We have investigated the effects of hyperglycemia on cardiomyocyte physiology and ventricular function. Our results indicate that defective Ca2+ handling is a critical component of the progressive deterioration of cardiac performance of the diabetic heart.
Keywords: diabetes, ventricular function, myocytes, Ca2+ handling
Abstract
Diabetes and other metabolic conditions characterized by elevated blood glucose constitute important risk factors for cardiovascular disease. Hyperglycemia targets myocardial cells rendering ineffective mechanical properties of the heart, but cellular alterations dictating the progressive deterioration of cardiac function with metabolic disorders remain to be clarified. In the current study, we examined the effects of hyperglycemia on cardiac function and myocyte physiology by employing mice with high blood glucose induced by administration of streptozotocin, a compound toxic to insulin-producing β-cells. We found that hyperglycemia initially delayed the electrical recovery of the heart, whereas cardiac function became defective only after ~2 mo with this condition and gradually worsened with time. Prolonged hyperglycemia was associated with increased chamber dilation, thinning of the left ventricle (LV), and myocyte loss. Cardiomyocytes from hyperglycemic mice exhibited defective Ca2+ transients before the appearance of LV systolic defects. Alterations in Ca2+ transients involved enhanced spontaneous Ca2+ releases from the sarcoplasmic reticulum (SR), reduced cytoplasmic Ca2+ clearance, and declined SR Ca2+ load. These defects have important consequences on myocyte contraction, relaxation, and mechanisms of rate adaptation. Collectively, our data indicate that hyperglycemia alters intracellular Ca2+ homeostasis in cardiomyocytes, hindering contractile activity and contributing to the manifestation of the diabetic cardiomyopathy.
NEW & NOTEWORTHY We have investigated the effects of hyperglycemia on cardiomyocyte physiology and ventricular function. Our results indicate that defective Ca2+ handling is a critical component of the progressive deterioration of cardiac performance of the diabetic heart.
prevalence of diabetes mellitus in the United States has increased in the last 2 decades and is currently estimated at 8.5% for individuals ≥ 20 yr of age (19), mirroring the worldwide occurrence of this disease (8). Systemic glucose elevation in diabetic patients affects various tissues and organs, comprising the heart, which is particularly vulnerable to this condition. Diabetes is a major risk factor for cardiovascular complications comprising coronary artery disease, atrial fibrillation, and heart failure (19). Restoration of glucose metabolism remains the major goal of therapeutic approaches for the treatment of diabetes, but clarification of the consequences of hyperglycemia on the heart may provide indication of putative protective and preventive strategies aimed at reducing morbidity and mortality associated with this disease.
Diabetes is coupled with protracted cardiac electrical recovery (5, 10, 20, 40, 42), a feature reiterated in rodent models of hyperglycemia (15, 17, 30). Lengthened repolarization and diastolic dysfunction are initial consequences of elevated glucose in experimental animals and are associated with prolongation of the action potential (AP) of cardiomyocytes (17). Delays of the repolarization phase of the AP are typically observed with cardiac pathologies and are symptomatic of concomitant modifications of electromechanical coupling and intracellular Ca2+ homeostasis (31, 39). Thus identification of the complex electrophysiological adaptations of cardiomyocytes to hyperglycemia may reveal corrective approaches to attenuate the detrimental effects of diabetes on the heart.
Excitation-contraction coupling in cardiomyocytes relies on intracellular Ca2+ fluctuations activating the contractile apparatus. Upon electrical excitation, the amount of Ca2+ released from the sarcoplasmic reticulum (SR) to the cytoplasm modulates the cross-bridge formation between myofilaments, thus determining the strength of force generated by the myocardium. In contrast, the rate of cytosolic Ca2+ clearance during diastole determines the pattern of muscle relaxation, by promoting the dissociation of Ca2+ from troponin C (1). Therefore, defective intracellular Ca2+ handling may be causative of the impaired systolic and diastolic performance of the diabetic heart.
In the current study we have evaluated early and chronic effects of hyperglycemia on cardiac function and established cellular alterations that accompany the progression of the diabetic cardiomyopathy. Using a mouse model of type 1 diabetes, we show that sustained hyperglycemia alters intracellular Ca2+ homeostasis in cardiomyocytes by interfering with mechanisms of Ca2+ release from the SR and clearance from the cytoplasm. These cellular alterations appear to be critical factors in the pathophysiology of the diabetic cardiomyopathy.
METHODS
In vivo studies.
Female C57Bl/6 and male FVB mice were studied in accordance with the Guide for Care and Use of Laboratory Animals; experiments were approved by the local Animal Care Committee (Institutional Animal Care and Use Committee). When needed, isoflurane (1–1.5%, inhalation) was employed as methodology of anesthesia.
To induce hyperglycemia, mice at 3–6 mo of age were treated with streptozotocin (STZ, Sigma-Aldrich) for 4–6 consecutive days (50–100 mg·kg−1·body wt,−1·day−1 ip). Age-, sex-, and strain-matched naïve mice served as control animals. STZ was dissolved in 0.9% saline solution containing 20 mM sodium citrate tribasic dihydrate (Sigma-Aldrich). Final STZ concentration was 5 mg/l. Glucose levels were assessed using TRUEtrack meter (Home Diagnostics) and test strips. Animals with blood glucose level >300 mg/dl were included in the study.
Echocardiography was performed in mice using a Visualsonics Vevo 2100 System equipped with a MS550D high-frequency (22–55 MHz) linear transducer. To evaluate cardiac functional and anatomical parameters, short- and long-axis views of the left ventricle (LV) were obtained in restrained, conscious mice (17, 31) using single-handed manual restraint method.
Electrocardiograms (ECGs) were recorded under isoflurane anesthesia by inserting needle electrodes subcutaneously into the mouse limbs. Electrical signals were amplified (Animal Bio Amp, ADInstruments), digitized using a 4 kHz A/D converter (MPVS-400, Millar Instruments), and recorded using LabChart software (ADInstruments) with low- and high-pass filtering at 100 Hz and 3 Hz, respectively. Surface ECG intervals were measured using LabChart (version 7 or 8) (17, 31). To reduce electrical noise and motion artifacts, consecutive beats were averaged by the software and employed to calculate heart rate and electrocardiographic parameters. QT interval was measured manually by determining the earliest onset and latest offset of ventricular deflections from the averaged cycles (17, 31). Rate-corrected QT interval (QTc) was normalized by the RR interval duration using Bazett formula (QTc = QT/√RR).
To record ECGs in freely roaming, unanesthetized animals, telemetric biopotential transmitters (ETA-F10, Data Science International) were implanted in mice anesthetized with isoflurane (1–1.5%, inhalation) (17, 31). The peritoneum was exposed with a midline incision, the implant was secured within the peritoneal cavity, and leads were secured subcutaneously corresponding to position II. Subsequently, signals were transmitted from implants to RPC-1 receivers and Data Exchange Matrix (Data Science International) and stored in a computer. Telemetric ECGs were evaluated during normal activity. All recordings were digitized at 2 kHz and analyzed off-line with Ponemah 5.10 software.
LV hemodynamics were obtained in anesthetized mice (isoflurane, ~1.5%) in the closed chest preparation with a MPVS-400 system for small animals (Millar Instruments) equipped with a PVR-1045 catheter (17, 31). The mouse was intubated and ventilated (MiniVent Type 845, Hugo Sachs Elektronik-Harvard Apparatus) with isoflurane anesthesia; the right carotid artery was then exposed, and the pressure transducer was inserted and advanced in the LV cavity. Data were acquired with Chart 5 or LabChart8 (ADInstruments) software (17, 31).
Ex vivo properties of the mouse heart.
To assess ex vivo electrical and functional properties, hearts were perfused through the aorta in a Langendorff apparatus (Radnoti) at a constant pressure of 80 mmHg with Krebs–Henseleit buffer (KHB, Sigma-Aldrich), containing (in mM) 118 NaCl, 4.7 KCl, 11 glucose, 1.2 MgSO4, 1.2 KH2PO4, 1.8 CaCl2, and 25 NaHCO3, gassed with 95% O2-5% CO2 (pH 7.4) at 37°C (17, 31, 32). Temperature was maintained by immersing the heart in a water-heated glassware reservoir (Radnoti), containing preheated KHB. Initially, data was acquired in sinus rhythm, and hearts were then stimulated at 125-ms basic cycle length with a 2-ms square pulse at ~1.5-fold its threshold level (4 channels stimulator, BMS 414, Crescent Electronics), using a mini-coaxial electrode (Harvard Apparatus). Pseudo-ECGs were obtained with a two-lead mini ECG system (Harvard Apparatus), in which electrodes were placed on the right atrium and apex of the heart. LV pressure was measured by a fluid-filled balloon catheter connected to a pressure transducer (Harvard Apparatus). Signals was amplified (Animal Bio Amp and Bridge Amp, ADInstruments), digitized using a 4 kHz A/D converter (Power Laboratory 8/30, ADInstruments), and recorded using LabChart 7 Pro software. The fluid-filled balloon, made of a small square of polyethylene film, was inserted into the left ventricle. Selective β-adrenergic stimulation was achieved with 10 nM isoproterenol (Sigma-Aldrich) delivered with an infusion pump (Pump 11 Elite, Harvard Apparatus) connected to the perfusion line. Data were analyzed with LabChart 7 Pro software.
Histological analysis.
With the animal under deep isoflurane anesthesia, the abdominal aorta was cannulated with a polyethylene catheter, PE-50, filled with a phosphate buffer solution and heparin, 100 U/ml. The heart was arrested in diastole by infusion of a solution containing ~100 mM CdCl2, via the aortic catheter. The thorax was then opened, and the inferior vena cava was perforated at the level of the right atrium to allow drainage of blood and perfusate. After an initial perfusion with phosphate buffer, the coronary vasculature was perfused for ~15 min with phosphate-buffered formalin (10%, Sigma-Aldrich). Perfusion pressure was set at ~80 mmHg. Subsequently, the heart was excised, kept for at least 24 h in phosphate-buffered formalin (10%), and then embedded in paraffin (31). Sections 4 µm thick of the LV were stained for vitronectin (Novus Biologicals) to label necrotic and late apoptotic cells (34). Myocytes were identified by α-sarcomeric actin (Sigma-Aldrich), and nuclei were stained by DAPI (Sigma-Aldrich). Sections were analyzed using an upright microscope (Olympus BX60), an inverted confocal microscope (Olympus Fluoview FV1000), and ImageJ software.
Myocyte isolation.
With the animal under deep anesthesia (isoflurane), thoracotomy was performed, heart was excised, and LV myocytes were enzymatically dissociated as previously reported (17, 31, 32). Briefly, the heart was connected to a plastic cannula for retrograde perfusion through the aorta in a Langendorff system (Radnoti) at 37°C. Perfusate consisted of a Ca2+-free solution gassed with 85% O2-15% N2. After 5 min, 0.1 mM CaCl2, 274 U/ml collagenase (type 2, Worthington Biochemical), and 0.57 U/ml protease (type XIV, Sigma-Aldrich) were added to the solution which contained (in mM) 126 NaCl, 4.4 KCl, 5 MgCl2, 20 HEPES, 22 glucose, 20 taurine, 5 creatine, 5 Na pyruvate, and 5 NaH2PO4 (pH 7.4). At completion of digestion, atria and right ventricle were dissected and the LV was cut in small pieces, and these fragments were shaken in resuspension solution and filtered using a 200-µm nylon mesh (Spectrum Laboratories). Only rod-shaped myocytes exhibiting cross striations and showing no spontaneous contractions or contractures were selected for physiological studies; cells were used within 8 h following enzymatic digestion.
Patch-clamp studies.
Isolated LV myocytes were placed in a bath on the stage of an IX53 inverted microscope (Olympus) for patch-clamp measurements (17, 26, 31–33). Experiments were conducted at 37°C. Data were acquired by means of the whole cell, patch-clamp technique in current-clamp mode using an Axoclamp 900A amplifier (Molecular Devices). Electrical signals were digitized using 250 kHz 16-bit resolution A/D converter (Digidata 1550, Molecular Devices) and recorded using pCLAMP 10 software (Molecular Devices) with low-pass filtering at 2 kHz. Pipettes (BF165-120-10, Sutter Instruments) were pulled by means of a horizontal (P-1000, Sutter Instruments) glass microelectrode puller.
For action potentials (AP) measurements, cells were stimulated at 4 Hz with current pulses 1–1.5-fold the AP threshold (17, 31–33). Myocytes were bathed with Tyrode solution containing (in mM) 140 NaCl, 5.4 KCl, 1 MgCl2, 5 HEPES, 5.5 glucose, and 1 CaCl2 (pH 7.4, adjusted with NaOH). The composition of the pipette solution was (in mM) 10 NaCl, 113 KCl, 0.5 MgCl2, 5 K2-ATP, 5.5 glucose, 5 EGTA, 10 HEPES (pH 7.2 with KOH). Data was analyzed using Clampfit (pCLAMP) software.
Ca2+ imaging in myocytes.
Isolated LV myocytes were placed in a bath on the stage of an Axiovert (Zeiss) inverted microscopes for the evaluation of Ca2+ transients (31, 32). Experiments were conducted at 37°C. Cells were bathed continuously with Tyrode solution. Measurements were collected in field-stimulated (SD9 Grass stimulators) cells by IonOptix fluorescence systems (IonOptix). Ca2+ transients were elicited by rectangular depolarizing pulses, ~2 ms in duration, and ~1.5-fold threshold in intensity, with platinum electrodes. Ca2+ transients were measured by epifluorescence after loading the myocytes with 2 µM Fluo-4 AM (Invitrogen). Excitation length was 480 nm with emission collected at 535 nm using a ×40 objective. Fluo signals were expressed as normalized fluorescence (F/F0), where F0 is the diastolic fluorescent level subtracted by the background signal measured in the region adjacent to the cell (31, 32).
Caffeine-induced Ca2+ transient.
To evaluate SR Ca2+ load in field-stimulated myocytes, stimulation was interrupted and a 2-s caffeine (20 mM, Sigma-Aldrich) spritz was delivered using glass micropipettes and a microinjector (Pico-liter Injector, Warner Instruments). Ca2+ transients were recorded by two-photon microscope (31, 32). Glass micropipettes (BF100-78-10, Sutter Instruments) were pulled by means of a horizontal (P-1000, Sutter Instrument) glass microelectrode puller. Myocytes were loaded with 5 µM Fluo-4 AM and placed on the stage of an upright microscope (BX51WI Olympus microscope coupled with a Bio-Rad Radiance 2100MP system). Cells were bathed with Tyrode’s solution and field stimulated using platinum electrodes. Fluo-4 was excited at 920 nm wavelength with mode-locked Ti:sapphire femtosecond laser (Tsunami, Spectra-Physics) and the emission signal was collected at 535 nm. Images were acquired in line-scan mode with the myocyte oriented in the long axis, at 6 ms sampling rate (31, 32). Data were analyzed with ImageJ software. Fluo signals were expressed as normalized fluorescence (F/F0).
Na+/Ca2+ exchange (NCX) activity was evaluated by the decay of the caffeine-induced Ca2+ transient, which was fitted with a monoexponential function (31, 37, 41). Data were analyzed with ImageJ software. Cells presenting prolonged time constant of Ca2+ transient decay (>8 s) and/or poor fitting (R2 < 0.98) were excluded from the study, without affecting statistical results.
Ca2+ sparks.
To evaluate diastolic Ca2+ releases from the SR, myocytes loaded with 5 µM Fluo-4 AM were paced at 1 Hz and a 30-s period of rest was introduced to assess the occurrence of Ca2+ sparks. Images were acquired by two-photon microscope in line-scan mode, with the myocyte oriented in the long axis, at 2 ms sampling rate. Images were analyzed with ImageJ software with Sparkmaster plugin following application of smoothing and median filters (22).
Data analysis.
Data are presented as means ± SE or means ± SE and scattered plots. Statistical analysis was performed using SigmaPlot 11.0 software. Data were initially tested for normality (Shapiro-Wilk) and equal variance for assignment to parametric or non-parametric analysis (17, 31, 33). Parametric test included Student’s t-test or ANOVA followed by Bonferroni test for non-paired comparison between two or among multiple groups, respectively. For paired statistical analysis, paired t-test or one-way, repeated-measures analysis of variance followed by Bonferroni test were employed for two groups or multiple comparisons, respectively. When normality or equal variance were not met, nonparametric analysis was performed using Mann-Whitney rank sum test or Kruskal-Wallis one way analysis of variance on ranks followed by Dunn’s method, for nonpaired comparison between two or among multiple groups, respectively. Wilcoxon-signed rank test or Friedman repeated-measures analysis of variance on ranks were employed for paired comparison between two or among multiple groups, respectively. P < 0.05 was considered significant.
RESULTS
Diabetes progressively affects ventricular function.
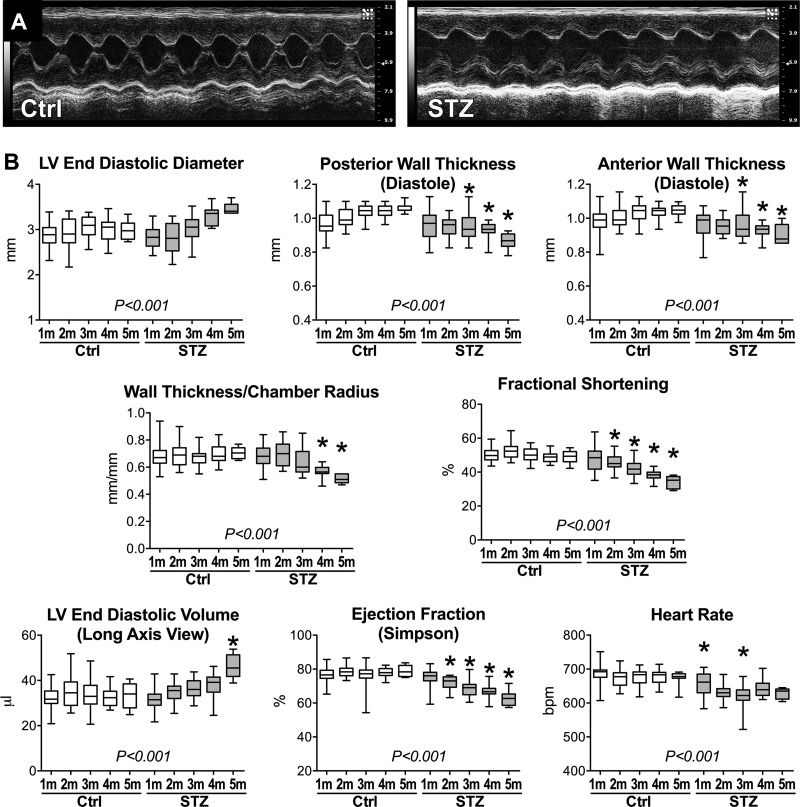
To define initial and long-term effects of elevated blood glucose on LV function, diabetes was induced in mice by STZ administration. Cardiac performance was then evaluated in vivo by echocardiography and electrocardiograms, together with LV hemodynamics at euthanasia. Strain-, age-, and sex-matched naïve mice were used as control (Ctrl). Approximately 1 mo after the induction of diabetes, FVB male mice treated with STZ had significantly elevated glucose level (≥500 mg/dl, n = 28) with respect to non-treated animals (172 ± 31 mg/dl, n = 19), as previously reported for C57Bl/6 female mice (17). After ~1 mo of hyperglycemia, no major alterations of LV function were observed by echocardiography. In contrast, beginning at ~2 mo after STZ administration, diabetic mice displayed depressed LV fractional shortening and ejection fraction, relative to control animals (Fig. 1). Thinning of the LV wall and ventricular dilation became then apparent with progression of the disease, resulting in severe reduction of wall thickness-to-chamber radius ratio at ~4 and ~5 mo after the onset of diabetes.
Fig. 1.
Cardiac function is progressively depressed with hyperglycemia. A: M-mode echocardiographic images for one naïve male FVB mouse control (Ctrl; left) and one streptozotocin (STZ)-treated male FVB mouse ~5 mo after induction of diabetes (right). LV, left ventricular. B: echocardiographic parameters obtained on a monthly basis in naïve male FVB mice (Ctrl, n = 46, 26, 28, 17, and 12 at 1, 2, 3, 4, and 5 mo, respectively) and STZ-treated male FVB mice (STZ, n = 50, 27, 25, 12, and 6 at 1, 2, 3, 4, and 5 mo after induction of diabetes, respectively) are shown as median and interquartile ranges. Analysis was performed in restrained, conscious mice. *P < 0.05 vs. Ctrl for the same time point using pairwise comparison. P < 0.001 refers to results of analysis of variance for multiple groups.
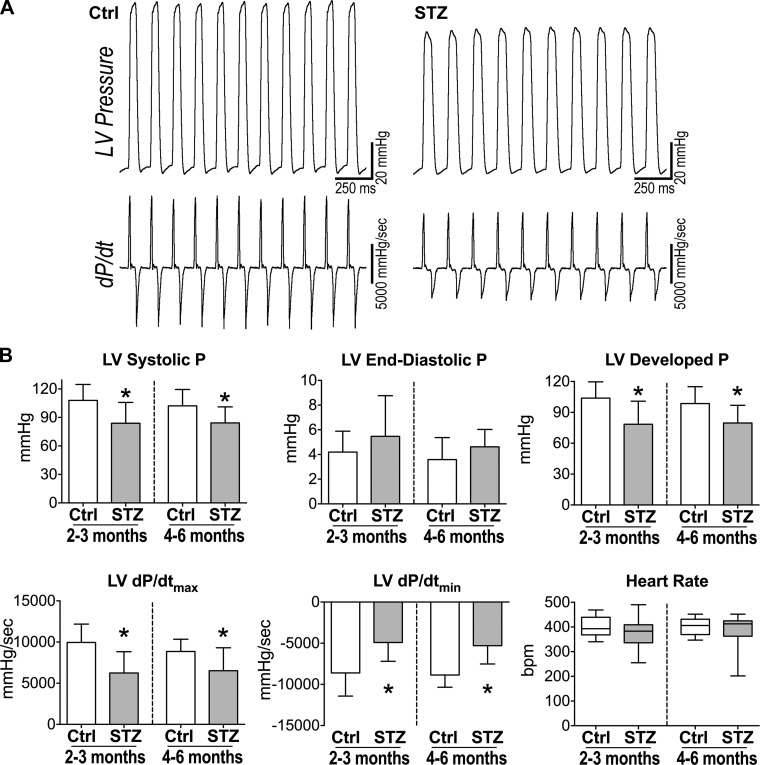
LV hemodynamic parameters, evaluated in the closed-chest preparation, were also depressed at 2 to 3 mo of diabetes. Systolic pressure, developed pressure, and maximal rates of contraction (dP/dtmax) and relaxation (dP/dtmin) were attenuated in STZ-treated mice, and these defects were maintained at 4–6 mo (Fig. 2).
Fig. 2.
Chronic hyperglycemia results in depressed LV hemodynamics. A: LV pressure (P) and first derivative of LV pressure (dP/dt) measurements for one naïve male FVB mouse (Ctrl; left) and one STZ-treated male FVB mouse ~3 mo after induction of diabetes (right). B: hemodynamic parameters under isoflurane anesthesia for naïve male FVB mice (Ctrl, n = 13–15) and STZ-treated male FVB mice at 2 to 3 and 4–6 mo after diabetes (STZ, n = 15) are shown as means ± SE *P < 0.05 vs. Ctrl for the same time point.
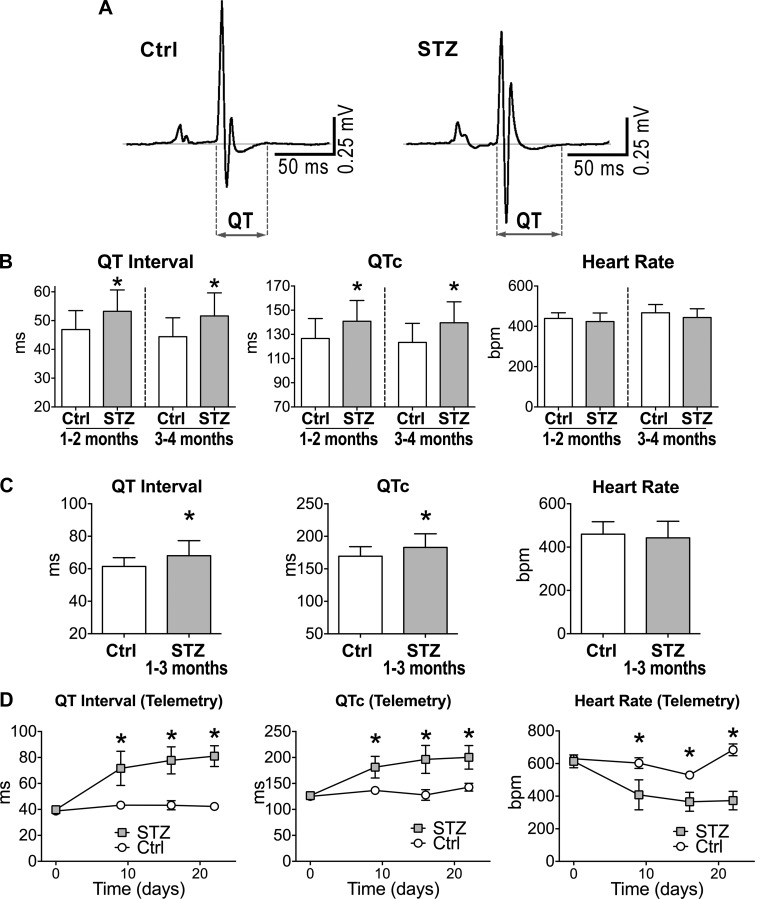
The declined performance of the diabetic heart was coupled with prolonged electrical recovery, assessed by the QT interval and rate-corrected QTc of the electrocardiogram (Fig. 3, A-C). As previously noted (17), telemetric measurements in unanesthetized mice revealed that delays in electrical recovery were already evident in the first 2 wk of diabetes (Fig. 3C). Hyperglycemia tended to reduce heart rate in the conscious state, a trend that was also observed during echocardiographic assessment (see Fig. 1B).
Fig. 3.
Hyperglycemia prolongs cardiac electrical recovery. A: electrocardiograms (ECGs) obtained in one naïve male FVB mouse (Ctrl; left) and one STZ-treated male FVB mouse 49 days after induction of diabetes (right). B: electrocardiographic parameters under anesthesia for naïve male FVB mice (Ctrl, n = 26–28) and STZ-treated male FVB mice at 1 to 2 and 3 to 4 mo after induction of diabetes (STZ, n = 20–26) are shown as means ± SE *P < 0.01 vs. Ctrl for the same time point. C: quantitative data for anesthetized naïve female C57Bl/6 mice (Ctrl, n = 20) and STZ-treated female C57Bl/6 mice at 38–99 days after diabetes (STZ, n = 26) are shown as means ± SE *P < 0.05 vs. Ctrl. D: quantitative data for serially acquired ECGs in nonrestrained naïve (Ctrl, n = 2) and STZ-treated (STZ, n = 6) female C57Bl/6 mice by telemetry are shown as mean ± SE *P < 0.05 vs. Ctrl for the same time point.
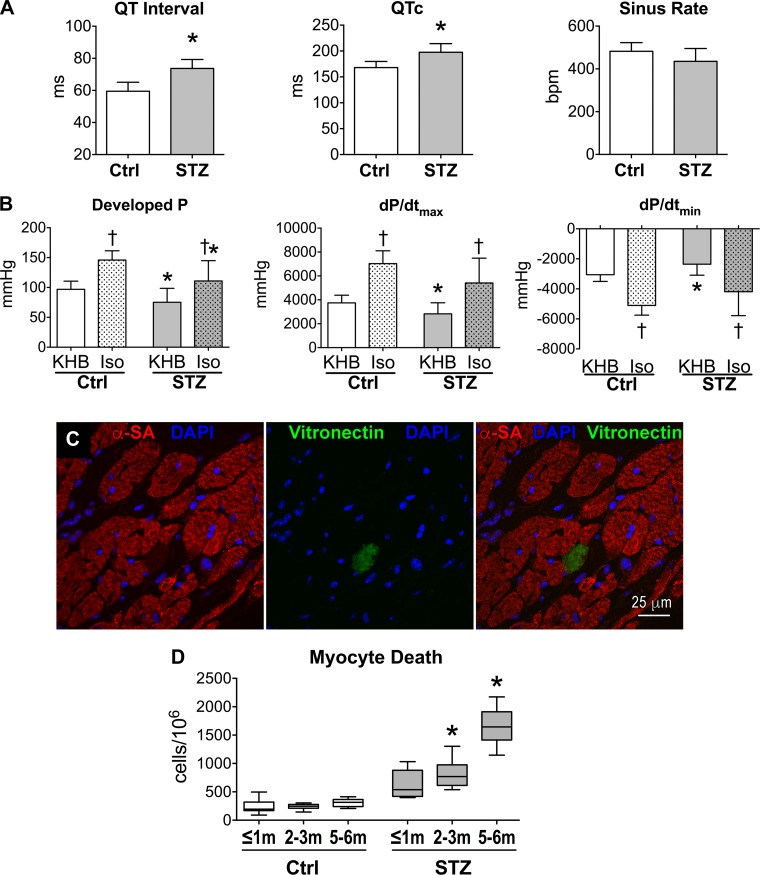
Diabetes is often associated with autonomic nervous imbalance (6, 28, 35, 38), a condition that may modulate mechanical and electrical properties of the heart. Thus, to establish whether intrinsic defects rather than extrinsic factors contributed to the altered cardiac function of hyperglycemic mice, ex vivo preparations and Langendorff-perfused organs were employed to reduce the influence of circulating neurohumoral signals. Additionally, effects of β-adrenergic stimulation on LV performance were tested to establish whether mechanisms of inotropic reserve are still operative in the diabetic myocardium and able to attenuate defects induced by hyperglycemia. In agreement with our electrocardiographic findings in vivo, electrical recovery was protracted in the perfused diabetic hearts (Fig. 4A). Moreover, LV developed pressure and maximal rate of contraction and relaxation were significantly reduced, compared with the heart from control animals (Fig. 4B). In both groups, β-adrenergic stimulation with isoproterenol enhanced LV developed pressure, but this agonist failed to abrogate differences between control and hyperglycemic organs.
Fig. 4.
Intrinsic defects of the diabetic heart. A: electrocardiographic parameters for perfused hearts from naïve female C57Bl/6 mice (Ctrl, n = 9) and STZ-treated female C57Bl/6 mice at 49–99 days after induction of diabetes (n = 9) are shown as means ± SE *P < 0.001 vs. Ctrl. B: LV pressure and maximal rate of contraction (+dP/dtmax) and relaxation (+dP/dtmin) in perfused hearts from naïve female C57Bl/6 mice (Ctrl, n = 9) and STZ-treated female C57Bl/6 mice at 49–99 days after induction of diabetes (n = 9) are shown as means ± SE. Data were obtained at baseline (Krebs-Henseleit buffer, KHB) and following perfusion with 10 nM isoproterenol (Iso). *P < 0.05 vs. Ctrl; †P < 0.01 vs. KHB. C: cell death identified by the expression of vitronectin in the myocardium of a STZ-treated FVB male mouse by confocal microscopy. D: quantitative data for myocyte death assessed in tissue sections from naïve FVB male mice (Ctrl, n = 8, 7, and 9 at ≤1, 2 to 3, and 4 to 5 mo, respectively) and STZ-treated male FVB mice (n = 5, 8, and 6 at ≤1, 2 to 3, and 4 to 5 mo after induction of diabetes, respectively) are shown as median and interquartile ranges. Analysis of variance for the 6 groups: P < 0.001. *P < 0.05 vs. Ctrl for the same time point using pairwise comparison.
Collagen accumulation and replacement fibrosis have been identified in the myocardium of diabetic patients (25), raising the possibility that hyperglycemia promotes necrotic cell death ultimately affecting cardiac performance. The progressive thinning of the LV free wall observed in hyperglycemic mice is also consistent with cumulative cell loss. Therefore, necrotic and late apoptotic events (34) in myocytes were evaluated in hearts exposed to sustained high levels of glucose throughout the duration of the study. By histological analysis of tissue sections (Fig. 4C), the fraction of dying myocytes was 2.6-fold higher in hearts from mice at 20–30 days after induction of diabetes, with respect to control organs. This fraction gradually increased with the progression of the hyperglycemic condition and reached a value 5.4-fold larger than control at 5 to 6 mo (Fig. 4D). Therefore, diabetes is associated with alterations in electrical and mechanical properties of the heart, together with thinning of the LV and increased myocyte death.
Diabetes leads to myocyte dysfunction.
To evaluate the effects of diabetes on myocyte behavior, Ca2+ transients were assessed in isolated cells, and these measurements were complemented by patch-camp analysis of AP properties, because the repolarization phase of the AP has important consequences on Ca2+ transient characteristics (3, 11, 27, 31–33).
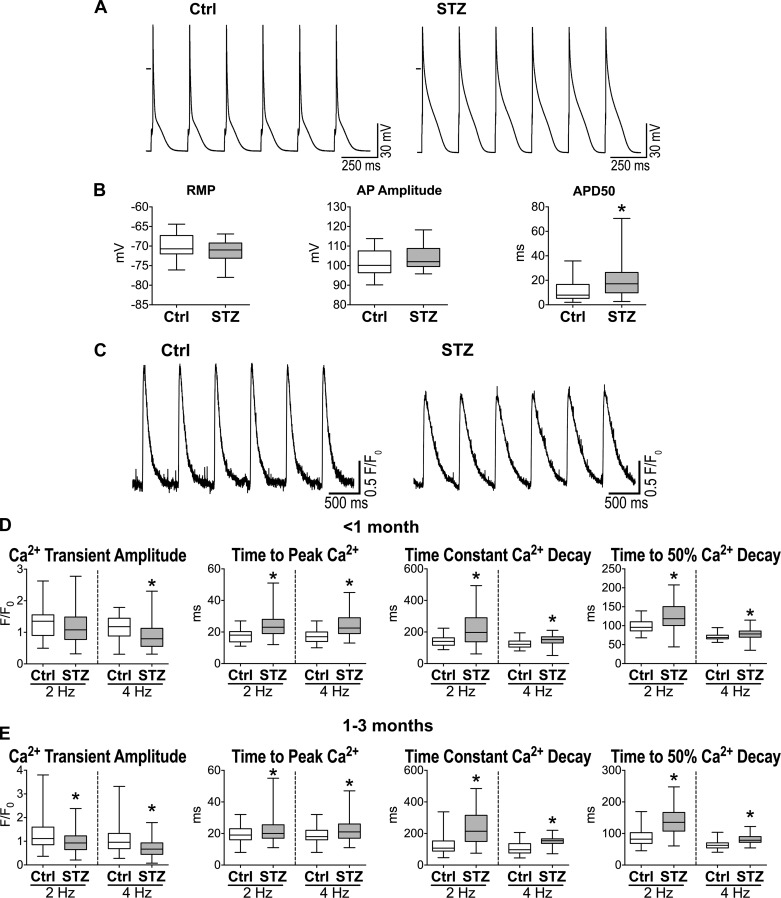
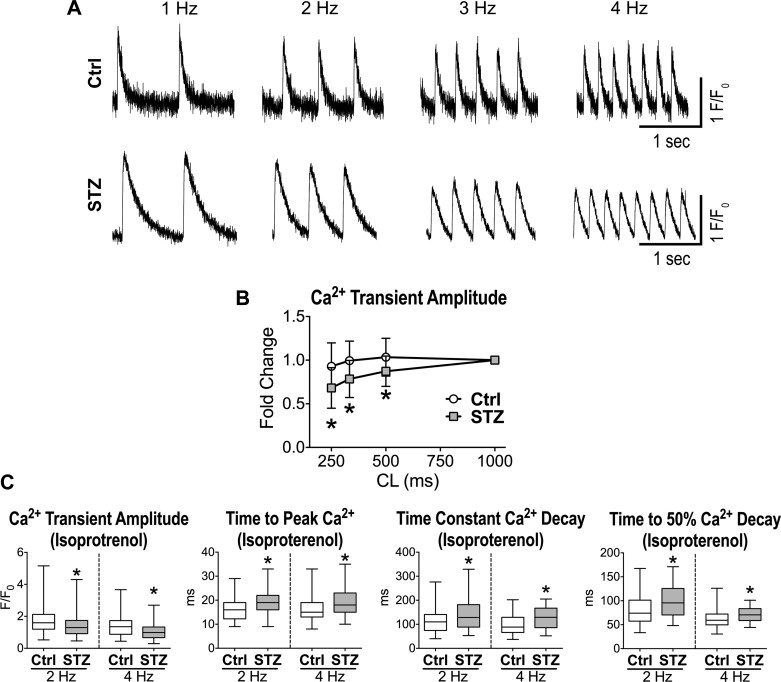
Cardiomyocytes from hyperglycemic mice at late stage (>2 mo) had protracted AP duration (Fig. 5, A and B), a feature that was also observed in the initial phases of the disease (17). Despite the inotropic support provided by the long AP, Ca2+ transient amplitude in STZ-myocytes paced at 2 Hz was comparable with control cells at <1 mo of diabetes, but declined at later stage (1–3 mo) (Fig. 5, C–E). In contrast, time to peak and decay of Ca2+ transient were lengthened in diabetic cells at both time points. Importantly, at 4-Hz stimulation rate, Ca2+ transient amplitude was also reduced in the early phase (<1 mo), suggesting that mechanisms of rate adaptation are affected by hyperglycemia. In fact, STZ-myocytes exposed to various stimulation rates presented a steeper negative Ca2+-frequency relationship compared with control cells (Fig. 6, A and B). These results indicate that Ca2+ transient defects are aggravated in diabetic myocytes at high pacing frequencies.
Fig. 5.
Diabetes induces defective myocyte function. A: action potentials (APs) of myocytes obtained from one naïve female C57Bl/6 mouse (Ctrl; left) and one STZ-treated female C57Bl/6 mouse 83 days after induction of diabetes (right). B: AP properties for isolated cardiomyocytes from naïve female C57Bl/6 mice (Ctrl, n = 27 cells from 13 animals) and STZ-treated female C57Bl/6 mice at 70–178 days after induction of diabetes (n = 28 cells from 8 animals) are shown as median and interquartile ranges. *P < 0.05 vs. Ctrl. RMP, resting membrane potential; APD50, AP duration at 50% repolarization. C: Ca2+ transients in myocytes obtained from one naïve female C57Bl/6 mouse (Ctrl; left) and one STZ-treated female C57Bl/6 mouse 60 days after induction of diabetes (right). D: Ca2+ transient properties obtained at 2- and 4-Hz pacing rates for isolated cardiomyocytes from naïve female C57Bl/6 mice (Ctrl, n = 35–38 cells from 2 animals) and STZ-treated female C57Bl/6 mice at 15–23 days after induction of diabetes (n = 64–71 cells from 4 animals) are shown as median and interquartile ranges. *P < 0.01 vs. Ctrl. E: Ca2+ transient properties obtained at 2- and 4-Hz pacing rate for isolated cardiomyocytes from naïve female C57Bl/6 mice (Ctrl, n = 153 cells from 9 animals) and STZ-treated female C57Bl/6 mice at 38–82 days after induction of diabetes (n = 188–189 cells from 9 animals) are shown as median and interquartile ranges. *P < 0.001 vs. Ctrl.
Fig. 6.
Diabetes affects rate adaptation and inotropic mechanisms of cardiomyocytes. A: Ca2+ transients at progressively faster pacing rate for one myocyte obtained from a naïve C57Bl/6 female mouse (Ctrl; top) and one myocyte from a STZ-treated female C57Bl/6 mouse 38 days after induction of diabetes (bottom). B: quantitative data for rate adaptation of amplitude and decay of Ca2+ transients for cardiomyocytes from naïve female C57Bl/6 mice (Ctrl, n = 24 cells from 5 animals) and STZ-treated female C57Bl/6 mice at 38–88 days after induction of diabetes (STZ, n = 16 cells from 4 animals) are shown as means ± SE. *P < 0.05 vs. Ctrl at the same cycle length (CL). C: Ca2+ transient properties obtained at 2- and 4-Hz pacing rates in the presence of 100 nM isoproterenol for myocytes from naïve female C57Bl/6 mice (Ctrl, n = 88 cells from 5 animals) and STZ-treated female C57Bl/6 mice at 40–82 days after induction of diabetes (n = 85–86 cells from 5 animals) are shown as median and interquartile ranges. F/F0, normalized fluorescence. *P < 0.01 vs. Ctrl.
To better define the behavior of myocytes from hyperglycemic mice, Ca2+ transient characteristics were measured following β-adrenergic stimulation, a transduction signaling that promotes phosphorylation and activity of Ca2+ handling proteins (2). This intervention potentiated Ca2+ transient properties but failed to abrogate differences between the two groups of cells (Fig. 6C). Collectively, these results indicate that hyperglycemia leads to dysfunctional Ca2+ cycling, contributing to the defective cardiac performance of the diabetic heart.
Diabetes alters intracellular Ca2+ homeostasis.
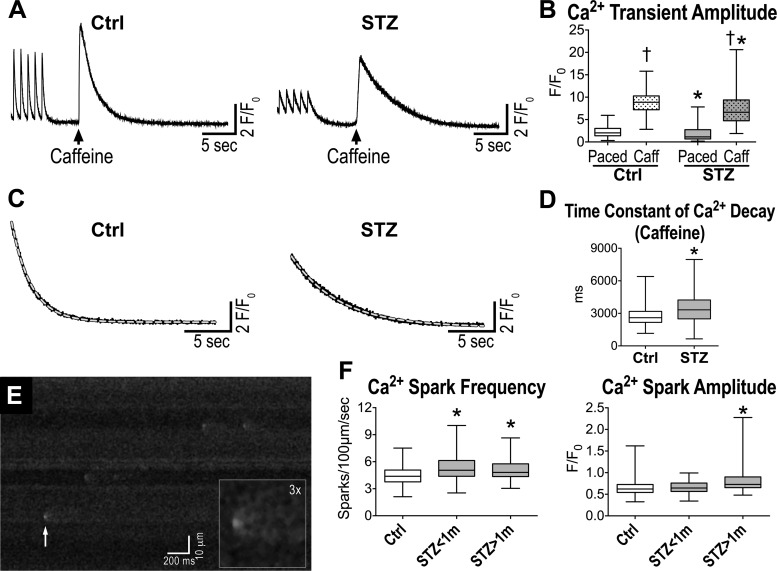
Complex alterations of Ca2+ fluxes may occur in diabetic myocytes attenuating amplitude and kinetics of Ca2+ transients, weakening mechanisms of rate adaptation, and blunting the inotropic consequences of the prolonged AP duration (3, 9, 11, 27, 31–33). The delayed time to peak of Ca2+ transient observed in diabetic myocytes is consistent with the protracted AP (31), but the reduced amplitude of the developed Ca2+ is indicative of concomitant defects in the release of Ca2+ from the SR. In this regard, Ca2+ load of the SR may represent an important variable conditioning the amount of Ca2+ released upon stimulation and its adaptations to faster pacing rates. To test this possibility, isolated cardiomyocytes were rapidly exposed to caffeine, which opens ryanodine receptors (RyRs), allowing the release of Ca2+ stored in the SR. The amplitude of the caffeine-induced Ca2+ transient was reduced in diabetic cells, indicating the SR Ca2+ content was affected by the hyperglycemic condition (Fig. 7, A and B). Moreover, the decay of the caffeine-induced Ca2+ transient, which reflects NCX activity (31, 41), was slower with respect to control cells (Fig. 7C), suggesting that diabetes affects this mechanism of Ca2+ extrusion.
Fig. 7.
Diabetes alters intracellular Ca2+ handling. A: caffeine-induced Ca2+ transients for one myocyte obtained from a naïve C57Bl/6 female mouse (Ctrl; left) and one myocyte from a STZ-treated female C57Bl/6 mouse 77 days after induction of diabetes (right). B: quantitative data for Ca2+ transient amplitude induced by electrical stimulation (Paced) or caffeine spritz (Caff) for cardiomyocytes from naïve female C57Bl/6 mice (Ctrl, n = 257 cells from 6 animals) and STZ-treated female C57Bl/6 mice at 63–97 days after induction of diabetes (n = 216 cells from 5 animals) are shown as median and interquartile ranges. †P < 0.001 vs. Paced; *P < 0.001 vs. Ctrl in the same experimental condition. C: fitting with monoexponential functions (gray dashed lines) of the decay phase of caffeine-induced Ca2+ transients shown in A. The following parameters characterize the 2 functions: Ctrl, time constant = 2075 ms, R2 = 0.997; STZ, time constant = 4994 ms, R2 = 0.995. D: quantitative data for the decay of the caffeine-induce Ca2+ transient, which is reflective of Na+/Ca2+ exchanger activity for cardiomyocytes from naïve female C57Bl/6 mice (Ctrl, n = 181 cells from 6 animals) and STZ-treated female C57Bl/6 mice at 63–97 days after induction of diabetes (n = 133 cells from 5 animals) are shown as median and interquartile ranges. *P < 0.001 vs. Ctrl. E: line-scan image of a myocyte from a STZ-treated female C57Bl/6 mouse 15 days after induction of diabetes. White arrow points to a spontaneous Ca2+ release event, which is magnified in inset. F: quantitative data for the occurrence and amplitude of Ca2+ sparks in cardiomyocytes from naïve female C57Bl/6 mice (Ctrl, n = 101 cells from 4 animals) and STZ-treated female C57Bl/6 mice at 15–19 and 45–83 days after induction of diabetes (STZ < 1 m; n = 53 cells from 3 animals; STZ > 1 m; n = 80 cells from 5 animals) are shown as median and interquartile ranges. *P < 0.05 vs. Ctrl.
Subsequently, to define putative factors responsible for the reduced Ca2+ load of the SR, spontaneous elementary Ca2+ releases from the SR were tested. Ca2+ sparks manifesting during diastole reflect the uncoordinated opening of clusters of RyR channels (Fig. 7D) and have important consequences on the preservation of SR Ca2+ content on the one hand and susceptibility of delayed afterdepolarizations on the other. Both in the early and chronic phases of diabetes, myocytes presented enhanced frequency of spontaneous Ca2+ releases, with increased amplitude at late time point (Fig. 7E). Thus defective function of RyRs may represent an important component of the perturbed Ca2+ cycling of hyperglycemic myocytes.
DISCUSSION
Results of the current study strengthen the notion that the progressive deterioration of cardiac performance with hyperglycemia is associated, at the cellular level, with prolongation of the AP and defective intracellular Ca2+ cycling. Spontaneous Ca2+ releases from the SR are enhanced, and Ca2+ sequestration is slowed in diabetic cells, and these two factors contribute to the attenuated SR Ca2+ content and decreased amplitude of Ca2+ transients.
Consistent with our previous findings in hyperglycemic female mice (17), male FVB animals show defective systolic function only at ~2 mo after induction of diabetes, whereas prolongation of the QT interval of the ECG becomes apparent at earlier stages of the disease. Also, alterations at the cellular level occur before performance of the entire organ is compromised, suggesting that defects in the myocyte compartment influence the progression of the diabetic cardiomyopathy. Additionally, hyperglycemia leads to myocyte death and the cumulative cell loss is consistent with the gradual decline of ventricular mechanics and thinning of the LV.
Myocytes from mice treated with STZ have reduced L-type Ca2+ current (17), but the prolonged duration of the AP appears to sustain Ca2+ influx and Ca2+ induced-Ca2+ release processes (3, 31), as documented by the delayed time to peak of Ca2+ transients. However, this mechanism of inotropic support fails to normalize Ca2+ transient in diabetic cells and the reduced SR Ca2+ load seems to be accountable for this defect. The slow Ca2+ transient decay is indicative of impaired sequestration of Ca2+ in the SR during diastole, a factor that may limit SR Ca2+ content and subsequent Ca2+ induced-Ca2+ release process (14, 23). Also, the attenuated rate-adaptation of Ca2+ transient in diabetic myocytes seems to be secondary to the ineffective Ca2+ reuptake (23). The high frequency of spontaneous Ca2+ sparks are consistent with leaky SR (43), contributing to the depletion of Ca2+ stores (29). Overall, perturbed Ca2+ translocation between SR and cytoplasmic compartments in diabetic cells favors Ca2+ overload with direct consequences to systolic and diastolic function of myocytes and whole heart. Moreover, the deregulated intracellular Ca2+ homeostasis may promote arrhythmias and beat-to-beat variability, which have been observed in patients (12, 16, 18, 36) and experimental models of diabetes (17, 24). Furthermore, excessive cytoplasmic Ca2+ load may initiate a cascade of events leading to dysfunction of mitochondria (13), representing the basis for the enhanced cell death (7) observed with diabetes.
Alterations in expression, post-translational status, and activity of Ca2+ handling proteins have been reported in experimental models of type 1 and type 2 diabetes (4, 21), and our observations offer an integrated overview of the detrimental consequences of sustained hyperglycemia on key processes regulating Ca2+ homeostasis in cardiomyocytes. Further studies are warranted to elucidate the molecular basis for these defects and to establish whether normalization of intracellular Ca2+ shuttling is a viable strategy to protect the diabetic heart.
GRANTS
This work was supported by National Institute of Health grants and intramural funds at New York Medical College.
DISCLOSURES
P. Anversa is a member of Autologous LLP, and P. Anversa and A. Leri are members of AAL Scientifics Corp. All other authors have nothing to disclose.
AUTHOR CONTRIBUTIONS
A.S., G.B., Y.Z. and M.R. conceived and designed the research; A.S., G.B., Y.Z., A.C., M.M., S.S., and M.R. prepared reagents, performed experiments, and/or acquired data; A.S., G.B., Y.Z., A.C., M.M., S.S., and M.R. analyzed data, performed statistical analysis, and/or interpreted results; A.L., P.A., T.H.H., and M.R. provided funding support; A.S. and M.R drafted the manuscript; A.S., G.B., Y.Z., A.C., M.M., S.S., P.A., A.L., P.G., K.Q., J.T.J, T.H.H., and M.R. edited and revised the manuscript; A.S., G.B., Y.Z., A.C., M.M., S.S., P.A., A.L., P.G., K.Q., J.T.J, T.H.H., and M.R. approved the manuscript.
REFERENCES
- 1.Bers DM. Calcium cycling and signaling in cardiac myocytes. Annu Rev Physiol 70: 23–49, 2008. doi: 10.1146/annurev.physiol.70.113006.100455. [DOI] [PubMed] [Google Scholar]
- 2.Bers DM. Cardiac excitation-contraction coupling. Nature 415: 198–205, 2002. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 3.Bouchard RA, Clark RB, Giles WR. Effects of action potential duration on excitation-contraction coupling in rat ventricular myocytes. Action potential voltage-clamp measurements. Circ Res 76: 790–801, 1995. doi: 10.1161/01.RES.76.5.790. [DOI] [PubMed] [Google Scholar]
- 4.Boudina S, Abel ED. Diabetic cardiomyopathy, causes and effects. Rev Endocr Metab Disord 11: 31–39, 2010. doi: 10.1007/s11154-010-9131-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cardoso CR, Salles GF, Deccache W. Prognostic value of QT interval parameters in type 2 diabetes mellitus: results of a long-term follow-up prospective study. J Diabetes Complications 17: 169–178, 2003. doi: 10.1016/S1056-8727(02)00206-4. [DOI] [PubMed] [Google Scholar]
- 6.Carnethon MR, Golden SH, Folsom AR, Haskell W, Liao D. Prospective investigation of autonomic nervous system function and the development of type 2 diabetes: the Atherosclerosis Risk In Communities study, 1987–1998. Circulation 107: 2190–2195, 2003. doi: 10.1161/01.CIR.0000066324.74807.95. [DOI] [PubMed] [Google Scholar]
- 7.Chiong M, Wang ZV, Pedrozo Z, Cao DJ, Troncoso R, Ibacache M, Criollo A, Nemchenko A, Hill JA, Lavandero S. Cardiomyocyte death: mechanisms and translational implications. Cell Death Dis 2: e244, 2011. doi: 10.1038/cddis.2011.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Danaei G, Finucane MM, Lu Y, Singh GM, Cowan MJ, Paciorek CJ, Lin JK, Farzadfar F, Khang YH, Stevens GA, Rao M, Ali MK, Riley LM, Robinson CA, Ezzati M; Global Burden of Metabolic Risk Factors of Chronic Diseases Collaborating Group (Blood Glucose) . National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2·7 million participants. Lancet 378: 31–40, 2011. doi: 10.1016/S0140-6736(11)60679-X. [DOI] [PubMed] [Google Scholar]
- 9.Grubb S, Aistrup GL, Koivumäki JT, Speerschneider T, Gottlieb LA, Mutsaers NA, Olesen SP, Calloe K, Thomsen MB. Preservation of cardiac function by prolonged action potentials in mice deficient of KChIP2. Am J Physiol Heart Circ Physiol 309: H481–H489, 2015. doi: 10.1152/ajpheart.00166.2015. [DOI] [PubMed] [Google Scholar]
- 10.Gruden G, Giunti S, Barutta F, Chaturvedi N, Witte DR, Tricarico M, Fuller JH, Cavallo Perin P, Bruno G. QTc interval prolongation is independently associated with severe hypoglycemic attacks in type 1 diabetes from the EURODIAB IDDM complications study. Diabetes Care 35: 125–127, 2012. doi: 10.2337/dc11-1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Janczewski AM, Spurgeon HA, Lakatta EG. Action potential prolongation in cardiac myocytes of old rats is an adaptation to sustain youthful intracellular Ca2+ regulation. J Mol Cell Cardiol 34: 641–648, 2002. doi: 10.1006/jmcc.2002.2004. [DOI] [PubMed] [Google Scholar]
- 12.Jouven X, Lemaître RN, Rea TD, Sotoodehnia N, Empana JP, Siscovick DS. Diabetes, glucose level, and risk of sudden cardiac death. Eur Heart J 26: 2142–2147, 2005. doi: 10.1093/eurheartj/ehi376. [DOI] [PubMed] [Google Scholar]
- 13.Lemasters JJ, Theruvath TP, Zhong Z, Nieminen AL. Mitochondrial calcium and the permeability transition in cell death. Biochim Biophys Acta 1787: 1395–1401, 2009. doi: 10.1016/j.bbabio.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lindner M, Erdmann E, Beuckelmann DJ. Calcium content of the sarcoplasmic reticulum in isolated ventricular myocytes from patients with terminal heart failure. J Mol Cell Cardiol 30: 743–749, 1998. doi: 10.1006/jmcc.1997.0626. [DOI] [PubMed] [Google Scholar]
- 15.Lu Z, Jiang YP, Wu CY, Ballou LM, Liu S, Carpenter ES, Rosen MR, Cohen IS, Lin RZ. Increased persistent sodium current due to decreased PI3K signaling contributes to QT prolongation in the diabetic heart. Diabetes 62: 4257–4265, 2013. doi: 10.2337/db13-0420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McNally PG, Lawrence IG, Panerai RB, Weston PJ, Thurston H. Sudden death in type 1 diabetes. Diabetes Obes Metab 1: 151–158, 1999. doi: 10.1046/j.1463-1326.1999.00025.x. [DOI] [PubMed] [Google Scholar]
- 17.Meo M, Meste O, Signore S, Sorrentino A, Cannata A, Zhou Y, Matsuda A, Luciani M, Kannappan R, Goichberg P, Leri A, Anversa P, Rota M. Reduction in Kv Current Enhances the Temporal Dispersion of the Action Potential in Diabetic Myocytes: Insights From a Novel Repolarization Algorithm. J Am Heart Assoc 5: e003078, 2016. doi: 10.1161/JAHA.115.003078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miki T, Tobisawa T, Sato T, Tanno M, Yano T, Akasaka H, Kuno A, Ogasawara M, Murase H, Saitoh S, Miura T. Does glycemic control reverse dispersion of ventricular repolarization in type 2 diabetes? Cardiovasc Diabetol 13: 125, 2014. doi: 10.1186/s12933-014-0125-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Després JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jiménez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER III, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB; Writing Group Members; American Heart Association Statistics Committee; Stroke Statistics Subcommittee . Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation 133: e38–e360, 2016. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 20.Okin PM, Devereux RB, Lee ET, Galloway JM, Howard BV, Strong Heart S; Strong Heart Study . Electrocardiographic repolarization complexity and abnormality predict all-cause and cardiovascular mortality in diabetes: the strong heart study. Diabetes 53: 434–440, 2004. doi: 10.2337/diabetes.53.2.434. [DOI] [PubMed] [Google Scholar]
- 21.Pereira L, Ruiz-Hurtado G, Rueda A, Mercadier JJ, Benitah JP, Gómez AM. Calcium signaling in diabetic cardiomyocytes. Cell Calcium 56: 372–380, 2014. doi: 10.1016/j.ceca.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 22.Picht E, Zima AV, Blatter LA, Bers DM. SparkMaster: automated calcium spark analysis with ImageJ. Am J Physiol Cell Physiol 293: C1073–C1081, 2007. doi: 10.1152/ajpcell.00586.2006. [DOI] [PubMed] [Google Scholar]
- 23.Pieske B, Maier LS, Bers DM, Hasenfuss G. Ca2+ handling and sarcoplasmic reticulum Ca2+ content in isolated failing and nonfailing human myocardium. Circ Res 85: 38–46, 1999. doi: 10.1161/01.RES.85.1.38. [DOI] [PubMed] [Google Scholar]
- 24.Rajab M, Jin H, Welzig CM, Albano A, Aronovitz M, Zhang Y, Park HJ, Link MS, Noujaim SF, Galper JB. Increased inducibility of ventricular tachycardia and decreased heart rate variability in a mouse model for type 1 diabetes: effect of pravastatin. Am J Physiol Heart Circ Physiol 305: H1807–H1816, 2013. doi: 10.1152/ajpheart.00979.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Regan TJ, Lyons MM, Ahmed SS, Levinson GE, Oldewurtel HA, Ahmad MR, Haider B. Evidence for cardiomyopathy in familial diabetes mellitus. J Clin Invest 60: 884–899, 1977. doi: 10.1172/JCI108843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rota M, Vassalle M. Patch-clamp analysis in canine cardiac Purkinje cells of a novel sodium component in the pacemaker range. J Physiol 548: 147–165, 2003. doi: 10.1113/jphysiol.2003.039263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sah R, Ramirez RJ, Kaprielian R, Backx PH. Alterations in action potential profile enhance excitation-contraction coupling in rat cardiac myocytes. J Physiol 533: 201–214, 2001. doi: 10.1111/j.1469-7793.2001.0201b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scognamiglio R, Avogaro A, Casara D, Crepaldi C, Marin M, Palisi M, Mingardi R, Erle G, Fasoli G, Dalla Volta S. Myocardial dysfunction and adrenergic cardiac innervation in patients with insulin-dependent diabetes mellitus. J Am Coll Cardiol 31: 404–412, 1998. doi: 10.1016/S0735-1097(97)00516-0. [DOI] [PubMed] [Google Scholar]
- 29.Shan J, Betzenhauser MJ, Kushnir A, Reiken S, Meli AC, Wronska A, Dura M, Chen BX, Marks AR. Role of chronic ryanodine receptor phosphorylation in heart failure and β-adrenergic receptor blockade in mice. J Clin Invest 120: 4375–4387, 2010. doi: 10.1172/JCI37649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shao CH, Rozanski GJ, Patel KP, Bidasee KR. Dyssynchronous (non-uniform) Ca2+ release in myocytes from streptozotocin-induced diabetic rats. J Mol Cell Cardiol 42: 234–246, 2007. doi: 10.1016/j.yjmcc.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 31.Signore S, Sorrentino A, Borghetti G, Cannata A, Meo M, Zhou Y, Kannappan R, Pasqualini F, O’Malley H, Sundman M, Tsigkas N, Zhang E, Arranto C, Mangiaracina C, Isobe K, Sena BF, Kim J, Goichberg P, Nahrendorf M, Isom LL, Leri A, Anversa P, Rota M. Late Na(+) current and protracted electrical recovery are critical determinants of the aging myopathy. Nat Commun 6: 8803, 2015. doi: 10.1038/ncomms9803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Signore S, Sorrentino A, Ferreira-Martins J, Kannappan R, Shafaie M, Del Ben F, Isobe K, Arranto C, Wybieralska E, Webster A, Sanada F, Ogórek B, Zheng H, Liu X, Del Monte F, D’Alessandro DA, Wunimenghe O, Michler RE, Hosoda T, Goichberg P, Leri A, Kajstura J, Anversa P, Rota M. Inositol 1, 4, 5-trisphosphate receptors and human left ventricular myocytes. Circulation 128: 1286–1297, 2013. doi: 10.1161/CIRCULATIONAHA.113.002764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sorrentino A, Signore S, Qanud K, Borghetti G, Meo M, Cannata A, Zhou Y, Wybieralska E, Luciani M, Kannappan R, Zhang E, Matsuda A, Webster A, Cimini M, Kertowidjojo E, D’Alessandro DA, Wunimenghe O, Michler RE, Royer C, Goichberg P, Leri A, Barrett EG, Anversa P, Hintze TH, Rota M. Myocyte repolarization modulates myocardial function in aging dogs. Am J Physiol Heart Circ Physiol 310: H873–H890, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stepanek O, Brdicka T, Angelisova P, Horvath O, Spicka J, Stockbauer P, Man P, Horejsi V. Interaction of late apoptotic and necrotic cells with vitronectin. PLoS One 6: e19243, 2011. doi: 10.1371/journal.pone.0019243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Straznicky NE, Grima MT, Sari CI, Eikelis N, Lambert EA, Nestel PJ, Esler MD, Dixon JB, Chopra R, Tilbrook AJ, Schlaich MP, Lambert GW. Neuroadrenergic dysfunction along the diabetes continuum: a comparative study in obese metabolic syndrome subjects. Diabetes 61: 2506–2516, 2012. doi: 10.2337/db12-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suys BE, Huybrechts SJ, De Wolf D, Op De Beeck L, Matthys D, Van Overmeire B, Du Caju MV, Rooman RP. QTc interval prolongation and QTc dispersion in children and adolescents with type 1 diabetes. J Pediatr 141: 59–63, 2002. doi: 10.1067/mpd.2002.125175. [DOI] [PubMed] [Google Scholar]
- 37.Tang M, Zhang X, Li Y, Guan Y, Ai X, Szeto C, Nakayama H, Zhang H, Ge S, Molkentin JD, Houser SR, Chen X. Enhanced basal contractility but reduced excitation-contraction coupling efficiency and beta-adrenergic reserve of hearts with increased Cav1.2 activity. Am J Physiol Heart Circ Physiol 299: H519–H528, 2010. doi: 10.1152/ajpheart.00265.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thorp AA, Schlaich MP. Relevance of Sympathetic Nervous System Activation in Obesity and Metabolic Syndrome. J Diabetes Res 2015: 341583, 2015. doi: 10.1155/2015/341583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tomaselli GF, Marbán E. Electrophysiological remodeling in hypertrophy and heart failure. Cardiovasc Res 42: 270–283, 1999. doi: 10.1016/S0008-6363(99)00017-6. [DOI] [PubMed] [Google Scholar]
- 40.Veglio M, Giunti S, Stevens LK, Fuller JH, Perin PC; EURODIAB IDDM Complications Study Group . Prevalence of Q-T interval dispersion in type 1 diabetes and its relation with cardiac ischemia: the EURODIAB IDDM Complications Study Group. Diabetes Care 25: 702–707, 2002. doi: 10.2337/diacare.25.4.702. [DOI] [PubMed] [Google Scholar]
- 41.Voigt N, Li N, Wang Q, Wang W, Trafford AW, Abu-Taha I, Sun Q, Wieland T, Ravens U, Nattel S, Wehrens XH, Dobrev D. Enhanced sarcoplasmic reticulum Ca2+ leak and increased Na+-Ca2+ exchanger function underlie delayed afterdepolarizations in patients with chronic atrial fibrillation. Circulation 125: 2059–2070, 2012. doi: 10.1161/CIRCULATIONAHA.111.067306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Whitsel EA, Boyko EJ, Rautaharju PM, Raghunathan TE, Lin D, Pearce RM, Weinmann SA, Siscovick DS. Electrocardiographic QT interval prolongation and risk of primary cardiac arrest in diabetic patients. Diabetes Care 28: 2045–2047, 2005. doi: 10.2337/diacare.28.8.2045. [DOI] [PubMed] [Google Scholar]
- 43.Yaras N, Ugur M, Ozdemir S, Gurdal H, Purali N, Lacampagne A, Vassort G, Turan B. Effects of diabetes on ryanodine receptor Ca release channel (RyR2) and Ca2+ homeostasis in rat heart. Diabetes 54: 3082–3088, 2005. doi: 10.2337/diabetes.54.11.3082. [DOI] [PubMed] [Google Scholar]