Abstract
We tested the hypothesis that female mice null for uncoupling protein 1 (UCP1) would have increased susceptibility to Western diet-induced “whitening” of brown adipose tissue (AT) and glucose intolerance. Six-week-old C57BL/6J wild-type (WT) and UCP1 knockout (UCP1−/−) mice, housed at 25°C, were randomized to either a control diet (10% kcal from fat) or Western diet (45% kcal from fat and 1% cholesterol) for 28 wk. Loss of UCP1 had no effect on energy intake, energy expenditure, spontaneous physical activity, weight gain, or visceral white AT mass. Despite similar susceptibility to weight gain compared with WT, UCP1−/− exhibited whitening of brown AT evidenced by a striking ~500% increase in mass and appearance of large unilocular adipocytes, increased expression of genes related to inflammation, immune cell infiltration, and endoplasmic reticulum/oxidative stress (P < 0.05), and decreased mitochondrial subunit protein (COX I, II, III, and IV, P < 0.05), all of which were exacerbated by Western diet (P < 0.05). UCP1−/− mice also developed liver steatosis and glucose intolerance, which was worsened by Western diet. Collectively, these findings demonstrate that loss of UCP1 exacerbates Western diet-induced whitening of brown AT, glucose intolerance, and induces liver steatosis. Notably, the adverse metabolic manifestations of UCP1−/− were independent of changes in body weight, visceral adiposity, and energy expenditure. These novel findings uncover a previously unrecognized metabolic protective role of UCP1 that is independent of its already established role in energy homeostasis.
Keywords: obesity, inflammation, insulin resistance, glucose intolerance
cumulative evidence indicates that obesity is an important contributor to the development of systemic insulin resistance, Type 2 diabetes, and cardiovascular disease (16, 41). Specifically, white adipose tissue (AT) inflammation has been implicated as a causal link between obesity and cardiometabolic complications (15, 21, 41, 60), including insulin resistance (51). By contrast, brown AT appears to be more protected against inflammation, and its quantity inversely associates with insulin resistance, Type 2 diabetes, and risk of cardiovascular disease (28). Brown adipocytes are unique in that they are characterized by multilocular lipid droplets and numerous mitochondria that contain uncoupling protein 1 (UCP1) in their inner-mitochondrial membrane. UCP1 allows brown AT to generate heat via uncoupling oxidative phosphorylation at the expense of ATP production. Like white AT, brown AT is also insulin-sensitive and has been identified as an important organ for glucose disposal (38, 42) despite only making up a small fraction of total body AT. Moreover, increased brown AT activity improves whole body insulin sensitivity and glucose homeostasis in overweight humans (6), and adipose tissue-specific overexpression of UCP1 protects against diet-induced obesity in mice (24). However, despite the confirmation of metabolically active brown AT in adult humans (9, 47, 59, 63, 67), not all individuals possess positive or active brown AT (47), which is one aspect that is driving human brown AT research.
“Browning” of white adipocytes occurs under certain environmental and pharmacological conditions. The most potent stimulus appears to be cold exposure (3, 64), but exercise training (55, 57) and β-adrenergic activation (40, 55) also have been shown to result in increased UCP1 activity and mitochondrial biogenesis in white AT, as well as improved insulin sensitivity and increased energy expenditure (53, 54). In contrast, obesity induces “whitening” of brown AT characterized by impaired β-adrenergic signaling, vascular rarefaction, larger lipid droplets, inflammation, and decreased mitochondrial respiration (8, 14, 37, 49, 50). Paradoxically, diet-induced whitening of brown AT is associated with an upregulation of UCP1 (4, 12, 48, 66) yet, whether this induction of UCP1 serves to “buffer” metabolic dysfunction is unknown. Herein, we tested the hypothesis that loss of UCP1 exacerbates Western diet-induced glycemic dysregulation and that this loss of protection is associated with increased whitening of brown AT depots, including interscapular and thoracic periaortic AT.
METHODS
Experimental design.
Heterozygote UCP1−/+ mice on a C57BL/6J background were purchased from Jackson Laboratory (Bar Harbor, ME), and they were bred at our facility to produce homozygote (UCP1−/−) and littermate wild-type (WT) mice. At 6 wk of age, mice were randomized to either a control low-fat diet or Western diet ad libitum for 28 wk, providing a total of four groups (n = 9–14/group). Control diet (CD; 3.85 kcal/g of food) contained 10% kcal fat, 70% kcal carbohydrate, and 20% kcal protein, with 3.5% kcal sucrose (D12110704, custom formulated; Research Diets, New Brunswick, NJ). Western diet (WD; 4.68 kcal/g of food) contained 44.9% kcal fat, 35.1% kcal carbohydrate, and 20% kcal protein, with 1% cholesterol and 17% kcal sucrose (D09071604, custom formulated; Research Diets). Food intake was measured over a two-week period 2 wk before euthanasia by assessing gram amount of food consumed (i.e., food in − food out). Subsequently, the energy per gram of food was computed (CD, 3.85 kcal/g of food; WD, 4.68 kcal/g of food) and presented as energy intake (kcal/day). All mice were pair-housed (within group) and kept with a light cycle from 0700 to 1900 and a dark cycle from 1900 to 0700. Importantly, prior investigations show that the environmental temperature directly influences the susceptibility of obesity in UCP1-deficient mice. Specifically, UCP1−/− mice are susceptible to diet-induced obesity when housed at 30°C (12), but not at 20–23°C (10, 23, 26). Recent reevaluation of animal housing temperatures recommends housing mice between 23°C and 25°C to best mimic human physiology (52), although this is debated (4). Thus, in an effort to translate our findings to human physiology, we studied mice housed at 25°C. At 34 wk of age, mice were euthanized following a 5-h fast, and tissues were harvested and either fixed in 10% formalin, snap-frozen in liquid nitrogen, and stored at −80°C until analysis, or immediately used in ex vivo experiments. All procedures were approved in advance by the University of Missouri Institutional Animal Care and Use Committee.
Body composition.
The percent body fat (BF%) was measured by a nuclear magnetic resonance imaging whole body composition analyzer (EchoMRI 4in1/1100; Echo Medical Systems, Houston, TX). This noninvasive measure was performed on conscious mice within 1 wk of euthanasia.
Total energy expenditure.
Using a metabolic monitoring system (Promethion, Sable Systems, Las Vegas, NV), we determined total energy expenditure during the 12:12-h light-dark cycles by monitoring oxygen consumption and carbon dioxide production over a 3-day period, as previously described (61). Total energy expenditure was calculated in absolute terms and relative to body weight. These measurements were performed at 30 wk of age.
Glucose tolerance testing.
Glucose tolerance tests were also performed at 30 wk of age. Briefly, after a 5-h fast, blood glucose was measured from the tail vein. The tail was nicked, and blood was sampled by a hand-held glucometer (Alpha Trak, Abbott Laboratories, Abbott Park, IL). A baseline measure of blood glucose was taken before giving a sterile solution of 50% dextrose [2 g/kg body weight (BW)] via intraperitoneal injection, as previously performed (65). Glucose measures were taken 15, 30, 45, 60, and 120 min after the glucose injection. Glucose area under curve (AUC) from baseline was calculated.
Fasting blood parameters.
Plasma glucose, cholesterol, triglycerides, and nonesterified fatty acids (NEFA) assays were performed by a commercial laboratory (Comparative Clinical Pathology Services, Columbia, MO) on an Olympus AU680 automated chemistry analyzer (Beckman-Coulter, Brea, CA) using assays according to manufacturer’s guidelines. Plasma insulin concentrations were determined using a commercially available, mouse-specific ELISA (Alpco Diagnostics, Salem, NH). Plasma lactate was measured via Yellow Springs Instruments 2700 SELECT analyzer (Yellow Springs, OH). The whole blood samples were analyzed for HbA1c using a boronate affinity HPLC method, Trinity ultra2 (Kansas City, MO), as previously described (65). The homeostasis model assessment of insulin resistance (HOMA-IR) was used as a surrogate measure of hepatic insulin resistance [(fasting insulin (μU/l) × fasting glucose (mg/dl)/405.1) (30)] and indices of adipose tissue insulin resistance (AT-IR) were calculated as the product of fasting insulin (μU/l) and fasting NEFAs (mmol/l) (27).
Histological assessments.
Formalin-fixed samples were processed through paraffin embedment, sectioned at 5 µm, and stained with hematoxylin and eosin (interscapular brown AT, thoracic periaortic AT), oil-red-O (liver), UCP1 antibody (thoracic aorta with surrounding periaortic AT; antibody: U6382, Sigma-Aldrich), and macrophage marker Mac-2 antibody (perigonadal white AT; antibody: CL8942AP, Cedarlane). Sections were evaluated via an Olympus BX34 photomicroscope (Olympus, Melville, NY), and images were taken via an Olympus SC30 Optical Microscope Accessory CMOS color camera. Objective quantification of macrophage infiltration in perigonadal white AT was done by determining the positive Mac-2 stained area per 10× fields of view using ImageJ software (NIH public domain; National Institutes of Health, Bethesda, MD). Crown-like structure density was defined as Mac-2 positive-stained area per adipocyte (17). The average of three 10× fields of view was used per animal. Adipocyte size was calculated on the basis of 100 adipocytes/animal obtained from the same three 10× fields. Briefly, cross-sectional areas of the adipocytes were obtained from perimeter tracings using ImageJ software, as performed previously (65). All procedures were performed by an investigator who was blinded to the experimental groups.
RNA extraction and quantitative real-time RT-PCR.
Perigonadal white AT, interscapular brown AT, and thoracic periaortic AT samples were homogenized in TRIzol solution using a tissue homogenizer (TissueLyser LT, Qiagen, Valencia, CA). Total RNA was isolated according to the Qiagen’s RNeasy lipid tissue protocol and assayed using a Nanodrop spectrophotometer (Thermo Scientific, Wilmington, DE) to assess purity and concentration. First-strand cDNA was synthesized from total RNA using the high-capacity cDNA reverse transcription kit (Applied Biosystems, Carlsbad, CA). Quantitative real-time PCR was performed, as previously described using the ABI StepOne Plus sequence detection system (Applied Biosystems) (36, 43). Primer sequences were designed using the NCBI Primer Design tool and have been previously published (43, 65). All primers were purchased from Integrated DNA Technologies (Coralville, IA). A 20-μl reaction mixture containing 10 μl iTaq UniverSYBR Green SMX (Bio-Rad, Hercules, CA), and the appropriate concentrations of gene-specific primers plus 4 μl of cDNA template were loaded in a single well of a 96-well plate. PCR reactions were performed in duplicate under thermal conditions as follows: 95°C for 10 min, followed by 40 cycles of 95°C for 15 s, and 60°C for 45 s. A dissociation melt curve analysis was performed to verify the specificity of the PCR products. GAPDH was used as housekeeping control gene. GAPDH cycle threshold (CT) was not different among the groups of animals. mRNA expression values are presented as 2ΔCT, whereby ΔCT = GAPDH CT − gene of interest CT. mRNA levels were expressed as fold change to the wild-type control diet group, which was set at 1.
Western blot analysis.
Triton X-100 tissue lysates were used to produce Western blot-ready Laemmli samples. Protein samples (10 µg/lane) were separated by SDS-PAGE, transferred to polyvinylidene difluoride membranes, and probed with primary antibodies. Akt (no. 4691, 1:500), phospho-specific Akt at Ser473 (no. 4060, 1:250), AMPK (no. 2532, 1:1,000), phosphospecific AMPK at Thr172 (no. 2531, 1:1,000), phosphospecific AMPK at Ser485 (no. 4185, 1:1,000), GLUT4 (no. 2213, 1:1,000), ACC (no. 3662, 1:1,000), phosphospecific ACC at Ser79 (no. 3661, 1:1,000), and β-tubulin (no. 2146, 1:1000) antibodies were purchased from Cell Signaling. Oxphos (no. ab110413, 1:2000) cocktail antibody was purchased from Abcam. UCP-1 (no. U6382, 1:1,000) antibody was purchased from Sigma-Aldrich. Intensity of individual protein bands were quantified using FluoroChem HD2 (AlphaView, version 3.4.0.0) and were expressed as a ratio to control band β-tubulin. Intensity of individual protein bands were quantified using FluoroChem HD2 (AlphaView, version 3.4.0.0) and were expressed as ratio to control band β-tubulin.
Liver triglycerides.
Hepatic triacylglycerol (TG) concentration was determined using a commercially available kit (Wako L-Type TG M; Wako Pure Chemical Industries, Osaka, Japan). A BioTek uQUANT microplate spectrophotometer (Biotek Instruments, Winooski, VT) was used to analyze the absorbance set at a wavelength of 600 nm. Data are expressed as milligrams TG/gram of liver (wet weight), as described previously (65).
Statistical analysis.
A 2×2 (genotype × diet) analysis of variance (ANOVA) was used to evaluate the effects of UCP1 deficiency and Western diet for all dependent variables. Main effects of genotype and diet, as well as genotype by diet interaction were examined, and LSD post hoc tests were used for pairwise comparisons. All data are presented as means ± SE. For all statistical tests, significance was accepted at P ≤ 0.05. All statistical analyses were performed with SPSS V20.0.
RESULTS
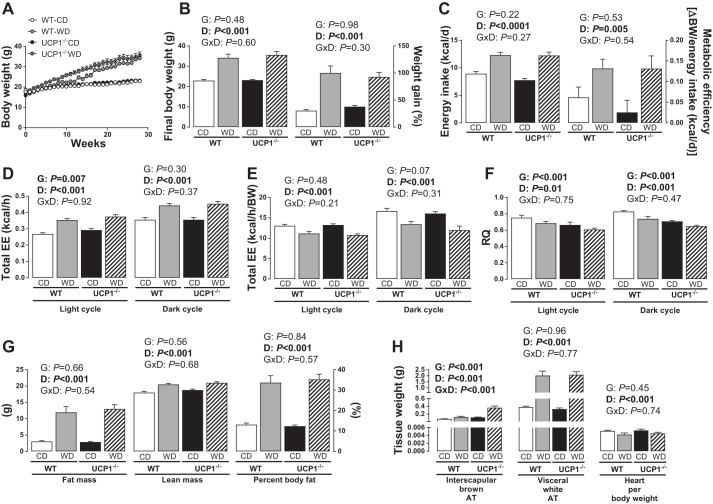
Body weight gain, food intake, energy expenditure, and body composition were unaffected by loss of UCP1.
No differences in body weight, body composition (e.g., BF%, fat mass, lean mass), food intake, metabolic efficiency, or relative energy expenditure were noted between UCP1−/− and WT mice on either diet (Fig. 1, A–C, E, and G). As expected, Western diet-fed animals, independent of genotype, were heavier and exhibited a greater rate of weight gain, BF%, fat mass, and lean mass compared with control diet-fed mice (Diet effect, P < 0.05; Fig. 1, A and B and G). Average daily energy intake and absolute energy expenditure were greater in Western diet-fed animals (Diet effect, P < 0.001; Fig. 1, C and D) in both the light and dark cycles; however, Western diet reduced energy expenditure when correcting for body weight (Diet effect, P < 0.001). In addition, we noted a small but significant increase in absolute energy expenditure during the light cycle in UCP1−/− mice compared with WT mice (Genotype effect, Fig. 1D, P = 0.03), which was eliminated by correcting for body weight (Genotype effect, Fig. 1E, P = 0.48). No differences in spontaneous physical activity were observed in the light or dark cycles by diet or genotype (Table 1). Substrate oxidation, estimated by RQ, was lower in UCP1−/− mice compared with WT mice, suggestive of higher fat oxidation (Diet effect, P < 0.001, Fig. 1F); it also decreased with Western diet.
Fig. 1.
Body weight, body composition, energy expenditure, kilocalories consumed, and tissue weights in WT and UCP1−/− mice fed a control diet vs. Western diet. A: weekly body weight. B: final body weight and % weight gain. C: energy intake in kilocalories per day. Total energy expenditure (EE) intake and feeding efficiency (D) and total (absolute) EE (E) total (relative) energy expenditure during light cycle and dark cycle. F: RQ during light and dark cycle. G: fat mass, lean mass, and % body fat. H: tissue weights. Data are expressed as means ± SE. WT, wild-type; CD, control diet; WD, Western diet; D, main effect of diet; G, main effect of genotype; DxG, diet by genotype interaction. Significant P values (≤0.05) are highlighted in bold; n = 9–14 per group.
Table 1.
Fasting blood characteristics and spontaneous physical activity
| Wild-Type (n = 7–14/group) |
UCP1−/− (n = 9–12/group) |
||||
|---|---|---|---|---|---|
| Variable | Control Diet (n = 13 or 14) |
Western Diet (n = 7–9) |
Control Diet (n = 9) |
Western Diet (n = 12 or 13) |
Two-Way ANOVA |
| Total cholesterol, mg/dl | 62.5 ± 4.1 | 119.9 ± 13.5 | 52.6 ± 3.2 | 84.1 ± 6.0 |
G: P = 0.02 D: P < 0.001 D × G: P = 0.06 |
| LDL cholesterol, mg/dl | 8.2 ± 0.3 | 16.2 ± 1.4 | 7.3 ± 0.5 | 11.8 ± 0.5 |
G: P = 0.001 D: P < 0.001 D × G: P = 0.02 |
| HDL cholesterol, mg/dl | 32.9 ± 2.5 | 52.4 ± 7.1 | 27.3 ± 1.6 | 40.7 ± 3.1 |
G: P = 0.02 D: P < 0.001 D × G: P = 0.41 |
| Triglycerides, mg/dl | 171.4 ± 7.3 | 165.3 ± 8.0 | 154.5 ± 7.6 | 127.4 ± 7.7 |
G: P = 0.001 D: P = 0.04 D × G: P = 0.19 |
| NEFA, mmol/l | 0.66 ± 0.02 | 0.45 ± 0.07 | 0.61 ± 0.07 | 0.55 ± 0.04 | G: P = 0.63 D: P = 0.01 DG: P = 0.14 |
| HOMA-IR | 7.9 ± 1.4 | 18.1 ± 3.8 | 7.4 ± 1.2 | 24.3 ± 3.9 | G: P = 0.35 D: P < 0.001 D × G: P = 0.27 |
| HbA1c | 3.9 ± 0.03 | 4.1 ± 0.08 | 4.0 ± 0.02 | 4.1 ± 0.06 | G: P = 0.14 D: P < 0.01 D × G: P = 0.50 |
| Lactate, mg/dl | 44.4 ± 1.5 | 51.8 ± 4.1 | 41.8 ± 5.7 | 44.5 ± 2.7 | G: P = 0.14 D: P = 0.13 D × G: P = 0.48 |
| SPA − light cycle (X+Y beam breaks) | 9,842.9 ± 1,730.3 | 7,676.7 ± 964.1 | 8,345.4 ± 1,193.6 | 10,838.5 ± 1,703.7 | G: P = 0.58 D: P = 0.91 D × G: P = 0.13 |
| SPA − dark cycle (X+Y beam breaks) | 20,498.2 ± 2,722.6 | 24,557.3 ± 3,025.0 | 23,144.6 ± 3,778.3 | 27,365.4 ± 3,091.4 | G: P = 0.40 D: P = 0.21 D × G: P = 0.98 |
Values are expressed as means ± SE. NEFA, nonesterified fatty acid; HOMA-IR, homeostasis model assessment of insulin resistance; HbA1c, hemoglobin A1c; SPA, spontaneous physical activity. Bolded values indicate significant difference. D, main effect of diet; G, main effect of genotype; DxG, diet by genotype interaction.
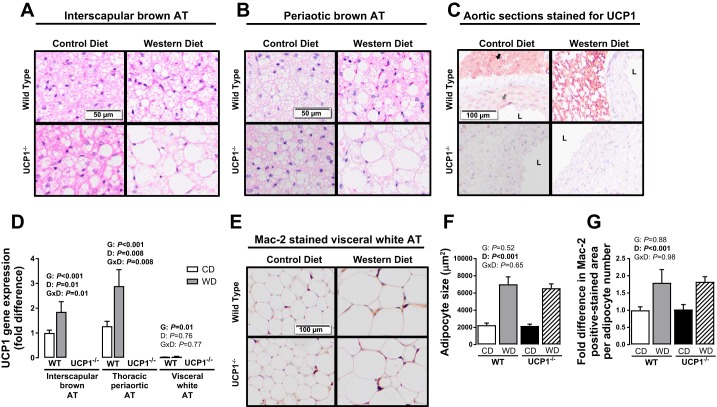
Ablation of UCP1 results in whitening of brown adipose tissue.
Loss of UCP1 increased interscapular brown AT mass, and this effect was exacerbated by Western diet (Genotype × Diet interaction, P < 0.001; Fig. 1H). Histological examination of interscapular and thoracic periaortic brown AT revealed evidence of increased lipid deposition in a genotype- and diet-dependent fashion (Fig. 2, A and B). In contrast to interscapular brown AT mass, visceral white perigonadal AT weight was not impacted by loss of UCP1 but was increased by Western diet (Diet effect, P < 0.001; Fig. 1H). Similarly, while in perigonadal AT Western diet increased adipocyte size and macrophage infiltration, assessed via Mac-2 staining (Fig. 2, E–G), UCP1 ablation had no effect on those measures.
Fig. 2.
Interscapular brown (A) and thoracic periaortic (B) AT representative histology images (×20) and histology images (×10) of aortic sections and adjacent periaortic AT stained for UCP1 identified as the maroon color (C). D: UCP1 expression in the designated ATs. E: histology images (×10) of visceral white perigonadal AT stained for Mac-2. Average adipocyte size (F) and fold differences in Mac-2 positive stained area in WT and UCP1−/− mice fed a control diet vs. Western diet (G). Data are expressed as means ± SE. Black arrow indicates strongly positive immunoreactivity of UCP1 in periaortic AT. Gray arrow indicates faintly positive immunoreactivity of UCP1 in the media of the aortic wall. Positive staining for UCP1 is absent in the UCP1−/− mice. WT, wild-type; CD, control diet; WD, Western diet; D, main effect of diet; G, main effect of genotype; DxG, diet by genotype interaction. Significant P values (≤0.05) are highlighted in bold; n = 9–14 per group.
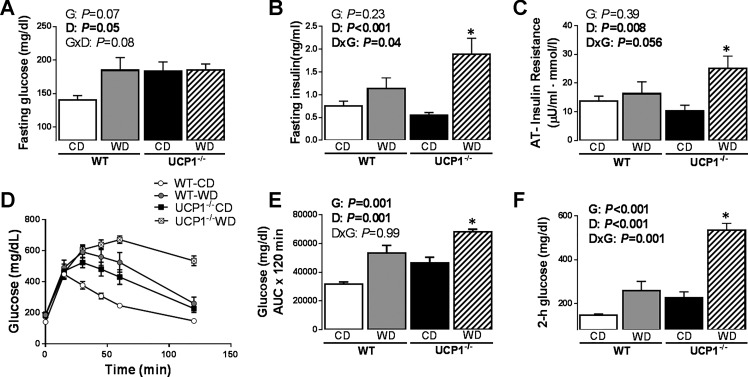
UCP1 ablation exacerbates Western diet-induced glycemic dysregulation.
Relative to WT, UCP1−/− mice fed a control diet exhibited increased fasting blood glucose concentrations (Fig. 3A, P = 0.009). As expected, fasting blood glucose concentrations and HbA1c were higher in Western diet compared with control diet-fed mice (Diet effect, P < 0.05 and P < 0.01; Fig. 3A, Table 1). While Western diet-fed animals were more insulin resistant (Diet effect, P < 0.01), as indicated by HOMA-IR (Table 1), fasting insulin levels were exacerbated by Western diet in UCP1−/− animals (Genotype × Diet interaction, P < 0.05; Fig. 3B). AT insulin resistance index was increased by Western diet (Diet effect, P < 0.05) and was worsened by diet in UCP1−/− animals (Genotype × Diet interaction, P = 0.056; Fig. 3C). Postprandial glucose AUC was increased by both genotype (Genotype effect, P < 0.0001; Fig. 3, D and E) and diet (Diet effect, P < 0.0001; Fig. 3, D and E). In addition, UCP1−/− mice exhibited higher 2-h glucose levels, a common clinical biomarker of Type 2 diabetes risk, compared with WT mice (Genotype effect, P < 0.0001), and this genotype effect was exacerbated by Western diet (Genotype × Diet interaction, P = 0.001; Fig. 3F). Taken together, these findings demonstrate that UCP1 deletion promotes glucose intolerance, and this effect is amplified by Western diet.
Fig. 3.
The effect of UCP1 on glucose homeostasis. Values for fasting glucose (A), fasting insulin (B), AT insulin resistance (C), glucose tolerance testing (D), glucose AUC (E), and 2-h glucose (F) in WT and UCP1−/− mice fed a control diet vs. Western diet. Data are expressed as means ± SE. AUC, area under the curve. Significant P values (≤0.05) are highlighted in bold. *P < 0.05 vs. all groups; n = 9–14 per group.
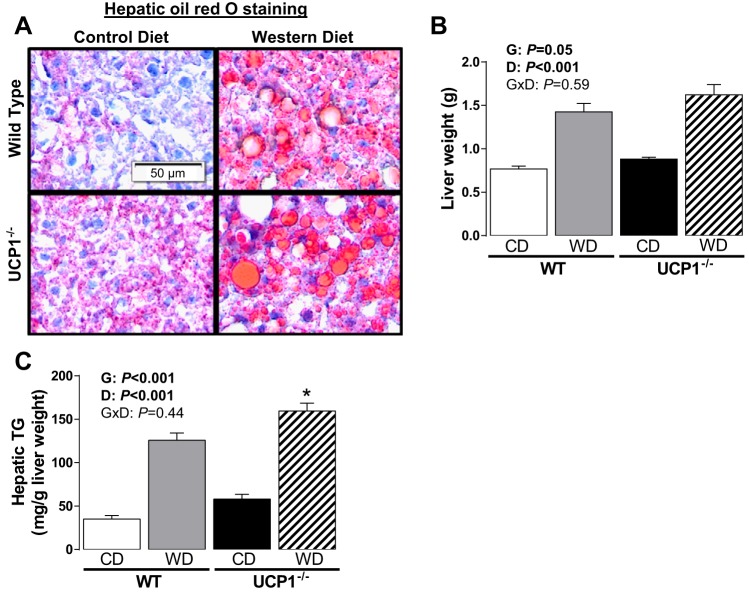
On the other hand, liver weight and TG content were increased by both loss of UCP1 and Western diet (Fig. 4, A–C). The increase in liver weight with loss of UCP1 was subtle in control diet-fed animals [i.e., 14.8% greater in UCP1−/− (Fig. 4B)], whereas the increase in liver TG was more notable in mice fed Western diet (i.e., 65.7% greater in UCP1−/−, P = 0.02). Oil-red-O staining of liver histologically validated the increased lipid deposition (Fig. 4A). Taken together, despite not affecting total adiposity or visceral adipose tissue immune cell infiltration, ablation of UCP1 induced whitening of brown AT depots and promoted a fatty liver phenotype.
Fig. 4.
Representative histology images (×20) of oil red O-stained liver (A), liver weight (B), and hepatic triglycerides (C) in WT and UCP1−/− mice fed a control diet vs. Western diet. Data are expressed as means ± SE. No significant interactions were found. Significant P values (≤0.05) are highlighted in bold. *P < 0.05 vs. all groups; n = 9–14 per group.
Fasting lipids.
Fasting lipid concentrations are reported in Table 1. As expected, total cholesterol, LDL-C, and HDL-C were increased by Western diet; however, UCP1−/− animals exhibited lower lipid levels (P < 0.05, Table 1). UCP1−/− and Western diet resulted in lower TG (Table 1). Fasting NEFAs were lower with Western diet without an effect of genotype.
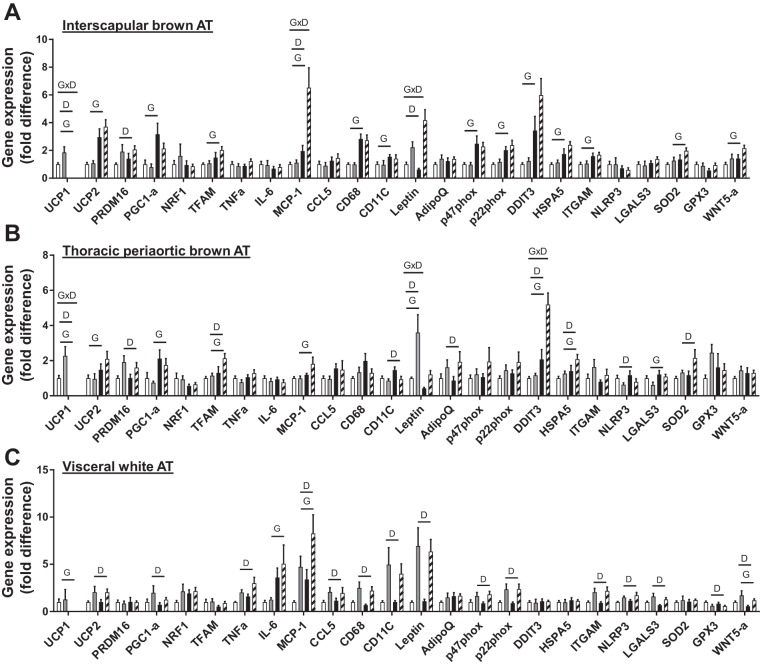
Gene expression in interscapular brown, thoracic periaortic, and visceral perigonadal white AT.
Gene expression data in interscapular and periaortic brown AT, as well as visceral white AT, are presented in Fig. 5. Confirming our model, UCP1 gene expression was undetectable in both interscapular and thoracic periaortic AT and visceral white AT in UCP1-null mice (Figs. 2D and 5, A–C). In interscapular brown AT, UCP1 ablation increased mRNA levels of mitochondrial-related genes (e.g., UCP2, PRDM16, PGC1-α, and TFAM), inflammation (MCP-1), immune cell infiltration (e.g., CD68, CD11C, and ITGAM), oxidative and endoplasmic reticulum (ER) stress (e.g., p22phox, p47phox, DDIT3, and HSPA5), Wnt5a, and the antioxidant marker, SOD2 (P < 0.05, Fig. 5A). Western diet increased UCP1 (in WT only) and PRDM16 (mitochondrial genes), and inflammation (e.g., MCP-1), whereas leptin and MCP-1 mRNA levels were exacerbated by Western diet (Genotype × Diet interaction, P < 0.05; Fig. 5A). Similar increases in thoracic periaortic AT mRNA levels were found with loss of UCP1 for mitochondria-related genes (e.g., UCP1, UCP2, PRDM16, PGC1-a, and TFAM), inflammatory adipokines (MCP-1, leptin), immune cell infiltration (e.g., LGALS3), and endoplasmic reticulum (ER) stress (e.g., DDIT3, HSPA5). Western diet increased mitochondrial genes [e.g., UCP1, PRDM16, TFAM], immune cell infiltration, and inflammation (e.g., CD11c, NLRP3), ER stress (e.g., DDIT3, HSPA5), and SOD2, whereas expression of leptin and DDIT3 were exacerbated by loss of UCP1 (Genotype × Diet interaction, P < 0.05, Fig. 5B).
Fig. 5.
Gene expression in interscapular brown (A), thoracic periaortic brown (B), and visceral white perigonadal (C) AT in WT and UCP1−/− mice fed a control diet vs. Western diet. Data are expressed as means ± SE. Significant P values (≤0.05) are highlighted in bold; n = 9–14 per group. Open bars, WT-CD; gray bars, WT-WD; black bars, UCP1−/− CD; striped bars, UCP1 WD.
In visceral white AT, mRNA markers of inflammation (e.g., MCP-1, TNF-α, CCL5), immune cell infiltration (e.g., CD68, ITGAM, NLRP3, LGALS3, and CD11c), oxidative stress (e.g., p22phox and p47phox), and the noncanonical Wnt5a signaling protein were elevated with Western diet, while loss of UCP1-induced MCP-1 and IL-6 expression (P < 0.05, Fig. 5C). Collectively, these changes in gene expression suggest that loss of UCP1 leads to immune cell infiltration, inflammation, oxidative stress, and ER stress in brown fat, whereas less pronounced effects occur in visceral white AT.
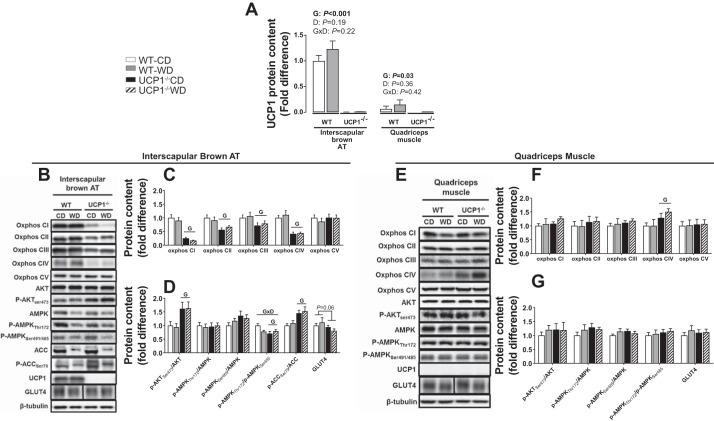
Akt and AMPK activity and oxidative phosphorylation in interscapular brown AT and quadriceps muscle.
UCP1 protein content was undetectable in both interscapular brown AT and quadriceps muscle of UCP1-null mice (Fig. 6A), while 18 of 23 WT animals also exhibited undetectable UCP1 content in muscle (Fig. 6E). In interscapular brown AT, the AMPK activation and inhibition sites pThr172 and pSer485 were not affected by either Western diet or UCP1 ablation, respectively (Fig. 6, B and D). However, the pThr172/pSer485 AMPK ratio was lower in UCP1−/− mice (Fig. 6D). The inhibitory site of ACC, a proxy for AMPK activity, pSer79 was increased in UCP1−/− mice, whereas the activation site of Akt, pSer473 was increased by UCP1 removal, with no effect of Western diet (Fig. 6, B and D). UCP1−/− mice, independent of diet, showed attenuated oxphos protein content compared with WT mice (Genotype effect, P < 0.05 in complex I, II, III, and IV, Fig. 6C). GLUT4 protein tended to be decreased by UCP1 deletion in interscapular brown AT (P = 0.06, Fig. 6D); however, there was no effect of diet.
Fig. 6.
Interscapular brown AT (A–D) and muscle protein content (A, B, E, F). A: UCP1 protein content (A), oxphos protein (C, E), Akt, AMPK, and GLUT4 protein (D, F) in WT and UCP1−/− mice fed a control diet vs. Western diet. Blots were normalized to β-tubulin. B: one representative β-tubulin blot is presented. p-Akt, and p-AMPK, p-ACC were normalized to total Akt, AMPK, and ACC, respectively. Protein levels are expressed as fold change (means ± SE) to the wild-type control diet group, which was set at 1. A: protein levels are expressed as fold change (means ± SE) to interscapular brown AT wild-type control diet group (i.e., 1). Representative bands for GLUT4 were obtained from the same gel but are noncontiguous, as denoted by separation with a black line. Significant P values (≤0.05) are highlighted in bold. (n = 9–13 per group).
In skeletal muscle, no effects of genotype or diet were found on AMPK or Akt phosphorylation sites, GLUT4, and oxphos CI–CIII, and CV protein content (Fig. 6, B, E–F). However, we did find an increase in oxphos CIV protein content in UCP1−/− mice. This is consistent with the idea that UCP1 plays a lesser role in modulating the skeletal muscle phenotype likely due to its low expression (Fig. 6A). However, it does not rule out the possibility for adaptive changes in skeletal muscle energy flux as a consequence of whole body UCP1 removal.
DISCUSSION
The most salient finding of this investigation was that, despite not affecting energy balance or total adiposity, deletion of UCP1 exacerbated Western diet-induced glucose intolerance, an effect that was associated with whitening of brown AT and development of hepatic steatosis. These findings support the new notion that UCP1 exerts protective metabolic effects that are not contingent upon alterations in energy expenditure and/or body composition.
Western diet led to increased expression of UCP1 mRNA in interscapular and thoracic periaortic brown fat, and this finding is consistent with prior work by our group (7, 61) and others (4, 12, 48, 66) using various rodent models of obesity (e.g., induced by ovariectomy, hyperphagia, and diet). Although the mechanisms responsible for this “paradoxical” upregulation of UCP1 in brown fat depots in obesity remain incompletely understood (reviewed in Ref. 13), our findings are consistent with the hypothesis that obesity-mediated induction of UCP1 represents a compensatory and protective response to buffer metabolic dysfunction during chronic energy overload. This hypothesis is supported by the fact that diet-induced thermogenesis is abrogated in UCP1-deficient animals (12). It is conceivable that the metabolic protection afforded by UCP1 in the setting of obesity may be attributed to the retention of multilocular brown adipocytes and abundant mitochondria (Fig. 2). Indeed, an important and novel finding of the present study was that, in mice lacking UCP1, Western diet produced a marked switch to unilocular, lipid-filled, adipocytes in brown fat depots, thus resembling white AT. This suggests that the absence of UCP1, which is needed for fatty acid-induced mitochondrial uncoupling, resulted in decreased fatty acid turnover and a shift from an oxidative to a storage phenotype in brown AT in our cohort of female mice. The observed whitening of brown AT caused by loss of UCP1 was also accompanied by decreased oxidative phosphorylation proteins, which further lends support for an AT storage (e.g., lipid) phenotype in the absence of UCP1. Western diet-induced whitening of brown AT that was exacerbated by ablation of UCP1 was also paralleled by increased expression of inflammatory genes in brown AT depots. The fact that the exacerbating effects of UCP1 deficiency on AT inflammation were more pronounced in brown than white AT depots is, to our knowledge, a new finding showing that anti-inflammatory effects of UCP1 are predominantly conferred within high UCP1-expressing fat depots.
Our data do not support changes in overall adiposity or energy expenditure with UCP1 removal; however, we found increased interscapular brown AT mass, histologically, attributed to increased lipid storage (Fig. 2). It does not appear that this increase in interscapular brown adiposity is associated with an increase in lipogenic machinery, given that the inhibitory site on ACC, which prevents malonyl-CoA formation (11, 46), was elevated in UCP1−/− animals. Notably, consistent with our findings, Inokuma et al. (19) demonstrated no changes in body weight or energy intake of UCP1−/− mice housed at 26°C compared with WT mice consuming a cafeteria diet despite increased brown AT mass as a percentage of total body weight. Furthermore, compared with the leptin-deficient mouse (ob/ob), the double-mutant ob/ob-UCP1−/− mouse exhibits indistinguishable adiposity and energy expenditure (housing conditions: 21°C–28°C) (58), indicating that UCP1 may not be required for the control of energy balance under these environmental conditions. In contrast, when UCP1 knockout mice are housed at a temperature exceeding 28°C, they become obesity prone, likely explained by the increase in metabolic efficiency (12). Thus, the uncoupled metabolic perturbations of UCP1 deficiency and obesity are not completely understood and require further investigation.
Our finding that loss of UCP1 worsened Western diet-induced liver steatosis is also of significance. Given that AT has been implicated in the development of fatty liver, it is possible that inflammation in brown AT and resulting “batokine” dysregulation (62) contributed to liver steatosis; however, more research is needed to establish this link between brown AT dysfunction and fatty liver. Alternatively, it is also possible that lack of UCP1 in the liver played a direct role in the development of fatty liver. In this regard, prior work indicates that hepatic adenoviral gene delivery of UCP1 prevents high-fat diet-induced liver steatosis, and this is associated with downregulation of genes related to fatty acid synthesis and a marked increase in the expression of genes related to fatty acid oxidation (20). In addition, transgenic upregulation of UCP1 in skeletal muscle prevents diet-induced obesity and insulin resistance in mice (25, 34); however, expression/content of UCP1 in muscle of WT rodents is often undetectable (22, 33, 34) or minimal (1) (Fig. 6A), questioning the relative role of muscle-specific UCP1 activity in normogenic animals.
In addition to the thermogenic role of brown AT, accumulating evidence in both animals (3, 53, 54) and humans (6, 29) has identified a role of brown AT in regulating glucose homeostasis and insulin sensitivity, whereby brown AT is inversely associated with insulin resistance and Type 2 diabetes (9, 35). One interpretation of the inverse association between Type 2 diabetes and lower presence of detectable brown AT posits that reduced activity of brown AT may predispose one to Type 2 diabetes via obesity and decreased glucose disposal in brown AT (32). Notably, our findings lend support to this concept but demonstrate that obesity is not required for loss of UCP1 to cause glucose intolerance. Indeed, independent of changes in body weight and total adiposity, we found that deletion of UCP1 impaired glucose homeostasis. To put it in perspective, the degree of glucose intolerance caused by deletion of UCP1 in control diet-fed mice was similar to that produced by long-term Western diet feeding-induced obesity. Glycemic dysregulation and hyperinsulinemia induced by Western diet were further exacerbated with loss of UCP1, raising the possibility that these effects were mediated by whitening of brown AT. More research is needed to establish whether “rebrowning” of whitened brown AT restores glucose homeostasis. Further investigation is also warranted to determine whether rebrowning of whitened brown periaortic AT corrects vascular dysfunction associated with obesity.
Our study design does not permit tissue-specific assessment of glucose intolerance. However, basal GLUT4 content was attenuated (P = 0.06) in interscapular brown AT of UCP1-deficient mice, despite increased pAktSer473, suggesting that important components of intracellular glucose trafficking are impaired. These data build on our previous findings that obese rats with low fitness exhibit decreased brown AT glucose disposal (38), as well as data from others demonstrating that high-fat feeding impairs brown AT insulin signaling (31). In addition, Inokuma et al. (18) revealed the importance of UCP1 in adrenergically stimulated glucose uptake, since UCP1 knockout prevented brown AT glucose uptake, and elegant data by Thoonen et al. (56) indicate that glucose intolerance induced via UCP1 deficiency is restored via functional brown AT transplant into UCP1−/− mice, supporting a direct role of brown AT function in glucose homeostasis.
Notably, in the present study, the effects of UCP1 deletion on systemic glucose homeostasis appear to be largely independent of skeletal muscle mitochondrial oxidative phosphorylation complexes, GLUT4 content, and components of insulin signaling and glucose trafficking (i.e., pAkt, pAMPK). However, complex IV of the respiratory chain was increased in skeletal muscle in response to UCP1 deficiency, which is likely a compensatory response to attenuated brown AT nonshivering thermogenesis. Thus, it is possible that skeletal muscle compensates for the loss of uncoupled metabolism due to UCP1 deficiency. Likewise, surgical ablation of brown AT or genetic deletion of UCP1 has been demonstrated to increase futile cycling in skeletal muscle in an effort to compensate for the loss of brown AT thermogenesis (2, 44). It is conceivable that in the setting of UCP1 deficiency compounded by excess energy intake, futile cycling in skeletal muscle may effectively regulate cellular energy status (2), although this hypothesis has recently been challenged (45). Accordingly, despite the potential adaptive increase in muscle thermogenic activity, it does not appear to be sufficient to prevent glucose intolerance and fatty liver in our cohort of UCP1-deficient female mice. Thus, further experiments should be done using in vivo glucose tracers or in vitro tests of insulin action to trace the organ or tissues (i.e., liver, skeletal muscle, adipose tissue) responsible for insulin resistance.
With respect to human obesity, the role of UCP1 and brown AT is not completely understood. Nonetheless, brown AT is reduced in human obesity and Type 2 diabetes, and elucidating the mechanisms and implications for these changes is an intense area of human research today. Sampling brown AT at multiple sites in obese vs. lean humans as done in mice has not been performed. There is no quantitative data demonstrating similar increases in brown AT mass in humans with obesity. In fact, the increased brown AT activity following exposure to cooling (15°C) is significantly attenuated when quantified by 18-FDG-PET fusion scans in healthy obese compared with lean men (59). Nevertheless, after cold exposure, UCP1 protein can still be demonstrated in overweight men in whom UCP1 immunohistochemical staining was 61 times higher in supraclavicular fat than subcutaneous white AT of the same subject (5). In addition, mitochondrial respiration of brown AT human biopsies were largely suppressed by the UCP1 inhibitor, GDP, an effect that was absent in white AT (5). These findings were corroborated by the same laboratory, who further indicated that the GDP inhibition of UCP1 was similar between human subclavicular brown AT and rodent interscapular brown AT (39). Thus, despite the lack of direct comparisons between brown AT from lean and obese humans, preliminary evidence supports a functional role of UCP1 in human subclavicular brown AT (39). Additional studies are required to determine the clinical significance of these findings and therapeutic potential.
Perspectives and Significance
Herein, we have provided a detailed analysis of the metabolic phenotype of the UCP1−/− female mouse at the specific environmental temperature of 25°C, where the adaptation of skeletal muscle and other muscle to generate heat results in an increase in energy expenditure that may compensate for the loss of functional brown AT. We found that deletion of UCP1 aggravated Western diet-induced disruption of glucose metabolism accompanied by a whitening of UCP1-expressing tissues, namely, interscapular and thoracic periaortic brown AT, as well as development of liver steatosis. Thus, our data support the hypothesis that expression of UCP1 in brown fat buffers metabolic derangements during chronic energy overload. Notably, loss of metabolic protection caused by UCP1 deficiency was independent of changes in body weight, total adiposity, and energy expenditure. The uncoupled metabolic perturbations of UCP1 loss and obesity underscore the importance of UCP1’s impact on glucose and lipid metabolism.
GRANTS
This study was supported by grants from the Cardiometabolic Disease Research Foundation (to J. Padilla), the Sears Trust Research Foundation (to J. Padilla), National Institutes of Health (NIH) K01 HL-125503 (to J. Padilla) and R21 DK-105368 (to J. Padilla), the MU Research Council (to V. Vieira-Potter), MU Richard Wallace Faculty Incentive Grant (to V. Vieira-Potter), and the NIH Initiative for Maximizing Student Diversity EXPRESS Fellows Program R25GM056901 (to T. Gaines).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
N.C.W., M.L.G., R.J.W., R.J.S., T.M.Z., T.L.G., M.L.W., N.G.K., and J.P. performed experiments; N.C.W., V.J.V.-P., M.L.G., R.J.W., and J.P. analyzed data; N.C.W., V.J.V.-P., H.S.S., and J.P. interpreted results of experiments; N.C.W. prepared figures; N.C.W. drafted manuscript; N.C.W., V.J.V.-P., T.M.Z., J.A.K., H.S.S., and J.P. edited and revised manuscript; N.C.W., V.J.V.-P., M.L.G., R.J.W., R.J.S., T.M.Z., M.L.W., N.G.K., J.A.K., H.S.S., and J.P. approved final version of manuscript.
ACKNOWLEDGMENTS
We kindly acknowledge Katherine S. Wainright for her technical assistance.
REFERENCES
- 1.Almind K, Manieri M, Sivitz WI, Cinti S, Kahn CR. Ectopic brown adipose tissue in muscle provides a mechanism for differences in risk of metabolic syndrome in mice. Proc Natl Acad Sci USA 104: 2366–2371, 2007. doi: 10.1073/pnas.0610416104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bal NC, Maurya SK, Sopariwala DH, Sahoo SK, Gupta SC, Shaikh SA, Pant M, Rowland LA, Bombardier E, Goonasekera SA, Tupling AR, Molkentin JD, Periasamy M. Sarcolipin is a newly identified regulator of muscle-based thermogenesis in mammals. Nat Med 18: 1575–1579, 2012. doi: 10.1038/nm.2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartelt A, Bruns OT, Reimer R, Hohenberg H, Ittrich H, Peldschus K, Kaul MG, Tromsdorf UI, Weller H, Waurisch C, Eychmüller A, Gordts PL, Rinninger F, Bruegelmann K, Freund B, Nielsen P, Merkel M, Heeren J. Brown adipose tissue activity controls triglyceride clearance. Nat Med 17: 200–205, 2011. doi: 10.1038/nm.2297. [DOI] [PubMed] [Google Scholar]
- 4.Cannon B, Nedergaard J. Metabolic consequences of the presence or absence of the thermogenic capacity of brown adipose tissue in mice (and probably in humans). Int J Obes 34, Suppl 1: S7–S16, 2010. doi: 10.1038/ijo.2010.177. [DOI] [PubMed] [Google Scholar]
- 5.Chondronikola M, Annamalai P, Chao T, Porter C, Saraf MK, Cesani F, Sidossis LS. A percutaneous needle biopsy technique for sampling the supraclavicular brown adipose tissue depot of humans. Int J Obes 39: 1561–1564, 2015. doi: 10.1038/ijo.2015.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chondronikola M, Volpi E, Børsheim E, Porter C, Annamalai P, Enerbäck S, Lidell ME, Saraf MK, Labbe SM, Hurren NM, Yfanti C, Chao T, Andersen CR, Cesani F, Hawkins H, Sidossis LS. Brown adipose tissue improves whole-body glucose homeostasis and insulin sensitivity in humans. Diabetes 63: 4089–4099, 2014. doi: 10.2337/db14-0746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crissey JM, Jenkins NT, Lansford KA, Thorne PK, Bayless DS, Vieira-Potter VJ, Rector RS, Thyfault JP, Laughlin MH, Padilla J. Adipose tissue and vascular phenotypic modulation by voluntary physical activity and dietary restriction in obese insulin-resistant OLETF rats. Am J Physiol Regul Integr Comp Physiol 306: R596–R606, 2014. doi: 10.1152/ajpregu.00493.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crissey JM, Padilla J, Vieira-Potter VJ, Thorne PK, Koch LG, Britton SL, Thyfault JP, Laughlin MH. Divergent role of nitric oxide in insulin-stimulated aortic vasorelaxation between low- and high-intrinsic aerobic capacity rats. Physiol Rep 3: e12459, 2015. doi: 10.14814/phy2.12459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo FC, Palmer EL, Tseng YH, Doria A, Kolodny GM, Kahn CR. Identification and importance of brown adipose tissue in adult humans. N Engl J Med 360: 1509–1517, 2009. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Enerbäck S, Jacobsson A, Simpson EM, Guerra C, Yamashita H, Harper ME, Kozak LP. Mice lacking mitochondrial uncoupling protein are cold-sensitive but not obese. Nature 387: 90–94, 1997. doi: 10.1038/387090a0. [DOI] [PubMed] [Google Scholar]
- 11.Fediuc S, Gaidhu MP, Ceddia RB. Regulation of AMP-activated protein kinase and acetyl-CoA carboxylase phosphorylation by palmitate in skeletal muscle cells. J Lipid Res 47: 412–420, 2006. doi: 10.1194/jlr.M500438-JLR200. [DOI] [PubMed] [Google Scholar]
- 12.Feldmann HM, Golozoubova V, Cannon B, Nedergaard J. UCP1 ablation induces obesity and abolishes diet-induced thermogenesis in mice exempt from thermal stress by living at thermoneutrality. Cell Metab 9: 203–209, 2009. doi: 10.1016/j.cmet.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 13.Fromme T, Klingenspor M. Uncoupling protein 1 expression and high-fat diets. Am J Physiol Regul Integr Comp Physiol 300: R1–R8, 2011. doi: 10.1152/ajpregu.00411.2010. [DOI] [PubMed] [Google Scholar]
- 14.Gao M, Ma Y, Liu D. High-fat diet-induced adiposity, adipose inflammation, hepatic steatosis and hyperinsulinemia in outbred CD-1 mice. PLoS One 10: e0119784, 2015. doi: 10.1371/journal.pone.0119784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ginsberg HN, MacCallum PR. The obesity, metabolic syndrome, and type 2 diabetes mellitus pandemic: Part I. Increased cardiovascular disease risk and the importance of atherogenic dyslipidemia in persons with the metabolic syndrome and type 2 diabetes mellitus. J Cardiometab Syndr 4: 113–119, 2009. doi: 10.1111/j.1559-4572.2008.00044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gutierrez DA, Puglisi MJ, Hasty AH. Impact of increased adipose tissue mass on inflammation, insulin resistance, and dyslipidemia. Curr Diab Rep 9: 26–32, 2009. doi: 10.1007/s11892-009-0006-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang ZH, Manickam B, Ryvkin V, Zhou XJ, Fantuzzi G, Mazzone T, Sam S. PCOS is associated with increased CD11c expression and crown-like structures in adipose tissue and increased central abdominal fat depots independent of obesity. J Clin Endocrinol Metab 98: E17–E24, 2013. doi: 10.1210/jc.2012-2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Inokuma K, Ogura-Okamatsu Y, Toda C, Kimura K, Yamashita H, Saito M. Uncoupling protein 1 is necessary for norepinephrine-induced glucose utilization in brown adipose tissue. Diabetes 54: 1385–1391, 2005. doi: 10.2337/diabetes.54.5.1385. [DOI] [PubMed] [Google Scholar]
- 19.Inokuma K, Okamatsu-Ogura Y, Omachi A, Matsushita Y, Kimura K, Yamashita H, Saito M. Indispensable role of mitochondrial UCP1 for antiobesity effect of beta3-adrenergic stimulation. Am J Physiol Endocrinol Metab 290: E1014–E1021, 2006. doi: 10.1152/ajpendo.00105.2005. [DOI] [PubMed] [Google Scholar]
- 20.Ishigaki Y, Katagiri H, Yamada T, Ogihara T, Imai J, Uno K, Hasegawa Y, Gao J, Ishihara H, Shimosegawa T, Sakoda H, Asano T, Oka Y. Dissipating excess energy stored in the liver is a potential treatment strategy for diabetes associated with obesity. Diabetes 54: 322–332, 2005. doi: 10.2337/diabetes.54.2.322. [DOI] [PubMed] [Google Scholar]
- 21.Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, Kitazawa R, Kitazawa S, Miyachi H, Maeda S, Egashira K, Kasuga M. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest 116: 1494–1505, 2006. doi: 10.1172/JCI26498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klaus S, Rudolph B, Dohrmann C, Wehr R. Expression of uncoupling protein 1 in skeletal muscle decreases muscle energy efficiency and affects thermoregulation and substrate oxidation. Physiol Genomics 21: 193–200, 2005. doi: 10.1152/physiolgenomics.00299.2004. [DOI] [PubMed] [Google Scholar]
- 23.Kontani Y, Wang Y, Kimura K, Inokuma KI, Saito M, Suzuki-Miura T, Wang Z, Sato Y, Mori N, Yamashita H. UCP1 deficiency increases susceptibility to diet-induced obesity with age. Aging Cell 4: 147–155, 2005. doi: 10.1111/j.1474-9726.2005.00157.x. [DOI] [PubMed] [Google Scholar]
- 24.Kopecký J, Rossmeisl M, Hodný Z, Syrový I, Horáková M, Kolárová P. Reduction of dietary obesity in aP2-Ucp transgenic mice: mechanism and adipose tissue morphology. Am J Physiol Endocrinol Metab 270: E776–E786, 1996. [DOI] [PubMed] [Google Scholar]
- 25.Li B, Nolte LA, Ju JS, Han DH, Coleman T, Holloszy JO, Semenkovich CF. Skeletal muscle respiratory uncoupling prevents diet-induced obesity and insulin resistance in mice. Nat Med 6: 1115–1120, 2000. doi: 10.1038/80450. [DOI] [PubMed] [Google Scholar]
- 26.Liu X, Rossmeisl M, McClaine J, Riachi M, Harper ME, Kozak LP. Paradoxical resistance to diet-induced obesity in UCP1-deficient mice. J Clin Invest 111: 399–407, 2003. doi: 10.1172/JCI200315737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lomonaco R, Ortiz-Lopez C, Orsak B, Webb A, Hardies J, Darland C, Finch J, Gastaldelli A, Harrison S, Tio F, Cusi K. Effect of adipose tissue insulin resistance on metabolic parameters and liver histology in obese patients with nonalcoholic fatty liver disease. Hepatology 55: 1389–1397, 2012. doi: 10.1002/hep.25539. [DOI] [PubMed] [Google Scholar]
- 28.Manolopoulos KN, Karpe F, Frayn KN. Gluteofemoral body fat as a determinant of metabolic health. Int J Obes 34: 949–959, 2010. doi: 10.1038/ijo.2009.286. [DOI] [PubMed] [Google Scholar]
- 29.Matsushita M, Yoneshiro T, Aita S, Kameya T, Sugie H, Saito M. Impact of brown adipose tissue on body fatness and glucose metabolism in healthy humans. Int J Obes 38: 812–817, 2014. doi: 10.1038/ijo.2013.206. [DOI] [PubMed] [Google Scholar]
- 30.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28: 412–419, 1985. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 31.Nadal-Casellas A, Bauzá-Thorbrügge M, Proenza AM, Gianotti M, Lladó I. Sex-dependent differences in rat brown adipose tissue mitochondrial biogenesis and insulin signaling parameters in response to an obesogenic diet. Mol Cell Biochem 373: 125–135, 2013. doi: 10.1007/s11010-012-1481-x. [DOI] [PubMed] [Google Scholar]
- 32.Nedergaard J, Bengtsson T, Cannon B. Three years with adult human brown adipose tissue. Ann N Y Acad Sci 1212: E20–E36, 2010. doi: 10.1111/j.1749-6632.2010.05905.x. [DOI] [PubMed] [Google Scholar]
- 33.Nedergaard J, Golozoubova V, Matthias A, Asadi A, Jacobsson A, Cannon B. UCP1: the only protein able to mediate adaptive non-shivering thermogenesis and metabolic inefficiency. Biochim Biophys Acta 1504: 82–106, 2001. doi: 10.1016/S0005-2728(00)00247-4. [DOI] [PubMed] [Google Scholar]
- 34.Neschen S, Katterle Y, Richter J, Augustin R, Scherneck S, Mirhashemi F, Schürmann A, Joost HG, Klaus S. Uncoupling protein 1 expression in murine skeletal muscle increases AMPK activation, glucose turnover, and insulin sensitivity in vivo. Physiol Genomics 33: 333–340, 2008. doi: 10.1152/physiolgenomics.00226.2007. [DOI] [PubMed] [Google Scholar]
- 35.Ouellet V, Routhier-Labadie A, Bellemare W, Lakhal-Chaieb L, Turcotte E, Carpentier AC, Richard D. Outdoor temperature, age, sex, body mass index, and diabetic status determine the prevalence, mass, and glucose-uptake activity of 18F-FDG-detected BAT in humans. J Clin Endocrinol Metab 96: 192–199, 2011. doi: 10.1210/jc.2010-0989. [DOI] [PubMed] [Google Scholar]
- 36.Padilla J, Jenkins NT, Roberts MD, Arce-Esquivel AA, Martin JS, Laughlin MH, Booth FW. Differential changes in vascular mRNA levels between rat iliac and renal arteries produced by cessation of voluntary running. Exp Physiol 98: 337–347, 2013. doi: 10.1113/expphysiol.2012.066076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Padilla J, Jenkins NT, Vieira-Potter VJ, Laughlin MH. Divergent phenotype of rat thoracic and abdominal perivascular adipose tissues. Am J Physiol Regul Integr Comp Physiol 304: R543–R552, 2013. doi: 10.1152/ajpregu.00567.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park YM, Rector RS, Thyfault JP, Zidon TM, Padilla J, Welly RJ, Meers GM, Morris ME, Britton SL, Koch LG, Booth FW, Kanaley JA, Vieira-Potter VJ. Effects of ovariectomy and intrinsic aerobic capacity on tissue-specific insulin sensitivity. Am J Physiol Endocrinol Metab 310: E190–E199, 2016. doi: 10.1152/ajpendo.00434.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Porter C, Herndon DN, Chondronikola M, Chao T, Annamalai P, Bhattarai N, Saraf MK, Capek KD, Reidy PT, Daquinag AC, Kolonin MG, Rasmussen BB, Borsheim E, Toliver-Kinsky T, Sidossis LS. Human and mouse brown adipose tissue mitochondria have comparable UCP1 function. Cell Metab 24: 246–255, 2016. doi: 10.1016/j.cmet.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Razzoli M, Frontini A, Gurney A, Mondini E, Cubuk C, Katz LS, Cero C, Bolan PJ, Dopazo J, Vidal-Puig A, Cinti S, Bartolomucci A. Stress-induced activation of brown adipose tissue prevents obesity in conditions of low adaptive thermogenesis. Mol Metab 5: 19–33, 2015. doi: 10.1016/j.molmet.2015.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reaven GM. Insulin resistance: the link between obesity and cardiovascular disease. Med Clin North Am 95: 875–892, 2011. doi: 10.1016/j.mcna.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 42.Roberts-Toler C, O’Neill BT, Cypess AM. Diet-induced obesity causes insulin resistance in mouse brown adipose tissue. Obesity (Silver Spring) 23: 1765–1770, 2015. doi: 10.1002/oby.21134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roseguini BT, Mehmet Soylu S, Whyte JJ, Yang HT, Newcomer S, Laughlin MH. Intermittent pneumatic leg compressions acutely upregulate VEGF and MCP-1 expression in skeletal muscle. Am J Physiol Heart Circ Physiol 298: H1991–H2000, 2010. doi: 10.1152/ajpheart.00006.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rowland LA, Bal NC, Kozak LP, Periasamy M. Uncoupling protein 1 and sarcolipin are required to maintain optimal thermogenesis, and loss of both systems compromises survivial of mice under cold stress. J Biol Chem 290: 12282–12289, 2015. doi: 10.1074/jbc.M115.637603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rowland LA, Maurya SK, Bal NC, Kozak L, Periasamy M. Sarcolipin and uncoupling protein 1 play distinct roles in diet-induced thermogenesis and do not compensate for one another. Obesity (Silver Spring) 24: 1430–1433, 2016. doi: 10.1002/oby.21542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ruderman NB, Saha AK, Vavvas D, Witters LA. Malonyl-CoA, fuel sensing, and insulin resistance. Am J Physiol Endocrinol Metab 276: E1–E18, 1999. [DOI] [PubMed] [Google Scholar]
- 47.Saito M, Okamatsu-Ogura Y, Matsushita M, Watanabe K, Yoneshiro T, Nio-Kobayashi J, Iwanaga T, Miyagawa M, Kameya T, Nakada K, Kawai Y, Tsujisaki M. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes 58: 1526–1531, 2009. doi: 10.2337/db09-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sakamoto T, Nitta T, Maruno K, Yeh YS, Kuwata H, Tomita K, Goto T, Takahashi N, Kawada T. Macrophage infiltration into obese adipose tissues suppresses the induction of UCP1 expression in mice. Am J Physiol Endocrinol Metab 310: 676-687, 2016. doi: 10.1152/ajpendo.00028.2015. [DOI] [PubMed] [Google Scholar]
- 49.Shimizu I, Aprahamian T, Kikuchi R, Shimizu A, Papanicolaou KN, MacLauchlan S, Maruyama S, Walsh K. Vascular rarefaction mediates whitening of brown fat in obesity. J Clin Invest 124: 2099–2112, 2014. doi: 10.1172/JCI71643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shimizu I, Walsh K. The whitening of brown fat and its implications for weight management in obesity. Curr Obes Rep 4: 224–229, 2015. doi: 10.1007/s13679-015-0157-8. [DOI] [PubMed] [Google Scholar]
- 51.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest 116: 1793–1801, 2006. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Speakman JR, Keijer J. Not so hot: Optimal housing temperatures for mice to mimic the thermal environment of humans. Mol Metab 2: 5–9, 2012. doi: 10.1016/j.molmet.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stanford KI, Middelbeek RJ, Townsend KL, An D, Nygaard EB, Hitchcox KM, Markan KR, Nakano K, Hirshman MF, Tseng YH, Goodyear LJ. Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. J Clin Invest 123: 215–223, 2013. doi: 10.1172/JCI62308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stanford KI, Middelbeek RJ, Townsend KL, Lee MY, Takahashi H, So K, Hitchcox KM, Markan KR, Hellbach K, Hirshman MF, Tseng YH, Goodyear LJ. A novel role for subcutaneous adipose tissue in exercise-induced improvements in glucose homeostasis. Diabetes 64: 2002–2014, 2015. doi: 10.2337/db14-0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sutherland LN, Bomhof MR, Capozzi LC, Basaraba SA, Wright DC. Exercise and adrenaline increase PGC-1alpha mRNA expression in rat adipose tissue. J Physiol 587: 1607–1617, 2009. doi: 10.1113/jphysiol.2008.165464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thoonen R, Ernande L, Cheng J, Nagasaka Y, Yao V, Miranda-Bezerra A, Chen C, Chao W, Panagia M, Sosnovik DE, Puppala D, Armoundas AA, Hindle A, Bloch KD, Buys ES, Scherrer-Crosbie M. Functional brown adipose tissue limits cardiomyocyte injury and adverse remodeling in catecholamine-induced cardiomyopathy. J Mol Cell Cardiol 84: 202–211, 2015. doi: 10.1016/j.yjmcc.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Trevellin E, Scorzeto M, Olivieri M, Granzotto M, Valerio A, Tedesco L, Fabris R, Serra R, Quarta M, Reggiani C, Nisoli E, Vettor R. Exercise training induces mitochondrial biogenesis and glucose uptake in subcutaneous adipose tissue through eNOS-dependent mechanisms. Diabetes 63: 2800–2811, 2014. doi: 10.2337/db13-1234. [DOI] [PubMed] [Google Scholar]
- 58.Ukropec J, Anunciado RV, Ravussin Y, Kozak LP. Leptin is required for uncoupling protein-1-independent thermogenesis during cold stress. Endocrinology 147: 2468–2480, 2006. doi: 10.1210/en.2005-1216. [DOI] [PubMed] [Google Scholar]
- 59.van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, Schrauwen P, Teule GJ. Cold-activated brown adipose tissue in healthy men. N Engl J Med 360: 1500–1508, 2009. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- 60.Vieira-Potter VJ. Inflammation and macrophage modulation in adipose tissues. Cell Microbiol 16: 1484–1492, 2014. doi: 10.1111/cmi.12336. [DOI] [PubMed] [Google Scholar]
- 61.Vieira-Potter VJ, Padilla J, Park YM, Welly RJ, Scroggins RJ, Britton SL, Koch LG, Jenkins NT, Crissey JM, Zidon T, Morris EM, Meers GM, Thyfault JP. Female rats selectively bred for high intrinsic aerobic fitness are protected from ovariectomy-associated metabolic dysfunction. Am J Physiol Regul Integr Comp Physiol 308: R530–R542, 2015. doi: 10.1152/ajpregu.00401.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Villarroya J, Cereijo R, Villarroya F. An endocrine role for brown adipose tissue? Am J Physiol Endocrinol Metab 305: E567–E572, 2013. doi: 10.1152/ajpendo.00250.2013. [DOI] [PubMed] [Google Scholar]
- 63.Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T, Taittonen M, Laine J, Savisto NJ, Enerbäck S, Nuutila P. Functional brown adipose tissue in healthy adults. N Engl J Med 360: 1518–1525, 2009. doi: 10.1056/NEJMoa0808949. [DOI] [PubMed] [Google Scholar]
- 64.Vitali A, Murano I, Zingaretti MC, Frontini A, Ricquier D, Cinti S. The adipose organ of obesity-prone C57BL/6J mice is composed of mixed white and brown adipocytes. J Lipid Res 53: 619–629, 2012. doi: 10.1194/jlr.M018846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wainright KS, Fleming NJ, Rowles JL, Welly RJ, Zidon TM, Park YM, Gaines TL, Scroggins RJ, Anderson-Baucum EK, Hasty AH, Vieira-Potter VJ, Padilla J. Retention of sedentary obese visceral white adipose tissue phenotype with intermittent physical activity despite reduced adiposity. Am J Physiol Regul Integr Comp Physiol 309: R594–R602, 2015. doi: 10.1152/ajpregu.00042.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yao L, Heuser-Baker J, Herlea-Pana O, Zhang N, Szweda LI, Griffin TM, Barlic-Dicen J. Deficiency in adipocyte chemokine receptor CXCR4 exacerbates obesity and compromises thermoregulatory responses of brown adipose tissue in a mouse model of diet-induced obesity. FASEB J 28: 4534–4550, 2014. doi: 10.1096/fj.14-249797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zingaretti MC, Crosta F, Vitali A, Guerrieri M, Frontini A, Cannon B, Nedergaard J, Cinti S. The presence of UCP1 demonstrates that metabolically active adipose tissue in the neck of adult humans truly represents brown adipose tissue. FASEB J 23: 3113–3120, 2009. doi: 10.1096/fj.09-133546. [DOI] [PubMed] [Google Scholar]