Abstract
In many mammals, including humans, removal of one lung (pneumonectomy) results in the compensatory growth of the remaining lung. Compensatory growth involves not only an increase in lung size, but also an increase in the number of alveoli in the peripheral lung; however, the process of compensatory neoalveolarization remains poorly understood. Here, we show that the expression of α-smooth muscle actin (SMA)—a cytoplasmic protein characteristic of myofibroblasts—is induced in the pleura following pneumonectomy. SMA induction appears to be dependent on pleural deformation (stretch) as induction is prevented by plombage or phrenic nerve transection (P < 0.001). Within 3 days of pneumonectomy, the frequency of SMA+ cells in subpleural alveolar ducts was significantly increased (P < 0.01). To determine the functional activity of these SMA+ cells, we isolated regenerating alveolar ducts by laser microdissection and analyzed individual cells using microfluidic single-cell quantitative PCR. Single cells expressing the SMA (Acta2) gene demonstrated significantly greater transcriptional activity than endothelial cells or other discrete cell populations in the alveolar duct (P < 0.05). The transcriptional activity of the Acta2+ cells, including expression of TGF signaling as well as repair-related genes, suggests that these myofibroblast-like cells contribute to compensatory lung growth.
Keywords: compensatory growth, gene expression, lung, myofibroblasts
in most mammals, including humans (3), removal of one lung (pneumonectomy) results in the compensatory growth of the remaining lung (20). After murine pneumonectomy, the remaining lung demonstrates an increase in the volume of all four lobes—with particular increase in the volume of the cardiac lobe (12). Coincident with the increase in lung volume, there is an increase in weight and cell number (4, 23). Moreover, the process of postpneumonectomy lung growth is rapid with compensatory growth occurring within weeks; most of the new alveoli (74%) are detectable within 7 days of surgery (10). The mechanism of compensatory growth is unknown.
There are few mechanical or morphological clues suggesting the mechanism of neoalveolarization. In development, the importance of mechanical forces in lung growth has been suggested by several in vivo observations. Limited mechanical stretch—associated with congenital diaphragmatic hernia (22), oligohydramnios (1), and phrenic nerve dysfunction (16)—has been associated with underdeveloped alveolar septa. Excessive mechanical stretch, commonly associated with neonatal mechanical ventilation, has been associated with disordered alveolar septa (29).
In compensatory lung growth, histology of the postpneumonectomy lung demonstrates no dominant cellular aggregates and only a modest increase in septal thickness (35, 42). Within days of pneumonectomy, ~20–30% of alveolar ducts are dilated as a result of septal retraction. Ysasi et al. (42) have suggested that new alveoli are formed by repartitioning of the alveolar septa within the dilated ducts.
A cell type potentially involved in the repartitioning of the alveolar duct is the myofibroblast. Myofibroblasts have been implicated in the alveolarization stage of lung development—a process involving the “lifting” of alveolar septa (24). Characterized by the cytoplasmic expression of α-smooth muscle actin (SMA) and the production of extracellular matrix components, myofibroblasts have been spatially associated with the development of alveolar septa during lung development (8, 39). In adult lungs, myofibroblasts have been associated with a variety of diseases including pulmonary fibrosis (26) and pulmonary hypertension (21).
In this report, we manipulated the in vivo forces applied to the remaining lung after pneumonectomy—namely, static and cyclic stretch—to show that postpneumonectomy deformation induces SMA+ cells in the pleura. We tracked the apparent migration of the SMA+ cells into subpleural alveolar ducts. The functional activity of the SMA+ cells in these regenerative “hot spots” was investigated by single-cell qPCR.
METHODS
Mice.
Male mice, 8- to 10-wk-old wild-type C57BL/6 (Jackson Laboratory, Bar Harbor, ME) were used for all experiments. The mice were anesthetized as previously described (14). The care of the animals was consistent with guidelines of the American Association for Accreditation of Laboratory Animal Care (Bethesda, MD) and approved by our Institutional Animal Care and Use Committee.
Pneumonectomy.
Each animal undergoing pneumonectomy was ventilated on a flexiVent (SciReq, Montreal, QC, Canada) at ventilator settings of 200 breaths/min, 10 ml/kg tidal volume, and positive end-expiratory pressure of 2 cmH2O with a pressure-limited constant flow profile (14). A left fifth intercostal space thoracotomy provided exposure for hilar ligation and left pneumonectomy. In some mice, sham thoracotomy (N = 4), plombage (N = 4), and phrenic nerve transection (N = 5) were performed as previously described (41). Postoperatively, the animal was weaned from mechanical ventilation and maintained on supplemental oxygen until normal spontaneous ventilation was observed.
Immunohistochemistry.
Cryostat sections (8 µm) were obtained from lung specimens perfused with OCT compound and snap frozen. Serial sections of the cardiac lobe ranged from 46 to 59 sections. After warming the slide to 27°C, the sections were fixed for 10 min (2% paraformaldehyde and PBS at pH 7.43). The slides were washed with buffer (PBS, 5% sheep serum, 0.1% azide, 1 mM MgCl2, 1 mM CaCl2) and blocked with 20% sheep serum, 20% goat serum, 0.1% azide in PBS. The slides were treated with anti-SMA polyclonal antibody (primary rabbit ab5694, Abcam; secondary goat anti-rabbit Texas Red; Life Technologies, Carlsbad, CA). Specificity controls were purified rabbit IgG (Abcam) or secondary antibody alone. The slides were incubated for 1 h at 27°C, washed three times, and mounted with either DAPI-containing medium (Vector Laboratories. Burlingame, CA) or Hoechst 33342 (Sigma-Aldrich, St. Louis, MO).
Fluorescence microscopy.
The tissue sections were imaged with a Nikon Eclipse TE2000 inverted epifluorescence microscope using Nikon objectives of ×10, ×20, and ×40 linear magnification with infinity correction. An X-Cite (Exfo, Vanier, QC, Canada) 120 W metal halide light source and a liquid light guide were used to illuminate the tissue samples. The tricolor excitation and emission filters (Chroma Technology, Bellows Falls, VT) were controlled by a MAC5000 controller (Ludl, Hawthorne, NY) and MetaMorph software 7.8 (Molecular Devices, Downington, PA). The fluorescence microscopy 16-bit fluorescent images were digitally recorded on a C9100-02 camera (Hamamatsu, Japan), digitally recombined and pseudocolored based on recording wavelength.
Image segmentation and cytometry.
Automated 16-bit fluorescent image acquisition was performed using MetaMorph 7.8 (Molecular Devices) and the MAC5000 controller (Ludl). The multiparameter images of the section of the cardiac lobe were combined into an image stack (*.stk file). The image stack contained positional metadata that allowed eventual reconstruction into an image montage. The image stack was processed using standard MetaMorph filters. The image stack was segmented into cell nuclei and cytoplasmic features using custom routines created with CellProfiler (Broad Institute, Cambridge, MA). Image parsing was used to manage memory overruns. The segmented images from CellProfiler were reconstructed into image montages of the cardiac lobe using FCS Express 5 software (De Novo Software, Los Angeles, CA). Using FCS Express 5 (De Novo), the high-expressing SMA cells on the dot plot were gated and the corresponding cells in the image montage were highlighted. This technique permitted quantitative and spatially localized single-cell immunohistochemistry analysis.
Precision-cut lung slices.
Agarose at 3% (wt/vol) or alginate (1% wt/vol) and gelatin (5% wt/vol) were thoroughly mixed and warmed to 37°C. The trachea was cannulated and the warm embedding medium was infused through the trachea using the lowest pressure necessary to inflate the peripheral lung. At total lung capacity, the trachea was clamped and the lung block placed in 34 mM calcium chloride solution (in deionized water reconstituted to isotonicity with NaCl) at 4°C for 30 min to allow for gelation. Sectioning was performed with the Leica VT1000 S vibrating blade microtome (Leica Biosystems, Nussloch, Germany) using stainless steel razor blades (Gillette, Boston, MA). The microtome was operated at the following adjustable settings: knife angle, 5–7°; sectioning speed, 0.05–0.2 mm/s; oscillation frequency, 80–100 Hz; and oscillation amplitude, 0.6 mm. Sections 200–300 µm thick were mounted on a polyethylene naphthalene membrane frame slide (Life Technologies) for laser microdissection.
Laser microdissection.
The Arcturus XT LCM System (Life Technologies) was used for all ultraviolet (UV) laser dissection of precision-cut lung slices. The UV laser was a specially adapted beta-test laser for wet tissue applications. The Arcturus XT software was used to target tissue for UV dissection. Peripheral alveolar ducts were identified in whole mounts as a minimum of six contiguous subpleural alveoli surrounding a central air space. Laser microdissection harvested alveolar ducts distal to the columnar-squamoid transition. The dissection included the contiguous pleura but excluded occasional large blood vessels (2). Dissected tissue was then placed into collagenase solution in a single well of a 96-well plate for enzymatic tissue dissociation.
Enzymatic digestion.
Enzymatic digestion of the lung reflected a previously published protocol (2). Briefly, 1 mg/ml collagenase Type IV (Worthington, Lakewood, NJ) and 0.01 mg/ml DNase I (Fisher Scientific, Pittsburgh, PA) in Dulbecco’s modified Eagle’s medium (Thermo Fisher Scientific, Waltham, MA) was used to dissociate the tissue. Dissociation was performed at 37°C under constant agitation for 30–45 min. The digest was filtered through 35-µm nylon mesh, and remaining debris was removed by centrifugation at 1,200 rpm for 3 min. The process of microfiltration and centrifugation was then repeated once more in preparation for microfluidic analysis. Of note, the efficiency of enzymatic digestion varied by postoperative day, suggesting differences in the extracellular matrix. Acta2+ cell capture frequency ranged from 4 to 10%.
Viability assessment.
A small aliquot of cell suspension (7.5–10 µl) was used to assess cell concentration and viability by Trypan blue exclusion. Trypan blue (Sigma-Aldrich) was added in 1:1 ratio and cell concentration was determined using a standard microscope hemocytometer. Each cell counted was determined to be alive or dead based on Trypan blue exclusion and viability was calculated as a percentage of total cells.
C1-specific target amplification.
Single mouse lung cell capture and STA (specific target amplification) were carried out using the Fluidigm C1 Single-Cell Auto Prep System and Single-Cell Auto Prep Array integrated fluidic circuits (IFCs) (Fluidigm, South San Francisco, CA). For these experiments, medium-sized (10–17 µm cell diameter) STA IFCs were used. Chip-priming, cell-loading, lysis, reverse transcription, and preamplification were performed in accordance with Fluidigm’s recommended protocol using lysis and preamplification reagents from the Single Cell-to-CT Kit (Ambion/Life Technologies) and pooled preamplification primers custom designed to enrich for 96 loci of interest (outer primer sequences provided in the Supplemental Material; Supplemental Material for this article is available online at the Journal website). Cells were loaded onto the chip at concentrations ranging from 120 to 370 cells/µl and captured cells were imaged using an Olympus IX71 microscope to assess cell number and quality. Fluidigm’s standard STA script was modified using C1 Script Builder (Fluidigm) so that the total capture volume was increased fourfold.
Single-cell multiplexed quantitative PCR.
Preamplified cDNA samples from single cells were analyzed by qPCR using 96.96 Dynamic Array IFCs and the Biomark HD System from Fluidigm. Processing of the IFCs and operation of the instruments were performed according to the manufacturer’s procedures. A Master Mix was prepared consisting of 420 µl SsoFast EvaGreen Supermix with Low ROX (Bio-Rad 172-5211), 42 µl 20 × DNA Binding Dye Sample Loading Reagent (Fluidigm 100-5360), plus 18 µl H2O, and 4 µl of this mix was dispensed to each well of a 96-well assay plate. Three microliters of preamplified cDNA sample were added to each well and the plate was briefly vortexed and centrifuged. Following priming of the IFC in the IFC Controller HX, 5 µl of the cDNA sample and Master Mix were dispensed to each sample inlet of the 96.96 IFC. Then, 4.5 µl of each 10 × assay (5 µM each primer, inner primer sequences in Supplementary Material) were dispensed to each detector inlet of the 96.96 IFC. After loading the assays and samples into the IFC in the IFC controller HX, the IFC was transferred to the Biomark HD system and PCR was performed using the thermal protocol GE Fast 96×96 PCR+Melt v2.pcl. This protocol consists of a thermal mix of 70°C, 40 min; 60°C, 30 s, hot start at 95°C, 1 min, PCR cycle of 30 cycles of (96°C, 5 s; 60°C, 20 s), and melting using a ramp from 60°C to 95°C at 1°C/3 s.
Data analysis and graphical display.
Data was analyzed with Fluidigm Real-Time PCR Analysis software using the linear (derivative) baseline correction method and the auto (global) Ct threshold method. The Cq values determined were exported to Excel for further processing using Singular Analysis Toolset (Fluidigm) in the R software environment. Principal component analysis and hierarchical clustering by Euclidean distance were performed for all cells. qPCR experiments were performed for control (4 biological replicates), day 1 (3 biological replicates), day 3 (5 biological replicates), and day 7 (4 biological replicates) time points. The single-cell data was converted into an FCS file standard (7) using available conversion software (GenePattern, Broad Institute). The FCS file was subsequently analyzed using hierarchical and combination gates using the FCS Express 5 (De Novo) and Cytobank (https://www.cytobank.org/) software. Statistical analyses were based on measurements in at least three different mice. The unpaired Student’s t-test for samples of unequal variances was used to calculate statistical significance. The data was expressed as mean ± one standard deviation. The significance level for the sample distribution was defined as P < 0.05.
RESULTS
α-Smooth muscle actin (SMA) expression in the lung.
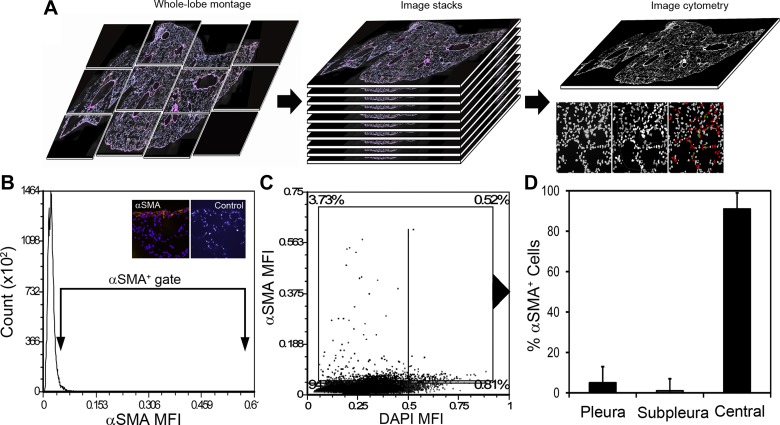
To identify resident myofibroblasts, we performed SMA immunohistochemistry on nonsurgical murine cardiac lobes. The immunostained lobes were imaged and whole-lobe montage image stacks were created (Fig. 1A). Control tissue sections, treated with purified rabbit IgG or secondary antibody alone, were used to define SMA positivity (Fig. 1B). As expected, SMA staining was prominent in the central lobar regions reflecting perivascular and peribronchial smooth muscle cells as well as a smaller population of central myofibroblasts. Gating in the subpleural regions, where septal remodeling occurs, demonstrated that less than 2% of the cells were SMA+; less than 1% of the SMA+ cells were localized to alveolar ducts (Fig. 1, C and D).
Fig. 1.
α-SMA+ cell distribution in the murine lung. A: tissue sections of the murine lung were stained with a nuclear intercalation dye (Hoechst or DAPI) as well as primary anti-SMA rabbit polyclonal antibody followed by fluorescent anti-rabbit detection antibody. Whole-lobe montages were created of the cardiac lobe for subsequent segmentation and image cytometry. B: paired tissue samples, stained with the detection antibody alone or purified rabbit IgG, served as negative controls (B, inset). C: the cells were displayed on a dot plot reflecting SMA and DAPI mean fluorescence intensity (MFI). Quadrant percentages are shown in the plot corners. D: the SMA+ cells were gated and the corresponding cells localized in the tissue image. Most of the SMA+ cells were localized to the central regions of the lung corresponding to large airways and blood vessels. Less than 5% localized to the pleura and less than 1% to subpleural regions of the lung. Areas of atelectasis or gross inflammation were excluded from the analysis. N = 7 mice; mean number of cells per montage = 1.53 × 105 cells.
Influence of mechanical forces on SMA expression.
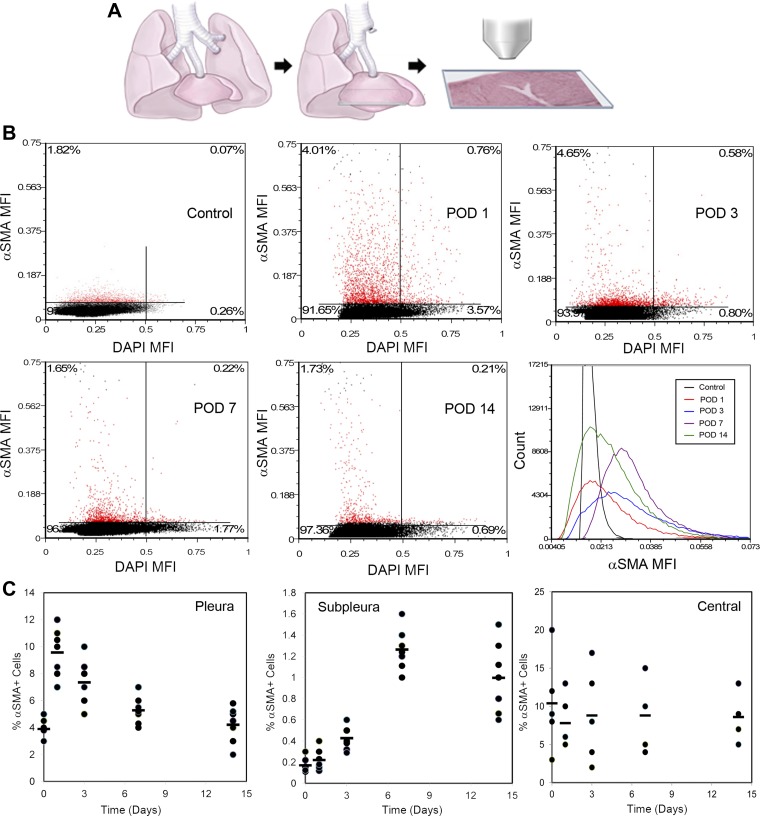
To investigate the influence of in vivo lung deformation on SMA expression, we examined the cardiac lobe for cells expressing SMA after pneumonectomy (Fig. 2A), sham thoracotomy (N = 5), pneumonectomy plus phrenic nerve transection (inhibition of cyclic stretch; N = 5), and pneumonectomy plus plombage (inhibition of static stretch; N = 5). Systematic whole-lobe immunohistochemical staining and image cytometry analyzed the pleural and subpleural regions of the cardiac lobe. Image cytometry demonstrated a significant rise in SMA expression within the first 3 days after pneumonectomy (Fig. 2B). The sham thoracotomy, phrenic nerve transection and plombage conditions were indistinguishable from nonsurgical controls; all three control conditions were significantly different from pneumonectomy alone (P < 0.001). In the pneumonectomy condition, image cytometry, modified by manual gating, permitted the localization of the enhanced SMA expression to the pleural and subpleural regions of the lung (Fig. 2C). The variability in the total number of central cells resulted in wide variability in the percentage central SMA+ cells. A frequency analysis of SMA+ cell location demonstrated a progressive increase in SMA+ cells in the subpleural regions of the lung (Fig. 3).
Fig. 2.
α-SMA expression in the cardiac lobe after murine pneumonectomy. A: in mice, a left pneumonectomy was performed and the remaining cardiac lobe was examined on postoperative days (POD) 1, 3, 7, and 14. B: a representative sequence of SMA expression in the pleura and subpleural region is shown. A significant increase in SMA+ cells was seen on POD 1 and 3 with subsequent decline on POD 7 and 14. Percentages in the upper quadrants reflect the percentage of SMA+ cells; the right upper quadrant reflects cells with enhanced SMA expression and increased DNA content. To demonstrate the variability in SMA expression, the histogram shows representative SMA expression in individual mice at different time points after pneumonectomy. C: using image cytometry, SMA+ cells were gated and localized to the corresponding segmented image. The initial rise in SMA+ cells was identified in the pleura with subsequent increase in SMA+ cells within the subpleural regions. To illustrate the variability and trends of SMA expression, the percent of SMA+ cells is shown for the pleura, subpleura, and central regions of the cardiac lobe (N = 5–7; bar = aggregate mean). The difference between pleura and subpleural SMA+ cells was highly significant on POD 1, 3, and 7 (P < 0.001). The variability in the total number of central cells resulted in wide variability in the percentage of central SMA+ cells.
Fig. 3.
Whole-lobe montages, segmented and analyzed by image cytometry, are shown for the cardiac lobe in a nonsurgical control and postpneumonectomy days 3, 7, and 14. A: a representative section of the midcardiac lobe is shown. B: regions of the pleura and subpleural regions are magnified. C: for each cardiac lobe, the frequency of SMA+ cells was plotted as a function of the distance from the pleura; the modal distribution of N = 5 animals is shown for Control, POD 3, and POD 7 time points; N = 3 for POD 14. Pleural SMA+ cells increased on day 3 with progressive increase in SMA+ cells in the subpleural and central regions. Note the increase in the size of the cardiac lobe, reflecting compensatory growth, on day 14. Bar = 200 µm.
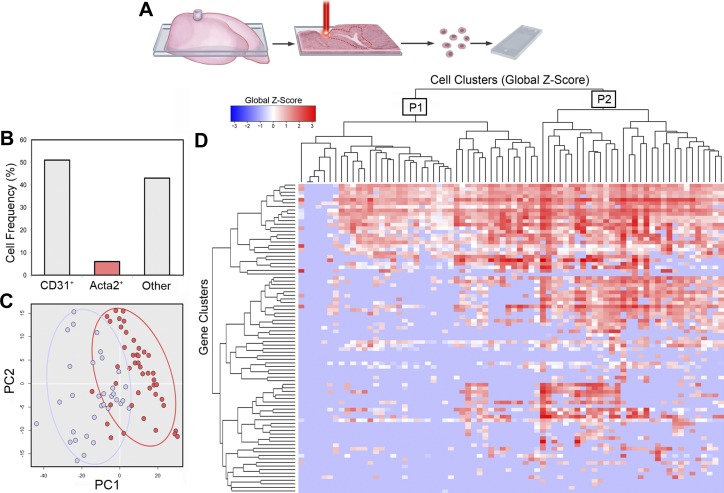
Transcriptional profile of subpleural cells.
The functional role of the SMA+ cells appearing in the subpleural region after pneumonectomy was explored using single-cell analysis. Laser microdissection of subpleural alveolar ducts facilitated single-cell isolation by microfluidics (C1 System, Fluidigm) (Fig. 4A). Of the 2,211 cells analyzed by microfluidics and qPCR, 1,107 passed rigorous criteria for single cell analysis. Consistent with image cytometry, 4% of control samples were positive for the SMA gene Acta2; a mean of 6.9% of the cells on postpneumonectomy days 1, 3, and 7 were Acta2+ (range 4–10% of captured cells). Because fewer than 3% of the cells in the phrenic nerve and plombage controls were positive for Acta2, these conditions were excluded from further analysis.
Fig. 4.
Single-cell qPCR analysis of Acta2+ cells from the cardiac lobe on POD 1, POD 3, and POD 7 after left pneumonectomy. A: laser microdissection isolated subpleural “hot spots” associated with SMA staining and lung regeneration (2). B: after laser microdissection, single cells were isolated by microfluidics and qPCR transcriptional analysis was performed (see methods). Representative isolation of a population of known prevalence was demonstrated by CD31 surface expression and Pecam1 gene expression (4); 30% were CD31+/Pecam1+ and 4% of total cells were Acta2+. Principal component analysis (PC) (C) and hierarchical clustering (D) identified two major populations of Acta2+ cells; a lower transcriptional activity population (designated P1) and a higher transcriptional activity population (designated P2).
The postpneumonectomy Acta2+ cells demonstrated two distinctive expression profiles by principal component analysis and hierarchical clustering. Population 1 (designated P1, Fig. 4C) was relatively less transcriptionally active but demonstrated a similar profile to Population 2 (P2, Fig. 4C). There was no statistical difference in transcriptional activity of the two Acta2+ cell populations between postoperative days 1, 3, and 7 (P > 0.05). The distinctive transcriptional profile of P1 and P2 was demonstrated by a comparison with the Acta2− cells in Population 3 (P3) (Fig. 5).
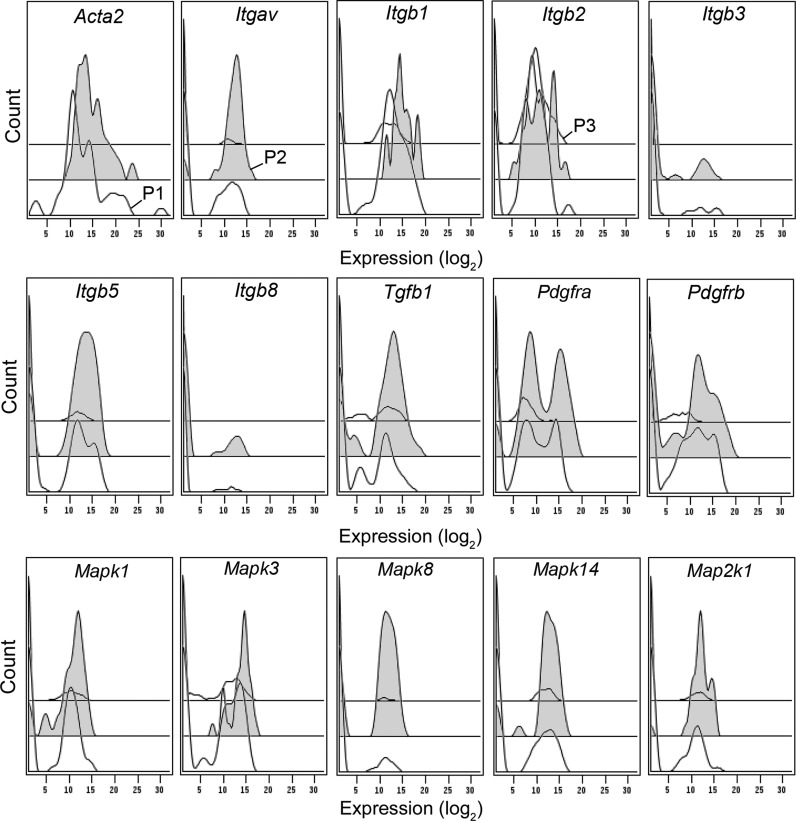
Fig. 5.
Tiered gene expression histograms of the transcriptional profile of cells isolated from alveolar ducts. Because of the transcriptional profile statistical identity on POD 1, POD 3, and POD 7, aggregate data is shown. The high transcriptional activity population (designated P2 and colored gray) is bracketed by the low transcriptional activity population (P1) and all Acta2− cells (P3). The majority of the Acta2+ cells in P2 coexpressed Itgav, Itgb1, and Itgb2. As expected, the P2 cells expressed high levels of Tgfb signaling molecules. Notably, Mapk expression was significantly higher in P2 than P1.
Most Acta2+ cells were also Itgav+ and Itgb1+ (61%). Gating hierarchies demonstrated that of the Itgav+ cells, 100% were Itgav+/Itgb1+, 95% were Itgav+/Itgb1+/Itgb2+, and 84% were Itgav+/Itgb1+/Itgb2+/Itgb5+. The broad gene coexpression of the integrin β-subunits was unexpected, particularly the β2-subunit commonly associated with leukocyte expression. As expected, the Acta2+ myofibroblasts expressed Tgfb1 (as well as intracellular signaling molecules Smad4 and Smad2), Notable was Pdgfra gene expression: 46% of all cells and 76% of Acta2+ cells in the remodeling alveolar duct expressed the Pdgfra gene (Fig. 5). The most prominent gene expression pathway was the mitogen-activated protein kinase (Mapk) expression in P2. Mapk expression effectively discriminated P1 and P2; similarly, the Acta2− cells (P3) demonstrated a much lower number of cells with detectable Mapk expression. Mapk1, Mapk3, Mapk8, Mapk14, and Map2k1 were coexpressed in 35% of Acta2+ cells.
Remodeling profile of the Acta2+ cells.
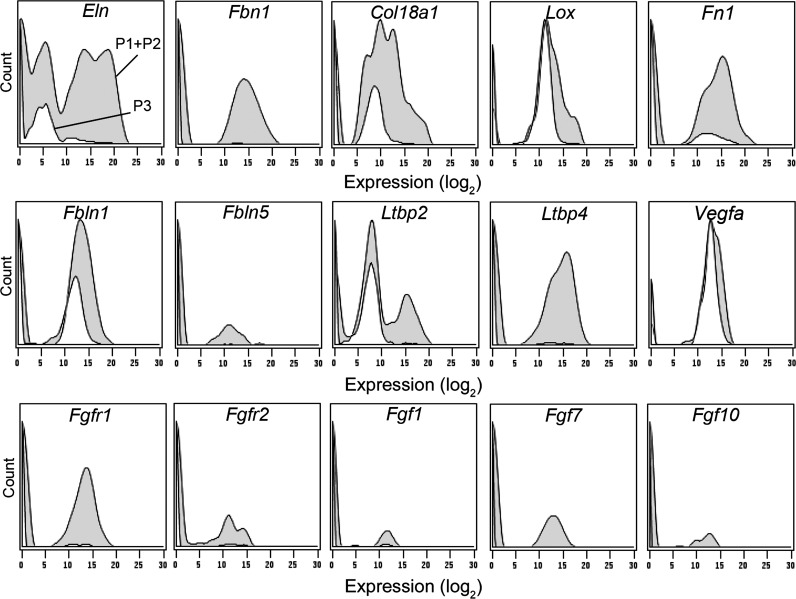
Since the Acta2+ cells were harvested from regenerative “hot spots,” we examined the cells for gene expression related to lung growth (Fig. 6). Nearly all Acta2+ cells coexpressed notable levels of the genes associated with lung remodeling: elastin (80%, Eln), the elastin scaffold protein fibrillin-1 (55%, Fbn1), collagen (83%, Col18a1), and fibronectin (64%, Fn1). The cross-linking enzyme lysyl oxidase (Lox) was prominently expressed in both Acta2+ and Acta2− cells.
Fig. 6.
Gene expression histograms of the transcriptional profile of cells isolated from alveolar ducts. Because of the transcriptional profile statistical identity on POD 1, POD 3 and POD 7, aggregate data is shown. All Acta2+ cells (P1+, P2+, gray) were compared with all Acta2− cells (P3, white). The Acta2+ cells consistently demonstrated expression of lung-related matrix genes (Eln, Fbn1, Col18a1, Lox, Fn1, Fbln1, Ltbp2, and Ltbp4). The Fgf gene family, commonly associated with lung development, demonstrated variable expression.
More specialized genes associated with lung remodeling were also expressed in the Acta2+ cells. Fibulins (Fbln) and latent transforming growth factor beta binding proteins (Ltbp) were prominently expressed in Acta2+ cells. Of note, 23% of the Acta2+ cells coexpressed Fbln1, Fbln5, Ltbp2, and Ltbp4. Other lung remodeling genes, such as those involved in angiogenesis (e.g., Vegfa, Epha2, Ephb4, and Eng), demonstrated significantly higher expression in the Acta2+ population than Acta2− cells (P < 0.001). Finally, the fibroblast growth factor (Fgf) gene family demonstrated variable expression; no clear pattern of Fgf coexpression was found (Fig. 6).
DISCUSSION
In this report, an in vivo analysis of myofibroblast population dynamics—defined by SMA+ protein expression or Acta2+ gene expression—produced four principal findings: 1) SMA+ cells were induced in the pleura by postpneumonectomy deformation. 2) The induction of SMA+ pleural cells preceded the accumulation of SMA+ cells in the subpleural alveolar ducts. 3) Cells in the subpleural alveolar ducts expressing the SMA gene (Acta2) demonstrated a broad and highly active transcriptional profile. 4) Cells expressing the SMA gene (Acta2) also demonstrated a transcriptional profile specific for lung repair and regeneration. We conclude that SMA+/Acta2+ cells induced in the peripheral lung contribute to postpneumonectomy lung growth.
A major contribution of this work is the in vivo characterization of a low-frequency and spatially distributed cell type during the process of lung remodeling and regeneration. Myofibroblasts, commonly defined as demonstrating fibroblast morphology, expressing cytoplasmic α-smooth muscle actin, and producing matrix components (17), are rare in tissues. The low-frequency and “enigmatic” phenotype of myofibroblasts (27) has led to acknowledged controversy regarding myofibroblast function (18). Here, we empirically defined our target cell population as expressing the SMA protein or the Acta2 gene. Optical tissue imaging and quantitative cytometry of SMA+ cells demonstrated the dynamic transitions of this cell-type—from the induction of SMA expression in pleural cells to their increased frequency in the region of regenerating alveolar ducts. Single-cell isolation and qPCR suggested the potential for these SMA+/Acta2+ cells to contribute to lung remodeling.
The pleural induction of SMA+ cells and the subsequent appearance of SMA+ cells in the subpleural region of the lung suggests a process of centripetal migration reminiscent of unipolar ingression during early development (31). Despite the suggestive temporal sequence, the pleural and subpleural cells may not be related. The appearance of SMA+ cells in the subpleural lung could be the result of 1) the local transition of resident fibroblasts into myofibroblasts (5), 2) the selective migration of blood-borne myofibroblasts into the peripheral lung, or 3) the centrifugal migration of central myofibroblasts into the peripheral lung. Our data suggests that tracking cell migration will be useful in discriminating these possibilities.
After murine pneumonectomy, the cardiac lobe is displaced into the empty hemithorax (13). Displacement of the cardiac lobe is associated with “static” deformation (12) and “cyclic” stretch (11). An intriguing observation in a growing number of studies is that preventing cardiac lobe displacement (plombage) (6, 19) or inhibiting cyclic stretch (phrenic nerve transection) (41) blocks lung growth (34, 41). Despite the common element of deformation or “stretch,” a mechanistic cellular explanation for these observations has remained elusive. Here, we show that pneumonectomy is associated with rapid (24 h) transition of the pleural mesothelium into SMA+ cells. Consistent with contemporary understanding of epithelial-mesenchymal transition (EMT) (34) (alternatively, mesothelial-mesenchymal transition), the transition of mesothelial cells into SMA+ cells required both static and cyclic stretch since both plombage and phrenic nerve transection blocked this transition. Also consistent with EMT, the SMA+ cells appeared to acquire migratory behavior (25). Within 3 days, SMA+ cells were detected in subpleural alveolar ducts.
Within the Acta2+ population, hierarchical clustering identified two populations (P1 and P2) of Acta2-positive cells. Although it is tempting to ascribe the clustering data to distinct cell populations, the single-cell transcription data suggests otherwise. Interestingly, both the P1 and P2 populations expressed similar transcriptional profiles. In addition, both populations demonstrated comparable transcriptional levels in those genes being expressed (e.g., Pdgfra). The distinction between the P1 and P2 populations was profile variability; that is, the cells in P2 expressed most, but not all, of the profile genes expressed by P1. Furthermore, there was variability in the genes that were not expressed in P2. These results suggest that both P1 and P2 were regulated by a comparable gene-regulatory program, but that the expression of individual genes within that program varied because of the network of gene regulatory interactions (38)—perhaps interactions that depend on cell context (e.g., cell-substratum adhesion) or intracellular processes (e.g., burst transcription, cell cycle-dependence).
An important observation of the single-cell qPCR analysis was the broad transcriptional activity of the Acta2+ cells. In addition to the production of matrix components commonly associated with myofibroblasts (e.g., elastin and collagen), the Acta2+ cells transcribed a variety of growth factors and cell surface receptors. PDGFRα, a receptor frequently implicated in lung repair (5), demonstrated high and low transcriptional levels consistent with prior in vivo observations (15). Similarly, the Acta2+ cells were found to express numerous genes associated with angiogenesis (e.g., Vegfa). Given this diverse transcriptional activity, we have revised our conception of “myofibroblast” to include a broader role in regeneration and repair.
The most convincing evidence that Acta2+ cells contribute to lung remodeling was the transcription of elastin (Eln) and the scaffold protein fibrillin-1 (Fbn1). Elastin is not only a fundamental structural protein in the lung parenchyma, but elastin, collagen, and fibrillin microfibrils constitute the core of the cable line element that supports the lung (36). In development, the cable line element “lifts” the septa that form alveolar walls. Analogously, postpneumonectomy neoalveolarization is associated with remodeling of the cable line element (20, 37, 42)—possibly due to stretch-dependent elastase activity demonstrated in the postpneumonectomy cardiac lobe (40). Despite the overall slow turnover of elastin in the adult human (30) and rodent (9, 28) lung, we speculate that the high levels of elastin transcription represent a selective and targeted contribution to both septal and line element remodeling.
Single-cell qPCR allowed us to avoid “Simpson’s paradox” (32); that is, the statistical observation that findings attributable to a particular subset (e.g., myofibroblasts) may diminish or even reverse when subset data is combined (e.g., bulk transcriptional analysis). In addition to these statistical benefits, single-cell qPCR allowed us to determine gene coexpression. When analyzed by combinatorial gating, our single-cell data demonstrated the coexpression of multiple integrin β subunits and Mapk genes. Gene coexpression suggests that these coexpressed genes are both functionally related and controlled by the same transcriptional regulatory program. In conventional gene coexpression networks, coexpression is inferred by correlation or dependency (33). Gene coexpression networks depend on large data sets—data sets in which low-frequency cell populations may be underrepresented. Complementing the “big data” approach, the single-cell approach uses actual gene coexpression within individual cells, harvested within a defined functional context, to infer a common transcriptional regulatory program. We anticipate that this approach will be usefully applied to other low-frequency populations in future studies.
GRANTS
This research was supported in part by National Institutes of Health Grants HL94567, CA009535, and ES000002, and by SCGI, Broad Institute.
DISCLOSURES
K. J. Livak and S. Li are employees of the Fluidigm Corporation; the other authors declare no competing financial interests.
AUTHOR CONTRIBUTIONS
R.D.B., A.B.Y., W.L.W., C.D.V., and P.P. performed experiments; R.D.B., A.B.Y., W.L.W., C.D.V., A.T., S.P., S.L., J.G., P.P., K.J.L., M.A., P.B., and S.J.M. analyzed data; R.D.B., A.B.Y., W.L.W., C.D.V., A.T., S.P., S.L., J.G., K.J.L., M.A., P.B., and S.J.M. interpreted results of experiments; R.D.B., S.L., J.G., and M.A. prepared figures; R.D.B. and A.T. drafted manuscript; R.D.B., W.L.W., C.D.V., A.T., S.P., S.L., J.G., P.P., K.J.L., M.A., P.B., and S.J.M. edited and revised manuscript; R.D.B., A.B.Y., W.L.W., C.D.V., A.T., S.P., S.L., J.G., P.P., K.J.L., M.A., P.B., and S.J.M. approved final version of manuscript.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Arne Kienzle for assistance with computational analysis.
REFERENCES
- 1.Adzick NS, Harrison MR, Glick PL, Villa RL, Finkbeiner W. Experimental pulmonary hypoplasia and oligohydramnios: relative contributions of lung fluid and fetal breathing movements. J Pediatr Surg 19: 658–665, 1984. doi: 10.1016/S0022-3468(84)80349-8. [DOI] [PubMed] [Google Scholar]
- 2.Bennett RD, Ysasi AB, Belle JM, Wagner WL, Konerding MA, Blainey PC, Pyne S, Mentzer SJ. Laser microdissection of the alveolar duct enables single-cell genomic analysis. Front Oncol 4: 260, 2014. doi: 10.3389/fonc.2014.00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Butler JP, Loring SH, Patz S, Tsuda A, Yablonskiy DA, Mentzer SJ. Evidence for adult lung growth in humans. N Engl J Med 367: 244–247, 2012. doi: 10.1056/NEJMoa1203983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chamoto K, Gibney BC, Lee GS, Lin M, Collings-Simpson D, Voswinckel R, Konerding MA, Tsuda A, Mentzer SJ. CD34+ progenitor to endothelial cell transition in post-pneumonectomy angiogenesis. Am J Respir Cell Mol Biol 46: 283–289, 2012. doi: 10.1165/rcmb.2011-0249OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen L, Acciani T, Le Cras T, Lutzko C, Perl AKT. Dynamic regulation of platelet-derived growth factor receptor α expression in alveolar fibroblasts during realveolarization. Am J Respir Cell Mol Biol 47: 517–527, 2012. doi: 10.1165/rcmb.2012-0030OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cowan MJ, Crystal RG. Lung growth after unilateral pneumonectomy: quantitation of collagen synthesis and content. Am Rev Respir Dis 111: 267–277, 1975. [DOI] [PubMed] [Google Scholar]
- 7.Dean PN, Bagwell CB, Lindmo T, Murphy RF, Salzman GC. Introduction to flow cytometry data file standard. Cytometry 11: 321–322, 1990. doi: 10.1002/cyto.990110302. [DOI] [PubMed] [Google Scholar]
- 8.Dickie R, Wang YT, Butler JP, Schulz H, Tsuda A. Distribution and quantity of contractile tissue in postnatal development of rat alveolar interstitium. Anat Rec (Hoboken) 291: 83–93, 2008. doi: 10.1002/ar.20622. [DOI] [PubMed] [Google Scholar]
- 9.Dubick MA, Rucker RB, Cross CE, Last JA. Elastin metabolism in rodent lung. Biochim Biophys Acta 672: 303–306, 1981. doi: 10.1016/0304-4165(81)90297-X. [DOI] [PubMed] [Google Scholar]
- 10.Fehrenbach H, Voswinckel R, Michl V, Mehling T, Fehrenbach A, Seeger W, Nyengaard JR. Neoalveolarisation contributes to compensatory lung growth following pneumonectomy in mice. Eur Respir J 31: 515–522, 2008. doi: 10.1183/09031936.00109407. [DOI] [PubMed] [Google Scholar]
- 11.Filipovic N, Gibney BC, Kojic M, Nikolic D, Isailovic V, Ysasi A, Konerding MA, Mentzer SJ, Tsuda A. Mapping cyclic stretch in the postpneumonectomy murine lung. J Appl Physiol (1985) 115: 1370–1378, 2013. doi: 10.1152/japplphysiol.00635.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Filipovic N, Gibney BC, Nikolic D, Konerding MA, Mentzer SJ, Tsuda A. Computational analysis of lung deformation after murine pneumonectomy. Comput Methods Biomech Biomed Engin 17: 838–844, 2014. doi: 10.1080/10255842.2012.719606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gibney BC, Houdek JP, Chamoto K, Lee GS, Ackermann M, Lin M, Collings-Simpson D, Konerding MA, Tsuda A, Mentzer SJ. Mechanostructural adaptations preceding postpneumonectomy lung growth. Exp Lung Res 38: 396–405, 2012. doi: 10.3109/01902148.2012.715364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gibney BC, Lee GS, Houdek JP, Lin M, Miele LF, Chamoto K, Konerding MA, Tsuda A, Mentzer SJ. Dynamic determination of oxygenation and lung compliance in murine pneumonectomy. Exp Lung Res 37: 301–309, 2011. doi: 10.3109/01902148.2011.561399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Green J, Endale M, Auer H, Perl AKT. Diversity of interstitial lung fibroblasts is regulated by platelet-derived growth factor receptor α kinase activity. Am J Respir Cell Mol Biol 54: 532–545, 2016. doi: 10.1165/rcmb.2015-0095OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harding R, Hooper SB. Regulation of lung expansion and lung growth before birth. J Appl Physiol (1985) 81: 209–224, 1996. [DOI] [PubMed] [Google Scholar]
- 17.Hinz B, Phan SH, Thannickal VJ, Galli A, Bochaton-Piallat ML, Gabbiani G. The myofibroblast: one function, multiple origins. Am J Pathol 170: 1807–1816, 2007. doi: 10.2353/ajpath.2007.070112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hinz B, Phan SH, Thannickal VJ, Prunotto M, Desmoulière A, Varga J, De Wever O, Mareel M, Gabbiani G. Recent developments in myofibroblast biology: paradigms for connective tissue remodeling. Am J Pathol 180: 1340–1355, 2012. doi: 10.1016/j.ajpath.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoffman AM, Shifren A, Mazan MR, Gruntman AM, Lascola KM, Nolen-Walston RD, Kim CF, Tsai L, Pierce RA, Mecham RP, Ingenito EP. Matrix modulation of compensatory lung regrowth and progenitor cell proliferation in mice. Am J Physiol Lung Cell Mol Physiol 298: L158–L168, 2010. doi: 10.1152/ajplung.90594.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsia CCW, Berberich MA, Driscoll B, Laubach VE, Lillehei CW, Massaro C, Perkett EA, Pierce RA, Rannels DE, Ryan RM, Tepper RS, Townsley MI, Veness-Meehan KA, Wang N, Warburton D; ad hoc Statement Committee, American Thoracic Society . Mechanisms and limits of induced postnatal lung growth. Am J Respir Crit Care Med 170: 319–343, 2004. doi: 10.1164/rccm.200209-1062ST. [DOI] [PubMed] [Google Scholar]
- 21.Kapanci Y, Burgan S, Pietra GG, Conne B, Gabbiani G. Modulation of actin isoform expression in alveolar myofibroblasts (contractile interstitial cells) during pulmonary hypertension. Am J Pathol 136: 881–889, 1990. [PMC free article] [PubMed] [Google Scholar]
- 22.Kitagawa M, Hislop A, Boyden EA, Reid L. Lung hypoplasia in congenital diaphragmatic hernia. A quantitative study of airway, artery, and alveolar development. Br J Surg 58: 342–346, 1971. doi: 10.1002/bjs.1800580507. [DOI] [PubMed] [Google Scholar]
- 23.Konerding MA, Gibney BC, Houdek JP, Chamoto K, Ackermann M, Lee GS, Lin M, Tsuda A, Mentzer SJ. Spatial dependence of alveolar angiogenesis in post-pneumonectomy lung growth. Angiogenesis 15: 23–32, 2012. doi: 10.1007/s10456-011-9236-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mund SI, Stampanoni M, Schittny JC. Developmental alveolarization of the mouse lung. Dev Dyn 237: 2108–2116, 2008. doi: 10.1002/dvdy.21633. [DOI] [PubMed] [Google Scholar]
- 25.Nieto MA. Epithelial plasticity: a common theme in embryonic and cancer cells. Science 342: 1234850, 2013. doi: 10.1126/science.1234850. [DOI] [PubMed] [Google Scholar]
- 26.Noble PW, Barkauskas CE, Jiang D. Pulmonary fibrosis: patterns and perpetrators. J Clin Invest 122: 2756–2762, 2012. doi: 10.1172/JCI60323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Orenstein JM. The “myofibroblast” that is omnipresent in pathology and key to the EMT concepts does not actually exist, since normal fibroblasts contain stress fibril organelles (SMA bundles with dense bodies) variably detected by TEM and IHC: conclusions by a diagnostic pathologist with decades of ultrastructural experience. Ultrastruct Pathol 38: 387–398, 2014. doi: 10.3109/01913123.2014.940231. [DOI] [PubMed] [Google Scholar]
- 28.Pierce JA, Resnick H, Henry PH. Collagen and elastin metabolism in the lungs, skin, and bones of adult rats. J Lab Clin Med 69: 485–493, 1967. [PubMed] [Google Scholar]
- 29.Pierce RA, Albertine KH, Starcher BC, Bohnsack JF, Carlton DP, Bland RD. Chronic lung injury in preterm lambs: disordered pulmonary elastin deposition. Am J Physiol Lung Cell Mol Physiol 272: L452–L460, 1997. [DOI] [PubMed] [Google Scholar]
- 30.Shapiro SD, Endicott SK, Province MA, Pierce JA, Campbell EJ. Marked longevity of human lung parenchymal elastic fibers deduced from prevalence of D-aspartate and nuclear weapons-related radiocarbon. J Clin Invest 87: 1828–1834, 1991. doi: 10.1172/JCI115204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shook D, Keller R. Mechanisms, mechanics and function of epithelial-mesenchymal transitions in early development. Mech Dev 120: 1351–1383, 2003. doi: 10.1016/j.mod.2003.06.005. [DOI] [PubMed] [Google Scholar]
- 32.Simpson EH. The interpretation of interaction in contingency tables. J R Stat Soc Series B Stat Methodol 13: 238–241, 1951. [Google Scholar]
- 33.Stuart JM, Segal E, Koller D, Kim SK. A gene-coexpression network for global discovery of conserved genetic modules. Science 302: 249–255, 2003. doi: 10.1126/science.1087447. [DOI] [PubMed] [Google Scholar]
- 34.Thiery JP, Acloque H, Huang RYJ, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell 139: 871–890, 2009. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 35.Voswinckel R, Motejl V, Fehrenbach A, Wegmann M, Mehling T, Fehrenbach H, Seeger W. Characterisation of post-pneumonectomy lung growth in adult mice. Eur Respir J 24: 524–532, 2004. doi: 10.1183/09031936.04.10004904. [DOI] [PubMed] [Google Scholar]
- 36.Wagner W, Bennett RD, Ackermann M, Ysasi AB, Belle J, Valenzuela CD, Pabst A, Tsuda A, Konerding MA, Mentzer SJ. Elastin cables define the axial connective tissue system in the murine lung. Anat Rec (Hoboken) 298: 1960–1968, 2015. doi: 10.1002/ar.23259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weibel ER. How to make an alveolus. Eur Respir J 31: 483–485, 2008. doi: 10.1183/09031936.00003308. [DOI] [PubMed] [Google Scholar]
- 38.Wills QF, Livak KJ, Tipping AJ, Enver T, Goldson AJ, Sexton DW, Holmes C. Single-cell gene expression analysis reveals genetic associations masked in whole-tissue experiments. Nat Biotechnol 31: 748–752, 2013. doi: 10.1038/nbt.2642. [DOI] [PubMed] [Google Scholar]
- 39.Yamada T, Suzuki E, Gejyo F, Ushiki T. Developmental changes in the structure of the rat fetal lung, with special reference to the airway smooth muscle and vasculature. Arch Histol Cytol 65: 55–69, 2002. doi: 10.1679/aohc.65.55. [DOI] [PubMed] [Google Scholar]
- 40.Young SM, Liu S, Joshi R, Batie MR, Kofron M, Guo J, Woods JC, Varisco BM. Localization and stretch-dependence of lung elastase activity in development and compensatory growth. J Appl Physiol (1985) 118: 921–931, 2015. doi: 10.1152/japplphysiol.00954.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ysasi AB, Belle JM, Gibney BC, Fedulov AV, Wagner W, Tsuda A, Konerding MA, Mentzer SJ. Effect of unilateral diaphragmatic paralysis on post-pneumonectomy lung growth. Am J Physiol Lung Cell Mol Physiol 305: L439–L445, 2013. doi: 10.1152/ajplung.00134.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ysasi AB, Wagner W, Bennett RD, Ackermann M, Belle JM, Valenzuela CD, Pabst AM, Tsuda A, Konerding MA, Mentzer SJ. Remodeling of alveolar septa in post-pneumonectomy lung growth. Am J Physiol Lung Cell Mol Physiol 308: L1237–L1244, 2015. doi: 10.1152/ajplung.00042.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.