Abstract
Buildups of ammonia can cause potentially fatal brain swelling in mammals, but such swelling is reversible in the anoxia- and ammonia-tolerant goldfish (Carassius auratus). We investigated brain swelling and its possible relationship to oxidative stress in the brain and liver of goldfish acutely exposed to high external ammonia (HEA; 5 mmol/l NH4Cl) at two different acclimation temperatures (14°C, 4°C). Exposure to HEA at 14°C for 72h resulted in increased internal ammonia and glutamine concentrations in the brain, and it caused cellular oxidative damage in the brain and liver. However, oxidative damage was most pronounced in brain, in which there was a twofold increase in thiobarbituric acid–reactive substances, a threefold increase in protein carbonylation, and a 20% increase in water volume (indicative of brain swelling). Increased activities of catalase, glutathione peroxidase, and glutathione reductase in the brain suggested that goldfish upregulate their antioxidant capacity to partially offset oxidative stress during hyperammonemia at 14°C. Notably, acclimation to colder (4°C) water completely attenuated the oxidative stress response to HEA in both tissues, and there was no change in brain water volume despite similar increases in internal ammonia. We suggest that ammonia-induced oxidative stress may be responsible for the swelling of goldfish brain during HEA, but further studies are needed to establish a mechanistic link between reactive oxygen species production and brain swelling. Nevertheless, a high capacity to withstand oxidative stress in response to variations in internal ammonia likely explains why goldfish are more resilient to this stressor than most other vertebrates.
Keywords: ammonia toxicity, reactive oxygen species (ROS), protein carbonyls, TBARS, catalase, superoxide dismutase, brain swelling
the deamination of excess amino acids following feeding, vigorous swimming, and prolonged periods of starvation can cause ammonia to accumulate to toxic levels in the blood and tissues of fish (8, 65, 74). Low millimolar (mmol/l) concentrations of ammonia can also build up in confined aquaculture pens and in aquatic ecosystems because of anthropogenic inputs that include agricultural runoff, industrial effluent, and sewage waste, causing ammonia excretion via the gills to be inhibited, leading to both the accumulation of endogenous ammonia and uptake of exogenous ammonia (8, 44, 69). Elevated water pH as a consequence of increased photosynthetic activity, or life in saline-alkaline lakes, may also subject fishes to elevated internal ammonia due to reductions in the blood-water NH3 partial pressure gradient across the gills (72, 73, 75).
Increased internal ammonia concentrations, or hyperammonemia, can lead to decreased growth rates, altered energy metabolism, disruption of ionic balance, variation in hormone regulation, and increased vulnerability to disease (2, 7, 8, 11, 44, 51, 52). Although fish are more tolerant of ammonia than mammals, the relative resistance to ammonia’s toxic effects varies considerably across different fish species (8, 19, 44). In mammals, hyperammonemia resulting from acute liver failure or congenital defects in the ornithine urea cycle, can cause hyperexcitability, glutamate excitotoxicity, coma, convulsions, and life-threatening brain swelling (10). Such brain swelling is accompanied by increased intracranial pressure, brain herniation, and death in hyperammonemic mammals (10, 36). Teleosts exhibit similar symptoms to toxic buildups of internal ammonia, including hyperventilation and hyperexcitability, convulsions, coma, and death (8). Wilkie et al. (71) recently demonstrated that the brains of goldfish (Carassius auratus) and crucian carp (Carassius carassius) undergo significant, albeit reversible, swelling in response to acute exposure to high environmental ammonia (HEA). However, the cellular mechanisms underlying such brain swelling and tolerance still remain poorly understood.
While the underlying mechanisms of ammonia-induced brain swelling remain elusive, some researchers have suggested that reactive oxygen species (ROS) and reactive nitrogen species, such as superoxide radicals (O2˙−), nitric oxide (˙NO), hydrogen peroxide (H2O2), peroxynitrite (ONOO−), and hydroxyl radicals (˙OH), may mediate the downstream events leading to ammonia neurotoxicity (for reviews see Refs. 1, 36, 38, 64). Oxidative stress is induced when cellular antioxidant defenses can no longer counterbalance the increase of steady-state ROS levels and/or repair oxidative damage to the lipid, protein, or nucleic acid components of the cell (31). ROS can cause damage to lipids and proteins in the plasma membrane of neurons and glial cells of the brain, which may disrupt ionic and osmotic gradients, ultimately leading to cellular water uptake and brain swelling (for reviews see Refs. 1, 36, 38).
To protect cells from free radical-mediated damage, vertebrates have evolved numerous enzymatic and nonenzymatic antioxidant defenses that scavenge and subsequently neutralize ROS and reactive nitrogen species in the cell (30, 33, 50). This study focused on one aspect of antioxidant defense: antioxidant enzymes. The key antioxidant enzymes in fish include the primary antioxidant enzymes, catalase (CAT) and superoxide dismutase (SOD), and the glutathione-dependent antioxidant enzymes glutathione peroxidase (GPX), glutathione reductase (GR), and glutathione-S-transferases. The induction of oxidative stress and the robustness of the antioxidant response can vary considerably between fish species depending on their tolerance to stressors inducing oxidative damage (9, 30, 33, 50). In response to anoxia and reoxygenation, goldfish upregulate antioxidant defenses to effectively manage the post-anoxic increase in ROS production, limiting the extent of oxidative damage occurring in the brain and other tissues (33). Similar increases in antioxidant capacity have also been observed in goldfish exposed to a wide array of stressors capable of inducing ROS production (32, 34, 49, 50).
Numerous studies exist on ammonia toxicity in different fishes, but ammonia-induced oxidative stress and antioxidant defenses in fish are not fully understood. A number of studies have characterized how hepatic tissue responds to ammonia-induced oxidative stress (e.g., 50, 70), but much less is known about the response of the fish brain to ammonia. The aim of the present study was to elucidate how the brain of the ammonia- and anoxia-tolerant goldfish responds to ammonia. Because of their known tolerance of post-anoxic oxidative stress (33), we predicted that the goldfish brain would exhibit a pronounced antioxidant response, which would limit ammonia-induced oxidative damage to lipids and proteins. Given the role of therapeutic hypothermia and/or brain cooling in managing neurotoxicity in mammals (for a review see 14), we also predicted that ammonia-induced brain swelling and oxidative stress would be attenuated at colder ambient temperatures.
MATERIALS AND METHODS
Experimental Animals and Holding
Juvenile goldfish (n = 150) of both sexes, weighing 25.0 ± 0.7 g, were purchased from a commercial supplier (Aquality Tropical Fish Wholesale, Mississauga, Canada) and transferred to the fish aquatics facility at Wilfrid Laurier University. Fish were held in 110-liter aquaria continuously receiving filtered, aerated well water (pH ∼8.0 ± 0.2, temperature ∼14 ± 1°C, dissolved oxygen content >85% saturation, ammonia <0.01 mmol/l) at a rate of 0.5–1.0 l/min. A subset of fish (n = 65) was held in an aquarium connected to a Delta Star 4 air-cooled water chiller (Aqua Logic, San Diego, CA), in which the water temperature was cooled gradually (1°C/day) to a set point of 4 ± 1°C. Goldfish were acclimated to these holding conditions for at least 3 wk before experimentation. Fish were fed three times weekly with commercially available pellets, but were starved 2 days before experimentation to minimize the confounding effects that ammonia accumulation and defecation could have on water quality during static HEA experiments (50). All experiments were approved by the Wilfrid Laurier University Animal Care Committee and followed guidelines of the Canadian Council on Animal Care.
Experimental Protocol
The day before experiments, goldfish were transferred one at a time to individual, rectangular fish-holding chambers that were ~1–3 liters in volume and receiving aerated well water at a rate of 0.5–1.0 l/min. The chambers were darkened to minimize stress to the fish and were contained within a 150-liter recirculating system comprising a head tank, which drained via a flow splitter into each individual fish-holding chamber. Fish were either held under control conditions (no ammonia) or exposed to HEA, which was initiated by temporarily cutting off water flow to each chamber and adding a sufficient volume of 5 mol/l NH4Cl stock solution to the water to achieve a nominal total ammonia (TAmm) concentration of 5 mmol/l. At the same time, 5 mol/l NH4Cl stock was also added to the head tank to yield a target total ammonia concentration of 5 mmol/l in the recirculating system. Once the TAmm concentration in the recirculating system equilibrated (1 h), water flow was restored to the individual containers, and fish were held under these conditions for 24 or 72 h. Water pH was continuously monitored in the head tank with a Radiometer PHM84 pH meter, connected to a Radiometer GK2401C pH electrode (Radiometer, Copenhagen, Denmark) and spot checked in each fish-holding container with a portable pH meter (Oakton Instruments, Vernon Hills, IL). Water pH was controlled (pH 8.07 ± 0.01) by the dropwise addition of 0.1 N HCl into the head tank with a solenoid valve connected to a TTT80 Autotitrator (Radiometer) and a PHM84 meter. Water samples were collected at different time intervals (2, 8, 24, 36, 48, and 72 h) throughout HEA exposure and water TAmm concentrations measured to ensure that target concentrations were maintained.
At each sampling period (Control, 24 h, 72 h), fish were euthanized with 1.0 g/l tricaine methanesulfonate buffered with 2.0 g/l NaHCO3 (Syndel Laboratories, Nanaimo, Canada). Blood was collected immediately by caudal puncture with a 1.0-ml syringe fitted with a 21-gauge needle prerinsed with sodium heparin (∼50.0 U/ml; Sigma-Aldrich, St. Louis, MO) to prevent coagulation. Whole brain and liver were then carefully removed from each animal, snap frozen in liquid N2, and stored at −80°C until analyzed for the determination of ammonia and glutamine concentrations, indices of oxidative stress, and antioxidant enzymes. To determine the effect of ammonia exposure on brain water volume at each temperature (14°C, 4°C), fresh, intact brains were collected from two additional groups of goldfish, but weighed and dried to constant mass in a laboratory oven.
Analytical Techniques
Tissue and water ammonia concentrations.
Whole brain and liver tissues were prepared for ammonia and glutamine analysis by homogenization, and subsequent deproteination, in 7% ice-cold perchloric acid (PCA) using a Precellys 24-bead tissue homogenizer (Bertin Technologies) (67). The resulting slurry was incubated on ice for 10 min and centrifuged for 10 min at 10,000 g at 4°C. The supernatant was then withdrawn and neutralized with 2 N KOH, followed by immediate quantification of tissue ammonia and glutamine content. The TAmm concentrations in untreated plasma and neutralized extracts of brain and liver were quantified enzymatically with a commercially available assay kit (Cat. No. AA0100, Sigma-Aldrich), in which [TAmm] quantification was based on the glutamate dehydrogenase–catalyzed oxidation of NADPH, measured at 340 nm. Water TAmm concentrations were measured colorimetrically at 650 nm via the salicylate-hypochlorite method (62).
Tissue glutamine concentrations.
Tissue extracts of whole brain and liver were deproteinated and neutralized for glutamine analysis as described above. Briefly, tissue glutamine content was quantified colorimetrically at 540 nm by usinglutamine synthetase (GS) followed by the addition of ferric chloride, according to Mecke (35).
Indices of oxidative damage.
The concentration of malondialdehyde (MDA), a naturally occurring end product of lipid peroxidation and peroxidative tissue injury, was measured by using the thiobarbituric acid-reactive substances (TBARS) assay (39). For this assay, whole brain or liver tissue was sonicated in ice-cold radioimmunoprecipitation buffer (10% wt/vol) and centrifuged at 1,600 g for 10 min at 4°C. The supernatant was withdrawn and used for the determination of tissue TBARS content. Homogenates were mixed with SDS and thiobarbituric acid (TBA) in an acetate buffer (pH 3.5). Butylated hydroxytoluene, an antioxidant, was added to the mixture to avoid oxidation during subsequent heating, as recommended by Halliwell and Chirico (15). After incubation at 95°C for 60 min, the pink-red chromogen produced by the TBA-MDA adduct was mixed vigorously with an equal volume of n-butanol and centrifuged at 10,000 g for 5 min at 4°C. The butanol phase was removed and used to quantify the level of TBARS by measuring its absorbance at 532 nm. The level of TBARS was calculated and expressed in nanomoles of TBARS per gram wet tissue mass (wet mass).
Protein carbonyl content, the most commonly used biomarker of protein oxidative damage in animal tissues (6), was quantified with a commercially available assay kit (Item No. 10005020, Cayman Chemical, Ann Arbor, MI). Briefly, frozen brain and liver samples were homogenized (1:5 wt/vol) in ice-cold potassium phosphate (KPi) buffer (50 mM KPi, 1.1 mM EDTA, 0.1 mM PMSF, pH 6.7) and then centrifuged at 10,000 g for 15 min at 4°C. Homogenates were mixed with 2,4-dinitrophenylhydrazine and TCA, incubated in the dark at room temperature for 60 min, and then centrifuged at 10,000 g for 10 min at 4°C. The resulting supernatant was discarded, and the precipitated protein pellets were washed three times with ethanol/ethyl acetate (1:1 vol/vol) mixture to remove any unreacted 2,4-dinitrophenylhydrazine. The protein pellets were finally dissolved in guanidine hydrochloride and centrifuged as above to remove insoluble debris. Protein carbonyl content in the resulting supernatants was quantified spectrophotometrically at 360 nm by using a molar extinction coefficient of 22,000 M−1·cm−1, and values expressed as nanomoles protein carbonyls per milligram protein. Protein concentration in the final, resuspended pellet was quantified based on its relative absorption at 280 nm, using BSA in guanidine HCl (Item No. 10005020, Cayman Chemical) as a standard.
Measurement of antioxidant enzymes.
The activities of SOD, CAT, glutathione peroxidase (GPX), and GR were determined from homogenates of goldfish brain and liver (1:20 wt/vol) prepared in ice-cold buffer (20 mM HEPES, 1 mM EDTA, 0.1% Triton X-100, pH 7.4) by using a Tenbroek all-glass tissue homogenizer and centrifuged for 2 min at 10,000 g in a refrigerated Eppendorf 5430R centrifuge (Eppendorf, Hamburg, Germany). Aliquots of the resulting homogenates were sonicated over ice for 15 s at 40 V with a Q125 tissue sonicator (QSonica, Newtown, CT). All enzyme activity measurements were initially optimized to attain linear time and concentration dependence.
SOD percent (%) inhibition rate was determined with a commercially available SOD assay kit (Cat No. 19160, Sigma-Aldrich). SOD activity was determined from a standard curve by using known units (U) of purified SOD enzyme (Cat No. S2515, Sigma-Aldrich) against the percent inhibition rate of water-soluble tetrazolium salt (WST-1) under identical conditions.
Catalase and glutathione peroxidase activities were determined in homogenates of brain and liver tissue according to the methods of Weydert and Cullen (68). The activity of CAT was determined by monitoring the initial rate of H2O2 decomposition at 240 nm. The final concentrations of the reaction components were 20 mM KPi buffer (pH 7.4), 20 mM H2O2, and 10 μl of appropriately diluted sample. Total GPX activity was determined by monitoring the reduction in NADPH absorbance at 340 nm in the presence of 0.25 mM tert-butyl hydroperoxide in a reaction medium containing (final concentrations) 50 mM KPi buffer (pH 7.0), 1 mM EDTA, 1 mM NaN3, 0.2 mM NADPH, 1 mM GSH, and 1 U/ml GR. The activity of GR was determined by monitoring the oxidation of NADPH in the presence of 10 mM GSSG at 340 nm in a medium containing (final concentrations) 50 mM KPi buffer (pH 7.0), 1 mM EDTA, and 0.25 mM NADPH. The activity of GPX and GR were calculated by using a molar extinction coefficient of 6220 M−1·cm−1. One unit (U) of CAT, GPX, and GR defined as the amount of enzyme consuming 1 μmole of substrate per minute at 25°C, with all antioxidant enzyme activities expressed in international units (or milliunits) per milligram of tissue protein. Protein content in homogenates of brain and liver were determined via the Bradford method, using BSA as a standard (3).
Calculations and Statistics
Brain water content was quantified by the wet weight/dry weight method, as previously described by Wilkie et al. (71). Briefly, whole brains were carefully excised, and the wet tissue mass was determined in preweighed 1.5-ml tubes, followed by determination of the dry tissue mass after drying the tissue to constant mass for 72 h at 65 ± 5°C, yielding brain water content in milliliters H2O per gram wet mass (ml H2O/g wet mass).
However, this was only a relative measure of tissue water content, not actual water volume. To determine the actual water volume, it was necessary to express the tissue water content as the volume of water per gram dry tissue mass (ml H2O/g dry mass). This was done by dividing the brain water content (ml H2O/g wet mass) by the mass of dry tissue (g dry mass) over the wet tissue mass (g wet mass).
With the use of this approach, it was possible to directly calculate how much the volume of H2O changed in the brain during HEA, as a function of brain swelling.
All values are presented as the means ± SE. Data were analyzed by one-way analysis of variance (ANOVA), followed by the Tukey-Kramer post hoc test. Student’s unpaired two-tailed t-test was used for single comparisons between tissues. In all determinations, the null hypothesis was rejected if P < 0.05.
RESULTS
Ammonia Concentrations
Goldfish acclimated to warmer (14°C) water were exposed to a measured TAmm concentration of 5.30 ± 0.10 mmol/l, with concentrations fluctuating between 5.10 ± 0.04 and 5.79 ± 0.16 mmol/l during the exposure period (Table 1). The goldfish acclimated to cooler (4°C) water were exposed to a similar measured TAmm concentration of 5.30 ± 0.20 mmol/l, with concentrations fluctuating between 5.16 ± 0.01 and 5.41 ± 0.02 mmol/l during the exposure period (Table 1). At 14°C, the goldfish survived HEA despite 10-fold increases in plasma [TAmm] from ~150 µmol/l under control conditions to ~1,440 µmol/l after 24 h, and ~1,325 µmol/l after 72 h (Fig. 1A). At 4°C, similar increases in plasma [TAmm] were observed during HEA, which increased to ~1,740 µmol/l after 72 h from control values of ~100 µmol/l (Fig. 1B). Mortality was not observed in control fish or in any experimental group throughout the experimental period.
Table 1.
Measured concentrations of TAmm to which 14°C-acclimated and 4°C-acclimated goldfish were exposed for 72 h
| 14°C |
4°C |
|||
|---|---|---|---|---|
| Time, h | Measured [TAmm], mM | pH | Measured [TAmm], mM | pH |
| 2 | 5.10 ± 0.09 (19) | 8.17 ± 0.01 (19) | 5.16 ± 0.01 (8) | 8.12 ± 0.00 (8) |
| 8 | 5.33 ± 0.06 (19) | 8.10 ± 0.01 (19) | 5.37 ± 0.05 (8) | 8.08 ± 0.00 (8) |
| 24 | 5.10 ± 0.04 (19) | 8.10 ± 0.01 (19) | 5.25 ± 0.03 (8) | 8.03 ± 0.00 (8) |
| 36 | 5.79 ± 0.16 (10) | 8.05 ± 0.01 (10) | 5.36 ± 0.04 (8) | 8.07 ± 0.00 (8) |
| 48 | 5.18 ± 0.15 (10) | 8.04 ± 0.01 (10) | 5.38 ± 0.03 (8) | 8.13 ± 0.00 (8) |
| 72 | 5.17 ± 0.20 (10) | 7.95 ± 0.03 (10) | 5.41 ± 0.02 (8) | 8.19 ± 0.00 (8) |
Measurements are expressed as means ± SE; number in parentheses is number of independent measurements of water [TAmm] and pH that were made throughout the exposure period. Nominal total ammonia concentration ([TAmm]) = 5 mmol/l.
Fig. 1.
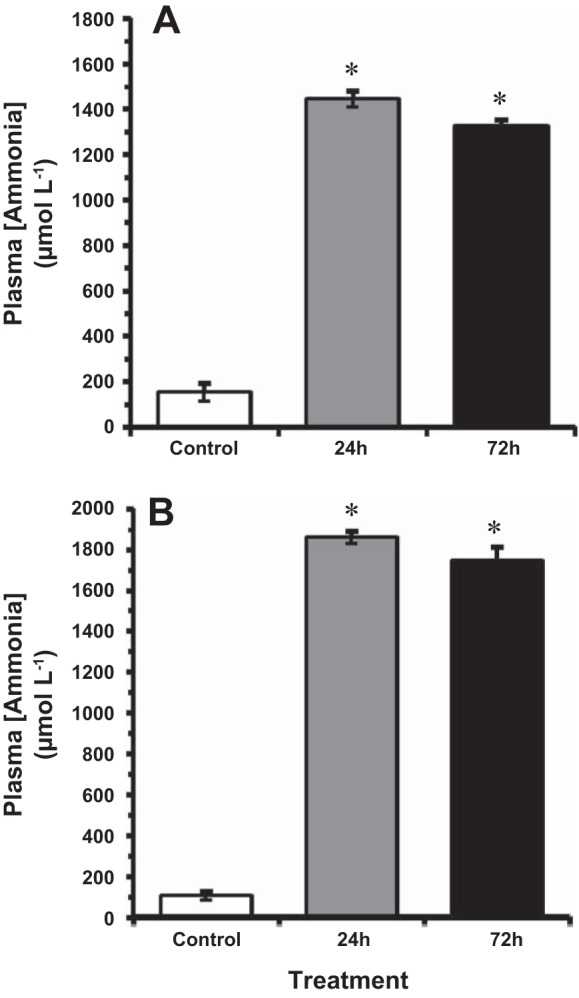
The effect of acute ammonia exposure (measured ammonia = 5.3 mmol/l) on plasma total ammonia levels [TAmm] in the goldfish at 14°C (A) and 4°C (B). Data expressed as means ± SE (n = 8). *Statistically significant differences from the corresponding control groups as assessed by ANOVA followed by Tukey-Kramer posttest, P < 0.05.
After exposure to HEA, ammonia concentrations in the goldfish brain significantly increased at both temperatures (Fig. 2). In the 14°C-acclimated goldfish, brain TAmm concentrations increased nearly sixfold to ~5.7 μmol/g wet mass after 72 h exposure to HEA, compared with control concentrations of ~1 μmol/g wet mass (Fig. 2A). In the 4°C-acclimated individuals, brain [TAmm] increased nearly sevenfold from control values of ~0.7 μmol/g wet mass to 5 μmol/g wet mass and 5.2 μmol/g wet mass after 24 h and 72 h, respectively (Fig. 2B).
Fig. 2.
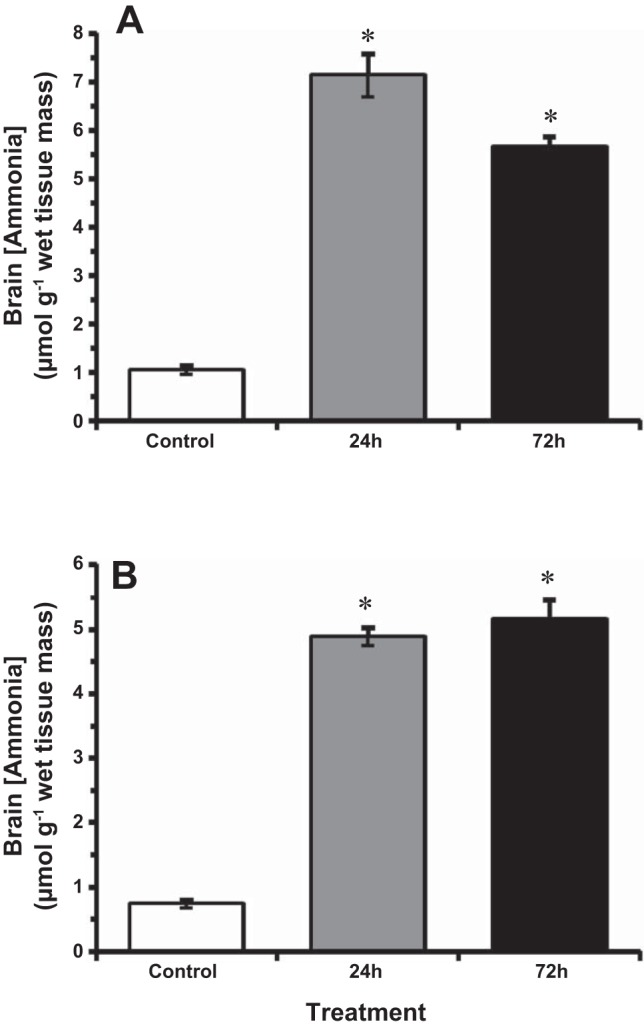
The effect of acute ammonia exposure (measured ammonia = 5.3 mmol/l) on ammonia concentration in goldfish brain at 14°C (A) and 4°C (B). Data expressed as means ± SE (n = 8). *Statistically significant differences from the corresponding control groups as assessed by ANOVA followed by Tukey-Kramer posttest, P < 0.05.
Glutamine Concentrations
At 14°C, brain glutamine (steady-state) concentrations were elevated five- to sixfold after 24 h and 72 h exposure to HEA from control values of ~4 μmol/g wet mass (Fig. 3A). Brain glutamine concentrations rose more slowly in 4°C-acclimated goldfish, however, increasing from control values of ~5 μmol/g wet mass to ~17 μmol/g wet tissue after 24 h, but continued to increase further to nearly 24 μmol/g wet mass after 72-h HEA exposure (Fig. 3B). By 72 h of HEA, no significant differences in glutamine concentration were noted between ammonia-exposed goldfish at 4°C and 14°C (Fig. 3).
Fig. 3.
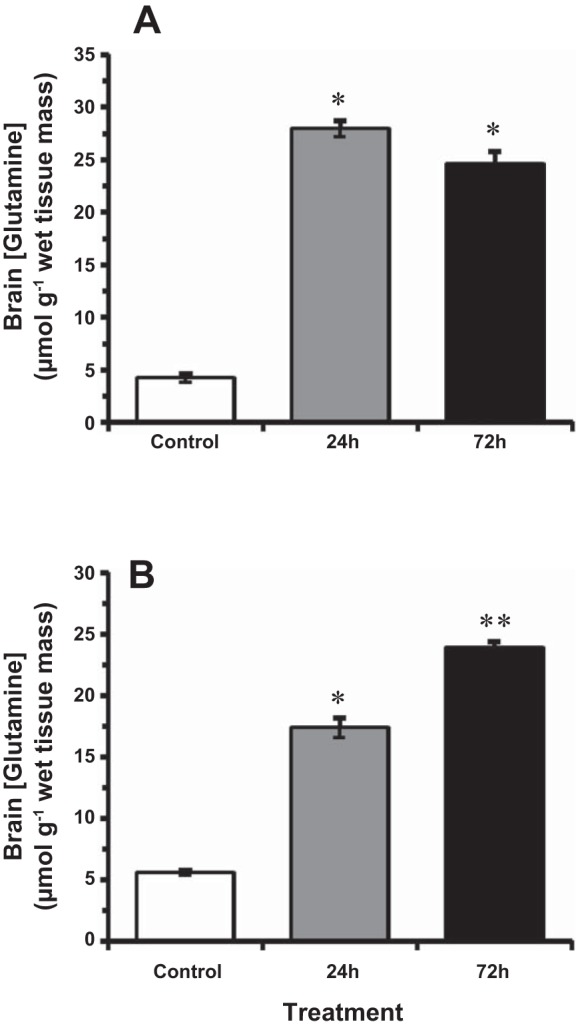
The effect of acute ammonia exposure (measured ammonia = 5.3 mmol/l) on glutamine concentration in goldfish brain at 14°C (A) and 4°C (B). Data expressed as means ± SE (n = 8). *Statistically significant differences from the corresponding control groups and **24-h treatment groups as assessed by ANOVA followed by Tukey-Kramer posttest, P < 0.05.
Brain Water Volume
Brain water volume increased significantly by 20% in 14°C-acclimated goldfish following 72 h of exposure to HEA from control values of ~5.14 ml H2O/g dry mass to ~6.38 ml H2O/g dry mass (Fig. 4A). In contrast, no brain swelling was observed in fish exposed to HEA in colder (4°C) water, as brain water content did not deviate significantly from control values of ~5 ml/g dry mass throughout the 72-h HEA exposure period (Fig. 4B). No significant differences in control (pre-HEA exposure) brain water volume were noted between goldfish acclimated to 4°C and those acclimated to 14°C (Fig. 4).
Fig. 4.
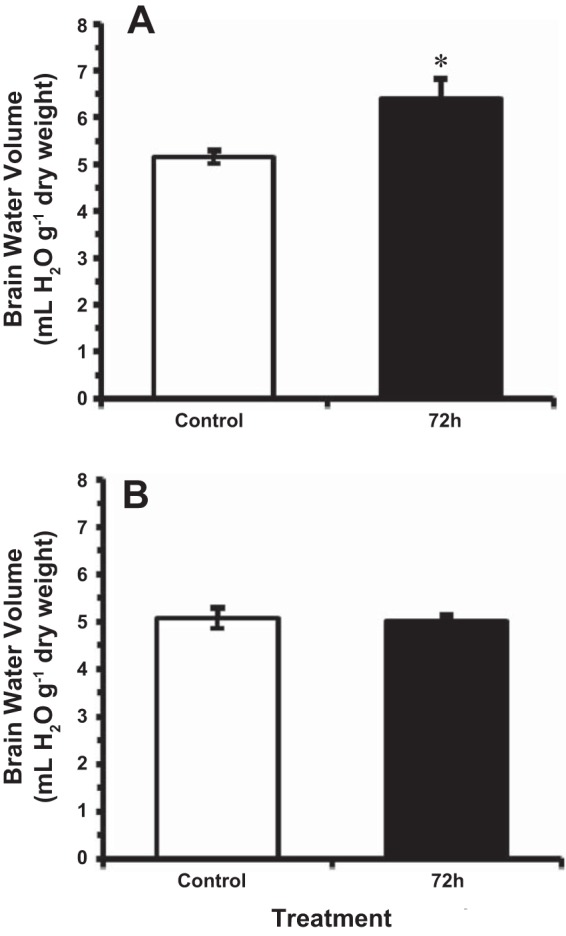
The effect of acute ammonia exposure (measured ammonia = 5.3 mmol/l) on water volume in goldfish brain at (A) 14°C, and (B) 4°C. Data are presented as means ± SE (n = 6–8). *Statistically significant differences from the corresponding control groups as assessed by ANOVA followed by Tukey-Kramer posttest, P < 0.05.
Indices of Oxidative Stress
In control goldfish, baseline concentrations of TBARS in the liver were six- and threefold higher than in the brain of 14°C- and 4°C-acclimated fish, respectively (Fig. 5). After 24 and 72 h exposure to HEA, the level of TBARS did not change significantly from control values in the liver of goldfish exposed to ammonia at 14°C. In the brain, however, there was a pronounced twofold increase in TBARS after 72 h of HEA (Fig. 5A). However, the level of TBARS remained unchanged from control values in the brain and liver of goldfish exposed to HEA at 4°C (Fig. 5B).
Fig. 5.
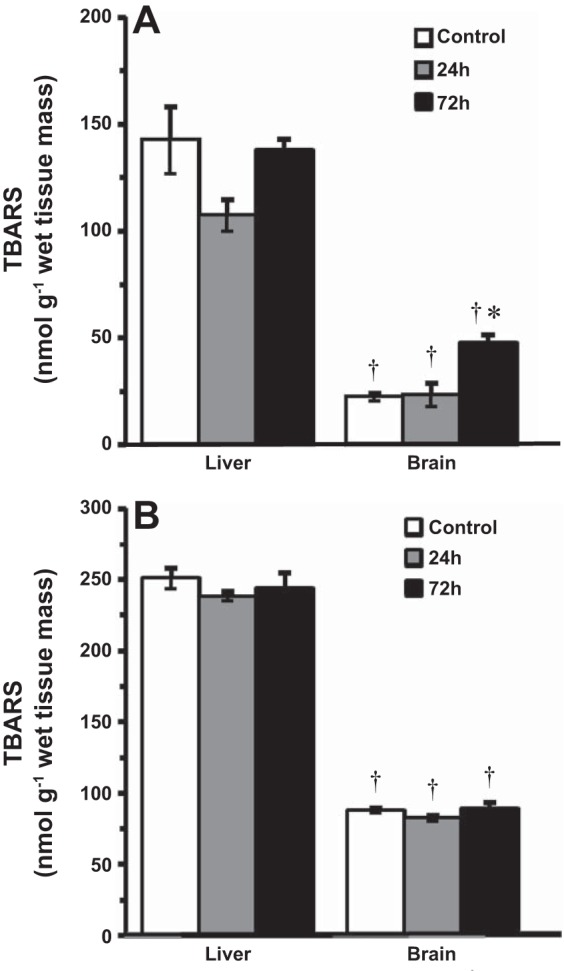
Level of thiobarbituric acid-reactive substances (TBARS) in tissues of goldfish exposed to acute ammonia toxicity (measured ammonia = 5.3 mmol/l) at 14°C (A) and 4°C (B). Data are presented as means ± SE (n = 6–8). *Statistically significant differences from the corresponding control groups as assessed by ANOVA followed by Tukey-Kramer posttest, P < 0.05. †Significantly different from corresponding control or experimental group in liver tissue, P < 0.05.
In contrast to lipid peroxidation, the liver and brain were much more susceptible to protein carbonylation after HEA exposure at 14°C. In control fish, protein carbonyl content was similar in the liver and the brain at both acclimation temperatures. After 24 h of HEA, however, protein carbonyl content in the liver increased by nearly threefold to 3.5 nmol/mg protein before returning to control values after 72 h of HEA in 14°C-acclimated goldfish (Fig. 6A). In the brain, protein carbonyl content increased in a time-dependent manner during HEA exposure at 14°C, increasing twofold from ~1 nmol/mg protein in control fish to ~2 nmol/mg protein after 24 h, and increasing further to 2.5 nmol/mg protein after 72 h exposure (Fig. 6A). At colder (4°C) temperatures, however, there was no increase in protein carbonyl content during HEA exposure, which remained similar to control values in both the liver and the brain (Fig. 6B).
Fig. 6.
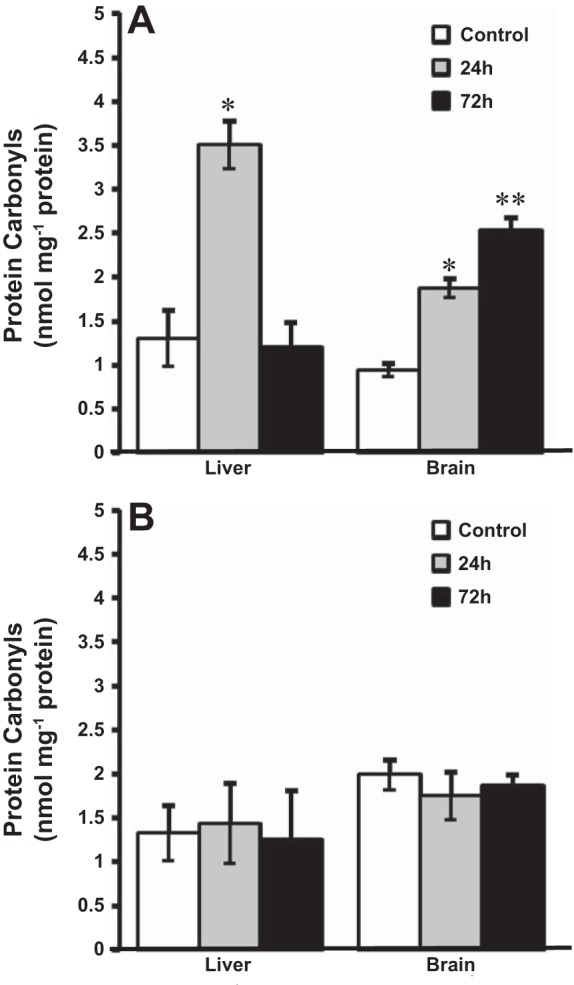
Level of protein carbonyls in tissues of goldfish exposed to acute ammonia toxicity (measured ammonia = 5.3 mmol/l) at 14°C (A) and 4°C (B). Data are presented as means ± SE (n = 6–8). *Statistically significant differences from the corresponding control groups or **24-h treatment groups as assessed by ANOVA followed by Tukey-Kramer posttest, P < 0.05.
Antioxidant Enzyme Activities
At 14°C, SOD activity did not change in either the brain or liver following 24 h of HEA exposure (Fig. 7A). By 72 h, however, SOD activity had increased by almost twofold in the liver, but not in the brain (Fig. 7A). HEA exposure at 14°C also caused differential changes in CAT activity between liver and brain in fish exposed to HEA (Fig. 7B). In the liver, CAT activity did not significantly change until 72 h of HEA, when activity was threefold greater than in control fish (Fig. 7B). In contrast, CAT activity in the brain increased twofold after 24 h of HEA and remained elevated through 72 h of HEA (Fig. 7B). In the 4°C-acclimated goldfish, however, SOD and CAT activities in the brain remained unchanged from control values throughout HEA (Table 2).
Fig. 7.
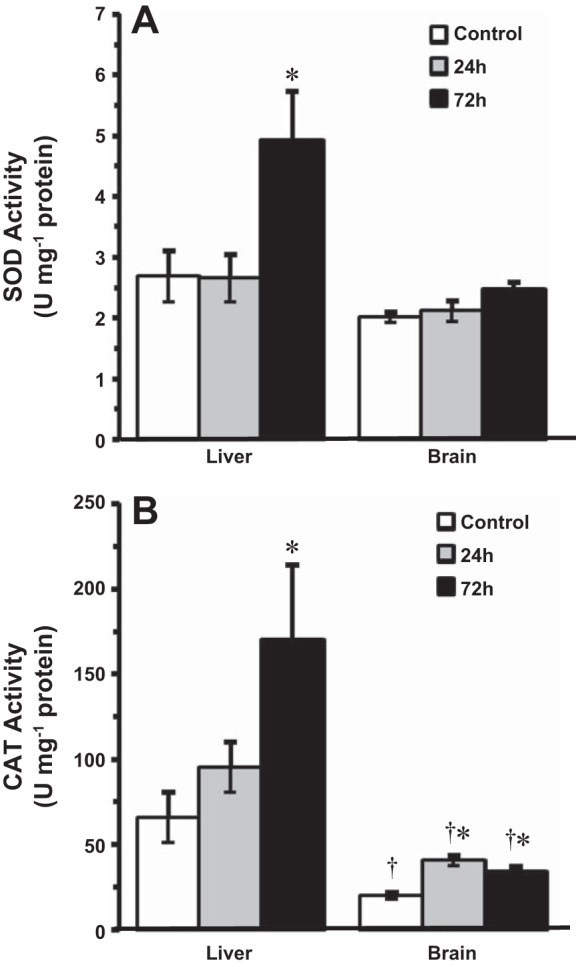
The effect of acute ammonia exposure (measured ammonia = 5.3 ± 0.1 mmol/l) on the specific activities of primary antioxidant enzymes in tissues of 14°C-acclimated goldfish, superoxide dismutase (SOD) (A) and catalase (CAT) (B). Data are presented as means ± SE (n = 5–7). *Statistically significant differences from the corresponding control groups or 24-h treatment groups as assessed by ANOVA followed by Tukey-Kramer posttest, P < 0.05. †Significantly different from corresponding liver groups, P < 0.05.
Table 2.
Effect of acute ammonia exposure on the specific activities of primary antioxidant enzymes in brain of 4°C-acclimated goldfish
| Specific Activity, U/mg protein | |
|---|---|
| Brain SOD | |
| Control | 3.4 ± 0.2 (7) |
| 24 h HEA | 3.2 ± 0.2 (7) |
| 72 h HEA | 3.3 ± 0.2 (7) |
| Brain CAT | |
| Control | 18.9 ± 0.80 (8) |
| 24 h HEA | 19.0 ± 1.50 (7) |
| 72 h HEA | 20.3 ± 0.90 (7) |
Values are means ± SE; number in parentheses is number of independent measurements. Measured ammonia = 5.3 ± 0.2 mmol/l. SOD, superoxide dismutase; SOD, superoxide dismutase; HEA, high environmental ammonia; CAT, catalase. No statistically significant differences from the corresponding control groups or 24-h treatment groups as assessed by ANOVA followed by Tukey-Kramer posttest, P < 0.05.
The activities of glutathione-dependent antioxidants were also measured in 14°C-acclimated goldfish brain and liver (Fig. 8, A and B). Baseline GPX activities in the liver were fourfold greater compared with brain (Fig. 8A). In the liver, HEA had no significant effect on GPX activity. The activity of GPX in the goldfish brain, however, was significantly elevated by almost 50% after 24 h HEA, followed by a complete recovery by 72 h of exposure (Fig. 8A). Baseline GR activity was also greater in the liver than in the brain by approximately twofold (Fig. 8B). Ammonia exposure induced differential changes in GR activity between liver and brain in these fish. In the liver, GR activity significantly increased by 75% following 72 h HEA. In contrast, GR activity in the brain increased significantly by 49% at 24 h and remained 38% higher than control values through 72 h of HEA exposure (Fig. 8B).
Fig. 8.
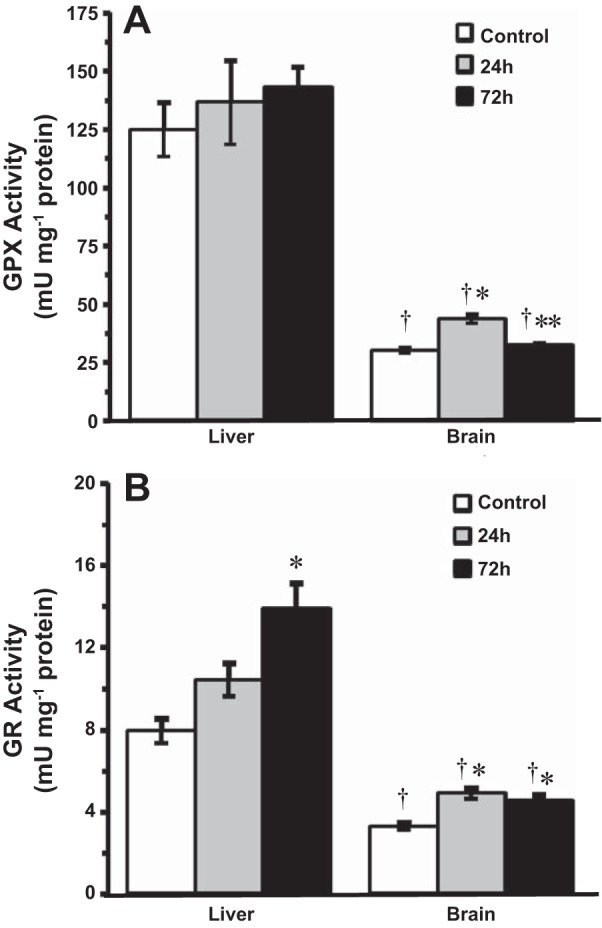
The effect of acute ammonia exposure (measured ammonia = 5.3 ± 0.1 mmol/l) on the specific activities of glutathione-dependent antioxidant enzymes in tissues of 14°C-acclimated goldfish, glutathione peroxidase (GPx) (A) and glutathione reductase (GR) (B). Data are presented as means ± SE (n = 6−8). *Statistically significant differences from the corresponding control groups or **24-h treatment groups as assessed by ANOVA followed by Tukey-Kramer posttest, P < 0.05. †Significantly different from corresponding liver groups, P < 0.05.
DISCUSSION
Ammonia-Induced Oxidative Stress in Goldfish
The present study demonstrated that increases in internal ammonia concentrations arising from HEA exposure led to a temperature-dependent and tissue-specific oxidative stress response in goldfish, with the brain experiencing significantly greater oxidative stress than the liver. Unlike other vertebrates (4, 25, 53), goldfish exhibit a significant upregulation in the activity of multiple key antioxidant enzymes and the maintenance of other antioxidant enzyme activities during acute hyperammonemia in the brain. This high neural antioxidant capacity likely offsets the extent of oxidative damage and swelling in the brain to a level that is physiologically manageable and even reversible (71).
In the present study, brain TAmm approached 6–7 μmol/g wet mass in goldfish exposed to HEA. These values are comparable to those tolerated by other ammonia-tolerant fishes, including toadfish (Opsanus beta), which readily withstand brain ammonia concentrations of 4–6 μmol/g wet mass during HEA (66). Similarly, immersed climbing perch (Anabas testudineus) and weather loach (Misgurnus anguillicaudatus) exposed to HEA had brain TAmm concentrations near 4 μmol/g wet mass (57, 59). These concentrations of brain TAmm greatly exceed those known to cause morbidity and death in mammals (10), suggesting that these fishes have a high neural tolerance of ammonia.
A major consequence of hyperammonemia in mammals is the generation of ROS, which can cause peroxidation of polyunsaturated fatty acids, yielding unstable lipid hydroperoxides that decompose into MDA (25, 36, 38). The marked increase in MDA content (quantified as TBARS) in the brain of ammonia-exposed goldfish at 14°C, suggests that peroxidative lipid damage is also a major adverse consequence of ammonia accumulation in the goldfish brain. However, lipid peroxidation was not observed in the goldfish liver. A similar study reported 40% increases in TBARS content in the goldfish liver during 48 h exposure to 1 mmol/l TAmm (50). However, this increase was only transient, and TBARS content was completely restored shortly thereafter. Thus, compared with the liver, the brain appears to be more susceptible to ammonia-induced oxidative stress, at least in normothermic (14°C) fish.
The greater sensitivity to HEA-induced lipid peroxidative injury observed in the brain may be because the liver has a greater relative antioxidant capacity. Moreover, brain tissue contains relatively high levels of peroxidation of polyunsaturated fatty acids compared with the liver, specifically docosahexaenoic acid, which is particularly prone to lipid peroxidation in the presence of ROS (12, 43, 54). Furthermore, high rates of O2 consumption, the relatively high amount of membrane lipids (i.e., high ratio of membrane surface area to cytosolic volume), the high Ca2+ flux across neuronal membranes observed in the brain, and the presence of ROS-producing microglia may also contribute to the greater susceptibility of brain tissue to ammonia-induced oxidative stress compared with the liver (12).
ROS can also oxidize amine groups on several amino acids residues, transforming them into carbonyls (6). Protein carbonyl content increased in a time-dependent manner in the 14°C-acclimated goldfish brain during HEA, suggesting that ROS-mediated damage to proteins is also substantial during acute hyperammonemia. In contrast, protein oxidation increased only transiently in the liver, returning to control levels by 72 h HEA in these goldfish, likely due to the activation of one or more antioxidant enzymes (see below). Similar increases in lipid and protein oxidation have been reported in Nile tilapia (Oreochromis niloticus) liver and white muscle (16); mudskipper (Boleophthalmus boddarti) gill and brain (4); big head carp larvae (Hypophthalmythys nobilis) (55); silver carp larvae (Hypophthalmichthys molitrix) (56): and European sea bass (Dicentrarchus labrax) brain, liver, and kidney (53) during exposure to HEA, suggesting that oxidative stress is a common response to variations in increased internal ammonia in teleosts.
Mechanism(s) of Ammonia-Induced Oxidative Stress and Brain Swelling
Much of the ammonia entering the goldfish brain during HEA exposure is likely converted to glutamine via the ATP-dependent GS-catalyzed addition of ammonia to glutamate (Fig. 3). In vitro mammalian studies even suggest that glutamine accumulation in the brain may be associated with cellular oxidative stress (1, 22, 28, 36, 38). Researchers have proposed that newly synthesized glutamine is transported in excess into mitochondria, in which it is subsequently hydrolyzed back into ammonia via mitochondrial phosphate-activated glutaminases, leading to significant mitochondrial ROS generation and induction of the mitochondrial permeability transition (1, 37). Free radicals and the mitochondrial permeability transition appear to initiate a cascade of events in mammalian cells, which may include bioenergetic failure, mitogen-activated protein kinase activation, Na-K-Cl cotransporter activation (i.e., NKCC-1), and/or NF-κB activation, ultimately resulting in the disruption of ionic/cell volume regulation and subsequent brain swelling (1, 20, 36).
Alternatively, glutamine accumulation could increase intracellular osmolarity leading to net water influx and cell swelling, independent of ROS production (64). Indeed, a strong correlation between intracranial pressure and glutamine concentration was demonstrated in human patients suffering from hyperammonemia (58). However, the observation in the present study that brain swelling was attenuated in goldfish exposed to ammonia in colder (4°C) water despite elevated brain glutamine concentrations argues against the hypothesis that glutamine accumulation is the proximate cause of brain swelling in the goldfish. In general agreement with our findings, pretreatment of goldfish with methionine sulfoximine (intraperitoneal injection), sufficient to decrease brain GS activity and intracellular glutamine concentrations, failed to attenuate ammonia-induced brain swelling in vivo (71). Moreover, mammalian studies have reported that brain swelling was attenuated in hyperammonemic rats made mildly hypothermic (35°C), despite elevated brain glutamine concentrations (5, 23, 46).
Cold Temperatures Attenuate Ammonia-Induced ROS Production and Brain Swelling
Acclimation and subsequent exposure to HEA at cold (4°C) temperatures not only prevented ammonia-induced brain swelling, but also completely attenuated the increases in protein carbonyl content and lipid peroxidation observed in goldfish brain during exposure to HEA in warmer (14°C) water. Moreover, the activity of primary antioxidant enzymes remained unaffected throughout HEA in the brain and liver of goldfish at 4°C, indicating that the lack of oxidative damage observed in these tissues was not due to an upregulation of antioxidant defenses (Table 2). This is the first direct evidence suggesting that temperature may have profound impacts on the pathophysiology of ammonia in fish.
The paucity of free radical-mediated damage and brain swelling in 4°C-acclimated goldfish was not mediated by an effect on blood and brain ammonia concentrations during HEA exposure. This contrasts the neuroprotective effects of mild hypothermia on ammonia toxicity in mammals, for which reductions in oxidative damage and brain swelling are attributed, at least in part, to a lowering of brain ammonia concentrations (23, 46). In the goldfish, however, we suggest that the neuroprotective effects of cold (4°C) acclimation on brain swelling are mediated by a reduction in ammonia- and/or glutamine-induced ROS production, most likely due to reductions in mitochondrial O2 consumption as a consequence of reduced metabolic rate (for a review see Ref. 14). However, more studies are needed to establish a direct, mechanistic link between ROS-mediated damage and brain swelling in the goldfish.
Upregulation of Antioxidant Defenses During Acute Hyperammonemia
Superoxide dismutase (SOD) catalyzes the conversion of highly toxic O2˙− to H2O2. Baseline levels of SOD were similar in liver and brain, suggesting that they have a similarly high basal capacity to scavenge O2˙−, as also observed by Lushchak et al. (33) in the same species. The marked increase in SOD activity in the goldfish liver might partly explain the restoration of protein carbonyl content to control values by 72 h HEA (Fig. 6). Similar elevations in hepatic SOD activity have been demonstrated in the liver of goldfish exposed to various metals (29, 49, 63) and 1 mmol/l NH4HCO3 (50).
The minimal change in SOD activity observed in the brain of goldfish during HEA exposure suggests that brain tissue may have sufficient antioxidant capacity to counter increased O2˙− production. Interestingly, Lushchak et al. (33) observed no increases in SOD activity in goldfish brain and liver during post-anoxic recovery, during which time there was ample evidence of ROS generation based on increases in conjugated dienes and the induction of other antioxidant enzymes in both tissues.
Catalase (CAT) is an important primary antioxidant that catalyzes the decomposition of H2O2 arising from the dismutation of O2˙−. As commonly observed for other lower vertebrates (17, 18, 24, 41), CAT activity in control goldfish was much higher in the liver (66 U/mg protein) than in the brain (19 U/mg protein), which likely renders the liver significantly more resistant to ammonia-induced H2O2 overproduction and peroxidative tissue injury. Surprisingly, despite high constitutive expression compared with the brain, liver CAT activity doubled during HEA exposure. Interestingly, hepatic CAT activity also undergoes significant activation in goldfish exposed to anoxia/reoxygenation (33), hyperoxia (32), and low concentrations of ammonia (50). This observation, in conjunction with marked elevations in SOD activity, likely explains the restoration of protein carbonyl content in the liver after 72 h of HEA (Fig. 6A).
Unlike SOD, CAT activity in the brain was activated at earlier exposure periods and remained elevated throughout HEA exposure. This observation supports the assumption that basal SOD activity in the brain may be sufficient to protect cells against increased production of O2˙− during hyperammonemia due to a relatively greater induction of brain CAT to counteract the resultant increases in H2O2. Similar increases in CAT activity have been observed in the goldfish brain during post-hyperoxic recovery and exposure to 2,4-dichlorophenoxyacetic acid (32, 34).
Glutathione peroxidase (GPX) catalyzes the reduction of H2O2 and lipid peroxides arising from the earlier stages of lipid peroxidation in the cell, converting reduced glutathione (GSH) to its oxidized form (GSSG) in the process (68). At first glance, the lack of GPX induction in the liver, and the minimal change in GPX activity in the brain during HEA exposure, suggests goldfish have a relatively low capacity for managing fluctuations in H2O2 and lipid peroxide levels. However, while the specific activity of GPX decreases in the brain of most ammonia-sensitive mammals during acute hyperammonemia (25, 26, 28), goldfish maintained constitutive GPX activity in both organs throughout HEA. The maintenance of constitutive GPX activities in both the brain and liver, along with the transient increase in the brain at 24 h of HEA, may limit oxidative stress in both organs to manageable and physiologically tolerable levels. This may explain why lipid peroxidation did not increase in the brain during the first 24 h of HEA (Fig. 5A).
GR catalyzes the reduction of GSSG to GSH, which maintains a strong reducing environment in the cell. Ammonia induced a significant increase in GR activity in both the goldfish liver and brain, suggesting that following acute exposure to hyperammonemia, hepatic and nervous tissue respond by increasing the capacity for GSH regeneration, thereby rendering the cell more effective at managing ROS production through GPX-catalyzed and spontaneous reduction of H2O2 and lipid peroxides. However, GR activity in the goldfish brain was upregulated at earlier (24 h) exposure periods and did not recover during HEA. This improved capacity for GSSG reduction in the goldfish brain likely explains why brain GPX activity increased, albeit transiently, but hepatic GPX remained largely unaffected. However, the concentration of cellular GSH was not measured to confirm if this is the case. Intracellular GSH concentrations in the goldfish brain and liver were maintained during exposure to anoxia/reoxygenation (33), 2,4-dichlorophenoxyacetic acid (34), and 1 mmol/l NH4HCO3 (50). Moreover, lipid peroxidation appears to be a more specific indicator of ROS-mediated cytotoxicity in fish than a change in cellular GSH concentration (45).
The goldfish liver was characterized by constitutively higher activities of GPX and GR, approximately two to four times more than in the brain. Moreover, hepatic CAT activity was approximately three times greater than in the brain. Similar findings have been observed for other lower vertebrates (17, 18, 24, 41), effectively rendering the liver more resistant to oxidative stress. The higher constitutive expression of antioxidant enzymes in the liver compared with the brain may be due to the fact that hepatic tissue is an important site of numerous ROS-generating reactions owing to high rates of cytochrome P450s and peroxisomal enzymes (13, 40, 47). It is also important to note that the liver is the most important site of ammoniogenesis in goldfish under normal physiological conditions (61). As a result, high constitutive antioxidant enzyme expression would limit oxidative cellular damage by rendering the cell less vulnerable to elevated ROS generation. This may also explain the relatively greater susceptibility of the brain to ammonia-induced oxidative damage in the goldfish and perhaps other fish species.
Collectively, our findings suggest that goldfish brains have a high capacity to withstand oxidative stress, and are more resilient to this disturbance of homeostasis than other vertebrate brains. Unlike other studies on ammonia neurotoxicity, we observed the upregulation of several key antioxidant enzymes (CAT, GPX, and GR) and the maintenance of other enzymatic activities in the goldfish brain during HEA. In ammonia-sensitive mammals, antioxidant defenses in the brain are overwhelmed by high rates of ROS production and become rapidly downregulated following injection with high doses of ammonium salts (25–27). Although few studies have addressed this question in teleosts, significant reductions in the activities of CAT and GR have been reported in the brain of mudskipper (B. boddarti) during HEA (4). Similarly, CAT and GPX activity decreased significantly in the brain of Dicentrarchus labrax following acute hyperammonemia (53). In these vertebrates, ammonia appears to induce strong oxidative stress, according to the recently proposed classification based on intensity (31).
Although the level of lipid peroxidation and protein carbonylation in the goldfish brain remained elevated after 72 h of HEA at 14°C, ammonia appears to induce only low intensity oxidative stress in this species (31). This challenge triggers the upregulation of antioxidant enzymes and the maintenance of other enzymatic activities, which limits oxidative cellular damage and associated brain swelling to a level that is physiologically tolerable and reversible in the goldfish brain (71). This adaptation has also been demonstrated in the brain of goldfish exposed to post-anoxic recovery, where rates of ROS production can be very high (33). While the antioxidant response in the brain of most animals is modest (12, 25, 27), the adaptive response by antioxidant enzymes in the goldfish brain reported here likely contributes to the extremely high tolerance of ammonia in the goldfish brain (70, 71).
Perspectives and Significance
While further studies are needed to establish a mechanistic link between ROS-mediated damage and brain swelling, this study shows that ammonia-induced brain swelling in the goldfish (Carassius auratus) is associated with significant oxidative stress and that this response is temperature dependent. Moreover, the high antioxidant capacity of the goldfish, marked by the upregulation and maintenance of key antioxidant enzymes, likely minimizes the degree of oxidative damage and associated brain swelling to a level that is physiologically tolerable and reversible for the goldfish brain. This adaptive response likely explains the high tolerance to ammonia and hypoxia in this species. An enhanced ability to limit oxidative damage and brain swelling in response to HEA, anoxia/hypoxia, and other environmental stressors likely provides goldfish with the capacity to inhabit, exploit, and thrive in a wide range of niches that are often ice covered, marginal, eutrophic, and unhabitable for potential competitors and predators.
GRANTS
D. F. J. Lisser was supported by a Natural Sciences and Engineering Research Council (NSERC) of Canada postgraduate scholarship and a Dean’s Scholarship (Wilfrid Laurier). Research funding for this work was provided by NSERC Discovery grants to G. R. Scott and M. P. Wilkie.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
D.F.L., Z.M.L., and P.Q.P.-H. performed experiments; D.F.L., Z.M.L., and P.Q.P.-H. analyzed data; D.F.L., G.R.S., and M.P.W. interpreted results of experiments; D.F.L. prepared figures; D.F.L. drafted manuscript; G.R.S. and M.P.W. edited and revised manuscript; M.P.W. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors acknowledge the invaluable technical assistance provided by Sherry Du (McMaster University), and Dr. Andrea Lister, Gena Braun, and Sanya Sidhu (Wilfrid Laurier University). We also thank Dr. Jonathan Wilson (Wilfrid Laurier) for access to the resources in his laboratory, especially the Precellys 24 bead tissue homogenizer. We are also grateful to Dr. Lillian DeBruin and Dr. Allison McDonald (Wilfrid Laurier) for input into the data analysis and interpretation.
REFERENCES
- 1.Albrecht J, Norenberg MD. Glutamine: a Trojan horse in ammonia neurotoxicity. Hepatology 44: 788–794, 2006. doi: 10.1002/hep.21357. [DOI] [PubMed] [Google Scholar]
- 2.Arillo A, Margiocco C, Melodia F, Mensi P, Schenone G. Ammonia toxicity mechanism in fish: studies on rainbow trout (Salmo gairdneri Rich). Ecotoxicol Environ Saf 5: 316–328, 1981. doi: 10.1016/0147-6513(81)90006-3. [DOI] [PubMed] [Google Scholar]
- 3.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254, 1976. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 4.Ching B, Chew SF, Wong WP, Ip YK. Environmental ammonia exposure induces oxidative stress in gills and brain of Boleophthalmus boddarti (mudskipper). Aquat Toxicol 95: 203–212, 2009. doi: 10.1016/j.aquatox.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 5.Córdoba J, Crespin J, Gottstein J, Blei AT. Mild hypothermia modifies ammonia-induced brain edema in rats after portacaval anastomosis. Gastroenterology 116: 686–693, 1999. doi: 10.1016/S0016-5085(99)70191-5. [DOI] [PubMed] [Google Scholar]
- 6.Dalle-Donne I, Rossi R, Giustarini D, Milzani A, Colombo R. Protein carbonyl groups as biomarkers of oxidative stress. Clin Chim Acta 329: 23–38, 2003. doi: 10.1016/S0009-8981(03)00003-2. [DOI] [PubMed] [Google Scholar]
- 7.Dosdat A, Person J, Coves D, Dutto G, Gasset E, Le Roux A, Lemarie G. Effect of chronic exposure to ammonia on growth, food utilisation and metabolism of the European sea bass (Dicentrarchus labrax). Aquat Living Resour 16: 509–520, 2003. doi: 10.1016/j.aquliv.2003.08.001. [DOI] [Google Scholar]
- 8.Eddy FB. Ammonia in estuaries and effects on fish. J Fish Biol 67: 1495–1513, 2005. doi: 10.1111/j.1095-8649.2005.00930.x. [DOI] [Google Scholar]
- 9.Eyckmans M, Celis N, Horemans N, Blust R, De Boeck G. Exposure to waterborne copper reveals differences in oxidative stress response in three freshwater fish species. Aquat Toxicol 103: 112–120, 2011. doi: 10.1016/j.aquatox.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 10.Felipo V, Butterworth RF. Neurobiology of ammonia. Prog Neurobiol 67: 259–279, 2002. doi: 10.1016/S0301-0082(02)00019-9. [DOI] [PubMed] [Google Scholar]
- 11.Foss A, Siikavuopio SI, Saether BS, Evenson TH. Effect of chronic ammonia exposure on growth in juvenile Atlantic cod. Aquaculture 237: 179–189, 2004. doi: 10.1016/j.aquaculture.2004.03.013. [DOI] [Google Scholar]
- 12.Friedman J. Why is the nervous system vulnerable to oxidative stress? In: Oxidative Stress and Free Radical Damage in Neurology, edited by Gadoth N, Göbel HH. New York: Humana, 2011. doi: 10.1007/978-1-60327-514-9_2 [DOI] [Google Scholar]
- 13.Goksøyr A, Förlin L. The cytochrome P450 system in fish, aquatic toxicology and environmental monitoring. Aquat Toxicol 22: 287–311, 1992. doi: 10.1016/0166-445X(92)90046-P. [DOI] [Google Scholar]
- 14.González-Ibarra FP, Varon J, López-Meza EG. Therapeutic hypothermia: critical review of the molecular mechanisms of action. Front Neurol 2: 4, 2011. doi: 10.3389/fneur.2011.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Halliwell B, Chirico S. Lipid peroxidation: its mechanism, measurement, and significance. Am J Clin Nutr 57, Suppl: 715S–724S, 1993. [DOI] [PubMed] [Google Scholar]
- 16.Hegazi MMA, Attia ZI, Ashour OA. Oxidative stress and antioxidant enzymes in liver and white muscle of Nile tilapia juveniles in chronic ammonia exposure. Aquat Toxicol 99: 118–125, 2010. doi: 10.1016/j.aquatox.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 17.Hermes-Lima M, Storey KB. Antioxidant defenses in the tolerance of freezing and anoxia by garter snakes. Am J Physiol Regul Integr Comp Physiol 265: R646–R652, 1993. [DOI] [PubMed] [Google Scholar]
- 18.Hermes-Lima M, Storey KB. Relationship between anoxia exposure and antioxidant status in the frog Rana pipiens. Am J Physiol Regul Integr Comp Physiol 271: R918–R925, 1996. [DOI] [PubMed] [Google Scholar]
- 19.Ip YK, Chew SF, Randall DJ. Ammonia toxicity, tolerance, and excretion. In: Nitrogen Excretion–Fish Physiology, edited by Wright PA, Anderson PM. New York: Academic, 2001. doi: 10.1016/S1546-5098(01)20005-3. [DOI] [Google Scholar]
- 20.Jayakumar AR, Liu M, Moriyama M, Ramakrishnan R, Forbush B III, Reddy PVB, Norenberg MD. Na-K-Cl Cotransporter-1 in the mechanism of ammonia-induced astrocyte swelling. J Biol Chem 283: 33874–33882, 2008. doi: 10.1074/jbc.M804016200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jayakumar AR, Panickar KS, Murthy CRK, Norenberg MD. Oxidative stress and mitogen-activated protein kinase phosphorylation mediate ammonia-induced cell swelling and glutamate uptake inhibition in cultured astrocytes. J Neurosci 26: 4774–4784, 2006. doi: 10.1523/JNEUROSCI.0120-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jayakumar AR, Rao KVR, Murthy CR, Norenberg MD. Glutamine in the mechanism of ammonia-induced astrocyte swelling. Neurochem Int 48: 623–628, 2006. doi: 10.1016/j.neuint.2005.11.017. [DOI] [PubMed] [Google Scholar]
- 23.Jiang W, Desjardins P, Butterworth RF. Hypothermia attenuates oxidative/nitrosative stress, encephalopathy and brain edema in acute (ischemic) liver failure. Neurochem Int 55: 124–128, 2009. doi: 10.1016/j.neuint.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 24.Joanisse DR, Storey KB. Oxidative damage and antioxidants in Rana sylvatica, the freeze-tolerant wood frog. Am J Physiol Regul Integr Comp Physiol 271: R545–R553, 1996. [DOI] [PubMed] [Google Scholar]
- 25.Kosenko E, Kaminsky Y, Kaminsky A, Valencia M, Lee L, Hermenegildo C, Felipo V. Superoxide production and antioxidant enzymes in ammonia intoxication in rats. Free Radic Res 27: 637–644, 1997. doi: 10.3109/10715769709097867. [DOI] [PubMed] [Google Scholar]
- 26.Kosenko E, Kaminski Y, Lopata O, Muravyov N, Felipo V. Blocking NMDA receptors prevents the oxidative stress induced by acute ammonia intoxication. Free Radic Biol Med 26: 1369–1374, 1999. doi: 10.1016/S0891-5849(98)00339-6. [DOI] [PubMed] [Google Scholar]
- 27.Kosenko E, Kaminsky Y, Lopata O, Muravyov N, Kaminsky A, Hermenegildo C, Felipo V. Nitroarginine, an inhibitor of nitric oxide synthase, prevents changes in superoxide radical and antioxidant enzymes induced by ammonia intoxication. Metab Brain Dis 13: 29–41, 1998. doi: 10.1023/A:1020626928259. [DOI] [PubMed] [Google Scholar]
- 28.Kosenko E, Venediktova N, Kaminsky Y, Montoliu C, Felipo V. Sources of oxygen radicals in brain in acute ammonia intoxication in vivo. Brain Res 981: 193–200, 2003. doi: 10.1016/S0006-8993(03)03035-X. [DOI] [PubMed] [Google Scholar]
- 29.Kubrak OI, Lushchak OV, Lushchak JV, Torous IM, Storey JM, Storey KB, Lushchak VI. Chromium effects on free radical processes in goldfish tissues: comparison of Cr(III) and Cr(VI) exposures on oxidative stress markers, glutathione status and antioxidant enzymes. Comp Biochem Physiol C Toxicol Pharmacol 152: 360–370, 2010. doi: 10.1016/j.cbpc.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 30.Lushchak VI. Environmentally induced oxidative stress in aquatic animals. Aquat Toxicol 101: 13–30, 2011. doi: 10.1016/j.aquatox.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 31.Lushchak VI. Free radicals, reactive oxygen species, oxidative stress and its classification. Chem Biol Interact 224: 164–175, 2014. doi: 10.1016/j.cbi.2014.10.016. [DOI] [PubMed] [Google Scholar]
- 32.Lushchak VI, Bagnyukova TV, Husak VV, Luzhna LI, Lushchak OV, Storey KB. Hyperoxia results in transient oxidative stress and an adaptive response by antioxidant enzymes in goldfish tissues. Int J Biochem Cell Biol 37: 1670–1680, 2005. doi: 10.1016/j.biocel.2005.02.024. [DOI] [PubMed] [Google Scholar]
- 33.Lushchak VI, Lushchak LP, Mota AA, Hermes-Lima M. Oxidative stress and antioxidant defenses in goldfish Carassius auratus during anoxia and reoxygenation. Am J Physiol Regul Integr Comp Physiol 280: R100–R107, 2001. [DOI] [PubMed] [Google Scholar]
- 34.Matviishyn TM, Kubrak OI, Husak VV, Storey KB, Lushchak VI. Tissue-specific induction of oxidative stress in goldfish by 2,4-dichlorophenoxyacetic acid: mild in brain and moderate in liver and kidney. Environ Toxicol Pharmacol 37: 861–869, 2014. doi: 10.1016/j.etap.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 35.Mecke D. L-Glutamine, colorimetric method with glutamine synthetase. In: Methods of Enzymatic Analysis, edited by Bergmeyer HU. New York: Academic, 1985. [Google Scholar]
- 36.Norenberg MD, Jayakumar AR, Rama Rao KV, Panickar KS. New concepts in the mechanism of ammonia-induced astrocyte swelling. Metab Brain Dis 22: 219–234, 2007. doi: 10.1007/s11011-007-9062-5. [DOI] [PubMed] [Google Scholar]
- 37.Norenberg MD, Rama Rao KV, Jayakumar AR. Ammonia neurotoxicity and the mitochondrial permeability transition. J Bioenerg Biomembr 36: 303–307, 2004. doi: 10.1023/B:JOBB.0000041758.20071.19. [DOI] [PubMed] [Google Scholar]
- 38.Norenberg MD, Rama Rao KV, Jayakumar AR. Signaling factors in the mechanism of ammonia neurotoxicity. Metab Brain Dis 24: 103–117, 2009. doi: 10.1007/s11011-008-9113-6. [DOI] [PubMed] [Google Scholar]
- 39.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95: 351–358, 1979. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 40.Orbea A, Ortiz-Zarragoitia M, Solé M, Porte C, Cajaraville MP. Antioxidant enzymes and peroxisome proliferation in relation to contaminant body burdens of PAHs and PCBs in bivalve molluscs, crabs and fish from the Urdaibai and Plentzia estuaries (Bay of Biscay). Aquat Toxicol 58: 75–98, 2002. doi: 10.1016/S0166-445X(01)00226-0. [DOI] [PubMed] [Google Scholar]
- 41.Pérez-Campo R, López-Torres M, Rojas C, Cadenas S, Barja G. A comparative study of free radicals in vertebrates. I. Antioxidant enzymes. Comp Biochem Physiol 105: 749–755, 1993. doi: 10.1016/0305-0491(93)90116-M. [DOI] [PubMed] [Google Scholar]
- 43.Pifferi F, Perret M, Guesnet P, Aujard F, Alessandri JM. Fatty acid composition of the brain, retina, liver and adipose tissue of the grey mouse lemur (Microcebus murinus, primate). Lipids 47: 793–801, 2012. doi: 10.1007/s11745-012-3686-x. [DOI] [PubMed] [Google Scholar]
- 44.Randall DJ, Tsui TKN. Ammonia toxicity in fish. Mar Pollut Bull 45: 17–23, 2002. doi: 10.1016/S0025-326X(02)00227-8. [DOI] [PubMed] [Google Scholar]
- 45.Rau MA, Whitaker J, Freedman JH, Di Giulio RT. Differential susceptibility of fish and rat liver cells to oxidative stress and cytotoxicity upon exposure to prooxidants. Comp Biochem Physiol C Toxicol Pharmacol 137: 335–342, 2004. doi: 10.1016/j.cca.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 46.Rose C, Michalak A, Pannunzio M, Chatauret N, Rambaldi A, Butterworth RF. Mild hypothermia delays the onset of coma and prevents brain edema and extracellular brain glutamate accumulation in rats with acute liver failure. Hepatology 31: 872–877, 2000. doi: 10.1053/he.2000.5923. [DOI] [PubMed] [Google Scholar]
- 47.Schlezinger JJ, Stegeman JJ. Induction and suppression of cytochrome P450 1A by 3,3′,4,4′,5-pentachlorobiphenyl and its relationship to oxidative stress in the marine fish scup (Stenotomus chrysops). Aquat Toxicol 52: 101–115, 2001. doi: 10.1016/S0166-445X(00)00141-7. [DOI] [PubMed] [Google Scholar]
- 48.Schliess F, Görg B, Fischer R, Desjardins P, Bidmon HJ, Herrmann A, Butterworth RF, Zilles K, Häussinger D. Ammonia induces MK-801-sensitive nitration and phosphorylation of protein tyrosine residues in rat astrocytes. FASEB J 16: 739–741, 2002. [DOI] [PubMed] [Google Scholar]
- 49.Shi H, Sui Y, Wang X, Luo Y, Ji L. Hydroxyl radical production and oxidative damage induced by cadmium and naphthalene in liver of Carassius auratus. Comp Biochem Physiol C Toxicol Pharmacol 140: 115–121, 2005. doi: 10.1016/j.cca.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 50.Sinha AK, AbdElgawad H, Giblen T, Zinta G, De Rop M, Asard H, Blust R, De Boeck G. Anti-oxidative defences are modulated differentially in three freshwater teleosts in response to ammonia-induced oxidative stress. PLoS One 9: e95319, 2014. doi: 10.1371/journal.pone.0095319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sinha AK, Liew HJ, Diricx M, Blust R, De Boeck G. The interactive effects of ammonia exposure, nutritional status and exercise on metabolic and physiological responses in gold fish (Carassius auratus L.). Aquat Toxicol 109: 33–46, 2012. doi: 10.1016/j.aquatox.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 52.Sinha AK, Liew HJ, Diricx M, Kumar V, Darras VM, Blust R, De Boeck G. Combined effects of high environmental ammonia, starvation and exercise on hormonal and ion-regulatory response in goldfish (Carassius auratus L.). Aquat Toxicol 114–115: 153–164, 2012. doi: 10.1016/j.aquatox.2012.02.027. [DOI] [PubMed] [Google Scholar]
- 53.Sinha AK, Zinta G, AbdElgawad H, Asard H, Blust R, De Boeck G. High environmental ammonia elicits differential oxidative stress and antioxidant responses in five different organs of a model estuarine teleost (Dicentrarchus labrax).Comp Biochem Physiol C Toxicol Pharmacol 174–175: 21–31, 2015. doi: 10.1016/j.cbpc.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 54.Sugihara N, Tsuruta Y, Date Y, Furuno K, Kohashi K. High peroxidative susceptibility of fish oil polyunsaturated fatty acid in cultured rat hepatocytes. Toxicol Appl Pharmacol 126: 124–128, 1994. doi: 10.1006/taap.1994.1098. [DOI] [PubMed] [Google Scholar]
- 55.Sun H, Lü K, Minter EJ, Chen Y, Yang Z, Montagnes DJS. Combined effects of ammonia and microcystin on survival, growth, antioxidant responses, and lipid peroxidation of bighead carp Hypophthalmythys nobilis larvae. J Hazard Mater 221-222: 213–219, 2012. doi: 10.1016/j.jhazmat.2012.04.036. [DOI] [PubMed] [Google Scholar]
- 56.Sun H, Yang W, Chen Y, Yang Z. Effect of purified microcystin on oxidative stress of silver carp Hypophthalmichthys molitrix larvae under different ammonia concentrations. Biochem Syst Ecol 39: 536–543, 2011. doi: 10.1016/j.bse.2011.08.001. [DOI] [Google Scholar]
- 57.Tay YL, Loong AM, Hiong KC, Lee SJ, Tng YYM, Wee NLJ, Lee SML, Wong WP, Chew SF, Wilson JM, Ip YK. Active ammonia transport and excretory nitrogen metabolism in the climbing perch, Anabas testudineus, during 4 days of emersion or 10 minutes of forced exercise on land. J Exp Biol 209: 4475–4489, 2006. doi: 10.1242/jeb.02557. [DOI] [PubMed] [Google Scholar]
- 58.Tofteng F, Hauerberg J, Hansen BA, Pedersen CB, Jørgensen L, Larsen FS. Persistent arterial hyperammonemia increases the concentration of glutamine and alanine in the brain and correlates with intracranial pressure in patients with fulminant hepatic failure. J Cereb Blood Flow Metab 26: 21–27, 2006. doi: 10.1038/sj.jcbfm.9600168. [DOI] [PubMed] [Google Scholar]
- 59.Tsui TKN, Randall DJ, Chew SF, Jin Y, Wilson JM, Ip YK. Accumulation of ammonia in the body and NH(3) volatilization from alkaline regions of the body surface during ammonia loading and exposure to air in the weather loach Misgurnus anguillicaudatus. J Exp Biol 205: 651–659, 2002. [DOI] [PubMed] [Google Scholar]
- 61.Van Waarde A. Aerobic and anaerobic ammonia production by fish. Comp Biochem Physiol B 74: 675–684, 1983. [DOI] [PubMed] [Google Scholar]
- 62.Verdouw H, Van Echteld CJA, Dekkers EMJ. Ammonia determination based on indophenol formation with sodium salicylate. Water Res 12: 399–402, 1978. doi: 10.1016/0043-1354(78)90107-0. [DOI] [Google Scholar]
- 63.Vieira MC, Torronteras R, Córdoba F, Canalejo A. Acute toxicity of manganese in goldfish Carassius auratus is associated with oxidative stress and organ specific antioxidant responses. Ecotoxicol Environ Saf 78: 212–217, 2012. doi: 10.1016/j.ecoenv.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 64.Walsh PJ, Veauvy CM, McDonald MD, Pamenter ME, Buck LT, Wilkie MP. Piscine insights into comparisons of anoxia tolerance, ammonia toxicity, stroke and hepatic encephalopathy. Comp Biochem Physiol A Mol Integr Physiol 147: 332–343, 2007. doi: 10.1016/j.cbpa.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang Y, Heigenhauser GJF, Wood CM. Integrated responses to exhaustive exercise and recovery in rainbow trout white muscle: acid-base, phosphogen, carbohydrate, lipid, ammonia, fluid volume and electrolyte metabolism. J Exp Biol 195: 227–258, 1994. [DOI] [PubMed] [Google Scholar]
- 66.Wang Y, Walsh PJ. High ammonia tolerance in fishes of the family Batrachoididae (Toadfish and Midshipmen). Aquat Toxicol 50: 205–219, 2000. doi: 10.1016/S0166-445X(99)00101-0. [DOI] [PubMed] [Google Scholar]
- 67.Wang YX, Wilkie MP, Heigenhauser GJF, Wood CM. The analysis of metabolites in rainbow trout white muscle: a comparison of different sampling and processing methods. J Fish Biol 45: 855–873, 1994. doi: 10.1111/j.1095-8649.1994.tb00950.x. [DOI] [Google Scholar]
- 68.Weydert CJ, Cullen JJ. Measurement of superoxide dismutase, catalase and glutathione peroxidase in cultured cells and tissue. Nat Protoc 5: 51–66, 2010. doi: 10.1038/nprot.2009.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wilkie MP. Mechanisms of ammonia excretion across fish gills. Comp Biochem Physiol 118: 39–50, 1997. doi: 10.1016/S0300-9629(96)00407-0. [DOI] [Google Scholar]
- 70.Wilkie MP, Pamenter ME, Duquette S, Dhiyebi H, Sangha N, Skelton G, Smith MD, Buck LT. The relationship between NMDA receptor function and the high ammonia tolerance of anoxia-tolerant goldfish. J Exp Biol 214: 4107–4120, 2011. doi: 10.1242/jeb.057513. [DOI] [PubMed] [Google Scholar]
- 71.Wilkie MP, Stecyk JAW, Couturier CS, Sidhu S, Sandvik GK, Nilsson GE. Reversible brain swelling in crucian carp (Carassius carassius) and goldfish (Carassius auratus) in response to high external ammonia and anoxia. Comp Biochem Physiol 184: 65–75, 2015. doi: 10.1016/j.cbpa.2014.12.038. [DOI] [PubMed] [Google Scholar]
- 72.Wilkie MP, Wood CM. Nitrogenous waste excretion, acid-base regulation, and ionoregulation in rainbow trout (Oncorynchus mykiss) exposed to extremely alkaline water. Physiol Zool 64: 1069–1086, 1991. doi: 10.1086/physzool.64.4.30157957. [DOI] [Google Scholar]
- 73.Wilkie MP, Wright PA, Iwama GK, Wood CM. The physiological responses of the Lahontan cutthroat trout (Oncorhynchus clarki henshawi), a resident of highly alkaline pyramid lake (pH 9.4), to challenge at pH 10. J Exp Biol 175: 173–194, 1993. [Google Scholar]
- 74.Wood CM. Influence of feeding, exercise, and temperature on nitrogen metabolism and excretion. In: Nitrogen Excretion–Fish Physiology, edited by Wright PA, Anderson PM. New York: Academic, 2001. doi: 10.1016/S1546-5098(01)20007-7 [DOI] [Google Scholar]
- 75.Wright PA, Iwama GK, Wood CM. Ammonia and urea excretion in Lahontan cutthroat trout (Oncorhynchus clarki henshawi) adapted to the highly alkaline pyramid lake (pH 9.4). J Exp Biol 175: 153–172, 1993. [Google Scholar]


