In this study we report a series of novel observations that underscore the importance of bone morphogenetic protein (BMP) signaling in the regulation of colonic homeostasis during the development of injury and inflammation. In particular, we present evidence that BMP signaling mitigates the response of the colonic epithelium to injury and inflammation and that cytokines, such as TNF-α and IL-1β, inhibit the expression of BMP-4.
Keywords: bone morphogenetic protein-responsive element, myofibroblasts, cytokines, chemokines
Abstract
The bone morphogenetic proteins (BMPs) regulate gastrointestinal homeostasis. We investigated the expression of BMP-4 and the localization and function of BMP signaling during colonic injury and inflammation. Mice expressing the β-galactosidase (β-gal) gene under the control of a BMP-responsive element (BRE), BMP-4-β-gal/ mice, and animals generated by crossing villin-Cre mice to mice with floxed alleles of BMP receptor 1A (villin-Cre;Bmpr1aflox/flox) were treated with dextran sodium sulfate (DSS) to induce colonic injury and inflammation. Expression of BMP-4, β-gal, BMPR1A, IL-8, α-smooth muscle actin, and phosphorylated Smad1, -5, and -8 was assessed by X-Gal staining, quantitative RT-PCR, and immunohistochemistry. Morphology of the colonic mucosa was examined by staining with hematoxylin and eosin. The effect of IFN-γ, TNF-α, IL-1β, and IL-6 on BMP-4 mRNA expression was investigated in human intestinal fibroblasts, whereas that of BMP-4 on IL-8 was assessed in human colonic organoids. BMP-4 was localized in α-smooth muscle actin-positive mesenchymal cells while the majority of BMP-generated signals targeted the epithelium. DSS caused injury and inflammation leading to reduced expression of BMP-4 and of BMPR1A mRNAs, and to decreased BMP signaling. Deletion of BMPR1A enhanced colonic inflammation and damage. Administration of anti-TNF-α antibodies to DSS-treated mice ameliorated colonic inflammation and increased the expression of BMP-4 and BMPR1A mRNAs. TNF-α and IL-1β inhibited both basal and IFN-γ-stimulated BMP-4 expression, whereas IL-6 had no effect. BMP-4 reduced TNF-α-stimulated IL-8 mRNA expressor IL-8 mRNA expression in the organoids. Inflammation and injury inhibit BMP-4 expression and signaling, leading to enhanced colonic damage and inflammation. These observations underscore the importance of BMP signaling in the regulation of intestinal inflammation and homeostasis.
NEW & NOTEWORTHY In this study we report a series of novel observations that underscore the importance of bone morphogenetic protein (BMP) signaling in the regulation of colonic homeostasis during the development of injury and inflammation. In particular, we present evidence that BMP signaling mitigates the response of the colonic epithelium to injury and inflammation and that cytokines, such as TNF-α and IL-1β, inhibit the expression of BMP-4.
the bone morphogenetic proteins (BMPs) are important regulators of a broad array of biological actions during both embryonic and postnatal vertebrate development (6, 7, 12, 13, 28, 29). Several BMPs, such as BMP-2, BMP-4, and BMP-7, appear to be expressed in gastrointestinal tissues where they have been shown to play a significant role in the regulation of epithelial cell differentiation and proliferation (2, 3, 10, 11, 21, 25, 33). Although BMP-4 is localized in the mesenchyme (26, 27), BMP2 and BMP7 appear to be expressed in the epithelium (11, 17, 18, 27).
One of the best-characterized signaling pathways that mediate the intracellular actions of the BMPs is that leading to the activation of the regulatory proteins Smad1, -5, and -8 (6, 7, 12, 29). In particular, binding of the BMPs to the BMP type I receptor (BMPR-I) leads to the dimerization of BMPR-I with the BMP type II receptor, a molecule that has serine/threonine kinase activity. This event triggers the phosphorylation of both BMPR-I and of Smad1, -5, and -8, allowing the association of Smad1, -5, and -8 with Smad4 in a heterodimeric complex that transclocates to the nucleus where it activates gene transcription (6, 7, 12, 29). Previous studies have shown that the colonic epithelium expresses BMP signaling molecules, confirming the notion that BMP signaling might represent an important mechanism for the regulation of colonic epithelial cell homeostasis (11, 17, 19).
The clinical relevance of these observations has been underscored by studies conducted in colon cancer cells that have demonstrated an important role for BMP signaling in the inhibition of colon cancer cell growth (3, 11).
Studies have also shown that the BMPs can exert anti-inflammatory actions (5, 17, 18, 26) and that they could therefore represent novel and hitherto poorly characterized regulators of gastrointestinal inflammation. Indeed, recent work from our laboratory has demonstrated that expression of the BMP signaling inhibitor noggin in the stomach increases Helicobacter-induced inflammation leading to enhanced gastric epithelial cell proliferation and to the accelerated development of dysplasia (26). In support of this hypothesis, BMP-7 has been shown to ameliorate the severity of colonic inflammation and to accelerate the healing of colitis in rats exposed to trinitrobenzenesulfonic acid, a well-established inducer of experimental colitis in rodents (17, 18).
The localization of the BMPs and the function of BMP-generated signals during colonic injury and inflammation have been only partially characterized. Moreover, the factors involved in the regulation of BMP expression in the inflamed and injured colon are unknown.
Accordingly, we took advantage of several lines of genetically engineered mice and of cultures of human fibroblast cells and of colonic organoids to test the hypothesis that BMP-4 expression and signaling are modulated by inflammation and that the BMP signal transduction pathway negatively regulates the response of the colonic mucosa to inflammatory stimuli. For these studies we employed the dextran sodium sulfate (DSS) model of colonic injury and inflammation, an experimental system that has been extensively studied and characterized (8, 23, 30). We also opted to focus our studies on BMP-4 since this peptide appears to be exclusively expressed in the gastrointestinal mesenchyme (26, 27), thus allowing us to examine how mesenchymal signals regulate the response of the colonic mucosa to inflammation.
METHODS
Mice.
BRE-β-gal mice (4), which express the β-galactosidase (β-gal) gene under the control of a BMP-responsive element (BRE), were obtained from L. Oxburgh (Maine Medical Center Research Institute). BMP-4-β-gal/+ mice (13), which have a β-galactosidase marked allele of the BMP-4 gene, were a gift of B. Hogan (Duke University). Villin-Cre mice, which express Cre recombinase in the intestine, including the colon (15), and Bmpr1aflox/flox (19) mice were received from the laboratories of Drs. Deborah Gumucio and Yuji Mishina, respectively (University of Michigan). To delete Bmpr1a in the colonic epithelium, villin-Cre;Bmpr1aflox/flox mutants were generated. Mice were maintained on a C57Bl/6 background. Specific-pathogen-free female C57BL/6 mice aged 8–10 wk were purchased from Jackson Laboratory (Bar Harbor, ME). Mouse genotyping was described elsewhere (15, 19). Animals were housed in the animal maintenance facility at the University of Michigan. All animal experiments were approved by the University of Michigan Animal Care and Use Committee.
Quantitative RT-PCR analysis.
Mouse and human BMP-4, mouse BMPR1A, and human IL-8 transcript levels were determined by quantitative RT-PCR (QRT-PCR). RNA was isolated from the colon of 3-mo-old mice, human fibroblasts, and colonoids using TRIzol reagent (Invitrogen, Carlsbad, CA), followed by DNase treatment and purification with the RNeasy Mini kit (Qiagen, Valencia, CA). RT reactions were conducted according to previously published reports (25, 26). Reactions were performed using the Icycler (Bio-Rad, Hercules, CA) with a 20-μl reaction in PCR buffer (Invitrogen) containing 2 μl of cDNA (RT product), 5.5 mM MgCl2, 100 nM primers [mouse BMP-4 forward: 5′-AGA GCA GAG CCA GGG AAC CG, mouse BMP-4 reverse: 5′-CCA AAA GTG ACC AGG AGG GG; mouse BMPR1A forward: 5′-CCGCTATGGAGAAGTATGGATGGG, mouse BMPR1A reverse: 5′-CAGACCACAAGCAGCAGAATAAGC; human BMP-4 and GAPDH primers were obtained from Qiagen; mouse TNF-α and GAPDH and human IL-8 primers were previously described (26)], 200 nM dNTPs, 0.1× SYBR Green, 10 nM fluorescein, and 0.025 units Platinum Taq (Invitrogen). Expression levels were normalized to glyceraldehyde-3-phosphate dehydrogenase expression. The cycle parameters were as follows: 95°C for 3 min, 40 cycles at 95, 60, and 72°C each for 30 s, and 72°C for 5 min. The annealing temperature for BMP-4 was 65°C and 60°C for all other genes. After amplification was completed, the samples were subjected to melt-curve analysis by increasing the temperature from 60 to 100°C, in 0.5°C intervals every 10 s for 80 steps to assess product purity. No signal was detected with control samples that were not treated with RT (data not shown).
Colon length, histochemical analysis, and image acquisition.
Tissue fixation, generation of paraffin sections, cryosectioning, β-galactosidase staining, and immunostaining were performed according to previously published methods (15, 25, 26). After mice were killed, the entire colon was removed from the cecum to the anus, and the colon length was assessed as an indirect indicator of inflammation (32). Frozen sections were stained with the following primary antibodies: anti-β-galactosidase (1:1,500) (gift from James Douglas Engel, University of Michigan), anti-actin, α-smooth muscle-Cy3 (1:500) (Sigma, St. Louis, MO), anti-F4/80 (1:100), and anti-Ki-67 (1:500) (Novocastra, Newcastle upon Tyne, UK). Paraffin sections were stained with anti-phosphorylated serine-463 and -465 of Smad1 (1:100). For immunohistochemistry with detection with diaminobenzidine as substrate, slides were rinsed and subsequently treated with biotin-conjugated secondary antibodies (1:200) (Vector Laboratories, Burlingame, CA) for 30 min at room temperature. To visualize biotin staining, the Vectastain Elite ABC kit was used (Vector Laboratories), followed by counterstaining with hematoxylin. For immunofluorescence analysis, FITC-donkey anti-rabbit (1:100), Alexa 555-donkey anti-rabbit (1:500), and Alexa 594-donkey anti-rat (1:500) secondary antibodies were used (Invitrogen). ProLong Gold Antifade reagent with DAPI (Molecular Probes, Eugene, OR) was used for nuclear counterstain and mounting medium. Control experiments were performed by incubating the slides in the presence of the secondary antibodies without the primary antibodies (data not shown). Both paraffin and frozen sections were also stained with hematoxylin and eosin (H&E) to visualize cellular morphology (25, 26). Visualization of slides was performed with a Nikon Eclipse E 800 fluorescence microscope.
For histological scoring of epithelial damage and inflammation, sections from the left colon of three to four separate animals from each treatment group were examined and scored as follows. Only fields that contained full-thickness mucosa that was oriented perpendicularly were scored. All well-oriented fields were scored blindly at ×200 magnification. Each ×200 field was scored separately for the presence or absence of neutrophilic and mononuclear cell infiltration and epithelial damage. The score was expressed as a percent of affected fields. In addition, the severity of the changes was graded using a scale from 1 to 4. Grade 1 was characterized by mild inflammatory changes [few polymorphonuclear neutrophils (PMNs)] and by the presence of dilated glands and of sloughed cells. Grade 2 showed moderate inflammation with PMNs clusters, flattened epithelium, and partial gland loss. Grade 3 exhibited severe inflammation with widespread PMNs and loss of entire crypts. Grade 4 was characterized by presence of ulcers and of spreading of the inflammatory changes to the submucosa. Each field was scored, and the final score was calculated as a weighted average of all fields (sum of the number of fields for each score × the score ÷ the total number of fields). In the TNF-α inhibition studies the histological score was calculated according to previously described criteria (22, 32).
NL-685 and CCD-18Co cell culture.
Human CCD-18Co colonic myofibroblasts were purchased from ATCC (Manassas, VA). Human primary NL-685 intestinal fibroblasts were a gift of Claudio Fiocchi (Cleveland Clinic). CCD-18Co cells were grown at 37°C in minimum essential medium (GIBCO, Grand Island, NY) with 10% heat-inactivated fetal bovine serum (FBS) in 5% CO2-95% O2. NL-685 cells were grown at 37°C in minimum essential medium, supplemented with 1× nonessential amino acid solution, 2 mM l-glutamine, 1 mM sodium pyruvate, and 25 mM HEPES. Cells were incubated for different times points with human Tnf-α (0.1–10 ng/ml), IFN-γ (3–300 ng/ml), IL-1β (0.5–50 ng/ml), and IL-6 (0.1–100 ng/ml) (R&D Systems, Minneapolis, MN).
DSS colitis.
Acute colonic injury and inflammation were induced using DSS according to standard previously reported protocols (8, 23, 30, 32). Briefly, 12-wk wild-type mice (32), BRE-β-gal, BMP-4-β-gal/+, Villin-Cre, and Villin-Cre;Bmpr1aflox/flox, mice were given 3% (wt/vol) DSS (mol wt 40 kDa) dissolved in drinking water, which was fed ad libitum for 4 days. Analysis of the animals was performed 3 days after DSS administration. Control C57BL/6 mice received the same drinking water without DSS.
Human colonic organoids (colonoids).
Human colonic organoids were generated from biopsies of human normal colonic mucosa as previously described (16, 24). Isolated colonic glands were pelleted at 150 g for 10 min at 4°C and resuspended in 200 μl of organoid culture media (50% L-WRN conditioned media, 10% FBS, 10 mM HEPES, 1 mM N-acetyl-l-cysteine, 100 ng/ml human epidermal growth factor, 2 mM l-glutamine, 2× B27, 2× N2 supplement, 20 mM nicotinamide, 1× penicillin-streptomycin, 1× fungizone, 1× gentamicin, and 10 μM Y-27532 in Advanced DMEM-F-12). Aliquots were diluted in Matrigel at a 1:4 ratio before plating in a prewarmed 24-well plate and overlaying with 500 μl culture media. Media was replaced every other day, and cultures were maintained via passaging one time per week. Studies were performed in organoids that had been passaged at least two to three times before analysis. Three days after splitting, the organoids were cultured in noggin-free media containing Wnt and R-spondin in the presence and absence of TNF-α (10 ng/ml), alone and in association with BMP-4 (20 ng/ml). Examination of the organoid cultures maintained in noggin-free media under light microscopy did not indicate the presence of any signs of toxicity. Human tissue was obtained under Institutional Review Board-approved protocols at the University of Michigan.
TNF-α inhibition studies.
These studies were conducted as previously described (32). Briefly, 6- to 8-wk-old mice were intraperitoneally injected with 10 mg/kg Remicade (Janssen Biotech, Horsham, PA) 1 day and 4 h before and every other day after DSS treatment. All mice were maintained in standard cages in a light- and temperature-controlled room and were given standard chow and water ad libitum.
Data analysis.
Data are expressed as means ± SE. Statistical analysis was performed using Student's t-test. P values <0.05 were considered to be significant.
RESULTS
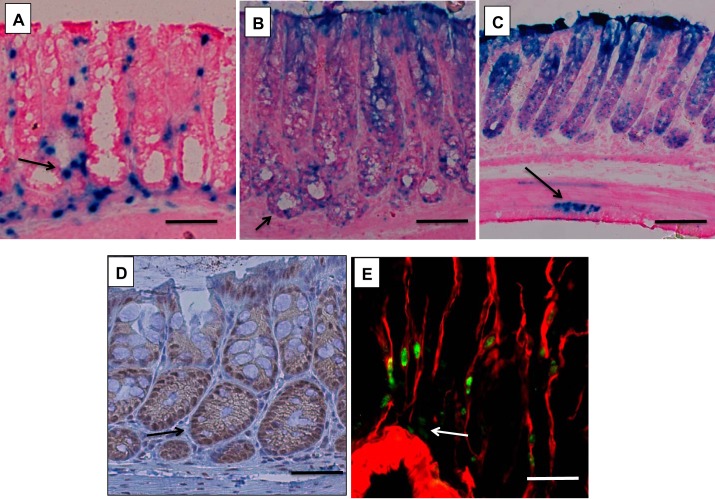
We examined the localization of BMP-4-expressing cells and of cells receiving BMP-generated signals in the colonic mucosa. As shown in Fig. 1A, X-gal staining of the colonic mucosa of BMP-4-β-gal/+ mice indicated that BMP-4 is expressed in mesenchymal cells located both under and between the glands. To define the localization of cells receiving BMP-generated signals, we used BRE-β-gal mice. As demonstrated in Fig. 1B, X-gal-positive cells could be detected mostly in the epithelium. However, review of slides derived from the colons of at least four separate mice also demonstrated the presence of rare X-gal-positive cells in the mesenchymal layers of the mucosa (Fig. 1C). Similar results were observed when we stained paraffin sections of the colonic mucosa with antibodies recognizing phosphorylated and active forms of the BMP-4 signal-transducing proteins Smad1, -5, and -8 (Fig. 1D). To better define the type of cells that express BMP-4 in the colon, we performed an immunohistochemical analysis of sections of the colonic mucosa of BMP-4-β-gal/+ mice using anti-β-galactosidase and anti-α-smooth muscle actin (SMA) antibodies. Although, as indicated in Fig. 1E, BMP-4 expression could be predominantly detected in α-SMA-positive cells, a few rare β-galactosidase-positive α-SMA-negative cells were also noted, suggesting that BMP-4 expression is not exclusively restricted to α-SMA-expressing mesenchymal cells. These findings are in agreement with our previously published observations generated in the gastric mucosa of mice (26).
Fig. 1.
Expression of bone morphogenetic protein (BMP)-4 and localization of BMP signaling in the colon. A–C: X-Gal-stained frozen sections from BMP-4-β-galactosidase (β-gal)/+ mice (A) and BMP-responsive element (BRE)-β-gal mice (B and C). D: paraffin sections from wild-type mice stained with anti-p-Smad1, -5, and -8 antibodies and biotin-conjugated secondary antibodies. E: frozen sections from BMP-4-β-gal/+ mice stained with anti-β-gal primary antibodies and FITC-conjugated secondary antibodies (green), together with Cy3-conjugated-anti-actin, α-smooth muscle antibodies (red). Arrow in A points to BMP-4-expressing cells. Arrows in B, C, and D point to cells receiving BMP-generated signals. Arrow in E points to β-gal-positive α- smooth muscle actin (SMA)-negative cells. Results similar to those depicted were observed in at least three other separate experiments. Size bar is 50 μm in A, B, and D, 100 μm in C, and 20 μm in E.
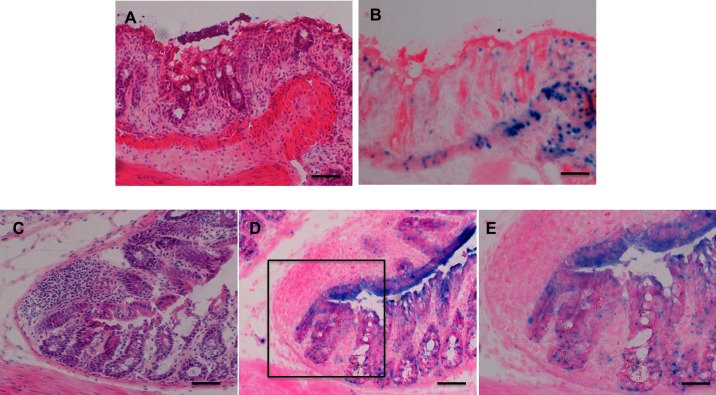
We investigated the pattern of expression of BMP-4 and the localization of cells receiving BMP-generated signals in the colonic mucosa after acute DSS treatment. The DSS-treated mice exhibited a significant increase in the expression of TNF-α (7.21 ± 1.74-fold induction over control, mean ± SE, n = 3, P < 0.05) and a significant reduction in the length of the colon [84.63 ± 0.57 (SE) mm, n = 3, in the absence and 70.25 ± 0.62 (SE) mm, n = 4, in the presence of DSS, P < 0.05]. As indicated in Fig. 2, A and C, H&E-stained frozen sections of the colonic mucosa of DSS-treated-BMP-4-β-gal/+ and of -BRE-β-gal mice revealed the presence of patches of injured and inflamed mucosa. X-gal staining of the slides demonstrated that both BMP-4-expressing cells (Fig. 2B) and epithelial cells receiving BMP-generated signals (Fig. 2, D and E) were located adjacent to, but not in, the areas of injury and inflammation.
Fig. 2.
Localization of BMP-4 and of BMP signaling in the dextran sodium sulfate (DDS)-treated colon. A and B: matching frozen sections from the left colon of DSS-treated BMP-4-β-gal/+ mice stained with hematoxylin and eosin (H&E, A) and X-gal (B). C and D: matching frozen sections from the left colon of DSS-treated BRE-β-gal mice stained with H&E (C) and X-gal (D). E: magnified window depicting X-gal-positive cells. Size bar is 100 μm in A–D and 50 μm in E. Results similar to those depicted were observed in at least three other separate experiments.
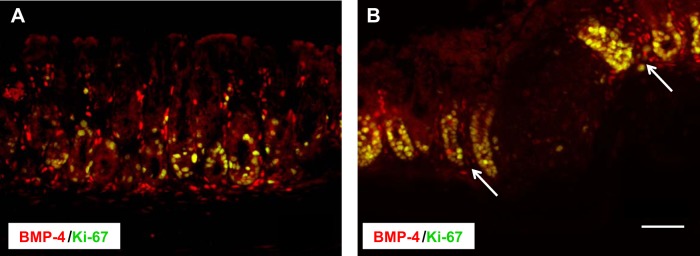
Immunohistochemical analysis of the colonic mucosa of both control (Fig. 3A) and DSS-treated (Fig. 3B) BMP-4-β-gal/+ mice with antibodies directed against both β-galactosidase and Ki-67, a well-established mitotic marker, indicated the presence of BMP-4-expressing cells in close contact with proliferating cells located mostly at the base of the glands (Fig. 3A) and in areas surrounding, but not in, patches of injured and inflamed mucosa (Fig. 3B).
Fig. 3.
Localization of BMP-4 and of Ki-67-positive cells in the colonic mucosa. Frozen sections from the left colon of BMP-4-β-gal/+ mice in the absence (A) and presence (B) of DSS were stained anti-β-gal primary antibodies and Cy3-conjugated secondary antibodies (red), together with anti-Ki-67 primary antibodies and Alexa 488-conjugated secondary antibodies (green). Arrows point to BMP-4-expressing cells. Size bar = 50 μm. Results similar to those depicted were observed in at least three other separate experiments.
Measurement by QRT-PCR of both BMP-4 and BMPR1A mRNAs demonstrated that treatment with DSS leads to decreased expression of these molecules, confirming the notion that inflammation and injury negatively regulate BMP expression and signaling (Fig. 4, A and B).
Fig. 4.
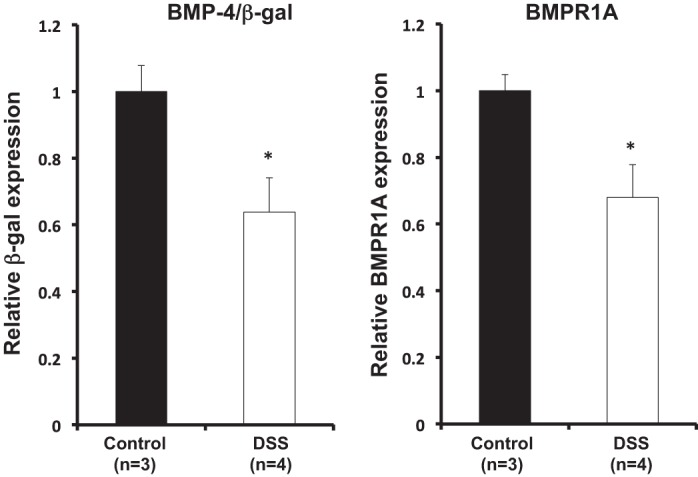
Expression of BMP-4 and of BMP receptor 1A (BMPR1A) in DSS-treated BMP-4-β-gal/+ mice. BMP-4 and BMPR1A mRNA signals in DSS-treated mice were compared with those detected in control untreated mice using quantitative RT-PCR (QRT-PCR) and displayed as fold change over controls. Values are shown as means ± SE. *P < 0.05.
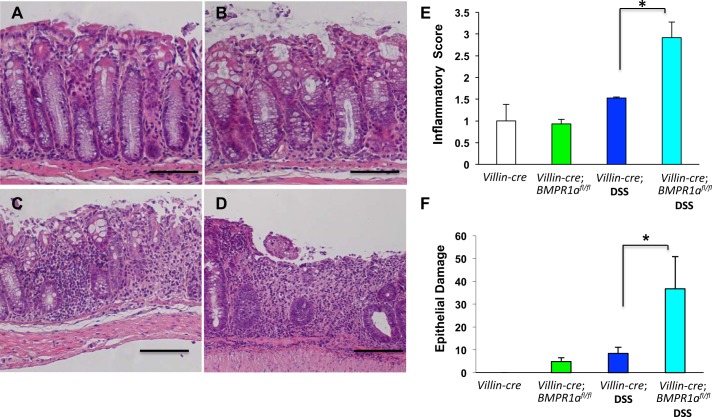
To better characterize the significance and function of BMP signaling during acute colonic injury and inflammation, we undertook studies in which we deleted bmpr1a in the colonic mucosa by crossing mice with floxed alleles of bmpr1a with animals expressing Cre-recombinase under the control of 12.4 kb of the villin promoter (15), a system that was previously characterized and employed to study the role of BMP signaling in intestinal homeostasis (2). For our experiments we opted to challenge the mice with 3% DSS, a well-established and characterized model of acute injury and inflammation of the colonic mucosa of rodents (8, 23, 30). Microscopic and morphometric analysis of H&E-stained sections of the colonic mucosa of DSS-treated villin-Cre;Bmpr1aflox/flox mice demonstrated a significant increase in the severity of injury and inflammation compared with nontransgenic/DSS-treated age-matched littermates (Fig. 5, A–F).
Fig. 5.
Loss of BMPR1A enhances inflammation and epithelial damage. A–D: representative H&E-stained gastric paraffin sections of the left colon of villin-Cre mice (A), villin-Cre;BMPR1Aflox/flox mice (B), DSS-treated villin-Cre mice (C), and of DSS-treated villin-Cre;BMPR1Aflox/flox mice (D). E and F: bar graphs represent inflammatory (E) and epithelial damage severity (F) scores calculated in both villin-Cre and villin-Cre;BMPR1Aflox/flox mice in the presence or absence of DSS. Values are shown as means ± SE, n = 3. *P < 0.05. Size bar = 100 μm.
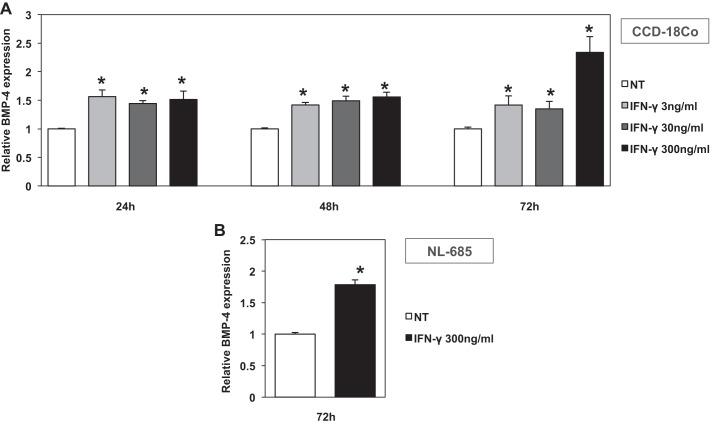
To gain more insight into the mechanisms that control the expression of BMP-4 during inflammation, we examined if cytokines regulate the expression of this peptide. We first investigated the effect of IFN-γ, a key mediator of colonic inflammation (8, 23), on the human colonic fibroblast cell line CCD-18Co. As shown in Fig. 6A, IFN-γ stimulated the expression of BMP-4 mRNA in these cells at doses ranging between 3 and 300 ng/ml after 24, 48, and 72 h of incubation. To confirm the significance of these observations, we tested the effect of IFN-γ in primary cultures of NL-685 human fibroblasts. As shown on the QRT-PCR assays, depicted in Fig. 6B, IFN-γ stimulated the expression of BMP-4 mRNA in NL-685 cells after 72 h of incubation.
Fig. 6.
Effect of IFN-γ on BMP-4 mRNA expression in human fibroblasts. BMP-4 mRNA abundance in CCD-18Co human colonic fibroblasts (A) stimulated with IFN-γ (3–300 ng/ml) for 24, 48, and 72 h and in human primary NL-685 fibroblasts (B) stimulated with IFN-γ (300 ng/ml) for 72 h was measured by QRT-PCR and displayed as fold change over controls. Values are shown as means ± SE, n = 4 for the 72-h time point and n = 3 for all others. *P < 0.05.
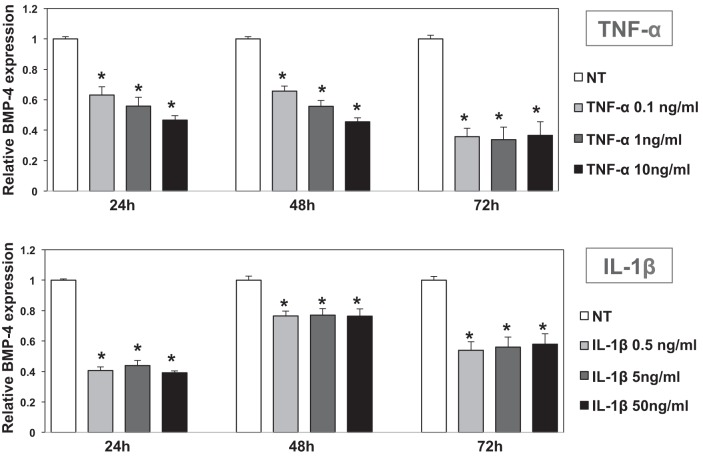
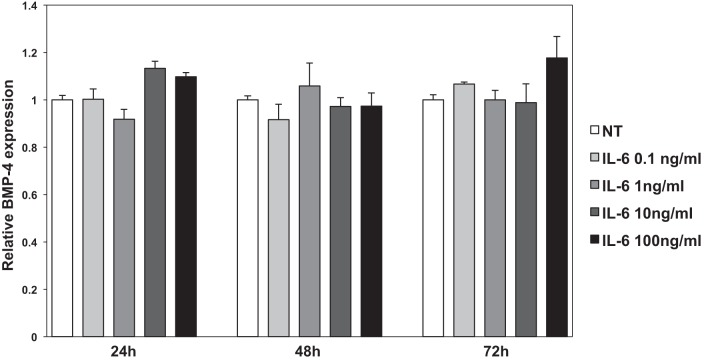
We examined if other cytokines that are known to mediate colonic inflammation, such as TNF-α, IL-1β, and IL-6 (23), regulate BMP-4 expression. As shown in Fig. 7, A and B, both TNF-α and IL-1β inhibited the expression of BMP-4 mRNA expression in CCD-18Co cells after 24, 48, and 72 h of incubation. In contrast to these observations, IL-6 had no significant effect on BMP-4 mRNA expression, underscoring the specificity of the actions of cytokines on the regulation of BMP-4 (Fig. 8). Identical results were seen in NL-685 cells treated with TNF-α, IL-1β, and IL-6 (data not shown).
Fig. 7.
Effect of TNF-α and IL-1β on BMP-4 mRNA expression in human CCD-18Co fibroblasts. BMP-4 mRNA abundance in CCD-18Co human colonic fibroblasts treated with TNF-α (0.1–10 ng/ml) and IL-1β (0.5–50 ng/ml) for 24, 48, and 72 h was measured by QRT-PCR and displayed as fold change over controls. Values are shown as means ± SE, n = 3. *P < 0.05.
Fig. 8.
Effect of IL-6 on BMP-4 mRNA expression in human CCD-18Co fibroblasts. BMP-4 mRNA abundance in CCD-18Co human colonic fibroblasts treated with IL-6 (0.1–100 ng/ml) for 24, 48, and 72 h was measured by QRT-PCR and displayed as fold change over controls. Values are shown as means ± SE, n = 3.
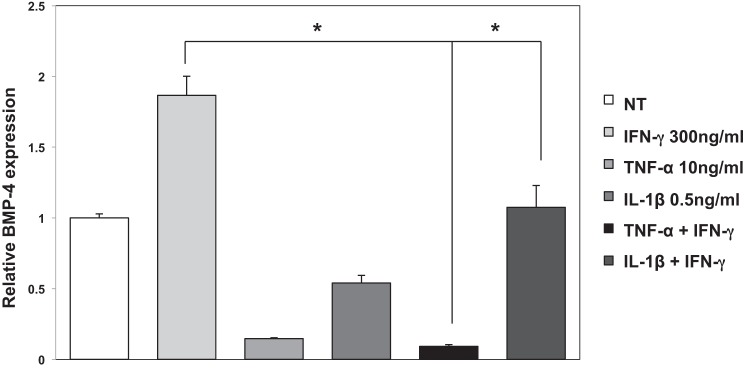
We investigated if TNF-α and IL-1β modulate the stimulatory effect of IFN-γ on BMP-4 mRNA expression. Accordingly, we cultured the CCD-18Co cells in the presence of IFN-γ, alone and in association with either TNF-α or IL-1β. As shown in Fig. 9, both cytokines exerted significant inhibitory effects on IFN-γ stimulation of BMP-4 expression, suggesting that the level of expression of BMP-4 is regulated by the coordinated effect of different types of inflammatory mediators. Identical results were seen in NL-685 cells (data not shown).
Fig. 9.
Effect of TNF-α and IL-1β on IFN-γ-stimulated BMP-4 mRNA expression in human CCD-18Co fibroblasts. BMP-4 mRNA abundance in CCD-18Co human colonic fibroblasts stimulated with IFN-γ (300 ng/ml) in the presence or absence of TNF-α (10 ng/ml) and IL-1β (0.5 ng/ml) for 72 h was measured by QRT-PCR and displayed as fold change over controls. Values are shown as means ± SE, n = 3. *P < 0.05.
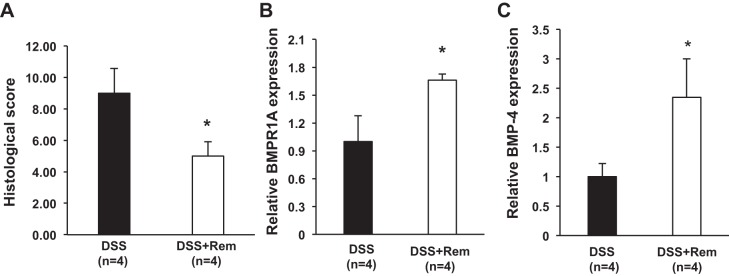
To confirm the relevance of TNF-α in the regulation of BMP-4 and of BMPR1A mRNA expression in vivo, we administered anti-TNF-α antibodies (Remicade) to DSS-treated mice. As shown in Fig. 10A, Remicade ameliorated the histological score, and it significantly increased the expression of both BMP-4 (Fig. 10B) and BMPR1A (Fig. 10C) mRNAs, confirming the notion that TNF-α negatively regulates BMP-4 expression and signaling.
Fig. 10.
Remicade ameliorates DSS-induced colitis and increases BMP-4 and BMPR1A mRNA expression. A: histological score calculated in DSS-treated mice in the presence and absence of Remicade (Rem). B and C: BMPR1A (B) and BMP-4 (C) mRNA abundance in DSS-treated mice in the presence or absence of Remicade was measured by QRT-PCR and displayed as fold change over controls. Values are shown as means ± SE. *P < 0.05.
We performed studies to better define the targets of BMP-4 in the human colonic epithelium. For these investigations we examined the effect of BMP-4 on the expression of IL-8, a chemokine that plays an important role in gastrointestinal inflammation (23, 26), using cultures of human colonoids derived from biopsies of normal human colonic mucosa. As indicated in Fig. 11, BMP-4 inhibited TNF-α-stimulated IL-8 expression in the human colonoids, underscoring the importance of BMP signaling in the response of the colonic epithelium to inflammatory stimuli.
Fig. 11.

Inhibition of basal and TNF-α-stimulated IL-8 gene expression by BMP-4 in human colonoids. IL-8 mRNA abundance in colonoids stimulated with TNF-α (10 ng/ml) in the presence or absence of BMP-4 (20 ng/ml) was measured by QRT-PCR. Values are shown as means ± SE, n = 3, where n represents the no. of separate individuals from whom the biopsies were obtained. *P < 0.05 vs. TNF-α-stimulated IL-8 gene expression.
DISCUSSION
In this manuscript we report a series of novel observations that underscore the importance of BMP signaling in the regulation of colonic homeostasis during the development of injury and inflammation. In particular, we present evidence that BMP signaling mitigates the response of the colonic epithelium to injury and inflammation and that cytokines, such as TNF-α and IL-1β, inhibit the expression of BMP-4.
Damage of the colonic epithelium is known to induce complex programs of cellular activation that lead to stimulation of stem cell proliferation and to tissue healing and regeneration (17, 18, 20). Recent reports have shown that fibroblasts are the site of production of peptides, such as Wnt5a and TGF-β, that regulate the process of wound healing and the reestablishment of intestinal epithelial homeostasis (20). In our study we show that, in the colonic mucosa, BMP-4 is predominantly expressed in α-SMA-positive mesenchymal cells located under and between glands. We also demonstrate that, after treatment with DSS, BMP-4-producing cells can be identified at the edge of areas of injury, in close contact with proliferating cells. It is therefore conceivable that BMP-4 might act in concert with TGF-β and Wnt5-a to regulate the process of tissue repair, contributing to the healing and restitution of the colonic mucosa. An attractive theory could be that BMP-4 controls the census and differentiation of colonic stem cells during the process of tissue regeneration and homeostasis. Another interesting possibility is that BMP signaling could contribute to the maintenance of the integrity of the intestinal epithelium. In support of this hypothesis, one study has shown that deletion of Smad5, a transducer of BMP-generated signals, leads to disruption of the apical junctional complex of the intestinal epithelium, suggesting that BMP signaling preserves the integrity of the epithelial barrier and that loss of this signaling mechanism contributes to the development of damage and inflammation (1). These observations are in agreement with our finding that inhibition of BMP signaling, achieved by deletion of Bmpr1a, causes an enhanced inflammatory response and more severe tissue damage.
In our studies we observed that, although the majority of BMP-generated signals target the epithelium, rare foci of X-gal-positive cells could be also seen in the mesenchyme. Although the significance of this observation is currently unclear, a recent study has shown that cells receiving BMP signals could be detected in the vasculature of embryonic mouse intestines (28). It is therefore possible that BMP signaling might target vascular structures and that it might be involved in the process of angiogenesis, a biological event that is crucial for the regeneration and healing of gastrointestinal epithelia. It is clear that additional studies will be necessary to further explore this intriguing possibility.
Exposure of the colonic epithelium to inflammatory stimuli is known to lead to the release of cytokines and chemokines, peptides known to play a prominent role in the induction and maintenance of mucosal damage (8, 23). IL-1β, TNF-α, IFN-γ, and IL-6 have all been shown to induce inflammation and tissue damage in several models of colonic injury, including that involving treatment with DSS (8, 23, 17, 18). An interesting finding of our study is that cytokines appear to have specific regulatory actions on the expression of BMP-4 in human intestinal fibroblasts. Interestingly, a previously published report conducted in neural cells in vitro has shown that TNF-α and IFN-γ stimulate the production of both BMP-2 and BMP-4 in astrocytes, but not in neural stem cells (14), indicating that cytokines can regulate the production of BMPs in some cell types, but not in others, during brain damage and inflammation. In addition, TNF-α has been shown to induce BMP-2 expression in chondrocytes (9) while it appears to inhibit the expression of BMP-4 in murine posterior eye cup explants (31), supporting the notion that cytokines regulate the expression of BMPs in a cell type-specific manner. Although our in vitro findings clearly show that IFN-γ stimulates the expression of BMP-4 in human fibroblasts, they do not allow us to establish if this effect has in vivo physiological and functional relevance. One attractive possibility is that IFN-γ-mediated induction of BMP-4 expression could represent a self-safe mechanism aimed at tampering the intensity of the inflammatory response. However, the observation that this effect is lost in the presence of both TNF-α and IL-1β suggests that the overall result of activation of inflammatory mechanisms in the colonic mucosa is inhibition of BMP-4 expression and signaling. The finding that Remicade increases the expression of both BMP-4 and BMPR1A in the colonic mucosa of DSS-treated mice appears to further support this hypothesis.
One additional significant finding of our manuscript is that induction of BMP signaling leads to inhibition of TNF-α-stimulated IL-8 production, a chemokine that plays a crucial role in the response of the epithelium to inflammatory stimuli (23, 26). The observation that this effect could be seen in normal human colonic epithelial cells underscores the relevance of these results in human pathophysiology.
In conclusion, our investigations propose the existence of a mechanism based on the release of a mesenchymal peptide, BMP-4, that specifically activates signaling pathways that exert inhibitory effects on the response of the colonic mucosa to injury and inflammation. Moreover, IL-1β- and TNF-α-mediated inhibition of BMP-4, a peptide that appears to exert anti-inflammatory effects, is likely to represent an important step for the development of inflammation and tissue damage. These findings underscore the importance of BMP signaling in the regulation of colonic inflammation and epithelial homeostasis, providing new clues for a better understanding of the pathophysiological mechanisms that lead to the development of inflammatory conditions of the colon. Additional investigations and possible manipulations of the BMP signal transduction pathway might therefore offer novel opportunities for the treatment of colonic inflammation.
GRANTS
This work was supported by National Institutes of Health (NIH) Grants RO1-DK-083373 (A. Todisco), by the University of Michigan Gastrointestinal Peptide Research Center (Grant P30-DK-34933), by NIH Grant CA-148828 (Y. M. Shah), by the Funderburg Award in Gastric Biology Related to Cancer (A. Todisco), from the Foundation for Digestive Health and Nutrition and by a Career Development Award from the Crohn's and Colitis Foundation (J. C. Brazil).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
T.J., H.T., M.M., X.H., X.X., and J.C.B. performed experiments; T.J., H.T., M.M., K.A.E., Y.M.S., and A.T. analyzed data; T.J., H.T., M.M., K.A.E., Y.M.S., and A.T. interpreted results of experiments; T.J., H.T., M.M., K.A.E., and A.T. prepared figures; T.J., H.T., M.M., X.H., X.X., J.C.B., K.A.E., Y.M.S., and A.T. approved final version of manuscript; M.M., J.C.B., K.A.E., Y.M.S., and A.T. edited and revised manuscript; A.T. conceived and designed research; A.T. drafted manuscript.
ACKNOWLEDGMENTS
We thank Kathy McClinchey for technical assistance.
REFERENCES
- 1.Allaire JM, Darsigny M, Marcoux SS, Roy SA, Schmouth JF, Umans L, Zwijsen A, Boudreau F, Perreault N. Loss of Smad5 leads to the disassembly of the apical junctional complex and increased susceptibility to experimental colitis. Am J Physiol Gastrointest Liver Physiol 300: G586–G597, 2011. [DOI] [PubMed] [Google Scholar]
- 2.Auclair BA, Benoit YD, Rivard N, Mishina Y, Perreault N. Bone morphogenetic protein signaling is essential for terminal differentiation of the intestinal secretory cell lineage. Gastroenterology 133: 887–896, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Beck SE, Jung BH, Fiorino A, Gomez J, Rosario ED, Cabrera BL, Huang SC, Chow JY, Carethers JM. Bone morphogenetic protein signaling and growth suppression in colon cancer. Am J Physiol Gastrointest Liver Physiol 291: G135–G145, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blank U, Seto ML, Adams DC, Wojchowski DM, Karolak MJ, Oxburgh L. An in vivo reporter of BMP signaling in organogenesis reveals targets in the developing kidney. BMC Dev Biol 8: 86, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blessing M, Nanney LB, King LE, Hogan BL. Chemical skin carcinogenesis is prevented in mice by the induced expression of a TGF-β related transgene. Teratog Carcinog Mutagen 15: 11–21, 1995. [DOI] [PubMed] [Google Scholar]
- 6.Bragdon B, Moseychuk O, Saldanha S, King D, Julian J, Nohe A. Bone morphogenetic proteins: a critical review. Cell Signal 23: 609–620, 2011. [DOI] [PubMed] [Google Scholar]
- 7.Brazil DP, Church RH, Surae S, Godson C, Martin F. BMP signalling: agony and antagony in the family. Trends Cell Biol 25: p249–264, 2015. [DOI] [PubMed] [Google Scholar]
- 8.Dieleman LA, Palmen MJ, Akol H, Bloemena E, Peña AS, Meuwissen SG, Van Rees EP. Chronic experimental colitis induced by dextran sulphate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin Exp Immunol 114: 385–391, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fukui N, Ikeda Y, Ohnuki T, Hikita A, Tanaka S, Yamane S, Suzuki R, Sandell LJ, Ochi T. Pro-inflammatory cytokine tumor necrosis factor-α induces bone morphogenetic protein-2 in chondrocytes via mRNA stabilization and transcriptional up-regulation. J Biol Chem 281: 27229–27241, 2006. [DOI] [PubMed] [Google Scholar]
- 10.Haramis AP, Begthel H, van den Born M, van Es J, Jonkheer S, Offerhaus GJ, Clevers H. De novo crypt formation and juvenile polyposis on BMP inhibition in mouse intestine. Science 303: 1684–1686, 2004. [DOI] [PubMed] [Google Scholar]
- 11.Hardwick JC, Van Den Brink GR, Bleuming SA, Ballester I, Van Den Brande JM, Keller JJ, Offerhaus GJ, Van Deventer SJ, Peppelenbosch MP. Bone morphogenetic protein 2 is expressed by, and acts upon, mature epithelial cells in the colon. Gastroenterology 126: 111–121, 2004. [DOI] [PubMed] [Google Scholar]
- 12.Kawabata M, Imamura T, Miyazono K. Signal transduction by bone morphogenetic proteins. Cytokine Growth Factor Rev 9: 49–61, 1998. [DOI] [PubMed] [Google Scholar]
- 13.Lawson KA, Dunn NR, Roelen BA, Zeinstra LM, Davis AM, Wright CV, Korving JP, Hogan BL. Bmp4 is required for the generation of primordial germ cells in the mouse embryo. Genes Dev 13: 424–436, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lü HZ, Hu JG. Expression of bone morphogenetic proteins-2/4 in neural stem cells and their lineages. Acta Neurobiol Exp 69: 441–447, 2009. [DOI] [PubMed] [Google Scholar]
- 15.Madison BB, Dunbar L, Qiao XT, Braunstein K, Braunstein E, Gumucio DL. Cis elements of the villin gene control expression in restricted domains of the vertical (crypt) and horizontal (duodenum, cecum) axes of the intestine. J Biol Chem 277: 33275–83, 2002. [DOI] [PubMed] [Google Scholar]
- 16.Mahe MM, Sundaram N, Watson CL, Shroyer NF, Helmrath MA. Establishment of human epithelial enteroids and colonoids from whole tissue and biopsy. J Vis Exp In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maric I, Poljak L, Zoricic S, Bobinac D, Bosukonda D, Sampath KT, Vukicevic S. Bone morphogenetic protein-7 reduces the severity of colon tissue damage and accelerates the healing of inflammatory bowel disease in rats. J Cell Physiol 196: 258–264, 2003. [DOI] [PubMed] [Google Scholar]
- 18.Maric I, Kucic N, Turk Wensveen T, Smoljan I, Grahovac B, Zoricic Cvek S, Celic T, Bobinac D, Vukicevic S. BMP signaling in rats with TNBS-induced colitis following BMP7 therapy. Am J Physiol Gastrointest Liver Physiol 302: G1151–G1162, 2012. [DOI] [PubMed] [Google Scholar]
- 19.Mishina Y, Hanks MC, Miura S, Tallquist MD, Behringer RR. Generation of Bmpr/Alk3 conditional knockout mice. Genesis 32: 69–72, 2002. [DOI] [PubMed] [Google Scholar]
- 20.Miyoshi H, Ajima R, Luo CT, Yamaguchi TP, Stappenbeck TS. Wnt5a potentiates TGF-β signaling to promote colonic crypt regeneration after tissue injury. Science 338: 108–113, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nitsche H, Ramamoorthy S, Sareban M, Pausawasdi N, Todisco A. Functional role of bone morphogenetic protein-4 in isolated canine parietal cells. Am J Physiol Gastrointest Liver Physiol 293: G607–G614, 2007. [DOI] [PubMed] [Google Scholar]
- 22.Peng XD, Wu XH, Chen LJ, Wang ZL, Hu XH, 
 Song LF, He CM, Luo YF, Chen ZZ, Jin K, Lin HG, Li XL, Wang YS, Wei YQ. Inhibition of phosphoinositide 3-kinase ameliorates dextran sodium sulfate-induced colitis in mice. J Pharm Exp Ther 332: 46–56, 2010. [DOI] [PubMed] [Google Scholar]
- 23.Perše M, Cerar A. Dextran sodium sulphate colitis mouse model: traps and tricks. J Biomed Biotechnol 718617: 1–13, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ, Clevers H. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459: 262–265, 2009. [DOI] [PubMed] [Google Scholar]
- 25.Shinohara M, Mao M, Keeley TM, El-Zaatari M, Lee HJ, Eaton KA, Samuelson LC, Merchant JL, Goldenring JR, Todisco A. Bone morphogenetic protein signaling regulates gastric epithelial cell development and proliferation in mice. Gastroenterology 139: 2050–2060, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takabayashi H, Shinohara M, Mao M, Phaosawasdi P, El-Zaatari M, Zhang M, Ji T, Eaton KA, Dang D, Kao J, Todisco A. Anti-inflammatory activity of bone morphogenetic protein signaling pathways in stomachs of mice. Gastroenterology 147: 396–406, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van den Brink GR, Hardwick JC, Tytgat GN, Brink MA, Ten Kate FJ, Van Deventer SJ, Peppelenbosch MP. Sonic hedgehog regulates gastric gland morphogenesis in man and mouse. Gastroenterology 121: 317–328, 2001. [DOI] [PubMed] [Google Scholar]
- 28.Walton KD, Whidden M, Kolterud Å, Shoffner SK, Czerwinski MJ, Kushwaha J, Parmar N, Chandhrasekhar D, Freddo AM, Schnell S, Gumucio DL. Villification in the mouse: Bmp signals control intestinal villus patterning. Development 143: 427–436, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang RN, Green J, Wang Z, Deng Y, Qiao M, Peabody M, Zhang Q, Ye J, Yan Z, Denduluri S, Idowu O, Li M, Shen C, Hu A, Haydon RC, Kang R, Mok J, Lee MJ, Luu HL, Shi LL. Bone morphogenetic protein (BMP) signaling in development and human diseases. Genes Dis 1: 87–105, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wirtz S, Neufert C, Weigmann B, Neurath MF. Chemically induced mouse models of intestinal inflammation. Nat Protoc 2: 541–546, 2007. [DOI] [PubMed] [Google Scholar]
- 31.Xu J, Zhu D, He S, Spee C, Ryan SJ, Hinton DR. Transcriptional regulation of bone morphogenetic protein 4 by tumor necrosis factor and its relationship with age-related macular degeneration. FASEB J 25: 2221–2231, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xue X, Ramakrishnan S, Anderson E, Taylor M, Zimmermann EM, Spence JR, Huang S, Greenson JK, Shah YM. Endothelial PAS domain protein 1 activates the inflammatory response in the intestinal epithelium to promote colitis in mice. Gastroenterology 145: 831–841, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou XP, Woodford-Richens K, Lehtonen R, Kurose K, Aldred M, Hampel H, Launonen V, Virta S, Pilarski R, Salovaara R, Bodmer WF, Conrad BA, Dunlop M, Hodgson SV, Iwama T, Järvinen H, Kellokumpu I, Kim JC, Leggett B, Markie D, Mecklin JP, Neale K, Phillips R, Piris J, Rozen P, Houlston RS, Aaltonen LA, Tomlinson IP, Eng C. Germline mutations in BMPR1A/ALK3 cause a subset of cases of juvenile polyposis syndrome and of Cowden and Bannayan-Riley-Ruvalcaba syndromes. Am J Hum Genet 69: 704–711, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]