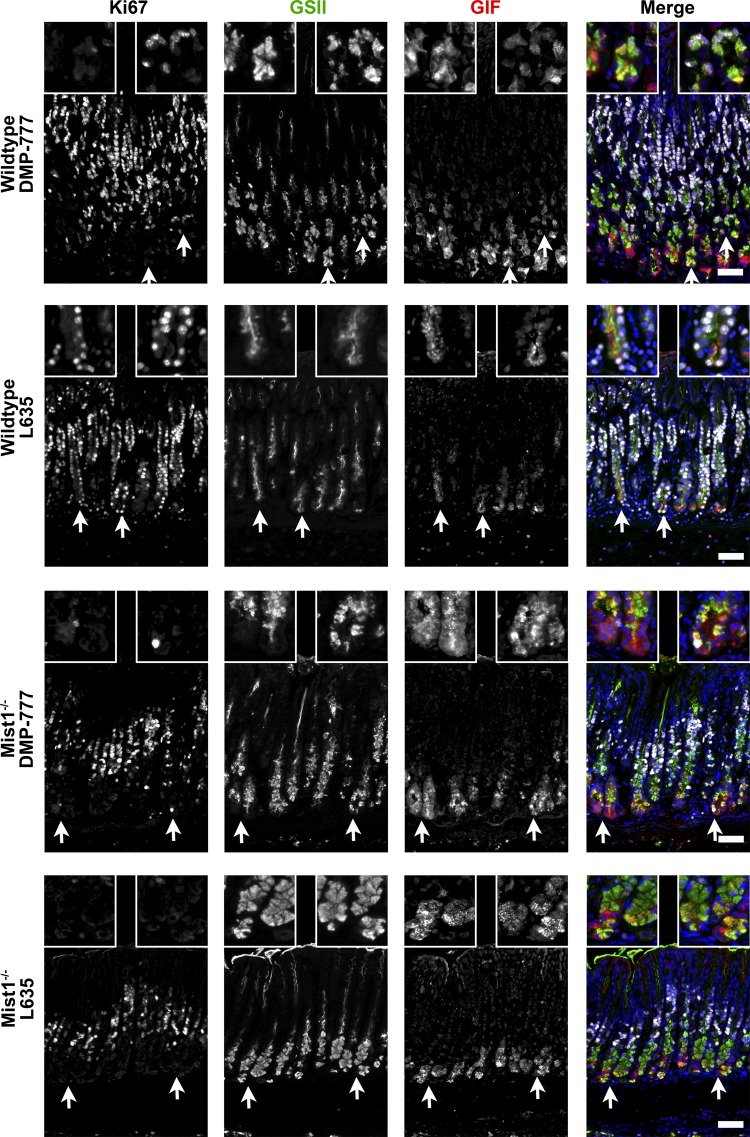
Fig. 3.
Proliferation of SPEM cells after DMP-777 or L635 administration of wild-type and Mist1−/− mice. Sections were immunostained for Ki67 (white), GSII lectin (green), and GIF (red). DMP-777-treated and L635-treated wild-type mice had an increase in GSII lectin and GIF dual-positive cells at the base of glands representing the induction of SPEM. Moreover, Ki67 immunostaining demonstrated an induction of proliferation of the SPEM cells (triple-positive cells) in drug-treated wild-type mice (most significantly in L635 treatment). DMP-777-treated and L635-treated Mist1−/− mice had a similar increase in GSII lectin and GIF dual-positive SPEM cells at the base of glands. However, only occasional Ki67-immunolabeled SPEM cells are observed in drug-treated Mist1−/− mice. Arrows mark higher magnification areas of the insets. DAPI (blue). Scale bar = 50 µm.
In untreated wild-type and Mist1−/− mouse gastric glands, as mucous neck cells (GSII lectin only) migrate toward the base of glands, they switch from expression of mucins to GIF expression during transition into chief cells (Figs. 2 and 3B). No significant difference between wild-type and Mist1−/− mice was found in the total number of mucous neck cells (7.2 vs. 6.3 cells per gland, respectively) or chief cells (8.6 vs. 6.4 cells per gland) (Fig. 4A). As previously reported (15, 20), DMP-777 and L635 administration to wild-type mice induced an increase of GSII+/GIF+-colabeled SPEM cells as well as proliferative SPEM cells (GSII+/GIF+/Ki67+) at the base of glands (Fig. 3 and 4B). DMP-777-treated and L635-treated Mist1−/− mice also showed an increase in GSII+/GIF+-colabeled cells localized at the base of the gland compared with untreated Mist1−/− mice (Figs. 3 and 4B). In DMP-777 or L635 treatment-matched wild-type vs. Mist1−/− comparisons, no significant difference was found in the total number of GSII only cells (DMP-777: 11.5 vs. 10.6 cells per gland; L635: 5.8 vs. 7.8 cells per gland) or GSII+/GIF+ cells (DMP-777: 7.7 vs. 4.9 cells per gland; L635: 3.5 vs. 3.8 cells per gland). However, DMP-777-treated Mist1−/− mice retained more chief cells (GIF+ only) than treated controls (1.4 vs. 3.3 cells per gland). While we did observe an increase in retained chief cells in L635-treated Mist1−/− mice (1.0 vs. 2.5 cells per gland), this difference did not reach statistical significance (Fig. 4A). As noted previously, DMP-777 produces less proliferative SPEM than L635 treatment (Fig. 4A) and while Mist1−/− mice showed less proliferation in SPEM after DMP-777 treatment, the differences was not statistically significant. However, there was a statistically significant 3.3-fold decrease in proliferative SPEM cells in L635-treated Mist1−/− mice compared with L635-treated wild-type mice (0.64 vs. 2.14 cells per gland, respectively) (Fig. 4A). Still, the localization of these proliferative SPEM cells to the base of the glands in L635-treated Mist1−/− mice was similar to L635-treated wild-type mice (Fig. 4B). Together, these findings suggest that the functional immaturity of Mist1−/− mouse chief cells impairs the progression of SPEM.
Chief cell transdifferentiation plasticity diminishes as chief cells age.
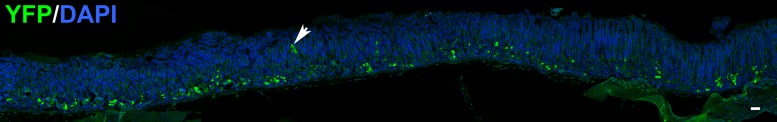
Chief cells are long-lived postmitotic zymogen-secreting cells that transdifferentiate from mucus neck cells during migration toward the base of corpus glands (2). Using the Mist1CreER/+;RosaYFP mouse to mark chief cells, we sought to determine if all chief cells, newly matured as well as aged, have a similar capacity for transdifferentiation into metaplasia. Chief cells were lineage labeled through tamoxifen administration to mice at 6 wk of age. To induce metaplasia, mice were treated with L635 at either 1, 2, or 3.5 mo after tamoxifen administration. Based on the clear differences found in Mist1−/− mice, we focused our lineage tracing study by using only L635 for metaplasia induction. In normal mice, the overwhelming majority of YFP-positive cells were observed in the gland base as expected, because chief cells are long lived and MIST1 protein is expressed exclusively in chief cells. Rare YFP-positive but GIF-negative cells could be seen at higher levels in the unit (Fig. 5).
Fig. 5.
Distribution of YFP-labeling in the gastric corpus of tamoxifen-treated Mist1-CreERT2;LSL-YFP mice. Mist1-CreERT2;LSL-YFP mice were treated with tamoxifen and then euthanized 2 wk following tamoxifen induction. A representative section of gastric corpus was immunostained for YFP (green) along with DAPI staining (blue) to demonstrate nuclei. Note that most of the labeling was observed in chief cells at the bases of glands. Only rare labeling of cells in the isthmus region was observed (arrowhead). Bar = 100 µm.
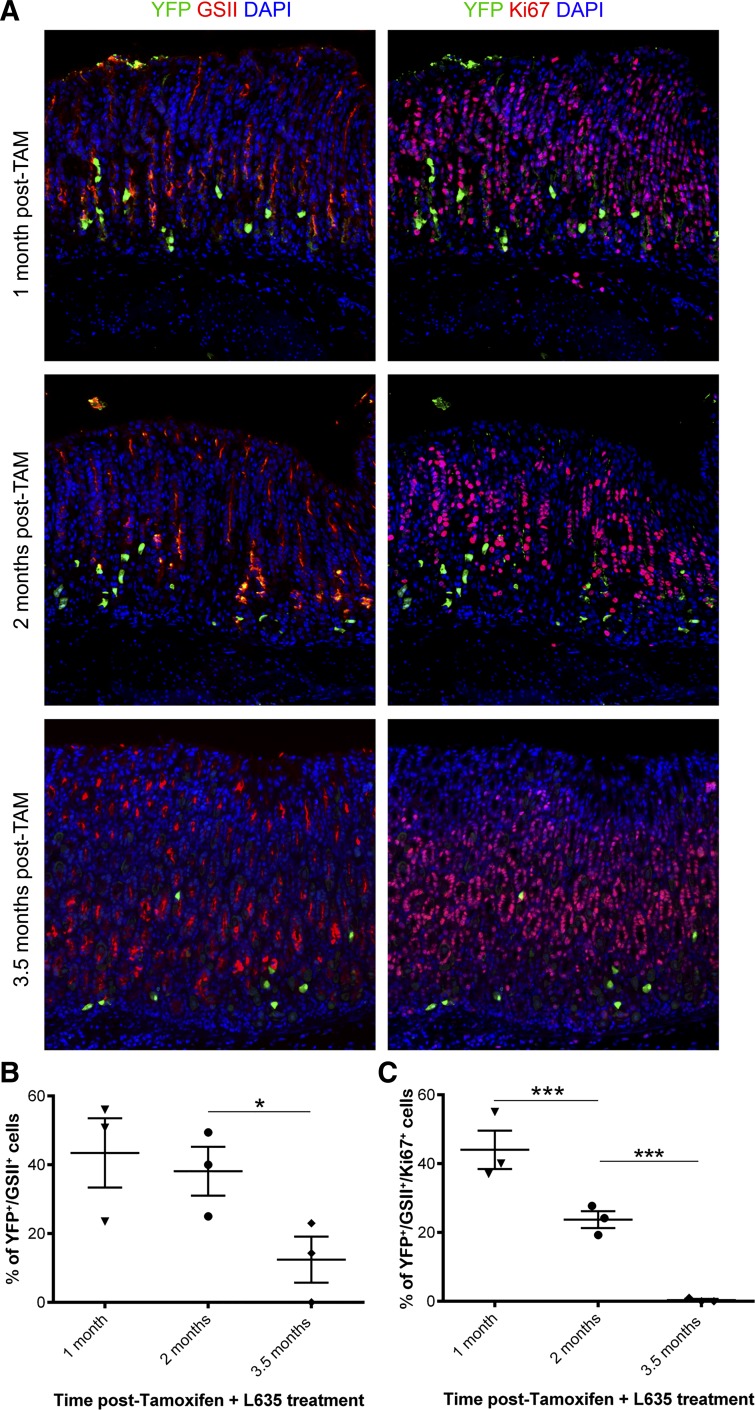
Immunostaining for YFP, GSII lectin, and Ki67 was used to determine the ability for lineage-marked YFP-positive cells to transdifferentiate into metaplasia and proliferate. We first examined the ability of aged chief cells to transdifferentiate into SPEM. Cells colabeled for YFP and GSII lectin were identified as lineage marked SPEM. Mice treated with L635 1 and 2 mo after tamoxifen induction showed ~40% of YFP-positive cells coexpressing GSII lectin, indicative of lineage marked SPEM (43% and 38%, respectively) (Fig. 6, A and B). However, at 3.5 mo posttamoxifen induction, we observed a significant decrease in the percent of YFP and GSII lectin-copositive cells (12%) (Fig. 6B).
Fig. 6.
Immunostaining and quantitation of lineage-traced SPEM and proliferation. A: sections of corpus from L635-treated Mist1CreER/+;RosaYFP mice at each time point (1, 2, and 3.5 mo posttamoxifen (TAM) induction) were stained for YFP (green), GSII (red on left), Ki67 (red on right), and DAPI (blue). Images on the left show YFP (green) and GSII lectin (red) staining with copositive cells at the base of the gland indicating SPEM formation in YFP-marked chief cells after L635-treatment. YFP (green) and Ki67 (red) staining in the same sections is shown on the right with copositive cells indicative of proliferative SPEM after L635-treatment. Scale bar = 100 µm. B and C: the total number of YFP-positive cells was assessed to the number of either YFP+/GSII lectin+ dual-positive or YFP+/GSII lectin+/Ki67+ triple-positive cells at each time point. B: the percent of dual-positive YFP and GSII lectin cells significantly decreased to 12% at 3.5 mo (*P < 0.05). C: proliferating marked SPEM cells decreased at each time point (44, 24, and 0.3%, respectively; ***P < 0.001).
We have previously shown L635-treated mice develop a highly proliferative metaplasia that is indicative of metaplasia with an intestinalized phenotype (27). To determine if YFP-positive SPEM cells reactivated their proliferative capacity upon L635-treatment, we analyzed tissue sections from all time points for triple-labeled cells (YFP+/GSII+/Ki67+) indicative of proliferative lineage traced SPEM cells (Fig. 6, A and C, GSII not shown in A). At the 1-mo time point, we observed ~44% of YFP-positive SPEM cells were proliferative in L635-treated mice. The number of proliferative YFP-positive SPEM cells significantly decreased to 24% after L635 treatment at 2 mo posttamoxifen induction. By 3.5 mo posttamoxifen, we did not observe any proliferative YFP-positive SPEM cells after L635 treatment. Taken together, these data show the ability of chief cells to transdifferentiate and progress to proliferative SPEM diminishes with age.
DISCUSSION
Previous studies have focused predominately on the elucidation of extrinsic influences involved in the metaplastic progression in the stomach such as immune cells and other signaling molecules (16, 17, 19, 26). These studies have extended our understanding of the complex networks necessary for chief cell transdifferentiation and progression to SPEM. However, the extent of the intrinsic chief cell plasticity remains unknown. In this study, we investigated the roles of two intrinsic chief cell characteristics, functional maturity and cellular age, in the metaplastic progression of SPEM.
Loss of Mist1 alters various mechanisms of acinar cell physiology including cellular organization and stress response (13, 21). In the stomach, Mist1−/− chief cells cannot properly maintain their secretory machinery (23). In this study, we have defined the term “functional immaturity” to encompass any undifferentiated or defective mechanisms resulting in an altered cellular functioning. Utilizing our two systems of parietal cell loss (DMP-777 and L635), we tested the ability for functionally immature chief cells in Mist1−/− mice to transdifferentiate into SPEM and progress to more proliferative metaplasia. Upon parietal cell loss, GSII+/GIF+ dual-positive cells were observed at the base of glands similar to SPEM cells found in treated wild-type mice. While both SPEM cells and “neck to chief” transition cells colabel with these two markers, SPEM cells are typically distinguished by their localization at the base of glands (18, 23). Thus while there may be a subset of accumulated transition cells that failed to complete differentiation into chief cells, the localization of the GSII+/GIF+ dual-positive cells in treated Mist1−/− mice identifies them as presumptive SPEM cells. However, DMP-777-treated Mist1−/− mice retained more chief cells than DMP-777-treated control mice. This retention suggests a limitation in the functionally immature Mist1−/− chief cells to transdifferentiate into SPEM. Additionally, L635-treated Mist1−/− mice showed significantly fewer proliferating SPEM cells than L635-treated wild-type mice. We have previously shown that the more proliferative SPEM phenotype observed after L635 treatment, in comparison with DMP-777 treatment, relates to a more prominent M2-macrophage infiltrate (20). Thus upon a prominent inflammatory infiltrate, functionally immature chief cells can transdifferentiate into SPEM cells but have impaired capabilities to efficiently reactivate the proliferative properties found in advanced SPEM. Because MIST1 protein is expressed only in chief cells in the stomach, this resistance to progress to proliferative SPEM is likely due to the innate cell-autonomous defect of loss of Mist1 function in chief cells. Of note, Mist1 is also expressed in mature plasma cells; however, previous studies have shown that loss of Mist1 does not cause defects in these cells (3). Future in-depth molecular studies will be needed to explore the specific innate chief cell molecular underpinnings necessary to reactivate proliferation. The prevailing assertion of previous studies is that external factors are the driving force of the metaplastic progression (20, 27). However, the present study demonstrates that chief cells are not purely passive participants but instead possess intrinsic cellular traits that contribute to the complex signaling involved in the metaplastic progression. Furthermore, these studies demonstrate the first intrinsic characteristic of chief cells necessary for the progression to advanced SPEM.
Acinar cell lineages from various tissues such as the pancreas are often considered to be long-lived cell populations. In the stomach, previous reports have shown that chief cells have the ability to live up to 100–200 days (12, 22). However, very little is known about the functional changes that occur in zymogenic cell lineages as they age. As chief cells appear to be the principal source of SPEM (15), we investigated the ability of aging chief cells to retain their plasticity to transdifferentiate. Our data show that as chief cells age, they lose the ability to transdifferentiate into SPEM and reenter the cell cycle. After 1 and 2 mo posttamoxifen, ~40% of the lineage-traced chief cells transdifferentiated decreasing to only 12% at 3.5 mo. Furthermore, proliferation of lineage traced SPEM progressively decreased at each time point. Compared with 44% at 1 mo and 24% at 2 mo, none of the labeled SPEM was proliferative at 3.5 mo. While some older chief cells (at 2 mo) may retain their ability to transdifferentiate, they more readily lose their ability to reactivate proliferation as their cellular age increases. Thus our data confirm that the origin of advanced proliferative SPEM is “young” chief cells as opposed to the aging long-lived chief cells.
The spatiotemporal organization of the gastric gland in which cell lineage differentiation can be tracked along the gland axis provides a considerable advantage in studying the events that occur within the gland. Previously, for quantification of specific cellular features, representative images selected to prove the spatial distribution within the gland were accompanied by total numerical counts reported in bar graph formats. The ability for statistical comparisons of frequency distribution in the gland is lost in this method. Furthermore, manual localization measurements do not account for varying glandular height as they lack the ability to normalize the heights of several glands. Thus they sorely lack an extensive frequency distribution. More recent studies have begun to address this need for comprehensive spatial quantification in various tissue systems (5, 8), but there is still a lack of extensive quantification that incorporates the ordered spatiotemporal organization of the stomach. Accordingly, we have developed a customized software that effectively utilizes this inherent spatiotemporal gastric organization to more systematically and comprehensively quantify the frequency distributions of selected cell characteristics within the gastric gland. Our software also normalizes the glandular height to accurately perform statistical comparisons of the distributions. Most notably, in our Mist1−/− experiments, we were able to visually and quantitatively distinguish the localization of retained chief cells in drug-treated Mist1−/− mice. In addition, the increase in GSII+/GIF+ cells in untreated Mist1−/− mice was confirmed to be the previously reported transition cells based on the same localization found in wild-type mice. While in our studies cell lineages were manually identified, future studies could further expand on this automated localization quantification. Identification of individual cell types can also be automated by utilizing cell identification software such as CellProfiler (14) as done in previous studies (25, 27). This would provide further streamlining of an automated method for thorough analysis from automated scanning of the tissue sections to identification of the immunostaining patterns to quantification (total counts and frequency distributions) and statistical comparison of these patterns. This spatiotemporal quantification method can be utilized to elucidate various other cellular and molecular characteristics within the gastric glands as well as in other spatiotemporal organized tissues such as the intestine and colon.
In summary, our studies have demonstrated that chief cell functional maturation and chief cell age both affect the ability of chief cells to transdifferentiate into SPEM and adopt a proliferative metaplastic phenotype. These findings suggest that younger chief cells present a higher capacity for transdifferentiation and thus likely represent the predominant lineage for production of metaplastic lineages under conditions of acute and chronic mucosal damage.
GRANTS
These studies were supported by Department of Veterans Affairs Merit Review Award Grant I01BX000930 and National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Grant R01-DK-071590. C. P. Petersen was supported by NIDDK National Research Service Award Predoctoral Fellowship F31-DK-104600. V. G. Weis was supported by NIDDK Institutional Postdoctoral Fellowship T32-DK-007673. A. R. Meyer was supported by National Institute of General Medical Sciences Institutional Training Grant T32-GM-008554. J. C. Mills was funded by NIDDK Grants R01-DK-094989 and R01-DK-105129 and the Siteman Cancer Center Investment Program. This work was supported by core resources of the Vanderbilt Digestive Disease Center (P30-DK-058404) and the Vanderbilt-Ingram Cancer Center (P30-CA-68485), and imaging was supported by the Vanderbilt Digital Histology Shared Resource.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
V.G.W., C.P.P., A.R.M., and E.C. performed experiments; V.G.W., C.P.P., J.A.W., A.R.M., E.C., and J.R.G. analyzed data; V.G.W., C.P.P., J.A.W., A.R.M., E.C., J.C.M., and J.R.G. interpreted results of experiments; V.G.W., C.P.P., J.A.W., A.R.M., and J.R.G. prepared figures; V.G.W. and C.P.P. drafted manuscript; V.G.W., C.P.P., J.A.W., A.R.M., E.C., J.C.M., and J.R.G. edited and revised manuscript; V.G.W., C.P.P., J.A.W., A.R.M., E.C., J.C.M., and J.R.G. approved final version of manuscript.
REFERENCES
- 1.Aihara E, Matthis AL, Karns RA, Engevik KA, Jiang P, Wang J, Yacyshyn BR, Montrose MH. Epithelial regeneration after gastric ulceration causes prolonged cell-type alterations. Cell Mol Gastroenterol Hepatol 2: 625–647, 2016. doi: 10.1016/j.jcmgh.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bredemeyer AJ, Geahlen JH, Weis VG, Huh WJ, Zinselmeyer BH, Srivatsan S, Miller MJ, Shaw AS, Mills JC. The gastric epithelial progenitor cell niche and differentiation of the zymogenic (chief) cell lineage. Dev Biol 325: 211–224, 2009. doi: 10.1016/j.ydbio.2008.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Capoccia BJ, Lennerz JK, Bredemeyer AJ, Klco JM, Frater JL, Mills JC. Transcription factor MIST1 in terminal differentiation of mouse and human plasma cells. Physiol Genomics 43: 174–186, 2011. doi: 10.1152/physiolgenomics.00084.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Correa P. A human model of gastric carcinogenesis. Cancer Res 48: 3554–3560, 1988. [PubMed] [Google Scholar]
- 5.Earle KA, Billings G, Sigal M, Lichtman JS, Hansson GC, Elias JE, Amieva MR, Huang KC, Sonnenburg JL. Quantitative imaging of gut microbiota spatial organization. Cell Host Microbe 18: 478–488, 2015. doi: 10.1016/j.chom.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Engevik AC, Feng R, Choi E, White S, Bertaux-Skeirik N, Li J, Mahe MM, Aihara E, Yang L, DiPasquale B, Oh S, Engevik KA, Giraud AS, Montrose MH, Medvedovic M, Hlemrath MA, Goldenirng JR, Zavros Y. The development of spasmolytic polypeptide/TFF2-expressing metaplasia (SPEM) during gastric repair is absent in the aged stomach. Cell Mol Gastroent Hepatol 2: 605–624, 2016. doi: 10.1016/j.jcmgh.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldenring JR, Nam KT, Wang TC, Mills JC, Wright NA. Spasmolytic polypeptide-expressing metaplasia and intestinal metaplasia: time for reevaluation of metaplasias and the origins of gastric cancer. Gastroenterology 138: 2207–2210.e1, 2010. doi: 10.1053/j.gastro.2010.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huh WJ, Khurana SS, Geahlen JH, Kohli K, Waller RA, Mills JC. Tamoxifen induces rapid, reversible atrophy, and metaplasia in mouse stomach. Gastroenterology 142: 21–24.e7, 2012. doi: 10.1053/j.gastro.2011.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karam SM. Dynamics of epithelial cells in the corpus of the mouse stomach. IV. Bidirectional migration of parietal cells ending in their gradual degeneration and loss. Anat Rec 236: 314–332, 1993. doi: 10.1002/ar.1092360205. [DOI] [PubMed] [Google Scholar]
- 10.Karam SM, Leblond CP. Dynamics of epithelial cells in the corpus of the mouse stomach. I. Identification of proliferative cell types and pinpointing of the stem cell. Anat Rec 236: 259–279, 1993. doi: 10.1002/ar.1092360202. [DOI] [PubMed] [Google Scholar]
- 11.Karam SM, Leblond CP. Dynamics of epithelial cells in the corpus of the mouse stomach. II. Outward migration of pit cells. Anat Rec 236: 280–296, 1993. doi: 10.1002/ar.1092360203. [DOI] [PubMed] [Google Scholar]
- 12.Karam SM, Leblond CP. Dynamics of epithelial cells in the corpus of the mouse stomach. III. Inward migration of neck cells followed by progressive transformation into zymogenic cells. Anat Rec 236: 297–313, 1993. doi: 10.1002/ar.1092360204. [DOI] [PubMed] [Google Scholar]
- 13.Kowalik AS, Johnson CL, Chadi SA, Weston JY, Fazio EN, Pin CL. Mice lacking the transcription factor Mist1 exhibit an altered stress response and increased sensitivity to caerulein-induced pancreatitis. Am J Physiol Gastrointest Liver Physiol 292: G1123–G1132, 2007. doi: 10.1152/ajpgi.00512.2006. [DOI] [PubMed] [Google Scholar]
- 14.Lamprecht MR, Sabatini DM, Carpenter AE. CellProfiler: free, versatile software for automated biological image analysis. Biotechniques 42: 71–75, 2007. doi: 10.2144/000112257. [DOI] [PubMed] [Google Scholar]
- 15.Nam KT, Lee HJ, Sousa JF, Weis VG, O’Neal RL, Finke PE, Romero-Gallo J, Shi G, Mills JC, Peek RM Jr, Konieczny SF, Goldenring JR. Mature chief cells are cryptic progenitors for metaplasia in the stomach. Gastroenterology 139: 2028–2037.e9, 2010. doi: 10.1053/j.gastro.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nam KT, Varro A, Coffey RJ, Goldenring JR. Potentiation of oxyntic atrophy-induced gastric metaplasia in amphiregulin-deficient mice. Gastroenterology 132: 1804–1819, 2007. doi: 10.1053/j.gastro.2007.03.040. [DOI] [PubMed] [Google Scholar]
- 17.Nomura S, Yamaguchi H, Ogawa M, Wang TC, Lee JR, Goldenring JR. Alterations in gastric mucosal lineages induced by acute oxyntic atrophy in wild-type and gastrin-deficient mice. Am J Physiol Gastrointest Liver Physiol 288: G362–G375, 2005. doi: 10.1152/ajpgi.00160.2004. [DOI] [PubMed] [Google Scholar]
- 18.Nozaki K, Ogawa M, Williams JA, Lafleur BJ, Ng V, Drapkin RI, Mills JC, Konieczny SF, Nomura S, Goldenring JR. A molecular signature of gastric metaplasia arising in response to acute parietal cell loss. Gastroenterology 134: 511–522, 2008. doi: 10.1053/j.gastro.2007.11.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ogawa M, Nomura S, Varro A, Wang TC, Goldenring JR. Altered metaplastic response of waved-2 EGF receptor mutant mice to acute oxyntic atrophy. Am J Physiol Gastrointest Liver Physiol 290: G793–G804, 2006. doi: 10.1152/ajpgi.00309.2005. [DOI] [PubMed] [Google Scholar]
- 20.Petersen CP, Weis VG, Nam KT, Sousa JF, Fingleton B, Goldenring JR. Macrophages promote progression of spasmolytic polypeptide-expressing metaplasia after acute loss of parietal cells. Gastroenterology 146: 1727–38.e8, 2014. doi: 10.1053/j.gastro.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pin CL, Rukstalis JM, Johnson C, Konieczny SF. The bHLH transcription factor Mist1 is required to maintain exocrine pancreas cell organization and acinar cell identity. J Cell Biol 155: 519–530, 2001. doi: 10.1083/jcb.200105060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Quante M, Marrache F, Goldenring JR, Wang TC. TFF2 mRNA transcript expression marks a gland progenitor cell of the gastric oxyntic mucosa. Gastroenterology 139: 2018–2027.e2, 2010. doi: 10.1053/j.gastro.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramsey VG, Doherty JM, Chen CC, Stappenbeck TS, Konieczny SF, Mills JC. The maturation of mucus-secreting gastric epithelial progenitors into digestive-enzyme secreting zymogenic cells requires Mist1. Development 134: 211–222, 2007. doi: 10.1242/dev.02700. [DOI] [PubMed] [Google Scholar]
- 24.Schmidt PH, Lee JR, Joshi V, Playford RJ, Poulsom R, Wright NA, Goldenring JR. Identification of a metaplastic cell lineage associated with human gastric adenocarcinoma. Lab Invest 79: 639–646, 1999. [PMC free article] [PubMed] [Google Scholar]
- 25.Sousa JF, Ham AL, Nam KT, Lee H, Kim WH, Yang HK, Zhang B, Li M, LaFleur BJ, Liebler DC, Goldenring JR. Proteomic profiling of metaplasia and cancer in the human stomach reveals metaplastic-specific biomarkers. Am J Pathol 181: 1560–1572, 2012. doi: 10.1016/j.ajpath.2012.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tu SP, Quante M, Bhagat G, Takaishi S, Cui G, Yang XD, Muthuplani S, Shibata W, Fox JG, Pritchard DM, Wang TC. IFN-γ inhibits gastric carcinogenesis by inducing epithelial cell autophagy and T-cell apoptosis. Cancer Res 71: 4247–4259, 2011. doi: 10.1158/0008-5472.CAN-10-4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weis VG, Sousa JF, LaFleur BJ, Nam KT, Weis JA, Finke PE, Ameen NA, Fox JG, Goldenring JR. Heterogeneity in mouse SPEM lineages identifies markers of metaplastic progression. Gut 62: 1270–1279, 2013. doi: 10.1136/gutjnl-2012-302401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoshizawa N, Takenaka Y, Yamaguchi H, Tetsuya T, Tanaka H, Tatematsu M, Nomura S, Goldenring JR, Kaminishi M. Emergence of spasmolytic polypeptide-expressing metaplasia in Mongolian gerbils infected with Helicobacter pylori. Lab Invest 87: 1265–1276, 2007. doi: 10.1038/labinvest.3700682. [DOI] [PubMed] [Google Scholar]