Abstract
The impact of omeprazole (OM), a widely used over-the-counter proton pump inhibitor, on weight gain has not been extensively explored. We examined what factors, e.g., diet composition, microbiota, genetic strain, and sex, might affect weight gain in mice fed a high caloric diet while on OM. Inbred C57BL/6J strain, a 50:50 hybrid (B6SJLF1/J) strain, and mice on a highly mixed genetic background were fed four diets: standard chow (STD, 6% fat), STD with 200 ppm OM (STD + O), a high-energy chow (HiE, 11% fat), and HiE chow with OM (HiE + O) for 17 wk. Metabolic analysis, body composition, and fecal microbiota composition were analyzed in C57BL/6J mice. Oral glucose tolerance tests were performed using mice on the mixed background. After 8 wk, female and male C57BL/6J mice on the HiE diets ate less, whereas males on the HiE diets compared with the STD diets gained weight. All diet treatments reduced energy expenditure in females but in males only those on the HiE + O diet. Gut microbiota composition differed in the C57BL/6J females but not the males. Hybrid B6SJLF1/J mice showed similar weight gain on all test diets. In contrast, mixed strain male mice fed a HiE + O diet gained ∼40% more weight than females on the same diet. In addition to increased weight gain, mixed genetic mice on the HiE + O diet cleared glucose normally but secreted more insulin. We concluded that sex and genetic background define weight gain and metabolic responses of mice on high caloric diets and OM.
Keywords: acid suppression, metabolism, gastrin, energy expenditure, microbiota
omeprazole (OM) is an over-the-counter proton-pump inhibitor (PPI) used in the treatment of excess gastric acid and gastroesophageal reflux disease (GERD), whose long-term use has increased without medical indications (17). The incidence of GERD is highly correlated with obesity (18), and, as a result, most studies addressing the effects of chronic OM use include only overweight, obese, or type II diabetic patients (20, 28, 30–32). As a result, weight changes are typically not followed during these studies. A recent study found that PPI users had a higher body mass index that correlated with changes in gut microbiota composition (21). However, whether the changes in gut microbiota were associated with PPI use or body mass was not investigated. With respect to nonobese individuals, there are discrepancies between reports in humans and animal models. Yoshikawa et al. (45) reported that nonobese patients under chronic OM treatment developed undesired weight gain without dietary or lifestyle modifications during the study. By contrast, studies using rats (9) and chickens (15, 16) report that chronic treatment with OM results in less weight gain. More recently, OM was shown to enhance weight loss in exendin-4-treated db/db mice (34). Therefore OM treatment in animals has not correlated consistently with the weight gain effect more often observed in human subjects, raising questions regarding factors other than species differences contributing to OM-induced weight gain.
With obesity increasing worldwide, identifying factors that mediate weight gain and weight control is an urgent health problem, and, as such, research into understanding weight gain and obesity has spawned multiple clinical studies and various animal models. Diet-induced obesity by feeding mice high-fat diets is probably the most common model reported in the literature (29). However, high-fat diets may not be the most appropriate approach to inducing diet-induced obesity in humans because typical Western diets are high in both fat and carbohydrates (5). To better mimic human subjects consuming calorie-rich diets while on chronic OM treatment, we analyzed the effect of chronic OM in mice fed a nonpurified, grain-based high-energy (HiE) diet formulated with higher amounts of fat and carbohydrates that more closely reflect the calorie-rich diet typically consumed by individuals. Most studies utilize only male mice, and a wide variety of mouse strains have been used, each of which have different nutritional requirements that could influence the metabolic outcome when exposed to different diets (33). As a result, the effect of sex and genetic background on weight gain, diet-induced obesity, and other metabolic changes has not been carefully examined. Therefore, the goal of our study was to evaluate the effect of low-dose OM and a high-calorie diet on both female and male mice with different genetic backgrounds.
MATERIALS AND METHODS
Animals.
The experiments presented in this report were performed in animal facilities at the University of Michigan, Ann Arbor and the University of Minnesota, Saint Paul Campus under standard specific pathogen-free conditions. All animal procedures were approved and performed according to the University of Michigan Institutional Animal Care and Use Committee and the Institutional Animal Care and Use Committee of the University of Minnesota.
All C57BL/6J (stock no. 000664) and B6SJLF1/J heterozygous mice, which are an F1 50:50 mix of two inbred strains: C57BL/6J and SJL/J (stock no. 100012) mice, were purchased from Jackson Laboratory at 6–8 wk of age and randomly assigned to the test groups upon arrival. Mice of mixed genetic background were produced through multiple crosses of mice with different genetic backgrounds (FVB, SJL, C57BL/6) resulting in a mouse line previously reported to be phenotypically normal (38). Commercial genotype profiling of this mixed genetic strain (Charles River Laboratories) showed 68.8–77.9% C57BL/6, with ∼20% of the tested loci contributed by the129, SJL, Balb/c, FVB, and DBA/2 inbred strains. No single inbred match was found for 14 out of the 110 alleles (∼12%) tested.
Mice were fed LabDiet 5001 (Site 1) or Harlan 2018 diet (Site 2) upon arrival and were allowed to acclimate to the research facilities for 3–5 days before we started the experiments. All mice were 8–9 wk old at the start of experiments and were group housed, with the same number of mice per cage for females and males (3 or 4 animals/cage), except during the performance of the metabolic analysis when the mice were single housed for 10 days. Mice were fed the treatment diets for up to 20 wk and weighed weekly to assess changes in weight. Food intake was determined daily by subtracting the weight of residual feed from provided feed for 2 wk except during metabolic analysis, as described below. Mice were weighed weekly and euthanized at the end of the experiment. The day before euthanasia, fecal samples were collected for microbiota analysis. At the time of euthanasia, total body and epididymal fat pad weights were measured.
Diets.
Treatment diets were standard chow (STD, LabDiet 5001; TestDiet), high-energy diet (HiE, LabDiet 5015; TestDiet). OM-supplemented diets were prepared by the same manufacturer by adding 200 ppm of OM (0.02%) to the STD and HiE diets (STD + O and HiE + O, respectively). Nutritional profiles of the diets are shown in Table 1.
Table 1.
Nutritional profile of diets utilized in the study
| Standard | HiE | Change | |
|---|---|---|---|
| Protein | 23.90% | 18.90% | −5.00% |
| Fat (EE) | 5.00% | 10.70% | +5.70% |
| Fat (acid hydrolysis) | 6.00% | 11.30% | +5.30% |
| Fiber (max) | 5.50% | 2.50% | −3.00% |
| Ash | 7.00% | 5.80% | −1.20% |
| Nitrogen-free extract | 48.60% | 52.10% | +3.50% |
| Sugars | |||
| Starch | 22.44% | 33.13% | +10.69% |
| Glucose | 0.21% | 0.11% | −0.10% |
| Fructose | 0.28% | 0.11% | −0.17% |
| Sucrose | 3.74% | 0.90% | −2.84% |
| Lactose | 2.01% | 2.70% | +0.69% |
| Total digestible nutrients | 76.4% | 88.30% | +11.90% |
| Energy from | |||
| Protein | 28.50% | 19.90% | −8.60% |
| Fat (EE) | 13.40% | 25.30% | +11.90% |
| Carbohydrates | 58.00% | 54.80% | −3.20% |
| Total, kcal/g | 3.35 | 3.80 | +0.45 |
Data sourced from the diet's manufacturer. Energy: sum of decimal fractions of protein, fat, and carbohydrate × 4, 9, 4 kcal/g, respectively. EE, ether extract; STD, standard diet; HiE, high-energy diet.
Metabolic analysis.
Metabolic characterization was performed by the University of Michigan Animal Phenotyping Core. Oxygen consumption (V̇o2), carbon dioxide production (V̇co2), spontaneous motor activity, and food intake were measured using the Comprehensive Laboratory Monitoring System (CLAMS; Columbus Instruments). Mice were weighed each time before the measurements and placed individually into sealed chambers (7.9″ × 4″ × 5″) with free access to food and water. The study was carried out in an experimentation room set at 20–23°C with 12-h:12-h light/dark cycles (6:00 PM to ∼6:00 AM). The measurements were carried out continuously for 72 h. The amount of food eaten by each animal was monitored through a precision balance attached below the chamber. V̇o2 and V̇co2 in each chamber were sampled sequentially for 5 s in 10-min intervals, and the motor activity was recorded every second in x and z dimensions. The air-flow rate through the chambers was adjusted to keep the oxygen differential around 0.3% at resting conditions. The respiratory quotient (RQ), also known as the respiratory exchange ratio, was calculated as V̇co2/V̇o2. The percent relative cumulative frequency was used for analysis of V̇o2 and RQ as previously described (35). Body fat, lean mass, and free fluids were measured in live nonanesthetized mice using a TD-NMR analyzer (Minispec LF90II; Bruker Optics).
Oral glucose tolerance test.
Mice on the mixed genetic background were fasted for 5 h before glucose administration via oral gavage at a dose of 2.0 g/kg. Tail vein blood samples were collected before and after the gavage at 0, 15, 30, 60, and 120 min. Glucose was measured using a glucometer (Accu-check; Roche Diagnostics), and insulin levels were determined using a commercial ELISA kit (Linco; EMD Millipore).
Microbiome analysis.
Feces were collected for microbiome analysis at the end of the experiment directly from the mouse anus into a 1.5-ml tube and snap frozen. Genomic DNA was extracted from the feces using a modified protocol of the Qiagen DNeasy Blood and Tissue kit. These modifications included the following: 1) adding a bead-beating step using UltraClean fecal DNA bead tubes (Mo Bio Laboratories,) that were shaken using a Mini-Beadbeater-16 (BioSpec Products) for 1.5 min, 2) increasing the amount of ATL buffer used in the initial steps of the protocol (from 180 μl to 360 μl), 3) increasing the volume of proteinase K used (from 20 μl to 40 μl), and 4) decreasing the amount of AE buffer used to elute the DNA at the end of the protocol (from 200 μl to 85 μl).
Samples were submitted to the University of Michigan Host Microbiome Initiative and processed using the MiSeq Illumina platform. 16S rRNA gene libraries were constructed using primers specific to the V4 region. Mothur (http://www.mothur.org) (39) was used for analysis. Sequences were assigned to operational taxonomic units (OTUs) using a cut-off value of 0.03 and classified against the Ribosomal Database Project 16S rRNA gene training set (version 9) using a naive Bayesian approach with an 80% confidence threshold. Curated OTU sequence data were converted to relative abundance ± SE. Within-community diversity (α-diversity) was calculated using Species Richness and the Shannon diversity index (H′). Between-community diversity (β-diversity) was determined using the Yue and Clayton (θYC) distance metric. Nonmetric multidimensional scaling (NMDS) was used to ordinate the β-diversity data. An analysis of molecular variation (AMOVA) was utilized to test for significant differences between the centroids of the different groups (24). Mann-Whitney U-test was used to test for differences in α-diversity.
Statistical analysis.
Group differences for weight gain were determined by two-way ANOVA followed by Fisher's least significant difference test. Glucose and insulin levels were analyzed using one-way ANOVA followed by Tukey's test for multiple comparisons. Differences between the areas under the curve (AUC) were analyzed using the Mann-Whitney U-test. All other differences were determined by one-way ANOVA followed by the Kruskal-Wallis test. P ≤ 0.05 was considered significant. All analyses were performed using GraphPad Prism 7.00 (GraphPad Software). A posteriori power calculations were performed using G*Power 3.1.9.2 software (Kiel University) post hoc power analysis given α (0.05), sample size, and effect size calculated using the data from the experiments.
RESULTS
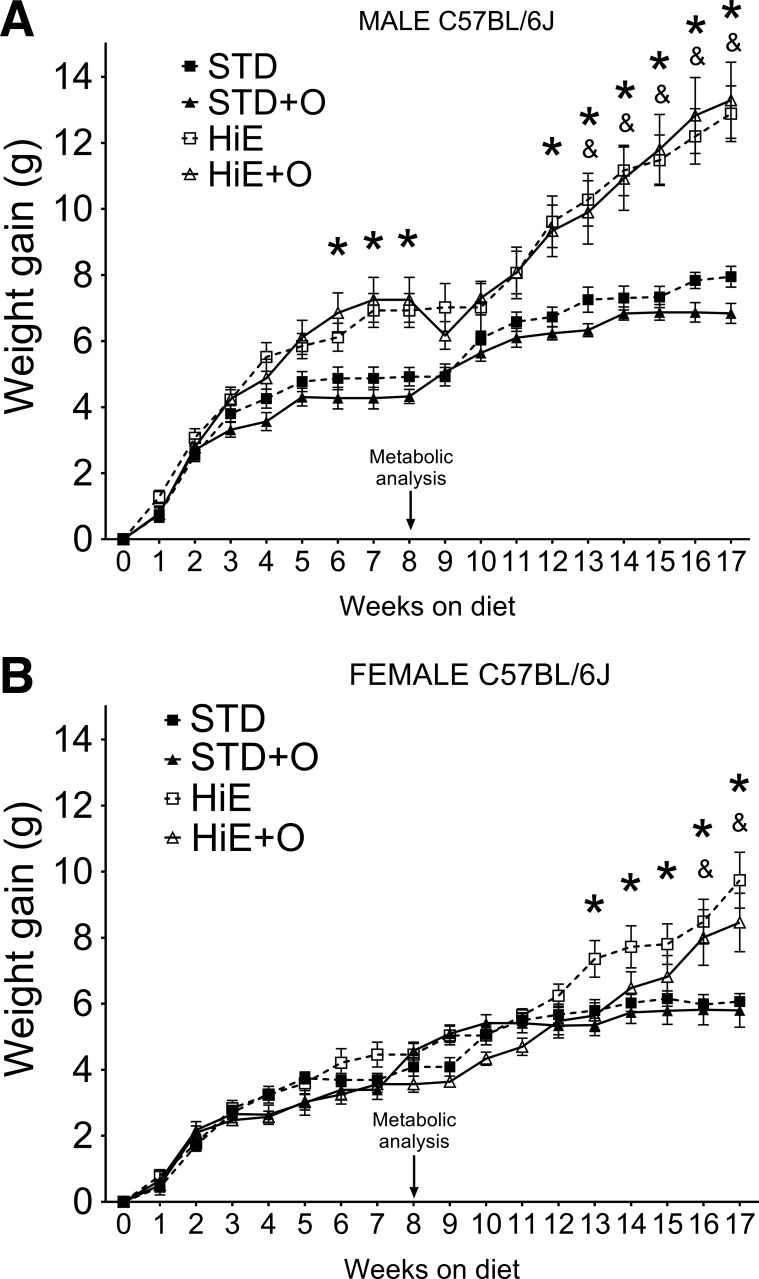
To determine whether mice on both an HiE diet and OM developed metabolic changes and eventually gained weight, C57BL/6J male and female mice were fed an STD diet or an HiE diet with and without OM (200 ppm). Six weeks after treatment initiation, male mice on the HiE + O diet showed significantly greater weight gain than mice on STD chow with or without OM (Fig. 1A). Metabolic analysis was performed at week 8 to identify early metabolic changes induced by the treatments. We observed that, once male mice were single housed for the metabolic analysis (Fig. 1A), they did not gain weight for about 2 wk once they were returned to cohousing status. Nevertheless, the male mice on either HiE diet regimen gained more weight than male mice on the STD diets even after they recovered from the metabolic analysis testing. Female mice on the HiE diet showed greater weight gain than females on all other diets after 12 wk, and females on HiE + O gained a similar amount of weight as females on HiE after 15 wk (Fig. 1B). The weight gain profiles clearly show that males were more responsive to a high-calorie diet than females. However, simultaneous OM treatment mice of either sex did not substantially affect their ability to gain weight on the HiE diets.
Fig. 1.
Weight gain of C57BL/6J mice fed a standard (STD) or a high-energy (HiE) diet with or without omeprazole supplementation (STD + O and HiE + O, respectively). A: male mice. B: female mice. Data presented are means ± SE. n = 8 per group. *P ≤ 0.05 vs. the STD, &P ≤ 0.05 vs. the STD + O diet.
After 8 wk on the diets, female mice on all diets exhibited similar fat and lean mass composition consistent with the lack of a significant increase in body weight (Table 2). However, male mice on the HiE + O diet significantly gained fat and lost lean mass when compared with males on STD and STD + O diets (Table 2). Although males on the HiE diet showed quantitative increases in fat and lean mass, they were not significantly different from males on the STD (P = 0.13 and P = 0.06 for lean and fat, respectively) or STD + O diets (P = 0.23 and P = 0.15 for lean and fat, respectively). Thus NMR analysis of body composition showed that, in male mice, OM promoted adiposity when combined with an HiE diet (Table 2).
Table 2.
Body composition of C57BL/6J mice after 8 wk of treatment
| Group | % Fat | % Lean |
|---|---|---|
| Female | ||
| STD | 6.50 ± 1.06 | 76.13 ± 1.98 |
| STD + O | 6.56 ± 1.66 | 75.81 ± 1.87 |
| HiE | 6.86 ± 2.09 | 75.09 ± 1.78 |
| HiE + O | 6.36 ± 1.89 | 75.65 ± 2.06 |
| Male | ||
| STD | 5.15 ± 0.96 | 76.81 ± 1.08 |
| STD + O | 5.60 ± 1.90 | 76.49 ± 2.36 |
| HiE | 8.78 ± 4.03 | 73.48 ± 4.02 |
| HiE + O | 10.40 ± 3.99* | 71.65 ± 3.71* |
Data are means ± SD; n = 8 mice per group.
Significantly different compared with STD and STD with omeprazole supplementation (STD + O).
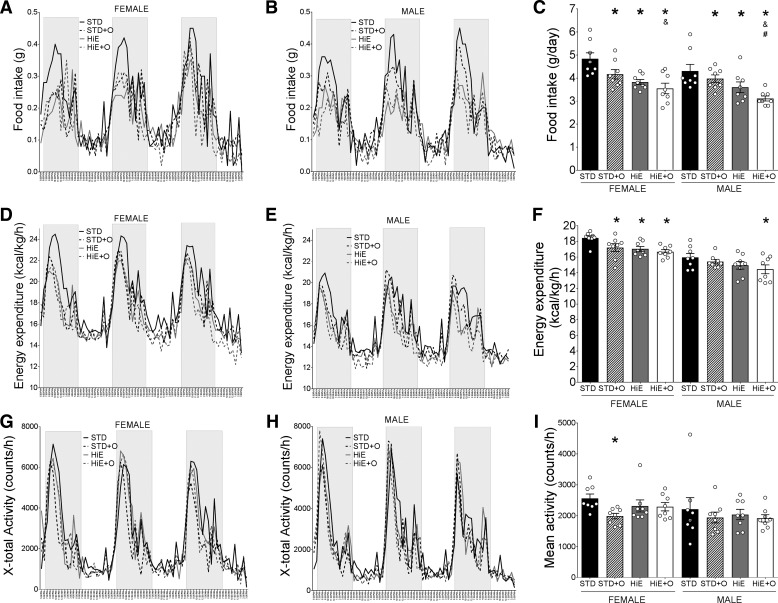
To identify the metabolic changes associated with weight gain and adiposity, we evaluated food intake, energy expenditure, activity, and the RQ of all mice after 8 wk on the treatment diets. Female and male mice on the same diets ate similar amounts (Fig. 2, A–C), and mice on the STD diet had a higher food intake than mice on all other diets (Fig. 2C), suggesting that the increased weight observed in males on the HiE diets was not due to increased food intake. Rather, mice of both sexes on the HiE diets restricted their food intake (Fig. 2C), which raised the issue of what metabolic factors might explain the sex differences in weight gain. Overall, energy expenditure was higher in females than males on the same diets (Fig. 2, D and E). Although females on any of the diets had a significantly lower energy expenditure than females on the STD diets (Fig. 2, D and F), only males on the HiE + O diet showed reduced energy expenditure compared with males on the STD diet (Fig. 2, E and F). We found no differences in the mean activity of females and males on the same diets or between mice of the same sex on different diets. The one exception was for the females on the STD + O diet, which showed less activity compared with females on the STD diet (Fig. 2, G–I). As expected, respiratory quotients were reduced on the HiE diets, with no effect of OM supplementation or sex (data not shown).
Fig. 2.
Metabolic analysis of C57BL/6J mice after 8 wk fed an STD or an HiE diet with or without omeprazole supplementation. Hourly food intake of female (A) and male (B) mice and daily average food intake (C) are shown. Hourly and average (F) energy expenditure of female (D) and male (E) mice are shown. Hourly and average (I) activity of female (G) and male (H) mice are shown. Data presented in A, B, D, E, G and H are means, bars in C, F, and I are means ± SE. n = 8 per group. *P ≤ 0.05 compared with the STD diet, &P ≤ 0.05 compared with the STD + O diet, #P ≤ 0.05 compared with the HiE diet.
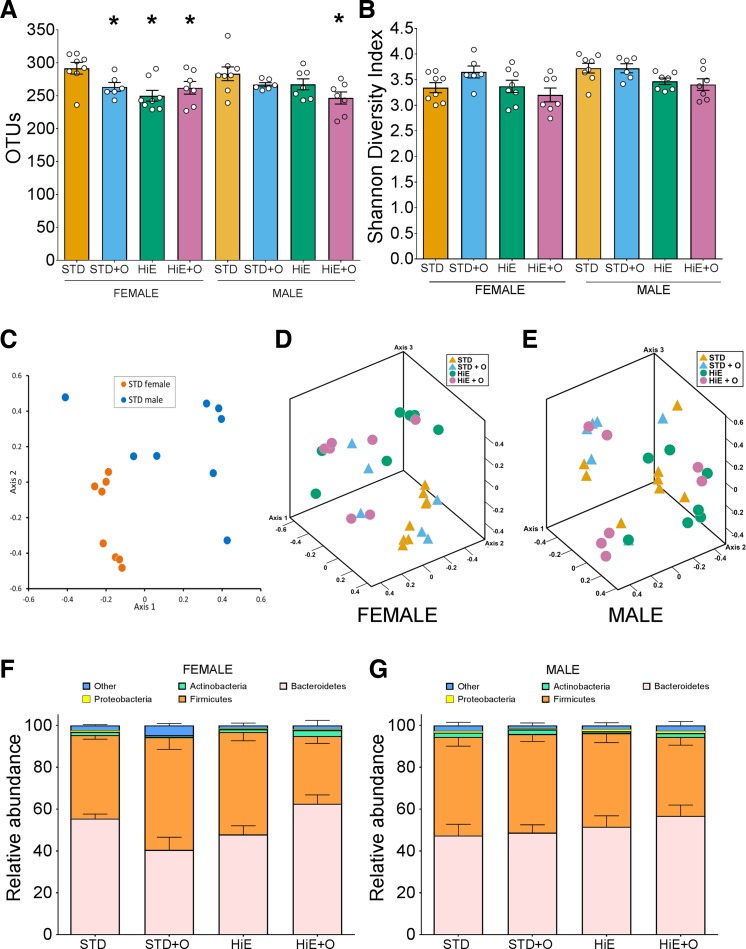
Changes in the gut microbiota have been associated with changes in diet (4), OM treatment (37), and weight control (1, 22, 42). Therefore, to assess whether there was an effect of our treatments on the gut microbiota, we analyzed the community structures and found that the bacterial composition between females and males was significantly different (Fig. 3C, AMOVA, P < 0.001). When analyzed for sex and diet, the OTU richness in female mice on the STD diet was higher than in females on all the other supplemented diets, whereas only males on the HiE + O diet showed a reduced number of OTUs compared with males on the STD diets (Fig. 3A). Although the microbial diversity of both females and males was not different between the different diets (Fig. 3B), the microbial community structures in the female groups were significantly different in STD + O (P = 0.026), HiE (P = 0.001), and HiE + O (P = 0.001) compared with the STD diet based on the NMDS plots of β-diversity (Fig. 3, D and F). No differences in community structures were observed in male mice (Fig. 3, E and G). These observations suggested that diet and OM altered the microbiome in the female but not in male mice (Fig. 3, E and F). Whether the changes in the microbial community structure of female mice contributed to the ability to maintain their weight requires further investigation.
Fig. 3.
Fecal microbiota richness (A) and diversity (B) in female and male C57BL/6J mice fed treatment diets for 8 wk. Nonmetric multidimensional scaling plots, using θYC, of gut microbial communities in females and males on STD diet (stress = 0.08, r2 = 0.95, C) as well as female (stress = 0.11, r2 = 0.9, D) and male mice (stress = 0.14, r2 = 0.82, E) on the treatment diets are shown. Major bacterial phyla compositions of female (F) and male (G) mice fed an STD or an HiE diet with or without omeprazole supplementation are shown. Data presented are means ± SE, n = 8 per group. *P ≤ 0.05 compared with the STD diet.
To evaluate whether the pattern of weight gain in our study was affected by the stress associated with single housing and transportation to the metabolic facilities for analysis, as was reported in another study (10), we performed a second independent experiment under the same conditions except that mice did not undergo metabolic analysis at week 8 and were not single housed. In the second experiment, females on the HiE + O diet gained more weight than females on the STD and STD + O after 8 wk (7.52 ± 3.14 g compared with 3.03 ± 0.91 g and 3.71 ± 0.89 g, respectively, P ≤ 0.001), and females on the HiE diet gained more weight (6.52 ± 2.08 g) (P ≤ 0.01) than females on the STD (3.23 ± 0.30 g) and STD + O (3.78 ± 0.58 g) diets after 10 wk. In the second experiment, by week 15, females on HiE and HiE + O diets (10.45 ± 3.65 g and 10.84 ± 2.65 g, respectively) gained more weight (P ≤ 0.001) than females in the first experiment (HiE: 7.81 ± 1.74 g, HiE + O: 6.82 ± 1.82 g), whereas females on STD and STD + O gained similar weight in both experiments. In males, the only difference found between both experiments was that males on the HiE and HiE + O energy diets in the second experiment showed significantly higher weight gain compared with males on STD diet at week 9, a week later than observed in the first experiment. These observations suggested that female mice might be more susceptible to stress induced by single housing and transportation than male mice.
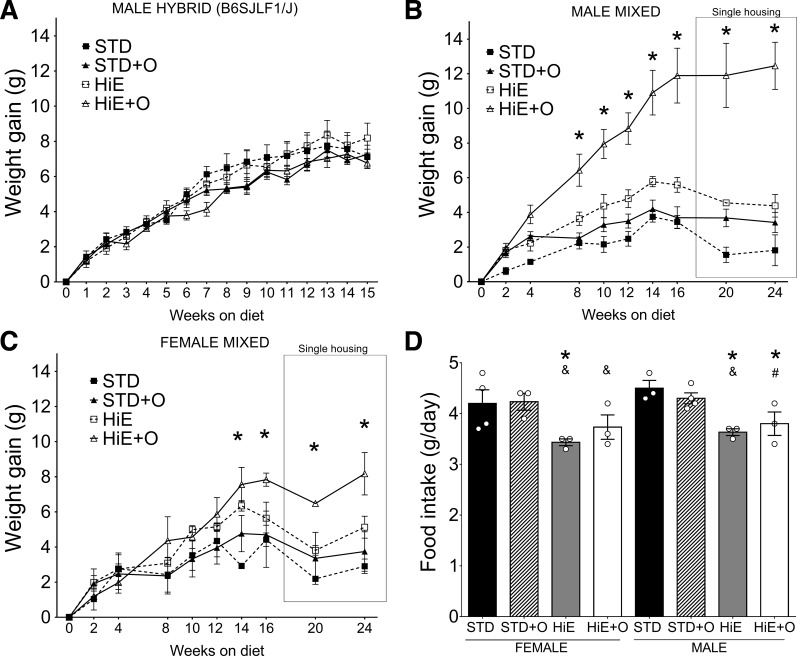
It is well recognized that genetic background can affect phenotype development in different animal models (11, 12, 25, 41, 43). Human populations are genetically mixed; therefore, we evaluated the role of genetic background to treatment diets in two different groups of genetically mixed mice. We fed our treatment diets to mice heterozygous for B6 and SJL alleles at all loci (B6SJLF1/J, JAX stock no. 100012) for 15 wk. In contrast to C57BL/6J mice, the hybrid mice did not show differences in weight gain on our test diets (Fig. 4A). We also tested a group of mice with a highly mixed genetic background. Both females and males on this mixed background showed significant weight gain when fed the HiE + O diet compared with all other diets (Fig. 4, B and C, post hoc power 0.97), a response that was not observed with mice on the pure C57BL/6J or the B6/SJL heterozygous mice. The weight gain observed in the males fed HiE + O (Fig. 4B) was significantly higher than that of females (Fig. 4C) and similar to what males on the C57BL/6J pure background gained on the HiE diets (Fig. 1A), suggesting a possible genetic component that mediates susceptibility to the HiE + O diet that is different between the inbred C57BL/6J, hybrid, and highly mixed mice strains. Females on pure C57BL/6J background in our second experiment (without metabolic analysis on week 8) gained as much weight as the males on the same diets. In contrast, by week 16, genetically mixed females on HiE + O gained 7.83 ± 0.648 g, whereas males gained 11.89 ± 2.74 g, ∼40% more than females, suggesting that, depending on genetic background, sex is also an important factor affecting weight gain. After 17 wk on the diets, single-housed genetically mixed mice showed no further weight gain in females and males, perhaps attributable to changes in energy expenditure to maintain body temperature (27) and other undetermined factors. The food intake of these highly mixed genetic mice was similar to that of all other strains studied (Figs. 2C and 4D). Although food intake was reduced in both mixed females and males on HiE and HiE + O diets, it was not different between females and males on the mixed background (Fig. 4D).
Fig. 4.
A: weight gain of hybrid B6SJLF1/J male on the test diets, n = 6 per group. Weight gain of male (B) and female (C) mice in a mixed genetic background fed an STD or an HiE diet with or without omeprazole supplementation is shown; n = 4 per group except for n = 3 females in HiE diet. D: daily food intake of female and male mice on mixed genetic background for the test diets. Bars represent means ± SE. *P ≤ 0.05 compared with the STD diet, &P ≤ 0.05 compared with STD + O diet, #P ≤ 0.05 compared with the HiE diet.
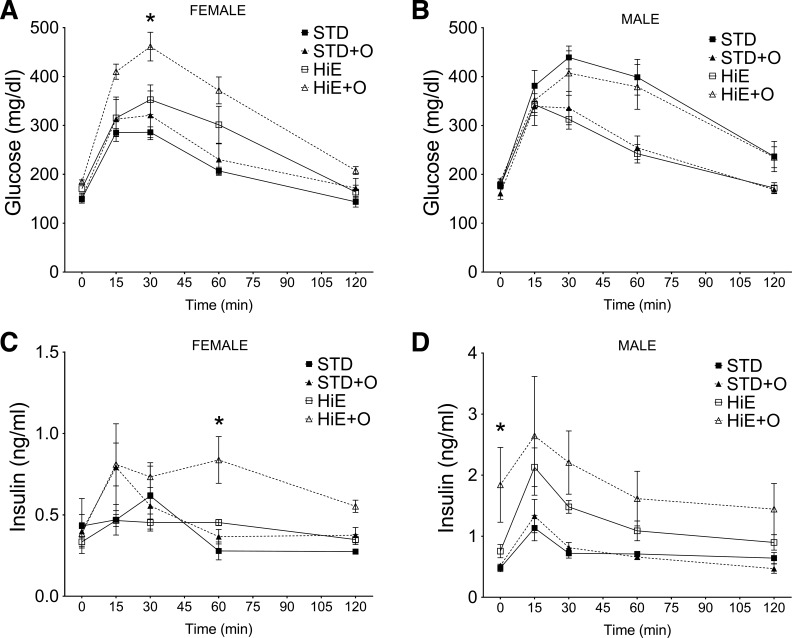
To define whether the weight gain in these genetically mixed mice showed any indicators of the metabolic syndrome, we performed oral glucose tolerance tests after 16 wk. All mice cleared glucose after 120 min (Fig. 5, A and B); however, the AUC for the glucose levels of females on the HiE + O diet was significantly higher than that of females on the STD and STD + O diets (P = 0.014 and P = 0.036, post hoc power 0.95 and 0.41, respectively). The AUC for glucose levels of males on a STD diet was higher than STD + O and HiE (P = 0.028 and P = 0.019, post hoc power 0.48 and 0.51, respectively) but not different from that of mice on the HiE + O diet. Insulin levels were significantly higher for males compared with females on both the HiE and HiE + O diets (P = 0.015 and P = 0.001, post hoc power 0.22 and 0.14, respectively) (Fig. 5, C and D). In females, only the HiE + O diet induced higher insulin levels compared with the STD diet (AUC, P = 0.048, post hoc power 0.52) (Fig. 5C). In contrast, males showed significantly higher AUC for insulin when on the HiE diet compared with the STD (P = 0.005, post hoc power 0.63) and showed a higher trend when on the HiE + O (P = 0.072). Of note, the basal level of insulin in the male mice on the HiE + O diet was significantly higher than that of mice on the STD (P = 0.029, post hoc power 0.33) or STD + O (P = 0.047, post hoc power 0.47) diets, further supporting that genetically mixed male mice on the HiE + O diet become obese and are prone to develop insulin resistance. After 24 wk on the experimental diets, genetically mixed female mice on the HiE (1.66 ± 0.97% of body mass) and HiE + O (3.14 ± 0.87% of body mass) diets showed larger epididymal fat pads than mice on STD and STD + O diets (0.95 ± 0.11% and 0.99 ± 0.38% of body mass, respectively), which were only statistically different between the STD and HiE + O diet (P = 0.008, post hoc power 0.89). In males, the epididymal fat pad of mice on the HiE (2.43 ± 0.64% of body mass) was not statistically greater than that of mice on the STD and STD + O diets (1.54 ± 0.24% and 1.72 ± 0.24% of body mass, respectively), whereas mice on the HiE + O (4.81 ± 0.97% of body mass) were statistically different than mice on the STD (P < 0.0001, post hoc power 0.98), STD + O (P = 0.0002, post hoc power 0.96), and HiE (P = 0.0036, post hoc power 0.71), further supporting the observations that OM combined with an HiE diet induced more adiposity in females and males of a mixed genetic background.
Fig. 5.
Glucose clearance and insulin levels observed in female (A and C) and male (B and D) mice on a mixed genetic background fed an STD or an HiE diet with or without omeprazole supplementation for 16 wk administered an oral glucose tolerance test; n = 4 per group except for n = 3 females on an HiE diet. Data presented are means ± SE. *P ≤ 0.05 compared with the STD diet.
DISCUSSION
In this study, we found that genetic background and sex are the major factors involved in the weight gain of mice fed HiE diets, with female mice being more resistant to weight gain than male mice when in a mixed genetic background. OM induced significant weight gain in both male and female mice only on a mixed genetic background, supporting a strong genetic contribution in the response to the drug.
We observed that sex was a factor that strongly affected weight gain. Female mice ate similar amounts of food as the male mice; however, they maintained a higher energy expenditure level when on a pure genetic background (C57BL/6J). Female and male mice on mixed genetic backgrounds gained more weight on the HiE + O diet although not to the same extent. Thus both sexes responded to the HiE + O diet by gaining weight once the contribution from other strains reached at least 30% but was not observed when the genetic background was completely inbred (100% C57BL/6J) or was a simple hybrid of two strains (50% each of C57BL/6J and SJL). Like many other animals, mice adjust food intake to their energy requirements (7, 23, 26), which might explain the reduced food intake of mice on the HiE diets. Interestingly, there was a significant effect of OM reducing food intake in both female and male C57BL/6J mice, which was not observed in the genetically mixed mice, suggesting a role for OM in food intake regulation that depends on the genetic background and not the sex. Previously, OM was reported to exhibit an inhibitory effect on food intake in calves (13, 14). A study in human volunteers who received a single dose of OM before eating a test meal were less hungry over the experimental period (6). Our results further suggest that this reduction in food intake may involve a genetic component because we did not observe this effect in mice on the mixed genetic background.
Our observations concur with previous studies that demonstrated sex differences in feeding behavior, activity, and weight gain in mice, with females showing changes not observed in males (2, 44). In a recent report by Horakova et al. (19), female and male C57BL/6 mice gained weight to the same extent and had similar responses to intraperitoneal glucose administration and insulin levels after 10 wk on a high-fat diet. One possible explanation for the differences in our results and their study might be that their mice were internally bred, whereas we purchased mice from a commercial vendor. Another significant difference between the two studies is the composition of the diets utilized. The Horakova study used a high-fat diet with 60% of calories from fat, whereas we used an HiE diet with only 25% fat and ∼55% carbohydrates.
There are only a limited number of published reports that use animal models of chronic OM treatment. Some of those studies show that doses higher than 40 mg/kg per day of OM for >2 wk are needed to suppress gastric acidity and induce hypergastrinemia in mice and dogs (3, 8, 36). Treating with OM at 200 ppm in STD chow is a relatively low dose, yet we found the dose sufficient to significantly suppress gastric acidity and increase plasma gastrin levels two- to threefold in mice fed an STD diet for 6 mo (40) or a high-fat diet (60% calories from fat) for 3 wk without OM (38). On that basis, we queried whether an HiE diet (12% higher energy from fat than STD chow) combined with OM would more dramatically effect gastric acid and gastrin secretion but did not observe significant differences in this study (data not shown), suggesting that higher dietary levels of fat and OM appear to modulate weight gain through independent mechanisms. Specifically, we show in the present study that sex and genetic background are the variables that exert a greater influence over diet-dependent weight gain on OM.
It is well established that mouse strains have different nutritional requirements that should be taken into consideration to formulate mouse diets for research. We verified that the diets used in this study were supplemented to cover and exceed the National Research Council requirements (33) for all of the strains and that our mice received adequate amounts of nutrients when adjusted for food intake. Another study reported that there is a clear difference in weight gain, adipose tissue deposition, and nonalcoholic fatty liver disease in mice on a 100% inbred genetic background when fed high-fat diets (11). Unfortunately only males were used in the study. Nevertheless, they found the C57BL/6 and CD-1 mice had the greatest weight gain on the high-fat diet compared with the 129Sv strain. The 129Sv strain exhibited more consistent fatty liver changes despite minimal weight gain, consistent with a significant contribution from genetic background. Overall our results underscore the importance of utilizing females and males in physiological studies because sex is an important factor, not only in defining weight gain, but also in energy utilization and gut microbiome composition.
In conclusion, we show here that responses to diet in mice depend on genetic background, underscoring the importance of using appropriate controls when using mouse models in those studies involving genetically modified mice. Lack of consistent genetic background as controls can lead to low reproducibility and inaccurate conclusions. Nevertheless, defining specific genetic variants that contribute to differential responses in weight gain while on OM will require further investigation.
GRANTS
This study was supported by Michigan Gastrointestinal Peptide Research Center NIDDK 2P30 DK034933 (M. Saqui-Salces), NIH/NIDDK P01 DK062041 (J. Merchant), P30 DK097153 (UMICH Metabolomics Core), and 5P30 DK034933-Microbiome Core.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.S.-S. and A.C.T. performed experiments; M.S.-S., A.C.T., and M.G.G. analyzed data; M.S.-S. and J.L.M. interpreted results of experiments; M.S.-S. and M.G.G. prepared figures; M.S.-S. drafted manuscript; M.S.-S., M.G.G., and J.L.M. edited and revised manuscript; M.S.-S., A.C.T., M.G.G., and J.L.M. approved final version of manuscript.
REFERENCES
- 1.Annalisa N, Alessio T, Claudette TD, Erald V, Antonino DL, Nicola DD. Gut microbioma population: An indicator really sensible to any change in age, diet, metabolic syndrome, and life-style. Mediators Inflamm 2014: 901308, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arndt SS, Laarakker MC, van Lith HA, van der Staay FJ, Gieling E, Salomons AR, van't Klooster J, Ohl F. Individual housing of mice—impact on behaviour and stress responses. Physiol Behav 97: 385–93, 2009. [DOI] [PubMed] [Google Scholar]
- 3.Betton GR, Dormer CS, Wells T, Pert P, Price CA, Buckley P. Gastric ECL-cell hyperplasia and carcinoids in rodents following chronic administration of H2-antagonists SK&F 93479 and oxmetidine and omeprazole. Toxicol Pathol 16: 288–98, 1988. [DOI] [PubMed] [Google Scholar]
- 4.Bolnick DI, Snowberg LK, Hirsch PE, Lauber CL, Org E, Parks B, Lusis AJ, Knight R, Caporaso JG, Svanbäck R. Individual diet has sex-dependent effects on vertebrate gut microbiota. Nat Commun 5: 4500, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borghjid S, Feinman RD. Response of C57Bl/6 mice to a carbohydrate-free diet. Nutr Metab (Lond) 9: 69, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cecil JE, Francis J, Read NW. Investigation into the role of cephalic stimulation of acid secretion on gastric emptying and appetite following a soup meal using the gastric acid inhibitor omeprazole. Appetite 42: 99–105, 2004. [DOI] [PubMed] [Google Scholar]
- 7.Chee KM, Romsos DR, Bergen WG. Effect of dietary fat on protein intake regulation in young obese and lean mice. J Nutr 111: 668–77, 1981. [DOI] [PubMed] [Google Scholar]
- 8.Cowan A, Earnest DL, Ligozio G, Rojavin MA. Omeprazole-induced slowing of gastrointestinal transit in mice can be countered with tegaserod. Eur J Pharmacol 517: 127–131, 2005. [DOI] [PubMed] [Google Scholar]
- 9.Cui GL, Syversen U, Zhao CM, Chen D, Waldum HL. Long-term omeprazole treatment suppresses body weight gain and bone mineralization in young male rats. Scand J Gastroenterol 36: 1011–1015, 2001. [DOI] [PubMed] [Google Scholar]
- 10.Faulk C, Barks A, Sánchez BN, Zhang Z, Anderson OS, Peterson KE, Dolinoy DC. Perinatal lead (Pb) exposure results in sex-specific effects on food intake, fat, weight, and insulin response across the murine life-course. PLoS One 9: e104273, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fengler VH, Macheiner T, Kessler SM, Czepukojc B, Gemperlein K, Müller R, Kiemer AK, Magnes C, Haybaeck J, Lackner C, Sargsyan K. Susceptibility of different mouse wild type strains to develop diet-induced NAFLD/AFLD-associated liver disease. PLoS One 11: e0155163, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Finn R, Evans CC, Lee L. Strain-dependent brain defects in mouse models of primary ciliary dyskinesia with mutations in Pcdp1 and Spef2. Neuroscience 277: 552–567, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fox M, Uche U, Vaillant C, Ganabadi S, Calam J. Effects of Ostertagia ostertagi and omeprazole treatment on feed intake and gastrin-related responses in the calf. Vet Parasitol 105: 285–301, 2002. [DOI] [PubMed] [Google Scholar]
- 14.Fox MT, Gerrelli D, Shivalkar P, Jacobs DE. Effect of omeprazole treatment on feed intake and blood gastrin and pepsinogen levels in the calf. Res Vet Sci 46: 280–282, 1989. [PubMed] [Google Scholar]
- 15.Gagnemo-Persson R, Håkanson R, Sundler F, Persson P. Growth of the parathyroid glands in omeprazole-treated chickens. Scand J Gastroenterol 29: 493–497, 1994. [DOI] [PubMed] [Google Scholar]
- 16.Gagnemo-Persson R, Samuelsson A, Hâkanson R, Persson P. Chicken parathyroid hormone gene expression in response to gastrin, omeprazole, ergocalciferol, and restricted food intake. Calcif Tissue Int 61: 210–215, 1997. [DOI] [PubMed] [Google Scholar]
- 17.Haastrup PF, Paulsen MS, Christensen RD, Søndergaard J, Hansen JM, Jarbøl DE. Medical and non-medical predictors of initiating long-term use of proton pump inhibitors: A nationwide cohort study of first-time users during a 10-year period. Aliment Pharmacol Ther 44: 78–87, 2016. [DOI] [PubMed] [Google Scholar]
- 18.Hampel H. Meta-analysis: Obesity and the risk for gastroesophageal reflux disease and its complications. Ann Intern Med 143: 199, 2005. [DOI] [PubMed] [Google Scholar]
- 19.Horakova O, Hansikova J, Bardova K, Gardlo A, Rombaldova M, Kuda O, Rossmeisl M, Kopecky J. Plasma acylcarnitines and amino acid levels as an early complex biomarker of propensity to high-fat diet-induced obesity in mice. PLoS One 11: e0155776, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hove KD, Færch K, Bödvarsdóttir TB, Karlsen AE, Petersen JS, Vaag A. Treatment with a proton pump inhibitor improves glycaemic control in type 2 diabetic patients—a retrospective analysis. Diabetes Res Clin Pract 90: e72–e74, 2010. [DOI] [PubMed] [Google Scholar]
- 21.Jackson MA, Goodrich JK, Maxan ME, Freedberg DE, Abrams JA, Poole AC, Sutter JL, Welter D, Ley RE, Bell JT, Spector TD, Steves CJ. Proton pump inhibitors alter the composition of the gut microbiota. Gut 65: 749–756, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Joyce AS, MacSharry J, Casey PG, Kinsella M, Murphy EF, Shanahan F, Hill C, Gahan CGM. Regulation of host weight gain and lipid metabolism by bacterial bile acid modification in the gut. Proc Natl Acad Sci USA 111: 7421–7426, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koza RA, Nikonova L, Hogan J, Rim JS, Mendoza T, Faulk C, Skaf J, Kozak LP. Changes in gene expression foreshadow diet-induced obesity in genetically identical mice. PLoS Genet 2: e81, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kozich JJ, Westcott SL, Baxter NT, Highlander SK, Schloss PD. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Appl Environ Microbiol 79: 5112–5120, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lawson GW, Van Winkle LS, Toskala E, Senior RM, Parks WC, Plopper CG. Mouse strain modulates the role of the ciliated cell in acute tracheobronchial airway injury-distal airways. Am J Pathol 160: 315–327, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin PY, Romsos DR, Leveille GA. Food intake, body weight gain, and body composition of the young obese (ob/ob) mouse. J Nutr 107: 1715–1723, 1977. [DOI] [PubMed] [Google Scholar]
- 27.Lin PY, Romsos DR, Vander Tuig JG, Leveille GA. Maintenance energy requirements, energy retention and heat production of young obese (ob/ob) and lean mice fed a high-fat or a high-carbohydrate diet. J Nutr 109: 1143–1153, 1979. [DOI] [PubMed] [Google Scholar]
- 28.Lundell L, Miettinen P, Myrvold HE, Hatlebakk JG, Wallin L, Engström C, Julkunen R, Montgomery M, Malm A, Lind T, Walan A. Comparison of outcomes twelve years after antireflux surgery or omeprazole maintenance therapy for reflux esophagitis. Clin Gastroenterol Hepatol 7: 1292–1298; quiz 1260, 2009. [DOI] [PubMed] [Google Scholar]
- 29.Lutz TA, Woods SC. Overview of animal models of obesity. Curr Protoc Pharmacol 5: 5.61, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mefford IN, Mefford JT, Burris AC. Improved diabetes control and pancreatic function in a type 2 diabetic after omeprazole administration. Case Rep Endocrinol 2012: 1–4, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meineche-Schmidt V. Empiric treatment with high and standard dose of omeprazole in general practice: Two-week randomized placebo-controlled trial and 12-mo follow-up of health-care consumption. Am J Gastroenterol 99: 1050–1058, 2004. [DOI] [PubMed] [Google Scholar]
- 32.Nagahara A, Hojo M, Asaoka D, Sasaki H, Watanabe S. A randomized prospective study comparing the efficacy of on-demand therapy versus continuous therapy for 6 mo for long-term maintenance with omeprazole 20 mg in patients with gastroesophageal reflux disease in Japan. Scand J Gastroenterol 49: 409–417, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.National Research Council. Nutrient Requirements of Laboratory Animals, 4th ed. Atlanta, GA: National Academies, 1995. [Google Scholar]
- 34.Patel V, Joharapurkar A, Gandhi T, Patel K, Dhanesha N, Kshirsagar S, Dhote V, Detroja J, Bahekar R, Jain M. Omeprazole improves the anti-obesity and antidiabetic effects of exendin-4 in db/db mice (−4 db/db)*. J Diabetes 5: 163–171, 2013. [DOI] [PubMed] [Google Scholar]
- 35.Riachi M, Himms-Hagen J, Harper ME. Percent relative cumulative frequency analysis in indirect calorimetry: application to studies of transgenic mice. Can J Physiol Pharmacol 82: 1075–1083, 2004. [DOI] [PubMed] [Google Scholar]
- 36.Säfholm C, Havu N, Forssell H, Sundell G, Mattsson H. Effect of 7 years' daily oral administration of omeprazole to beagle dogs. Digestion 55: 139–147, 1994. [DOI] [PubMed] [Google Scholar]
- 37.Sands SA, Tsau S, Yankee TM, Parker BL, Ericsson AC, LeVine SM. The effect of omeprazole on the development of experimental autoimmune encephalomyelitis in C57BL/6J and SJL/J mice. BMC Res Notes 7: 605, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saqui-Salces M, Dowdle WE, Reiter JF, Merchant JL. A high-fat diet regulates gastrin and acid secretion through primary cilia. FASEB J 26: 3127–3139, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, Lesniewski RA, Oakley BB, Parks DH, Robinson CJ, Sahl JW, Stres B, Thallinger GG, Van Horn DJ, Weber CF. Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol 75: 7537–7541, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sundaresan S, Kang AJ, Hayes MM, Choi EYK, Merchant JL. Deletion of Men1 and somatostatin induces hypergastrinemia and gastric carcinoids. Gut. gutjnl-2015-310928. doi: 10.1136/gutjnl-2015-310928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sundberg JP, Berndt A, Sundberg BA, Silva KA, Kennedy V, Smith RS, Cooper TK, Schofield PN. Approaches to investigating complex genetic traits in a large-scale inbred mouse aging study. Vet Pathol 53: 456–467, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ussar S, Griffin NW, Bezy O, Fujisaka S, Vienberg S, Softic S, Deng L, Bry L, Gordon JI, Kahn CR. Interactions between gut microbiota, host genetics and diet modulate the predisposition to obesity and metabolic syndrome. Cell Metab 22: 516–530, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.West DB, Boozer CN, Moody DL, Atkinson RL. Dietary obesity in nine inbred mouse strains. Am J Physiol Regul Integr Comp Physiol 262: R1025–R1032, 1992. [DOI] [PubMed] [Google Scholar]
- 44.Yamada C, Saegusa Y, Nahata M, Sadakane C, Hattori T, Takeda H. Influence of aging and gender differences on feeding behavior and ghrelin-related factors during social isolation in mice. PLoS One 10: e0140094, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yoshikawa I, Nagato M, Yamasaki M, Kume K, Otsuki M. Long-term treatment with proton pump inhibitor is associated with undesired weight gain. World J Gastroenterol 15: 4794–4798, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]