The differential impact of α- and β-myosin heavy chain (MHC) on contractile dynamics causes a mutant cardiac troponin T (TnTA30V) to differently modulate cardiac contractile function. TnTA30V attenuated Ca2+-activated maximal tension and length-mediated cross-bridge recruitment against α-MHC but augmented these parameters against β-MHC, suggesting divergent contractile phenotypes.
Keywords: hypertrophic cardiomyopathy, dilated cardiomyopathy, troponin T, myosin heavy chain, cross-bridge recruitment
Abstract
The present study investigated the functional consequences of the human hypertrophic cardiomyopathy (HCM) mutation A28V in cardiac troponin T (TnT). The A28V mutation is located within the NH2 terminus of TnT, a region known to be important for full activation of cardiac thin filaments. The functional consequences of the A28V mutation in TnT remain unknown. Given how α- and β-myosin heavy chain (MHC) isoforms differently alter the functional effect of the NH2 terminus of TnT, we hypothesized that the A28V-induced effects would be differently modulated by α- and β-MHC isoforms. Recombinant wild-type mouse TnT (TnTWT) and the mouse equivalent of the human A28V mutation (TnTA30V) were reconstituted into detergent-skinned cardiac muscle fibers extracted from normal (α-MHC) and transgenic (β-MHC) mice. Dynamic and steady-state contractile parameters were measured in reconstituted muscle fibers. Step-like length perturbation experiments demonstrated that TnTA30V decreased the magnitude of the muscle length-mediated recruitment of new force-bearing cross bridges (ER) by 30% in α-MHC fibers. In sharp contrast, TnTA30V increased ER by 55% in β-MHC fibers. Inferences drawn from other dynamic contractile parameters suggest that directional changes in ER in TnTA30V + α-MHC and TnTA30V + β-MHC fibers result from a divergent impact on cross bridge-regulatory unit (troponin-tropomyosin complex) cooperativity. TnTA30V-mediated effects on Ca2+-activated maximal tension and instantaneous muscle fiber stiffness (ED) were also divergently affected by α- and β-MHC. Our study demonstrates that TnTA30V + α-MHC and TnTA30V + β-MHC fibers show contrasting contractile phenotypes; however, only the observations from β-MHC fibers are consistent with the clinical data for A28V in humans.
NEW & NOTEWORTHY The differential impact of α- and β-myosin heavy chain (MHC) on contractile dynamics causes a mutant cardiac troponin T (TnTA30V) to differently modulate cardiac contractile function. TnTA30V attenuated Ca2+-activated maximal tension and length-mediated cross-bridge recruitment against α-MHC but augmented these parameters against β-MHC, suggesting divergent contractile phenotypes.
myofilament activation is a highly regulated process that involves coordinated interactions between sarcomeric proteins. A thin filament regulatory protein that is essential for optimal expression of such protein-protein interactions is cardiac troponin T (TnT). Within TnT, there are defined regions that regulate myofilament activation through distinct mechanisms. For example, a specialized domain identified as the NH2 terminus (1–43 residues) of TnT has previously been shown to be required for full activation of thin filaments (7, 30). Two hypertrophic cardiomyopathy (HCM) mutations, A27V and A28V (38a, 36), have been mapped to the NH2 terminus. However, the functional consequences of these HCM-related mutations are still unknown.
The importance of studying A28V becomes clear given that the A28 residue is highly conserved among numerous species (human, rat, mouse, guinea pig, and rabbit). Our recent work has shown that deletion of NH2-terminal 1–43 residues in rat TnT (TnT1–43Δ) attenuates maximal activation of thin filaments (30), thereby suggesting that the NH2 terminus is required for full activation. We have previously suggested that the NH2 terminus brings about its effect via an intramolecular interaction with the central region (CR: residues 77–193) of TnT (22). Because the CR of TnT directly interacts with tropomyosin, the influence of the NH2 terminus on CR-tropomyosin activity may have important ramifications for the activation of regulatory units (RU: tropomyosin-troponin complex) and thin filaments. Moreover, given that CR-tropomyosin activity is differently altered by α- and β-myosin heavy chain (MHC) isoforms (19, 21, 23), we hypothesized that the effect of the A28V mutation on thin filament activation would be differently altered by α- and β-MHC.
The differential impact of α- and β-MHC is important to consider because rodent (rat and mice) hearts predominantly express the faster cycling α-MHC, whereas human hearts express the slower cycling β-MHC. Additionally, our previous study showed that the effect of the NH2 terminus on contractile function was differentially altered by α- and β-MHC isoforms; for instance, the attenuation of maximal tension by TnT1–43Δ was more severe in α-MHC fibers than in β-MHC fibers (30). This observation suggests that the functional consequences due to structural alterations in the NH2 terminus of TnT, such as disease-causing mutations, would be differently modified by α- and β-MHC. This notion was also supported by previous studies of mutations in TnT, which showed diverse effects on contractile function when studied against α- and β-MHC (11, 21, 23, 30). Notably, a recent study from our laboratory showed that the HCM-linked mutation F70L in TnT induced a dilated cardiomyopathy (DCM)-like contractile phenotype in α-MHC fibers, but it induced an HCM-like contractile phenotype in β-MHC fibers (11). These observations highlight the importance of testing the effects of the A28V mutation in α- and β-MHC-expressing models to assess whether the contractile phenotype differs between rodent (α-MHC) and human hearts (β-MHC).
To test our hypothesis, we generated a recombinant mouse wild-type TnT (TnTWT) and a mouse analog of the human A28V mutation (TnTA30V). TnT variants were reconstituted into detergent-skinned left ventricular papillary muscle fibers from wild-type (α-MHC) and transgenic (β-MHC) mice to measure various indexes of contractile function. The magnitude of muscle length (ML)-mediated cross-bridge (XB) recruitment (ER), also referred to as stretch activation (5, 43, 44), decreased in TnTA30V + α-MHC fibers but increased in TnTA30V + β-MHC fibers. Ca2+-activated maximal tension and the magnitude of instantaneous muscle fiber stiffness (ED) corroborated ER. Our findings suggest that TnTA30V induces a DCM-like phenotype in α-MHC expressing hearts, whereas it induces an HCM-like phenotype in β-MHC-expressing hearts. We will discuss the molecular mechanism by which TnTA30V induces such divergent contractile phenotypes against α- and β-MHC backgrounds.
MATERIALS AND METHODS
Animal Protocols
Wild-type (WT) C57BL/6N mice expressing α-MHC were obtained from Simonsen Laboratories (Gilroy, CA). Transgenic mice expressing β-MHC were generated and characterized as described previously (19, 23, 27, 28, 40). The level of β-MHC expression in transgenic mouse hearts is ~70% of the total cardiac MHC (23). Overexpression of β-MHC in cardiac muscle does not affect the stoichiometry or phosphorylation status of other sarcomeric proteins, as previously described (23). The transgenic line was maintained by backcrossing to WT mice, and offspring were genotyped using PCR (28). All animals (3- to 5-mo-old male mice) used in this study received proper care and treatment. Experimental protocols were approved by the Washington State University Institutional Animal Care and Use Committee. Mice were euthanized according to recommended procedures from the Euthanasia of the American Veterinary Medical Association.
Recombinant Mouse Cardiac Troponin Subunits
Recombinant c-myc-tagged mouse TnT (TnTWT and TnTA30V), troponin I (TnI), and troponin C (TnC) were cloned into the T7 promoter-based pSBETa vector and expressed in BL21 STAR [DE3] cells (Invitrogen, Carlsbad, CA) for protein synthesis. Cardiac troponin subunits were purified using ion-exchange chromatography techniques (7, 24, 38). For protein preparations, cultured cells were spun down and sonicated in buffer containing 50 mM Tris base (pH 8.0 at 4°C), 6 M urea, and 5 mM EDTA. Sonication buffer contained the following protease inhibitors: 0.4 mM PMSF, 4 mM benzamidine HCl (Benz HCl), 1 mM DTT, and 0.01% sodium azide (NaN3). The sonicated preparation was centrifuged to remove insoluble cellular material. TnT variants were purified as follows: supernatant from sonicated preparation was used for ammonium sulfate fractionation. The pellet from 70% ammonium sulfate fractionation was dissolved in 50 mM Tris base (pH 8.0 at 4°C), 6 M urea, 1 mM EDTA, 0.4 mM PMSF, 4 mM Benz HCl, 1 mM DTT, and 0.01% NaN3. TnT variants were purified by ion-exchange chromatography on a DEAE-fast Sepharose column and eluted using a 0–0.4 M NaCl gradient (7). TnI was purified on a CM fast Sepharose cation exchange column and eluted using a 0–0.3 M KCl gradient (24). TnC was partially purified on a DE-52 anion exchange column and then further purified on a phenyl Sepharose column (38). All fractions containing pure proteins were pooled and dialyzed thoroughly against deionized water containing 0.25% β-mercaptoethanol, lyophilized, and stored at −80°C.
Detergent-Skinned Mouse Cardiac Muscle Fibers
Left ventricular papillary muscle fibers from WT and transgenic mouse hearts were isolated and detergent skinned, as described previously (8, 9). Briefly, mice were deeply anesthetized using isoflurane and their hearts were quickly excised and placed into ice-cold high-relaxing solution (pH 7.0 at 4°C). High-relaxing solution contained the following: 50 mM N,N-bis(2-hydroxyethyl)-2-amino-ethane-sulfonic acid (BES), 30.83 mM potassium propionate (K-propionate), 10 mM NaN3, 20 mM EGTA, 6.29 mM magnesium chloride (MgCl2), 6.09 mM sodium adenosine triphosphate (Na2ATP), 20 mM 2,3-butanedione monoxime (BDM), 4 mM Benz HCl, 1 mM DTT, and a cocktail of protease inhibitors (5 µM bestatin, 2 µM E-64, 10 µM leupeptin, 1 µM pepstatin, and 200 µM PMSF). Left ventricular papillary muscle bundles were quickly removed and dissected into thinner sections (2–2.5 mm in length and 150–200 µm in thickness) and detergent-skinned overnight at 4°C in high-relaxing solution containing 1% Triton X-100.
Western Blot Analysis
Muscle fibers were solubilized in 2.5% SDS solution (10 µl/fiber), and an equal volume of protein loading dye [125 mM Tris·HCl (pH 6.8), 20% glycerol, 2% SDS, 0.01% bromophenol blue, and 50 mM β-mercaptoethanol] was added. Proteins were separated on an 8% SDS gel and transferred to a polyvinylidene difluoride membrane for Western blot analysis using a Trans-Blot Turbo transfer system (Bio-Rad Laboratories, Hercules, CA). Incorporation level of TnTWT or TnTA30V in reconstituted muscle fibers was assessed using a monoclonal anti-TnT primary antibody (M401134; Fitzgerald Industries, Concord, MA) followed by a horseradish peroxidase-labeled anti-mouse secondary antibody (RPN2132, Amersham Biosciences, Piscataway, NJ). Densitometric analysis was performed to quantify the level of recombinant TnT incorporation using National Institutes of Health ImageJ software (https://imagej.nih.gov/ij/), as described previously (22, 32).
Reconstitution of Recombinant Troponin into Detergent-Skinned Muscle Fibers
Recombinant troponin was reconstituted into detergent-skinned muscle fibers, as described previously (8, 9, 11, 23). In brief, excess amounts of TnTWT or TnTA30V (0.9 mg/ml) and TnI (1.0 mg/ml) were dissolved in an extraction solution containing 50 mM Tris·HCl (pH 8.0), 6 M urea, 1 M KCl, and 1 mM DTT. Successive dialysis (at 4°C) against two buffers, with decreasing concentrations of salt and urea (0.7 M KCl, 4 M urea; and 0.5 M KCl, 2 M urea), was performed to gradually remove the high salt and urea in the extraction solution. The extraction solution was then dialyzed overnight (at 4°C) against buffer containing 50 mM BES (pH 7.0 at 20°C), 200 mM KCl, 10 mM BDM, 5 mM EGTA, and 6.27 mM MgCl2. All buffers used for dialysis, and the extraction solution, contained a cocktail of protease inhibitors. Dialyzed extraction solution was spun at 3,000 rpm for 15 min to remove any undissolved protein. Skinned fibers were treated with the extraction solution containing TnTWT + TnI or TnTA30V + TnI for ~3 h at room temperature (22°C), with gentle shaking. To finish the reconstitution procedure, fibers were incubated in high-relaxing solution containing TnC (3 mg/ml) overnight at 4°C.
Isometric Steady-State Tension
Isometric tension was measured as described previously (8, 11). T-shaped aluminum clips were used to suspend reconstituted muscle fibers between a motor arm (model 322C; Aurora Scientific, Aurora, ON, Canada) and a force transducer (AE 801; Sensor One Technologies, Sausalito, CA). Sarcomere length was set to 2.3 µm under relaxing conditions, as described previously (8). After two cycles of maximal activation (pCa 4.3) and relaxation (pCa 9.0), sarcomere length was readjusted to 2.3 µm, if needed. Muscle fibers were exposed to various Ca2+ solutions ranging from pCa 4.3 to 9.0. The compositions of pCa solutions were calculated using methods described previously (16). The composition of pCa 4.3 solution is as follows (in mM): 31 K propionate, 5.95 Na2ATP, 6.61 MgCl2, 10 EGTA, 10.11 CaCl2, 50 BES, 5 NaN3, and 10 phosphoenolpyruvate (PEP). The composition of pCa 9.0 is as follows (in mM): 51.14 K propionate, 5.83 Na2ATP, 6.87 MgCl2, 10 EGTA, 0.024 CaCl2, 50 BES, 5 NaN3, and 10 PEP. Both activating and relaxing solutions contained 0.5 mg/ml pyruvate kinase (500 U/mg), 0.05 mg/ml lactate dehydrogenase (870 U/mg), 20 µM diadenosine pentaphosphate, 10 µM oligomycin, and a cocktail of protease inhibitors. pH and ionic strength of maximal activating and relaxing solutions were adjusted to 7.0 and 180 mM, respectively. All experiments were performed at 20°C.
Muscle Fiber Mechanodynamics
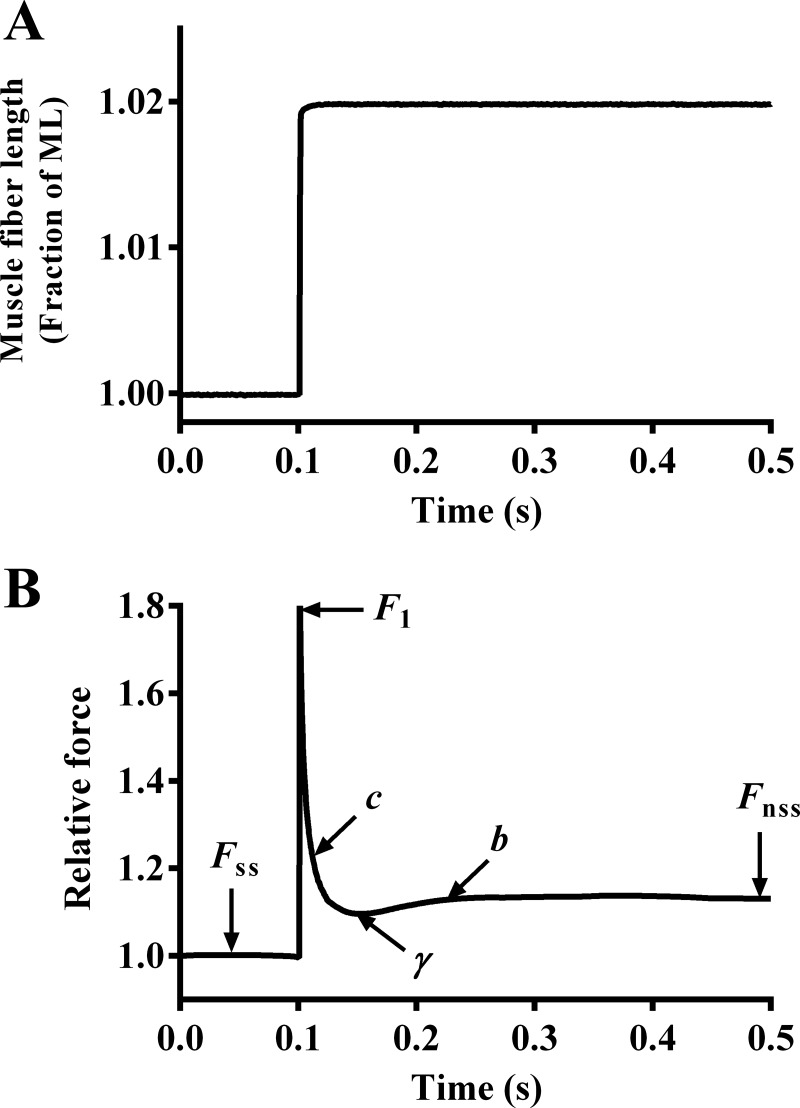
Maximally activated muscle fibers were allowed to attain steady-state force (Fss) and subsequently subjected to various amplitude stretch/release perturbations (±0.5, ±1.0, ±1.5, and ±2.0% of the initial ML) (18). A nonlinear recruitment distortion (NLRD) model was fit to the resulting family of force responses to estimate the following model parameters: the magnitude of instantaneous stiffness caused by a sudden change in ML (ED), the rate at which strained cross bridges detach, following a sudden change in ML (c), the negative impact of strained XBs on other force-bearing XBs (γ), the rate at which new XBs are recruited into the force-bearing state, following a sudden change in ML (b), and the sudden stretch-mediated increase in the magnitude of XB recruitment (ER; stretch activation). The physiological relevance of NLRD model parameters is explained below.
ED.
Following a sudden 2% stretch in ML (Fig. 1A), there is an instantaneous increase in force (F1; Fig. 1B) due to the rapid distortion of elastic elements within strongly bound XBs. F1 is strongly correlated to the number of force-bearing XBs before ML change (33). Therefore, the magnitude of F1 increases with an increase in the number of force-bearing XBs and vice versa. ED is estimated as the slope of the linear relationship between changes in force (F1 − Fss) and the corresponding changes in ML (ΔL) (18).
Fig. 1.
Force response to a sudden 2% stretch in a maximally activated α-MHC fiber reconstituted with wild-type mouse troponin T (TnTWT). A sudden 2% stretch in muscle length (ML) imposed on a maximally-activated muscle fiber (A) and the corresponding force response elicited (B) is shown. The force was normalized to the isometric steady-state force value (Fss) before ML change. The nonlinear recruitment distortion (NLRD) model was fit to the resulting force responses to estimate various model parameters (18). F1 is the instantaneous increase in force due to a sudden increase in ML, c is the rate of exponential force decline, γ is the magnitude of force decline from F1 to the minimum force point (nadir), and b is the rate at which force rises from nadir to a new steady-state (Fnss). The physiological significance of these parameters is described in materials and methods.
c.
As the fiber is maintained at the increased ML, there is a rapid exponential decline in force from F1 to a minimum point (Fig. 1B). This decay in force is due to the rapid detachment of strained XBs at a characteristic rate, c. We have previously shown that c is a valuable index of XB detachment rate, g (6, 23).
γ.
The magnitude of force decline from F1 to a minimum point (nadir) represents the negative effect of strained XBs on other XBs. When this negative effect is more pronounced, the magnitude of force decline is greater; that is, the nadir is more pronounced and γ is augmented. Because there is no direct interaction between neighboring XBs, the effect of strained XBs on other XBs is transduced via thin filament-based cooperative/allosteric mechanisms.
b.
Force rises gradually from nadir at a characteristic rate, b, due to recruitment of additional force-bearing XBs (Fig. 1B).
ER.
Force will eventually reach a new steady-state (Fnss; Fig. 1B) that is higher than Fss due to the ML-mediated recruitment of additional force-bearing XBs. ER is estimated as the slope of the relationship between changes in force (Fnss − Fss) and ΔL (18).
Rate of Tension Redevelopment
The rate of tension redevelopment (ktr) was estimated using a modification to the original large slack-restretch maneuver described by Brenner and Eisenberg (2). Upon attainment of the steady-state at maximal activation (pCa 4.3), the motor arm was commanded to rapidly (0.5 ms) slacken the muscle fiber by 10% of the initial ML, using a high-speed length-control device (model 322C; Aurora Scientific). After a brief (25 ms) period at the decreased length, the motor arm was commanded to rapidly (within 0.5 ms) swing past the original ML by a 10% stretch and was then brought back to the initial ML. This 10% stretch was applied to disengage any remaining strongly bound XBs. Once the fiber was returned to the initial ML, it was allowed to redevelop force. ktr was estimated by fitting the following monoexponential function to the force redevelopment phase:
where Fss is the steady-state isometric force and Fres is the residual force from which the fiber starts to redevelop force. The residual force was no more than ~10% of the initial Fss in all cases.
Data Analysis
The Hill equation was fitted to normalized pCa-tension relationships to derive two parameters, pCa50 (an index of myofilament Ca2+ sensitivity) and nH (an index of myofilament cooperativity). All parameters were analyzed using a two-way ANOVA, with one factor being TnT (TnTWT and TnTA30V) and the second factor being the MHC isoform (α-MHC and β-MHC). We first assessed whether there was a significant two-way interaction effect on a given contractile parameter. A significant MHC-TnT interaction effect does not mean that there is a direct interaction between TnT and MHC, but it suggests that the effect of TnT on a given contractile parameter was dissimilar in α- and β-MHC fibers. If the two-way interaction effect was not significant, then the main effect of TnT was assessed. To probe the cause for a significant interaction effect or a main effect, post hoc multiple t-tests were performed using Fisher’s uncorrected least significant differences method. Statistical significance was set at P < 0.05. Data for each group are expressed as means ± SE.
RESULTS
Western Blot Analysis of Reconstituted Muscle Fibers
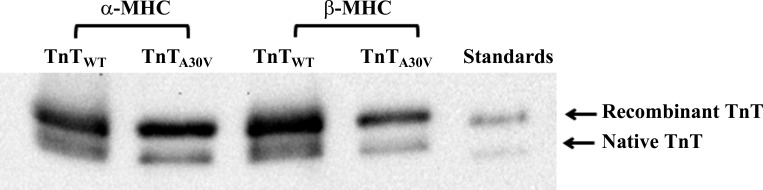
To assess the extent of recombinant troponin incorporation in α- and β-MHC fibers, Western blot analysis was used. Addition of an 11-amino acid c-myc tag at the NH2 terminus of recombinant TnT allowed us to separate recombinant and endogenous TnT on an SDS gel. This enabled us to quantify the level of recombinant TnT incorporation in reconstituted fibers. Previous studies have demonstrated that inclusion of the c-myc tag has no impact on the TnT-mediated function in cardiac muscle (10, 11, 35). Densitometric analysis of the Western blot (Fig. 2) showed that, in α-MHC fibers, the level of incorporation was 75 ± 2% (n = 3; Fig. 2, lane 1) for TnTWT and 69 ± 3% (n = 3; Fig. 2, lane 2) for TnTA30V. Likewise, in β-MHC fibers, the level of incorporation was 73 ± 1% (n = 3; Fig. 2, lane 3) for TnTWT and 72 ± 2% (n = 3; Fig. 2, lane 4) for TnTA30V. Similar incorporation levels of TnTWT or TnTA30V in both α- and β-MHC fibers provided a good model to study the interplay between TnTA30V- and MHC-mediated effects on various contractile parameters.
Fig. 2.
Western blot analysis of reconstituted fibers. Reconstituted muscle fibers were solubilized in 2.5% SDS solution, as described in materials and methods. Protein samples were loaded and run on an 8% SDS gel to separate various proteins. Proteins were transferred onto a polyvinylidene difluoride membrane and TnT was probed using anti-TnT primary and secondary antibodies. Lanes 1–4: TnTWT + α-MHC, TnTA30V + α-MHC, TnTWT + β-MHC, and TnTA30V + β-MHC, respectively. Lane 5: purified c-myc-tagged recombinant TnT and endogenous (native) TnT proteins.
Fig. 3.
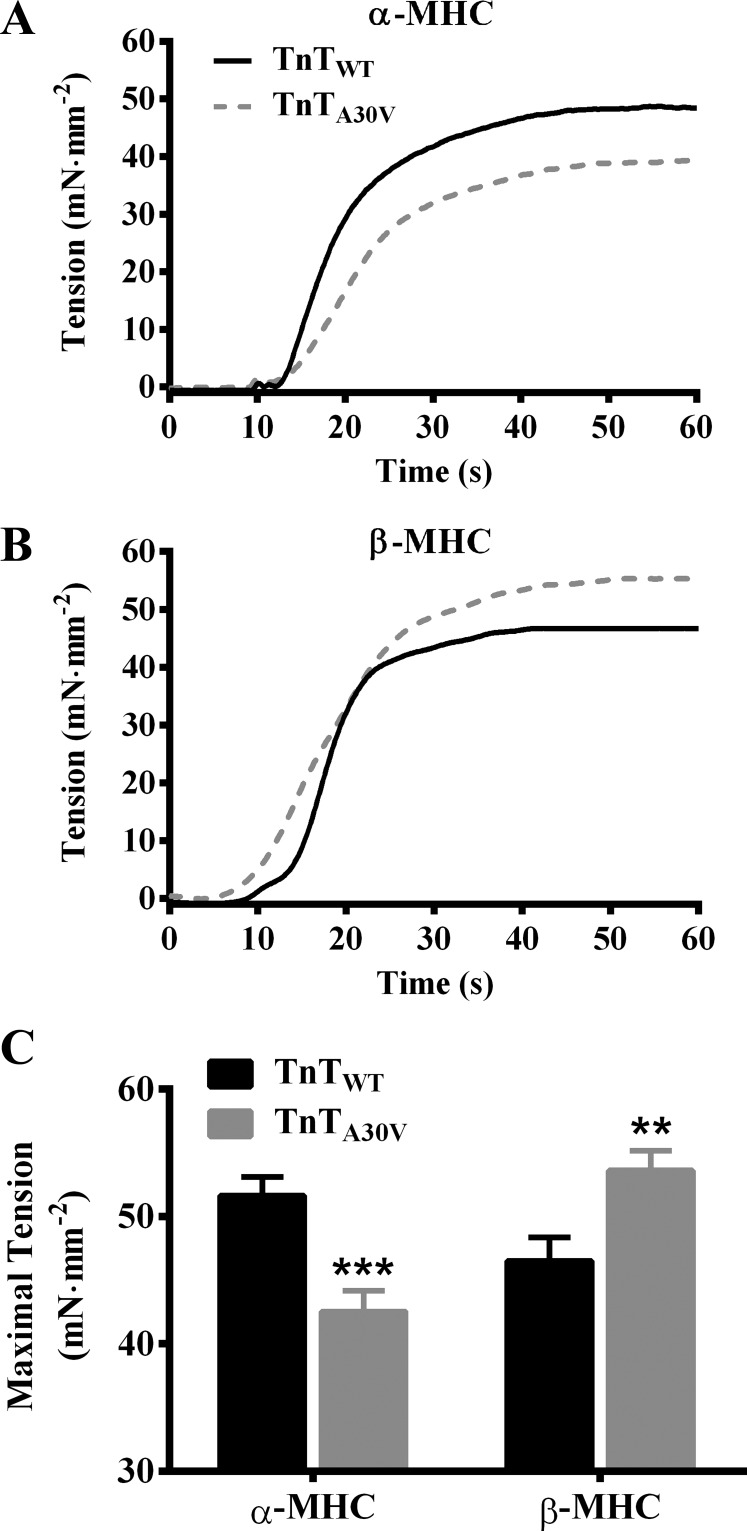
TnTA30V-mediated impact on steady-state maximal tension in α- and β-MHC fibers. Measurements of steady-state tension were made in muscle fibers at maximal Ca2+ activation (pCa 4.3), as described previously (15, 45). The effect of TnTA30V on the force response (normalized to the cross-sectional area) during isometric contraction at pCa 4.3 in α-MHC (A) and β-MHC fibers (B) is shown. The effect of TnTA30V on maximal tension in α- and β-MHC fibers (C). Data for each group are expressed as means ± SE. Statistical differences were analyzed for TnTA30V, relative to TnTWT, using two-way ANOVA and post hoc Fisher’s LSD tests (**P < 0.01; ***P < 0.001). The number of fibers measured (from 3 hearts) were as follows: TnTWT + α-MHC (n = 12), TnTA30V + α-MHC (n = 14), TnTWT + β-MHC (n = 11), and TnTA30V + β-MHC (n = 12).
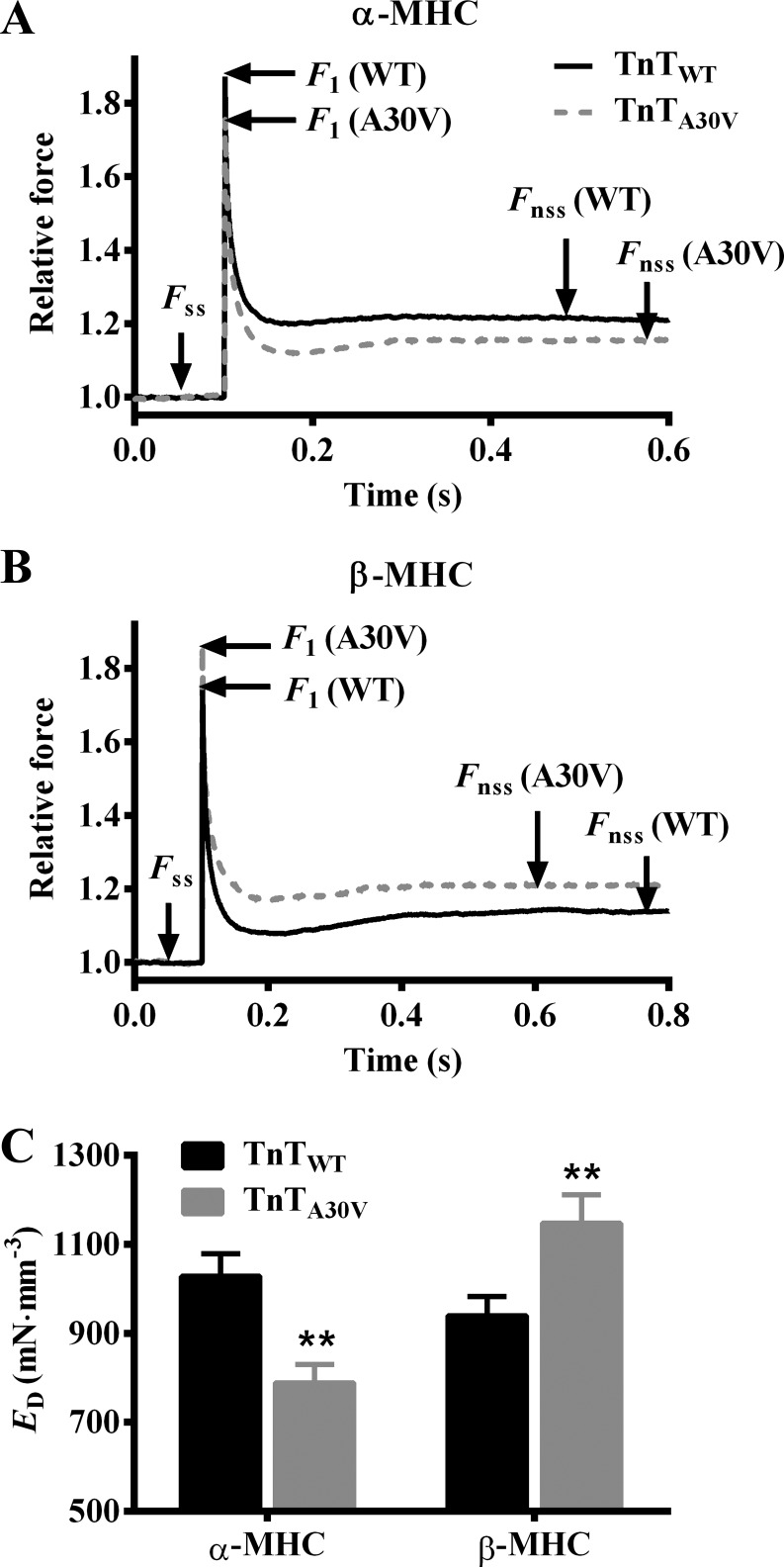
Fig. 4.
TnTA30V-mediated impact on ED in α- and β-MHC fibers. A family of step-like length perturbations (±0.5, ±1.0, ±1.5, and ±2.0% of the initial muscle length, ML) were used to elicit force responses in maximally activated fibers. Effect of TnTA30V on the force response to a 2% quick stretch in α-MHC (A) and β-MHC fibers (B) is shown. Forces were normalized to the isometric steady state force, Fss, before ML change. ED is an approximate measure of the number of strongly bound cross bridges (XBs) (6, 18), and is estimated as the slope of the relationship between (F1−Fss) and corresponding ML changes (ΔL). The effect of TnTA30V on ED in α- and β-MHC fibers (C). Data for each group are expressed as means ± SE. Statistical differences were analyzed for TnTA30V, relative to TnTWT, using two-way ANOVA and post hoc Fisher’s LSD tests (**P < 0.01). The number of fibers measured (from 3 hearts) were as follows: TnTWT + α-MHC (n = 12), TnTA30V + α-MHC (n = 14), TnTWT + β-MHC (n = 11), and TnTA30V + β-MHC (n = 12).
Effect of α- and β-MHC Isoforms on the TnTA30V-Mediated Impact on Maximal Tension and ED
To determine whether the TnTA30V-mediated effect on the thin filament was differently altered by α- and β-MHC isoforms, we analyzed differences in steady-state tension measured at maximal Ca2+ activation (pCa 4.3). A comparison of the representative force traces during isometric contractions showed that TnTA30V decreased tension in α-MHC fibers (Fig. 3A) but increased tension in β-MHC fibers (Fig. 3B). These dissimilar effects of TnTA30V on maximal tension in α- and β-MHC fibers gave rise to a significant two-way interaction effect (P < 0.001). To determine the cause of this significant interaction effect, we performed post hoc multiple t-tests. Post hoc analysis confirmed that TnTA30V significantly attenuated maximal tension by 18% (P < 0.001; Fig. 3C) in α-MHC fibers, while it significantly augmented maximal tension by 15% (P < 0.01; Fig. 3C) in β-MHC fibers. These observations demonstrate that the TnTA30V-mediated effects on maximal activation are divergently modulated by α- and β-MHC.
To support our findings in maximal tension, we assessed instantaneous muscle fiber stiffness, ED. We previously showed that ED is strongly correlated to maximal tension (11, 18, 32) and is an approximate index of the number of strongly bound XBs. ED is estimated as the slope of the linear relationship between changes in force (F1 − Fss) and ΔL, as described in materials and methods. Therefore, ED increases when F1 increases and vice versa. Figure 4, A and B, shows the effect of TnTA30V on the force responses to a sudden 2% stretch in α- and β-MHC fibers, respectively. It is evident that TnTA30V attenuated F1 in α-MHC fibers (Fig. 4A), which suggested a decrease in ED. On the other hand, TnTA30V augmented F1 in β-MHC fibers (Fig. 4B), which suggested an increase in ED. Two-way ANOVA revealed a significant MHC-TnT interaction effect on ED (P < 0.001), which confirmed that α- and β-MHC isoforms differently modulated the TnTA30V-mediated effect on ED. Post hoc t-tests demonstrated that TnTA30V significantly attenuated ED by 23% (P < 0.01; Fig. 4C) in α-MHC fibers, whereas it significantly augmented ED by 22% (P < 0.01; Fig. 4C) in β-MHC fibers. Similar findings in maximal tension and ED confirm that a decrease in tension in TnTA30V + α-MHC fibers is due to attenuation of the number of strongly bound XBs, while an increase in tension in TnTA30V + β-MHC fibers is due to augmentation of the number of strongly bound XBs.
Effect of α- and β-MHC Isoforms on the TnTA30V-Mediated Impact on the pCa-Tension Relationship
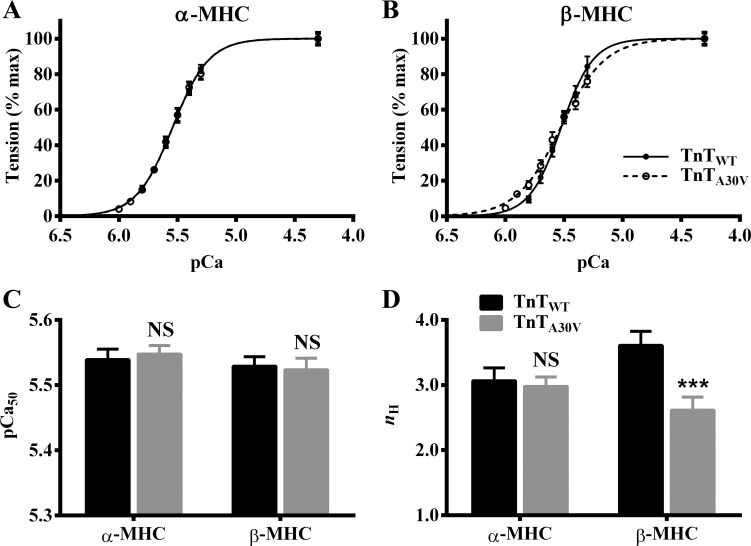
To determine whether TnTA30V affected myofilament Ca2+ sensitivity (pCa50) and cooperativity (nH) in α- and β-MHC fibers, we assessed the pCa-tension relationship. A comparison of pCa-tension relationships in α-MHC (Fig. 5A) and β-MHC fibers (Fig. 5B) showed that TnTA30V did not alter pCa50, regardless of the MHC isoform. As expected, two-way ANOVA did not reveal a significant MHC-TnT interaction effect (P = 0.65) or a main effect of TnT (P = 0.92) on pCa50. Post hoc analysis confirmed that TnTA30V had no effect on pCa50 (Fig. 5C), demonstrating that TnTA30V does not affect myofilament Ca2+ sensitivity, regardless of the MHC isoform.
Fig. 5.
TnTA30V-mediated impact on pCa-tension relationships in α- and β-MHC fibers. A comparison of pCa-tension relationships in α-MHC (A) and β-MHC (B) fibers is shown. The Hill equation was fit to pCa-tension relationships to estimate pCa50 and nH, as described previously (15, 45). The effect of TnTA30V on pCa50 (C) and nH (D) in α- and β-MHC fibers. Data for each group are expressed as means ± SE. Error bars are smaller than symbols in some cases. Statistical differences were analyzed for TnTA30V, relative to TnTWT, using two-way ANOVA and post hoc Fisher’s LSD tests (***P < 0.001; NS, not significant). The number of fibers measured (from 3 hearts) were as follows: TnTWT + α-MHC (n = 12), TnTA30V + α-MHC (n = 14), TnTWT + β-MHC (n = 11), and TnTA30V + β-MHC (n = 12).
A comparison of pCa-tension relationships also showed that TnTA30V did not alter nH in α-MHC fibers (Fig. 5A), while it attenuated nH in β-MHC fibers (Fig. 5B). Two-way ANOVA revealed a significant MHC-TnT interaction effect (P < 0.05) on nH, demonstrating that α- and β-MHC isoforms differently altered the TnTA30V-mediated effect on nH. Post hoc t-tests revealed that TnTA30V had no impact on nH in α-MHC fibers, but it significantly decreased nH by 28% (P < 0.001; Fig. 5D) in β-MHC fibers. These observations suggest that α- and β-MHC isoforms have differential effects on the TnTA30V-mediated impact on myofilament cooperativity.
Effect of α- and β-MHC Isoforms on the TnTA30V-Mediated Impact on XB Detachment Kinetics
We compared differences in the rate constant of XB distortion (c) between groups to determine whether the TnTA30V-mediated impact on the XB detachment rate (g) was differently altered by α- and β-MHC. We have previously shown that c is a valuable index of g (6, 23). Two-way ANOVA of c did not show a significant MHC-TnT interaction effect (P = 0.19) or main effect of TnT (P = 0.54). This is because TnTA30V did not alter c in either α- or β-MHC fibers (Table 1). These observations confirm that TnTA30V does not affect XB detachment kinetics, regardless of the MHC isoform.
Table 1.
Effect of TnTA30V on dynamic contractile parameters in α- and β-MHC fibers
| α-MHC |
β-MHC |
|||
|---|---|---|---|---|
| TnTWT | TnTA30V | TnTWT | TnTA30V | |
| c, s−1 | 47.09 ± 1.67 | 43.48 ± 2.49 | 19.32 ± 0.99 | 20.61 ± 1.26 |
| b, s−1 | 34.74 ± 1.94 | 33.64 ± 1.91 | 12.00 ± 0.51 | 11.30 ± 0.43 |
| γ, s−1 | 58.00 ± 5.52 | 55.59 ± 4.41 | 42.02 ± 2.36 | 42.21 ± 3.61 |
| ktr, s−1 | 12.25 ± 0.49 | 12.42 ± 0.44 | 5.77 ± 0.21 | 5.31 ± 0.26 |
Data for each group are expressed as means ± SE. Rate parameters, c, b, and γ, were estimated by fitting the nonlinear recruitment distortion model to the family of force responses to various amplitude step-like length perturbations (18). ktr was estimated by fitting a monoexponential function to the force response to a large release-restretch procedure, as described in materials and methods. Statistical differences were analyzed for mouse troponin-T equivalent of the human A28V mutation (TnTA30V) relative to wild-type mouse troponin T (TnTWT), using two-way ANOVA and post hoc Fisher’s least significant differences tests. For a given myosin heavy chain (MHC) isoform, none of the parameters listed in the table were significantly different between TnTA30V and TnTWT. The number of fibers measured (from 3 hearts) were as follows: TnTWT + α-MHC (n = 12), TnTA30V + α-MHC (n = 14), TnTWT + β-MHC (n = 11), and TnTA30V + β-MHC (n = 12).
Effect of α- and β-MHC Isoforms on the TnTA30V-Mediated Impact on XB Turnover Rate
To determine whether TnTA30V altered XB turnover rate in an MHC-dependent manner, we assessed two contractile parameters, ktr and b. ktr is a measure of the rate of force redevelopment following a large slack-restretch maneuver (3, 17). b is the rate constant of delayed force rise following a sudden increase in ML (18). We have previously shown that ktr is correlated to b and that both parameters are useful indexes of XB turnover rate (6, 18). Two-way ANOVA of ktr did not reveal a significant MHC-TnT interaction effect (P = 0.40) or a main effect of TnT (P = 0.69), which indicated that TnTA30V had no effect on ktr, regardless of the MHC isoform (Table 1). Our observations on ktr were corroborated by b. Two-way ANOVA did not show a significant MHC-TnT interaction effect (P = 0.89) or a main effect of TnT (P = 0.56) on b. Post hoc analysis showed that TnTA30V had no impact on b in either α- or β-MHC fibers (Table 1). No effect of TnTA30V on ktr and b in both α- and β-MHC fibers suggests that TnTA30V does not affect XB turnover rate, regardless of the MHC isoform.
Effect of α- and β-MHC Isoforms on the TnTA30V-Mediated Impact on γ
We compared estimates of γ to test whether the TnTA30V-mediated impact on thin filament cooperativity was differently altered by α- and β-MHC isoforms. As described earlier (18), γ represents the negative impact of strained XBs on other XBs, an effect that is transduced via allosteric/cooperative mechanisms within thin filaments. Therefore, if α- and β-MHC differently altered the TnTA30V-mediated effect on thin filament regulatory mechanisms that underlie XB-XB cooperativity, it would manifest as a change in γ. Two-way ANOVA of γ did not reveal a significant MHC-TnT interaction effect (P = 0.76) or a main effect of TnT (P = 0.79). Post hoc t-tests indicated that TnTA30V did not affect γ, regardless of the MHC isoform (Table 1). These observations suggest that TnTA30V does not affect the thin filament allosteric/cooperative mechanisms that mediate the negative impact of strained XBs on other XBs, regardless of the MHC isoform.
Effect of α- and β-MHC Isoforms on the TnTA30V-Mediated Impact on ER
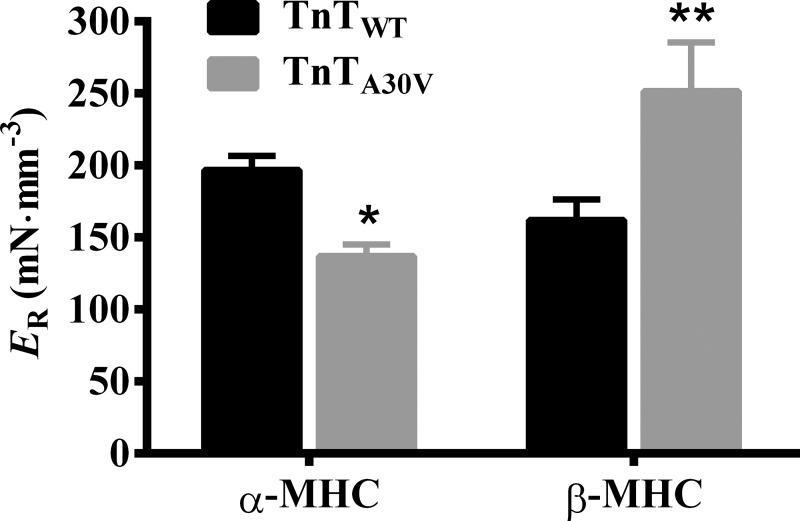
We assessed ER to determine whether TnTA30V differentially altered the magnitude of ML-mediated recruitment of new force-bearing cross bridges in α- and β-MHC fibers. ER is estimated as the slope of the relationship between changes in force (Fnss − Fss) and ΔL. Therefore, ER increases when Fnss is augmented and vice versa. A comparison of force responses to a 2% stretch showed that TnTA30V decreased Fnss in α-MHC fibers (Fig. 4A), which suggested attenuation of ER. In contrast, TnTA30V increased Fnss in β-MHC fibers (Fig. 4B), which suggested augmentation of ER. Two-way ANOVA showed a significant MHC-TnT interaction effect on ER (P < 0.001), which demonstrated that α- and β-MHC differently altered the TnTA30V-mediated impact on ER. Post hoc t-tests showed that TnTA30V significantly attenuated ER by 30% in α-MHC fibers (P < 0.05; Fig. 6), while it significantly augmented ER by 55% in β-MHC fibers (P < 0.01; Fig. 6). Our data demonstrate that TnTA30V- and α-MHC-induced changes in the thin filaments interact to attenuate ER, while those induced by TnTA30V and β-MHC interact to augment ER.
Fig. 6.
TnTA30V-mediated impact on ER in α- and β-MHC fibers. ER is an approximate measure of the number of newly recruited XBs, following an increase in ML (6, 18). ER is estimated as the slope of the relationship between (Fnss − Fss) and the corresponding ML changes (ΔL) (see Fig. 4, A and B). Data for each group are expressed as means ± SE. Statistical differences were analyzed for TnTA30V, relative to TnTWT, using two-way ANOVA and post hoc Fisher’s LSD tests (*P < 0.05; **P < 0.01). The number of fibers measured (from 3 hearts) were as follows: TnTWT + α-MHC (n = 12), TnTA30V + α-MHC (n = 14), TnTWT + β-MHC (n = 11), and TnTA30V + β-MHC (n = 12).
DISCUSSION
To our knowledge, this is the first study to characterize the effect of the mouse analog (TnTA30V) of human HCM mutation A28V on contractile function against α- and β-MHC backgrounds. Novel findings from our study demonstrate that the magnitude of XB recruitment is attenuated by TnTA30V in α-MHC fibers but is augmented by TnTA30V in β-MHC fibers. Therefore, our study suggests that TnTA30V induces DCM-like contractile changes in rodent hearts (α-MHC), whereas TnTA30V induces HCM-like contractile changes in human hearts (β-MHC).
TnTA30V-Mediated Effect on Maximal Tension Is Divergently Altered by α- and β-MHC
One major finding was that TnTA30V attenuated maximal tension in α-MHC fibers but augmented maximal tension in β-MHC fibers (Fig. 3C). The highly conserved A30 residue is located within the NH2 terminus of TnT, a region known to be important for full activation of thin filaments (7, 30). Previously, we have shown that rat TnT lacking the NH2 terminus (1–43 residues; TnT1–43Δ) induces a more prominent attenuation of maximal tension in α-MHC fibers (46%) than in β-MHC fibers (18%) (30). Therefore, we posit that the A30 residue is important for the NH2 terminus to fully activate thin filaments. This raises the following question: “how does A30V impact maximal thin filament activation?”
The mechanism by which A30V affects activation may be gleaned from a previously proposed model (22), which suggests that the NH2 terminus (1–43 residues) makes intramolecular contacts with the CR (residues 77–193) of TnT. It is well established that a major portion of the CR of TnT interacts directly with tropomyosin to modulate RU and thin filament activation (22, 37). Therefore, the modulatory role of the NH2 terminus on CR-tropomyosin interactions has important implications for the regulation of thin filament activation. The A30V mutation may modify CR-tropomyosin interactions by perturbing intramolecular interactions between the NH2 terminus and CR, leading to attenuation of RU activation. Because RU activation is critical for XB entry into the cycling pool, attenuation of RU activation may decrease the number of strongly bound XBs. This notion is substantiated by a significant attenuation of maximal tension (Fig. 3C) and ED (Fig. 4C) in TnTA30V + α-MHC fibers. In contrast, augmentation of these parameters in TnTA30V + β-MHC fibers suggests that the slower cycling β-MHC counteracts the attenuating effect of A30V on RU activation. We suspect this effect is due to enhanced XB-based cooperativity by β-MHC because of its longer dwell time in the strongly bound state. Collectively, these observations suggest that the A30V-mediated effects on the thin filament interact differently with those induced by α- and β-MHC to divergently alter the magnitude of XB recruitment.
TnTA30V Attenuates Myofilament Cooperativity in β-MHC Fibers
Another interesting observation in our study was that TnTA30V showed no effect on nH in α-MHC fibers but decreased nH in β-MHC fibers. This is somewhat surprising given that the slow cycling rate of β-MHC tends to elevate XB-based cooperativity (17). To reconcile this discrepancy, we refer to the study by Razumova et al. (39), which shows that RU-RU cooperativity dominates the contribution to nH over XB-based (XB-XB and XB-RU) cooperativity. From unaltered nH in TnTA30V + α-MHC fibers, we infer that RU-RU cooperativity is not altered. On the other hand, a decrease in nH in TnTA30V + β-MHC fibers suggests that RU-RU cooperativity is attenuated. Enhanced XB-based cooperativity in β-MHC fibers, specifically XB-RU cooperativity, may decrease the pool of RUs from which RU-RU cooperativity could recruit (39). This effect is expected to decrease the contribution of RU-RU cooperativity to nH, which is reflected as attenuation of nH in TnTA30V + β-MHC fibers. Enhanced XB-based cooperativity is also expected to increase thin filament activation (39), which is validated by a significant increase in maximal tension (Fig. 3C) and ED (Fig. 4C).
TnTA30V Does Not Affect the Rate Constants of XB Recruitment or XB Detachment Regardless of the MHC Isoform
Previous modeling studies have shown strong dependence of XB recruitment dynamics on XB-XB cooperativity. Enhanced XB-XB cooperativity decreases ktr, whereas attenuated XB-XB cooperativity increases ktr (3, 39); these predictions are also valid for b because it is strongly correlated to ktr (6, 18). Thus, a significant change in XB-XB cooperativity is expected to manifest as a change in b and ktr. However, unaffected b and ktr in TnTA30V + α-MHC or TnTA30V + β-MHC fibers (Table 1) suggest that TnTA30V does not alter XB-XB cooperativity, regardless of the MHC isoform. Because γ is also affected by XB-XB cooperativity (17, 18), unaltered γ in TnTA30V + α-MHC or TnTA30V + β-MHC fibers (Table 1) substantiates our conclusion that TnTA30V does not alter XB-XB cooperativity.
Additionally, TnTA30V showed no effect on c in α- and β-MHC fibers (Table 1), suggesting that TnTA30V does not affect the XB detachment rate, g. According to the two-state XB model (1), ktr = f + g, where f is the rate of XB attachment; therefore, any change in f would be reflected by a change in ktr. Because both ktr and g were unaffected by TnTA30V in α- and β-MHC fibers (Table 1), we infer that TnTA30V does not alter f, regardless of the MHC isoform. Collectively, our observations on b, ktr, and c suggest that TnTA30V does not affect XB cycling kinetics.
α- and β-MHC Divergently Alter the TnTA30V-Mediated Effect on ER
Ano ther striking finding was that TnTA30V attenuated ER in α-MHC fibers but augmented ER in β-MHC fibers (Fig. 6). The magnitude of ER is affected by ML-mediated XB recruitment mechanisms that operate within thin filaments (6). One such mechanism that drives changes in ER is XB-based (XB-XB/XB-RU) cooperativity (5, 6, 43). For example, a decrease in XB-based cooperativity has been shown to speed up b but attenuate ER (5, 6, 43). In contrast, an increase in XB-based cooperativity has been shown to slow down b but augment ER (5, 6). Collectively these findings from the aforementioned studies highlight the importance of XB-based cooperativity in the ML-mediated recruitment of strong XBs. Findings from our study suggest that XB-XB cooperativity is unaffected by TnTA30V, regardless of the MHC isoform. Thus directional changes in ER in TnTA30V + α-MHC and TnTA30V + β-MHC fibers may be attributed to comparable changes in XB-RU cooperativity. That is, a decrease in ER in TnTA30V + α-MHC fibers may be linked to attenuation of XB-RU cooperativity, while an increase in ER in TnTA30V + β-MHC fibers may be linked to augmentation of XB-RU cooperativity. Therefore, the mechanistic basis for our observations is that TnTA30V-mediated effects on the thin filament are differently altered by α- and β-MHC, leading to divergent effects on XB-RU cooperativity.
Summary and Conclusion
Divergent effects on ER in TnTA30V + α-MHC and TnTA30V + β-MHC fibers have significant implications for heart function in rodents (α-MHC) and humans (β-MHC). Previous studies have suggested that ER (stretch activation) is important for enhancing ventricular force output during the late phase of ejection (14, 42, 44); therefore, a decrease in ER is expected to lead to a lower than normal ventricular force output and vice versa. Thus attenuation of ER in TnTA30V + α-MHC fibers may lead to systolic dysfunction. On the other hand, augmentation of ER in TnTA30V + β-MHC fibers translates to an increased ventricular force during the late phase of ejection and a consequent delay in ventricular relaxation (20, 26), suggesting diastolic dysfunction. Our assessment based on ER is further supported by our observations in maximal tension and ED because both these parameters were attenuated in TnTA30V + α-MHC fibers but augmented in TnTA30V + β-MHC fibers. Previous studies have shown that reduced force output and systolic dysfunction are hallmarks of DCM-causing mutations (29, 41, 47), whereas increased force output and diastolic dysfunction are hallmarks of HCM (25, 31, 46). Thus our data suggest that TnTA30V mimics a DCM-like phenotype in rodent hearts (α-MHC), whereas it mimics an HCM-like phenotype in human hearts (β-MHC). Although remodeling at the whole heart level may include a myriad of changes, our results have identified potential contributors to an overall cardiac phenotype. Our finding that TnTA30V induces an HCM-like phenotype is also consistent with the clinical data that associate the A28V mutation with HCM (12, 13, 34).
GRANTS
This work was supported, in part, by National Heart, Lung, and Blood Institute Grant HL-075643 (to M. Chandra).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
A.V.M. and M.C. conceived of and designed research; A.V.M. and S.K.G. performed experiments; A.V.M., S.K.G., and M.C. analyzed data; A.V.M., S.K.G., and M.C. interpreted results of experiments; A.V.M. and S.K.G. prepared figures; A.V.M., S.K.G., and M.C. drafted manuscript; A.V.M., S.K.G., and M.C. edited and revised manuscript; A.V.M., S.K.G., and M.C. approved final version of manuscript.
REFERENCES
- 1.Brenner B. Effect of Ca2+ on cross-bridge turnover kinetics in skinned single rabbit psoas fibers: implications for regulation of muscle contraction. Proc Natl Acad Sci USA 85: 3265–3269, 1988. doi: 10.1073/pnas.85.9.3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brenner B, Eisenberg E. Rate of force generation in muscle: correlation with actomyosin ATPase activity in solution. Proc Natl Acad Sci USA 83: 3542–3546, 1986. doi: 10.1073/pnas.83.10.3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campbell K. Rate constant of muscle force redevelopment reflects cooperative activation as well as cross-bridge kinetics. Biophys J 72: 254–262, 1997. doi: 10.1016/S0006-3495(97)78664-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campbell KB, Chandra M. Functions of stretch activation in heart muscle. J Gen Physiol 127: 89–94, 2006. doi: 10.1085/jgp.200509483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campbell KB, Chandra M, Kirkpatrick RD, Slinker BK, Hunter WC. Interpreting cardiac muscle force-length dynamics using a novel functional model. Am J Physiol Heart Circ Physiol 286: H1535–H1545, 2004. doi: 10.1152/ajpheart.01029.2003. [DOI] [PubMed] [Google Scholar]
- 7.Chandra M, Montgomery DE, Kim JJ, Solaro RJ. The N-terminal region of troponin T is essential for the maximal activation of rat cardiac myofilaments. J Mol Cell Cardiol 31: 867–880, 1999. doi: 10.1006/jmcc.1999.0928. [DOI] [PubMed] [Google Scholar]
- 8.Chandra M, Tschirgi ML, Ford SJ, Slinker BK, Campbell KB. Interaction between myosin heavy chain and troponin isoforms modulate cardiac myofiber contractile dynamics. Am J Physiol Regul Integr Comp Physiol 293: R1595–R1607, 2007. doi: 10.1152/ajpregu.00157.2007. [DOI] [PubMed] [Google Scholar]
- 9.Chandra M, Tschirgi ML, Rajapakse I, Campbell KB. Troponin T modulates sarcomere length-dependent recruitment of cross-bridges in cardiac muscle. Biophys J 90: 2867–2876, 2006. doi: 10.1529/biophysj.105.076950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chandra M, Tschirgi ML, Tardiff JC. Increase in tension-dependent ATP consumption induced by cardiac troponin T mutation. Am J Physiol Heart Circ Physiol 289: H2112–H2119, 2005. doi: 10.1152/ajpheart.00571.2005. [DOI] [PubMed] [Google Scholar]
- 11.Chandra V, Gollapudi SK, Chandra M. Rat cardiac troponin T mutation (F72L)-mediated impact on thin filament cooperativity is divergently modulated by α- and β-myosin heavy chain isoforms. Am J Physiol Heart Circ Physiol 309: H1260–H1270, 2015. doi: 10.1152/ajpheart.00519.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Curila K, Benesova L, Penicka M, Minarik M, Zemanek D, Veselka J, Widimsky P, Gregor P. Low prevalence and variable clinical presentation of troponin I and troponin T gene mutations in hypertrophic cardiomyopathy. Genet Test Mol Biomarkers 13: 647–650, 2009. doi: 10.1089/gtmb.2009.0041. [DOI] [PubMed] [Google Scholar]
- 13.Curila K, Benesova L, Penicka M, Minarik M, Zemanek D, Veselka J, Widimsky P, Gregor P. Spectrum and clinical manifestations of mutations in genes responsible for hypertrophic cardiomyopathy. Acta Cardiol 67: 23–29, 2012. doi: 10.2143/AC.67.1.2146562. [DOI] [PubMed] [Google Scholar]
- 14.Davis JS, Hassanzadeh S, Winitsky S, Lin H, Satorius C, Vemuri R, Aletras AH, Wen H, Epstein ND. The overall pattern of cardiac contraction depends on a spatial gradient of myosin regulatory light chain phosphorylation. Cell 107: 631–641, 2001. doi: 10.1016/S0092-8674(01)00586-4. [DOI] [PubMed] [Google Scholar]
- 15.de Tombe PP, Stienen GJM. Protein kinase A does not alter economy of force maintenance in skinned rat cardiac trabeculae. Circ Res 76: 734–741, 1995. doi: 10.1161/01.RES.76.5.734. [DOI] [PubMed] [Google Scholar]
- 16.Fabiato A, Fabiato F. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J Physiol (Paris) 75: 463–505, 1979. [PubMed] [Google Scholar]
- 17.Ford SJ, Chandra M. Length-dependent effects on cardiac contractile dynamics are different in cardiac muscle containing α- or β-myosin heavy chain. Arch Biochem Biophys 535: 3–13, 2013. doi: 10.1016/j.abb.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ford SJ, Chandra M, Mamidi R, Dong W, Campbell KB. Model representation of the nonlinear step response in cardiac muscle. J Gen Physiol 136: 159–177, 2010. doi: 10.1085/jgp.201010467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ford SJ, Mamidi R, Jimenez J, Tardiff JC, Chandra M. Effects of R92 mutations in mouse cardiac troponin T are influenced by changes in myosin heavy chain isoform. J Mol Cell Cardiol 53: 542–551, 2012. doi: 10.1016/j.yjmcc.2012.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gillebert TC, Leite-Moreira AF, De Hert SG. Load dependent diastolic dysfunction in heart failure. Heart Fail Rev 5: 345–355, 2000. doi: 10.1023/A:1026563313952. [DOI] [PubMed] [Google Scholar]
- 21.Gollapudi SK, Chandra M. The effect of cardiomyopathy mutation (R97L) in mouse cardiac troponin T on the muscle length-mediated recruitment of crossbridges is modified divergently by α- and β-myosin heavy chain. Arch Biochem Biophys 601: 105–112, 2016. doi: 10.1016/j.abb.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gollapudi SK, Gallon CE, Chandra M. The tropomyosin binding region of cardiac troponin T modulates crossbridge recruitment dynamics in rat cardiac muscle fibers. J Mol Biol 425: 1565–1581, 2013. doi: 10.1016/j.jmb.2013.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gollapudi SK, Tardiff JC, Chandra M. The functional effect of dilated cardiomyopathy mutation (R144W) in mouse cardiac troponin T is differently affected by α- and β-myosin heavy chain isoforms. Am J Physiol Heart Circ Physiol 308: H884–H893, 2015. doi: 10.1152/ajpheart.00528.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guo X, Wattanapermpool J, Palmiter KA, Murphy AM, Solaro RJ. Mutagenesis of cardiac troponin I. Role of the unique NH2-terminal peptide in myofilament activation. J Biol Chem 269: 15210–15216, 1994. [PubMed] [Google Scholar]
- 25.Gwathmey JK, Warren SE, Briggs GM, Copelas L, Feldman MD, Phillips PJ, Callahan M Jr, Schoen FJ, Grossman W, Morgan JP. Diastolic dysfunction in hypertrophic cardiomyopathy. Effect on active force generation during systole. J Clin Invest 87: 1023–1031, 1991. doi: 10.1172/JCI115061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hunter WC. Role of myofilaments and calcium handling in left ventricular relaxation. Cardiol Clin 18: 443–457, 2000. doi: 10.1016/S0733-8651(05)70155-7. [DOI] [PubMed] [Google Scholar]
- 27.Krenz M, Robbins J. Impact of beta-myosin heavy chain expression on cardiac function during stress. J Am Coll Cardiol 44: 2390–2397, 2004. doi: 10.1016/j.jacc.2004.09.044. [DOI] [PubMed] [Google Scholar]
- 28.Krenz M, Sanbe A, Bouyer-Dalloz F, Gulick J, Klevitsky R, Hewett TE, Osinska HE, Lorenz JN, Brosseau C, Federico A, Alpert NR, Warshaw DM, Perryman MB, Helmke SM, Robbins J. Analysis of myosin heavy chain functionality in the heart. J Biol Chem 278: 17466–17474, 2003. doi: 10.1074/jbc.M210804200. [DOI] [PubMed] [Google Scholar]
- 29.Lu QW, Wu XY, Morimoto S. Inherited cardiomyopathies caused by troponin mutations. J Geriatr Cardiol 10: 91–101, 2013. doi: 10.3969/j.issn.1671-5411.2013.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mamidi R, Chandra M. Divergent effects of α- and β-myosin heavy chain isoforms on the N terminus of rat cardiac troponin T. J Gen Physiol 142: 413–423, 2013. doi: 10.1085/jgp.201310971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maron BJ, Maron MS. Hypertrophic cardiomyopathy. Lancet 381: 242–255, 2013. doi: 10.1016/S0140-6736(12)60397-3. [DOI] [PubMed] [Google Scholar]
- 32.Michael JJ, Gollapudi SK, Chandra M. Effects of pseudo-phosphorylated rat cardiac troponin T are differently modulated by α- and β-myosin heavy chain isoforms. Basic Res Cardiol 109: 442, 2014. doi: 10.1007/s00395-014-0442-9. [DOI] [PubMed] [Google Scholar]
- 33.Michael JJ, Gollapudi SK, Ford SJ, Kazmierczak K, Szczesna-Cordary D, Chandra M. Deletion of 1-43 amino acids in cardiac myosin essential light chain blunts length dependency of Ca2+ sensitivity and cross-bridge detachment kinetics. Am J Physiol Heart Circ Physiol 304: H253–H259, 2013. doi: 10.1152/ajpheart.00572.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Millat G, Bouvagnet P, Chevalier P, Sebbag L, Dulac A, Dauphin C, Jouk PS, Delrue MA, Thambo JB, Le Metayer P, Seronde MF, Faivre L, Eicher JC, Rousson R. Clinical and mutational spectrum in a cohort of 105 unrelated patients with dilated cardiomyopathy. Eur J Med Genet 54: e570–e575, 2011. doi: 10.1016/j.ejmg.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 35.Montgomery DE, Tardiff JC, Chandra M. Cardiac troponin T mutations: correlation between the type of mutation and the nature of myofilament dysfunction in transgenic mice. J Physiol 536: 583–592, 2001. doi: 10.1111/j.1469-7793.2001.0583c.xd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morita H, Larson MG, Barr SC, Vasan RS, O’Donnell CJ, Hirschhorn JN, Levy D, Corey D, Seidman CE, Seidman JG, Benjamin EJ. Single-gene mutations and increased left ventricular wall thickness in the community: the Framingham Heart Study. Circulation 113: 2697–2705, 2006. doi: 10.1161/CIRCULATIONAHA.105.593558. [DOI] [PubMed] [Google Scholar]
- 37.Oliveira DM, Nakaie CR, Sousa AD, Farah CS, Reinach FC. Mapping the domain of troponin T responsible for the activation of actomyosin ATPase activity. Identification of residues involved in binding to actin. J Biol Chem 275: 27513–27519, 2000. doi: 10.1074/jbc.M002735200. [DOI] [PubMed] [Google Scholar]
- 38.Pan BS, Johnson RG Jr. Interaction of cardiotonic thiadiazinone derivatives with cardiac troponin C. J Biol Chem 271: 817–823, 1996. doi: 10.1074/jbc.271.2.817. [DOI] [PubMed] [Google Scholar]
- 38a.Rani DS, Nallari P, Dhandapani PS, Tamilarasi S, Narasimhan C, Rakshak D, Singh L, Thangaraj K. Genomics of complex disorders I. Genomic Med 2: 303–330, 2008. doi: 10.1007/s11568-009-9104-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Razumova MV, Bukatina AE, Campbell KB. Different myofilament nearest-neighbor interactions have distinctive effects on contractile behavior. Biophys J 78: 3120–3137, 2000. doi: 10.1016/S0006-3495(00)76849-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rice R, Guinto P, Dowell-Martino C, He H, Hoyer K, Krenz M, Robbins J, Ingwall JS, Tardiff JC. Cardiac myosin heavy chain isoform exchange alters the phenotype of cTnT-related cardiomyopathies in mouse hearts. J Mol Cell Cardiol 48: 979–988, 2010. doi: 10.1016/j.yjmcc.2009.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Spudich JA. Hypertrophic and dilated cardiomyopathy: four decades of basic research on muscle lead to potential therapeutic approaches to these devastating genetic diseases. Biophys J 106: 1236–1249, 2014. doi: 10.1016/j.bpj.2014.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stelzer JE, Fitzsimons DP, Moss RL. Ablation of myosin-binding protein-C accelerates force development in mouse myocardium. Biophys J 90: 4119–4127, 2006. doi: 10.1529/biophysj.105.078147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stelzer JE, Larsson L, Fitzsimons DP, Moss RL. Activation dependence of stretch activation in mouse skinned myocardium: implications for ventricular function. J Gen Physiol 127: 95–107, 2006. doi: 10.1085/jgp.200509432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stelzer JE, Patel JR, Moss RL. Protein kinase A-mediated acceleration of the stretch activation response in murine skinned myocardium is eliminated by ablation of cMyBP-C. Circ Res 99: 884–890, 2006. doi: 10.1161/01.RES.0000245191.34690.66. [DOI] [PubMed] [Google Scholar]
- 45.Stienen GJ, Zaremba R, Elzinga G. ATP utilization for calcium uptake and force production in skinned muscle fibres of Xenopus laevis. J Physiol 482: 109–122, 1995. doi: 10.1113/jphysiol.1995.sp020503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wigle ED, Rakowski H, Kimball BP, Williams WG. Hypertrophic cardiomyopathy. Clinical spectrum and treatment. Circulation 92: 1680–1692, 1995. doi: 10.1161/01.CIR.92.7.1680. [DOI] [PubMed] [Google Scholar]
- 47.Willott RH, Gomes AV, Chang AN, Parvatiyar MS, Pinto JR, Potter JD. Mutations in Troponin that cause HCM, DCM AND RCM: what can we learn about thin filament function? J Mol Cell Cardiol 48: 882–892, 2010. doi: 10.1016/j.yjmcc.2009.10.031. [DOI] [PubMed] [Google Scholar]