Abstract
Small airway fibrosis is a major pathological feature of chronic obstructive pulmonary disease (COPD) and is refractory to current treatments. Chronic inflammatory cells accumulate around small airways in COPD and are thought to play a major role in small airway fibrosis. Mice deficient in α/β T cells have recently been shown to be protected from both experimental airway inflammation and fibrosis. In these models, CD4+Th17 cells and secretion of IL-17A are increased. However, a pathogenic role for IL-17 in specifically mediating fibrosis around airways has not been demonstrated. Here a role for IL-17A in airway fibrosis was demonstrated using mice deficient in the IL-17 receptor A (il17ra). Il17ra-deficient mice were protected from both airway inflammation and fibrosis in two different models of airway fibrosis that employ COPD-relevant stimuli. In these models, CD4+ Th17 are a major source of IL-17A with other expressing cell types including γδ T cells, type 3 innate lymphoid cells, polymorphonuclear cells, and CD8+ T cells. Antibody neutralization of IL-17RA or IL-17A confirmed that IL-17A was the relevant pathogenic IL-17 isoform and IL-17RA was the relevant receptor in airway inflammation and fibrosis. These results demonstrate that the IL-17A/IL-17 RA axis is crucial to murine airway fibrosis. These findings suggest that IL-17 might be targeted to prevent the progression of airway fibrosis in COPD.
Keywords: fibrosis, inflammation, airway, interleukin-17, cigarette smoke
chronic obstructive pulmonary disease (COPD) is now the third leading cause of death in the United States (46). Peribronchiolar fibrosis surrounding the small airways contributes to the obstructive pathophysiology in COPD and is refractory to currently available therapies (29, 51). Mechanisms of airway fibrosis in COPD pathogenesis are incompletely understood.
Progress has recently been made in therapeutic development for idiopathic pulmonary fibrosis (36, 52). This progress has been facilitated by investigating mechanisms of fibrosis using preclinical models, largely based on employing bleomycin as a fibrogenic agent. Similarly, an understanding of mechanisms specifically driving airway fibrosis using mouse models that recapitulate key features of COPD may facilitate therapeutic development for COPD (4). Specific airway fibrosis models are needed due to the dramatic differences in pathological features between airway fibrosis and pulmonary fibrosis, which reflects their vastly different etiologies and pathophysiology (57, 67).
Cigarette smoke (CS) causes airway inflammation by inducing cellular injury and increasing susceptibility to respiratory pathogens, in particular viruses (49). CS and viruses together stimulate similar host danger responses, both leading to inflammasome activation and interleukin-1 (IL-1β) secretion by immune and airway epithelial cells (20, 34, 37, 43). Inflammation and fibrosis are inexorably linked, but how individual immune cell types, airway epithelial cells, and their secretory products contribute to airway fibrosis remains largely undefined. When delivered in supraphysiological (μg) quantities as a recombinant protein to the mouse lung, IL-1β causes pulmonary fibrosis (19, 65). When IL-1β is delivered to mouse airways by an adenoviral vector (Ad-IL-1β), expression is limited to airway epithelial cells and expression levels are in the physiological range seen in patients with acute exacerbations of COPD (8). Ad-IL-1β induces fibrosis in mice limited to the airways and shares similar pathological features to those seen in COPD patients (37). IL-1 signaling is involved in experimental CS-induced airway fibrosis and inflammation (13, 15). In mice exposed to CS in combination with a viral mimetic, polyinosinic:polycytidylic acid (PIC), airway fibrosis with similar features as seen in the Ad-IL-1β model occurs (45). In the of CS+PIC model, IL-1β levels peak at day 15–18 after exposure, close to the levels seen in the Ad-IL-1β model (45). This suggests that there are shared mechanistic features of airway fibrosis in the Ad-IL-1β and CS+PIC models. Indeed, both are dependent on the conversion of the latent to the active form of TGF-β (37, 45).
TGF-β1 is a multifunctional cytokine that is widely implicated in both pathological immunity and fibrosis. TGF-β plays a complex role in airway disease influencing multiple airway cell types: mesenchymal cells [(matrix deposition and fibrosis (5, 6, 37), smooth muscle reactivity (40), cytokine secretion (10)], airway epithelium [(differentiation (4), senescence (30), proliferation (16)], and immune cells [(dendritic cell recruitment (37), CD4+ Th17 differentiation (40)]. These proinflammatory and profibrotic effects of TGF-β in airway biology are integrated into a highly dynamic network that can potentially be targeted at multiple levels (48). At the most proximal level, TGF-β-dependent experimental airway fibrosis might be inhibited by inhibiting TGF-β activation, since TGF-β must be activated to function (48), or by neutralizing TGF-β itself (37). However, global targeting of TGF-β has shown evidence of toxicity in preclinical studies making other methods of targeting downstream effectors of TGF-β function potentially safer (2, 62).
TGF-β cooperates with other proinflammatory pathways to increase the recruitment or differentiation of immune cells that can potentially contribute to airway fibrosis. As one example, IL-1β and TGF-β conspire to mediate airway fibrosis (10, 37, 38). IL-1β and TGF-β are important in differentiation of CD4+ Th17 cells (3, 7). CD4+ Th17 cells by their secretion of IL-17A have been shown to mediate a number of pathological effects that could be indirectly involved in airway fibrosis including smooth muscle hypercontraction (40) and neutrophil recruitment (56, 63).
IL-1β and TGF-β together amplify innate and adaptive immune responses through mechanisms such as increasing the expression of the chemokine CCL20 from airway fibroblasts (10). CCL20 is critical for the recruitment and migration of dendritic cells (DCs), which express the chemokine receptor for CCL20, CCR6 (24). DCs are required for amplification of adaptive immune responses (23). A role for DCs and DC-mediated adaptive T cell immunity in murine airway fibrosis has recently been demonstrated (23). Using ccr6-deficient mice, a transgenic diphtheria toxin receptor dendritic cell ablation strategy, or T cell receptor α-deficient mice, it was determined that ccr6 expressing DCs and α/β T cells are required for IL-1β-mediated airway inflammation and fibrosis (23). The exact α/β T cell subsets involved in airway fibrosis in COPD remain to be fully elucidated, but in several murine CS exposure models CD4+ Th17 cells have been shown to be increased and involved in inflammatory cell recruitment, possibly through the increased expression of chemokines such as CCL2 and CCL20 (11, 45). In humans, a number of studies suggest the importance of CD4+ Th17 cells in COPD pathogenesis (14, 33, 54, 60). In addition, in CS-exposed mice other immune cell types might be important sources of IL-17 such as γδ T cells (9), polymorphonuclear cells (PMNs) (64), natural killer (NK) cells (55), CD8+ T cells (41), and type 3 innate lymphoid cells (ILC3) (35). While a role of IL-17 in COPD-related inflammation has been established both in humans (44) and in mice, the contribution of IL-17A in mediating airway fibrosis in response to COPD-relevant stimuli has yet to be elucidated.
As a first step in understanding the relevance of adaptive CD4+ Th17 cells in airway fibrosis, we elucidate the role that IL-17A plays in fibrotic airway pathology studying mice treated with COPD-relevant stimuli. We use two well-characterized airway disease systems that have airway fibrosis as a component either utilizing upper airway exposure to an adenoviral-IL-1β vector, which causes secretion of IL-1β by airway epithelial cells at levels similar as seen in hospitalized patients with COPD exacerbations (21, 37) or CS in combination with a viral mimetic, PIC (45). We report that IL-17A and its receptor IL-17RA play an essential role in mediating fibrosis in both of these murine airway fibrosis models.
MATERIALS AND METHODS
Mice.
All mice were bred and housed in specific pathogen–free housing under an IRB-approved protocol (IACUC AN098258) and in accordance with the guidelines of the Laboratory Animal Resource Center of the University of California, San Francisco (San Francisco, CA). il17ra−/− on a C57BL/6 background and C57BL/6 wild-type (WT) mice were obtained from Amgen (Thousand Oaks, CA) or the Jackson Laboratory (Bar Harbor, ME), respectively.
Antibodies and dosing.
Anti-mouse IL-17 Receptor A (IL-17RA) antibody (M751), anti-mouse IL-17A antibody (M210), and murine IgG1 control (4D2) were obtained from Amgen. Antibodies were formulated in PBS and administered intraperitoneally (IP) (250 μg/mouse in 200 μl).
Recombinant adenovirus.
The recombinant E1–E3 deleted type 5 adenovirus, either empty (Ad-C) or expressing human active IL-1β (Ad-IL-1β), has been described in detail elsewhere (38). The replication-deficient virus was commercially amplified and purified by cesium chloride gradient centrifugation and PD-10 Sephadex chromatography, plaque titered on 293 cells, and checked for WT contamination (ViraQuest, North Liberty, IA).
Intratracheal injections and antibody dosing protocol.
Mice were anesthetized with IP injection of Avertin (250 mg/kg IP). Then Ad-hIL-1β or Ad-LacZ (2.5 × 108 pfu in 75 μl sterile PBS) was instilled intratracheally with a needle (Popper 24G-1′ Straight 1.25-mm ball) using the direct visualized instillation technique (18). The control was Ad-LacZ. Antibodies were administered on day 1, 4, 8, 11, and 14.
CS and PIC exposure and antibody dosing protocol.
Mice were exposed using a whole body CS exposure system (Teague Enterprises, Woodland, CA) within a barrier facility. Mice are acclimated using increasing smoke exposures for 5 days starting at a TSP (total suspended particulates) of 40 mg/m3 for 2 h, and increasing incrementally to final smoke exposures of 100 TSP using 3R4F cigarettes. Full-dose exposures begin in week 1 with 5 h of continuous exposure/day, with rest on weekends. In week 2, intranasal doses of PIC (Invivogen, 50 μg/dose) are given on days 8 and 11, and again in week 3 on days 15, 18, and 21. Antibodies were administered on days 8, 11, 15, 18, and 21.
Morphometric analysis.
Airway morphometry was performed essentially as previously described (37). The right lung was removed and inflated in a syringe under −7.5 mb pressure for 1 min before fixation in 10% formalin for 24 h. Each of the four lobes of the right lung were bisected and paraffin embedded. Histological sections of were stained by hematoxylin and eosin, to assess airway inflammation, or by the Gomori Trichrome method to assess for fibrosis. Random digital images (n = 5) were taken using the ×4 objective from five of the eight sections from a blinded investigator. All airways in each section were assessed for the area of inflammatory cell infiltration or fibrosis surrounding airways and expressed as a fraction of airway basement membrane length, by a blinded investigator using digital imaging software (ImageJ). The number of airways examined/mouse always exceeded 12, the number of airways/mouse that we have shown to be needed to achieve a 95% confidence level (37).
Cell analysis.
Lung cell collection, staining, and gating were performed essentially as described (37). Live cells were stained without stimulation and cytokine capture assays for IL-17A and IFN-γ were performed using cytokine secretion assay kits, as per the manufacturer’s instructions (Miltenyi Biotec, Auburn, CA). For ILC3 staining, cells were fixed, permeabilized, and stained essentially as described (37). The antibodies in the two panels were as follows. Panel 1: B220 FITC, clone RA3-6B2 (Becton Dickinson, BD Biosciences, San Jose, CA); IL17a PE, Mouse IL-17 Secretion Assay-Detection Kit (Miltenyi Biotec, San Diego, CA); NK1.1 PerCP-Cy5.5, clone PK136 (Becton Dickinson, BD Biosciences); TCRγδ PECy7, clone eBioGL3 (eBioscience, San Diego, CA); TCRβ BV421, clone H57-597 (Becton Dickinson, BD Biosciences); CD8 Pacific Orange, clone 3B5 (Thermo Fisher Scientific, Invitrogen, Pleasanton, CA); GR1 650, clone RB6-8C5 (Biolegend, San Diego, CA); MHCII BV711, clone M5/114.15.2 (Becton Dickinson, BD Biosciences); IFNγ APC, Mouse IFN-γ Secretion Assay-Detection Kit (Miltenyi Biotec); CD45 Alexa Fluor 700, clone 30-F11 (Biolegend); CD4 APCCy7, clone GK1.5 (Becton Dickinson, BD Biosciences). Panel 2: IL33R/ST2 biotin, clone DJ8 (MDbiosciences, St. Paul, MN); Streptavidin FITC (eBioscience). IL17a PE, Mouse IL-17 Secretion Assay – Detection Kit (Miltenyi Biotec); TCRβ PerCP-Cy5.5, clone H57-597 (Affymetrix eBioscience, San Diego, CA); CD3ε PECy7, clone 145-2C11 (eBioscience); CD127 BV421, clone A7R34 (Biolegend); NK1.1 BV510, clone PK136 (Biolegend); CD90 BV605, clone 53-2.1 (Biolegend); GR1 650, clone RB6-8C5 (Biolegend); B220 BV711, clone RA3-6B2 (Biolegend); RORγt APC, clone B2D (Affymetrix, eBioscience); CD45 Alexa Fluor 700, clone 30-F11 (Biolegend); CD4 APCCy7, clone GK1.5 (Becton Dickinson, BD Biosciences).
Statistical analysis.
All data are reported as means ± SE. Comparisons between two different groups were determined by Student's t-test for parametric data or Mann-Whitney test for nonparametric data. One-way analysis of variance was used for multiple comparisons and Tukey's or Bonferroni’s post hoc tests used to test for statistical significance. Significance was defined as P < 0.05. Logistic regression analysis was performed using Stata (v12.1). All other statistical analyses were performed using the software package Prism 4.0b (GraphPad Software, San Diego, CA).
RESULTS
Absence of IL17RA protects against adenoviral IL-1β-induced airway inflammation and fibrosis.
Intratracheal delivery of adenoviral (Ad) vector expressing active human IL-1β induces robust airway inflammation and fibrosis, which is accompanied by increased numbers of CD4+ Th17 cells and increased expression of IL-17A (37). We first assessed the role of IL-17A in Ad-IL-1β-induced airway disease using il17ra−/− mice. WT mice exhibited increases in total cell, macrophage, and neutrophil counts in bronchoalveolar lavage (BAL) fluid 14 days after Ad-IL-1β treatment. These increases were not observed in il17ra−/− mice (Fig. 1, A–C). Morphometric analysis of airway inflammation revealed increased inflammatory cell localization around airways of WT mice treated with Ad-IL-1β compared with mice treated with control Ad-LacZ (Figs. 1D and 2, A and C). The Ad-IL-1β-dependent increase in airway inflammation was significantly reduced in il17ra−/− mice (Figs. 1D and 2, B and D). Airway fibrosis was assessed by Trichrome staining which stains fibrillar collagen (37). Morphometric analysis of airway fibrosis revealed significantly increased collagen staining around airways of WT mice treated with Ad-IL-1β compared with mice treated with control Ad-LacZ (Figs. 1E and 2E). The Ad-IL-1β-dependent increase in airway fibrosis was significantly reduced in il17ra−/− mice (Figs. 1E and 2F).
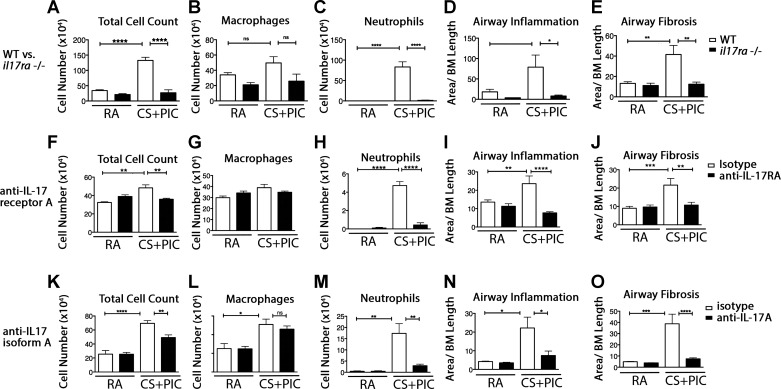
Fig. 1.
Lung and airway inflammation and airway wall fibrosis induced by Ad-IL-1β is dependent on IL-17RA/IL-17A. Wild-type (WT) or il17ra-deficient (il17ra−/−) mice were treated with intratracheal Ad-IL-1β or Ad-LacZ, as a control (A–E). After 14 days, bronchoalveolar lavage (BAL) was performed and total cell counts (A), macrophage (B), or neutrophil numbers (C) were assessed. Morphometric analysis of airway inflammation (D) or airway wall fibrosis (E) was performed from histological sections. WT mice treated with anti-IL-17RA (F–J), or anti-IL-17A (K–O) underwent the same BAL and morphometric analysis. Experiments were repeated a minimum of 3 times with at least 3 mice/group. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Fig. 2.
Airway wall inflammation and fibrosis induced by Ad-IL-1β is dependent on IL-17RA/IL-17A. WT (A, C, E) or il17ra−/− mice (B, D, F) were treated with intratracheal Ad-LacZ (A and B) or Ad-IL-1β (C–F). After 14 days, lungs were harvested and assessed histologically by hematoxylin and eosin (H&E, A–D) or Trichrome staining (E, F). WT mice were intratracheally injected with Ad-LacZ (G, H, M, N) or Ad-IL-1β (I–L, O–R) and treated with isotype control (G, I, K, M, O, Q), anti-IL-17RA (H, J, L) or anti-IL-17A (N, P, R). After 14 days airway inflammation and fibrosis were assessed by H&E (G–J, M–P) or Trichrome staining (K, L, Q, R). Experiments were repeated a minimum of 3 times with at least 3 mice/group. Bar = 100 μm.
Neutralization of IL-17RA protects against adenoviral IL-1β-induced airway inflammation and fibrosis.
Treatment with a neutralizing antibody to mouse IL-17RA significantly inhibited Ad-IL-1β induced increases in total cell and neutrophil counts in BAL fluid 14 days after Ad-IL-1β treatment (Fig. 1, F–H). Morphometric analysis of airway inflammation revealed that neutralizing IL-17RA significantly reduced the Ad-IL-1β -induced increase in inflammatory cells localized around airways of WT mice (Figs. 1I and 2, G–J). Neutralizing antibody to IL-17RA significantly inhibited Ad-IL-1β-induced airway fibrosis as assessed by morphometric analysis of Trichrome-stained sections (Figs. 1J and 2, K and L).
Neutralization of IL-17A protects against adenoviral IL-1β-induced airway inflammation and fibrosis.
IL-17A uses a heteromeric receptor complex comprised of IL-17RA and IL-17RC and is the main IL-17 family cytokine implicated in fibroinflammatory lung pathology (17, 32). Treatment with a neutralizing antibody to mouse IL-17A significantly inhibited Ad-IL-1β induced increases in total cell and neutrophil counts in BAL fluid 14 days after Ad-IL-1β treatment (Fig. 1, K–M). Morphometric analysis of airway inflammation revealed that neutralizing IL-17A significantly reduced the Ad-IL-1β -induced increase in inflammatory cells localized around airways of WT mice (Figs. 1N and 2, M–P). Neutralizing antibody to IL-17A significantly inhibited Ad-IL-1β-induced airway fibrosis as assessed by morphometric analysis of Trichrome-stained sections (Figs. 1O and 2, Q and R).
Role of IL-17RA in CS-induced airway inflammation and fibrosis.
We next sought to determine the role of IL-17RA in models that incorporate CS, a more physiologically relevant stimulus. We used CS in combination with intranasal administration of the viral-mimetic PIC, which synergistically amplifies CS-induced airway remodeling and mimics the proinflammatory environment that occurs during acute exacerbations of COPD (53). Three weeks of CS exposure with 1 wk intranasal PIC stimulation caused a similar increase as seen in Ad-IL-1β-treated animals in total cell, and neutrophil counts in BAL fluid (Fig. 3, A–C). Morphometric analysis of airway inflammation revealed that il17ra−/− mice were significantly protected against CS+PIC-induced increased inflammatory cell localization and fibrosis around airways (Figs. 3, D and E, and 4, A–F).
Fig. 3.
Lung and airway inflammation, and airway wall fibrosis induced by cigarette smoke in combination with intranasal polyinosinic:polycytidylic acid is dependent on IL-17RA/IL-17A. WT or il17ra-deficient (il17ra−/−) mice were treated with cigarette smoke (CS) in combination with polyinosinic:polycytidylic acid (PIC) or room air (RA) as a control (A–E). After 14 days, bronchoalveolar lavage (BAL) was performed and total cell counts (A), macrophage (B), or neutrophil numbers (C) were assessed. Morphometric analysis of airway inflammation (D) or airway wall fibrosis was performed from histological sections (E). WT mice treated with either RA or CS+PIC with isotype, or anti-IL-17RA (F–J), or anti-IL-17A (K–O) underwent the same BAL (F–H, K–M) and morphometric analysis (I, J, N, O), as above. Experiments were repeated a minimum of 3 times with at least 3 mice/group. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Fig. 4.
Airway wall inflammation and fibrosis induced by CS+PIC is dependent on IL-17RA/IL-17A. WT (A, C, E) or il17ra−/− mice (B, D, F) were treated with RA (A, B) or CS+PIC (C–F). After 21 days, lungs were harvested and assessed histologically by H&E (A–D) or Trichrome staining (E, F). WT mice were treated with RA (G, H, M, N) or CS+PIC (I–L, O–R) and treated with isotype control (G, I, K, M, O, Q), anti-IL-17RA (H, J, L) or anti-IL-17A (N, P, R). After 21 days airway inflammation and fibrosis were assessed by H&E (G–J, M–P) or Trichrome staining (K, L, Q, R). Experiments were repeated a minimum of 3 times with at least 3 mice/group. Bar = 100 μm.
Inhibition of IL-17RA protects against CS-induced airway inflammation and fibrosis.
Treatment with a neutralizing antibody to mouse IL-17RA significantly inhibited CS+PIC-induced increases in total cell, and neutrophil counts in BAL fluid (Fig. 3, F–H). Morphometric analysis of airway inflammation and fibrosis revealed that antibody inhibition of IL-17RA significantly reduced the CS+PIC-induced increases in inflammatory cell localization and fibrosis around airways seen in control antibody-treated WT mice (Figs. 3, I and J, and 4, G–L).
Inhibition of IL-17A protects against CS-induced airway inflammation and fibrosis.
Neutralizing antibody to IL-17A significantly inhibited CS-PIC induced increases in total cell and neutrophil counts in BAL fluid (Fig. 3, K–M), morphometric airway inflammation (Figs. 3N and 4, M–P), and fibrosis (Figs. 3O and 4, Q and R).
CD4+ IL-17A-secreting cells are significantly increased in CS +PIC-exposed mice.
Total lung immune cell numbers were increased 2.6 fold in mice exposed to CS+PIC compared with room air (2.3×106 ± 0.6×106, room air; 6.1×106 ± 0.5×106, CS+PIC; P = 0.01). Using multicolor cell staining, we detected IL-17A in subsets of PMNs, NK cells, CD4 and CD8+ T cells, γδ T cells, and innate lymphoid (ILC3) cells (Fig. 5), all cell types that have been reported to express IL-17A (9, 35, 41, 55, 64). Of these, only CD4+ IL-17 cells (Th17) and CD8+ IL-17 were significantly increased in CS+PIC-exposed mice compared with room air-exposed mice (Fig. 5M). There was a nonsignificant trend for increased IL-17A expressing γδ T cells (Fig. 5M). Innate lymphoid cells expressing IL-17 represent the ILC3 subset. The total numbers of ILC3 cells was not significantly different in CS+PIC-exposed compared with room air-exposed mice (Fig. 5, J–M). However, the percentage of these cells was decreased in CS+PIC-exposed compared with room air-exposed mice (0.28% ± 0.09, room air, 0.10% ± 0.04; P = 0.02). Taken together, these results demonstrate diverse populations of immune cells that produce IL-17A and show that IL-17A is induced by CS+PIC exposure by subsets of CD4+ cells and to a lesser extent by CD8+ cells. The γδ T cell subset appeared to show a variable induction of IL-17A by CS+PIC exposure that did not reach statistical significance (P = 0.0.15).
Fig. 5.
CD4 + IL-17-expressing cells (Th17) are significantly increased in the lung by exposure to CS+PIC. WT C57B/6 mice were exposed to CS+PIC and the lungs harvested on day 21 after the initiation of smoke exposure. Lung immune cells were analyzed by multicolor flow cytometry. Cells were stained in a single panel for Gr1, NK1.1, B220, TCRβ, TCRγδ, CD8, CD4, IL17A secretion, and INFγ secretion (A–I) or for CD3ε, CD90, CD127, Rorγt, IL17A, and INFγ (J–L). A: cells expressing Gr1, Nk1.1, or neither were gated and percent positive staining with IL-17A shown as total positive cell number in M. In B, Gr1, Nk1.1 double-negative cells were gated into Tcrγδ (D–F)- and Tcrβ (C)-positive populations, which were then gated into IFNγ-negative and IL-17A-positive populations in room air (E) compared with CS+PIC (F)-treated mice. IL-17A-positive cells were defined using pooled cells stained without surface capture for IL-17A (D). In C, Tcrβ-positive cells were gated into CD8- and CD4-positive populations. Shown in in G–J, Tcrβ, CD4+ or CD8 (not shown) cells were gated into IL-17A-positive and IL-17-negative populations, which were enumerated as shown in M. J: lung cells were gated into CD3ε and CD90-positive and -negative populations. Innate lymphoid cells (CD3ε neg, CD90 positive) were gated into CD127 positive/IL-17-negative (ILC1) and CD127-negative/IL-17A-positive (ILC3) populations in room air (K) and CS+PIC-exposed mice (L) and enumerated in M. IL-17A-positive cells were defined by pooled cells stained without IL-17A surface capture. IL-17A-positive ILC3 cells were also positive for Rorγt (not shown). In M, total numbers of IL-17A-expressing cell types (PMNs, NK, CD4, CD8, γδ, and ILC3) in room air (open bars) and CS+PIC-exposed (solid bars) mice (n = 3 in each group) are shown. *P < 0.05, **P < 0.01 as determined by Student’s t-test.
DISCUSSION
This study has provided evidence that IL-17A and its receptor IL-17RA are specifically involved in murine peribronchiolar airway fibrosis caused by COPD-relevant stimuli, specifically CS and IL-1β. This evidence is based on studies using IL-17RA-deficient mice and verified using neutralizing antibodies to either IL-17RA or IL-17A. Taken together these studies support a mechanistic role of IL-17A/IL-17RA in murine airway fibrosis while providing a proof-of-concept that antibody targeting of this pathway might be used to prevent airway fibrosis in humans.
Our findings suggest that airway fibrosis shares common mechanistic features with pulmonary fibrosis since both can be experimentally linked to IL-1β and IL-17 (65). However, airway fibrosis is etiologically, clinically, and pathologically distinct from parenchymal lung fibrosis [i.e., idiopathic pulmonary fibrosis (IPF)] and vice versa. Airway fibrosis in COPD is driven by injury to the airway epithelium from chronic smoke exposure, which leads to reactive epithelial changes, chronic inflammation, and mucus hypersecretion, which together predispose to bacterial and viral infection and a self-sustaining cycle of injury and repair (6). The underlying cause of IPF is less clear and while smoking and infection are associated with increased risk, they have not yet been shown to be causative (42, 47). The predominant theory of the pathogenesis of IPF is that it is caused by injury, from an unknown source, to alveolar epithelial cells, which stimulates an aberrant mesenchymal response (26). In COPD inflammation plays an established role in airway fibrosis; in IPF the role of inflammation remains controversial (23, 25, 26). Thus when envisioning new therapeutic targets for airway fibrosis it is crucial to use preclinical models, which employ COPD relevant stimuli and recapitulate key features of airway fibrosis in humans.
The study of airway fibrosis has been hindered by lack of robust animal models. Airway fibrosis is challenging to induce in mice by cigarette exposure alone (45). Murine airway remodeling systems utilizing intratracheal application of Ad-IL-1β mimic key pathological, chemokine, and cytokine profiles that occur in COPD, while at the same time delivering a physiological relevant dose expressed by a physiologically relevant cell type (37). Epithelial expression of IL-1β models what is found in airway disease in humans since airway epithelium is a major source of IL-1β in airway remodeling in COPD (37). We use an adenoviral dose where peak IL-1β expression at 1 wk postinjection is similar or slightly below the peak physiological range (~1 ng/ml) found in humans during acute exacerbations (8). The murine airway remodeling system utilizing CS+PIC demonstrates similar airway fibrosis as Ad-IL-1β (45), while CS exposure alone causes minimal or no airway fibrosis (13, 45, 50). Both the Ad-IL-1β and CS+PIC models share concomitant increases in proinflammatory CD4+ Th17 cells and the profibrogenic cytokines that are increased in human COPD disease biospecimens [i.e., IL-1β (16), CCL2 (40), CCL20 (17), and IL-17A (41)]. The similar airway phenotype between the Ad-IL-1β and CS+PIC systems suggests the importance of IL-1β in CS-related lung pathology (15). The importance of inflammasome activation and subsequent cleavage of pro-IL-1β and secretion of IL-1β in response to PIC or live influenza A virus has been well documented (1). In studies from other laboratories a single supraphysiological dose (≥1 μg) of recombinant IL-1β causes alveolar inflammation and fibrosis, which mimics the pathology of IPF (19, 65). Despite the different pathologies that result from different delivery modes and doses of IL-1β, both are IL-17 dependent. We believe that our data showing that IL-17 plays a role in airway fibrosis is an important independent finding that complements and extends experiments performed with extreme doses of recombinant IL-1β or bleomycin that model IPF, a completely different disease process.
In the CS+PIC system there is a temporal increase in IL-1β levels 15–18 day after the initiation of CS coinciding with a maximal increase in IL-17A, which is temporally related to the maximum influx of inflammatory cells and the development of airway fibrosis (45). Here we find that at day 15 after the initiation of CS exposure, IL-17A is secreted by a variety of innate and adaptive immune cell types including PMNs, CD4+ and CD8+T cells, γδ T cells, and ILC3 cells (Fig. 5M). The highest expression levels of IL-17A were from CD4+T cells, γδ T cells, and ILC3 cells. Of all the IL-17A-expressing cell types, only the increase in IL-17A expression in response to CS+PIC by CD4+ and CD8+ T cells reached statistical significance. Our previous finding that mice lacking α/β Τ-cells were protected against Ad-IL1β-induced airway fibrosis, together with our present findings, suggests that both CD4+ and CD8+T cells may be important sources of IL-17A in airway fibrosis (23). However, since CD4+T cells express much more IL-17A than CD8+T cells, the former are most likely the more important α/β T cell subset in airway fibrosis. Interestingly, a high percentage of ILC3 cells expressed high levels of IL-17A in room air-exposed mice, which decreased upon CS+PIC exposure. Because the overall lung immune cell numbers increased with CS+PIC exposure the total numbers of ILC3 expressing IL-17A cells did not change. This suggests that IL-17A expressing ILC3 cells play a distinct role from IL-17A expressing α/β T cells in airway pathology with the former dominating in homeostatic and the later in pathological conditions. Finally, there was also was a nonsignificant trend for increased IL-17A expression by γδ T cells by CS+PIC exposure. It is possible that a critical threshold of IL-17A expression derived from multiple cell sources mediates airway fibrosis, and that multiple IL-17A-expressing cell types (i.e., CD4+, CD8+, γδ T cells) could be targeted to influence airway fibrosis in COPD patients.
The previous observation that CD4+ Th17 cells and IL-17A were increased in both the Ad-IL-1β and CS+PIC models was consistent with the known roles for IL-1β and TGF-β in directly promoting CD4+ Th17 cell differentiation from naive T cells (3, 7, 12, 37, 45, 61). IL-1β and TGF-β also play indirect roles that are important in mediating early events leading to CD4+ Th17 cell differentiation. We have previously determined that IL-1β and TGF-β together are required for efficient transcription and secretion of CCL20 from fibroblasts, which binds to CCR6 expressed by immature dendritic cells (10). The CCL20/CCR6 interaction increases DC chemokinesis, which increases overall DC influx, trafficking to draining lymph nodes, and promotion of CD4+ Th17 cell differentiation (23, 24, 37). Deficiency of ccr6, DC depletion, or absence of α/β T cells all protected mice from Ad-IL-1β-induced airway fibrosis (23, 24). Together, these data prompted us to hypothesize that IL-17A was an important downstream effector in airway fibrosis.
The IL-17 cytokine family consists of six members (A–F) while the IL-17 receptor family consists of five members (A–E) (39). IL-17A and IL-17F are highly homologous, form both functional homo- and heterodimers, and bind to the same IL-17 receptor complex(s) (22, 31). IL-17A, IL-17F, and the IL-17A/IL-17F heterodimer bind to and signal through a heteromeric receptor complex comprised of IL-17RA and IL-17RC (68). IL-17A and IL-17F are widely implicated in a variety of autoimmune diseases (66); however, in fibroinflammatory lung disease IL-17A appears to be mainly implicated (19, 65). Our data support a role for IL-17A in murine models of airway fibrosis. However, we have not specifically addressed the role for IL-17F or IL-17A/IL-17F heterodimers in our models. The fibroinflammatory role that we have found for IL-17A and its receptor IL-17RA in murine airway fibrosis is consistent with other studies which have identified roles for IL-17A and IL-17RA, in other fibrotic lung disease models (65).
Several examples of mechanisms by which IL-17 could promote airway fibrosis are 1) through inhibition of autophagic degradation of collagen by epithelial cells (44), 2) through differentiation and activation of mesenchymal cells (58), or 3) through epithelial-mesenchymal transition (27). It is possible that these and other yet to be identified mechanisms all contribute to airway fibrosis. It is unlikely that the main fibrogenic mechanism is via direct effects on lung fibroblasts since we have found that IL-17 treatment of primary lung fibroblasts has minimal effects on col1A1 and col1A2 gene expression and on soluble collagen production (Moermans C and Ito S, unpublished data). Therefore, the effects of IL-17 are likely to be indirect and require engagement of several cell types and their associated secretory products.
It should be noted that we have not quantitatively detected any fixed obstructive physiological effects of the airway fibrosis that occurs in the CS+PIC model (34). Therefore, we do not know the significance that the airway fibrotic phenotype that we observe in mice has for the obstructive physiology found in fibrotic airways of COPD patients (45). The distinct anatomic and physiological differences between mouse and human airways (i.e., shortened bronchial tree, differences in species-related differences in airway caliber) make such comparisons difficult (28, 59). Therefore, while our findings support the hypothesis that increased IL-17A levels found in COPD leads to airway wall fibrosis and obstructive airway physiology, this remains speculative (69).
In conclusion, our data supports that airway fibrosis is a pathological consequence of increased IL-17 expression and prompts further investigation into the targeting of IL-17A or IL-17RA to modify the progression of airway fibrosis in COPD.
GRANTS
This work was supported by the grants from the NIH HL113032, HL063993, HL090662, NS044155, UCOP, TRDRP, UCSF Academic Senate, UCOP POC award (S. L. Nishimura), and UCSF Liver Center (P30DK026743) (to S. L. Nishimura and J. L. Baron).
DISCLOSURES
A. Budelsky was employed by Amgen during the research period.
AUTHOR CONTRIBUTIONS
H.Y., M.H., S.M., N.T., R.M., C.M., S.I., J.A., and A.G. performed experiments; H.Y., M.H., S.M., N.T., C.M., S.I., J.A., A.G., and S.L.N. analyzed data; H.Y., M.H., A.G., and S.L.N. interpreted results of experiments; H.Y., J.L.B., and S.L.N. edited and revised manuscript; H.Y., M.H., S.M., N.T., R.M., C.M., S.I., A.B., J.L.B., and S.L.N. approved final version of manuscript; C.M. and S.L.N. prepared figures; S.L.N. conceived and designed research; S.L.N. drafted manuscript.
REFERENCES
- 1.Allen IC, Scull MA, Moore CB, Holl EK, McElvania-TeKippe E, Taxman DJ, Guthrie EH, Pickles RJ, Ting JP. The NLRP3 inflammasome mediates in vivo innate immunity to influenza A virus through recognition of viral RNA. Immunity 30: 556–565, 2009. doi: 10.1016/j.immuni.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderton MJ, Mellor HR, Bell A, Sadler C, Pass M, Powell S, Steele SJ, Roberts RR, Heier A. Induction of heart valve lesions by small-molecule ALK5 inhibitors. Toxicol Pathol 39: 916–924, 2011. doi: 10.1177/0192623311416259. [DOI] [PubMed] [Google Scholar]
- 3.Annunziato F, Cosmi L, Santarlasci V, Maggi L, Liotta F, Mazzinghi B, Parente E, Filì L, Ferri S, Frosali F, Giudici F, Romagnani P, Parronchi P, Tonelli F, Maggi E, Romagnani S. Phenotypic and functional features of human Th17 cells. J Exp Med 204: 1849–1861, 2007. doi: 10.1084/jem.20070663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Araya J, Cambier S, Markovics JA, Wolters P, Jablons D, Hill A, Finkbeiner W, Jones K, Broaddus VC, Sheppard D, Barzcak A, Xiao Y, Erle DJ, Nishimura SL. Squamous metaplasia amplifies pathologic epithelial-mesenchymal interactions in COPD patients. J Clin Invest 117: 3551–3562, 2007. doi: 10.1172/JCI32526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Araya J, Cambier S, Morris A, Finkbeiner W, Nishimura SL. Integrin-mediated transforming growth factor-beta activation regulates homeostasis of the pulmonary epithelial-mesenchymal trophic unit. Am J Pathol 169: 405–415, 2006. doi: 10.2353/ajpath.2006.060049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Araya J, Nishimura SL. Fibrogenic reactions in lung disease. Annu Rev Pathol 5: 77–98, 2010. doi: 10.1146/annurev.pathol.4.110807.092217. [DOI] [PubMed] [Google Scholar]
- 7.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 441: 235–238, 2006. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 8.Botelho FM, Bauer CM, Finch D, Nikota JK, Zavitz CC, Kelly A, Lambert KN, Piper S, Foster ML, Goldring JJ, Wedzicha JA, Bassett J, Bramson J, Iwakura Y, Sleeman M, Kolbeck R, Coyle AJ, Humbles AA, Stämpfli MR. IL-1α/IL-1R1 expression in chronic obstructive pulmonary disease and mechanistic relevance to smoke-induced neutrophilia in mice. PLoS One 6: e28457, 2011. doi: 10.1371/journal.pone.0028457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bozinovski S, Seow HJ, Chan SP, Anthony D, McQualter J, Hansen M, Jenkins BJ, Anderson GP, Vlahos R. Innate cellular sources of interleukin-17A regulate macrophage accumulation in cigarette- smoke-induced lung inflammation in mice. Clin Sci (Lond) 129: 785–796, 2015. doi: 10.1042/CS20140703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brand OJ, Somanath S, Moermans C, Yanagisawa H, Hashimoto M, Cambier S, Markovics J, Bondesson AJ, Hill A, Jablons D, Wolters P, Lou J, Marks JD, Baron JL, Nishimura SL. Transforming growth factor-β and interleukin-1β signaling pathways converge on the chemokine CCL20 promoter. J Biol Chem 290: 14717–14728, 2015. doi: 10.1074/jbc.M114.630368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen K, Pociask DA, McAleer JP, Chan YR, Alcorn JF, Kreindler JL, Keyser MR, Shapiro SD, Houghton AM, Kolls JK, Zheng M. IL-17RA is required for CCL2 expression, macrophage recruitment, and emphysema in response to cigarette smoke. PLoS One 6: e20333, 2011. doi: 10.1371/journal.pone.0020333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chung Y, Chang SH, Martinez GJ, Yang XO, Nurieva R, Kang HS, Ma L, Watowich SS, Jetten AM, Tian Q, Dong C. Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immunity 30: 576–587, 2009. doi: 10.1016/j.immuni.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Churg A, Zhou S, Wang X, Wang R, Wright JL. The role of interleukin-1beta in murine cigarette smoke-induced emphysema and small airway remodeling. Am J Respir Cell Mol Biol 40: 482–490, 2009. doi: 10.1165/rcmb.2008-0038OC. [DOI] [PubMed] [Google Scholar]
- 14.Doe C, Bafadhel M, Siddiqui S, Desai D, Mistry V, Rugman P, McCormick M, Woods J, May R, Sleeman MA, Anderson IK, Brightling CE. Expression of the T helper 17-associated cytokines IL-17A and IL-17F in asthma and COPD. Chest 138: 1140–1147, 2010. doi: 10.1378/chest.09-3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doz E, Noulin N, Boichot E, Guénon I, Fick L, Le Bert M, Lagente V, Ryffel B, Schnyder B, Quesniaux VF, Couillin I. Cigarette smoke-induced pulmonary inflammation is TLR4/MyD88 and IL-1R1/MyD88 signaling dependent. J Immunol 180: 1169–1178, 2008. doi: 10.4049/jimmunol.180.2.1169. [DOI] [PubMed] [Google Scholar]
- 16.Fjellbirkeland L, Cambier S, Broaddus VC, Hill A, Brunetta P, Dolganov G, Jablons D, Nishimura SL. Integrin alphavbeta8-mediated activation of transforming growth factor-beta inhibits human airway epithelial proliferation in intact bronchial tissue. Am J Pathol 163: 533–542, 2003. doi: 10.1016/S0002-9440(10)63681-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gaffen SL. Structure and signalling in the IL-17 receptor family. Nat Rev Immunol 9: 556–567, 2009. doi: 10.1038/nri2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ganter MT, Roux J, Miyazawa B, Howard M, Frank JA, Su G, Sheppard D, Violette SM, Weinreb PH, Horan GS, Matthay MA, Pittet JF. Interleukin-1β causes acute lung injury via αvβ5 and αvβ6 integrin-dependent mechanisms. Circ Res 102: 804–812, 2008. doi: 10.1161/CIRCRESAHA.107.161067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gasse P, Riteau N, Vacher R, Michel ML, Fautrel A, di Padova F, Fick L, Charron S, Lagente V, Eberl G, Le Bert M, Quesniaux VF, Huaux F, Leite-de-Moraes M, Ryffel B, Couillin I. IL-1 and IL-23 mediate early IL-17A production in pulmonary inflammation leading to late fibrosis. PLoS One 6: e23185, 2011. doi: 10.1371/journal.pone.0023185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geraghty P, Dabo AJ, D’Armiento J. TLR4 protein contributes to cigarette smoke-induced matrix metalloproteinase-1 (MMP-1) expression in chronic obstructive pulmonary disease. J Biol Chem 286: 30211–30218, 2011. doi: 10.1074/jbc.M111.238824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gessner C, Scheibe R, Wötzel M, Hammerschmidt S, Kuhn H, Engelmann L, Hoheisel G, Gillissen A, Sack U, Wirtz H. Exhaled breath condensate cytokine patterns in chronic obstructive pulmonary disease. Respir Med 99: 1229–1240, 2005. doi: 10.1016/j.rmed.2005.02.041. [DOI] [PubMed] [Google Scholar]
- 22.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol 6: 1123–1132, 2005. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 23.Hashimoto M, Yanagisawa H, Minagawa S, Sen D, Goodsell A, Ma R, Moermans C, McKnelly KJ, Baron JL, Krummel MF, Nishimura SL. A critical role for dendritic cells in the evolution of IL-1β-mediated murine airway disease. J Immunol 194: 3962–3969, 2015. doi: 10.4049/jimmunol.1403043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hashimoto M, Yanagisawa H, Minagawa S, Sen D, Ma R, Murray LA, Tsui P, Lou J, Marks JD, Baron JL, Krummel MF, Nishimura SL. TGF-β-dependent dendritic cell chemokinesis in murine models of airway disease. J Immunol 195: 1182–1190, 2015. doi: 10.4049/jimmunol.1500348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, Cherniack RM, Rogers RM, Sciurba FC, Coxson HO, Paré PD. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med 350: 2645–2653, 2004. doi: 10.1056/NEJMoa032158. [DOI] [PubMed] [Google Scholar]
- 26.Homer RJ, Elias JA, Lee CG, Herzog E. Modern concepts on the role of inflammation in pulmonary fibrosis. Arch Pathol Lab Med 135: 780–788, 2011. [DOI] [PubMed] [Google Scholar]
- 27.Huang Q, Han J, Fan J, Duan L, Guo M, Lv Z, Hu G, Chen L, Wu F, Tao X, Xu J, Jin Y. IL-17 induces EMT via Stat3 in lung adenocarcinoma. Am J Cancer Res 6: 440–451, 2016. [PMC free article] [PubMed] [Google Scholar]
- 28.Irvin CG, Bates JH. Measuring the lung function in the mouse: the challenge of size. Respir Res 4: 1, 2003. doi: 10.1186/rr199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ito K, Ito M, Elliott WM, Cosio B, Caramori G, Kon OM, Barczyk A, Hayashi S, Adcock IM, Hogg JC, Barnes PJ. Decreased histone deacetylase activity in chronic obstructive pulmonary disease. N Engl J Med 352: 1967–1976, 2005. doi: 10.1056/NEJMoa041892. [DOI] [PubMed] [Google Scholar]
- 30.Ito S, Araya J, Kurita Y, Kobayashi K, Takasaka N, Yoshida M, Hara H, Minagawa S, Wakui H, Fujii S, Kojima J, Shimizu K, Numata T, Kawaishi M, Odaka M, Morikawa T, Harada T, Nishimura SL, Kaneko Y, Nakayama K, Kuwano K. PARK2-mediated mitophagy is involved in regulation of HBEC senescence in COPD pathogenesis. Autophagy 11: 547–559, 2015. doi: 10.1080/15548627.2015.1017190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iwakura Y, Ishigame H. The IL-23/IL-17 axis in inflammation. J Clin Invest 116: 1218–1222, 2006. doi: 10.1172/JCI28508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jin W, Dong C. IL-17 cytokines in immunity and inflammation. Emerg Microbes Infect 2: e60, 2013. doi: 10.1038/emi.2013.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jin Y, Wan Y, Chen G, Chen L, Zhang MQ, Deng L, Zhang JC, Xiong XZ, Xin JB. Treg/IL-17 ratio and Treg differentiation in patients with COPD. PLoS One 9: e111044, 2014. doi: 10.1371/journal.pone.0111044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kang MJ, Lee CG, Lee JY, Dela Cruz CS, Chen ZJ, Enelow R, Elias JA. Cigarette smoke selectively enhances viral PAMP- and virus-induced pulmonary innate immune and remodeling responses in mice. J Clin Invest 118: 2771–2784, 2008. doi: 10.1172/JCI32709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim HY, Lee HJ, Chang YJ, Pichavant M, Shore SA, Fitzgerald KA, Iwakura Y, Israel E, Bolger K, Faul J, DeKruyff RH, Umetsu DT. Interleukin-17-producing innate lymphoid cells and the NLRP3 inflammasome facilitate obesity-associated airway hyperreactivity. Nat Med 20: 54–61, 2014. doi: 10.1038/nm.3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.King TE Jr, Bradford WZ, Castro-Bernardini S, Fagan EA, Glaspole I, Glassberg MK, Gorina E, Hopkins PM, Kardatzke D, Lancaster L, Lederer DJ, Nathan SD, Pereira CA, Sahn SA, Sussman R, Swigris JJ, Noble PW; ASCEND Study Group . A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med 370: 2083–2092, 2014. doi: 10.1056/NEJMoa1402582. [DOI] [PubMed] [Google Scholar]
- 37.Kitamura H, Cambier S, Somanath S, Barker T, Minagawa S, Markovics J, Goodsell A, Publicover J, Reichardt L, Jablons D, Wolters P, Hill A, Marks JD, Lou J, Pittet JF, Gauldie J, Baron JL, Nishimura SL. Mouse and human lung fibroblasts regulate dendritic cell trafficking, airway inflammation, and fibrosis through integrin αvβ8-mediated activation of TGF-β. J Clin Invest 121: 2863–2875, 2011. doi: 10.1172/JCI45589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kolb M, Margetts PJ, Anthony DC, Pitossi F, Gauldie J. Transient expression of IL-1beta induces acute lung injury and chronic repair leading to pulmonary fibrosis. J Clin Invest 107: 1529–1536, 2001. doi: 10.1172/JCI12568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kolls JK, Lindén A. Interleukin-17 family members and inflammation. Immunity 21: 467–476, 2004. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 40.Kudo M, Melton AC, Chen C, Engler MB, Huang KE, Ren X, Wang Y, Bernstein X, Li JT, Atabai K, Huang X, Sheppard D. IL-17A produced by αβ T cells drives airway hyper-responsiveness in mice and enhances mouse and human airway smooth muscle contraction. Nat Med 18: 547–554, 2012. doi: 10.1038/nm.2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lendermon EA, Dodd-o JM, Coon TA, Miller HL, Ganguly S, Popescu I, O’Donnell CP, Cardenes N, Levine M, Rojas M, Weathington NM, Zhao J, Zhao Y, McDyer JF. CD8(+)IL-17(+) T cells mediate neutrophilic airway obliteration in T-bet-deficient mouse lung allograft recipients. Am J Respir Cell Mol Biol 52: 622–633, 2015. doi: 10.1165/rcmb.2014-0059OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ley B, Collard HR. Epidemiology of idiopathic pulmonary fibrosis. Clin Epidemiol 5: 483–492, 2013. doi: 10.2147/CLEP.S54815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lucattelli M, Cicko S, Müller T, Lommatzsch M, De Cunto G, Cardini S, Sundas W, Grimm M, Zeiser R, Dürk T, Zissel G, Sorichter S, Ferrari D, Di Virgilio F, Virchow JC, Lungarella G, Idzko M. P2X7 receptor signaling in the pathogenesis of smoke-induced lung inflammation and emphysema. Am J Respir Cell Mol Biol 44: 423–429, 2011. doi: 10.1165/rcmb.2010-0038OC. [DOI] [PubMed] [Google Scholar]
- 44.Mi S, Li Z, Yang HZ, Liu H, Wang JP, Ma YG, Wang XX, Liu HZ, Sun W, Hu ZW. Blocking IL-17A promotes the resolution of pulmonary inflammation and fibrosis via TGF-beta1-dependent and -independent mechanisms. J Immunol 187: 3003–3014, 2011. doi: 10.4049/jimmunol.1004081. [DOI] [PubMed] [Google Scholar]
- 45.Minagawa S, Lou J, Seed RI, Cormier A, Wu S, Cheng Y, Murray L, Tsui P, Connor J, Herbst R, Govaerts C, Barker T, Cambier S, Yanagisawa H, Goodsell A, Hashimoto M, Brand OJ, Cheng R, Ma R, McKnelly KJ, Wen W, Hill A, Jablons D, Wolters P, Kitamura H, Araya J, Barczak AJ, Erle DJ, Reichardt LF, Marks JD, Baron JL, Nishimura SL. Selective targeting of TGF-β activation to treat fibroinflammatory airway disease. Sci Transl Med 6: 241ra79, 2014. doi: 10.1126/scitranslmed.3008074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miniño AM, Xu J, Kochanek KD. Deaths: preliminary data for 2008. Natl Vital Stat Rep 59: 1–52, 2010. [PubMed] [Google Scholar]
- 47.Molyneaux PL, Maher TM. The role of infection in the pathogenesis of idiopathic pulmonary fibrosis. Eur Respir Rev 22: 376–381, 2013. doi: 10.1183/09059180.00000713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nishimura SL. Integrin-mediated transforming growth factor-beta activation, a potential therapeutic target in fibrogenic disorders. Am J Pathol 175: 1362–1370, 2009. doi: 10.2353/ajpath.2009.090393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Papi A, Bellettato CM, Braccioni F, Romagnoli M, Casolari P, Caramori G, Fabbri LM, Johnston SL. Infections and airway inflammation in chronic obstructive pulmonary disease severe exacerbations. Am J Respir Crit Care Med 173: 1114–1121, 2006. doi: 10.1164/rccm.200506-859OC. [DOI] [PubMed] [Google Scholar]
- 50.Phillips B, Veljkovic E, Peck MJ, Buettner A, Elamin A, Guedj E, Vuillaume G, Ivanov NV, Martin F, Boué S, Schlage WK, Schneider T, Titz B, Talikka M, Vanscheeuwijck P, Hoeng J, Peitsch MC. A 7-month cigarette smoke inhalation study in C57BL/6 mice demonstrates reduced lung inflammation and emphysema following smoking cessation or aerosol exposure from a prototypic modified risk tobacco product. Food Chem Toxicol 80: 328–345, 2015. doi: 10.1016/j.fct.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 51.Postma DS, Timens W. Remodeling in asthma and chronic obstructive pulmonary disease. Proc Am Thorac Soc 3: 434–439, 2006. doi: 10.1513/pats.200601-006AW. [DOI] [PubMed] [Google Scholar]
- 52.Richeldi L, du Bois RM, Raghu G, Azuma A, Brown KK, Costabel U, Cottin V, Flaherty KR, Hansell DM, Inoue Y, Kim DS, Kolb M, Nicholson AG, Noble PW, Selman M, Taniguchi H, Brun M, Le Maulf F, Girard M, Stowasser S, Schlenker-Herceg R, Disse B, Collard HR; INPULSIS Trial Investigators . Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med 370: 2071–2082, 2014. doi: 10.1056/NEJMoa1402584. [DOI] [PubMed] [Google Scholar]
- 53.Saika S, Kono-Saika S, Tanaka T, Yamanaka O, Ohnishi Y, Sato M, Muragaki Y, Ooshima A, Yoo J, Flanders KC, Roberts AB. Smad3 is required for dedifferentiation of retinal pigment epithelium following retinal detachment in mice. Lab Invest 84: 1245–1258, 2004. doi: 10.1038/labinvest.3700156. [DOI] [PubMed] [Google Scholar]
- 54.Shan M, Cheng HF, Song LZ, Roberts L, Green L, Hacken-Bitar J, Huh J, Bakaeen F, Coxson HO, Storness-Bliss C, Ramchandani M, Lee SH, Corry DB, Kheradmand F. Lung myeloid dendritic cells coordinately induce TH1 and TH17 responses in human emphysema. Sci Transl Med 1: 4ra10, 2009. doi: 10.1126/scitranlsmed.3000154. [DOI] [PubMed] [Google Scholar]
- 55.Sharma AK, LaPar DJ, Zhao Y, Li L, Lau CL, Kron IL, Iwakura Y, Okusa MD, Laubach VE. Natural killer T cell-derived IL-17 mediates lung ischemia-reperfusion injury. Am J Respir Crit Care Med 183: 1539–1549, 2011. doi: 10.1164/rccm.201007-1173OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sichelstiel A, Yadava K, Trompette A, Salami O, Iwakura Y, Nicod LP, Marsland BJ. Targeting IL-1β and IL-17A driven inflammation during influenza-induced exacerbations of chronic lung inflammation. PLoS One 9: e98440, 2014. doi: 10.1371/journal.pone.0098440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stockley RA, Mannino D, Barnes PJ. Burden and pathogenesis of chronic obstructive pulmonary disease. Proc Am Thorac Soc 6: 524–526, 2009. doi: 10.1513/pats.200904-016DS. [DOI] [PubMed] [Google Scholar]
- 58.Tan Z, Qian X, Jiang R, Liu Q, Wang Y, Chen C, Wang X, Ryffel B, Sun B. IL-17A plays a critical role in the pathogenesis of liver fibrosis through hepatic stellate cell activation. J Immunol 191: 1835–1844, 2013. doi: 10.4049/jimmunol.1203013. [DOI] [PubMed] [Google Scholar]
- 59.Vanoirbeek JA, Rinaldi M, De Vooght V, Haenen S, Bobic S, Gayan-Ramirez G, Hoet PH, Verbeken E, Decramer M, Nemery B, Janssens W. Noninvasive and invasive pulmonary function in mouse models of obstructive and restrictive respiratory diseases. Am J Respir Cell Mol Biol 42: 96–104, 2010. doi: 10.1165/rcmb.2008-0487OC. [DOI] [PubMed] [Google Scholar]
- 60.Vargas-Rojas MI, Ramírez-Venegas A, Limón-Camacho L, Ochoa L, Hernández-Zenteno R, Sansores RH. Increase of Th17 cells in peripheral blood of patients with chronic obstructive pulmonary disease. Respir Med 105: 1648–1654, 2011. doi: 10.1016/j.rmed.2011.05.017. [DOI] [PubMed] [Google Scholar]
- 61.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity 24: 179–189, 2006. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 62.Vitsky A, Waire J, Pawliuk R, Bond A, Matthews D, Lacasse E, Hawes ML, Nelson C, Richards S, Piepenhagen PA, Garman RD, Andrews L, Thurberg BL, Lonning S, Ledbetter S, Ruzek MC. Homeostatic role of transforming growth factor-beta in the oral cavity and esophagus of mice and its expression by mast cells in these tissues. Am J Pathol 174: 2137–2149, 2009. doi: 10.2353/ajpath.2009.080723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Weaver CT, Elson CO, Fouser LA, Kolls JK. The Th17 pathway and inflammatory diseases of the intestines, lungs, and skin. Annu Rev Pathol 8: 477–512, 2013. doi: 10.1146/annurev-pathol-011110-130318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Werner JL, Gessner MA, Lilly LM, Nelson MP, Metz AE, Horn D, Dunaway CW, Deshane J, Chaplin DD, Weaver CT, Brown GD, Steele C. Neutrophils produce interleukin 17A (IL-17A) in a dectin-1- and IL-23-dependent manner during invasive fungal infection. Infect Immun 79: 3966–3977, 2011. doi: 10.1128/IAI.05493-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wilson MS, Madala SK, Ramalingam TR, Gochuico BR, Rosas IO, Cheever AW, Wynn TA. Bleomycin and IL-1beta-mediated pulmonary fibrosis is IL-17A dependent. J Exp Med 207: 535–552, 2010. doi: 10.1084/jem.20092121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Witowski J, Ksiazek K, Jörres A. Interleukin-17: a mediator of inflammatory responses. Cell Mol Life Sci 61: 567–579, 2004. doi: 10.1007/s00018-003-3228-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wolters PJ, Collard HR, Jones KD. Pathogenesis of idiopathic pulmonary fibrosis. Annu Rev Pathol 9: 157–179, 2014. doi: 10.1146/annurev-pathol-012513-104706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wright JF, Bennett F, Li B, Brooks J, Luxenberg DP, Whitters MJ, Tomkinson KN, Fitz LJ, Wolfman NM, Collins M, Dunussi-Joannopoulos K, Chatterjee-Kishore M, Carreno BM. The human IL-17F/IL-17A heterodimeric cytokine signals through the IL-17RA/IL-17RC receptor complex. J Immunol 181: 2799–2805, 2008. doi: 10.4049/jimmunol.181.4.2799. [DOI] [PubMed] [Google Scholar]
- 69.Zhang L, Cheng Z, Liu W, Wu K. Expression of interleukin (IL)-10, IL-17A and IL-22 in serum and sputum of stable chronic obstructive pulmonary disease patients. COPD 10: 459–465, 2013. doi: 10.3109/15412555.2013.770456. [DOI] [PubMed] [Google Scholar]