Abstract
Heart failure (HF) is an end point resulting from a number of disease states. The prognosis for HF patients is poor with survival rates precipitously low. Energy metabolism is centrally linked to the development of HF, and it involves the proteomic remodeling of numerous pathways, many of which are targeted to the mitochondrion. microRNAs (miRNA) are noncoding RNAs that influence posttranscriptional gene regulation. miRNA have garnered considerable attention for their ability to orchestrate changes to the transcriptome, and ultimately the proteome, during HF. Recently, interest in the role played by miRNA in the regulation of energy metabolism at the mitochondrion has emerged. Cardiac proteome remodeling during HF includes axes impacting hypertrophy, oxidative stress, calcium homeostasis, and metabolic fuel transition. Although it is established that the pathological environment of hypoxia and hemodynamic stress significantly contribute to the HF phenotype, it remains unclear as to the mechanistic underpinnings driving proteome remodeling. The aim of this review is to present evidence highlighting the role played by miRNA in these processes as a means for linking pathological stimuli with proteomic alteration. The differential expression of proteins of substrate transport, glycolysis, β-oxidation, ketone metabolism, the citric acid cycle (CAC), and the electron transport chain (ETC) are paralleled by the differential expression of miRNA species that modulate these processes. Identification of miRNAs that translocate to cardiomyocyte mitochondria (miR-181c, miR-378) influencing the expression of the mitochondrial genome-encoded transcripts as well as suggested import modulators are discussed. Current insights, applications, and challenges of miRNA-based therapeutics are also described.
Keywords: heart failure, metabolism, miRNA, mitochondria
heart failure (HF) currently plagues more than 5 million Americans, has a 50% survival rate after 5 years, and is a contributing factor in over 10% of deaths in America (91). The pressing need for treatment strategies to halt the progression of early-stage HF to late-stage HF is clearly evident when looking at the dismal prognosis. With two-thirds of patients given a diagnosis of HF having a history of ischemic heart disease, the focus of this review is the role of microRNAs (miRNA) in facilitating the adaptation of a shift in energy metabolism in early-stage ischemic HF (65). However, studies using various HF animal models of different stages and levels of ischemia are referenced to provide similar metabolic characteristics in each, but this also may account for variability in miRNA and protein expression and activity.
The pathophysiological stimuli of hypertension and atherosclerosis, which lead to a state of chronic cardiac hypoxia and hemodynamic stress, provide the impetus for a host of compensatory adaptations (3). As the heart begins to lose functional capacity, increased neurohormonal stimulation is the first mechanism employed to restore homeostasis (46). Over time this chronic stimulation, in addition to heightened molecular and mechanical stress, results in dysregulated kinase and phosphatase activity leading to dysfunctional hypertrophic growth of the heart (3, 46). Additionally, calcium homeostasis, essential for optimal contraction and relaxation of myofilaments, is negatively impacted by HF (99). A significant downregulation of sarcoplasmic reticulum calcium ATPase (SERCA2) protein and ryanodine receptor 2 (RYR2) protein was observed in a volume overload rat aortocaval fistula model of HF (99). Shah and Mann (117) also speak to the observed disruption of calcium homeostasis in their review of potential therapeutic targets of HF.
The metabolic transition from fatty acid oxidation (FAO) to glycolysis in the hypoxic environment of HF is well established in the literature (3, 15, 27, 28, 44, 49, 99, 110, 113, 136, 150). Depressed levels of FAO enzymes have been shown in many unique models of HF (15, 28, 99, 150). In some of the same studies, compensatory increases in enzymes of glycolysis were also found (28, 150). This dramatic shift in fuel utilization is a catalytic event in the progression of HF, and it is the aim of this review to elucidate the role of miRNA in its regulation.
Reactive Oxygen Species, miRNA, and Metabolic Shift in Heart Failure
While one might expect less oxidative stress in a state of hypoxia, the reality is quite the contrary. The reduction of flavin mononucleotide and respiratory cytochromes, along with the auto-oxidation of ubisemiquinone, defects in Complex II, and phosphorylation of Complex IV during ischemia in cardiac mitochondria, enhances their ability to transfer electrons to oxygen, with Complex III thought to contribute greatest to the reactive oxygen species (ROS) burden (9, 21, 135). The pathological stimuli of hypoxia in ischemic HF leads to an increase in oxygen radicals, which working synchronously, may activate hypoxia-inducible factors (HIF) to modulate the transcriptional response to hypoxia (135). In normoxic conditions HIF-1α is hydroxylated and degraded, so not only does hypoxia prevent normal hydroxylation and degradation, but others have suggested increased ROS contribute to the stabilization of HIF-1α (135). The action of HIF-1α in the oxidative stress response is vast in that it induces the transcription of over 100 genes, at least one of which contains an intronic sequence that codes for a miRNA that contributes to the oxidative stress response (19). The specifics of this mechanism by which miRNA-210 is generated and elicits its regulatory function is described later.
In agreement with the upregulation of miRNA found in response to hypoxia and increased ROS, Cheng et al. (24) showed that exposing cardiomyocytes to H2O2 for a short time (6 h) upregulated miRNA-21. Additionally, Simone et al. (121) propose a miRNA expression signature in fibroblasts when exposed to H2O2 which is prevented by pretreatment with cysteine, therefore confirming the role of ROS in altering miRNA expression. A complementary mechanism of ROS detection in the mitochondria is the redox state of glutathione (21). Increased ROS causes excess oxidation of glutathione, which leads to greater S-glutathionylation of ETC Complex I, enhancing its propensity to leak electrons to oxygen and further perpetuate the pro-oxidant cycle (21). These oxygen radicals produced are also thought to modulate the function of citric acid cycle enzyme (CAC), aconitase, to contribute to ROS generation (21). The contributory role of aconitase to oxidative stress is interesting to note, because aconitase has been found to be upregulated in pressure-overload-induced HF and aconitase 2 upregulated in myocardial infarction-induced HF (15, 150). Increased ROS are expected to modify and impair the function of calcium handling and contractile components in HF, and it looks to be the case with additional enzymes of energy production (45). In yet another mechanism of ROS detection and implication in ATP generation, Wang et al. (152) show that cysteine 294 of the ATP synthase α subunit acts as an oxidative stress sensor through its selective S-glutathionylation during HF, which is associated with a decrease in activity when compared with cardiac resynchronization therapy (CRT) that reverses this oxidative modification.
The NADPH oxidase protein family plays a pivotal role in the production of excess ROS within the cardiomyocyte. NADPH oxidase 4 (NOX4), the isoform with constitutive baseline expression, has been shown to be upregulated by the action of hypercholesterolemia downregulating miRNA-25, which targets it (143). However, the regulation of miRNA-25 is complicated, because its observed increased expression in HF directly inhibits the SERCA2a transcript leading to impaired calcium handling (61, 146). While the induction of NOX2 and NOX4 by HIF-1α in response to hypoxia and increased ROS surely exasperates the oxidative phenotype, the responsibility of the former has been shown to be critical to the development of the pathological state (78). In a pressure-overload model of HF, Parajuli et al. (97) showed that the loss of NOX2 prevented the oxidative stress, hypertrophy, fibrosis, and increased matrix metalloprotease activity of the myocardium. As for potential agents targeting the NOX2 transcript, miRNA-34 and miRNA-17 have shown promise (56, 73). In fact, the upregulation of NOX2 in HF could be partially attributed to the downregulation of miRNA-17 (35, 54, 56).
The foundational role of oxidative stress in HF is evidenced by the treatment with Szeto-Schiller (SS) 31, a mitochondrial-targeted antioxidant, following transverse aortic constriction (TAC)-induced HF, preventing the pathological remodeling of 84% of mitochondrial proteins and 69% of nonmitochondrial proteins (27). Numerous groups have provided additional evidence for possible mechanisms contributing to the elevated oxidative damage in HF (27, 28, 37, 49, 60, 75, 99, 110). Mitochondrial proteome remodeling and posttranslational modification may very well play a part in ROS generation during HF evidenced by the upregulation of pro-oxidant aconitase, downregulation of antioxidant superoxide dismutase (SOD), and hypersensitivity of the ETC and ATP synthase to S-glutathionylation (15, 21, 49, 150, 152). When the levels of ROS and damaged intracellular components climb to a point of unsustainability in the heart, autophagy and apoptosis are induced, leading to end-stage failure or myocardial infarction (27, 28, 37). Dai et al. (27, 28) characterize this state by observing a greater than twofold increase of autophagosomes and upregulation of apoptotic proteins, including VDAC, in TAC-induced HF. We support the hypothesis that a variety of pathological stimuli or conditions implicated in HF, especially hypoxia and increased ROS, contribute not only to the differential expression of mRNA and miRNA that modulate the oxidative stress response including the shift in energy metabolism, but also to oxidative posttranslational modifications that directly affect the activity of metabolic enzymes.
MiRNA Processing and Translocation
Understanding the mechanisms of miRNA synthesis and action helps to provide a greater framework in which to consider the significance of their differential expression during the development of disease. RNA Polymerase II is responsible for the transcription of long primary miRNA transcripts (pri-miRNA) (68, 69). These pri-miRNAs are further processed to ~70 nucleotide stem-loop precursor miRNAs (pre-miRNA) by the microprocessor complex composed of Drosha, a nuclear RNase III, Pasha, a double-stranded RNA binding protein, and other components adding to cleavage specificity (33, 68). Export of pre-miRNA from the nucleus has been shown to be attributed to Exportin-5 in conjunction with Ran-GTP (77). Once in the cytosol, the pre-miRNA is targeted by another RNase III, Dicer, which cleaves its loop, resulting in a double-stranded RNA (dsRNA) (53). The dimerization of Dicer with R2D2 imparts the ability of the complex to thermodynamically determine which strand will be kept to use as mature miRNA and which strand will be degraded (132). The function of the human immunodeficiency virus transactivating response RNA-binding protein (TRBP), also in association with Dicer, recruits Argonaute 2 (Ago2) to the bound miRNA, completing the RNA-induced silencing complex (RISC) (23, 42). The guide miRNA strand of the completed RISC binds a complementary or partially complementary sequence in the 3′-untranslated region (UTR) of its target mRNA, followed by translocation to a processing body (P-body) for mRNA degradation (74). Current evidence shows mammalian mRNAs are usually first repressed by miRNA via one or more of three mechanisms involving the action of GW182 and/or eIF4A, followed by deadenylation and degradation (36, 52, 55, 84). While much of the data assert that miRNA-mediated repression of translation occurs at the initiation stage, multiple reviews have highlighted that the exact regulatory mechanisms have not been completely worked out (52, 55, 81, 84, 137).
Recent work has characterized the localization of miRNA to the mitochondria, nucleus, and nucleolus (7, 41, 100). While importin 8 (IPO8) interacts with Ago2 to transport specific miRNAs into the nucleus, mitochondrial miRNA import is less clear (154). Barrey et al. (7) were the first to show the presence of both pre-miRNA and miRNA in the mitochondria, so this begs the question of whether pre-miRNAs have been processed from mitochondrial-encoded pri-miRNA or imported from the cytosol to possibly be further refined to mature miRNA (7). Although some reports dispute the potential for miRNA processing in the mitochondria due to lack of evidence for Dicer and Ago2 presence at the time of their study, recent advances from Jagannathan et al. and Das et al. of miRNA regulation of mitochondrial gene expression necessitate the presence of Ago2 (30, 57, 108). It is plausible that import of pre-miRNAs could be mediated by polynucleotide phosphorylase (PNPASE) due to its localization to the mitochondrial intermembrane space and its mechanism of recognizing sequences within the hairpin loop of RNA species to initiate import (5, 147, 148). However, further experiments to determine the presence of miRNA processing enzymes in the mitochondria are critical before concluding that mitochondrial-encoded miRNA posttranscriptionally regulate mitochondrial gene expression.
miRNA Impact on Metabolic Pathways in Heart Failure
miRNA play a prominent role in orchestrating the multitude of proteomic adaptations observed in HF. The modulation of a variety of pathways contributes to the resulting shift in energy metabolism from FAO to glycolysis. Below are some of the essential axes that, through differentially expressed miRNA, facilitate this transition. For the sake of this review, neither these pathways nor the miRNA that target them are an exhaustive list. Our approach in identifying the miRNA-regulation of energy metabolism was to first clearly characterize the shift in levels of proteins involved in substrate transport and the catabolism of various substrates in producing ATP. We reasoned that not only would an increase in miRNA expression decrease translation and a decrease in miRNA expression increase translation of proteins of interest in each pathway, but that even those transcription factors that elicit transcriptional regulation would be regulated by the differential expression of miRNA in HF. Careful analysis of previous data led us to identify miRNA species targeting mRNA transcripts of interest, and through the consultation of transcriptomic studies of the failing heart we were able to quantify the upregulation or downregulation of miRNAs implicated in a given pathway.
The transcriptomic studies of miRNA expression in the failing human myocardium have utilized a variety of methods including Northern blotting, microarrays, and deep sequencing, and the most relevant and inclusive of these can be found here (39, 54, 67, 83, 126, 131, 139, 164). Significantly, 14 of the 15 miRNA species differentially expressed in human HF that we highlight in Table 1 were either found to be differentially expressed in only one study or their differential expression in HF did not conflict in direction of regulation in any of the 8 major transcriptomic studies referenced above. The exception was miRNA-1 being downregulated in dilated cardiomyopathy and aortic stenosis but not ischemic cardiomyopathy in one study, but upregulated in patients with HF in five other studies (54, 67, 72, 83, 126, 131). The consistency of expression patterns of miRNAs targeting enzymes involved in energy metabolism during HF throughout the existing transcriptomic studies provides encouragement for the characterization of shared miRNA expression profiles in HF that may be useful in developing therapies targeting these similarities in HF patients as a group. Table 1 represents a list of miRNAs differentially expressed in human HF as well as protein targets with their respective functions, while Fig. 1 provides a graphical representation in a cellular context.
Table 1.
Differential Expression of miRNA in Human Heart Failure
| miRNA Species | ΔExpression | Targeted Protein/Function | Refs. |
|---|---|---|---|
| miRNA-208a | Upregulated in HF | MED13/systemic metabolism | (8, 16, 43, 82, 114, 140) |
| miRNA-17 | Downregulated in HF | NOX2/ROS production | (35, 54, 56) |
| miRNA-29a | Upregulated in HF | VDAC1, VDAC2/ATP transport, apoptosis | (6, 35, 49, 83, 131) |
| miRNA-424 | Upregulated in HF | Arl2/ATP transport | (29, 35, 93, 131) |
| miRNA-320 | Upregulated in HF; downregulated in I/R | PFKm/glycolysis; Hsp20/cardioprotection | (35, 54, 72, 106, 129, 131) |
| miRNA-199a | Upregulated in HF; downregulated in ischemia | Hk2, Pkm2/glycolysis; PPARδ/FAO | (3, 35, 103, 104, 139, 162) |
| miRNA-21 | Upregulated in HF | PPARα/FAO | (19, 35, 67, 72, 83, 131, 158) |
| miRNA-26a | Upregulated in HF | PDHX/aerobic respiration | (20, 72, 83) |
| miRNA-27b | Upregulated in HF and cardiac remodeling | PPARγ/lipid metabolism | (35, 54, 83, 151) |
| miRNA-27a | Upregulated in HF | PPARγ/lipid metabolism | (19, 35, 54, 83) |
| miRNA-24 | Upregulated in HF | FABP4/lipid metabolism | (35, 54, 63, 83, 139) |
| miRNA-210 | Upregulated in HF and hypoxia | ISCU1/2/redox | (40, 48, 72) |
| miRNA-181c | Upregulated in HF and hypoxia | Mt-COX1/OXPHOS; BCL2/apoptosis | (30, 31, 38, 149, 164) |
| miRNA-1 | Upregulated in HF and excessive glucose insult | Hsp60/apoptosis; Hsp70/apoptosis | (35, 54, 67, 72, 83, 118, 126, 131, 165) |
| miRNA-378 | Upregulated in HF and diabetes | ATP6/ATP synthesis | (59, 72, 83) |
Differential miRNA expression in human heart failure (HF). List of differentially regulated microRNAs (miRNA) and target proteins during heart failure. Protein function is indicated, as well as reference for differential miRNA expression and targeting of protein. FABP4, fatty acid binding protein subunit 4; VDAC1 and VDAC2, voltage-dependent anion-selective proteins 1 and 2; Mt-COX1, mitochondrial cytochrome c oxidase 1; BCL2, B-cell lymphoma 2; ROS, reactive oxygen species; I/R, ischemia/reperfusion; Arl2, ADP-ribosylation factor-like 2; PDHX, pyruvate dehydrogenase subunit X.
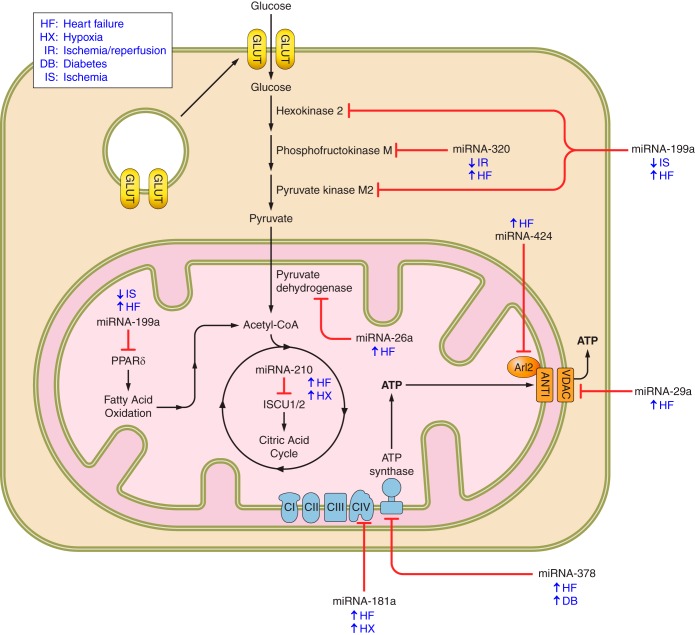
Fig. 1.
microRNA (miRNA)-mediated metabolic transition in human heart failure. Metabolic targets of differentially expressed miRNA in human heart failure. Depicted are miRNAs implicated in the proteomic remodeling of energy metabolism during heart failure. The upregulation or downregulation of each miRNA is shown beside the condition in which their differential expression is observed. The bars with marked ends connecting miRNA with protein indicates targeting and is not specific to condition.
Substrate transport.
To provide context to the proteomic shift in energy metabolism during HF as described above, let us first begin with metabolite transport both into and out of the failing cardiomyocyte. Whereas insulin-mediated glucose uptake may be impaired, the transport of glucose and flux of glycolysis in the nondiabetic, failing cardiomyocyte nonetheless remains constant or even increased in early-stage HF (71, 107, 119). Ischemia, contraction, catecholamines, increased ROS, increased intracellular calcium concentration, and decreased intracellular ATP content can all signal for the subcellular translocation to the membrane of multiple glucose transporter isoforms (4, 34, 47, 76, 112, 119, 160, 161). An ischemic environment, increased oxidative stress, and lower ATP content serve to activate AMP-activated protein kinase (AMPK) which indirectly signals for the translocation of sequestered GLUT1 and GLUT4 to the membrane while simultaneously accelerating glycolysis by phosphorylating and activating PFK2 (34, 119). Liver kinase B1 (LKB1) senses the increased AMP/ATP ratio and associates with the scaffold protein Sestrin2 (90, 119). AMPK is recruited to the newly formed complex where it is activated and phosphorylates AS160, which activates Rab to signal to the sequestered GLUT vesicles for translocation to the sarcolemma (4, 76, 90, 119, 161). Once at the membrane, internalization of GLUT4 has been suggested to be inhibited by the continued activity of AMPK (157). While the upregulation of glucose transport and/or glycolysis in early-stage ischemic HF is definitely shown to be mediated by translocation and activation of key proteins, not necessarily by differential expression, the potential translational regulation of these proteins by miRNA during HF remains an open question for the field (71, 125).
Due to the high dependence of cardiomyocytes on adequate ATP levels for a host of functions, it is essential to consider the transport of this nucleotide from the mitochondrial matrix where it is synthesized to the cytosol where it is utilized by a variety of enzymatic processes. Interestingly, miRNA may be partly responsible in the direct and indirect regulation of mitochondrial ATP transporter expression in HF. For example, voltage-dependent anion-selective protein 1 (VDAC1), the major outer mitochondrial membrane importer of ATP constituents and exporter of ATP, has been found to be downregulated in end-stage HF (49). A possible mechanism behind this observation can be found by looking at the upregulation of miRNA-29a in HF, a miRNA transcript that targets the 3′-UTR and represses the translation of VDAC1 and VDAC2 (6, 35, 83, 131). VDAC1 may also be a player in the mitochondrial permeability transition leading to apoptosis, which could account for spikes in VDAC expression in the failing heart (133). The inner mitochondrial membrane transporter of ADP and ATP, adenine nucleotide transporter 1 (ANT1), is also indirectly negatively regulated by the upregulation of a miRNA species during HF (93). miRNA-424, which is upregulated during HF, targets and suppresses ADP-ribosylation factor-like 2 (Arl2), a protein which complexes with binder of Arl2 (BART) and localizes to ANT1 (35, 93, 131).
Glycolysis.
The metabolic transition from β-oxidation to glycolysis is an important compensatory adaptation observed during the transition from a healthy heart to a failing heart (49). This signature response is well documented by a variety of proteomic studies (28, 49, 150). Downregulation of fatty acid oxidation with concurrent upregulation of glycolysis in a mouse model of HF induced by TAC has been characterized (28). Similar responses in an alternative method of inducing HF in rats by myocardial infarction revealed the same response of decreased fatty acid oxidation and a shift towards increased glycolysis (150). More evidence to support this shift to a more robust glycolytic phenotype comes from Urbonavicius et al. (136) who elucidated that glycolytic enzymes l-lactate dehydrogenase A chain and triose phosphate isomerase were significantly upregulated in human chronic reversibly dysfunctional myocardium (136). In a comprehensive review of proteomic remodeling in HF, Hollander et al. (49) make the argument that the majority of available literature on the subject reaches a consensus on the transition from using fatty acids to glucose as the predominant fuel.
While the shift in energy metabolism is clearly illustrated, the underlying molecular mechanisms facilitating the transition are less evident. In response to hypoxia and other deleterious environmental stimuli, differential miRNA expression in the heart suggests that it may be a significant contributing factor (38). The observation that the failing heart adopts a Warburg-like metabolic state and a more fetal gene program in which the pyruvate kinase M2 (Pkm2) isoform predominates, necessitates the regulatory flexibility permitted (104). And this dynamic state of regulation is indeed observed. It has been reported that miRNA-199a targets and downregulates hexokinase-2 (Hk2) and Pkm2 in hepatocellular carcinoma (HCC) cells, but during hypoxia, RNA-binding protein HuR complexes with miRNA-199a, silencing it, to facilitate the upregulation of glycolysis (162). Not surprisingly, it is observed that miRNA-199a is quickly downregulated in cardiomyocytes subjected to ischemia, explaining the enhanced expression of these two critical enzymes of glycolysis (103). Another compelling case for the regulation of glycolysis by miRNA during HF lies in miRNA-320 targeting its rate-limiting enzyme, phosphofructokinase (PFK) (129). Reduced expression of miRNA-320 is associated with increased expression of PFK in muscle, just as the reduced expression of miRNA-320 in hearts subjected to ischemia/reperfusion (I/R) in vivo and ex vivo, indicative of early HF, is associated with upregulation of glycolysis (35, 129). The preferential and stage-dependent regulation of a variety of critical proteins involved in numerous cellular processes by miRNA-320 could explain its upregulation in HF (35, 54, 72, 131). Following glycolysis, pyruvate dehydrogenase (PDH) subunit X has been found to be downregulated in HF, and a potential mechanism by which this regulation occurs is the enhanced expression of miRNA-26a, which targets and suppresses the PDH subunit X transcript (20, 49, 72, 83).
Fatty acid oxidation.
The healthy heart derives most of its basal ATP from FAO, whereas in the failing heart the ratiometric difference in contribution between FAO and glycolysis is not as pronounced (128). In a study assessing proteome dynamics of mitochondrial oxidative pathways using pressure overload-induced HF, Bugger et al. (15) found that 6 of 11 fatty acid oxidation proteins were significantly reduced, 1 was significantly increased, and 4 showed no significant changes. Recent reviews on this subject support this notion of a general decrease in proteins involved in FAO during proteomic remodeling of mitochondria in HF (49, 150). Sack et al. (113) propose a gene regulatory pathway involved in this metabolic shift, which may very well include differential expression of introns that contain regulatory miRNAs. While the upregulation of glycolysis in HF was shown to be facilitated by differential miRNA expression, the case can also be made for the downregulation of β-oxidation.
The peroxisome proliferator-activated receptor (PPAR) family of proteins are nuclear receptors, transcription factors that regulate the expression of fatty acid anabolic and catabolic genetic programs (39, 145, 158). PPARα functions to promote the transcription of genes involved in fatty acid oxidation, so the upregulation of miRNA-21, which targets and suppresses the PPARα transcript, during HF aligns with the results of the previously mentioned proteomic studies (35, 67, 72, 83, 131, 158). To support the observed downregulation of this nuclear-localized transcriptional activator of β-oxidation, Osorio et al. (96) found a concomitant decrease in retinoid X receptor α (RXRα), which PPARα binds, to elicit its function. el Azzouzi et al. (39) propose a mechanism by which PPARδ, another protein involved in the regulation of fatty acid catabolism, is downregulated during mitochondrial hemodynamic stress. These authors suggest that a hypoxic environment in the heart activates DNM3os, a RNA transcript composed of the miRNA-199a~214 cluster, and that these miRNA species target and suppress PPARδ (39). This claim has been supported by the observation that miRNA-199a and miRNA-214 are upregulated, both in transgenic mice expressing activated calcineurin A (CnA), which mimics severe cardiac hypertrophy, and in end-stage failing human hearts (139). This repression of miRNA-199a in response to cardiac hypoxia followed by its upregulation in end-stage HF may represent a precisely regulated mechanism by which glycolysis is first induced, but eventually the depression of fatty acid oxidation poses a more critical objective for the cardiomyocyte. An additional protein with reduced expression in the failing heart is peroxisome proliferator-activated receptor-gamma coactivator-1α (PGC-1α), which is regulated by miRNA-696 and found to be upregulated in hypoxia (1, 3). PGC-1 powerfully induces the expression of nuclear respiratory factor 1 (NRF1) and nuclear respiratory factor 2 (NRF2) which contribute to mitochondrial number and respiratory function (156). The reduced expression of this essential coactivator decreases the amount and activity of mitochondria, as would be expected by depressed FAO (156). This summarization of PGC-1 playing a vital role in mitochondrial biogenesis and cellular respiration is documented in cardiac myocytes as well (70). The regulation of PPARγ, a transcription factor involved in activating the fatty acid synthesis genetic program, may also be contributed to by miRNA in HF as miRNA-27a and miRNA-27b are both shown to target PPARγ and be upregulated in HF (19, 35, 54, 83, 151). More evidence to support that fatty acid synthesis is restricted in HF comes from the observation that miRNA-24, which targets fatty acid binding protein subunit 4 (FABP4), is also upregulated during HF (54, 63, 83, 139).
Citric acid cycle.
Despite some diversity in findings concerning the expression levels of CAC enzymes during HF, the general consensus seems to side with overall downregulation due to the hypoxic state (44, 49). Hollander et al. reference the reduced expression of malate dehydrogenase (MDH) during HF, and Gudjarnason et al. support this assertion of decreased flux through the observation of decreased isocitrate dehydrogenase (IDH) activity in coronary HF (CHF) as well (44, 49). As is discussed in more depth in the upcoming section, a potential posttranscriptional regulatory mechanism to explain the decrease in CAC activity is the upregulation of miRNA-210, which targets and suppresses ISCU1/2, proteins essential for the production of iron-sulfur clusters used by CAC enzymes for reduction-oxidation reactions (40, 48, 72).
ETC and ATP synthesis.
A significant decrease in activity levels of Complexes III and V in a canine pacing-induced cardiac failure model is supported by evidence of a reduced cytochrome content and reduced Complex III and IV activities in the hearts of transplant recipients with dilated cardiomyopathy (DCM) (14, 80). The observation that proteins from ETC Complex I are downregulated as well as mitochondrial ATP synthase D chain being downregulated in end-stage chronic HF strengthens these suggestions (49, 136). In an anaerobic environment these alterations are expected, but the fine-tuning by differential miRNA expression once again provides a more detailed mechanistic understanding.
Azzouzi et al. indicate that hypoxic conditions of HF activate the expression of miRNA-181c, which is responsible for targeting and suppressing cytochrome c oxidase subunit 1 (mt-COX1) involved in ETC Complex IV (3, 164). Das et al. (31) were the first to characterize this mitochondrial genome targeting by the nuclear-encoded miRNA-181c transcript. Another potential regulatory mechanism of the ETC during heart disease is the diminished availability of iron-sulfur clusters essential for oxidation-reduction reactions of ETC Complexes I, II, and III (48). Studies illustrate the stepwise process by which a hypoxic environment, one clearly implicated in HF, activates hypoxia-inducible factor 1 (HIF1) to induce the expression of miRNA-210, which targets and suppresses the iron-sulfur cluster assembly proteins ISCU1/2 (17, 38, 40, 48, 72). He et al. (48) highlight the essential role ISCU1/2 plays as a chaperone in the assembly of iron-sulfur clusters as well as the transport of these clusters to their functional location in the cell. Although others have reported no change in activity levels of ETC Complexes I, II, and IV in pacing-induced HF as well as no change in activity levels of Complexes II and V in human DCM, it has been argued that it is the functional capacity of a respirasome supercomplex composed of Complex I, Complex III dimer, and Complex IV that is most indicative of overall oxidative phosphorylation (14, 80, 111).
Ketone metabolism.
The impairment of FAO during HF naturally results in an increase in circulating free fatty acid (FFA) levels. The liver senses this increase, and begins to undergo ketogenesis using the FFAs as substrates (58). Increased systemic ketone levels reach the heart via the vasculature where they are uptaken by cardiomyocytes, with rates of oxidation matching rates of delivery (26). As a viable fuel in this condition, ketone bodies are metabolized in the heart via 3-hydroxybutyrate dehydrogenase 1 (BDH1), succinyl CoA:3-oxoacid CoA transferase (SCOT), and acetoacetyl CoA thiolase to produce acetyl-CoA molecules (26). Flux through the competing pathways of ketolysis and FAO that both produce acetyl-CoA in the mitochondria is altered in HF, and this is supported by their molecular phenotypes (155). Aubert et al. found the increased expression of 3-hydroxybutyrate dehydrogenase in HF, and Bedi et al. found the increased expression of succinyl CoA: 3-oxoacid CoA transferase in HF (2, 10). The systemic metabolic milieu of HF is rather complicated, so tissue-specific regulatory mechanisms are required to balance the production and use of ketone bodies. Since the liver is undergoing ketogenesis and the heart is undergoing ketolysis, one would expect the enzymes of ketone anabolism and catabolism to be differentially expressed accordingly. Thorrez et al. have shown that the expression of SCOT, the rate-limiting enzyme of ketolysis, is downregulated in the liver due to both epigenetic modifications in its promotor region and upregulation of miRNA-122, which targets it (116, 130). While no miRNA-mediated regulatory mechanisms have been found to date, increased expression of SCOT in the heart could very well be partially attributed to downregulation of one or multiple miRNA targeting it during HF. The role of miRNA in regulating cardiac ketone metabolism remains an unexplored and open question for this field. The relative ease of quantification of ketone body metabolism is especially advantageous clinically, as it has been shown that levels of both exhaled acetone and urinary ketone may correlate to severity of HF (25, 159).
Heat shock proteins.
Due to the incredible resiliency of the heart to adapt to harmful stimuli, evidenced by the ability to completely shift metabolic programs, the failing cardiomyocyte possesses protective mechanisms to aid in survival. One such family of enzymes devoted to reversing cell injury is that of the heat shock proteins (HSPs). In response to I/R, Ren et al. (106) found murine hearts significantly underexpressed miRNA-320, which targets and suppresses heat shock protein 20 (Hsp20). This observation could indicate an allocation by the cell to express more Hsp20, a cardioprotective protein, during an environmental insult to stay viable. miRNA-320 is upregulated in the failing heart however, and this may be explained by stage dependency in which the cardiomyocyte eventually reaches an apoptotic state of no possible return to homeostasis (35, 54, 72, 131). Shan et al. characterize the upregulation of miRNA-1, which regulates heat shock protein 60 (Hsp60), during excessive glucose insult that exhibits a toxicity beyond repair by the heart (118, 165). This increased expression of miRNA-1 during extreme conditions is reflective of the upregulation of miRNA-1 in HF (35, 67, 72, 83, 118, 126, 131).
Mitochondrial Genome Regulation and miRNA Therapeutics
Some of the most groundbreaking heart disease research is uncovering the role of miRNA to not only regulate nuclear-encoded mRNA of proteins destined for the mitochondria, but also its role in regulating mitochondrial genome expression (7, 13, 22, 30, 31, 38, 50, 57, 124, 134, 149). The mitochondrial genome is composed of 37 genes of which only 13 code for ETC proteins (98, 120). Due to the production of a polycistronic transcript encompassing most of the genome, mitochondrial gene expression is very weakly regulated transcriptionally (85, 127). This nature of mitochondrial gene expression necessitates extremely complex regulation posttranscriptionally, and it is found to be the case. Following splicing of the polycistronic transcript into its component mRNAs, tRNAs, and rRNAs, individual mRNA transcripts are subject to polyadenylation by mitochondrial poly(A) polymerase (PAPD1) (18). Those RNA molecules deemed as unimportant to the cell in its current environment or pathophysiological state are degraded by the action of human RNA helicase Suv3 along with other unidentified contributory proteins (127). Further regulation is conferred by pentatricopeptide repeat (PPR) domain proteins, which bind directly to mitochondrial mRNA (101). Proteins, including translation factors, involved in mitochondrial translation are shown to be regulated by a host of modifications including acetylation and phosphorylation (66).
The prominent role of miRNA regulation of the mitochondrial-encoded oxidative phosphorylation protein subunits is just beginning to be elucidated (7, 13, 22, 124). It has been shown by Das et al. that miRNA-181c, which targets and represses mt-COX1 in the mitochondria and B cell lymphoma 2 (BCL2) in the cytoplasm, is present in the mitochondria of cardiac myocytes during conditions of HF (30, 31, 38, 149, 164). Jagannathan et al. (57) were the first to characterize the redistribution of miRNA-378, which targets and suppresses F0 component ATP6, to the interfibrillar mitochondria in response to diabetic insult. Their findings of ATP6 being posttranscriptionally regulated by snRNA have been supported by others who successfully modulated the expression of ATP6 using chimeric antisense RNA (134). miRNA-378 has also been found to be upregulated in human HF (72, 83). The role of miRNA in mitochondria is rapidly growing in popularity as its appeal to cancer and cardiovascular health becomes more evident and its potential to be targeted using precision therapeutics expands (12, 123).
This era of molecular medicine is continuing to see an evolution in the diagnosis, prognosis, and treatment of disease. Because of the high degree of stability of miRNA in blood serum and plasma samples, circulating miRNA levels have the potential to aid in better characterizing various diseases including HF, as well as provide targets for pharmaceutical intervention (115, 144, 153). An example of this method in practice is illustrated by the observations that miRNA levels of HF patients who responded to cardiac resynchronization therapy (CRT) showed favorable changes while the nonresponders did not (79). Similarly, the use of miRNA signature as a prognostic marker for recovery in nonischemic human HF following left ventricular assist device (LVAD) removal has also been proposed, as Ramani et al. (102) show that significant downregulation of miRNAs 23a and 195 at time of placement predicts the ability to successfully recover following removal. miRNA-based therapies for cardiovascular disease are attracting attention as the failing cardiomyocyte transcriptome is being more clearly characterized and drug delivery systems are becoming more advanced (62).
Bernardo and Blaxall (11) present many exciting applications of these techniques in their review, including restoring expression of miRNA using oligonucleotide-based miRNA mimics, blocking miRNA-25 to restore SERCA2a activity and homeostasis in a mouse model of HF, and inhibiting miRNA-34, miRNA-34a, or miRNA-652 to improve angiogenesis, decrease fibrosis, and enhance healthy gene expression in mouse models of heart disease. Interestingly, cardiac fibrosis could be partially attenuated by injecting hearts with mimic miRNA-29b, which targets proteins of fibrosis, to counteract the natural downregulation of the miRNA-29 family in tissue adjacent to the infarction (141). Along the same lines, using an anti-miR to inhibit the action of miRNA-21, which facilitates fibrosis upon overexpression, elicits an antihypertrophic and antifibrotic response (29). Among the most promising targets for therapeutic intervention is miRNA-208a which is encoded by an intron of the α-myosin heavy chain (αMHC) gene and negatively regulates MED13, a component of the Mediator complex (8, 16, 43, 140). miRNA-208a has been shown to be solely expressed in the heart, and its upregulation is implicated in HF (51, 82, 114). Both anti-miR-208a-treated and αMHC-Med13 TG mice have displayed a lean phenotype on a high-fat diet compared with controls (8, 43). While thyromimetics have shown promise in improving lipid profiles and modulating whole body energy homeostasis, upregulation of MED13 by the above-mentioned strategies has improved cardiac function and significantly enhanced metabolic rates in white adipose tissue (WAT) and liver by increasing mitochondrial quantity and metabolic gene expression via a currently unidentified cardiac-derived molecule, without inducing tachycardia and arrhythmias as observed with thyroid hormone (TH) analogs (8, 43, 89, 122). Further, the overexpression of miRNA-208a in the heart is associated with downregulation of αMHC, upregulation of βMHC, collagen accumulation, decreased left ventricular ejection fraction (LVEF), and adverse clinical outcomes in human DCM (94, 114). The challenges of interorgan specificity and intracellular off-target side effects are mostly circumvented by anti-miR-208a; however, liver and renal toxicity due to anti-miR accumulation as well as immune-related adverse events remain a concern (89, 95).
While there are currently no clinical trials of miRNA-based therapeutics targeted to hearts of human patients, there are an abundance of promising results using this treatment strategy in HF models of mice, minipigs, and nonhuman primates as reviewed previously (109, 144). To date, few studies of miRNA-based treatments in humans have taken place, but not without promise. The first of which, initiated in 2013, was MRX34, a miRNA-34a mimic delivered via liposomal injection (86). Still ongoing, the Phase I trial has shown tolerable side effects with partial responses in two patients: one with advanced hepatocellular carcinoma (HCC) and one with melanoma (10a).1 MesomiR 1 is another Phase I study looking at the effect of delivering a miRNA mimic of a known miRNA tumor suppressor to the tum or, except it is focused on miRNA-16 packaged in an EnGeneIC Delivery Vehicle (EDV) containing an anti-EGFR-specific antibody (142). An alternative strategy to increasing downregulated miRNAs in disease is to administer antisense oligonucleotides to decrease upregulated miRNAs, and this was shown to be effective in human patients by targeting miRNA-122 in the liver with the drug Miravirsen (59). Since miRNA-122 overexpression in the liver serves to bind to and stabilize the hepatitis C virus, its inhibition led to dose-dependent prolonged depression of HCV RNA expression (59). The overexpression of miRNA-122 exclusively in the liver makes it an ideal target, so Vegter et al. (144) proposed that it is most advantageous to modulate the levels of cardiac-specific miRNAs including miR-1, miR-133, and miR-208 to reduce undesired side effects. Additionally, animal studies have shown that combining a miRNA mimic with a standard therapy as well as combining multiple miRNA mimics have elicited better outcomes than a single miRNA mimic alone (64, 105, 163). The success of miRNA-based therapies in preclinical trials and Phase I clinical trials suggests a potential future in which miRNA-based drugs will be used to treat human HF.
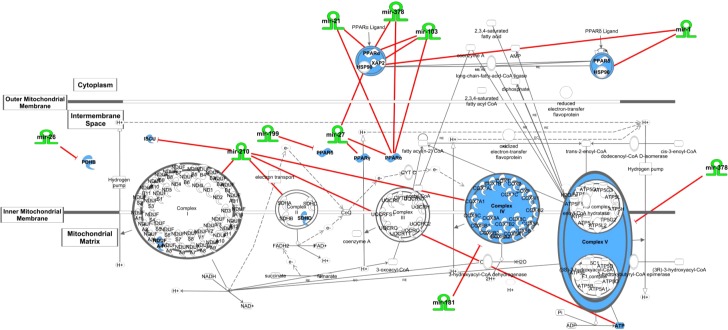
In a recent review of miRNA transport in cardiovascular disease, the authors show that the paracrine transport of miRNA from surrounding cells to cardiomyocytes via exosomes at least partially mediates the heart disease phenotype (32). Another testament to the substantial influence the surrounding environment imparts on the failing cardiomyocyte comes from Mishra et al. (87) who found that the deletion of matrix metalloproteinase 9 (MMP-9) upregulated the expression of pathologically downregulated miRNA-133, resulting in favorable changes in cardiac hypertrophy, fibrosis, and epigenetic modification. Thus, modulation of certain miRNA species may prove more successful by targeting the smooth muscle cells, endothelial cells, fibroblasts, and extracellular matrix (ECM) of the heart (88). The promising advances of this rapidly expanding field are undoubtedly vast; however, it also presents many challenges. Although the multiplicity of targets of miRNA species makes them attractive candidates for therapeutics in that they can impact multiple targets in the same axis providing a multipoint interventional strategy, they are also prone to off-target effects. Use of Ingenuity Pathway Analysis (IPA) provides evidence of the interconnectedness of regulatory miRNAs and their relationship to the ETC, β-oxidation, and PPAR signaling, illustrating their role at multiple axes as well as the participation of several miRNAs acting on the same axis point (Fig. 2). Further, the natural degradation of nucleic acids within the cell and the requirement of complicated precision delivery mechanisms present challenges for the researcher (138).
Fig. 2.
Ingenuity Pathway Analysis (IPA) overview. IPA network demonstrating the complexity and interconnectedness of miRNAs and their relationship to the ETC, beta-oxidation, and PPAR signaling. The mitochondrial dysfunction network was generated through the use of QIAGEN’s Ingenuity Pathway Analysis (IPA*, QIAGEN Redwood City, http://www.ingenuity.com).
Conclusion
The differential expression of miRNA species in HF has been characterized, as has the accompanying proteomic remodeling. In most of the pathways contributing to the transition in energy metabolism, differential expression of miRNAs that specifically target key components of these axes provides a regulatory platform to facilitate this transition (Table 1). While the mechanisms by which some miRNAs are differentially expressed in, and translocated to, the diseased cardiomyocyte are known, the elucidation of additional miRNA transcriptional regulatory mechanisms will undoubtedly provide further clarity to HF pathogenesis. The pathological stimuli of hypoxia and ischemia in early-stage ischemic HF mediate certain changes in miRNA expression that influence downstream protein targets facilitating a shift in intermediary metabolism. However, it should be noted that other miRNA alterations may be the consequence, rather than the cause, of impaired contractile function and metabolism in the heart. While there are a multitude of obstacles, an era of miRNA-based therapeutics for a variety of diseases including HF is nearer than ever before.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant R56-HL-128485.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
M.V.P., Q.A.H., and J.M.H. prepared figures; M.V.P. drafted manuscript; M.V.P. and J.M.H. edited and revised manuscript; M.V.P., Q.A.H., and J.M.H. approved final version of manuscript.
Footnotes
Prior to final publication of this article, the Phase I Study of MRX34 conducted by Mirna Therapeutics (86) was terminated because of serious immune-related events.
REFERENCES
- 1.Aoi W, Naito Y, Mizushima K, Takanami Y, Kawai Y, Ichikawa H, Yoshikawa T. The microRNA miR-696 regulates PGC-1α in mouse skeletal muscle in response to physical activity. Am J Physiol Endocrinol Metab 298: E799–E806, 2010. doi: 10.1152/ajpendo.00448.2009. [DOI] [PubMed] [Google Scholar]
- 2.Aubert G, Martin OJ, Horton JL, Lai L, Vega RB, Leone TC, Koves T, Gardell SJ, Krüger M, Hoppel CL, Lewandowski ED, Crawford PA, Muoio DM, Kelly DP. The failing heart relies on ketone bodies as a fuel. Circulation 133: 698–705, 2016. doi: 10.1161/CIRCULATIONAHA.115.017355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Azzouzi HE, Leptidis S, Doevendans PA, De Windt LJ. HypoxamiRs: regulators of cardiac hypoxia and energy metabolism. Trends Endocrinol Metab 26: 502–508, 2015. doi: 10.1016/j.tem.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 4.Bairwa SC, Parajuli N, Dyck JR. The role of AMPK in cardiomyocyte health and survival. Biochim Biophys Acta S0925-4439(16)30161-2; Epub ahead of print, 2016. doi: 10.1016/j.bbadis.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 5.Bandiera S, Matégot R, Girard M, Demongeot J, Henrion-Caude A. MitomiRs delineating the intracellular localization of microRNAs at mitochondria. Free Radic Biol Med 64: 12–19, 2013. doi: 10.1016/j.freeradbiomed.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 6.Bargaje R, Gupta S, Sarkeshik A, Park R, Xu T, Sarkar M, Halimani M, Roy SS, Yates J, Pillai B. Identification of novel targets for miR-29a using miRNA proteomics. PLoS One 7: e43243, 2012. doi: 10.1371/journal.pone.0043243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barrey E, Saint-Auret G, Bonnamy B, Damas D, Boyer O, Gidrol X. Pre-microRNA and mature microRNA in human mitochondria. PLoS One 6: e20220, 2011. doi: 10.1371/journal.pone.0020220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baskin KK, Grueter CE, Kusminski CM, Holland WL, Bookout AL, Satapati S, Kong YM, Burgess SC, Malloy CR, Scherer PE, Newgard CB, Bassel-Duby R, Olson EN. MED13-dependent signaling from the heart confers leanness by enhancing metabolism in adipose tissue and liver. EMBO Mol Med 6: 1610–1621, 2014. doi: 10.15252/emmm.201404218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Becker LB. New concepts in reactive oxygen species and cardiovascular reperfusion physiology. Cardiovasc Res 61: 461–470, 2004. doi: 10.1016/j.cardiores.2003.10.025. [DOI] [PubMed] [Google Scholar]
- 10.Bedi KC Jr, Snyder NW, Brandimarto J, Aziz M, Mesaros C, Worth AJ, Wang LL, Javaheri A, Blair IA, Margulies KB, Rame JE. Evidence for intramyocardial disruption of lipid metabolism and increased myocardial ketone utilization in advanced human heart failure. Circulation 133: 706–716, 2016. doi: 10.1161/CIRCULATIONAHA.115.017545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10a.Beg MS, Brenner A, Sachdev J, Ejadi S, Borad M, Kang YK, Lim H, Kim TY, Bader A, Stoudemire J, Smith S, Kim S, Hong DS. Safety, tolerability, and clinical activity of MRX34, the first-in-class liposomal miR-34 mimic, in patients with advanced solid tumors. Mol Cancer Ther 14, Suppl 2: C43, 2015. doi: 10.1158/1535-7163.TARG-15-C43. [DOI] [Google Scholar]
- 11.Bernardo BC, Blaxall BC. From bench to bedside: new approaches to therapeutic discovery for heart failure. Heart Lung Circ 25: 425–434, 2016. doi: 10.1016/j.hlc.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bienertova-Vasku J, Sana J, Slaby O. The role of microRNAs in mitochondria in cancer. Cancer Lett 336: 1–7, 2013. doi: 10.1016/j.canlet.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 13.Borralho PM, Rodrigues CM, Steer CJ. microRNAs in mitochondria: an unexplored niche. Adv Exp Med Biol 887: 31–51, 2015. doi: 10.1007/978-3-319-22380-3_3. [DOI] [PubMed] [Google Scholar]
- 14.Buchwald A, Till H, Unterberg C, Oberschmidt R, Figulla HR, Wiegand V. Alterations of the mitochondrial respiratory chain in human dilated cardiomyopathy. Eur Heart J 11: 509–516, 1990. [DOI] [PubMed] [Google Scholar]
- 15.Bugger H, Schwarzer M, Chen D, Schrepper A, Amorim PA, Schoepe M, Nguyen TD, Mohr FW, Khalimonchuk O, Weimer BC, Doenst T. Proteomic remodelling of mitochondrial oxidative pathways in pressure overload-induced heart failure. Cardiovasc Res 85: 376–384, 2010. doi: 10.1093/cvr/cvp344. [DOI] [PubMed] [Google Scholar]
- 16.Callis TE, Pandya K, Seok HY, Tang RH, Tatsuguchi M, Huang ZP, Chen JF, Deng Z, Gunn B, Shumate J, Willis MS, Selzman CH, Wang DZ. MicroRNA-208a is a regulator of cardiac hypertrophy and conduction in mice. J Clin Invest 119: 2772–2786, 2009. doi: 10.1172/JCI36154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chan SY, Loscalzo J. MicroRNA-210: a unique and pleiotropic hypoxamir. Cell Cycle 9: 1072–1083, 2010. doi: 10.4161/cc.9.6.11006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang JH, Tong L. Mitochondrial poly(A) polymerase and polyadenylation. Biochim Biophys Acta 1819: 992–997, 2012. doi: 10.1016/j.bbagrm.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen B, Li H, Zeng X, Yang P, Liu X, Zhao X, Liang S. Roles of microRNA on cancer cell metabolism. J Transl Med 10: 228, 2012. doi: 10.1186/1479-5876-10-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen B, Liu Y, Jin X, Lu W, Liu J, Xia Z, Yuan Q, Zhao X, Xu N, Liang S. MicroRNA-26a regulates glucose metabolism by direct targeting PDHX in colorectal cancer cells. BMC Cancer 14: 443, 2014. doi: 10.1186/1471-2407-14-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen YR, Zweier JL. Cardiac mitochondria and reactive oxygen species generation. Circ Res 114: 524–537, 2014. doi: 10.1161/CIRCRESAHA.114.300559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen Z, Li Y, Zhang H, Huang P, Luthra R. Hypoxia-regulated microRNA-210 modulates mitochondrial function and decreases ISCU and COX10 expression. Oncogene 29: 4362–4368, 2010. doi: 10.1038/onc.2010.193. [DOI] [PubMed] [Google Scholar]
- 23.Chendrimada TP, Gregory RI, Kumaraswamy E, Norman J, Cooch N, Nishikura K, Shiekhattar R. TRBP recruits the Dicer complex to Ago2 for microRNA processing and gene silencing. Nature 436: 740–744, 2005. doi: 10.1038/nature03868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheng Y, Liu X, Zhang S, Lin Y, Yang J, Zhang C. MicroRNA-21 protects against the H2O2-induced injury on cardiac myocytes via its target gene PDCD4. J Mol Cell Cardiol 47: 5–14, 2009. doi: 10.1016/j.yjmcc.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chung JH, Kim JS, Kim OY, Kang SM, Hwang GS, Shin MJ. Urinary ketone is associated with the heart failure severity. Clin Biochem 45: 1697–1699, 2012. doi: 10.1016/j.clinbiochem.2012.08.013. [DOI] [PubMed] [Google Scholar]
- 26.Cotter DG, Schugar RC, Crawford PA. Ketone body metabolism and cardiovascular disease. Am J Physiol Heart Circ Physiol 304: H1060–H1076, 2013. doi: 10.1152/ajpheart.00646.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dai DF, Hsieh EJ, Chen T, Menendez LG, Basisty NB, Tsai L, Beyer RP, Crispin DA, Shulman NJ, Szeto HH, Tian R, MacCoss MJ, Rabinovitch PS. Global proteomics and pathway analysis of pressure-overload-induced heart failure and its attenuation by mitochondrial-targeted peptides. Circ Heart Fail 6: 1067–1076, 2013. doi: 10.1161/CIRCHEARTFAILURE.113.000406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dai DF, Hsieh EJ, Liu Y, Chen T, Beyer RP, Chin MT, MacCoss MJ, Rabinovitch PS. Mitochondrial proteome remodelling in pressure overload-induced heart failure: the role of mitochondrial oxidative stress. Cardiovasc Res 93: 79–88, 2012. doi: 10.1093/cvr/cvr274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dangwal S, Thum T. microRNA therapeutics in cardiovascular disease models. Annu Rev Pharmacol Toxicol 54: 185–203, 2014. doi: 10.1146/annurev-pharmtox-011613-135957. [DOI] [PubMed] [Google Scholar]
- 30.Das S, Bedja D, Campbell N, Dunkerly B, Chenna V, Maitra A, Steenbergen C. miR-181c regulates the mitochondrial genome, bioenergetics, and propensity for heart failure in vivo. PLoS One 9: e96820, 2014. doi: 10.1371/journal.pone.0096820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Das S, Ferlito M, Kent OA, Fox-Talbot K, Wang R, Liu D, Raghavachari N, Yang Y, Wheelan SJ, Murphy E, Steenbergen C. Nuclear miRNA regulates the mitochondrial genome in the heart. Circ Res 110: 1596–1603, 2012. doi: 10.1161/CIRCRESAHA.112.267732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Das S, Halushka MK. Extracellular vesicle microRNA transfer in cardiovascular disease. Cardiovasc Pathol 24: 199–206, 2015. doi: 10.1016/j.carpath.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 33.Denli AM, Tops BB, Plasterk RH, Ketting RF, Hannon GJ. Processing of primary microRNAs by the Microprocessor complex. Nature 432: 231–235, 2004. doi: 10.1038/nature03049. [DOI] [PubMed] [Google Scholar]
- 34.Depre C, Vanoverschelde JL, Taegtmeyer H. Glucose for the heart. Circulation 99: 578–588, 1999. doi: 10.1161/01.CIR.99.4.578. [DOI] [PubMed] [Google Scholar]
- 35.Divakaran V, Mann DL. The emerging role of microRNAs in cardiac remodeling and heart failure. Circ Res 103: 1072–1083, 2008. doi: 10.1161/CIRCRESAHA.108.183087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Djuranovic S, Nahvi A, Green R. miRNA-mediated gene silencing by translational repression followed by mRNA deadenylation and decay. Science 336: 237–240, 2012. doi: 10.1126/science.1215691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Doenst T, Nguyen TD, Abel ED. Cardiac metabolism in heart failure: implications beyond ATP production. Circ Res 113: 709–724, 2013. doi: 10.1161/CIRCRESAHA.113.300376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Duarte FV, Palmeira CM, Rolo AP. The role of microRNAs in mitochondria: small players acting wide. Genes (Basel) 5: 865–886, 2014. doi: 10.3390/genes5040865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.el Azzouzi H, Leptidis S, Dirkx E, Hoeks J, van Bree B, Brand K, McClellan EA, Poels E, Sluimer JC, van den Hoogenhof MM, Armand AS, Yin X, Langley S, Bourajjaj M, Olieslagers S, Krishnan J, Vooijs M, Kurihara H, Stubbs A, Pinto YM, Krek W, Mayr M, da Costa Martins PA, Schrauwen P, De Windt LJ. The hypoxia-inducible microRNA cluster miR-199a∼214 targets myocardial PPARδ and impairs mitochondrial fatty acid oxidation. Cell Metab 18: 341–354, 2013. doi: 10.1016/j.cmet.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 40.Endo K, Naito Y, Ji X, Nakanishi M, Noguchi T, Goto Y, Nonogi H, Ma X, Weng H, Hirokawa G, Asada T, Kakinoki S, Yamaoka T, Fukushima Y, Iwai N. MicroRNA 210 as a biomarker for congestive heart failure. Biol Pharm Bull 36: 48–54, 2013. doi: 10.1248/bpb.b12-00578. [DOI] [PubMed] [Google Scholar]
- 41.Földes-Papp Z, König K, Studier H, Bückle R, Breunig HG, Uchugonova A, Kostner GM. Trafficking of mature miRNA-122 into the nucleus of live liver cells. Curr Pharm Biotechnol 10: 569–578, 2009. doi: 10.2174/138920109789069332. [DOI] [PubMed] [Google Scholar]
- 42.Gregory RI, Chendrimada TP, Cooch N, Shiekhattar R. Human RISC couples microRNA biogenesis and posttranscriptional gene silencing. Cell 123: 631–640, 2005. doi: 10.1016/j.cell.2005.10.022. [DOI] [PubMed] [Google Scholar]
- 43.Grueter CE, van Rooij E, Johnson BA, DeLeon SM, Sutherland LB, Qi X, Gautron L, Elmquist JK, Bassel-Duby R, Olson EN. A cardiac microRNA governs systemic energy homeostasis by regulation of MED13. Cell 149: 671–683, 2012. doi: 10.1016/j.cell.2012.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gudbjarnason S, Deschryver C, Hunn G, Bing RJ. Changes in myocardial enzyme patterns in human heart disease. J Lab Clin Med 64: 796–801, 1964. [PubMed] [Google Scholar]
- 45.Hafstad AD, Nabeebaccus AA, Shah AM. Novel aspects of ROS signalling in heart failure. Basic Res Cardiol 108: 359, 2013. doi: 10.1007/s00395-013-0359-8. [DOI] [PubMed] [Google Scholar]
- 46.Hamdani N, de Waard M, Messer AE, Boontje NM, Kooij V, van Dijk S, Versteilen A, Lamberts R, Merkus D, Dos Remedios C, Duncker DJ, Borbely A, Papp Z, Paulus W, Stienen GJ, Marston SB, van der Velden J. Myofilament dysfunction in cardiac disease from mice to men. J Muscle Res Cell Motil 29: 189–201, 2008. doi: 10.1007/s10974-008-9160-y. [DOI] [PubMed] [Google Scholar]
- 47.Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol 13: 251–262, 2012. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.He M, Lu Y, Xu S, Mao L, Zhang L, Duan W, Liu C, Pi H, Zhang Y, Zhong M, Yu Z, Zhou Z. MiRNA-210 modulates a nickel-induced cellular energy metabolism shift by repressing the iron-sulfur cluster assembly proteins ISCU1/2 in Neuro-2a cells. Cell Death Dis 5: e1090, 2014. doi: 10.1038/cddis.2014.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hollander JM, Baseler WA, Dabkowski ER. Proteomic remodeling of mitochondria in heart failure. Congest Heart Fail 17: 262–268, 2011. doi: 10.1111/j.1751-7133.2011.00254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huang D, Li C, Zhang H. Hypoxia and cancer cell metabolism. Acta Biochim Biophys Sin (Shanghai) 46: 214–219, 2014. doi: 10.1093/abbs/gmt148. [DOI] [PubMed] [Google Scholar]
- 51.Huang Y, Li J. MicroRNA208 family in cardiovascular diseases: therapeutic implication and potential biomarker. J Physiol Biochem 71: 479–486, 2015. doi: 10.1007/s13105-015-0409-9. [DOI] [PubMed] [Google Scholar]
- 52.Huntzinger E, Izaurralde E. Gene silencing by microRNAs: contributions of translational repression and mRNA decay. Nat Rev Genet 12: 99–110, 2011. doi: 10.1038/nrg2936. [DOI] [PubMed] [Google Scholar]
- 53.Hutvágner G, McLachlan J, Pasquinelli AE, Bálint E, Tuschl T, Zamore PD. A cellular function for the RNA-interference enzyme Dicer in the maturation of the let-7 small temporal RNA. Science 293: 834–838, 2001. doi: 10.1126/science.1062961. [DOI] [PubMed] [Google Scholar]
- 54.Ikeda S, Kong SW, Lu J, Bisping E, Zhang H, Allen PD, Golub TR, Pieske B, Pu WT. Altered microRNA expression in human heart disease. Physiol Genomics 31: 367–373, 2007. doi: 10.1152/physiolgenomics.00144.2007. [DOI] [PubMed] [Google Scholar]
- 55.Iwakawa HO, Tomari Y. The Functions of MicroRNAs: mRNA Decay and Translational Repression. Trends Cell Biol 25: 651–665, 2015. doi: 10.1016/j.tcb.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 56.Jadhav VS, Krause KH, Singh SK. HIV-1 Tat C modulates NOX2 and NOX4 expressions through miR-17 in a human microglial cell line. J Neurochem 131: 803–815, 2014. doi: 10.1111/jnc.12933. [DOI] [PubMed] [Google Scholar]
- 57.Jagannathan R, Thapa D, Nichols CE, Shepherd DL, Stricker JC, Croston TL, Baseler WA, Lewis SE, Martinez I, Hollander JM. Translational regulation of the mitochondrial genome following redistribution of mitochondrial microRNA in the diabetic heart. Circ Cardiovasc Genet 8: 785–802, 2015. doi: 10.1161/CIRCGENETICS.115.001067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Janardhan A, Chen J, Crawford PA. Altered systemic ketone body metabolism in advanced heart failure. Tex Heart Inst J 38: 533–538, 2011. [PMC free article] [PubMed] [Google Scholar]
- 59.Janssen HL, Reesink HW, Lawitz EJ, Zeuzem S, Rodriguez-Torres M, Patel K, van der Meer AJ, Patick AK, Chen A, Zhou Y, Persson R, King BD, Kauppinen S, Levin AA, Hodges MR. Treatment of HCV infection by targeting microRNA. N Engl J Med 368: 1685–1694, 2013. doi: 10.1056/NEJMoa1209026. [DOI] [PubMed] [Google Scholar]
- 60.Jüllig M, Hickey AJ, Chai CC, Skea GL, Middleditch MJ, Costa S, Choong SY, Philips AR, Cooper GJ. Is the failing heart out of fuel or a worn engine running rich? A study of mitochondria in old spontaneously hypertensive rats. Proteomics 8: 2556–2572, 2008. doi: 10.1002/pmic.200700977. [DOI] [PubMed] [Google Scholar]
- 61.Kalozoumi G, Yacoub M, Sanoudou D. MicroRNAs in heart failure: Small molecules with major impact. Glob Cardiol Sci Pract 2014: 79–102, 2014. doi: 10.5339/gcsp.2014.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kamps JA, Krenning G. Micromanaging cardiac regeneration: Targeted delivery of microRNAs for cardiac repair and regeneration. World J Cardiol 8: 163–179, 2016. doi: 10.4330/wjc.v8.i2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kang M, Yan LM, Li YM, Zhang WY, Wang H, Tang AZ, Ou HS. Inhibitory effect of microRNA-24 on fatty acid-binding protein expression on 3T3-L1 adipocyte differentiation. Genet Mol Res 12: 5267–5277, 2013. doi: 10.4238/2013.November.7.1. [DOI] [PubMed] [Google Scholar]
- 64.Kasinski AL, Kelnar K, Stahlhut C, Orellana E, Zhao J, Shimer E, Dysart S, Chen X, Bader AG, Slack FJ. A combinatorial microRNA therapeutics approach to suppressing non-small cell lung cancer. Oncogene 34: 3547–3555, 2015. doi: 10.1038/onc.2014.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Katz A, editor. Heart Failure: Pathophysiology, Molecular Biology, and Clinical Management. Philadelphia, PA: Lippincott Williams & Williams, 2000. [Google Scholar]
- 66.Koc EC, Koc H. Regulation of mammalian mitochondrial translation by post-translational modifications. Biochim Biophys Acta 1819: 1055–1066, 2012. doi: 10.1016/j.bbagrm.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 67.Lai KB, Sanderson JE, Izzat MB, Yu CM. Micro-RNA and mRNA myocardial tissue expression in biopsy specimen from patients with heart failure. Int J Cardiol 199: 79–83, 2015. doi: 10.1016/j.ijcard.2015.07.043. [DOI] [PubMed] [Google Scholar]
- 68.Lee Y, Ahn C, Han J, Choi H, Kim J, Yim J, Lee J, Provost P, Rådmark O, Kim S, Kim VN. The nuclear RNase III Drosha initiates microRNA processing. Nature 425: 415–419, 2003. doi: 10.1038/nature01957. [DOI] [PubMed] [Google Scholar]
- 69.Lee Y, Kim M, Han J, Yeom KH, Lee S, Baek SH, Kim VN. MicroRNA genes are transcribed by RNA polymerase II. EMBO J 23: 4051–4060, 2004. doi: 10.1038/sj.emboj.7600385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lehman JJ, Barger PM, Kovacs A, Saffitz JE, Medeiros DM, Kelly DP. Peroxisome proliferator-activated receptor gamma coactivator-1 promotes cardiac mitochondrial biogenesis. J Clin Invest 106: 847–856, 2000. doi: 10.1172/JCI10268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lei B, Lionetti V, Young ME, Chandler MP, d’Agostino C, Kang E, Altarejos M, Matsuo K, Hintze TH, Stanley WC, Recchia FA. Paradoxical downregulation of the glucose oxidation pathway despite enhanced flux in severe heart failure. J Mol Cell Cardiol 36: 567–576, 2004. doi: 10.1016/j.yjmcc.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 72.Leptidis S, El Azzouzi H, Lok SI, de Weger R, Olieslagers S, Kisters N, Silva GJ, Heymans S, Cuppen E, Berezikov E, De Windt LJ, da Costa Martins P. A deep sequencing approach to uncover the miRNOME in the human heart. PLoS One 8: e57800, 2013. doi: 10.1371/journal.pone.0057800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li SZ, Hu YY, Zhao J, Zhao YB, Sun JD, Yang YF, Ji CC, Liu ZB, Cao WD, Qu Y, Liu WP, Cheng G, Fei Z. MicroRNA-34a induces apoptosis in the human glioma cell line, A172, through enhanced ROS production and NOX2 expression. Biochem Biophys Res Commun 444: 6–12, 2014. doi: 10.1016/j.bbrc.2013.12.136. [DOI] [PubMed] [Google Scholar]
- 74.Liu J, Valencia-Sanchez MA, Hannon GJ, Parker R. MicroRNA-dependent localization of targeted mRNAs to mammalian P-bodies. Nat Cell Biol 7: 719–723, 2005. doi: 10.1038/ncb1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu T, Chen L, Kim E, Tran D, Phinney BS, Knowlton AA. Mitochondrial proteome remodeling in ischemic heart failure. Life Sci 101: 27–36, 2014. doi: 10.1016/j.lfs.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Luiken JJ, Glatz JF, Neumann D. Cardiac contraction-induced GLUT4 translocation requires dual signaling input. Trends Endocrinol Metab 26: 404–410, 2015. doi: 10.1016/j.tem.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 77.Lund E, Güttinger S, Calado A, Dahlberg JE, Kutay U. Nuclear export of microRNA precursors. Science 303: 95–98, 2004. doi: 10.1126/science.1090599. [DOI] [PubMed] [Google Scholar]
- 78.Manea SA, Constantin A, Manda G, Sasson S, Manea A. Regulation of Nox enzymes expression in vascular pathophysiology: Focusing on transcription factors and epigenetic mechanisms. Redox Biol 5: 358–366, 2015. doi: 10.1016/j.redox.2015.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Marfella R, Di Filippo C, Potenza N, Sardu C, Rizzo MR, Siniscalchi M, Musacchio E, Barbieri M, Mauro C, Mosca N, Solimene F, Mottola MT, Russo A, Rossi F, Paolisso G, D’Amico M. Circulating microRNA changes in heart failure patients treated with cardiac resynchronization therapy: responders vs. non-responders. Eur J Heart Fail 15: 1277–1288, 2013. doi: 10.1093/eurjhf/hft088. [DOI] [PubMed] [Google Scholar]
- 80.Marín-García J, Goldenthal MJ, Moe GW. Abnormal cardiac and skeletal muscle mitochondrial function in pacing-induced cardiac failure. Cardiovasc Res 52: 103–110, 2001. doi: 10.1016/S0008-6363(01)00368-6. [DOI] [PubMed] [Google Scholar]
- 81.Mathonnet G, Fabian MR, Svitkin YV, Parsyan A, Huck L, Murata T, Biffo S, Merrick WC, Darzynkiewicz E, Pillai RS, Filipowicz W, Duchaine TF, Sonenberg N. MicroRNA inhibition of translation initiation in vitro by targeting the cap-binding complex eIF4F. Science 317: 1764–1767, 2007. doi: 10.1126/science.1146067. [DOI] [PubMed] [Google Scholar]
- 82.Matkovich SJ, Hu Y, Eschenbacher WH, Dorn LE, Dorn GW II. Direct and indirect involvement of microRNA-499 in clinical and experimental cardiomyopathy. Circ Res 111: 521–531, 2012. doi: 10.1161/CIRCRESAHA.112.265736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Matkovich SJ, Van Booven DJ, Youker KA, Torre-Amione G, Diwan A, Eschenbacher WH, Dorn LE, Watson MA, Margulies KB, Dorn GW II. Reciprocal regulation of myocardial microRNAs and messenger RNA in human cardiomyopathy and reversal of the microRNA signature by biomechanical support. Circulation 119: 1263–1271, 2009. doi: 10.1161/CIRCULATIONAHA.108.813576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Meijer HA, Kong YW, Lu WT, Wilczynska A, Spriggs RV, Robinson SW, Godfrey JD, Willis AE, Bushell M. Translational repression and eIF4A2 activity are critical for microRNA-mediated gene regulation. Science 340: 82–85, 2013. doi: 10.1126/science.1231197. [DOI] [PubMed] [Google Scholar]
- 85.Mercer TR, Neph S, Dinger ME, Crawford J, Smith MA, Shearwood AM, Haugen E, Bracken CP, Rackham O, Stamatoyannopoulos JA, Filipovska A, Mattick JS. The human mitochondrial transcriptome. Cell 146: 645–658, 2011. doi: 10.1016/j.cell.2011.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mirna Therapeutics Inc. A Multicenter Phase I Study of MRX34, MicroRNA miR-RX34 Liposomal Injection. ClinicalTrials.gov Identifier NCT01829971 https://clinicaltrials.gov/ct2/show/NCT01829971, 2016.
- 87.Mishra PK, Givvimani S, Chavali V, Tyagi SC. Cardiac matrix: a clue for future therapy. Biochim Biophys Acta 1832: 2271–2276, 2013. doi: 10.1016/j.bbadis.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mishra PK, Tyagi N, Kumar M, Tyagi SC. MicroRNAs as a therapeutic target for cardiovascular diseases. J Cell Mol Med 13: 778–789, 2009. doi: 10.1111/j.1582-4934.2009.00744.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Montgomery RL, Hullinger TG, Semus HM, Dickinson BA, Seto AG, Lynch JM, Stack C, Latimer PA, Olson EN, van Rooij E. Therapeutic inhibition of miR-208a improves cardiac function and survival during heart failure. Circulation 124: 1537–1547, 2011. doi: 10.1161/CIRCULATIONAHA.111.030932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Morrison A, Chen L, Wang J, Zhang M, Yang H, Ma Y, Budanov A, Lee JH, Karin M, Li J. Sestrin2 promotes LKB1-mediated AMPK activation in the ischemic heart. FASEB J 29: 408–417, 2015. doi: 10.1096/fj.14-258814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Després JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jiménez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER III, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB; Writing Group Members; American Heart Association Statistics Committee; Stroke Statistics Subcommittee . Heart Disease and Stroke Statistics—2016 Update: A Report From the American Heart Association. Circulation 133: e38–e360, 2016. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 93.Nishi H, Ono K, Iwanaga Y, Horie T, Nagao K, Takemura G, Kinoshita M, Kuwabara Y, Mori RT, Hasegawa K, Kita T, Kimura T. MicroRNA-15b modulates cellular ATP levels and degenerates mitochondria via Arl2 in neonatal rat cardiac myocytes. J Biol Chem 285: 4920–4930, 2010. doi: 10.1074/jbc.M109.082610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Oliveira-Carvalho V, Carvalho VO, Bocchi EA. The emerging role of miR-208a in the heart. DNA Cell Biol 32: 8–12, 2013. doi: 10.1089/dna.2012.1787. [DOI] [PubMed] [Google Scholar]
- 95.Olson EN. MicroRNAs as therapeutic targets and biomarkers of cardiovascular disease. Sci Transl Med 6: 239ps3, 2014. doi: 10.1126/scitranslmed.3009008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Osorio JC, Stanley WC, Linke A, Castellari M, Diep QN, Panchal AR, Hintze TH, Lopaschuk GD, Recchia FA. Impaired myocardial fatty acid oxidation and reduced protein expression of retinoid X receptor-alpha in pacing-induced heart failure. Circulation 106: 606–612, 2002. doi: 10.1161/01.CIR.0000023531.22727.C1. [DOI] [PubMed] [Google Scholar]
- 97.Parajuli N, Patel VB, Wang W, Basu R, Oudit GY. Loss of NOX2 (gp91phox) prevents oxidative stress and progression to advanced heart failure. Clin Sci (Lond) 127: 331–340, 2014. doi: 10.1042/CS20130787. [DOI] [PubMed] [Google Scholar]
- 98.Peralta S, Wang X, Moraes CT. Mitochondrial transcription: lessons from mouse models. Biochim Biophys Acta 1819: 961–969, 2012. doi: 10.1016/j.bbagrm.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Petrak J, Pospisilova J, Sedinova M, Jedelsky P, Lorkova L, Vit O, Kolar M, Strnad H, Benes J, Sedmera D, Cervenka L, Melenovsky V. Proteomic and transcriptomic analysis of heart failure due to volume overload in a rat aorto-caval fistula model provides support for new potential therapeutic targets - monoamine oxidase A and transglutaminase 2. Proteome Sci 9: 69, 2011. doi: 10.1186/1477-5956-9-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Politz JC, Zhang F, Pederson T. MicroRNA-206 colocalizes with ribosome-rich regions in both the nucleolus and cytoplasm of rat myogenic cells. Proc Natl Acad Sci USA 103: 18957–18962, 2006. doi: 10.1073/pnas.0609466103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rackham O, Filipovska A. The role of mammalian PPR domain proteins in the regulation of mitochondrial gene expression. Biochim Biophys Acta 1819: 1008–1016, 2012. doi: 10.1016/j.bbagrm.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 102.Ramani R, Vela D, Segura A, McNamara D, Lemster B, Samarendra V, Kormos R, Toyoda Y, Bermudez C, Frazier OH, Moravec CS, Gorcsan J III, Taegtmeyer H, McTiernan CF. A micro-ribonucleic acid signature associated with recovery from assist device support in 2 groups of patients with severe heart failure. J Am Coll Cardiol 58: 2270–2278, 2011. doi: 10.1016/j.jacc.2011.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rane S, He M, Sayed D, Vashistha H, Malhotra A, Sadoshima J, Vatner DE, Vatner SF, Abdellatif M. Downregulation of miR-199a derepresses hypoxia-inducible factor-1alpha and Sirtuin 1 and recapitulates hypoxia preconditioning in cardiac myocytes. Circ Res 104: 879–886, 2009. doi: 10.1161/CIRCRESAHA.108.193102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rees ML, Subramaniam J, Li Y, Hamilton DJ, Frazier OH, Taegtmeyer H. A PKM2 signature in the failing heart. Biochem Biophys Res Commun 459: 430–436, 2015. doi: 10.1016/j.bbrc.2015.02.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Reid G, Kao SC, Pavlakis N, Brahmbhatt H, MacDiarmid J, Clarke S, Boyer M, van Zandwijk N. Clinical development of TargomiRs, a miRNA mimic-based treatment for patients with recurrent thoracic cancer. Epigenomics 8: 1079–1085, 2016. doi: 10.2217/epi-2016-0035. [DOI] [PubMed] [Google Scholar]
- 106.Ren XP, Wu J, Wang X, Sartor MA, Qian J, Jones K, Nicolaou P, Pritchard TJ, Fan GC. MicroRNA-320 is involved in the regulation of cardiac ischemia/reperfusion injury by targeting heat-shock protein 20. Circulation 119: 2357–2366, 2009. doi: 10.1161/CIRCULATIONAHA.108.814145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Riehle C, Abel ED. Insulin signaling and heart failure. Circ Res 118: 1151–1169, 2016. doi: 10.1161/CIRCRESAHA.116.306206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ro S, Ma HY, Park C, Ortogero N, Song R, Hennig GW, Zheng H, Lin YM, Moro L, Hsieh JT, Yan W. The mitochondrial genome encodes abundant small noncoding RNAs. Cell Res 23: 759–774, 2013. doi: 10.1038/cr.2013.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Romaine SP, Tomaszewski M, Condorelli G, Samani NJ. MicroRNAs in cardiovascular disease: an introduction for clinicians. Heart 101: 921–928, 2015. doi: 10.1136/heartjnl-2013-305402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rosca MG, Tandler B, Hoppel CL. Mitochondria in cardiac hypertrophy and heart failure. J Mol Cell Cardiol 55: 31–41, 2013. doi: 10.1016/j.yjmcc.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rosca MG, Vazquez EJ, Kerner J, Parland W, Chandler MP, Stanley W, Sabbah HN, Hoppel CL. Cardiac mitochondria in heart failure: decrease in respirasomes and oxidative phosphorylation. Cardiovasc Res 80: 30–39, 2008. doi: 10.1093/cvr/cvn184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Russell RR III, Bergeron R, Shulman GI, Young LH. Translocation of myocardial GLUT-4 and increased glucose uptake through activation of AMPK by AICAR. Am J Physiol Heart Circ Physiol 277: H643–H649, 1999. [DOI] [PubMed] [Google Scholar]
- 113.Sack MN, Rader TA, Park S, Bastin J, McCune SA, Kelly DP. Fatty acid oxidation enzyme gene expression is downregulated in the failing heart. Circulation 94: 2837–2842, 1996. doi: 10.1161/01.CIR.94.11.2837. [DOI] [PubMed] [Google Scholar]
- 114.Satoh M, Minami Y, Takahashi Y, Tabuchi T, Nakamura M. Expression of microRNA-208 is associated with adverse clinical outcomes in human dilated cardiomyopathy. J Card Fail 16: 404–410, 2010. doi: 10.1016/j.cardfail.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 115.Sayed AS, Xia K, Salma U, Yang T, Peng J. Diagnosis, prognosis and therapeutic role of circulating miRNAs in cardiovascular diseases. Heart Lung Circ 23: 503–510, 2014. doi: 10.1016/j.hlc.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 116.Shafqat N, Kavanagh KL, Sass JO, Christensen E, Fukao T, Lee WH, Oppermann U, Yue WW. A structural mapping of mutations causing succinyl-CoA:3-ketoacid CoA transferase (SCOT) deficiency. J Inherit Metab Dis 36: 983–987, 2013. doi: 10.1007/s10545-013-9589-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Shah AM, Mann DL. In search of new therapeutic targets and strategies for heart failure: recent advances in basic science. Lancet 378: 704–712, 2011. doi: 10.1016/S0140-6736(11)60894-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Shan ZX, Lin QX, Deng CY, Zhu JN, Mai LP, Liu JL, Fu YH, Liu XY, Li YX, Zhang YY, Lin SG, Yu XY. miR-1/miR-206 regulate Hsp60 expression contributing to glucose-mediated apoptosis in cardiomyocytes. FEBS Lett 584: 3592–3600, 2010. doi: 10.1016/j.febslet.2010.07.027. [DOI] [PubMed] [Google Scholar]
- 119.Shao D, Tian R. Glucose transporters in cardiac metabolism and hypertrophy. Compr Physiol 6: 331–351, 2015. doi: 10.1002/cphy.c150016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Shutt TE, Shadel GS. A compendium of human mitochondrial gene expression machinery with links to disease. Environ Mol Mutagen 51: 360–379, 2010. doi: 10.1002/em.20571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Simone NL, Soule BP, Ly D, Saleh AD, Savage JE, Degraff W, Cook J, Harris CC, Gius D, Mitchell JB. Ionizing radiation-induced oxidative stress alters miRNA expression. PLoS One 4: e6377, 2009. doi: 10.1371/journal.pone.0006377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Song Y, Yao X, Ying H. Thyroid hormone action in metabolic regulation. Protein Cell 2: 358–368, 2011. doi: 10.1007/s13238-011-1046-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Srinivasan H, Das S. Mitochondrial miRNA (MitomiR): a new player in cardiovascular health. Can J Physiol Pharmacol 93: 855–861, 2015. doi: 10.1139/cjpp-2014-0500. [DOI] [PubMed] [Google Scholar]
- 124.Sripada L, Tomar D, Singh R. Mitochondria: one of the destinations of miRNAs. Mitochondrion 12: 593–599, 2012. doi: 10.1016/j.mito.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 125.Stanley WC, Recchia FA, Lopaschuk GD. Myocardial substrate metabolism in the normal and failing heart. Physiol Rev 85: 1093–1129, 2005. doi: 10.1152/physrev.00006.2004. [DOI] [PubMed] [Google Scholar]
- 126.Sucharov C, Bristow MR, Port JD. miRNA expression in the failing human heart: functional correlates. J Mol Cell Cardiol 45: 185–192, 2008. doi: 10.1016/j.yjmcc.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Szczesny RJ, Borowski LS, Malecki M, Wojcik MA, Stepien PP, Golik P. RNA degradation in yeast and human mitochondria. Biochim Biophys Acta 1819: 1027–1034, 2012. doi: 10.1016/j.bbagrm.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 128.Taegtmeyer H, Golfman L, Sharma S, Razeghi P, van Arsdall M. Linking gene expression to function: metabolic flexibility in the normal and diseased heart. Ann N Y Acad Sci 1015: 202–213, 2004. doi: 10.1196/annals.1302.017. [DOI] [PubMed] [Google Scholar]
- 129.Tang H, Lee M, Sharpe O, Salamone L, Noonan EJ, Hoang CD, Levine S, Robinson WH, Shrager JB. Oxidative stress-responsive microRNA-320 regulates glycolysis in diverse biological systems. FASEB J 26: 4710–4721, 2012. doi: 10.1096/fj.11-197467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Thorrez L, Laudadio I, Van Deun K, Quintens R, Hendrickx N, Granvik M, Lemaire K, Schraenen A, Van Lommel L, Lehnert S, Aguayo-Mazzucato C, Cheng-Xue R, Gilon P, Van Mechelen I, Bonner-Weir S, Lemaigre F, Schuit F. Tissue-specific disallowance of housekeeping genes: the other face of cell differentiation. Genome Res 21: 95–105, 2011. doi: 10.1101/gr.109173.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Thum T, Galuppo P, Wolf C, Fiedler J, Kneitz S, van Laake LW, Doevendans PA, Mummery CL, Borlak J, Haverich A, Gross C, Engelhardt S, Ertl G, Bauersachs J. MicroRNAs in the human heart: a clue to fetal gene reprogramming in heart failure. Circulation 116: 258–267, 2007. doi: 10.1161/CIRCULATIONAHA.107.687947. [DOI] [PubMed] [Google Scholar]
- 132.Tomari Y, Matranga C, Haley B, Martinez N, Zamore PD. A protein sensor for siRNA asymmetry. Science 306: 1377–1380, 2004. doi: 10.1126/science.1102755. [DOI] [PubMed] [Google Scholar]
- 133.Tomasello F, Messina A, Lartigue L, Schembri L, Medina C, Reina S, Thoraval D, Crouzet M, Ichas F, De Pinto V, De Giorgi F. Outer membrane VDAC1 controls permeability transition of the inner mitochondrial membrane in cellulo during stress-induced apoptosis. Cell Res 19: 1363–1376, 2009. doi: 10.1038/cr.2009.98. [DOI] [PubMed] [Google Scholar]
- 134.Towheed A, Markantone DM, Crain AT, Celotto AM, Palladino MJ. Small mitochondrial-targeted RNAs modulate endogenous mitochondrial protein expression in vivo. Neurobiol Dis 69: 15–22, 2014. doi: 10.1016/j.nbd.2014.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Turrens JF. Mitochondrial formation of reactive oxygen species. J Physiol 552: 335–344, 2003. doi: 10.1113/jphysiol.2003.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Urbonavicius S, Wiggers H, Bøtker HE, Nielsen TT, Kimose HH, Østergaard M, Lindholt JS, Vorum H, Honoré B. Proteomic analysis identifies mitochondrial metabolic enzymes as major discriminators between different stages of the failing human myocardium. Acta Cardiol 64: 511–522, 2009. doi: 10.2143/AC.64.4.2041617. [DOI] [PubMed] [Google Scholar]
- 137.Valencia-Sanchez MA, Liu J, Hannon GJ, Parker R. Control of translation and mRNA degradation by miRNAs and siRNAs. Genes Dev 20: 515–524, 2006. doi: 10.1101/gad.1399806. [DOI] [PubMed] [Google Scholar]
- 138.van Rooij E, Marshall WS, Olson EN. Toward microRNA-based therapeutics for heart disease: the sense in antisense. Circ Res 103: 919–928, 2008. doi: 10.1161/CIRCRESAHA.108.183426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.van Rooij E, Sutherland LB, Liu N, Williams AH, McAnally J, Gerard RD, Richardson JA, Olson EN. A signature pattern of stress-responsive microRNAs that can evoke cardiac hypertrophy and heart failure. Proc Natl Acad Sci USA 103: 18255–18260, 2006. doi: 10.1073/pnas.0608791103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.van Rooij E, Sutherland LB, Qi X, Richardson JA, Hill J, Olson EN. Control of stress-dependent cardiac growth and gene expression by a microRNA. Science 316: 575–579, 2007. doi: 10.1126/science.1139089. [DOI] [PubMed] [Google Scholar]
- 141.van Rooij E, Sutherland LB, Thatcher JE, DiMaio JM, Naseem RH, Marshall WS, Hill JA, Olson EN. Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proc Natl Acad Sci USA 105: 13027–13032, 2008. doi: 10.1073/pnas.0805038105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.van Z and wijk N . Mesomi R. 1: A Phase I Study of TargomiRs as 2nd or 3rd Line Treatment for Patients With Recurrent MPM and NSCLC. ClinicalTrials.gov. U.S. National Institutes of Health, ClinicalTrials.gov Identifier NCT02369198. https://clinicaltrials.gov/ct2/show/NCT02369198, 2016.
- 143.Varga ZV, Kupai K, Szu″cs G, Gáspár R, Pálóczi J, Faragó N, Zvara A, Puskás LG, Rázga Z, Tiszlavicz L, Bencsik P, Görbe A, Csonka C, Ferdinandy P, Csont T. MicroRNA-25-dependent up-regulation of NADPH oxidase 4 (NOX4) mediates hypercholesterolemia-induced oxidative/nitrative stress and subsequent dysfunction in the heart. J Mol Cell Cardiol 62: 111–121, 2013. doi: 10.1016/j.yjmcc.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 144.Vegter EL, van der Meer P, de Windt LJ, Pinto YM, Voors AA. MicroRNAs in heart failure: from biomarker to target for therapy. Eur J Heart Fail 18: 457–468, 2016. doi: 10.1002/ejhf.495. [DOI] [PubMed] [Google Scholar]
- 145.Vidal-Puig A, Jimenez-Liñan M, Lowell BB, Hamann A, Hu E, Spiegelman B, Flier JS, Moller DE. Regulation of PPAR gamma gene expression by nutrition and obesity in rodents. J Clin Invest 97: 2553–2561, 1996. doi: 10.1172/JCI118703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wahlquist C, Jeong D, Rojas-Muñoz A, Kho C, Lee A, Mitsuyama S, van Mil A, Park WJ, Sluijter JP, Doevendans PA, Hajjar RJ, Mercola M. Inhibition of miR-25 improves cardiac contractility in the failing heart. Nature 508: 531–535, 2014. doi: 10.1038/nature13073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wang G, Chen HW, Oktay Y, Zhang J, Allen EL, Smith GM, Fan KC, Hong JS, French SW, McCaffery JM, Lightowlers RN, Morse HC III, Koehler CM, Teitell MA. PNPASE regulates RNA import into mitochondria. Cell 142: 456–467, 2010. doi: 10.1016/j.cell.2010.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wang G, Shimada E, Koehler CM, Teitell MA. PNPASE and RNA trafficking into mitochondria. Biochim Biophys Acta 1819: 998–1007, 2012. doi: 10.1016/j.bbagrm.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wang H, Li J, Chi H, Zhang F, Zhu X, Cai J, Yang X. MicroRNA-181c targets Bcl-2 and regulates mitochondrial morphology in myocardial cells. J Cell Mol Med 19: 2084–2097, 2015. doi: 10.1111/jcmm.12563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wang J, Bai L, Li J, Sun C, Zhao J, Cui C, Han K, Liu Y, Zhuo X, Wang T, Liu P, Fan F, Guan Y, Ma A. Proteomic analysis of mitochondria reveals a metabolic switch from fatty acid oxidation to glycolysis in the failing heart. Sci China C Life Sci 52: 1003–1010, 2009. doi: 10.1007/s11427-009-0140-2. [DOI] [PubMed] [Google Scholar]
- 151.Wang J, Song Y, Zhang Y, Xiao H, Sun Q, Hou N, Guo S, Wang Y, Fan K, Zhan D, Zha L, Cao Y, Li Z, Cheng X, Zhang Y, Yang X. Cardiomyocyte overexpression of miR-27b induces cardiac hypertrophy and dysfunction in mice. Cell Res 22: 516–527, 2012. doi: 10.1038/cr.2011.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wang SB, Foster DB, Rucker J, O’Rourke B, Kass DA, Van Eyk JE. Redox regulation of mitochondrial ATP synthase: implications for cardiac resynchronization therapy. Circ Res 109: 750–757, 2011. doi: 10.1161/CIRCRESAHA.111.246124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Weckbach LT, Grabmaier U, Clauss S, Wakili R. MicroRNAs as a diagnostic tool for heart failure and atrial fibrillation. Curr Opin Pharmacol 27: 24–30, 2016. doi: 10.1016/j.coph.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 154.Wei Y, Li L, Wang D, Zhang CY, Zen K. Importin 8 regulates the transport of mature microRNAs into the cell nucleus. J Biol Chem 289: 10270–10275, 2014. doi: 10.1074/jbc.C113.541417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wentz AE, d’Avignon DA, Weber ML, Cotter DG, Doherty JM, Kerns R, Nagarajan R, Reddy N, Sambandam N, Crawford PA. Adaptation of myocardial substrate metabolism to a ketogenic nutrient environment. J Biol Chem 285: 24447–24456, 2010. doi: 10.1074/jbc.M110.100651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, Troy A, Cinti S, Lowell B, Scarpulla RC, Spiegelman BM. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 98: 115–124, 1999. doi: 10.1016/S0092-8674(00)80611-X. [DOI] [PubMed] [Google Scholar]
- 157.Yang J, Holman GD. Insulin and contraction stimulate exocytosis, but increased AMP-activated protein kinase activity resulting from oxidative metabolism stress slows endocytosis of GLUT4 in cardiomyocytes. J Biol Chem 280: 4070–4078, 2005. doi: 10.1074/jbc.M410213200. [DOI] [PubMed] [Google Scholar]
- 158.Yang Z, Cappello T, Wang L. Emerging role of microRNAs in lipid metabolism. Acta Pharm Sin B 5: 145–150, 2015. doi: 10.1016/j.apsb.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Yokokawa T, Sugano Y, Shimouchi A, Shibata A, Jinno N, Nagai T, Kanzaki H, Aiba T, Kusano K, Shirai M, Takeishi Y, Yasuda S, Ogawa H, Anzai T. Exhaled acetone concentration is related to hemodynamic severity in patients with non-ischemic chronic heart failure. Circ J 80: 1178–1186, 2016. doi: 10.1253/circj.CJ-16-0011. [DOI] [PubMed] [Google Scholar]
- 160.Young LH, Renfu Y, Russell R, Hu X, Caplan M, Ren J, Shulman GI, Sinusas AJ. Low-flow ischemia leads to translocation of canine heart GLUT-4 and GLUT-1 glucose transporters to the sarcolemma in vivo. Circulation 95: 415–422, 1997. doi: 10.1161/01.CIR.95.2.415. [DOI] [PubMed] [Google Scholar]
- 161.Zaha VG, Young LH. AMP-activated protein kinase regulation and biological actions in the heart. Circ Res 111: 800–814, 2012. doi: 10.1161/CIRCRESAHA.111.255505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zhang LF, Lou JT, Lu MH, Gao C, Zhao S, Li B, Liang S, Li Y, Li D, Liu MF. Suppression of miR-199a maturation by HuR is crucial for hypoxia-induced glycolytic switch in hepatocellular carcinoma. EMBO J 34: 2671–2685, 2015. doi: 10.15252/embj.201591803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zhao J, Kelnar K, Bader AG. In-depth analysis shows synergy between erlotinib and miR-34a. PLoS One 9: e89105, 2014. doi: 10.1371/journal.pone.0089105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zhu X, Wang H, Liu F, Chen L, Luo W, Su P, Li W, Yu L, Yang X, Cai J. Identification of micro-RNA networks in end-stage heart failure because of dilated cardiomyopathy. J Cell Mol Med 17: 1173–1187, 2013. doi: 10.1111/jcmm.12096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zorio E, Medina P, Rueda J, Millán JM, Arnau MA, Beneyto M, Marín F, Gimeno JR, Osca J, Salvador A, España F, Estellés A. Insights into the role of microRNAs in cardiac diseases: from biological signalling to therapeutic targets. Cardiovasc Hematol Agents Med Chem 7: 82–90, 2009. doi: 10.2174/187152509787047676. [DOI] [PubMed] [Google Scholar]