We found that metaboreflex-induced increases in coronary blood flow and ventricular contractility are attenuated in hypertension. α1-Adrenergic blockade restored these parameters toward normal levels. These findings indicate that the primary mechanism mediating impaired metaboreflex-induced increases in ventricular function in hypertension is accentuated coronary vasoconstriction.
Keywords: exercise pressor reflex, coronary blood flow, contractility, hypertension
Abstract
Increases in myocardial oxygen consumption during exercise mainly occur via increases in coronary blood flow (CBF) as cardiac oxygen extraction is high even at rest. However, sympathetic coronary constrictor tone can limit increases in CBF. Increased sympathetic nerve activity (SNA) during exercise likely occurs via the action of and interaction among activation of skeletal muscle afferents, central command, and resetting of the arterial baroreflex. As SNA is heightened even at rest in subjects with hypertension (HTN), we tested whether HTN causes exaggerated coronary vasoconstriction in canines during mild treadmill exercise with muscle metaboreflex activation (MMA; elicited by reducing hindlimb blood flow by ~60%) thereby limiting increases in CBF and ventricular performance. Experiments were repeated after α1-adrenergic blockade (prazosin; 75 µg/kg) and in the same animals following induction of HTN (modified Goldblatt 2K1C model). HTN increased mean arterial pressure from 97.1 ± 2.6 to 132.1 ± 5.6 mmHg at rest and MMA-induced increases in CBF, left ventricular dP/dtmax, and cardiac output were markedly reduced to only 32 ± 13, 26 ± 11, and 28 ± 12% of the changes observed in control. In HTN, α1-adrenergic blockade restored the coronary vasodilation and increased in ventricular function to the levels observed when normotensive. We conclude that exaggerated MMA-induced increases in SNA functionally vasoconstrict the coronary vasculature impairing increases in CBF, which limits oxygen delivery and ventricular performance in HTN.
NEW & NOTEWORTHY We found that metaboreflex-induced increases in coronary blood flow and ventricular contractility are attenuated in hypertension. α1-Adrenergic blockade restored these parameters toward normal levels. These findings indicate that the primary mechanism mediating impaired metaboreflex-induced increases in ventricular function in hypertension is accentuated coronary vasoconstriction.
Listen to this article’s corresponding podcast at http://ajpheart.podbean.com/e/metaboreflex-induced-functional-coronary-vasoconstriction/.
when oxygen demand of working skeletal muscle exceeds oxygen supply, the muscle releases metabolites [e.g., hydrogen ion (13, 116), potassium ion (72), diprotonated phosphate (13, 107), lactic acid (21, 63, 96, 105, 108), and ATP (43, 70, 112)], which stimulate metabolically sensitive group III/IV afferents (2, 5, 15, 63, 76, 96), reflexively increasing sympathetic outflow from the brainstem, termed the muscle metaboreflex (12). When engaged during submaximal dynamic exercise, the metaboreflex increases heart rate (HR) and ventricular contractility resulting in large increases in cardiac output (CO) and mean arterial pressure (MAP) (3, 7, 19, 25, 71, 89, 97, 111, 117). The rise in CO is driven by marked increases in HR (4, 19, 29, 50, 88, 99, 117) coupled with a sustained or slight increase in stroke volume (SV) (16, 19, 89, 99, 106, 111), which is supported via enhanced ventricular contractility (dP/dtmax) (16, 18, 51, 89, 99), lusitropy (dP/dtmax) (51, 111), and central blood volume mobilization (103). The increase in CO is the primary mechanism mediating the rise in arterial blood pressure as little if any net peripheral vasoconstriction is observed (8, 18, 42, 51, 106, 111, 117). Therefore, the muscle metaboreflex has been described as a flow-sensitive, flow-raising reflex (92) that works to improve oxygen supply to the ischemic working muscle (90). However, the capacity of the metaboreflex to restore blood blow to the ischemic working muscle may be self-limiting (56).
During exercise, increased cardiac oxygen demand is met primarily via increases in coronary blood flow (CBF) inasmuch as oxygen extraction is already ~80% at rest (24, 26, 115). The rise in CBF is achieved via coronary metabolic and feed-forward, β2-adrenergic vasodilation (24, 26, 115), which is restrained by the vasoconstrictor actions of the rise in cardiac sympathetic nerve activity (SNA) (41). Muscle metaboreflex activation increases CBF solely via the large increase in MAP; despite large increases in cardiac work (greater CO pumped against a higher afterload), little if any coronary vasodilation occurs due to enhanced α1-adrenergic-mediated coronary vasoconstriction (6, 16, 91). Blockade of the constrictor tone increases CBF during muscle metaboreflex activation as now substantial vasodilation is accompanied by the large pressor response resulting in improved ventricular performance and CO (16, 17, 91).
Resting SNA is heightened in hypertension (34, 38, 53, 74, 75, 78, 114). Moreover, an enhanced sympathetically mediated coronary constrictor tone and restrained coronary metabolic vasodilation during dynamic exercise have been demonstrated in hypertension (39). However, recent studies support (14, 22, 35, 80, 101) and refute (95) accentuated muscle metaboreflex function in hypertension. We previously reported attenuated metaboreflex-induced chronotropic and inotropic responses in hypertension (98). In the present study, we hypothesized that exaggerated coronary vasoconstriction contributes to the impaired ability to increase ventricular performance during muscle metaboreflex activation in hypertension.
METHODS
Experimental subjects.
Six adult mongrel canines (20–25 kg) were selected for their willingness to run on a motor-driven treadmill. Due to availability issues with our vendor all animals were female. We have previously shown that gender does not affect the strength or mechanisms of the muscle metaboreflex (67). The animals exercised voluntarily and no negative reinforcement techniques were utilized. All protocols were approved by the Institutional Animal Care and Use Committee (IACUC) of Wayne State University and complied with the National Institutes of Health Guide to the Care and Use of Laboratory Animals.
Surgical procedures.
Each animal was instrumented with chronic, indwelling cardiovascular devices following two sterile surgical procedures: left thoracotomy followed by a left flank retroperitoneal surgery. The animals recovered a minimum of 10 days before the second surgery and a minimum of 7 days before the first experiment. During preoperative care, the animals were initially sedated with acepromazine (0.4–0.5 mg/kg im) followed by combined treatment of ketamine and diazepam (5.0 and 0.22 mg/kg iv, respectively). Anesthesia was maintained with isoflurane gas (1–3%). The animals received preoperative administration of cefazolin (30 mg/kg iv), carprofen (analgesic, 2.0 mg/kg iv), buprenorphine (analgesic, 0.01 mg/kg im), and fentanyl [analgesic, 125–175 µg/h (72 h) TDD). Before the left thoracotomy, animals received selective intercostal nerve blockade with bupivacaine HCl (2.0 mg/kg). Following each surgical procedure, animals received cefazolin (30 mg/kg iv) and prophylactic cephalexin [antibiotic, 30 mg/kg (b.i.d.) po] therapy for the term of the experimental protocol. During the 12 h postoperative period, animals were closely monitored and received buprenorphine and acepromazine (0.05 and 0.5 mg/kg iv, respectively) as needed to control any potential discomfort. For the following 10 days, animals received carprofen [4 mg/kg (OPD) po].
In the first surgical procedure, the thoracic cavity was opened via a left thoracotomy (4th intercostal space). The pericardium was cut and reflected to expose the heart. An ultrasonic perivascular flow probe (20PAU; Transonic Systems) was positioned around the ascending aorta to measure CO. Approximately 10 cm caudal to the thoracotomy incision, an implantable telemetry blood pressure transmitter (TA11 PA-D70; Data Sciences International) was tethered subcutaneously. The catheter of the transmitter was tunneled into the thoracic cavity through the 7th intercostal space and the tip was inserted and secured inside the left ventricle for measuring left ventricular pressure (LVP). Lastly, an ultrasonic perivascular flow probe (3PSB; Transonic Systems) was positioned around the circumflex artery to measure CBF. The pericardium was loosely reapproximated, the cables were tunneled subcutaneously and exteriorized between the scapulae and the chest was closed in layers.
In the second surgical procedure, an incision was made in the left flank cranial to the iliac crest. An ultrasonic perivascular flow probe (10PAA; Transonic Systems) was positioned around the terminal aorta for measuring hindlimb blood flow (HLBF). All side branches of the terminal aorta, between the common iliacs and the flow probe, were ligated and severed. In addition, two perivascular hydraulic occluders (8–10 mm; DocXS Biomedical Products) were positioned around the terminal aorta (distal to the flow probe) to provide the means to incrementally reduce HLBF. A 19-gauge polyvinyl catheter (Tygon, S54-HL; Norton) was advanced through a ligated lumbar artery and secured into the terminal aorta cranial to the probe and occluders to measure arterial pressure. Lastly, the left renal artery was exposed and an ultrasonic perivascular flow probe (4PSB; Transonic Systems) was positioned around the vessel to measure renal blood flow (RBF). A perivascular hydraulic occluder (4–6 mm; DocXS Biomedical Products) was positioned around the renal artery (distal to the flow probe) to provide the means to reduce RBF. The cables and vascular occluder tubing were tunneled subcutaneously and exteriorized between the scapulae and the abdomen was closed in layers.
Data acquisition.
After complete postoperative recovery, each animal was brought into the laboratory and allowed to roam freely and acclimate for approximately 15–20 min. Following this period, the animal was directed onto the treadmill. The CO, RBF, CBF, and HLBF flow probe cables were connected to transit-time perivascular flow meters (TS420; Transonic Systems). The LVP transmitter was turned on and the signal was collected via telemetry (Data Sciences International). The arterial catheter was connected to a pressure transducer (Transpac IV; ICU Medical). All aforementioned hemodynamic variables, in addition to MAP (calculated) and HR (triggered by the CO signal), were monitored as beat-by-beat averages and real-time waveforms by a data acquisition system (LabScribe; iWorx) and recorded for subsequent offline analysis.
Induction of hypertension.
Hypertension was induced via a modified (2K1C) Goldblatt model (31). After competition of control experiments, blood flow to the left kidney was reduced to a target level of ~30% of baseline via partial inflation of the renal vascular occluder. The level of RBF was checked at least two times per day and the vascular occluder adjusted until flow was stable. Hypertension gradually developed over the next several weeks. We defined hypertension as a systolic pressure ≥140 mmHg and a diastolic pressure ≥90 mmHg. The experiments were repeated after 34.4 ± 1.6 days of sustained hypertension. Thus the experiments were longitudinal in nature as each animal served as its own control.
Experimental procedures.
Both control and experimental procedures began with the animal standing unrestrained and still on the treadmill until all resting hemodynamic data were stable (typically 5–10 min). The treadmill was turned on and the speed was gradually increased to 3.2 km/h at 0% grade (mild exercise for a canine). Steady state was generally reached within 3–5 min. The muscle metaboreflex was engaged via partial reductions in HLBF during mild, dynamic exercise.
Data analysis.
CO, CBF, HLBF, RBF, LVP, HR, and MAP data were continuously recorded during each experimental procedure. Other hemodynamic parameters were calculated during offline data analysis [e.g., SV, dP/dtmax, dP/dtmin, total peripheral resistance (TPR; MAP/CO), nonischemic vascular conductance (NIVC), vascular conductance to all beds except the ischemic hindlimbs (CO-HLBF/MAP), coronary vascular conductance (CVC; CBF/MAP), and cardiac power (CP; MAPxCO/451)] (28). Muscle metaboreflex responses were quantified via analysis of the stimulus-response relationships via multiple linear regression analysis as described by Wyss et. al (117). Due to technical difficulties, we were only able to obtain dP/dtmax data for five animals. One-minute averages of steady-state data were calculated at rest, during exercise, and during metaboreflex activation. Mean values were averaged across all animals to obtain the population mean of the study.
Statistical analysis.
Averaged responses for each animal were analyzed with Systat software (Systat 11.0). P < 0.05 was set to determine statistical significance. A two-way repeated-measures ANOVA was used to compare hemodynamic data for time and/or conditional effects. In the event of a significant time-condition interaction, a C-matrix test for simple effects was performed. Data are reported as means ± SE.
RESULTS
Chronic reduction in RBF caused systemic hypertension, which was due to increased TPR as CO remained unchanged from normal levels (Table 1).
Table 1.
MAP, CO, and TPR at rest in the same animals before (normal) and after induction of hypertension
| Normal |
Hypertension |
|||||
|---|---|---|---|---|---|---|
| MAP, mmHg | CO, l/max | TPR, mmHg·l−1·max−1 | MAP, mmHg | CO, l/max | TPR, mmHg·l−1·max−1 | |
| Rest | 97.1 ± 2.6 | 3.44 ± 0.18 | 28.7 ± 1.9 | 132.1 ± 5.6* | 3.56 ± 0.17 | 37.6 ± 2.4* |
Values are means ± SE. MAP, mean arterial pressure; CO, cardiac output; TPR, total peripheral resistance.
P < 0.05 between normal and hypertension.
Average steady-state MAP, HR, SV, CO, and NIVC (Fig. 1) and CBF, CVC, dP/dtmax, and CP (Fig. 2) values are shown during rest, mild exercise, and muscle metaboreflex activation in control and after α1-adrenergic blockade in normal animals and in the same animals after induction of hypertension.
Fig. 1.
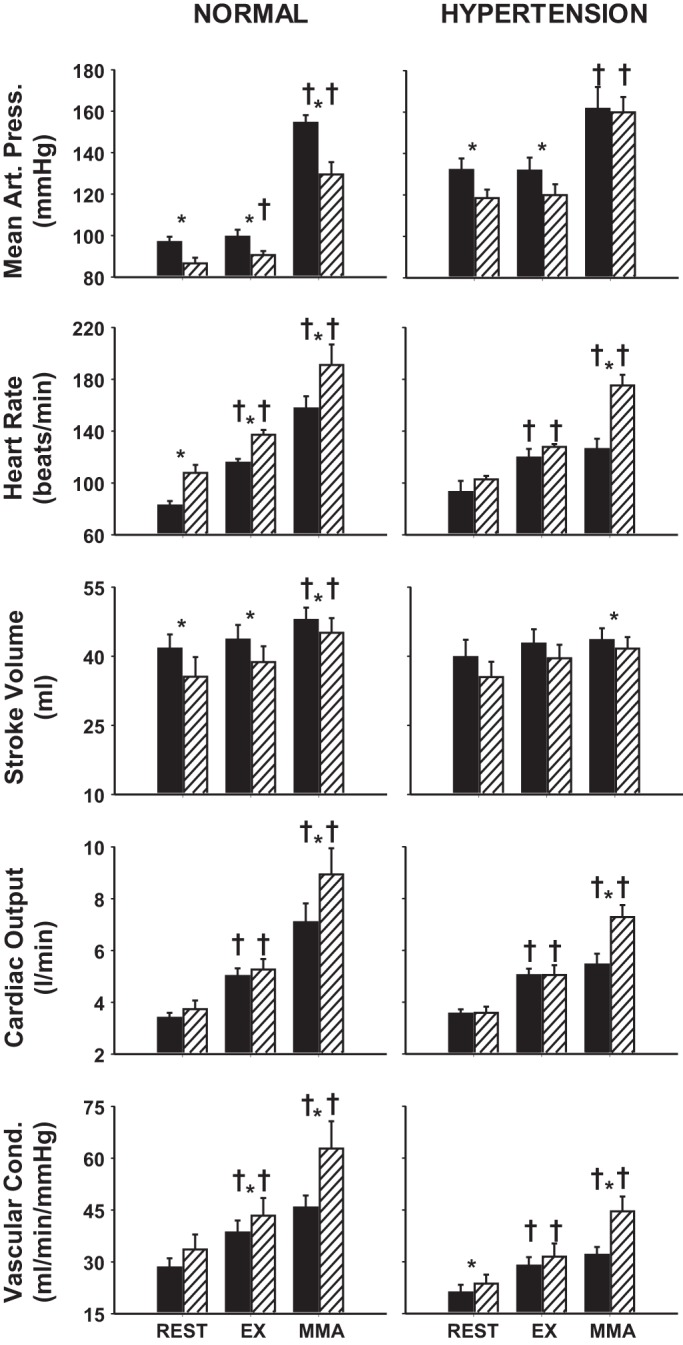
Average steady-state mean arterial pressure (MAP), heart rate (HR), stroke volume (SV), cardiac output (CO), and nonischemic vascular conductance (NIVC) values during rest, mild exercise (EX), and muscle metaboreflex activation (MMA) in control (filled bars) and after α1-adrenergic blockade (striped bars) in the same animals before (normal) after induction of hypertension. *P < 0.05 between control and α1-adrenergic blockade; †P < 0.05 from previous setting.
Fig. 2.
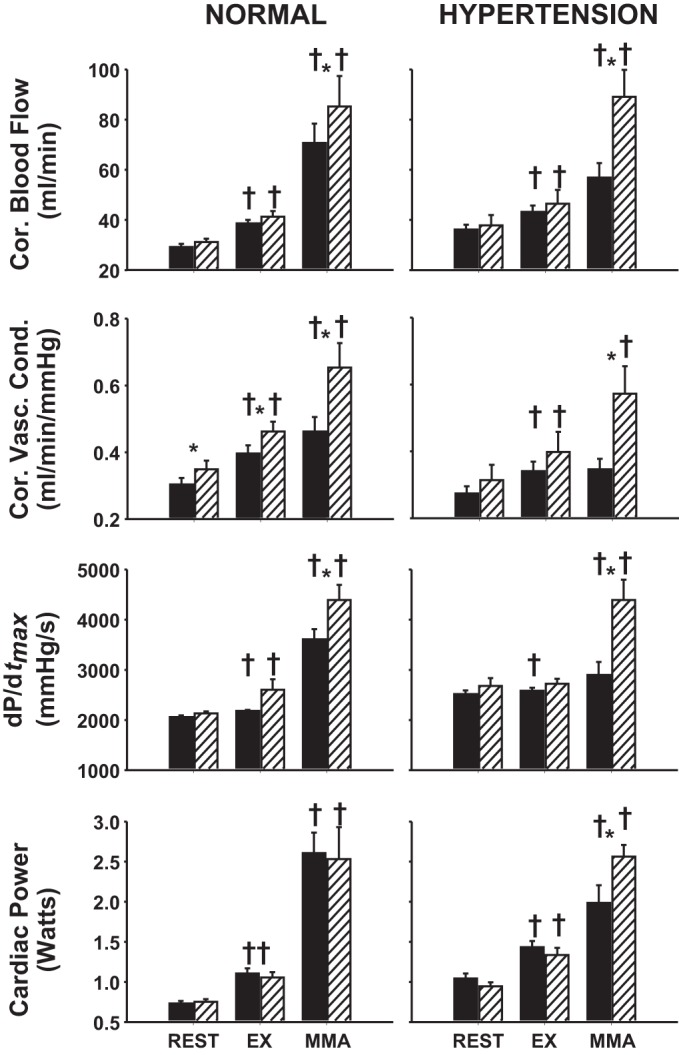
Average steady-state coronary blood flow (CBF), coronary vascular conductance (CVC), dP/dtmax, and cardiac power (CP) values during rest, mild exercise (EX, and muscle metaboreflex activation (MMA) in control (filled bars) and after α1-adrenergic blockade (striped bars) in the same animals before (normal) after induction of hypertension. *P < 0.05 between control and α1-adrenergic blockade; †P < 0.05 from previous setting.
Normal: Control vs. α1-adrenergic blockade.
The effects of α1-adrenergic blockade on cardiovascular parameters at rest, during exercise, and during peak metaboreflex activation in control experiments were similar to those we have previously observed in other studies (16, 55, 91). Briefly, HR, CO, NIVC, CBF, CVC, dP/dtmax, and CP increased from rest to exercise, while MAP and SV remained unchanged. Metaboreflex activation elicited increases in all cardiovascular parameters. Following α1-adrenergic blockade, resting MAP and SV were lower; HR and CVC were higher; and CO, NIVC, CBF, dP/dtmax, and CP were similar with respect to control. MAP, HR, CO, NIVC, CBF, CVC, dP/dtmax, and CP increased from rest to exercise, while SV remained unchanged. Metaboreflex activation increased all cardiovascular parameters. MAP and SV were lower; HR, CO, NIVC CBF, CVC, and dP/dtmax were higher; and CP was similar with respect to control during metaboreflex activation.
Hypertension: Control vs. α1-adrenergic blockade.
After induction of hypertension, HR, CO, NIVC, CBF, CVC, dP/dtmax, and CP increased from rest to exercise, while MAP and SV remained unchanged. Metaboreflex activation elicited increases in MAP, HR, CO, NIVC, CBF, dP/dtmax, and CP but did not affect SV or CVC. Following α1-adrenergic blockade, resting MAP was lower; NIVC was higher; and HR, SV, CO, CBF, CVC, dP/dtmax, and CP were similar with respect to control. HR, CO, NIVC, CBF, CVC, and CP increased from rest to exercise, while MAP, SV, and dP/dtmax remained unchanged. Metaboreflex activation increased MAP, HR, CO, NIVC, CBF, CVC, dP/dtmax, and CP but did not affect SV. HR, CO, NIVC, CBF, CVC, dP/dtmax, and CP were higher, SV lower, and MAP similar with respect to control during metaboreflex activation.
Average steady-state changes in MAP, HR, SV, CO, and NIVC (Fig. 3) and CBF, CVC, dP/dtmax, and CP (Fig. 4) are shown from mild exercise to muscle metaboreflex activation in control and after α1-adrenergic blockade in normal animals and in the same animals after induction of hypertension.
Fig. 3.
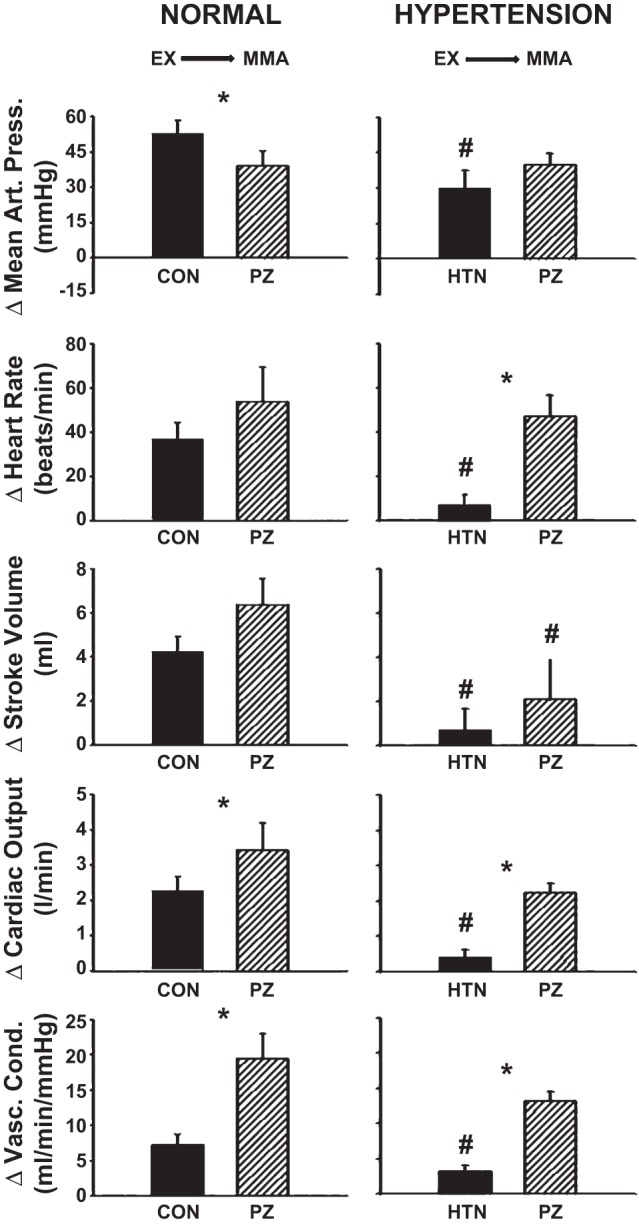
Average steady-state changes in MAP, HR, SV, CO, and NIVC from mild exercise (EX) to muscle metaboreflex activation (MMA) in control (filled bars) and after α1-adrenergic blockade [striped bars: prazosin (PZ)] in the same animals before (normal) after induction of hypertension (HTN). *P < 0.05 between control and prazosin; #P < 0.05 between normal and hypertension.
Fig. 4.
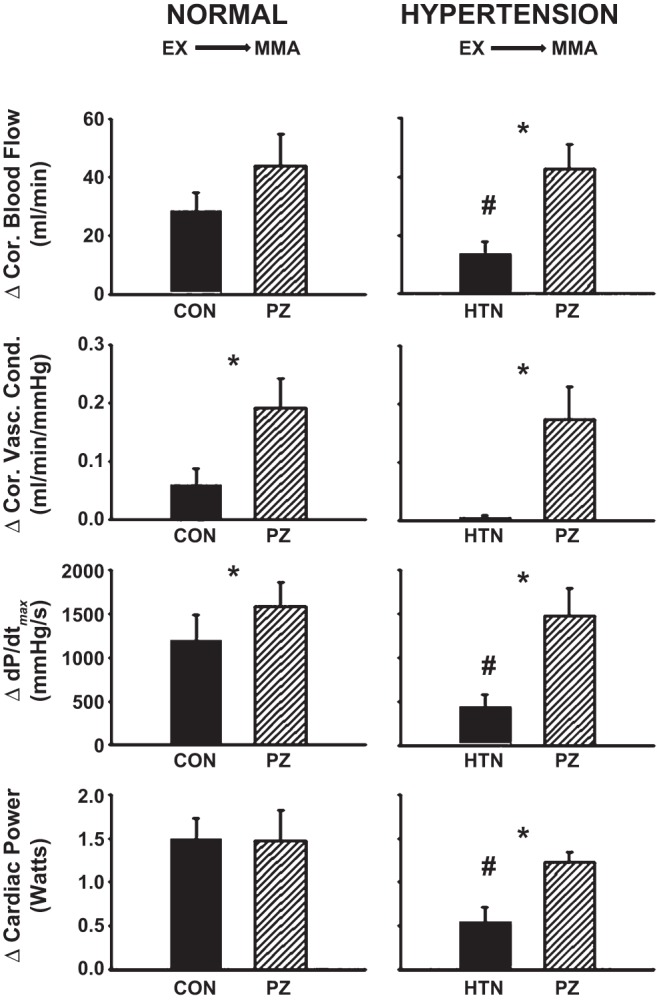
Average steady-state changes in CBF, CVC, dP/dtmax, and CP from mild exercise (EX) to muscle metaboreflex activation (MMA) in control (filled bars) and after α1-adrenergic blockade [striped bars: prazosin (PZ)] in the same animals before (normal) after induction of hypertension. *P < 0.05 between control and prazosin; #P < 0.05 between normal and hypertension.
Normal: Control vs. α1-adrenergic blockade.
Metaboreflex-induced increases in MAP were attenuated; CO, NIVC, CBF, CVC, and dP/dtmax were augmented; and HR, SV, and CP were not significantly affected following α1-adrenergic blockade.
Hypertension: Control vs. α1-adrenergic blockade.
Metaboreflex-induced increases in HR, CO, NIVC, CBF, CVC, dP/dtmax, and CP were significantly greater, and MAP and SV were not significantly affected following α1-adrenergic blockade.
Normal vs. hypertension.
Metaboreflex-induced increases in MAP, HR, SV, CO, NIVC, CBF, dP/dtmax, and CP were significantly attenuated following induction of hypertension, while CVC was unaffected. Following metaboreflex activation after α1-adrenergic blockade, increases in SV were significantly lower and MAP, HR, CO, NIVC, CBF, CVC, dP/dtmax, and CP were not significantly different from normal levels.
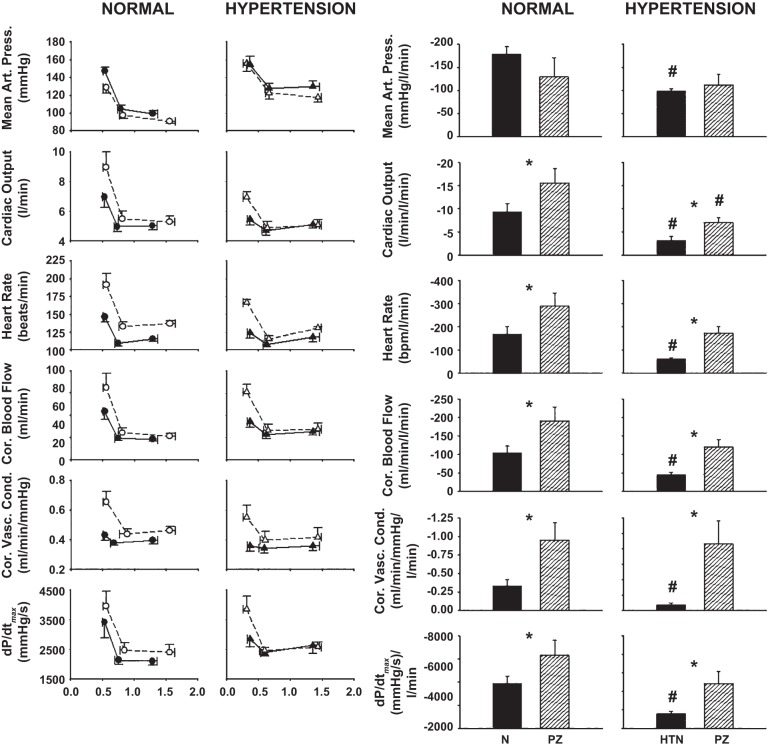
Figure 5, left, shows stimulus-response relationships for MAP, CO, HR, CBF, CVC, and dP/dtmax during free-flow exercise (mild exercise with no reduction in HLBF), threshold (intersection of the 2 linear regression lines), and maximal metaboreflex activation (metaboreflex-induced response at lowest imposed level of HLBF). The slopes of the metaboreflex-response lines are indicative of the gain of the metaboreflex for each individual cardiovascular parameter. Figure 5, right, shows slopes of the metaboreflex-induced response lines for MAP, CO, HR, CBF, CVC, and dP/dtmax in control and after α1-adrenergic blockade in normal animals and in the same animals after induction of hypertension. Following α1-adrenergic blockade, the slopes of the metaboreflex-induced response lines for CO, HR, CBF, CVC, and dP/dtmax were significantly higher while MAP was unchanged. These slopes were significantly lower for all cardiovascular parameters following induction of hypertension, while α1-adrenergic blockade fully restored the slopes (except CO; P = 0.042) to normal levels following α1-adrenergic blockade.
Fig. 5.
Left: stimulus-response relationships for MAP, CO, HR, CBF, CVC, and dP/dtmax as a function of graded reductions in hindlimb blood flow (HLBF). Data plotted are free-flow exercise, threshold, and max (from right to left, respectively) in control (solid lines/filled symbols) and after α1-adrenergic blockade (dashed lines/open symbols) in the same animals before (normal) after induction of hypertension. The slopes of the metaboreflex-response lines are indicative of the gain (or strength) of the metaboreflex for each individual cardiovascular parameter. Right: changes in the slopes of the metaboreflex-induced response lines for MAP, CO, HR, CBF, CVC and dP/dtmax in control (filled bars) and after α1-adrenergic blockade [striped bars: prazosin (PZ)] in the same animals before (normal) and after induction of hypertension. *P < 0.05 between control and prazosin; #P < 0.05 between normal and hypertension.
Figure 6 shows the relationship between CVC and CP in one animal. A straight line was ascribed from rest to free-flow exercise. Linear regression analysis was used fit a straight line through free-flow exercise and all subsequent reductions in HLBF. Following graded reductions, muscle metaboreflex activation elicited substantial increases in CP with only small increases in CVC.
Fig. 6.
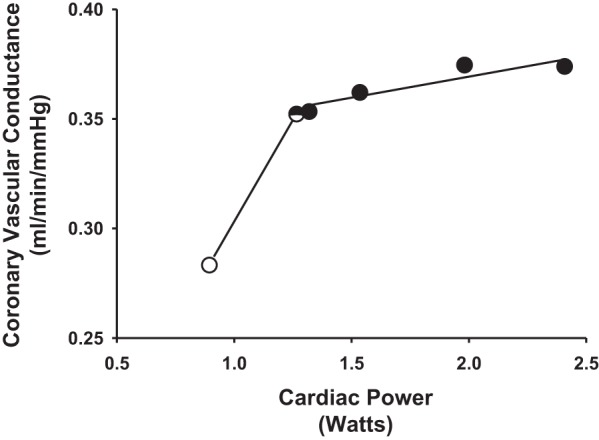
The relationship between CVC and CP in one animal during rest (open circle), free-flow exercise (half-filled circle), and all subsequent reductions in HLBF (filled circles). A straight line was ascribed from rest to free-flow exercise. Linear regression analysis was used fit a straight line through free-flow exercise and all subsequent reductions in HLBF. The relationship from rest to max was exceedingly nonlinear. Therefore, resting data were excluded and a straight line was ascribed to the remaining data points (i.e., free-flow exercise and all HLBF reductions) via linear regression analysis to appropriately compare the slopes of the relationship before and after induction of hypertension and with and without α1-adrenergic blockade (see Fig. 7).
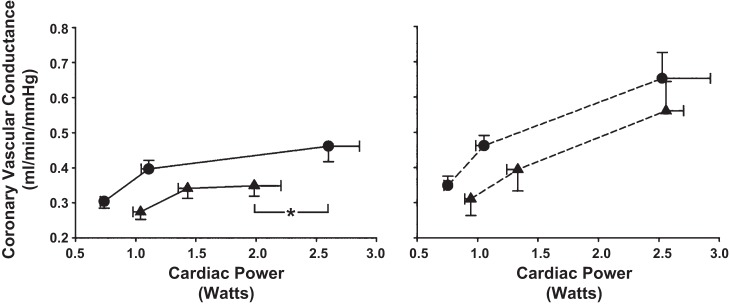
Figure 7 shows the relationship between CVC and CP before (circles) and after (triangles) induction of hypertension in control (left: solid lines) and following α1-adrenergic blockade (right: dashed lines) during rest, free-flow exercise, and max. After induction of hypertension, the slope of the relationship (from free-flow exercise to max) was significantly lower and the increase in CP was markedly attenuated. Following α1-adrenergic blockade, the slope of the relationship was significantly shifted upward and not any different than during α1-adrenergic blockade before induction of hypertension; however, the entire line was shifted toward lower CVC. Moreover, the increase in CP was not any different following α1-adrenergic blockade before and after induction of hypertension.
Fig. 7.
The relationships between CVC and CP before (circles) and after (triangles) induction of hypertension in control (left: solid lines) and following α1-adrenergic blockade (right: dashed lines) during rest, free-flow exercise, and max (left to right, respectively). The slope of the relationships from free-flow exercise to max was significantly lower following induction of hypertension and the increase in CP was markedly attenuated. Following α1-adrenergic blockade, the slope of this relationship and the increase in CP were not any different than during α1-adrenergic blockade before induction of hypertension. *P < 0.05 max CP before and after induction of hypertension.
Figure 8 shows the ratio of the changes in CVC and CP from free-flow exercise to max (i.e., ΔCVC:ΔCP) in control and following α1-adrenergic blockade before and after induction of hypertension. Following α1-adrenergic blockade, ΔCVC:ΔCP was significantly greater than in control. Following induction of hypertension, ΔCVC:ΔCP was significantly reduced. Following α1-adrenergic blockade, ΔCVC:ΔCP was not any different than during α1-adrenergic blockade before induction of hypertension.
Fig. 8.
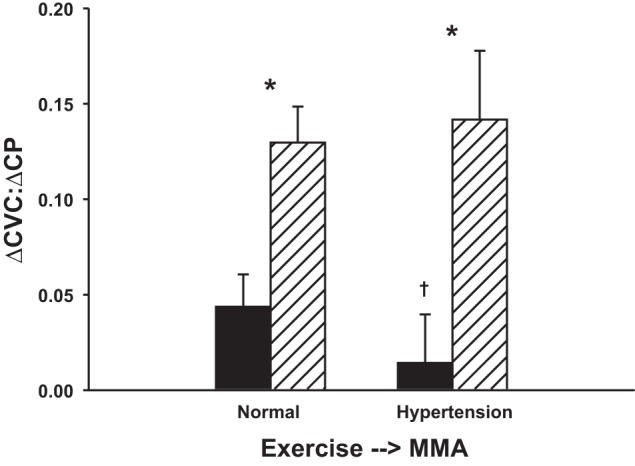
Ratio of changes in CVC and CP from free-flow exercise to max (i.e., ΔCVC:ΔCP) in control (filled bars) and following α1-adrenergic blockade (striped bars) in the same animals before (normal) and after induction of hypertension. Following induction of hypertension, ΔCVC:ΔCP was significantly reduced. Following α1-adrenergic blockade, ΔCVC:ΔCP was not any different than during α1-adrenergic blockade before induction of hypertension. *P < 0.05 between control and α1-adrenergic blockade; †P < 0.05 between normal and hypertension.
Figure 9 shows the relationship between dP/dtmax and CBF in control and after α1-adrenergic blockade in normal animals and in the same animals after induction of hypertension. The slope of the relationship between dP/dtmax and CBF was exceedingly linear with all data points considered. After induction of hypertension, metaboreflex-induced increases in both dP/dtmax and CBF were significantly attenuated. However, metaboreflex-induced increases in both dP/dtmax and CBF were not significantly different following α1-adrenergic blockade before and after induction of hypertension.
Fig. 9.
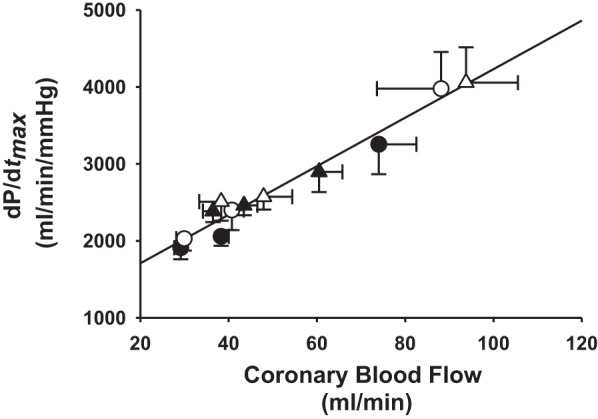
Relationship between dP/dtmax and CBF in control (filled symbols) and after α1-adrenergic blockade (open symbols) in the same animals before (normal) (circles) and after induction of hypertension (triangles). The slope of the relationship between dP/dtmax and CBF was exceedingly linear with all data points considered. After induction of hypertension, metaboreflex-induced increases in both dP/dtmax and CBF were significantly attenuated. Following α1-adrenergic blockade, metaboreflex-induced increases in both dP/dtmax and CBF were not any different before and after induction of hypertension.
DISCUSSION
Our major new findings are that increases in CBF during metaboreflex activation are attenuated and the ability to increase ventricular contractility is reduced after induction of hypertension. Blockade of α1-adrenergic receptors restored the increases in CBF and ventricular function toward normal levels indicating that the primary mechanism mediating the impaired metaboreflex-induced increases in ventricular function in hypertension is accentuated coronary vasoconstriction. In addition, metaboreflex-induced increases in CO and MAP are also attenuated in hypertension, and therefore the ability of the reflex to restore blood flow to ischemic working muscle is also likely impaired.
Coronary blood flow during exercise.
The increase in myocardial oxygen demand during submaximal dynamic exercise is principally met by increases in CBF inasmuch as myocardial oxygen consumption is highly blood flow dependent since oxygen extraction is already near maximal at rest (24, 26, 115). The rise in CBF is largely mediated via vasodilation as only modest increases in MAP occur (32). The rise in cardiac SNA with exercise acts both to promote coronary vasodilation via increasing cardiac work (as well as feed-forward, β2-adrgenergic receptor-mediated vasodilation) as well as coronary vasoconstriction via activation of vascular α1-adrenergic receptors. Gwirtz and colleagues demonstrated an α1-adrenergic-mediated coronary constrictor tone during dynamic exercise in normal animals (23, 41) and in animals with renovascular hypertension (39). Moreover, a coronary constrictor tone during exercise has also been shown to attenuate increases in CBF (40, 41, 47, 60, 85, 91), myocardial oxygen delivery (47), ventricular function (16, 40, 41, 60), and CO (16, 60). Paradoxically, in normal animals, this constrictor tone has been suggested to play a beneficial role in redistributing left-ventricular CBF from the epicardium to the more metabolically challenged subendocardium (27, 49); however, this hypothesis is not broadly accepted (10, 44).
Following muscle metaboreflex activation, substantial increases in cardiac SNA, CO, and afterload increase myocardial work and oxygen demand which is met by increases in CBF. These increases in CBF are solely driven by increases in MAP as little, if any, coronary vasodilation occurs. Coutsos et al. (16) showed in normal dogs that metaboreflex-mediated increases in CVC and CBF markedly improved following α1-adrenergic blockade and the reflex increases in ventricular function were accentuated. Metaboreflex activation in heart failure causes frank coronary vasoconstriction (17). Pharmacological blockade of this metaboreflex-induced neurogenic vasoconstriction partially restores ventricular function in heart failure (17).
Effect of hypertension on the muscle metaboreflex.
Is muscle metaboreflex function attenuated in hypertension? While few studies have investigated the effects of hypertension on metaboreflex control of cardiovascular function, most report that its function is accentuated (22, 35, 69, 79, 101) rather than attenuated (95). We analyzed the gain (or strength) of metaboreflex-mediated cardiovascular responses in hypertension by determining the slope of the metaboreflex-induced response line for each parameter measured. After induction of hypertension, the slopes were significantly lower for all cardiovascular parameters (see Fig. 5) indicating that metaboreflex function is attenuated in hypertension. However, following α1-adrenergic blockade the slopes of all metaboreflex-induced response lines were fully restored to the levels observed in control experiments following α1-adrenergic blockade. These data suggest that inotropic function is attenuated during metaboreflex activation in hypertension due to a restriction in CBF stemming from exaggerated coronary constrictor tone, not an attenuation in metaboreflex function per se nor any inherent reduction in intrinsic myocardial function (e.g., heart failure). Reduced CBF may also impair ventricular diastolic function and thereby limit increases in SV via impaired ventricular relaxation.
Coronary blood flow: Ventricular function interrelationship.
While changes in CBF can directly influence ventricular function, changes in ventricular function can indirectly influence CBF via changes in coronary metabolic vasodilation. As an increase in perfusion pressure can increase CBF independent of any change in coronary vasomotor tone (6), the extent of coronary vasodilation can only accurately be determined by calculating changes in conductance (or resistance) (87). Feigl and colleagues (32, 33) have shown the utility of quantifying coronary vasodilation as a function of myocardial oxygen consumption. As in our previous studies (16, 17), we used CP, which is well correlated to myocardial oxygen consumption (58). In normal animals, CVC increased from rest to exercise and a small, further increase occurred with metaboreflex activation similar to the response observed previously (with exception of the small increase in CVC, which was not statistically significant in our previous study). After induction of hypertension, the slope of the relationship (from free-flow exercise to maximal metaboreflex activation) was significantly lower and the increase in CP was markedly attenuated indicating reduced myocardial oxygen delivery as a result of a restrained coronary vasodilation. Following α1-adrenergic blockade, the slope of the relationship was significantly shifted upward and not significantly different than during α1-adrenergic blockade before induction of hypertension. Moreover, the increase in CP was not significantly different following α1-adrenergic blockade before and after induction of hypertension. These findings demonstrate that impaired metaboreflex-induced increases in CP are largely, if not solely, caused by heightened α1-adrenergic-mediated coronary vasoconstriction in hypertension.
An increase in CBF can improve ventricular function via an increase in myocardial oxygen delivery (86). We analyzed the relationship between ventricular function and coronary perfusion via linear regression of the relationship between dP/dtmax vs. CBF as described previously (17). This relationship was exceptionally linear. After induction of hypertension, metaboreflex-induced increases in both dP/dtmax and CBF were significantly attenuated, without any change in the slope of the relationship. Following α1-adrenergic blockade, metaboreflex-induced increases in dP/dtmax and CBF were restored to the same levels following α1-adrenergic blockade before induction of hypertension. These data support our hypothesis that exaggerated coronary vasoconstriction in hypertension with metaboreflex activation restrains coronary perfusion thereby limiting myocardial oxygen delivery and increases in ventricular function. While the highly linear relationship between dP/dtmax and CBF suggests that ventricular function is intimately dependent on changes in CBF, the fact that the slope of this relationship did not change after induction of hypertension suggests that most, if not all, of the ventricular impairment observed in this study was solely due to exaggerated coronary vasoconstriction, not myocardial dysfunction per se. Coutsos et al. (17) reported that exaggerated metaboreflex-mediated coronary vasoconstriction attenuates the rise in CBF and impairs increases in ventricular function in heart failure. Furthermore, they demonstrated that this impairment in ventricular function was partially ameliorated following α1-adrenergic blockade. They concluded that the impaired ventricular function in heart failure was due to both intrinsic ventricular dysfunction as well as exaggerated coronary vasoconstriction.
Perspectives.
The pathology of hypertension is wide-ranging. Structural and/or functional changes have been well documented in the heart and the vasculature (93). Moreover, alterations in arterial baroreflex (34, 118) and muscle metaboreflex function (22, 35, 68, 80, 95, 101) as well as brainstem processing of this reflex information have been demonstrated (36). The attenuation of chronotropic and inotropic function after induction of hypertension observed in the present study is likely not due to overt pathology of the heart and the vasculature as α1-adrenergic blockade fully restored chronotropic and inotropic function to the same levels observed after α1-adrenergic blockade before induction of hypertension. The arterial baroreflex buffers muscle metaboreflex-mediated pressor responses by attenuating metaboreflex-induced peripheral vasoconstriction (59, 104). There are reports of reduced baroreflex function in hypertension (54, 81, 110, 118); however, to the best of our knowledge, there are no studies demonstrating accentuated baroreflex function. Therefore, it is unlikely that the attenuated cardiovascular responses reported in the present study are a result of enhanced arterial baroreflex buffering of the muscle metaboreflex. Moreover, Smith et al. (109) demonstrated that impaired baroreflex function in hypertension has little, if any, effect on skeletal muscle reflex function. Dysfunctional metaboreflex afferent signal processing could at least partially explain the attenuated reflex-mediated increases in cardiovascular function; however, data suggest that metaboreceptors are actually sensitized in hypertension (79). Previous studies have demonstrated impaired cardiac responses potentially stemming from reduced β-adrenergic receptor function (11, 120) and density (77).
Hypertensive subjects have higher resting sympathetic activity, which can increase to dangerously high levels during strenuous exercise causing exaggerated increases in arterial pressure and heart rate and intense peripheral vasoconstriction. These pathological responses to exercise can be used as independent risk factors for cardiovascular mortality (57, 61, 62, 64, 82–84, 100). Indeed, “the ACC/AHA 2002 Guideline Update for Exercise Testing” warns that “exercise tolerance is decreased in patients with poor blood pressure control and severe systemic hypertension may cause exercise-induced ST depression in the absence of atherosclerosis” (30). Exaggerated coronary vasoconstriction during metaboreflex activation could have devastating consequences for hypertensive patients leading to arrhythmias, coronary artery vasospasm, and sudden cardiac death. Therapies aimed at lowering sympathetic activation in response to exercise or at limiting the resultant coronary vasoconstriction could help in preventing adverse responses to exercise in these patients.
Potential limitations.
All animals in the present study were female due to availability with our vendor. Data were not collected from animals during estrus. Our laboratory has shown that muscle metaboreflex function is not influenced by gender in normal canines (67).
Systemic α1-adrenergic blockade can alter loading conditions via reduced total peripheral resistance. Gwirtz and colleagues (23, 40, 60) administered prazosin via the intracoronary route. However, results from such studies on changes in ventricular function following α1-adrenergic blockade are limited to the particular segment of the left ventricle infused with drug. In the present study, we were interested in the effects of α1-adrenergic blockade on global left-ventricular function, and therefore, we infused prazosin systemically. We employed a selective, α1-adrenergic blocker as several studies have demonstrated that α2-adrenergic receptors have negligible effects on coronary vasomotor tone during exercise (9, 20, 40, 41, 113). However, some studies have shown that, along with α1-adrenergic receptors, α2-adrenergic receptors also play an important role in the modulation of coronary vasomotor tone (46, 102). The relative roles of α1- and α2-receptors as well as others mediating vasoactive responses from other neurotransmitters and circulating hormones (e.g., neuropeptide Y, vasopressin, angiotensin II, etc.) have yet to be elucidated.
Following α1-adrenergic blockade in normal animals, MAP was lower at rest, during exercise and during exercise with metaboreflex activation with respect to control. After induction of hypertension in the same animals, MAP was lower at rest and during exercise with respect to control. Interestingly, during exercise with metaboreflex activation, MAP following α1-adrenergic blockade was not any different than control (likely due to the blunted rise NIVC in hypertension). Our hypothesis was that increases in ventricular function would be impaired due to exaggerated coronary vasoconstriction during metaboreflex activation in hypertension. Therefore, the major potential concern with systemic prazosin administration would be its effects on our index of contractility (dP/dtmax) as this measurement is sensitive to loading conditions. However, while changes in preload can significantly affect dP/dtmax (94), changes in afterload have been shown to have little, if any, direct effect on this parameter (1, 52, 73, 94).
While nonselective α-adrenergic blockers reduce vasomotor tone by antagonizing both coronary α1- and α2-adrenergic receptors, it is well established that these agents also antagonize α2-adrenergic receptors on presynaptic nerve terminals, countering the negative feedback inhibitor effect of norepinephrine on the receptor, thereby leading to enhanced norepinephrine release (65, 66, 119). Heyndrickx et al. (48) reported elevated coronary sinus norepinephrine following selective α2-adrenergic blockade (yohimbine) in conscious canines causing enhanced chronotropic and inotropic effects during exercise. Cardiac presynaptic α1-adrenergic receptors have not been characterized. However, there are whole animal studies that support (37) and refute (41, 48) the existence of a negative feedback inhibitor effect of norepinephrine on a purported cardiac presynaptic α1-adrenergic receptor. As we employed a selective α1-adrenergic blocker (prazosin) in the present study, we do not expect that our chronotropic and inotropic data were influenced by this phenomenon. With that said, the perplexing rise in heart rate we observed following prazosin could be explained by this mechanism, and so we cannot entirely rule it out. Furthermore, the relative roles of α1- and α2-adrenergic receptors (as well as the receptors for other potential neurotransmitters, neuromodulators, and circulating vasoactive hormones such as NPY, angiotensin II, adenosine, vasopressin, etc.) may change during the development of hypertension. Clearly, further studies are necessary to determine the relative roles of all major vasoactive substances in the control of coronary blood flow during exercise and the effects of hypertension on these mechanisms.
In summary, we found that during metaboreflex activation coronary vasoconstriction is accentuated in hypertension, which markedly restrains coronary perfusion and thereby limits myocardial oxygen delivery and increases in ventricular function during submaximal dynamic exercise. Coronary vasoconstriction in hypertension could partially explain exercise intolerance and potentially precipitate adverse cardiovascular events during exercise such as coronary vasospasms, arrhythmias, myocardial ischemia, or sudden cardiac death (45).
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grants HL-55743 and HL-126706.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
M.D.S., J.K., A.C.K., R.A.-H., A.A., T.M.M., and R.A.A. performed experiments; M.D.S., J.K., J.A.S.-M., A.C.K., R.A.-H., A.A., T.M.M., R.A.A., and D.S.O'L. analyzed data; M.D.S., J.K., J.A.S.-M., R.A.-H., A.A., T.M.M., R.A.A., and D.S.O'L. interpreted results of experiments; M.D.S., J.K., A.C.K., T.M.M., and D.S.O'L. prepared figures; M.D.S. and D.S.O'L. drafted manuscript; M.D.S., J.K., R.A.A., and D.S.O'L. edited and revised manuscript; M.D.S., J.K., J.A.S.-M., A.C.K., R.A.-H., A.A., T.M.M., R.A.A., and D.S.O'L. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Jody Helme-Day for expert animal care and technical assistance.
REFERENCES
- 1.Adler D, Nikolic SD, Pajaro O, Sonnenblick EH, Yellin EL. Time to dP/dtmax reflects both inotropic and chronotropic properties of cardiac contraction: a conscious dog study. Physiol Meas 17: 287–295, 1996. doi: 10.1088/0967-3334/17/4/006. [DOI] [PubMed] [Google Scholar]
- 2.Adreani CM, Kaufman MP. Effect of arterial occlusion on responses of group III and IV afferents to dynamic exercise. J Appl Physiol (1985) 84: 1827–1833, 1998. [DOI] [PubMed] [Google Scholar]
- 3.Alam M, Smirk FH. Observations in man upon a blood pressure raising reflex arising from the voluntary muscles. J Physiol 89: 372–383, 1937. doi: 10.1113/jphysiol.1937.sp003485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alam M, Smirk FH. Observations in man on a pulse-accelerating reflex from the voluntary muscles of the legs. J Physiol 92: 167–177, 1938. doi: 10.1113/jphysiol.1938.sp003592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amann M, Runnels S, Morgan DE, Trinity JD, Fjeldstad AS, Wray DW, Reese VR, Richardson RS. On the contribution of group III and IV muscle afferents to the circulatory response to rhythmic exercise in humans. J Physiol 589: 3855–3866, 2011. doi: 10.1113/jphysiol.2011.209353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ansorge EJ, Shah SH, Augustyniak RA, Rossi NF, Collins HL, O’Leary DS. Muscle metaboreflex control of coronary blood flow. Am J Physiol Heart Circ Physiol 283: H526–H532, 2002. doi: 10.1152/ajpheart.00152.2002. [DOI] [PubMed] [Google Scholar]
- 7.Asmussen E, Nielsen M. Experiments on nervous factors controlling respiration and circulation during exercise employing blocking of the blood flow. Acta Physiol Scand 60: 103–111, 1964. doi: 10.1111/j.1748-1716.1964.tb02873.x. [DOI] [PubMed] [Google Scholar]
- 8.Augustyniak RA, Collins HL, Ansorge EJ, Rossi NF, O’Leary DS. Severe exercise alters the strength and mechanisms of the muscle metaboreflex. Am J Physiol Heart Circ Physiol 280: H1645–H1652, 2001. [DOI] [PubMed] [Google Scholar]
- 9.Bache RJ, Dai XZ, Herzog CA, Schwartz JS. Effects of nonselective and selective alpha 1-adrenergic blockade on coronary blood flow during exercise. Circ Res 61: II36–II41, 1987. [PubMed] [Google Scholar]
- 10.Baumgart D, Ehring T, Kowallik P, Guth BD, Krajcar M, Heusch G. Impact of alpha-adrenergic coronary vasoconstriction on the transmural myocardial blood flow distribution during humoral and neuronal adrenergic activation. Circ Res 73: 869–886, 1993. doi: 10.1161/01.RES.73.5.869. [DOI] [PubMed] [Google Scholar]
- 11.Böhm M, Gierschik P, Knorr A, Larisch K, Weismann K, Erdmann E. Desensitization of adenylate cyclase and increase of Gi alpha in cardiac hypertrophy due to acquired hypertension. Hypertension 20: 103–112, 1992. doi: 10.1161/01.HYP.20.1.103. [DOI] [PubMed] [Google Scholar]
- 12.Boushel R. Muscle metaboreflex control of the circulation during exercise. Acta Physiol (Oxf) 199: 367–383, 2010. doi: 10.1111/j.1748-1716.2010.02133.x. [DOI] [PubMed] [Google Scholar]
- 13.Boushel R, Madsen P, Nielsen HB, Quistorff B, Secher NH. Contribution of pH, diprotonated phosphate and potassium for the reflex increase in blood pressure during handgrip. Acta Physiol Scand 164: 269–275, 1998. doi: 10.1046/j.1365-201X.1998.00429.x. [DOI] [PubMed] [Google Scholar]
- 14.Choi HM, Stebbins CL, Lee OT, Nho H, Lee JH, Chun JM, Kim KA, Kim JK. Augmentation of the exercise pressor reflex in prehypertension: roles of the muscle metaboreflex and mechanoreflex. Appl Physiol Nutr Metab 38: 209–215, 2013. doi: 10.1139/apnm-2012-0143. [DOI] [PubMed] [Google Scholar]
- 15.Coote JH, Hilton SM, Perez-Gonzalez JF. The reflex nature of the pressor response to muscular exercise. J Physiol 215: 789–804, 1971. doi: 10.1113/jphysiol.1971.sp009498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coutsos M, Sala-Mercado JA, Ichinose M, Li Z, Dawe EJ, O’Leary DS. Muscle metaboreflex-induced coronary vasoconstriction functionally limits increases in ventricular contractility. J Appl Physiol (1985) 109: 271–278, 2010. doi: 10.1152/japplphysiol.01243.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coutsos M, Sala-Mercado JA, Ichinose M, Li Z, Dawe EJ, O’Leary DS. Muscle metaboreflex-induced coronary vasoconstriction limits ventricular contractility during dynamic exercise in heart failure. Am J Physiol Heart Circ Physiol 304: H1029–H1037, 2013. doi: 10.1152/ajpheart.00879.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crisafulli A, Scott AC, Wensel R, Davos CH, Francis DP, Pagliaro P, Coats AJS, Concu A, Piepoli MF. Muscle metaboreflex-induced increases in stroke volume. Med Sci Sports Exerc 35: 221–228, 2003. doi: 10.1249/01.MSS.0000048639.02548.24. [DOI] [PubMed] [Google Scholar]
- 19.Crisafulli A, Piras F, Filippi M, Piredda C, Chiappori P, Melis F, Milia R, Tocco F, Concu A. Role of heart rate and stroke volume during muscle metaboreflex-induced cardiac output increase: differences between activation during and after exercise. J Physiol Sci 61: 385–394, 2011. doi: 10.1007/s12576-011-0163-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dai XZ, Sublett E, Lindstrom P, Schwartz JS, Homans DC, Bache RJ. Coronary flow during exercise after selective α1- and α2-adrenergic blockade. Am J Physiol Heart Circ Physiol 256: H1148–H1155, 1989. [DOI] [PubMed] [Google Scholar]
- 21.Darques JL, Decherchi P, Jammes Y. Mechanisms of fatigue-induced activation of group IV muscle afferents: the roles played by lactic acid and inflammatory mediators. Neurosci Lett 257: 109–112, 1998. doi: 10.1016/S0304-3940(98)00816-7. [DOI] [PubMed] [Google Scholar]
- 22.Delaney EP, Greaney JL, Edwards DG, Rose WC, Fadel PJ, Farquhar WB. Exaggerated sympathetic and pressor responses to handgrip exercise in older hypertensive humans: role of the muscle metaboreflex. Am J Physiol Heart Circ Physiol 299: H1318–H1327, 2010. doi: 10.1152/ajpheart.00556.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dodd-o JM, Gwirtz PA. Coronary alpha 1-adrenergic constrictor tone varies with intensity of exercise. Med Sci Sports Exerc 28: 62–71, 1996. doi: 10.1097/00005768-199601000-00015. [DOI] [PubMed] [Google Scholar]
- 24.Duncker DJ, Bache RJ. Regulation of coronary blood flow during exercise. Physiol Rev 88: 1009–1086, 2008. doi: 10.1152/physrev.00045.2006. [DOI] [PubMed] [Google Scholar]
- 25.Eiken O, Bjurstedt H. Dynamic exercise in man as influenced by experimental restriction of blood flow in the working muscles. Acta Physiol Scand 131: 339–345, 1987. doi: 10.1111/j.1748-1716.1987.tb08248.x. [DOI] [PubMed] [Google Scholar]
- 26.Feigl EO. Coronary physiology. Physiol Rev 63: 1–205, 1983. [DOI] [PubMed] [Google Scholar]
- 27.Feigl EO. The paradox of adrenergic coronary vasoconstriction. Circulation 76: 737–745, 1987. doi: 10.1161/01.CIR.76.4.737. [DOI] [PubMed] [Google Scholar]
- 28.Fincke R, Hochman JS, Lowe AM, Menon V, Slater JN, Webb JG, LeJemtel TH, Cotter G; SHOCK Investigators . Cardiac power is the strongest hemodynamic correlate of mortality in cardiogenic shock: a report from the SHOCK trial registry. J Am Coll Cardiol 44: 340–348, 2004. doi: 10.1016/j.jacc.2004.03.060. [DOI] [PubMed] [Google Scholar]
- 29.Fisher JP, Adlan AM, Shantsila A, Secher JF, Sørensen H, Secher NH. Muscle metaboreflex and autonomic regulation of heart rate in humans. J Physiol 591: 3777–3788, 2013. doi: 10.1113/jphysiol.2013.254722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gibbons RJ, Balady GJ, Bricker JT, Chaitman BR, Fletcher GF, Froelicher VF, Mark DB, McCallister BD, Mooss AN, O’Reilly MG, Winters WL Jr, Gibbons RJ, Antman EM, Alpert JS, Faxon DP, Fuster V, Gregoratos G, Hiratzka LF, Jacobs AK, Russell RO, Smith SC Jr; American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1997 Exercise Testing Guidelines) . ACC/AHA 2002 guideline update for exercise testing: summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1997 Exercise Testing Guidelines). Circulation 106: 1883–1892, 2002. doi: 10.1161/01.CIR.0000034670.06526.15. [DOI] [PubMed] [Google Scholar]
- 31.Goldblatt H, Lynch J, Hanzal RF, Summerville WW. Studies on experimental hypertension: I. the production of persistent elevation of systolic blood pressure by means of renal ischemia. J Exp Med 59: 347–379, 1934. doi: 10.1084/jem.59.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gorman MW, Tune JD, Richmond KN, Feigl EO. Feedforward sympathetic coronary vasodilation in exercising dogs. J Appl Physiol (1985) 89: 1892–1902, 2000. [DOI] [PubMed] [Google Scholar]
- 33.Gorman MW, Tune JD, Richmond KN, Feigl EO. Quantitative analysis of feedforward sympathetic coronary vasodilation in exercising dogs. J Appl Physiol (1985) 89: 1903–1911, 2000. [DOI] [PubMed] [Google Scholar]
- 34.Grassi G. Role of the sympathetic nervous system in human hypertension. J Hypertens 16, Suppl: 1979–1987, 1998. doi: 10.1097/00004872-199816121-00019. [DOI] [PubMed] [Google Scholar]
- 35.Greaney JL, Matthews EL, Boggs ME, Edwards DG, Duncan RL, Farquhar WB. Exaggerated exercise pressor reflex in adults with moderately elevated systolic blood pressure: role of purinergic receptors. Am J Physiol Heart Circ Physiol 306: H132–H141, 2014. doi: 10.1152/ajpheart.00575.2013. [DOI] [PubMed] [Google Scholar]
- 36.Guo GB, Abboud FM. Impaired central mediation of the arterial baroreflex in chronic renal hypertension. Am J Physiol Heart Circ Physiol 246: H720–H727, 1984. [DOI] [PubMed] [Google Scholar]
- 37.Guth BD, Thaulow E, Heusch G, Seitelberger R, Ross J Jr. Myocardial effects of selective alpha-adrenoceptor blockade during exercise in dogs. Circ Res 66: 1703–1712, 1990. doi: 10.1161/01.RES.66.6.1703. [DOI] [PubMed] [Google Scholar]
- 38.Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci 7: 335–346, 2006. doi: 10.1038/nrn1902. [DOI] [PubMed] [Google Scholar]
- 39.Gwirtz PA. Coronary alpha 1-constrictor tone during renovascular hypertension. Circulation 92: 1576–1581, 1995. doi: 10.1161/01.CIR.92.6.1576. [DOI] [PubMed] [Google Scholar]
- 40.Gwirtz PA, Dodd-o JM, Brandt MA, Jones CE. Augmentation of coronary flow improves myocardial function in exercise. J Cardiovasc Pharmacol 15: 752–758, 1990. doi: 10.1097/00005344-199005000-00010. [DOI] [PubMed] [Google Scholar]
- 41.Gwirtz PA, Overn SP, Mass HJ, Jones CE. α1-Adrenergic constriction limits coronary flow and cardiac function in running dogs. Am J Physiol Heart Circ Physiol 250: H1117–H1126, 1986. [DOI] [PubMed] [Google Scholar]
- 42.Hammond RL, Augustyniak RA, Rossi NF, Churchill PC, Lapanowski K, O’Leary DS. Heart failure alters the strength and mechanisms of the muscle metaboreflex. Am J Physiol Heart Circ Physiol 278: H818–H828, 2000. [DOI] [PubMed] [Google Scholar]
- 43.Hanna RL, Kaufman MP. Role played by purinergic receptors on muscle afferents in evoking the exercise pressor reflex. J Appl Physiol (1985) 94: 1437–1445, 2003. doi: 10.1152/japplphysiol.01011.2002. [DOI] [PubMed] [Google Scholar]
- 44.Heusch G. The paradox of α-adrenergic coronary vasoconstriction revisited. J Mol Cell Cardiol 51: 16–23, 2011. doi: 10.1016/j.yjmcc.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 45.Heusch G, Baumgart D, Camici P, Chilian W, Gregorini L, Hess O, Indolfi C, Rimoldi O. alpha-adrenergic coronary vasoconstriction and myocardial ischemia in humans. Circulation 101: 689–694, 2000. doi: 10.1161/01.CIR.101.6.689. [DOI] [PubMed] [Google Scholar]
- 46.Heusch G, Deussen A, Schipke J, Thämer V. Alpha 1- and alpha 2-adrenoceptor-mediated vasoconstriction of large and small canine coronary arteries in vivo. J Cardiovasc Pharmacol 6: 961–968, 1984. doi: 10.1097/00005344-198409000-00034. [DOI] [PubMed] [Google Scholar]
- 47.Heyndrickx GR, Muylaert P, Pannier JL. α1-Adrenergic control of oxygen delivery to myocardium during exercise in conscious dogs. Am J Physiol Heart Circ Physiol 242: H805–H809, 1982. [DOI] [PubMed] [Google Scholar]
- 48.Heyndrickx GR, Vilaine JP, Moerman EJ, Leusen I. Role of prejunctional alpha 2-adrenergic receptors in the regulation of myocardial performance during exercise in conscious dogs. Circ Res 54: 683–693, 1984. doi: 10.1161/01.RES.54.6.683. [DOI] [PubMed] [Google Scholar]
- 49.Huang AH, Feigl EO. Adrenergic coronary vasoconstriction helps maintain uniform transmural blood flow distribution during exercise. Circ Res 62: 286–298, 1988. doi: 10.1161/01.RES.62.2.286. [DOI] [PubMed] [Google Scholar]
- 50.Ichinose M, Delliaux S, Watanabe K, Fujii N, Nishiyasu T. Evaluation of muscle metaboreflex function through graded reduction in forearm blood flow during rhythmic handgrip exercise in humans. Am J Physiol Heart Circ Physiol 301: H609–H616, 2011. doi: 10.1152/ajpheart.00076.2011. [DOI] [PubMed] [Google Scholar]
- 51.Ichinose MJ, Sala-Mercado JA, Coutsos M, Li Z, Ichinose TK, Dawe E, O’Leary DS. Modulation of cardiac output alters the mechanisms of the muscle metaboreflex pressor response. Am J Physiol Heart Circ Physiol 298: H245–H250, 2010. doi: 10.1152/ajpheart.00909.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ishikawa K, Chemaly ER, Tilemann L, Fish K, Ladage D, Aguero J, Vahl T, Santos-Gallego C, Kawase Y, Hajjar RJ. Assessing left ventricular systolic dysfunction after myocardial infarction: are ejection fraction and dP/dtmax complementary or redundant? Am J Physiol Heart Circ Physiol 302: H1423–H1428, 2012. doi: 10.1152/ajpheart.01211.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Johansson M, Elam M, Rundqvist B, Eisenhofer G, Herlitz H, Lambert G, Friberg P. Increased sympathetic nerve activity in renovascular hypertension. Circulation 99: 2537–2542, 1999. doi: 10.1161/01.CIR.99.19.2537. [DOI] [PubMed] [Google Scholar]
- 54.Jones JV, Floras JS. Baroreflex sensitivity changes during the development of Goldblatt two-kidney one-clip hypertension in rats. Clin Sci (Lond) 59: 347–352, 1980. doi: 10.1042/cs0590347. [DOI] [PubMed] [Google Scholar]
- 55.Kaur J, Spranger MD, Hammond RL, Krishnan AC, Alvarez A, Augustyniak RA, O’Leary DS. Muscle metaboreflex activation during dynamic exercise evokes epinephrine release resulting in β2-mediated vasodilation. Am J Physiol Heart Circ Physiol 308: H524–H529, 2015. doi: 10.1152/ajpheart.00648.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kaur J, Machado TM, Alvarez A, Krishnan AC, Hanna HW, Altamimi YH, Senador D, Spranger MD, O’Leary DS. Muscle metaboreflex activation during dynamic exercise vasoconstricts ischemic active skeletal muscle. Am J Physiol Heart Circ Physiol 309: H2145–H2151, 2015. doi: 10.1152/ajpheart.00679.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kazatani Y, Hamada M, Shigematsu Y, Hiwada K, Kokubu T. Beneficial effect of a long-term antihypertensive therapy on blood pressure response to isometric handgrip exercise in patients with essential hypertension. Am J Ther 2: 165–169, 1995. doi: 10.1097/00045391-199503000-00003. [DOI] [PubMed] [Google Scholar]
- 58.Khouri EM, Gregg DE, Rayford CR. Effect of exercise on cardiac output, left coronary flow and myocardial metabolism in the unanesthetized dog. Circ Res 17: 427–437, 1965. doi: 10.1161/01.RES.17.5.427. [DOI] [PubMed] [Google Scholar]
- 59.Kim JK, Sala-Mercado JA, Hammond RL, Rodriguez J, Scislo TJ, O’Leary DS. Attenuated arterial baroreflex buffering of muscle metaboreflex in heart failure. Am J Physiol Heart Circ Physiol 289: H2416–H2423, 2005. doi: 10.1152/ajpheart.00654.2005. [DOI] [PubMed] [Google Scholar]
- 60.Kim S-J, Kline G, Gwirtz PA. Limitation of cardiac output by a coronary α 1-constrictor tone during exercise in dogs. Am J Physiol Heart Circ Physiol 271: H1125–H1131, 1996. [DOI] [PubMed] [Google Scholar]
- 61.Kjeldsen SE, Mundal R, Sandvik L, Erikssen G, Thaulow E, Erikssen J. Exercise blood pressure predicts cardiovascular death and myocardial infarction. Blood Press Monit 2: 147–153, 1997. [PubMed] [Google Scholar]
- 62.Kjeldsen SE, Mundal R, Sandvik L, Erikssen G, Thaulow E, Erikssen J. Supine and exercise systolic blood pressure predict cardiovascular death in middle-aged men. J Hypertens 19: 1343–1348, 2001. doi: 10.1097/00004872-200108000-00001. [DOI] [PubMed] [Google Scholar]
- 63.Kniffki KD, Mense S, Schmidt RF. Responses of group IV afferent units from skeletal muscle to stretch, contraction and chemical stimulation. Exp Brain Res 31: 511–522, 1978. doi: 10.1007/BF00239809. [DOI] [PubMed] [Google Scholar]
- 64.Kokkinos PF, Andreas PE, Coutoulakis E, Colleran JA, Narayan P, Dotson CO, Choucair W, Farmer C, Fernhall B. Determinants of exercise blood pressure response in normotensive and hypertensive women: role of cardiorespiratory fitness. J Cardiopulm Rehabil 22: 178–183, 2002. doi: 10.1097/00008483-200205000-00009. [DOI] [PubMed] [Google Scholar]
- 65.Langer SZ. Sixth gaddum memorial lecture, National Institute for Medical Research, Mill Hill, January 1977. Presynaptic receptors and their role in the regulation of transmitter release. Br J Pharmacol 60: 481–497, 1977. doi: 10.1111/j.1476-5381.1977.tb07526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Langer SZ, Shepperson NB. Prejunctional modulation of noradrenaline release by alpha 2-adrenoceptors: physiological and pharmacological implications in the cardiovascular system. J Cardiovasc Pharmacol 4, Suppl 1: S35–S40, 1982. doi: 10.1097/00005344-198200041-00008. [DOI] [PubMed] [Google Scholar]
- 67.Laprad SL, Augustyniak RA, Hammond RL, O’Leary DS. Does gender influence the strength and mechanisms of the muscle metaboreflex during dynamic exercise in dogs? Am J Physiol Regul Integr Comp 276: R1203–R1208, 1999. [DOI] [PubMed] [Google Scholar]
- 68.Leal AK, Williams MA, Garry MG, Mitchell JH, Smith SA. Evidence for functional alterations in the skeletal muscle mechanoreflex and metaboreflex in hypertensive rats. Am J Physiol Heart Circ Physiol 295: H1429–H1438, 2008. doi: 10.1152/ajpheart.01365.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Leal AK, McCord JL, Tsuchimochi H, Kaufman MP. Blockade of the TP receptor attenuates the exercise pressor reflex in decerebrated rats with chronic femoral artery occlusion. Am J Physiol Heart Circ Physiol 301: H2140–H2146, 2011. doi: 10.1152/ajpheart.00403.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li J, Sinoway LI. ATP stimulates chemically sensitive and sensitizes mechanically sensitive afferents. Am J Physiol Heart Circ Physiol 283: H2636–H2643, 2002. doi: 10.1152/ajpheart.00395.2002. [DOI] [PubMed] [Google Scholar]
- 71.Lorentsen E. Systemic arterial blood pressure during exercise in patients with atherosclerosis obliterans of the lower limbs. Circulation 46: 257–263, 1972. doi: 10.1161/01.CIR.46.2.257. [DOI] [PubMed] [Google Scholar]
- 72.MacLean DA, Imadojemu VA, Sinoway LI. Interstitial pH, K+, lactate, and phosphate determined with MSNA during exercise in humans. Am J Physiol Regul Integr Comp Physiol 278: R563–R571, 2000. [DOI] [PubMed] [Google Scholar]
- 73.Mahler F, Ross J Jr, O’Rourke RA, Covell JW. Effects of changes in preload, afterload and inotropic state on ejection and isovolumic phase measures of contractility in the conscious dog. Am J Cardiol 35: 626–634, 1975. doi: 10.1016/0002-9149(75)90048-X. [DOI] [PubMed] [Google Scholar]
- 74.Malpas SC. Sympathetic nervous system overactivity and its role in the development of cardiovascular disease. Physiol Rev 90: 513–557, 2010. doi: 10.1152/physrev.00007.2009. [DOI] [PubMed] [Google Scholar]
- 75.Matsukawa T, Mano T, Gotoh E, Ishii M. Elevated sympathetic nerve activity in patients with accelerated essential hypertension. J Clin Invest 92: 25–28, 1993. doi: 10.1172/JCI116558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McCloskey DI, Mitchell JH. Reflex cardiovascular and respiratory responses originating in exercising muscle. J Physiol 224: 173–186, 1972. doi: 10.1113/jphysiol.1972.sp009887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Michel MC, Brodde OE, Insel PA. Peripheral adrenergic receptors in hypertension. Hypertension 16: 107–120, 1990. doi: 10.1161/01.HYP.16.2.107. [DOI] [PubMed] [Google Scholar]
- 78.Miyajima E, Yamada Y, Yoshida Y, Matsukawa T, Shionoiri H, Tochikubo O, Ishii M. Muscle sympathetic nerve activity in renovascular hypertension and primary aldosteronism. Hypertension 17: 1057–1062, 1991. doi: 10.1161/01.HYP.17.6.1057. [DOI] [PubMed] [Google Scholar]
- 79.Mizuno M, Murphy MN, Mitchell JH, Smith SA. Antagonism of the TRPv1 receptor partially corrects muscle metaboreflex overactivity in spontaneously hypertensive rats. J Physiol 589: 6191–6204, 2011. doi: 10.1113/jphysiol.2011.214429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mizuno M, Murphy MN, Mitchell JH, Smith SA. Skeletal muscle reflex-mediated changes in sympathetic nerve activity are abnormal in spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol 300: H968–H977, 2011. doi: 10.1152/ajpheart.01145.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Moreira ED, Ida F, Oliveira VL, Krieger EM. Early depression of the baroreceptor sensitivity during onset of hypertension. Hypertension 19, Suppl: II198–II201, 1992. doi: 10.1161/01.HYP.19.2_Suppl.II198. [DOI] [PubMed] [Google Scholar]
- 82.Mundal R, Kjeldsen SE, Sandvik L, Erikssen G, Thaulow E, Erikssen J. Exercise blood pressure predicts cardiovascular mortality in middle-aged men. Hypertension 24: 56–62, 1994. doi: 10.1161/01.HYP.24.1.56. [DOI] [PubMed] [Google Scholar]
- 83.Mundal R, Kjeldsen SE, Sandvik L, Erikssen G, Thaulow E, Erikssen J. Exercise blood pressure predicts mortality from myocardial infarction. Hypertension 27: 324–329, 1996. doi: 10.1161/01.HYP.27.3.324. [DOI] [PubMed] [Google Scholar]
- 84.Mundal R, Kjeldsen SE, Sandvik L, Erikssen G, Thaulow E, Erikssen J. Clustering of coronary risk factors with increasing blood pressure at rest and during exercise. J Hypertens 16: 19–22, 1998. doi: 10.1097/00004872-199816010-00004. [DOI] [PubMed] [Google Scholar]
- 85.Murray PA, Vatner SF. alpha-Adrenoceptor attenuation of the coronary vascular response to severe exercise in the conscious dog. Circ Res 45: 654–660, 1979. doi: 10.1161/01.RES.45.5.654. [DOI] [PubMed] [Google Scholar]
- 86.Nozawa T, Cheng CP, Noda T, Little WC. Relation between left ventricular oxygen consumption and pressure-volume area in conscious dogs. Circulation 89: 810–817, 1994. doi: 10.1161/01.CIR.89.2.810. [DOI] [PubMed] [Google Scholar]
- 87.O’Leary DS. Regional vascular resistance vs. conductance: which index for baroreflex responses? Am J Physiol Heart Circ Physiol 260: H632–H637, 1991. [DOI] [PubMed] [Google Scholar]
- 88.O’Leary DS. Autonomic mechanisms of muscle metaboreflex control of heart rate. J Appl Physiol (1985) 74: 1748–1754, 1993. [DOI] [PubMed] [Google Scholar]
- 89.O’Leary DS, Augustyniak RA. Muscle metaboreflex increases ventricular performance in conscious dogs. Am J Physiol Heart Circ Physiol 275: H220–H224, 1998. [DOI] [PubMed] [Google Scholar]
- 90.O’Leary DS, Augustyniak RA, Ansorge EJ, Collins HL. Muscle metaboreflex improves O2 delivery to ischemic active skeletal muscle. Am J Physiol Heart Circ Physiol 276: H1399–H1403, 1999. [DOI] [PubMed] [Google Scholar]
- 91.O’Leary DS, Sala-Mercado JA, Hammond RL, Ansorge EJ, Kim JK, Rodriguez J, Fano D, Ichinose M. Muscle metaboreflex-induced increases in cardiac sympathetic activity vasoconstrict the coronary vasculature. J Appl Physiol (1985) 103: 190–194, 2007. doi: 10.1152/japplphysiol.00139.2007. [DOI] [PubMed] [Google Scholar]
- 92.O’Leary DS, Sheriff DD. Is the muscle metaboreflex important in control of blood flow to ischemic active skeletal muscle in dogs? Am J Physiol Heart Circ Physiol 268: H980–H986, 1995. [DOI] [PubMed] [Google Scholar]
- 93.Oparil S, Zaman MA, Calhoun DA. Pathogenesis of hypertension. Ann Intern Med 139: 761–776, 2003. doi: 10.7326/0003-4819-139-9-200311040-00011. [DOI] [PubMed] [Google Scholar]
- 94.Perlini S, Meyer TE, Foëx P. Effects of preload, afterload and inotropy on dynamics of ischemic segmental wall motion. J Am Coll Cardiol 29: 846–855, 1997. doi: 10.1016/S0735-1097(96)00569-4. [DOI] [PubMed] [Google Scholar]
- 95.Rondon MU, Laterza MC, de Matos LD, Trombetta IC, Braga AM, Roveda F, Alves MJ, Krieger EM, Negrão CE. Abnormal muscle metaboreflex control of sympathetic activity in never-treated hypertensive subjects. Am J Hypertens 19: 951–957, 2006. doi: 10.1016/j.amjhyper.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 96.Rotto DM, Kaufman MP. Effect of metabolic products of muscular contraction on discharge of group III and IV afferents. J Appl Physiol (1985) 64: 2306–2313, 1988. [DOI] [PubMed] [Google Scholar]
- 97.Rowell LB, Freund PR, Hobbs SF. Cardiovascular responses to muscle ischemia in humans. Circ Res 48: I37–I47, 1981. [PubMed] [Google Scholar]
- 98.Sala-Mercado JA, Spranger MD, Abu-Hamdah R, Kaur J, Coutsos M, Stayer D, Augustyniak RA, O’Leary DS. Attenuated muscle metaboreflex-induced increases in cardiac function in hypertension. Am J Physiol Heart Circ Physiol 305: H1548–H1554, 2013. doi: 10.1152/ajpheart.00478.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sala-Mercado JA, Hammond RL, Kim JK, Rossi NF, Stephenson LW, O’Leary DS. Muscle metaboreflex control of ventricular contractility during dynamic exercise. Am J Physiol Heart Circ Physiol 290: H751–H757, 2006. doi: 10.1152/ajpheart.00869.2005. [DOI] [PubMed] [Google Scholar]
- 100.Sandvik L, Erikssen J, Ellestad M, Erikssen G, Thaulow E, Mundal R, Rodahl K. Heart rate increase and maximal heart rate during exercise as predictors of cardiovascular mortality: a 16-year follow-up study of 1960 healthy men. Coron Artery Dis 6: 667–679, 1995. doi: 10.1097/00019501-199508000-00012. [DOI] [PubMed] [Google Scholar]
- 101.Sausen MT, Delaney EP, Stillabower ME, Farquhar WB. Enhanced metaboreflex sensitivity in hypertensive humans. Eur J Appl Physiol 105: 351–356, 2009. doi: 10.1007/s00421-008-0910-8. [DOI] [PubMed] [Google Scholar]
- 102.Seitelberger R, Guth BD, Heusch G, Lee JD, Katayama K, Ross J Jr. Intracoronary alpha 2-adrenergic receptor blockade attenuates ischemia in conscious dogs during exercise. Circ Res 62: 436–442, 1988. doi: 10.1161/01.RES.62.3.436. [DOI] [PubMed] [Google Scholar]
- 103.Sheriff DD, Augustyniak RA, O’Leary DS. Muscle chemoreflex-induced increases in right atrial pressure. Am J Physiol Heart Circ Physiol 275: H767–H775, 1998. [DOI] [PubMed] [Google Scholar]
- 104.Sheriff DD, O’Leary DS, Scher AM, Rowell LB. Baroreflex attenuates pressor response to graded muscle ischemia in exercising dogs. Am J Physiol Heart Circ Physiol 258: H305–H310, 1990. [DOI] [PubMed] [Google Scholar]
- 105.Sheriff DD, Wyss CR, Rowell LB, Scher AM. Does inadequate oxygen delivery trigger pressor response to muscle hypoperfusion during exercise? Am J Physiol Heart Circ Physiol 253: H1199–H1207, 1987. [DOI] [PubMed] [Google Scholar]
- 106.Shoemaker JK, Mattar L, Kerbeci P, Trotter S, Arbeille P, Hughson RL. WISE 2005: stroke volume changes contribute to the pressor response during ischemic handgrip exercise in women. J Appl Physiol (1985) 103: 228–233, 2007. doi: 10.1152/japplphysiol.01334.2006. [DOI] [PubMed] [Google Scholar]
- 107.Sinoway LI, Smith MB, Enders B, Leuenberger U, Dzwonczyk T, Gray K, Whisler S, Moore RL. Role of diprotonated phosphate in evoking muscle reflex responses in cats and humans. Am J Physiol Heart Circ Physiol 267: H770–H778, 1994. [DOI] [PubMed] [Google Scholar]
- 108.Sinoway LI, Wroblewski KJ, Prophet SA, Ettinger SM, Gray KS, Whisler SK, Miller G, Moore RL. Glycogen depletion-induced lactate reductions attenuate reflex responses in exercising humans. Am J Physiol Heart Circ Physiol 263: H1499–H1505, 1992. [DOI] [PubMed] [Google Scholar]
- 109.Smith SA, Williams MA, Leal AK, Mitchell JH, Garry MG. Exercise pressor reflex function is altered in spontaneously hypertensive rats. J Physiol 577: 1009–1020, 2006. doi: 10.1113/jphysiol.2006.121558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Souza HC, Martins-Pinge MC, Dias da Silva VJ, Borghi-Silva A, Gastaldi AC, Blanco JH, Tezini GC. Heart rate and arterial pressure variability in the experimental renovascular hypertension model in rats. Auton Neurosci 139: 38–45, 2008. doi: 10.1016/j.autneu.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 111.Spranger MD, Sala-Mercado JA, Coutsos M, Kaur J, Stayer D, Augustyniak RA, O’Leary DS. Role of cardiac output versus peripheral vasoconstriction in mediating muscle metaboreflex pressor responses: dynamic exercise versus postexercise muscle ischemia. Am J Physiol Regul Integr Comp Physiol 304: R657–R663, 2013. doi: 10.1152/ajpregu.00601.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Stone AJ, Yamauchi K, Kaufman MP. Purinergic 2X receptors play a role in evoking the exercise pressor reflex in rats with peripheral artery insufficiency. Am J Physiol Heart Circ Physiol 306: H396–H404, 2014. doi: 10.1152/ajpheart.00762.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Strader JR, Gwirtz PA, Jones CE. Comparative effects of alpha-1 and alpha-2 adrenoceptors in modulation of coronary flow during exercise. J Pharmacol Exp Ther 246: 772–778, 1988. [PubMed] [Google Scholar]
- 114.Tuck ML. The sympathetic nervous system in essential hypertension. Am Heart J 112: 877–886, 1986. doi: 10.1016/0002-8703(86)90497-7. [DOI] [PubMed] [Google Scholar]
- 115.Tune JD, Richmond KN, Gorman MW, Feigl EO. Control of coronary blood flow during exercise. Exp Biol Med (Maywood) 227: 238–250, 2002. [DOI] [PubMed] [Google Scholar]
- 116.Victor RG, Bertocci LA, Pryor SL, Nunnally RL. Sympathetic nerve discharge is coupled to muscle cell pH during exercise in humans. J Clin Invest 82: 1301–1305, 1988. doi: 10.1172/JCI113730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wyss CR, Ardell JL, Scher AM, Rowell LB. Cardiovascular responses to graded reductions in hindlimb perfusion in exercising dogs. Am J Physiol Heart Circ Physiol 245: H481–H486, 1983. [DOI] [PubMed] [Google Scholar]
- 118.Xie PL, Chapleau MW, McDowell TS, Hajduczok G, Abboud FM. Mechanism of decreased baroreceptor activity in chronic hypertensive rabbits. Role of endogenous prostanoids. J Clin Invest 86: 625–630, 1990. doi: 10.1172/JCI114754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yamaguchi N, de Champlain J, Nadeau RA. Regulation of norepinephrine release from cardiac sympathetic fibers in the dog by presynaptic alpha- and beta-receptors. Circ Res 41: 108–117, 1977. doi: 10.1161/01.RES.41.1.108. [DOI] [PubMed] [Google Scholar]
- 120.Yurenev AP, Parfyonova EV, Krasnikova TL, Aripova NA. Alteration of beta-adrenoceptor function in hypertensive patients with different degrees of left ventricular hypertrophy. Am J Hypertens 5: 164S–168S, 1992. [DOI] [PubMed] [Google Scholar]