Abstract
We discuss the role of multiple cell types involved in rhythmic motor patterns in the large intestine that include tonic inhibition of the muscle layers interrupted by rhythmic colonic migrating motor complexes (CMMCs) and secretomotor activity. We propose a model that assumes these motor patterns are dependent on myenteric descending 5-hydroxytryptamine (5-HT, serotonin) interneurons. Asynchronous firing in 5-HT neurons excite inhibitory motor neurons (IMNs) to generate tonic inhibition occurring between CMMCs. IMNs release mainly nitric oxide (NO) to inhibit the muscle, intrinsic primary afferent neurons (IPANs), glial cells, and pacemaker myenteric pacemaker interstitial cells of Cajal (ICC-MY). Mucosal release of 5-HT from enterochromaffin (EC) cells excites the mucosal endings of IPANs that synapse with 5-HT descending interneurons and perhaps ascending interneurons, thereby coupling EC cell 5-HT to myenteric 5-HT neurons, synchronizing their activity. Synchronized 5-HT neurons generate a slow excitatory postsynaptic potential in IPANs via 5-HT7 receptors and excite glial cells and ascending excitatory nerve pathways that are normally inhibited by NO. Excited glial cells release prostaglandins to inhibit IMNs (disinhibition) to allow full excitation of ICC-MY and muscle by excitatory motor neurons (EMNs). EMNs release ACh and tachykinins to excite pacemaker ICC-MY and muscle, leading to the simultaneous contraction of both the longitudinal and circular muscle layers. Myenteric 5-HT neurons also project to the submucous plexus to couple motility with secretion, especially during a CMMC. Glial cells are necessary for switching between different colonic motor behaviors. This model emphasizes the importance of myenteric 5-HT neurons and the likely consequence of their coupling and uncoupling to mucosal 5-HT by IPANs during colonic motor behaviors.
Keywords: 5-HT, colon, enteric, ICC, mucosa
compared with the small intestine, there is quite a variation in the anatomy of the large intestine, between herbivores that eat mostly plants (e.g., guinea pigs, mice, and rabbits); carnivores that eat mostly meat (e.g., cat and dog), and omnivores that eat both plants and meat (e.g., human, pig, most primates, and rat) (11,24). Herbivores and omnivores generally have a longer colon than carnivores, reflecting the greater time needed to digest vegetation. Christensen (11) suggested that the human colon has more in common with a herbivore and represents humans' more primitive diet. Another significant difference between these categories of mammals is the structure of the longitudinal muscle coat. In the human and primate colon the longitudinal muscle is gathered into three bands called taenia coli (∼5 mm wide in humans) that merge into a continuous muscle coat in the rectum. In dogs and cats, which have a short colon, the longitudinal muscle coat is continuous around the colon, whereas in guinea pigs and mice, the longitudinal muscle covers ∼⅔ of the proximal colon, exposing a hypoganglionic region without longitudinal muscle, although it merges to become continuous around the rest of the colon (11, 15, 24, 37, 68, 69, 75). (In the large cecum of the guinea pig the longitudinal muscle is also gathered into three bands of taenia caeci.) However, the circular muscle coat in these animals appears to be continuous around the entire colon. Despite these anatomical differences, organization of the enteric nervous system and pacemaker cells have many similarities (1, 2, 11, 36, 66, 68, 75).
The colon is necessary for the recovery of water and electrolytes from the semifluid contents that result from digestion and secretions in the small intestine, which enters the colon through the ileocecal valve (11, 60, 69). The colon also uses bacteria to break down and absorb carbohydrates and process some vitamins (B group), and stores or accommodates fecal contents before evacuation through the anal sphincter. To do this, the colon relies on a series of functionally different compartments that eventually convert this liquid chyme into stools, or discreet fecal pellets in some mammals (e.g., guinea pigs, mice, rabbits, and rats) (11, 24, 37, 60, 99). Colonic motility likely involves complex interactions between pacemaker activity and enteric neurons that cause the slow progression of fecal material along the colon (2, 36, 68, 69). To perform these functions, transit of intraluminal contents through the human colon (∼1.5 m) is very slow, taking ≥30 h, whereas transit through the much longer small intestine (∼7 m) takes 2–4 h (86).
A possible mechanism underlying accommodation and slow transit through the colon involves enteric neural reflexes triggered by colonic elongation rather than circumferential stretch (19, 20, 32, 85). It has been shown in guinea pigs and mice that as the colon empties of fecal pellets it gradually shortens as each fecal pellet is expelled (up to 40% in guinea pig distal colon and ∼20% in murine colon). Elongation activates a powerful inhibitory neural reflex that excites long descending mechanosensitive nNOS interneurons triggered by longitudinal stretch. These interneurons release nitric oxide (NO) to reduce propulsion by suppressing the activity of myenteric neurons and intrinsic primary afferent neurons (IPANs) and pacemaker activity and as a consequence suppresses muscle activity (19,20,32,85). This inhibitory neural reflex has been referred to as the “occult reflex” (hidden from view), since it appears not to have a direct output to the muscle but inhibits mainly neurons involved in peristaltic nerve circuits. Colonic elongation also reduces the amplitude and propagation direction of rhythmic colonic migrating motor complexes (CMMCs), which, essentially, are peristaltic waves (32, 34).
The human ascending colon and guinea pig, rabbit, and rat proximal colon differ from other parts of the large intestine because it generates antiperistaltic waves that mix contents to absorb water and electrolytes to begin stool or pellet formation, and thereby involves pacemaker activity and neural activity (11, 13, 16, 36, 37, 103). Slow waves likely propagate in an antegrade direction along the colon because of a slow wave frequency gradient that drives antiperistaltic waves toward the cecum (11, 103). In guinea pigs and rats, this antiperistaltic activity (referred to as ripple contractions) appears to be generated in a specialized area referred to as the “pacemaker zone or area” at the colonic flexure, between the junction of the proximal colon and distal colon (11, 13, 15, 16, 36, 44). This pacemaker zone absorbs more water and electrolytes from the viscous contents and generates discreet fecal pellets of uniform size, even in vitro (15), that become more solid as they pass down the distal colon toward the rectum.
Colonic Peristaltic Waves
A variety of different motor patterns have been described in the large intestine including segmental activity, antiperistaltic and peristaltic waves, and tonic inhibition (10–16, 37, 50). Tonic inhibition of the muscle layers is interrupted by strong, robust, rhythmic, neurally mediated propagating peristaltic contractions, called CMMCs (Fig. 1) (1, 6–9, 11). CMMC-like activity has been observed in the colons of most mammals, including cats, dogs, guinea pigs, mice, rabbits, rats, and primates (Fig. 2) (1, 11–15, 50). CMMCs and high-amplitude propagating contractions (HAPCs) in the human colon are involved in the propulsion of fecal matter (82). HAPCs are rare (7–10 per 24 h) in the human colon, and are normally activated by vagal pathways during the gastrocolonic reflex and upon waking (82). However, in isolated human and murine colons, HAPCs and CMMCs have been observed to occur continuously with a similar frequency (every 3–4 min) and duration [30–60 s (82)]. Fecal pellet propulsion down the murine colon occurs at the same speed as the apparent conduction velocity of the CMMC contraction (∼0.8 mm/s), suggesting that the CMMC drives pellet propulsion (31). These motor patterns are generated primarily by myenteric neurons in the large intestine, because hexamethonium (a nicotinic receptor antagonist) can block CMMCs and HAPCs and suppress tonic inhibition (1, 18, 30, 31, 82).
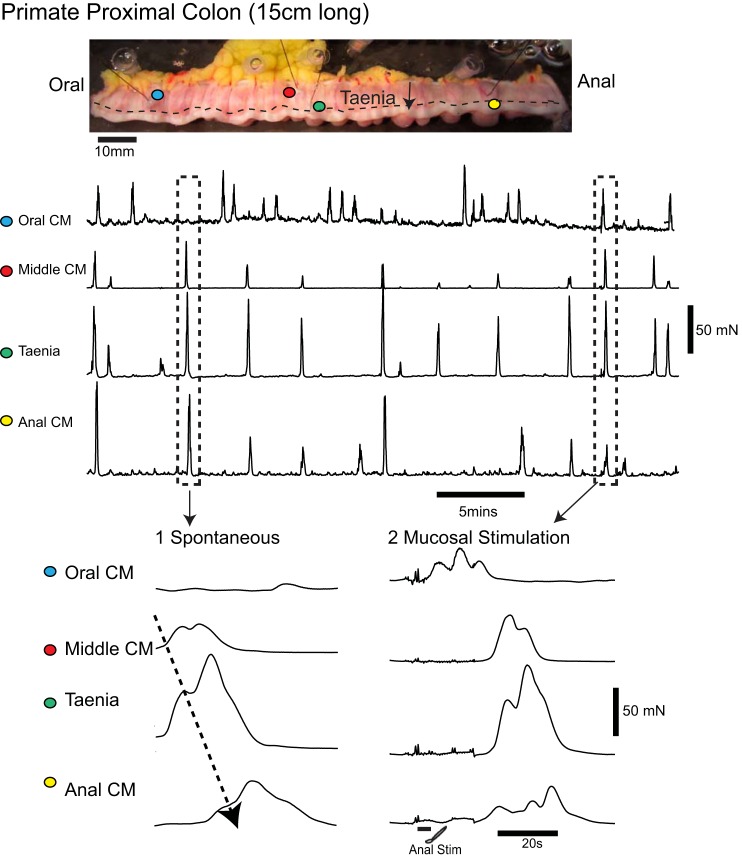
Fig. 1.
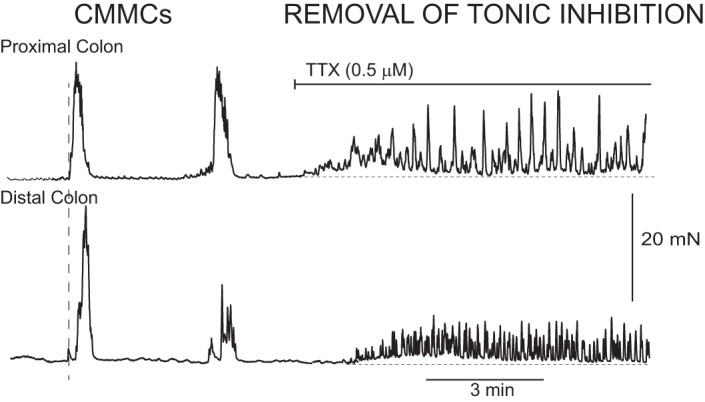
Colonic migrating motor complexes (CMMCs) in the murine colon and the effects of tetrodotoxin (TTX). A fixed fecal pellet in the mid murine colon generated CMMCs that propagated down the colon. TTX (0.5 μM) blocked murine CMMCs and increased muscle tone and contractions in both the proximal and distal colons. Contractions with TTX are likely due to increased pacemaker activity that is normally suppressed by inhibitory nerve activity between CMMCs.
Fig. 2.
CMMC activity in the primate proximal colon. The Cynomolgus primate colon has a similar structure to that of the human colon, including three bands of teania coli (dotted line). Tension transducers were attached to circular muscle (blue, red, and yellow dots on circular muscle) at regular intervals along the isolated empty proximal colon (length of segment ∼15 cm). One transducer was attached to a thick taenia (green dot). The colon generated rhythmic contractions (CMMCs) that propagated largely in an oral-to-anal direction (see 1). The taenia often contracted at the same time as the circular muscle. Stimulating the mucosa by inserting a brush into the anal end of the segment generated a CMMC that propagated down the colon (see 2).
Much of what we know regarding the mechanisms underlying tonic inhibition and CMMCs has been deduced from studies in canine, rat, and especially murine large intestines (6, 8, 9, 13, 18, 19, 36, 48, 68, 69, 71). In the rat colon, CMMCs have been separated into a long-distance contraction and a rhythmic propulsive motor complex, both of which are mediated by nerves and likely involve pacemaker cells (10, 36, 104). Propagating and nonpropagating CMMC contractions have been observed in canine and murine colons (31, 71). However, in mice, when a fecal pellet is held in a fixed location in the midcolon, the enteric neural circuitry generates only one type of propagating contraction (31), which is a CMMC that propagates from the proximal colon to the anus (31).
For CMMC propagation down the colon the contraction is preceded by a burst of inhibitory nerve activity (descending inhibition) in both the longitudinal and circular muscles followed by contraction (ascending excitation) (1, 18, 31, 91). The propagating CMMC viably involves classic polarized descending inhibitory and ascending excitatory peristaltic nerve pathways (1, 34, 91). Without descending inhibition, the CMMC does not propagate, but it occurs synchronously down the colon or propagates in an anal-to-oral direction (33, 34). Moreover, stimulating the mucosa at the anal end of the colon elicits a CMMC that can propagate in either an anal-to-oral direction or in the reverse direction (Fig. 2), suggesting that chains of ascending interneurons are important in carrying neural information up the colon (30, 32). It appears that ascending and descending interneurons synapse with each other to synchronize their activity to produce a propagating CMMC (91). As described below, these descending interneurons likely include 5-hydroxytryptamine (5-HT) neurons.
Pattern Generators and 5-HT Neurons
It has been proposed that rhythmic motor behaviors in the intestine are generated by central pattern generators (CPGs) rather than enteric neural reflexes (51, 100), as has been traditionally believed (14, 16, 20, 50, 80–85): “Central pattern generators rather than afferent-evoked reflexes are postulated to be responsible for the patterns of propulsive contractile behavior and secretion, which recur rhythmically…” (100). CPGs are neural circuits or groups of neurons capable of generating rhythmic activity in the absence of sensory input (27, 100). In invertebrates and vertebrates, central 5-HT neurons are either modulators of or part of CPGs in brain, spinal cord, and segmental ganglia: “A chief function of 5-HT in both vertebrates and invertebrates is to facilitate motor output. Similar roles for 5-HT in potentiating actions of both reflex activity and CPGs are well established in mollusks, leech, and vertebrates” (27). Studies using sheep intestine have also revealed the importance of myenteric 5-HT neurons in rhythmic motor activity in the gut: “the mechanism of cycling of motor events in the small intestine involved serotonergic myenteric neurons located in the duodenal bulb. The data were consistent with the regulation of the enteric biological clock via 5-HT neural receptors mediating inhibition” (65).
A number of myenteric interneurons in guinea pig and murine colons are mechanosensitive and require neural reflexes elicited either by circumferential stretch or longitudinal stretch to increase their activity, suggesting they are unlikely to be a type of CPG (85). Enteric neurons that may possibly constitute a modulator of activity or part of a pattern generator in the colon are myenteric serotonergic 5-HT descending interneurons that appear to be spontaneously active (Fig. 3) (19, 85). Despite their small numbers, colonic myenteric 5-HT interneurons (approximately 1 to 2% of all neurons) in the murine colon appear to be involved in tonic inhibition, CMMCs and secretomotor activity, and blood flow (19, 58). They have extensive projections throughout the myenteric plexus, submucous plexus, and submucosal arterioles, but not venules, suggesting that they comprise a type of central processing unit in the large bowel that coordinates these motor activities (57). 5-HT neurons are more numerous in the proximal colon, where most CMMCs are generated (57).
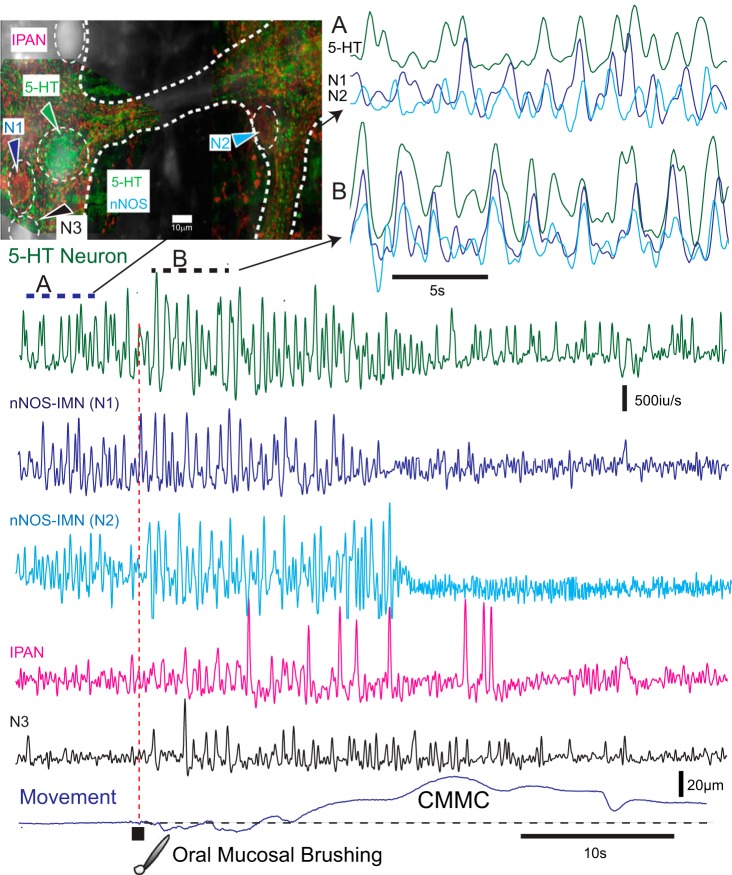
Fig. 3.
Activity in 5-hydroxytryptamine (5-HT) neurons and inhibitory motor neurons in Wnt1-GCaMP3 murine colon. In Wnt1-GCaMP3 mice, which labels all enteric neurons, subsequent 5-HT and neuronal nitric oxide synthase (nNOS) immunochemistry revealed a 5-HT neuron and two nNOS inhibitory motor neurons (IMNs, N1 and N2) in two different ganglia. These ganglia were in the midcolon. The myenteric neurons exhibited spontaneous Ca2+ transients that were differentiated to show Ca2+ spikes. Before the CMMC these neurons exhibited poorly coordinated activity (A, top right). Following brushing of the mucosa (five strokes with a brush in 1 s) at the oral end of the preparation, the 5-HT neuron and two nNOS-IMNs in the midcolon increased their activity, and their Ca2+ transients appeared more coordinated (B, bottom of top right) and coincided with the relaxation phase preceding the CMMC contraction. The nNOS-IMNs reduced their activity during the CMMC contraction. Following mucosal reflex stimulation, an intrinsic primary afferent neuron (IPAN; recognized by its ovoid shape and firing patterns) was transiently activated, as was another neuron (N3).
5-HT interneurons in the murine colon appear to innervate many cells, including other 5-HT neurons, inhibitory motor neurons (IMNs), IPANs (afterhyperpolarizing AH neurons), glia cells, and pacemaker cells in the colon (57). Furthermore, 5-HT neurons are unusual because they are immunoreactive for the Ca2+-binding protein calbindin, which was thought to be an exclusive marker only for IPANs (1, 45, 49, 57). They are also cholinergic neurons (49), although only a small portion (⅓) of their varicosities contain the vesicular acetylcholine (ACh) transporter (VAChT), suggesting that not all 5-HT varicosities synthesize and release ACh as a cotransmitter (72).
5-HT interneurons in large mammals.
In the colon of a large mammal such as the pig, which is a model of the human colon, 5-HT interneurons also have predominantly descending projections. They are not just in the myenteric plexus but also in the extensive Henle’s plexus located just below the circular muscle (99). The Henle’s plexus, which also contains muscle motor neurons and IPANs, provides a motor innervation to pacemaker cells that generate slow waves that lay on the surface of the outer circular muscle (68). 5-HT neurons in the Henle’s plexus also project to the deeper underlying and distinct Meissner plexus, which contains secretomotor neurons that supply the mucosal epithelium.
Also, some 5-HT interneurons in both the myenteric and Henle’s plexuses also project to prevertebral ganglia, suggesting they are also viscerofugal neurons synapsing with postganglionic sympathetic neurons (99). It has been shown that 5-HT neurons in the guinea pig small intestine are preferentially surrounded by baskets of tyrosine hydroxylase nerve terminals (98), suggesting they are likely targets for postganglionic sympathetic neurons that may provide negative feedback to 5-HT neurons. Furthermore, descending 5-HT interneurons in the pig are multipolar with a long descending axon, up to 8 mm in the colon but 50 mm in the small intestine (99).
Sources of 5-HT.
5-HT has two sources in the large intestine, 5-HT interneurons (∼5% of all 5-HT) and enterochromaffin (EC) cells (which contain ∼95% of 5-HT) in the mucosal lining (3, 25, 26, 79, 82). These two pools of 5-HT appear to be connected together by IPANs, which have sensory endings in the mucosa, and synapse with descending 5-HT interneurons (19, 57, 82). Serotonergic neurons and EC cells can be distinguished by their different tryptophan hydroxylase (TPH) enzymes that are rate limiting in the synthesis of 5-HT from the amino acid L-tryptophan (TPH2 in serotonergic neurons and TPH1 in EC cells) (25, 26, 33). 5-HT neurons protect the enteric nervous system from inflammatory damage, whereas the mucosa can release proinflammatory mediators (25). Gershon (25) suggested that 5-HT (TPH2) neurons are likely “more important for constitutive gastrointestinal motility than is that of enterochromaffin cells” (25).
5-HT receptors.
5-HT neurons can exert their diverse effects on many cell types in the large intestine (Table 1) by activating numerous 5-HT receptors (5-HT1 to 5-HT7 receptors, ∼14 receptor subtypes) (3, 26, 79). The often striking inhibitory effects of 5-HT1A, 5-HT2B, 5-HT3, 5-HT4, and 5-HT7 antagonists on colonic motility and secretomotor activity suggests that many of these receptors are largely activated by myenteric 5-HT interneurons (14, 19, 22, 26, 43, 53, 57, 58, 65, 70, 74, 79, 104), although 5-HT4 receptors and 5-HT3 receptors are also found on EC cells and on the mucosal endings of IPANs, respectively (1, 33, 35, 40). However, the role of 5-HT in colonic motor patterns is unclear and will be addressed below, as will the large number of different cell types across the colonic wall involved in tonic inhibition and peristaltic contractions.
Table 1.
Cell types in the large intestine
| Cells Involved in Tonic Inhibition | Cells Involved in CMMC Contractions |
|---|---|
| Longitudinal smooth muscle | Longitudinal smooth muscle |
| Circular smooth muscle | Circular smooth muscle |
| Inhibitory motor neurons | Intrinsic primary afferent neurons |
| Serotonergic interneurons | Ascending interneurons |
| Glia | Excitatory motor neurons |
| ICC-IM | Inhibitory motor neurons |
| PDGFRα+ | Serotonergic interneurons |
| ICC-MY* | Glia |
| ICC-SMP† | PDGFRα+ |
| Submucosal neurons | ICC-IM |
| ICC-MY* | |
| ICC-SMP† | |
| Submucosal neurons | |
| Enterochrommaffin cells |
CMCC, colonic migrating motor complex; ICC-IM, intramuscular interstitial cells of Cajal; ICC-MY, myenteric pacemaker interstitial cells of Cajal; ICC-SMP, submucosal pacemaker interstitial cells of Cajal; PDGFRα+, platelet-derived growth factor-receptor α-positive cells.
ICC-MY cells generate myenteric potential oscillations.
ICC-SMP cells generate electrical slow waves.
Pacemaker Cells and CMMCs
Pacemaker networks and enteric networks are usually treated as independent systems, and how these systems might interact to regulate colonic motor patterns will be addressed below.
Remarkably, the large intestine of most mammals, including mice, cats, dogs, rats, and humans, exhibit two similar dense networks of pacemaker interstitial cells of Cajal (ICC) at the level of the adjacent myenteric plexus called ICC-MY, whereas at the submucosal border of the circular muscle another distinct network of pacemaker ICC are located, called ICC-SM (2, 4, 11, 36, 57, 62, 64, 67–69, 83, 103). The myenteric and submucosal pacemaker networks do not appear to be connected anatomically to each other, although they can interact via the circular muscle electrically (68, 83).
ICC-MY appear to generate fast sinusoidal electrical oscillations called myenteric potential oscillations (MPOs) that can wax and wane in mice, dogs, and humans at a frequency of about 13–60/min, whereas ICC-SM generate electrical slow waves at a frequency of 5–16/min, depending on species (2, 36, 46, 51, 57, 64, 66–69, 82). Large mammals likely have slow-wave-generating ICC-SM penetrating into septa between muscle bundles (46, 47, 64). ICC-MY and ICC-SM are closely associated with inhibitory and excitatory nerve varicosities (2, 36, 67). MPOs generated by ICC-MY conduct into both longitudinal and circular muscle layers at the same time (83). Slow waves from the ICC-SM border propagate toward the myenteric border where they summate with MPOs in the circular muscle to give rise to complex contractile patterns (68, 83).
Although the ICC-MY appears like a regular ICC network, the cells do not seem to be effectively coupled, because MPO activity does not propagate through the ICC-MY network, but only from ICC-MY cells to adjacent longitudinal and circular muscle cells (2, 46). Neighboring ICC-MY cells have no corresponding activity in either murine or canine colons, even after tetrodotoxin (TTX) or Nω-nitro-l-arginine (l-NA) (2, 46). In contrast, slow waves propagate smoothly through the ICC-SM network at the submucosal border of circular muscle (46). Unlike MPOs, slow waves recorded near the submucosal border have a fast upstroke phase preceding the plateau phase that appears to be responsible for their propagation (2, 46, 47).
Surprisingly, ICC-MY activity appears to be coupled together by activation of excitatory motor neurons (EMNs) rather than propagation through the network of ICC-MY cells (2, 36, 46).
MPOs recorded in the muscle are often abolished by L-type Ca2+ channel antagonists (nicardipine and nifedipine), whereas ICC-MY activity appears to be unaffected by these drugs, suggesting that MPOs recorded in the muscle represent a response activity generated in ICC-MY cells (2, 18, 46, 62, 63).
Other researchers have observed rhythmic bursts of action potentials in the longitudinal and circular muscles of short preparations from mice, rats, and humans, suggesting there might be another type of rhythmic activity generated by ICC-MY cells, different from that of MPOs (51, 63, 64, 103). However, these rhythmic bursts of activity could represent periodic coupling of ICC-MY cells, perhaps by stretch usually imposed to facilitate intracellular recording. In fact, phasic depolarizations within these bursts in the muscle have a similar duration to MPOs (64, 103). Furthermore, following l-NA only continuous action potential firing is observed in the muscle and of control mice, in the W/Wv colon, which have deficient ICC, implying that NO acting on ICC-MY creates this burst activity (66).
MPOs during the CMMC.
The CMMC contraction (duration, approximately 30–60 s) consists of an underlying slow depolarization of the muscle upon which are superimposed rapid oscillations in membrane potential (up to 2 Hz; duration, ∼400 ms) that are identical to MPOs (2, 8, 18, 51). MPOs are generated by ICC-MY cells that are coupled by gap junctions to muscle cells (2, 46). MPOs propagate into longitudinal and circular smooth muscle cells, where they can give rise to muscle Ca2+ action potentials (spikes) that cause the muscle layers to contract together (2, 11, 38). Almost identical waveforms to the electrical events underlying the CMMC are observed with calcium imaging [i.e., fast Ca2+ transients (MPOs) on a sustained increase in calcium] (2, 17). Interestingly, morphine abolishes the slow depolarization and fast oscillations, but does not affect membrane potential or inhibitory junction potential (IJPs) (8), suggesting that it inhibits the components of the CMMC that depend on the release of neurotransmitters from EMNs. MPOs are, however, synchronized over distances of at least 15 mm along the colon during the CMMC, suggesting they are activated by synchronized activity in ascending interneurons that activate EMNs (2, 91).
Whether pacemakers cells without functionally active neurons can cause propulsion along the colon is unclear. In TTX, carbachol (a muscarinic agonist) can cause propagation through the colonic muscle of rats and rabbits, suggesting that slow waves, and perhaps MPOs, can propagate down the colon under the influence of excitatory neurotransmitters that excite the pacemaker networks along the colon (10, 13, 36, 51). However, the application of carbachol is not a physiological stimulus.
Tonic Inhibition and its Electrical Correlates
In newborn infants with Hirschsprung disease, the aganglionic rectum remains tonically constricted and generates a pseudo-obstruction to the normal propulsion of colonic content, suggesting that tonic inhibition of the muscle by IMNs is absent (11, 87, 101). It is this pseudo-obstruction that is believed to contribute to chronic constipation and the onset of megacolon behind the aganglionic segment. This suggests that regions with an absence of IMNs supplying the muscle leading to increased pacemaker activity could produce a constriction or blockage in the colon. Below, we examine how enteric neurons are involved in inhibiting colonic motility and the junction potentials and neurotransmitters involved.
Blocking enteric neural activity with TTX or NO synthesis with l-NA excites the colon, suggesting the muscle layers of the large bowel of mammals, including humans, is always subjected to ongoing inhibitory nerve activity (tonic inhibition) (Fig. 1) (1, 11, 12, 18, 21, 30, 46, 48, 51, 66). TTX blocks CMMCs and tonic inhibition, and unmasks pacemaker activity normally suppressed between CMMCs by inhibitory nerve activity (Fig. 1). In contrast, l-NA increases the frequency and amplitude of CMMCs in both the longitudinal and circular muscle layers, suggesting that both muscles are subject to the inhibitory actions of NO (18, 21, 61, 82). However, following l-NA administration, CMMCs do not propagate, but they occur synchronously down the colon (18, 32) and are therefore not technically CMMCs, but are referred to below as CMMC-like contractions.
Tonic inhibition normally occurs between CMMC contractions (every 3–4 min) and requires an intact myenteric plexus and fairly long segments of colon (>20 mm in mouse) to preserve interneuronal nerve pathways (1, 2, 19, 88, 89). Tonic inhibition is dependent upon ongoing activity in myenteric IMNs (1, 2, 19, 30, 89, 91) (Figs. 1 and 3) that release both NO and purines to cause resting membrane hyperpolarization, or slow IJPs and spontaneous fast IJPs (∼15/min in mouse) in the circular muscle, respectively (Fig. 4A) (18, 19, 88, 89, 91). Spontaneous IJPs are rare in longitudinal muscle, in which spontaneous action potential firing predominates (1, 18, 92). Although in circumferentially stretched preparations or following evoked peristaltic reflexes IJPs in the longitudinal muscle occur synchronously with those in circular muscle, as do excitatory junction potentials (EJPs) (93, 97). IJPs in longitudinal muscle are mediated by NO, whereas those in circular muscle are mediated by both purines and NO (93, 97).
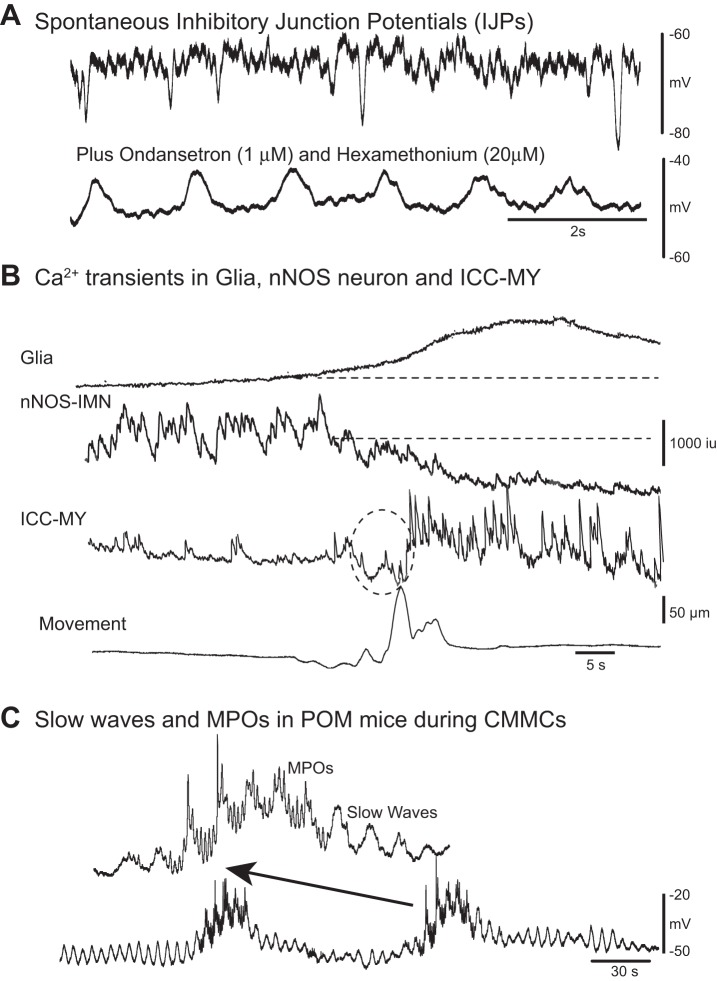
Fig. 4.
Spontaneous pacemaker activity and inhibitory junction potentials (IJPs). A: spontaneous fast IJPs in the circular muscle of murine colon recorded with intracellular microelectrodes. Following ondansetron (1 μM, a 5-HT3 receptor antagonist) and hexamethonium (20 μM, a nicotinic antagonist) administration tonic inhibition was abolished; that is, the membrane potential depolarized and spontaneous IJPs were abolished. These antagonists unmasked slow wave activity in the circular muscle. B: following a spontaneous contraction (bottom trace) Fluo-4 Ca2+ imaging revealed Ca2+ transients in a glial cell, adjacent IMNs, and neighboring myenteric pacemaker interstitial cells of Cajal (ICC-MY). The prolonged Ca2+ transient in the glial cell was associated with a reduction in spontaneous Ca2+ activity in an nNOS-IMN. During inhibition of the IMN, pacemaker myenteric potential oscillation (MPO) activity in ICC-MY turned on because it was under tonic inhibition (dotted circle indicates possible movement artifact). C: spontaneous CMMC-like activity in partially obstructed mice (POM) that lack tonic inhibition. Intracellular electrical recordings from circular muscle demonstrated that the circular muscle was depolarized and spontaneous IJPs were suppressed. Slow waves generated at the submucosal border by submucosal pacemaker interstitial cells of Cajal (ICC-SM) were observed to occur both between and during spontaneous CMMC-like activity. MPOs that originate in ICC-MY are readily observed during CMMC-like activity, where they summate with electrical slow waves originating in ICC-SM. However, CMMC-like contractions do not propagate in these mice, but occur synchronously along the colon, because they lack descending inhibition. (Dr. Dante Heredia performed the electrical recordings).
Inhibitory neurotransmission.
Inhibitory neurotransmission to the circular muscle in the colon is complex, and appears heterogeneous along the large intestine (75). Transmural nerve stimulation evokes a fast IJP followed by a slow IJP in the circular muscle of the murine proximal/midcolon, but only a fast IJP in the cecum and distal colon (75). The fast IJP in colons of mice, rats, and humans is generated by purines released by IMNs, and the slow IJP by the release of NO (38, 39, 51, 63, 93). Motor neurons have short projections along the murine colon (much less than 2.8 mm), whereas interneurons have projections up to 13 mm (91). Spontaneous fast IJPs recorded at closely spaced sites within circular muscle (as little as 1 mm apart) are not well coordinated, suggesting a lack of synchrony between interneurons driving IMNs between CMMCs (91). However, just preceding the CMMC, fast and slow IJPs become larger (31, 91), more frequent, and well coordinated even at sites 15 mm apart, suggesting that there is a synchronization of interneurons driving IMNs (Fig. 3) (91). The increase in synchronized firing of IMNs is necessary for the propagation of CMMCs in both circular and longitudinal muscle layers (17, 91). This inhibitory phase preceding the CMMC can be blocked by 5-HT3 receptor antagonists, suggesting that 5-HT interneurons are important in descending inhibition (19, 31).
Importantly, IMNs in the murine colon do not appear to have inherent activity in themselves but are largely driven by spontaneously active 5-HT interneurons, which are also cholinergic, and perhaps other cholinergic interneurons, because tonic inhibition (spontaneous IJPs and membrane hyperpolarization) is substantially reduced by 5-HT3 antagonists and is completely blocked by the addition of hexamethonium (Fig. 4A), suggesting that IMNs have both 5-HT3 and nicotinic receptors on their cell bodies (19).
Removing the mucosa does not affect tonic inhibition, but it normally blocks CMMCs (1, 17, 19, 31). Without the mucosa, tonic inhibition is ongoing rather than occurring just between CMMCs (18).
Smooth muscle/ICC-IM/PDGFRα+ syncytium.
NO and purines released by IMN nerve terminals in the circular muscle act primarily indirectly via intramuscular interstitial cells of Cajal (ICC-IM) and platelet-derived growth factor-receptor α-positive (PDGFRα+) cells, which are innervated by motor nerves (39, 66). ICC-IM mediate the slow IJP/membrane hyperpolarization, whereas PDGFRα+ cells mediate the fast IJP in circular muscle. The fast IJP is generated by activation of P2Y1 receptors on PDGFRα+ cells that activate Ca2+-activated SK channels (small-conductance K+ channels) on PDGFRα+ cells. ICC-IM and PDGFRα+ cells are connected to each other and to muscle cells by gap junctions that form the so-called smooth muscle/ICC-IM/PDGFRα+ (SIP) syncytium, producing, when activated, tonic and phasic hyperpolarization of circular muscle, respectively. PDGFRα+ cells are unlikely to be involved in regulating neurotransmission to the longitudinal muscle because inhibitory neurotransmission is dependent on NO (93, 97), which activates ICC-IM cells.
P2Y1 receptor knockout mouse.
In P2Y1 receptor knockout (KO) mice (38), which lack the fast IJP, fecal pellets propagate through the isolated proximal colon but are inhibited/blocked through the distal colon, suggesting that fast IJPs are important for propagation in the distal colon, but NO is more important for neuromuscular transmission in the proximal colon. In fact, CMMCs propagate along the proximal colon but occur synchronously down the distal colon in the P2Y1 receptor KO mouse, suggesting that CMMC propagation is necessary for normal fecal pellet propulsion (Fig. 5).
Fig. 5.
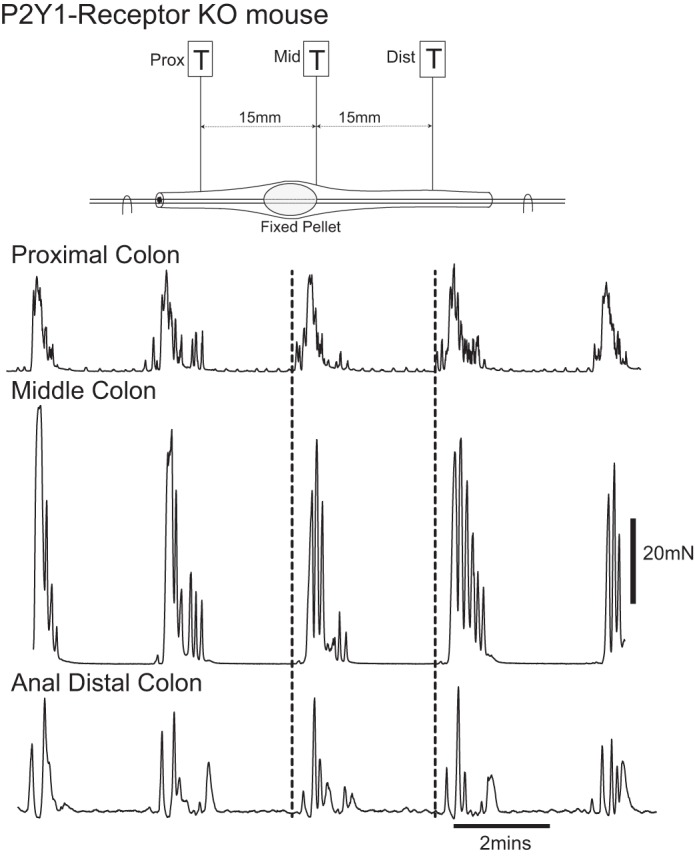
CMMCs in P2Y1 receptor knockout mice. An artificial fecal pellet was held in a fixed position in the midcolon. Spontaneous CMMCs originated in the proximal colon and propagated toward the midcolon. CMMC contractions occurred synchronously down the distal colon.
nNOS KO mouse.
In the colons of nNOS KO mice (17), which lacks NO release from IMNs but not purine release, the muscles are depolarized and fast IJPs still occur, but the colon is constricted and has very slow transit. CMMC-like contractions in the colons of nNOS KO mice do not propagate, but rather, they occur synchronously down the entire colon as they do in control colons following l-NA administration (18, 32). Therefore, CMMC-like contractions in nNOS KO mice are not CMMCs because they do not propagate down the colon, but occur simultaneously down the colon, causing very slow transit. NO maybe important in delaying the onset of the CMMC by inhibiting cholinergic neurons (73). However, severe phenotypic changes occur in nNOS KO mice because the colons are 1) constricted, 2) elongated, 3) have a depolarized circular muscle, and 4) exhibit slow transit (18). Fecal pellets in these mice are smaller in diameter, usually longer, and loosely formed. Therefore, the NO component of tonic inhibition is important for 1) helping to maintain the membrane potential in a hyperpolarized state, 2) dilating the colon, 3) regulating the frequency and propagation of CMMCs, 4) suppressing pacemaker activity between CMMCs, 5) normal stool formation, 6) normal transit and 7) accommodation.
NO within the myenteric plexus.
Moreover, apart from neuromuscular transmission, NO released by IMNs, and perhaps some nNOS interneurons, may also feasibly act within the myenteric plexus (73, 80, 84) to suppress activity in myenteric IPANs, EMNs, some interneurons, glial cells, and myenteric pacemaker cells (ICC-MY) that exhibit little activity between CMMCs, but are very active during a CMMC (Figs. 3 and 4B) (1, 2, 7, 30, 32). ICC-MY that generate MPOs are surrounded by nitrergic nerve varicosities that release NO between CMMCs to inhibit their activity (2). Also, calcium influx into glia, which exhibit prolonged Ca2+ transients (up to ∼40 s) during CMMCs (6), are also reduced by NO (108), as are IPANs (32).
Glial cells.
Activation of glial cells, which are innervated by nerves, follows neural activity during the CMMC and sometimes persists after neural activity declines, whereas between CMMCs glia exhibit little activity, suggesting they may also be under tonic inhibition (Fig. 4B) (7, 30). IMNs turn off during CMMC contractions (1, 2, 30), likely because glial cells release prostaglandins (PGE2) that suppress activity in IMNs (disinhibition) (34, 55), allowing other cells depressed by NO to be activated (Fig. 4B).
Disinhibition.
Because of the excitatory effects of TTX and lidocaine, Christensen and colleagues (12), who studied propagating CMMC-like activity in isolated cat colons deduced that “the migrating spike burst represents periodic release of the muscle from the tonic influence of nonadrenergic inhibitory nerves in the intramural plexuses.” Certainly, during disinhibition of IMNs, MPOs can turn on because they were under tonic inhibition (Fig. 4B). However, disinhibition is unlikely to be the sole reason for contraction, because TTX produces much smaller contractions than those occurring during the neurally mediated CMMC (Fig. 1). Although IMNs turn off during the CMMC, their suppression usually outlasts the CMMC contraction (Fig. 4B) (1, 2, 30). However, as IMNs turn off they can unmask MPO activity (Fig. 4B). Furthermore, CMMC-like activity is also observed in partially obstructed (POM) mice that have suppressed inhibitory nerve activity but robust MPO and slow wave activity (Fig. 4C) (34).
CMMCs and Excitatory Motor Neurons
Both the longitudinal and circular muscle layers contract together during the CMMC and during peristaltic reflexes (1, 18, 80, 87, 95, 97). Hexamethonium blocks CMMCs and HAPCs, likely by acting on nicotinic receptors between ascending interneurons and EMNs (1, 8, 18, 31). There is a slow propagation of neural activity during the CMMC, lasting up to ∼30 s, through the myenteric plexus, with some of the first neurons to be activated being IPANs that have a prolonged activation from the initial stimulus, suggesting they are excited by slow excitatory postsynaptic potentials (sEPSPs) (30). Recently, it has been shown using Ca2+ imaging of ChAT-GCaMP3 neurons that cholinergic EMNs exhibit coordinated activation during the CMMC (30), suggesting they are mostly responsible for contraction, rather than disinhibition, as was previously proposed (12, 89).
During the CMMC, IMNs and nitrergic nerve varicosities around ICC-MY turn off (i.e., disinhibition) to allow full excitation of ICC-MY and muscle directly by EMNs (2). In the murine proximal/midcolon, EMNs release ACh and tachykinins (6), which activate muscarinic and NKI receptors, respectively, on ICC-MY to generate MPOs and activate circular muscle cells by muscarinic and NKII receptors (2, 18).
5-HT Interneurons, Mucosal 5-HT, and CMMCs
Adjacent to the mucosa, IPANs in the guinea pig small intestine exhibit a background firing that leads to an increased excitability of S neurons (motor neurons and interneurons). This increased excitability of IPANs and S neurons was not observed when the mucosa was removed (45). This suggests that the excitability of IPANs is influenced by spontaneous release of 5-HT from mucosal EC cells (45). However, IPANs in the murine colon exhibit little activity between CMMCs, but are activated in response to mucosal stimulation (Fig. 3) (1, 30).
Removing the mucosa from the murine colon in more than 80 preparations abolished spontaneous CMMCs, although tonic inhibition remained intact (1, 18, 19, 31). Significant amounts of circumferential stretch can generate CMMCs without mucosal stimulation (31, 42, 106), although in preparations without the mucosa they do not appear to propagate and are therefore CMMC-like contractions (43, 106). This suggests that the release of 5-HT from mucosal EC cells is usually necessary for activating IPANs that trigger descending inhibition, which is necessary for CMMC propagation (34). Release of 5-HT from the mucosa may be a result of pressure exerted by a fecal pellet, longitudinal muscle contractions, and pacemaker contractile activity that distort EC cells to release 5-HT (31, 54, 59).
Removing not just the mucosa but also 5-HT3 antagonists normally blocks CMMCs by 1) disrupting the mucosal endings of IPANs and 2) blocking transduction at the EC cell-IPAN junction, respectively (1, 31, 33, 79). Therefore, EC cell 5-HT activates 5-HT3 receptors on the mucosal endings of myenteric IPANs (79, 82), which are necessary for raising the excitability of these sensory neurons, which in turn, are necessary for triggering the myenteric neural circuitry (Fig. 6).
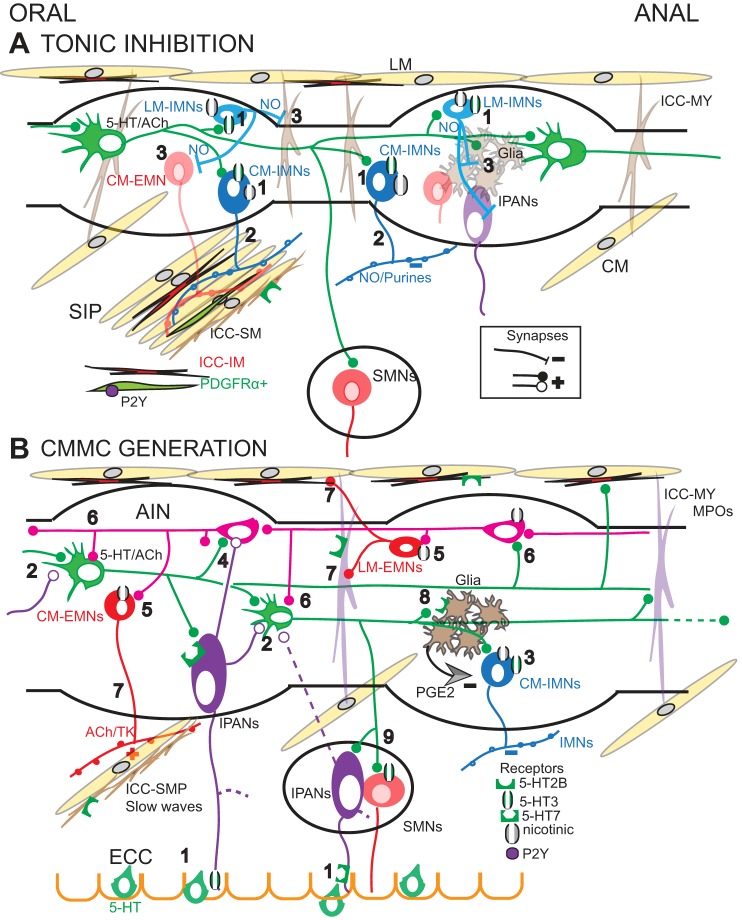
Fig. 6.
Neural circuit involved in colonic motor patterns. A: tonic inhibition. 1) 5-HT neurons (green) activate 5-HT3 and nicotinic receptors on myenteric IMNs to the longitudinal (LM) and circular muscle (CM). 2) CM-IMNs release nitric oxide (NO) and purines that activate the SIP syncytium [CM, intramuscular interstitial cells of Cajal (ICC-IM), and platelet-derived growth factor-receptor α-positive (PDGFRα+) cells]. 3) NO, perhaps released from LM-IMNs inhibit EMNs, ICC-MY, glial cells, and IPANs [note submucosal neurons (SMNs)] B: CMMC generation. 1) Mucosal release from enterochromaffin (EC) cells activates the mucosal endings of myenteric and submucosal IPANS via 5-HT3 and 5-HT7 receptors, respectively. 2) IPANs synapse with and synchronize 5-HT neurons via 5-HT7 receptors. 3) IMNs are strongly activated just prior to the CMMC, and which are necessary for its oral-to-anal propagation. 4) IPANs and 5-HT interneurons likely activate ascending interneurons (AINs). 5) AINs excite excitatory motor neurons (EMNs) to produce the CMMC contraction. 6) AINs also likely synapse with 5-HT neurons and vice versa. 7) EMNs excite the LM and CM, ICC-MY, and ICC-SMP. 8) Synchronized 5-HT neurons activate glial cells via 5-HT2B receptors that release PGE2 to inhibit IMNs, thereby disinhibiting cell types inhibited by NO. 9) During the CMMC, 5-HT neurons activate SMNs.
Without the mucosa, transmural nerve stimulation evokes a CMMC that is preceded by hyperpolarization, suggesting that the neural circuitry for generating the CMMC is in the myenteric plexus (18, 19). Furthermore, 5-HT3 receptor antagonists block the preceding hyperpolarization but they do not affect the evoked CMMC in these preparations without the mucosa (19). Although 5-HT7 receptor antagonists block the CMMC, they do not affect tonic inhibition or the preceding hyperpolarization (19).
5-HT7 antagonists feasibly suppress CMMCs by blocking an sEPSP in myenteric IPANs generated by 5-HT, which is released from descending serotonergic interneurons (52). The sEPSP in IPANs is likely associated with rises in cAMP (106) because activation of 5-HT7 receptors is known to increase cAMP (3). The sEPSP plausibly determines the duration of the CMMC, and its absolute and relative refractoriness following a preceding CMMC (32).
5-HT interneurons and IPANs also probably synapse with ascending interneurons, which excite EMNs to generate the CMMC contraction (34). Ascending interneurons synapse with each other and with EMNs, and also possibly with 5-HT neurons (Fig. 6) (91).
Coupling of Myenteric and Submucous Plexus
In rodents, the myenteric plexus is joined to the submucous plexus by myenteric descending serotonergic, nNOS, and cholinergic interneurons, as well as myenteric and submucosal IPANs (49, 57). Myenteric serotonergic interneurons also project to the submucous secretomotor/vasodilator neurons (SMNs) and submucosal arterioles. During tonic inhibition submucosal neurons exhibit rhythmic activity (58).
The strong activation of SMNs, especially during the robust inhibitory phase necessary for propagating the CMMC, is substantially inhibited by blocking 5-HT3 receptors on SMNs, suggesting that 5-HT neurons are important in connecting together the activity in the two plexuses (58).
Clearly, the arrangement of the myenteric plexus and the extensive submucosal plexuses (Henle’s and Meissner’s plexuses) in large mammals such as dogs, pigs, and primates is more complex than in rodents and needs to be studied in more detail (69, 99).
The myenteric and the submucosal plexuses likely form an integrated nervous system rather than being independent neural networks that couple rhythmic motility patterns to secretomotor activity (58, 82) (Fig. 6).
CMMC-Like Contractions in Constipated Colon Without Mucosal 5-HT Release
P2Y receptor KO mice and nNOS KO mice exhibit a phenotype of constipation. Other mice with this phenotype include POM mice, which have a sutured perianal region that does not directly affect the gut, and TPH1 KO mice, which have suppressed and no mucosal 5-HT release, respectively (Fig. 4C) (33, 34). Both of these mice have enlarged fecal pellets, slow transit of colon content, and an elongated colon. Moreover, in POM mice, l-NA increases contractile activity in the elongated, impacted, isolated colon (34), likely because it blocks NO release from mechanosensitive nNOS descending interneurons that are activated by elongation (longitudinal stretch) (this is referred to as the occult inhibitory reflex) (32). Therefore, even though IMNs do not appear to fire in POM mice, nNOS interneurons mediating the occult reflex are intact (32). Furthermore, outlet obstruction in POM mice leads to significant muscle hypertrophy, similar to that of mice with Hirschprung disease, which have rectal aganglionosis (87).
CMMC-like contractions also occur in the colons of POM mice and TPH1 KO mice, which have suppressed and compromised tonic inhibition and descending inhibition, respectively. CMMC-like contractions do not propagate in these mice; rather, they occur simultaneously down the colon (33, 34), suggesting that mucosal 5-HT release is necessary for activating descending inhibition by coupling IPANs to descending 5-HT interneurons, which excite IMNs. Although 5-HT3 antagonists can block CMMCs in control mice, they have no effect on CMMC-like contractions in the colons of TPH1 KO mice, confirming that they normally act primarily on the EC-IPAN junction (33).
Slow waves and MPOs generated by ICC-SM and ICC-MY cells, respectively, are prominent in POM mice, especially during CMMC-like activity, because tonic inhibition is suppressed (Fig. 4C). These results suggest that NO release is suppressed in the colon of POM mice, and perhaps TPH1 KO mice, probably because glial cells, which are more active without NO release (our unpublished observations), likely release prostaglandins that depress activity in IMNs (Fig. 4B) (34, 55). In POM mice, cyclooxygenase 2 (COX-2) is upregulated in the mucosa and in some nNOS neurons and myenteric glial cells (34), which we propose release PGE2 (34). A COX-2 inhibitor restores tonic inhibition and mucosal reflexes and CMMC propagation in POM mice (34). Conversely, PGE2 suppresses tonic inhibition in control mice (34).
However, EC cell 5-HT does not appear to set the clock for contractile rhythmicity, because the CMMC rhythm occurs in TPH1 KO mice and POM mice, which lack mucosal 5-HT release.
Furthermore, in human slow transit constipation (STC), CMMCs/HAPCs also occur synchronously along the isolated colon (taken from patients with colectomy) because descending inhibitory nerve pathways are absent, whereas ascending excitatory nerve pathways are intact (94, 106), conceivably because of a lack of mucosal 5-HT release. Therefore, the presence of CMMC-like contractions in humans with STC implies that pacemaker ICC are functional, as are excitatory nerve pathways. Like POM mice, in humans with STC the colon also exhibits rhythmic contractions between CMMC-like contractions.
Nonpropagating synchronized CMMCs implies that the entire colon is contracting and shortening at the same time.
Mucosal Reflexes Are Necessary for Normal Peristalsis
We know from studies of the small intestine and colon that low-threshold mucosal reflexes and higher-threshold stretch reflexes both exist (31, 76, 77). Mucosal reflexes are initiated by pressure or mechanical stimulation of the mucosa that likely causes the release 5-HT from EC cells, which stimulates the mucosal endings of IPANs (1, 31). In contrast, stretch reflexes can be activated by circumferential stretch applied to the muscle without significant involvement of the mucosa (76, 77). Mucosal reflexes dramatically enhance stretch reflexes, feasibly because IPANs synapse with stretch-sensitive interneurons (76). However, stretch reflexes do not appear to affect mucosal reflexes (76).
The isolated guinea pig distal colon exhibits occasional CMMCs (14), suggesting that mucosal 5-HT is not effectively released from EC cells without mucosal stimulation. In fact, mucosal stimulation readily activates a propagating CMMC in distal colon of the guinea pig (80, 95). When a fecal pellet is inserted in the colon it generates rhythmic CMMCs, feasibly because of pressure exerted on the mucosa by the presence of a fecal pellet (31).
In contrast, Spencer and colleagues (42, 74, 96, 106) came to a much different conclusion when they suggested that the mucosa was unnecessary for CMMCs and fecal pellet propulsion. They found that “spontaneous” CMMCs appear to occur in the isolated murine colon in preparations without the mucosa and that large fecal pellets could be propelled down the isolated guinea pig distal colon in the absence of the mucosa. This suggested that “dissection studies demonstrated that EC cell derived 5-HT is not responsible for initiating gut peristalsis or motility (i.e., CMMCs) but may modulate the frequency of such events” (42). However, their rake method of recording may have introduced significant amounts of circumferential stretch to the preparation, suggesting that their CMMCs were unlikely to be spontaneous, but rather activated by stretch (78). Moreover, CMMCs in their preparations without the mucosa do not appear to propagate but often occur simultaneously down the colon, suggesting they are more likely CMMC-like contractions rather than CMMCs (42, 106). Observations by Spencer et al. (90) in preparations without the mucosa gave rise to their “mechanical loop hypothesis,” in which stretch-activated reflexes by mechanosensory interneurons are sufficient to explain peristalsis and CMMCs, whereas mucosal reflexes are only modulatory (90).
The “mechanical loop hypothesis” seems unlikely as a general principle because high-threshold stretch reflexes are fairly insensitive, whereas mucosal reflexes activated by pressure or mechanical stimulation of the mucosa are very sensitive (1, 2, 31, 33). In fact, mucosal release of 5-HT appears to be normally involved in pellet propulsion in the distal colon of guinea pigs because, using a 5-HT sensor incorporated into an artificial fecal pellet, it was found that “The onset of mucosal 5-HT was observed prior to the movement of the fecal pellet. The release of mucosal 5-HT occurred oral to the fecal pellet and was linked to the contraction of the bowel wall that drove pellet propulsion” (54). However, if mucosal reflexes lower the threshold for stretch reflexes, then both IPANs and stretch-sensitive interneurons may both function together during normal peristalsis (76).
As noted by Bozler (5) in 1949, in the canine small intestine in vivo, “That distension is not necessary is indicated by the fact that a bolus so small that it does not distend the wall of the relaxed intestine can elicit peristalsis.” Moreover, 1) uninflated balloons can be propelled down the isolated distal colon of guinea pigs (23); 2) small fecal pellets in the absence of significant distension are readily propelled down the murine colon, suggesting they are propelled by mucosal reflexes alone (33); 3) removing a ring of the mucosa can block the progression of an inflated balloon at the lesion (23); and 4) localized application of 5-HT3 antagonists, acting at the EC-IPAN junction around a fecal pellet, blocks CMMCs and tension on a fecal pellet (31). In the in vivo intestine with a competent blood supply, the mucosa is likely to be healthier and more sensitive to stimulation (5).
Without mucosal 5-HT the propagation of a fecal pellet is likely to be compromised because IPANs are not sufficiently excited, and descending inhibition is likely compromised (33).
Repetitive pellet transits down the intact, isolated distal colon of a guinea pig resulted in increased immunoreactivity for 5-HT in myenteric ganglia (42), suggesting that mucosal 5-HT released by the pellet activates IPANs to couple this activity to myenteric 5-HT neurons (Fig. 6).
However, under certain inflammatory conditions when mucosal 5-HT is not released, then presumably, distension reflexes triggered by the colon likely play a role in propulsion (43).
Propagation of the CMMC
How the delay in CMMC down the colon is produced is unclear. The delay could be produced by differences in postjunctional inhibitory mechanisms at the level of the muscle along the colon as occurs in P2Y1 KO and nNOS KO mice, perhaps due to differences in the distribution of ICC-IM or PDGFRα+ cells down the colon.
Also, NO acting at the level of the myenteric plexus (80) likely plays a large part, because CMMCs do not propagate in nNOS KO mice, or in control mice after l-NA administration, which increases the frequency and amplitude of CMMC contractions (18, 32, 61). Along the colon, differences in the amount of NO release from IMNs, which can inhibit cholinergic neurons (73), could be due to increasing recruitment of 5-HT neurons that converge onto IMNs, thereby intensifying their release of NO.
In the esophagus, propagating contractions during secondary peristalsis, which has some similarities to that in the colon, maybe due to both activation of myenteric cholinergic neurons and delayed rebound contraction/excitation of the muscle (28). Rebound contraction results from the preceding hyperpolarization generated by NO released from IMNs (28). It appears that there is an increasing prolonged activation of IMNs down the esophagus that progressively release NO onto the muscle, which causes the increased latency in rebound contraction (28). Similar to what occurs in the colon, peristaltic contractions occur synchronously down the esophagus after blocking NO (102).
Whether a mechanism such as delayed rebound excitation is important in CMMC propagation in the colon is unknown, although it has been observed in the distal colon of the guinea pig following distension-evoked anal IJPs (97).
Model Nerve Circuits for Generating Colonic Motor Patterns
We provide a tentative model that can be readily tested for how motor behaviors in the rodent colon are generated. Serotonergic myenteric neurons are important not only for tonic inhibition but also for CMMCs; many cell types across the colonic wall appear to be involved in these motor behaviors.
Tonic inhibition relies on activity in nNOS IMNs that supply the longitudinal muscle (LM-IMN) and circular muscle (CM-IMN), respectively (49). 5-HT neurons can excite 5-HT3 and nicotinic receptors on IMNs (Fig. 6A). CM-IMNs activate the neuromuscular apparatus in circular muscle (SIP syncytium). During tonic inhibition, many cell types appear to have suppressed activity because of NO released from nNOS neurons, likely by LM-IMNs.
Myenteric 5-HT interneurons and EC cell 5-HT stores appear to be connected together by IPANs that have not just sensory endings in the mucosa, but they also synapse with 5-HT neurons (Fig. 6B). 5-HT released from EC cells likely stimulates myenteric and submucosal IPANs via 5-HT3 and 5-HT7 receptors on their mucosal endings, respectively (82). IPANs coordinate firing in many 5-HT neurons. In turn, 5-HT neurons synapse with IPANs (via 5-HT7 receptors) and glial cells. IPANs and 5-HT neurons likely activate ascending interneurons (AIN) that in turn excite EMNs that supply the SIP syncytium. Activated glial cells, which appear to be excited by 5-HT2B receptors (our unpublished observations), release prostaglandins that reduce activity in IMNs (disinhibition). 5-HT2B receptors are also present on pacemaker ICC (57).
During the inhibitory phase just preceding a CMMC, submucosal neurons are more intensely activated by myenteric 5-HT neurons, perhaps to cause secretion, which lubricates the passage of a fecal pellet (58).
Conclusions
Removing the colon from the body removes extrinsic neural inputs and allows CMMCs and HAPCs to occur rhythmically. CMMCs in isolated murine and primate colons are similar in frequency and duration to HAPCs in isolated human colons, and also similar to HAPCs in vivo.
Descending inhibition, which is coupled to mucosal 5-HT via IPANs, is essential for the orderly propagation of CMMCs, whereas tonic inhibition of the muscle and pacemaker activity is suppressed between CMMCs to prevent unproductive contractions.
During the generation of the CMMC, EMNs excite pacemaker ICC-MY cells to produce a high-frequency burst of MPOs, which couples activity in the longitudinal and circular muscle, causing them to strongly contract together. Despite their relative rarity, 5-HT neurons appear to play a central role in coordinating tonic inhibition, CMMCs, and secretion, whereas glial cells, by suppressing IMNs, appear to be important in switching between these motor behaviors. Mucosal 5-HT and neuronal 5-HT are normally synergistic because they appear to be necessary for the initiation, generation, and effective propagation of CMMCs, and consequently, propulsion of contents.
Future Directions
It will be important to determine how the myenteric and Henle plexuses in the colons of large mammals such as humans, primates, dogs, and pigs coordinate to produce colonic motility patterns. In particular: 1) how do 5-HT neurons in both plexuses interact to create CMMCs, tonic inhibition, and secretomotor activity; 2) what is the role of different 5-HT receptors in regulating these plexuses; 3) what is the role of mucosal 5-HT and IPANs in both plexuses; 4) are nNOS inhibitory motor neurons in these two plexuses necessary for both tonic inhibition and propagating CMMCs; 5) what is the role of enteric glia in regulating the activity of both plexuses; and 6) do extrinsic sympathetic reflexes inhibit enteric 5-HT neurons?
We also need to examine whether the colon has a repertoire of mechanisms for propelling fecal contents in the absence of mucosal 5-HT release, perhaps involving stretch-activated CMMC contractions as well as the role of pacemaker ICC in driving propulsion if enteric nerves are compromised.
Optogenetic techniques could be used to study activity in neural and ICC networks down the colon, including the behavior of 5-HT (TPH2) neurons and other neurons, glia, or pacemaker networks. These cells could be more readily studied by inserting genetically encoded Ca2+ reporters such as GCaMP3 into specific populations of cells (30). Also, channel opsins (56), which can be turned on (channel rhodopsin) or off (halorhodopsin) by blue and yellow light, respectively, could be genetically encoded into specific neurons such as TPH2 neurons, IPANs, glia, or pacemaker ICC to determine their importance and contributions to colonic motor activity and secretion.
GRANTS
This project was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants 1R56 DK-109277 to T. K. Smith and PO1 DK-41315-26 to S. D. Koh.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
T.K.S. and S.D.K. conceived and designed research, performed experiments, analyzed data, interpreted results of experiments, prepared figures, drafted the manuscript, and revised and approved the final version of the manuscript.
REFERENCES
- 1.Bayguinov PO, Hennig GW, Smith TK. Calcium activity in different classes of myenteric neurons underlying the migrating motor complex in the murine colon. J Physiol 588, Pt 3: 399–421, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bayguinov PO, Hennig GW, Smith TK. Ca2+ imaging of activity in ICC-MY during local mucosal reflexes and the colonic migrating motor complex in the murine large intestine. J Physiol 588: 4453–4474, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beattie DT, Smith JA. Serotonin pharmacology in the gastrointestinal tract: a review. Naunyn Schmiedebergs Arch Pharmacol 377: 181–203, 2008. [DOI] [PubMed] [Google Scholar]
- 4.Berezin I, Huizinga JD, Daniel EE. Structural characterization of interstitial cells of Cajal in myenteric plexus and muscle layers of canine colon. Can J Physiol Pharmacol 68: 1419–1431, 1990. [DOI] [PubMed] [Google Scholar]
- 5.Bozler E. Myenteric reflex. Am J Physiol 157: 329–337, 1949. [DOI] [PubMed] [Google Scholar]
- 6.Brierley SM, Nichols K, Grasby DJ, Waterman SA. Neural mechanisms underlying migrating motor complex formation in mouse isolated colon. Br J Pharmacol 132: 507–517, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Broadhead MJ, Bayguinov PO, Okamoto T, Heredia DJ, Smith TK. Ca2+ transients in myenteric glial cells during the colonic migrating motor complex in the isolated murine large intestine. J Physiol 590, Pt 2: 335–350, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bywater RA, Small RC, Taylor GS. Neurogenic slow depolarizations and rapid oscillations in the membrane potential of circular muscle of mouse colon. J Physiol 413: 505–519, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bywater RA, Spencer NJ, Fida R, Taylor GS. Second-, minute- and hour-metronomes of intestinal pacemakers. Clin Exp Pharmacol Physiol 25: 857–861, 1999. [DOI] [PubMed] [Google Scholar]
- 10.Chen J, Zhang Q, Yu Y, Li K, Liao H, Jiang L, Hong L, Du X, Hu X, Chen X, Yin S, Gao Q, Yin X, Luo H, Huizinga JD. Neurogenic and myogenic properties of pan-colonic motor patterns and their spatiotemporal organization in rats. PLoS One 8: e60474, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Christensen J. Colonic motility. In: Physiology of the Gastrointestinal Tract (4th ed.), edited by Johnson LR. New York: Raven, 1994, vol. 1, sect. 2, p. 991–1024. [Google Scholar]
- 12.Christensen J, Anuras S, Arthur C. Influence of intrinsic nerves on the electromyogram of cat colon. Am J Physiol Endocrinol Metab Gastrointest Physiol 234: E641–E647, 1978. [DOI] [PubMed] [Google Scholar]
- 13.Costa M, Dodds KN, Wiklendt L, Spencer NJ, Brookes SJ, Dinning PG. Neurogenic and myogenic motor activity in the colon of the guinea pig, mouse, rabbit, and rat. Am J Physiol Gastrointest Liver Physiol 305: G749–G759, 2013. [DOI] [PubMed] [Google Scholar]
- 14.Costa M, Furness JB. The peristaltic reflex: an analysis of the nerve pathways and their pharmacology. Naunyn Schmiedebergs Arch Pharmacol 294: 47–60, 1976. [DOI] [PubMed] [Google Scholar]
- 15.Costa M, Wiklendt L, Simpson P, Spencer NJ, Brookes SJ, Dinning PG. Neuromechanical factors involved in the formation and propulsion of fecal pellets in the guinea-pig colon. Neurogastroenterol Motil 27: 1466–1477, 2015. [DOI] [PubMed] [Google Scholar]
- 16.D'Antona G, Hennig GW, Costa M, Humphreys CM, Brookes SJ. Analysis of motor patterns in the isolated guinea-pig large intestine by spatio-temporal maps. Neurogastroenterol Motil 13: 483–492, 2001. [DOI] [PubMed] [Google Scholar]
- 17.Dickson EJ, Hennig GW, Heredia DJ, Lee HT, Bayguinov PO, Spencer NJ, Smith TK. Polarized intrinsic neural reflexes in response to colonic elongation. J Physiol 586, Pt 17: 4225–4240, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dickson EJ, Heredia DJ, McCann CJ, Hennig GW, Smith TK. The mechanisms underlying the generation of the colonic migrating motor complex in both wild-type and nNOS knockout mice. Am J Physiol Gastrointest Liver Physiol 298: G222–G232, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dickson EJ, Heredia DJ, Smith TK. Critical role of 5-HT1A, 5-HT3, and 5-HT7 receptor subtypes in the initiation, generation, and propagation of the murine colonic migrating motor complex. Am J Physiol Gastrointest Liver Physiol 299: G144–G157, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dickson EJ, Spencer NJ, Hennig GW, Bayguinov PO, Ren J, Heredia DJ, Smith TK. An enteric occult reflex underlies accommodation and slow transit in the distal large bowel. Gastroenterology 132: 1912–1924, 2007. [DOI] [PubMed] [Google Scholar]
- 21.Dinning PG, Szczesniak M, Cook IJ. Removal of tonic nitrergic inhibition is a potent stimulus for human proximal colonic propagating sequences. Neurogastroenterol Motil 18: 37–44, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Fida R, Bywater RA, Lyster DJ, Taylor GS. Chronotropic action of 5-hydroxytryptamine (5-HT) on colonic migrating motor complexes (CMMCs) in the isolated mouse colon. J Auton Nerv Syst 80: 52–63, 2000. [DOI] [PubMed] [Google Scholar]
- 23.Frigo GM, Lecchini S. An improved method for studying the peristaltic reflex in the isolated colon. Br J Pharmacol 39: 346–356, 1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gary RC. The movements of the large intestine. Physiol Rev 14: 103, 1934. [DOI] [PubMed] [Google Scholar]
- 25.Gershon MD. Serotonin is a sword and a shield of the bowel: serotonin plays offense and defense. Trans Am Clin Climatol Assoc 123: 268–280, 2012. [PMC free article] [PubMed] [Google Scholar]
- 26.Gershon MD, Tack J. The serotonin signaling system: from basic understanding to drug development for functional GI disorders. Gastroenterology 132: 397–414, 2007. [DOI] [PubMed] [Google Scholar]
- 27.Gillette R. Evolution and function in serotonergic systems. Integr Comp Biol 46: 838–846, 2006. [DOI] [PubMed] [Google Scholar]
- 28.Goyal RK, Chaudhury A. Physiology of normal esophageal motility. J Clin Gastroenterol 42: 610–619, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grasa L, Gil V, Gallego D, Martín MT, Jiménez M. P2Y1 receptors mediate inhibitory neuromuscular transmission in the rat colon. Br J Pharmacol 158: 1641–1652, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hennig GW, Gould TW, Koh SD, Corrigan RD, Heredia DJ, Shonnard MC, Smith TK. Use of genetically encoded calcium indicators (GECIs) combined with advanced motion tracking to examine the behavior of neurons and glia in the enteric nervous system of the intact murine colon. Front Cell Neurosci 9: 436, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heredia DJ, Dickson EJ, Bayguinov PO, Hennig GW, Smith TK. Localized release of serotonin (5-hydroxytryptamine) by a fecal pellet regulates migrating motor complexes in murine colon. Gastroenterology 136: 1328–1338, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heredia DJ, Dickson EJ, Bayguinov PO, Hennig GW, Smith TK. Colonic elongation inhibits pellet propulsion and migrating motor complexes in the murine large bowel. J Physiol 588, Pt 15: 2919–2934, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heredia DJ, Gershon MD, Koh SD, Corrigan RD, Okamoto T, Smith TK. Important role of mucosal serotonin in colonic propulsion and peristaltic reflexes: in vitro analyses in mice lacking tryptophan hydroxylase 1. J Physiol 591, Pt 23: 5939–5957, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heredia DJ, Grainger N, McCann CJ, Smith TK. Insights from a novel model of slow-transit constipation generated by partial outlet obstruction in the murine large intestine. Am J Physiol Gastrointest Liver Physiol 303: G1004–G1016, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hoffman JM, Tyler K, MacEachern SJ, Balemba OB, Johnson AC, Brooks EM, Zhao H, Swain GM, Moses PL, Galligan JJ, Sharkey KA, Greenwood-Van Meerveld B, Mawe GM. Activation of colonic mucosal 5-HT4 receptors accelerates propulsive motility and inhibits visceral hypersensitivity. Gastroenterology 142, 844–854, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huizinga JD, Martz S, Gil V, Wang XY, Jimenez M, Parsons S. Two independent networks of interstitial cells of Cajal work cooperatively with the enteric nervous system to create colonic motor patterns. Front Neurosci 5: 93, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hukuhara T, Neya T. The movements of the colon of rats and guinea pigs. Jpn J Physiol 18: 551–562, 1968. [DOI] [PubMed] [Google Scholar]
- 38.Hwang SJ, Blair PJ, Durnin L, Mutafova-Yambolieva V, Sanders KM, Ward SM. P2Y1 purinoreceptors are fundamental to inhibitory motor control of murine colonic excitability and transit. J Physiol 590, Pt 8: 157–197, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jiménez M, Clavé P, Accarino A, Gallego D. Purinergic neuromuscular transmission in the gastrointestinal tract; functional basis for future clinical and pharmacological studies. Br J Pharmacol 171: 4360–4375, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jin JG, Foxx-Orenstein AE, Grider JR. Propulsion in guinea pig colon induced by 5-hydroxytryptamine (HT) via 5-HT4 and 5-HT3 receptors. J Pharmacol Exp Ther 288: 93–97, 1999. [PubMed] [Google Scholar]
- 41.Keating DJ, Peiris H, Kyloh M, Brookes SJ, Spencer NJ. The presence of 5-HT in myenteric varicosities is not due to uptake of 5-HT released from the mucosa during dissection: use of a novel method for quantifying 5-HT immunoreactivity in myenteric ganglia. Neurogastroenterol Motil 25: 849–853, 2013. [DOI] [PubMed] [Google Scholar]
- 42.Keating DJ, Spencer NJ. Release of 5-hydroxytryptamine from the mucosa is not required for the generation or propagation of colonic migrating motor complexes. Gastroenterology 138: 659–670, 2010. [DOI] [PubMed] [Google Scholar]
- 43.Kendig DM, Grider JR. Serotonin and colonic motility. Neurogastroenterol Motil 27: 899–905, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kobayashi S, Chowdhury JU, Tokuno H, Nahar NS, Iino S. A smooth muscle nodule producing regular contractions at the mesenteric border of the pacemaker area in the guinea-pig colon. Arch Histol Cytol 59: 159–168, 1996. [DOI] [PubMed] [Google Scholar]
- 45.Kunze WA, Bertrand PP, Furness JB, Bornstein JC. Influence of the mucosa on the excitability of myenteric neurons. Neuroscience 76: 619–634, 1997. [DOI] [PubMed] [Google Scholar]
- 46.Lee HT, Hennig GW, Park KJ, Bayguinov PO, Ward SM, Sanders KM, Smith TK. Heterogeneities in ICC Ca2+ activity within canine large intestine. Gastroenterology 136: 2226–2236, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee HT, Hennig GW, Fleming NW, Keef KD, Spencer NJ, Ward SM, Sanders KM, Smith TK. Septal interstitial cells of Cajal conduct pacemaker activity to excite muscle bundles in human jejunum. Gastroenterology 133: 907–917, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li M, Johnson CP, Adams MB, Sarna SK. Cholinergic and nitrergic regulation of in vivo giant migrating contractions in rat colon. Am J Physiol Gastrointest Liver Physiol 283: G544–G552, 2002. [DOI] [PubMed] [Google Scholar]
- 49.Lomax AE, Furness JB. Neurochemical classification of enteric neurons in the guinea-pig distal colon. Cell Tissue Res 302: 59–72, 2000. [DOI] [PubMed] [Google Scholar]
- 50.Mackenna BR, McKirdy HC. Peristalsis in the rabbit distal colon. J Physiol 220: 33–54, 1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mañé N, Martínez-Cutillas M, Gallego D, Jimenez M. Enteric motor pattern generators involve both myogenic and neurogenic mechanisms in the human colon. Front Physiol 6: 205, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Monro RL, Bornstein JC, Bertrand PP. Slow excitatory post-synaptic potentials in myenteric AH neurons of the guinea-pig ileum are reduced by the 5-hydroxytryptamine7 receptor antagonist SB 269970. Neuroscience 134: 975–986, 2005. [DOI] [PubMed] [Google Scholar]
- 53.Morita M, Mochiki E, Takahashi N, Kawamura K, Watanabe A, Sutou T. Effects of 5-HT2B, 5-HT3 and 5-HT4 receptor antagonists on gastrointestinal motor activity in dogs. World J Gastroenterol 19: 6604–6612, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Morris R, Fagan-Murphy A, MacEachern SJ, Covill D, Patel BA. Electrochemical fecal pellet sensor for simultaneous real-time ex vivo detection of colonic serotonin signaling and motility. Sci Rep 6: 23442, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Murakami M, Ohta T, Otsuguro KI, Ito S. Involvement of prostaglandin E(2) derived from enteric glial cells in the action of bradykinin in cultured rat myenteric neurons. Neuroscience 145: 642–653, 2007. [DOI] [PubMed] [Google Scholar]
- 56.Nagel G, Brauner M, Liewald JF, Adeishvili N, Bamberg E, Gottschalk A. Light activation of channelrhodopsin-2 in excitable cells of Caenorhabditis elegans triggers rapid behavioral responses. Curr Biol 15: 2279–2284, 2005. [DOI] [PubMed] [Google Scholar]
- 57.Okamoto T, Barton MJ, Hennig GW, Birch GC, Grainger N, Corrigan RD, Koh SD, Sanders KM, Smith TK. Extensive projections of myenteric serotonergic neurons suggest they comprise the central processing unit in the colon. Neurogastroenterol Motil 26: 556–570, 2014. [DOI] [PubMed] [Google Scholar]
- 58.Okamoto T, Bayguinov PO, Broadhead MJ, Smith TK. Ca2+ transients in submucous neurons during the colonic migrating motor complex in the isolated murine large intestine. Neurogastroenterol Motil 24: 769–778, 2012. [DOI] [PubMed] [Google Scholar]
- 59.Patel BA. Mucosal serotonin overflow is associated with colonic stretch rather than phasic contractions. Neurogastroenterol Motil 28: 914–923, 2016. [DOI] [PubMed] [Google Scholar]
- 60.Phillips SF. Functions of the large bowel: an overview. Scand J Gastroenterol Suppl 93: 1–12, 1984. [PubMed] [Google Scholar]
- 61.Powell AK, Bywater RA. Murine intestinal migrating motor complexes: longitudinal components. Neurogastroenterol Motil 15: 245–56, 2003. [DOI] [PubMed] [Google Scholar]
- 62.Plujà L, Albertí E, Fernández E, Mikkelsen HB, Thuneberg L, Jiménez M. Evidence supporting presence of two pacemakers in rat colon. Am J Physiol Gastrointest Liver Physiol 281: G255–G266, 2001. [DOI] [PubMed] [Google Scholar]
- 63.Plujà L, Fernández E, Jiménez M. Neural modulation of the cyclic electrical and mechanical activity in the rat colonic circular muscle: putative role of ATP and NO. Br J Pharmacol 126: 883–892, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rae MG, Flaeming N, McGregor DB, Sanders KM, Keef KD. Control of motility patterns in the human colonic circular muscle layer by pacemaker activity. J Physiol 510: 309–320, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ruckebusch Y, Bardon T. Involvement of serotonergic mechanisms in initiation of small intestine cyclic motor events. Dig Dis Sci 29: 520, 1984. [DOI] [PubMed] [Google Scholar]
- 66.Sanders KM, Hwang SJ, Ward SM. Neuroeffector apparatus in gastrointestinal smooth muscle organs. J Physiol 588, Pt 23: 4621–4639, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sanders KM, Smith TK. Motor neurons of the submucous plexus regulate electrical activity of the circular muscle of canine proximal colon. J Physiol 280: 293–310, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sanders KM, Smith TK. Electrophysiology of colonic smooth muscle. In: Handbook of Physiology. The Gastrointestinal System. Motility and Circulation. Bethesda, MD: Am. Physiol. Soc; 1989, sect. 6, vol. I, pt. 2, chapt. 7, p. 251–271. [Google Scholar]
- 69.Sanders KM, Smith TK. Neural regulation of colonic motor function. In: Colonic Diseases, edited by Koch T. Totowa, NJ: Humana, 2003, chapt. 3, p. 35–52. [Google Scholar]
- 70.Sanger GJ. 5-Hydroxytryptamine and the gastrointestinal tract: where next? Trends Pharmacol Sci 29: 465–471, 2008. [DOI] [PubMed] [Google Scholar]
- 71.Sarna SK, Condon R, Cowles V. Colonic migrating and non-migrating motor complexes in dogs. Am J Physiol Gastrointest Liver Physiol 246: G355–G360, 1984. [DOI] [PubMed] [Google Scholar]
- 72.Sharrad DF, Chen BN, Brookes SJ. Neurochemical coding compared between varicose axons and cell bodies of myenteric neurons in the guinea-pig ileum. Neurosci Lett 534: 171–176, 2013. [DOI] [PubMed] [Google Scholar]
- 73.Shuttleworth CW, Xue C, Ward SM, de Vente J, Sanders KM. Immunohistochemical localization of 3′, 5′-cyclic guanosine monophosphate in the canine proximal colon: responses to nitric oxide and electrical stimulation of enteric inhibitory neurons. Neuroscience 56: 513–522, 1993. [DOI] [PubMed] [Google Scholar]
- 74.Sia TC, Whiting M, Kyloh M, Nicholas SJ, Oliver J, Brookes SJ, Dinning PG, Wattchow DA, Spencer NJ. 5-HT3 and 5-HT4 antagonists inhibit peristaltic contractions in guinea-pig distal colon by mechanisms independent of endogenous 5-HT. Front Neurosci 7: 136, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sibaev A, Hartmut F, Vanderwinden JM, Allescher HD, Storr M. Structural differences in the enteric neural network in murine colon: impact on electrophysiology. Am J Physiol Gastrointest Liver Physiol 285: G1325–G1334, 2003. [DOI] [PubMed] [Google Scholar]
- 76.Smith TK, Bornstein JC, Furness JB. Interactions between reflexes evoked by distension and mucosal stimulation: electrophysiological studies of guinea-pig ileum. J Auton Nerv Syst 34: 69–75, 1991. [DOI] [PubMed] [Google Scholar]
- 77.Smith TK, Bornstein JC, Furness JB. Convergence of reflex pathways excited by distension and mechanical stimulation of the mucosa onto the same myenteric neurons of the guinea pig small intestine. J Neurosci 12: 1502–1510, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Smith TK, Dickson EJ, Heredia DJ, Hennig GW, Bayguinov PO. Controversies involving the role of 5-hydroxytryptamine (5-HT) in generating colonic migrating motor complexes: what is spontaneous? Gastroenterology 138: 659–670, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Smith TK, Gershon MD. Cross talk proposal: 5-HT is necessary for peristalsis. J Physiol 593: 3225–3227, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Smith TK, McCarron SL. Nitric oxide modulates cholinergic reflex pathways in the guinea-pig distal colon. J Physiol 512: 898–906, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Smith TK, Oliver GR, Hennig GW, O'Shea DM, Vanden Berghe P, Kang SH, Spencer NJ. A smooth muscle tone-dependent stretch-activated migrating motor pattern in isolated guinea-pig distal colon. J Physiol 551, Pt 3: 955–969, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Smith TK, Park KJ, Hennig GW. Colonic migrating motor complexes, high amplitude propagating contractions, neural reflexes and the importance of neuronal and mucosal serotonin. Neurogastroenterol Motil 20: 423–446, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Smith TK, Reed JB, Sanders KM. Interaction of two electrical pacemakers in muscularis of canine proximal colon. Am J Physiol Cell Physiol 252: C290–C299, 1987. [DOI] [PubMed] [Google Scholar]
- 84.Smith TK, Robertson WJ. Synchronous movements of the longitudinal and circular muscle during peristalsis in the isolated guinea-pig distal colon. J Physiol 506, Pt 2: 563–577, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Smith TK, Spencer NJ, Hennig GW, Dickson EJ. Recent advances in enteric neurobiology: mechanosensitive interneurons. Neurogastroenterol Motil 19: 869–878, 2007. [DOI] [PubMed] [Google Scholar]
- 86.Southwell BR, Clarke MC, Sutcliffe J, Hutson JM. Colonic transit studies: normal values for adults and children with comparison of radiological and scintigraphic methods. Pediatr Surg Int 25: 559–572, 2009. [DOI] [PubMed] [Google Scholar]
- 87.Spencer NJ, Bayguinov P, Hennig GW, Park KJ, Lee HT, Sanders KM, Smith TK. Activation of neural circuitry and Ca2+ waves in longitudinal and circular muscle during CMMCs and the consequences of rectal aganglionosis in mice. Am J Physiol Gastrointest Liver Physiol 292: G546–G555, 2007. [DOI] [PubMed] [Google Scholar]
- 88.Spencer NJ, Bywater RA, Holman ME, Taylor GS. Spontaneous and evoked inhibitory junction potentials in the circular muscle layer of mouse colon. J Auton Nerv Syst 69: 115–121, 1998. [DOI] [PubMed] [Google Scholar]
- 89.Spencer NJ, Bywater RA, Taylor GS. Disinhibition during myo-electric complexes in the mouse colon. J Auton Nerv Syst 71: 37–47, 1998. [DOI] [PubMed] [Google Scholar]
- 90.Spencer NJ, Dinning PG, Brookes SJ, Costa M. Insights into the mechanisms underlying colonic motor patterns. J Physiol 594: 4099–4116, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Spencer NJ, Hennig GW, Dickson E, Smith TK. Synchronization of enteric neuronal firing during the murine colonic MMC. J Physiol 564: 829–847, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Spencer NJ, Hennig GW, Smith TK. Electrical rhythmicity and spread of action potentials in longitudinal muscle of guinea pig distal colon. Am J Physiol Gastrointest Liver Physiol 282: G904–G917, 2002. [DOI] [PubMed] [Google Scholar]
- 93.Spencer NJ, Hennig GW, Smith TK. Stretch-activated neuronal pathways to longitudinal and circular muscle in guinea pig distal colon. Am J Physiol Gastrointest Liver Physiol 284: G231–G241, 2003. [DOI] [PubMed] [Google Scholar]
- 94.Spencer NJ, Kyloh M, Wattchow DA, Thomas A, Sia TC, Brookes SJ, Nicholas SJ. Characterization of motor patterns in isolated human colon: are there differences in patients with slow-transit constipation? Am J Physiol Gastrointest Liver Physiol 302: G34–G43, 2012. [DOI] [PubMed] [Google Scholar]
- 95.Spencer NJ, McCarron SL, Smith TK. Sympathetic inhibition of ascending and descending interneurones during the peristaltic reflex in the isolated guinea-pig distal colon. J Physiol 519, Pt 2: 539–550, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Spencer NJ, Nicholas SJ, Robinson L, Kyloh M, Flack N, Brookes SJ, Zagorodnyuk VP, Keating DJ. Mechanisms underlying distension-evoked peristalsis in guinea pig distal colon: is there a role for enterochromaffin cells? Am J Physiol Gastrointest Liver Physiol 301: G519–G527, 2011. [DOI] [PubMed] [Google Scholar]
- 97.Spencer NJ, Smith TK. Simultaneous intracellular recordings from longitudinal and circular muscle during the peristaltic reflex in guinea-pig distal colon. J Physiol 533, Pt 3: 787–799, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tassicker BC, Hennig GW, Costa M, Brookes SJ. Rapid anterograde and retrograde tracing from mesenteric nerve trunks to the guinea-pig small intestine in vitro. Cell Tissue Res 295: 437–452, 1999. [DOI] [PubMed] [Google Scholar]
- 99.Timmermans JP, Hens J, Adriaensen D. Outer submucous plexus: an intrinsic nerve network involved in both secretory and motility processes in the intestine of large mammals and humans. Anat Rec 262: 71–78, 2001. [DOI] [PubMed] [Google Scholar]
- 100.Wood JD. Enteric nervous system: reflexes, pattern generators and motility. Curr Opin Gastroenterol 24: 149–158, 2008. [DOI] [PubMed] [Google Scholar]
- 101.Wood JD, Brann LR, Vermillion DL. Electrical and contractile behavior of large intestinal musculature of piebald mouse model for Hirschsprung's disease. Dig Dis Sci 31: 38–50, 1986. [DOI] [PubMed] [Google Scholar]
- 102.Yamato S, Spechler SJ, Goyal RK. Role of nitric oxide in esophageal peristalsis in the opossum. Gastroenterology 103: 197–204, 1992. [DOI] [PubMed] [Google Scholar]
- 103.Yoneda S, Takano H, Takaki M, Suzuki H. Properties of spontaneously active cells distributed in the submucosal layer of mouse proximal colon. J Physiol 542: 887–897, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yu Y, Chen JH, Li H, Yang Z, Du X, Hong L, Liao H, Jiang L, Shi J, Zhao L, Tan S, Luo H, Huizinga JD. Involvement of 5-HT3 and 5-HT4 receptors in colonic motor patterns in rats. Neurogastroenterol Motil 27: 914–928, 2015. [DOI] [PubMed] [Google Scholar]
- 105.Zafirov DH, Palmer JM, Nemeth PR, Wood JD. Cyclic 3′, 5′-adenosine monophosphate mimics slow synaptic excitation in myenteric plexus neurons. Brain Res 347: 368–371, 1985. [DOI] [PubMed] [Google Scholar]
- 106.Zagorodnyuk VP, Spencer NJ. Localization of the sensory neurons and mechanoreceptors required for stretch-evoked colonic migrating motor complexes in mouse colon. Front Physiol 2: 98, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zarate N, Spencer NJ. Chronic constipation: lessons from animal studies. Best Pract Res Clin Gastroenterol 25: 59–71, 2011. [DOI] [PubMed] [Google Scholar]
- 108.Zhang W, Sarosi GA, Barnhart DC, Mulholland MW. Endothelin-stimulated capacitative calcium entry in enteric glial cells: synergistic effects of protein kinase C activity and nitric oxide. J Neurochem 71: 205–212, 1998. [DOI] [PubMed] [Google Scholar]