Abstract
Skeletal muscle satellite cells (SC) play an important role in muscle adaptation. In untrained individuals, SC content and activation status have been observed to increase in response to a single bout of exercise. Muscle fiber characteristics change considerably when resistance exercise is performed chronically, but whether training status affects the activity of SC in response to a single bout of exercise remains unknown. We examined the changes in SC content and activation status following a single bout of resistance exercise, before and following a 16-wk progressive resistance training (RT) program in 14 young (25 ± 3 yr) men. Before and after RT, percutaneous biopsies from the vastus lateralis muscle were taken before a single bout of resistance exercise and after 24 and 72 h of postexercise recovery. Muscle fiber size, capillarization, and SC response were determined by immunohistochemistry. Following RT, there was a greater activation of SC after 24 h in response to a single bout of resistance exercise (Pre, 1.4 ± 0.3; 24 h, 3.1 ± 0.3 Pax7+/MyoD+ cells per 100 fibers) compared with before RT (Pre, 1.4 ± 0.3; 24 h, 2.2 ± 0.3 Pax7+/MyoD+ cells per 100 fibers, P < 0.05); no difference was observed 72 h postexercise. Following 16 wk of RT, MyoD mRNA expression increased from basal to 24 h after the single bout of exercise (P < 0.05); this change was not observed before training. Individual capillary-to-fiber ratio (C/Fi) increased in both type I (1.8 ± 0.3 to 2.0 ± 0.3 C/Fi, P < 0.05) and type II (1.7 ± 0.3 to 2.2 ± 0.3 C/Fi, P < 0.05) fibers in response to RT. After RT, enhanced activation of SC in response to resistance exercise is accompanied by increases in muscle fiber capillarization.
Keywords: muscle stem cells, Pax7, MyoD, capillaries, perfusion
the activation, proliferation, and/or differentiation of satellite cells (SC) are important events in postexercise recovery leading to muscle fiber adaptation, remodeling, and repair. After a single bout of damage (21, 22) or resistance exercise (37) in humans, expansion of the SC pool is observed by 24 h, peaking at 72 h postexercise (36). Irrespective of the model employed, these aforementioned studies (21, 22, 37) were primarily performed on exercise-naive participants. Presumably then, the typically observed increase in SC content may be a result of general stress rather than a refined adaptive response to an exercise bout. It is well established that repeated bouts of exercise result in markedly reduced indexes of muscle damage and stress following subsequent bouts (20). Similarly, exercise-trained individuals typically demonstrate an attenuated damage or stress response to a habitual exercise challenge (28, 29, 44), suggesting that adaptation has occurred. However, whether the acute SC response following a single bout of exercise is altered in exercise-trained individuals (i.e., individuals who are accustomed to the exercise stimulus) compared with exercise-naive individuals following a single exercise session remains unknown. Consequently, comparing the change in SC content in the untrained and trained state following a single bout of exercise can provide insight to the nature of adaptation.
The progression of SC through the myogenic program is orchestrated by a transcriptional network collectively known as the myogenic regulatory factors (i.e., MyoD, Myf5, Myogenin and MRF4). There is relatively little known regarding adaptation in the myogenic program following exercise-training. In addition, various regulatory factors such as hepatocyte growth factor (HGF), interleukin 6 (IL-6), myostatin, insulin-like growth factor-1 (IGF-1) have been shown to be key regulators in the process of activation, proliferation and/or differentiation (21–23, 26). Some of these factors are produced locally by skeletal muscle (27, 39). As an ‘endocrine organ’, skeletal muscle tissue produces and releases various cytokines that act in a paracrine, autocrine, or endocrine fashion (27). Consistent with this notion, it has been shown that the systemic environment plays a critical role in SC function (3, 9). Although regulatory signals may originate locally, they may also be derived from other organs and the broader circulatory system (42). Therefore, it has been hypothesized that muscle fiber capillarization may play an important role in the regulation of SC (5).
In healthy young men, RT is sufficient to promote capillarization (11). The increase in capillary number, induced by training, likely reflects the necessity to match the demand for oxygen (15) and nutrients (6, 7) to support growing/adapting muscle fibers. Furthermore, the increase in capillary number is larger as compared with the increase in muscle fiber size, leading to a greater number of capillaries per area muscle, which suggests a more efficient perfusion of the muscle fiber following prolonged resistance exercise training (14). Whether increased muscle fiber capillarization influences SC regulation in healthy young adults remains unknown.
We assessed the activation of the SC pool in response to a single bout of resistance exercise in a group of healthy young men before (untrained state response; UTSR) and following (trained state response; TSR) 16 wk of resistance training (RT). We hypothesized that, following RT there would be an augmented activation of muscle SC in response to a single bout of resistance exercise and that this would be associated with enhanced muscle fiber perfusion.
METHODS
Participants
Fourteen healthy young men (YM: 25 ± 3 yr; mean ± SE) were recruited to participate in this study. All participants were recreationally active with no formal weight training experience in the previous 6 mo. The participants in this study were a subset of a larger project investigating the adaptation of skeletal muscle tissue to prolonged resistance exercise training in healthy young men and included data relating to fiber cross-sectional area, strength changes with training, and expansion of the quiescent satellite cell pool (1, 24). The participant selection for the present study was based upon the availability of tissue for all time points for which to perform immunohistochemical analysis. Exclusion criteria included smoking, diabetes, the use of nonsteroidal anti-inflammatory drugs (NSAIDs), and/or statins, and history of respiratory disease and/or any major orthopedic disability. The study was approved by the Hamilton Health Sciences Integrated Research Ethics Board and conformed to the guidelines outlined in the Declaration of Helsinki. Participants gave their informed written consent before their inclusion to the study.
Muscle Biopsy Sampling
Percutaneous needle biopsies were taken after an (~10 h) overnight fast, from the midportion of the vastus lateralis under local anesthetic using a 5-mm Bergstrom needle adapted for manual suction (2). Subjects had not participated in any physical activity for at least 96 h before the biopsy collection before the bout of resistance exercise in the untrained condition (i.e., before resistance training) and the trained condition (i.e., following resistance training). The muscle biopsy procedure was repeated under the same fasted condition (~10 h) 24 h and 72 h following the single bout of resistance exercise detailed below. Incisions for the repeated muscle biopsy sampling were spaced ~3 cm apart to minimize any effect of the previous biopsy. Upon excision, muscle samples were immediately mounted in optimal cutting temperature (OCT) compound, frozen in liquid nitrogen-cooled isopentane, and stored at −80°C until further analyses.
Exercise Training
Exercise training was performed four times per week, divided into two upper and two lower body sessions under strict supervision as described previously (24). The lower body session consisted of five exercises: leg press, leg extension, leg curl, calf press, and plank exercise. The upper body session consisted of six exercises: chest press, shoulder press, lat pull down, row, biceps curl and triceps extension. Training progressed from two sets performed at 70% of 1 repetition maximum (RM) to four sets performed at 85% of 1RM, with the final set performed to the point of momentary muscle exhaustion. At the conclusion of each workout, and on the mornings of non-training days, participants consumed a beverage containing 30 g of whey protein, 25.9 g of carbohydrates and 3.4 g of fat (Musashi p30, Notting Hill Victoria, Australia).
Single Bout of Resistance Exercise
To determine the impact of resistance exercise on SC content and activation status in relation to RT, participants performed a single bout of resistance exercise both before and following 16 wk of RT. In short, the participants completed four sets of eight repetitions each at 80% of 1RM on leg press (Maxam, Hamilton, Ontario), leg extension (Atlantis, Laval, Quebec), calf press and leg curl (Hur, Kokkola Finland). The single bout of exercise was performed at the same relative intensity both before and following RT. The final set of each exercise was performed to volitional failure (1). A resting period of 2 min between sets was allowed. All participants were verbally encouraged during the exercise session to complete the entire protocol. Prior to and following the resistance exercise, a 5 min warm up was performed on a cycle ergometer.
Immunofluorescence
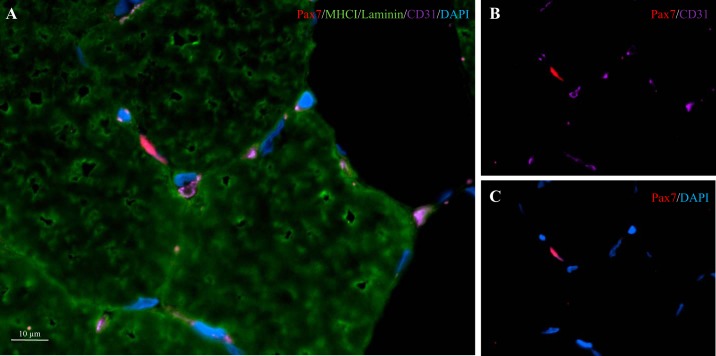
Muscle cross sections (7 µm) were prepared from unfixed OCT-embedded samples, allowed to air dry for 30 min and stored at −80°C. Samples were stained with antibodies against appropriate primary and secondary antibodies, found in Table 1, as previously described (25). Nuclei were labeled with DAPI (4′,6-diamidino-2-phenylindole) (1:20,000, Sigma-Aldrich, Oakville, ON, Canada), before being coverslipped with fluorescent mounting media (DAKO, Burlington, ON, Canada). The staining procedures were verified using negative controls, to ensure appropriate specificity of staining. Slides were viewed with the Nikon Eclipse Ti Microscope (Nikon Instruments), equipped with a high-resolution Photometrics CoolSNAP HQ2 fluorescent camera (Nikon Instruments, Melville, NY). Images were captured and analyzed using the Nikon NIS Elements AR 3.2 software (Nikon Instruments). All images were obtained with the ×20 objective, and ≥200 muscle fibers per subject per time point were included in the analyses for SC content/activation status (i.e., Pax7+/MyoD+ or Pax7+/MyoD−), and fiber cross-sectional area (CSA), and perimeter. The activation status of SCs was determined via the colocalization of Pax7+ and DAPI (Pax7+/MyoD−) and/or the colocalization of Pax7, MyoD and DAPI (i.e., Pax7+/MyoD+). Slides were blinded for both group and time point. The quantification of muscle fiber capillaries was performed on 50 muscle fibers per subject per time point (30). Based on the work of Hepple et al. (15), quantification of 1) capillary contacts (CC; the number of capillaries around a fiber), 2) the capillary-to-fiber ratio on an individual fiber basis (C/Fi), 3) the number of fibers sharing each capillary (i.e., the sharing factor), and 4) the capillary density (CD) was performed. The CD was calculated by using the cross-sectional area (m2) as the reference space. The capillary-to-fiber perimeter exchange index (CFPE) was calculated as an estimate of the capillary-to-fiber surface area (15). The SC-to-capillary distance measurements were performed on all SC that were enclosed by other muscle fibers, and has been described previously as well as in Fig. 1 (25). All immunofluorescent analysis were completed in a blinded fashion.
Table 1.
Antibody information
| Antibody | Species | Source | Clone | Primary | Secondary |
|---|---|---|---|---|---|
| Anti-Pax7 | Mouse | DSHB | Pax7 | 1:1 | Alexa 594, 488 goat-anti mouse 1:500 |
| Anti-laminin | Rabbit | Abcam | ab11575 | 1:500 | Alexa Fluor 488, 647 goat anti-rabbit, 1:500 |
| Anti-MHCI | Mouse | DSHB | A4.951 Slow isoform | 1:1 | Alexa Fluor 488 goat anti-mouse, 1:500 |
| Anti-CD31 | Rabbit | Abcam | ab28364 | 1:30 | Alexa Fluor 647 goat anti-rabbit, 1:500 |
| Anti-MyoD | Mouse | Dako | 5.8A | 1:50 | goat anti-mouse biotinylated secondary antibody, 1:200; streptavidin-594 fluorochrome, 1:250 |
Detailed information on primary and secondary antibodies and dilutions used for immunofluorescent staining of the frozen muscle cross sections.
Fig. 1.
Fiber type-specific staining with muscle capillaries. A: representative image of a MHCI/laminin/CD31/Pax7/DAPI stain of a muscle cross section. Channel views of CD31/Pax7 (B) and Pax7/DAPI (C).
RNA Isolation
RNA was isolated from 15 to 25 mg of muscle using the TRIzol/RNeasy method. All samples were homogenized with 1 ml of TRIzol Reagent (Life Technologies, Burlington, ON, Canada), in Lysing Maxtrix D tubes (MP Biomedicals, Solon, OH), with the FastPrep-24 Tissue and Cell Homogenizer (MP Biomedicals) for a duration of 40 s at a setting of 6 m/s. After a 5-min room temperature incubation, homogenized samples were stored at −80°C for 1 mo until further processing. After thawing on ice was completed, 200 ml of chloroform (Sigma-Aldrich, Oakville, ON, Canada) were added to each sample, mixed vigorously for 15 s, incubated at RT for 5 min, and spun at 12,000 g for 10 min at 4°C. The RNA (aqueous) phase was purified using the E.Z.N.A. Total RNA Kit 1 (Omega Bio-Tek, Norcross, GA) as per manufacturer’s instructions. RNA concentration (ng/ml) and purity (260/280) were determined with the Nano-Drop 1000 Spectrophotometer (Thermo Fisher Scientific, Rockville, MD). RNA integrity was determined using the Agilent 2100 Bioanalyzer (Agilent Technologies, Toronto, ON, Canada). Samples were reverse transcribed using a high-capacity cDNA reverse transcription kit (Applied Biosystems, Foster City, CA) in 20-μl reaction volumes, as per manufacturer’s instructions, using an Eppendorf Mastercycler epGradient Thermal Cycler (Eppendorf, Mississauga, ON, Canada) to obtain cDNA for gene expression analysis.
Quantitative Real-Time RT-PCR
All QPCR reactions were run in duplicate in 25-µl volumes containing RT Sybr Green qPCR Master Mix (Qiagen Sciences, Valencia, CA), prepared with the epMotion 5075 Eppendorf automated pipetting system (Eppendorf), and carried out using an Eppendorf Realplex2 Master Cycler epgradient (Eppendorf). Primers are listed in Table 2 and were resuspended in 1× TE buffer (10 mM Tris·HCl and 0.11 mM EDTA) and stored at −20°C before use. mRNA expression was calculated using the 2−ΔΔCt method, and fold changes from baseline were calculated using the ΔΔCt method (18). Gene expression was normalized to the housekeeping gene β-2-microglobulin (β2M). Expression of β2M did not differ between time points.
Table 2.
Primer sequences for quantitative real-time PCR
| Gene Name | Forward Sequence (5′→3′) | Reverse Sequence (5′→3′) |
|---|---|---|
| Myf5 | ATGGACGTGATGGATGGCTG | GCGGCACAAACTCGTCCCCAA |
| MyoD | GGTCCCTCGCGCCCAAAAGAT | CAGTTCTCCCGCCTCTCCTAC |
| MRF4 | CCCCTTCAGCTACAGACCCAA | CCCCCTGGAATGATCGGAAAC |
| β-2-m | ATGAG TATGCCTGCCGTGTGA | GGCATCTTCAAACCTCCATG |
MyoD, myogenic determination factor; Myf5, myogenic factor-5; MRF4, myogenic regulatory factor-4; β-2-m, β-2-microglobulin.
Statistical Analysis
Statistical analysis was performed using Sigma Stat 3.1.0 analysis software (Systat Software, Chicago, IL). To assess the long-term changes in muscle fiber characteristics in response to 16 wk of RT, two way ANOVA was performed with time (pre- and postexercise training) and fiber type (type I and II) as within-subject factors, and appropriate post hoc analysis was performed if interactions were detected. Separate one-way repeated-measures ANOVA, with time (Pre, 24 h, and 72 h) as a within factor, were performed to assess the following: the acute change in satellite cell activity status (i.e., Pax7+/MyoD− and/or Pax7+/MyoD+ cells); the acute change in distance of activated SC to nearest capillary following a single bout of resistance type exercise; the acute change in MRF mRNA expression, before and following 16 wk of RT. In the one-way repeated-measures ANOVA design for the acute SC response, postexercise time points were only compared with baseline, and Bonferonni corrections were applied to account for multiple comparisons. In addition, to assess the difference in the acute SC response before and following 16 wk of exercise training, a paired sample Student’s t-test was utilized to compare the change in SC content and activation status (Pre vs. 24 h, and Pre vs. 72 h), before and following 16 wk of RT. Statistical significance was accepted at P < 0.05. All results were presented as means ± SE.
RESULTS
Muscle Fiber CSA and Fiber-Type Distribution
Muscle fiber CSA was significantly greater in type II compared with type I, both before and after RT (P < 0.05, Table 3). We previously reported a significant increase in muscle fiber CSA in a larger cohort (1). Analysis of this subset of subjects resulted in similar statistically significant changes to those observed in the larger cohort previously reported (1). The percentage of type II muscle fibers was significantly greater than type I fibers (P < 0.05, Table 3); muscle fiber type distribution did not change with RT. After 16 wk of RT, there was a significant increase in both type I and type II muscle fiber CSA and perimeter (P < 0.05, Table 3). Furthermore, after16 wk of RT, type II muscle fiber CSA was greater than type I (P < 0.05, Table 3).
Table 3.
Skeletal muscle fiber characteristics before and after 16 wk of resistance exercise training in young men
| Fiber Type | Pre | Post | |
|---|---|---|---|
| Fiber area, µm2 | I | 5,621 ± 409 | 6,263 ± 413# |
| II | 5,771 ± 381* | 7,725 ± 519*# | |
| Fiber perimeter, µm2 | I | 294 ± 9 | 309 ± 11# |
| II | 319 ± 10* | 359 ± 18*# | |
| Fiber type distribution, fiber % | I | 33 ± 3 | 38 ± 2 |
| II | 67 ± 3* | 62 ± 2* |
Values are means ± SE.
Significant difference between fiber types (P < 0.05);
significant effect of exercise training (P < 0.05).
Muscle Fiber Capillarization
There was greater CC (the number of capillaries around a fiber), C/Fi ratio (capillary-to-fiber ratio), CFPE (capillary-to-fiber perimeter exchange index), and CD (capillary density) in type I compared with type II muscle fibers (P < 0.05, Table 4). In both type I and type II muscle fibers, CFPE and C/Fi was significantly greater following RT (all P < 0.05, Table 4). In contrast, no differences in type I and type II muscle fiber CC and CD were observed with RT.
Table 4.
Skeletal muscle fiber capillarization characteristics before and following 16 wk of resistance exercise training in young men
| Fiber Type | Pre | Post | |
|---|---|---|---|
| Capillary contacts | I | 3.18 ± 0.17 | 3.78 ± 0.22 |
| II | 2.12 ± 0.16* | 2.95 ± 0.21* | |
| Individual capillary-to-fiber ratio (C/Fi) | I | 1.71 ± 0.08 | 1.94 ± 0.03# |
| II | 1.64 ± 0.09 | 2.07 ± 0.09# | |
| Capillary density, capillaries/mm2 | I | 586 ± 32 | 640 ± 54 |
| II | 383 ± 34* | 400 ± 33* | |
| CFPE, capillaries/1,000 µm | I | 5.89 ± 0.21 | 6.45 ± 0.22# |
| II | 5.07 ± 0.19* | 5.95 ± 0.18*# |
Values are means ± SE. CFPE, capillary to fiber perimeter exchange index.
Significantly different compared with type I muscle fibers (P < 0.05);
significant effect for exercise training (P < 0.05).
Fiber Type-Specific Satellite Cell Content and Distance to Nearest Capillary
In resting muscle, SC content was greater in type II than type I muscle fibers (P < 0.05, Table 5) both before and after RT, as previously reported (1). Type II-associated SC were located at a greater distance to their nearest capillary as compared with type I-associated SC (P < 0.05, Table 5) both before and after RT. Both the number of type I- and type II-associated SC increased after RT (P < 0.05, Table 5). There was no change in distance to the nearest capillary from either type I- or type II-associated SC after 16 wk RT (Table 5).
Table 5.
Fiber type-associated SC content and distance to nearest capillary before and after 16 wk of resistance exercise training in young men
| Fiber Type | Pre | Post | |
|---|---|---|---|
| SC, Pax7+ cells/100 myofibers | I | 10.9 ± 0.8 | 13.4 ± 0.6# |
| II | 11.9 ± 0.8* | 15.6 ± 0.9*# | |
| SC distance to capillary, µm | I | 15.2 ± 1.0 | 13.9 ± 0.7 |
| II | 16.8 ± 0.7* | 15.9 ± 0.9* |
Values are means ± SE. SC: satellite cell.
Significant effect of fiber type (P < 0.05);
significant effect for exercise training (P < 0.05).
Satellite Cell Content and Activation Status in Response to an Acute Bout of Exercise
UTSR.
Response to a single bout of exercise resulted in total Pax7+ cells per 100 myofibers remaining unchanged at 24 h (11.9 ± 0.9 cells/100 myofibers) but increased significantly at 72 h (15.2 ± 1.3 cells/100 myofibers) compared with Pre (11.8 ± 1.1 cells/100 myofibers) (P < 0.05, Fig. 2A). Pax7+/MyoD+ cells per 100 myofibers were significantly higher at 24 h (2.2 ± 0.3 cells/100 myofibers) and 72 h (2.3 ± 0.4 cells/100 myofibers) after the single bout of exercise as compared with Pre (1.4 ± 0.3 cells/100 myofibers) (P < 0.05, Fig. 2B). Pax7+/MyoD− cells per 100 myofibers did not change from Pre (10.4 ± 1.0 cells/100 myofibers) to 24 h (9.7 ± 0.8 cells/100 myofibers), but was trending toward significance at 72 h (12.9 ± 1.2 cells/100 myofibers) after the single bout of exercise (P = 0.06, Fig. 2C).
Fig. 2.
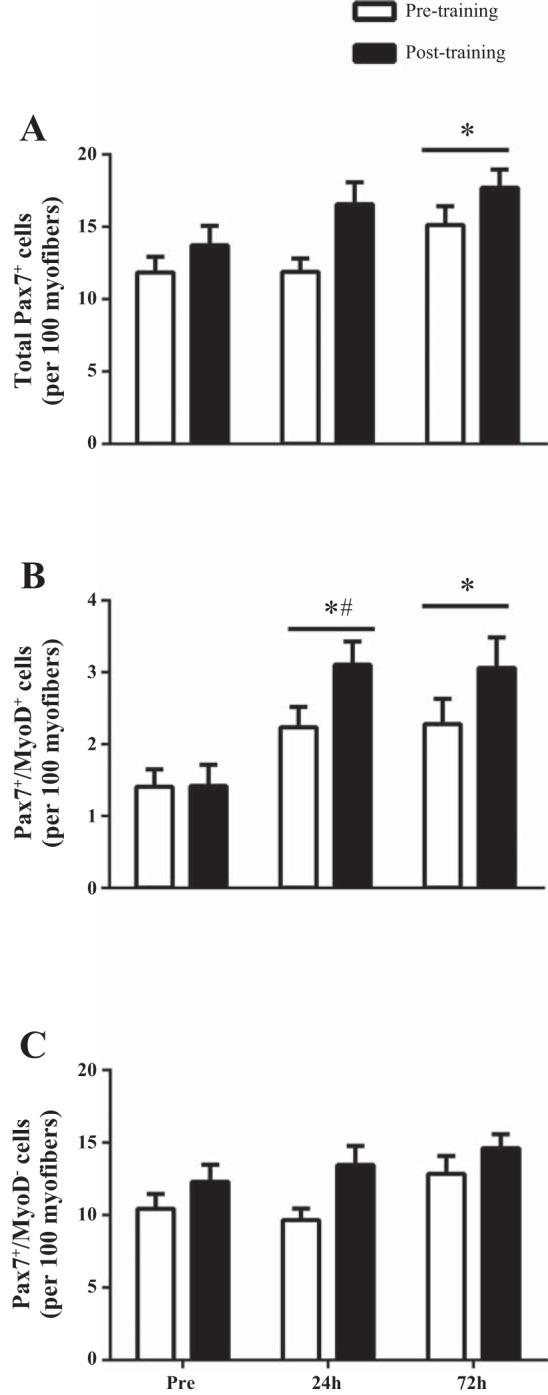
Characterization of the activity status of satellite cell (SC) after a single bout of resistance exercise before (UTSR; open bars) and after 16 wk of RT (TSR; filled bars). Quantification of these cell populations as total number of Pax7+ SC (A), number of MyoD+/Pax7+ (active SC; B), number of MyoD−/Pax7+ (quiescent SC; C) per 100 myofiber before, 24 h, and 72 h postexercise recovery. *Time effect vs. Pre (P < 0.05); bar indicates that effect of time is present for both before and after 16 wk of RT. #Significantly greater (P < 0.05) increase with time TSR vs. UTSR. Values are means ± SE.
TSR.
In response to a single bout of resistance exercise of the same relative intensity following 16 wk of RT, total Pax7+ cells/100 myofibers was unchanged 24 h (16.6 ± 1.5 cells/100 myofibers) and increased significantly at 72 h (17.7 ± 1.3 cells/100 myofibers) compared with Pre (13.7 ± 1.4 cells/100 myofibers) (P < 0.05, Fig. 2A). Pax7+/MyoD+ cells per 100 myofibers were significantly increased at 24 h (3.1 ± 0.2 cells/100 myofibers) and 72 h (3.1 ± 0.4 cells/100 myofibers) after the single bout of exercise compared with Pre (1.4 ± 0.4 cells/100 myofibers) (P < 0.05, Fig. 2B). Pax7+/MyoD− cells per 100 myofibers were unchanged from Pre (12.3 ± 1.2 cells/100 myofibers) to 24 h (13.5 ± 1.3 cells/100 myofibers), but was trending toward significance at 72 h (14.6 ± 1.0 cells/100 myofibers) after the single bout of exercise (P = 0.08, Fig. 2C).
UTSR vs. TSR.
In comparing the UTSR and TSR responses we discovered that there was a greater change in the number of Pax7+/MyoD+ cells from Pre to 24 h postexercise recovery compared with UTSR (Fig. 2B).
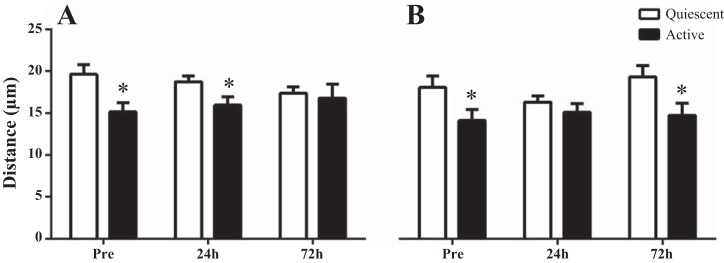
Distance of SC to Nearest Capillary in Response to an Acute Bout of Resistance Exercise
UTSR.
Pax7+/MyoD+ cells were closer to their nearest capillary compared with Pax7+/MyoD− cells both before the single bout of exercise (Pre) and at 24 h postexercise recovery (P < 0.05, Fig. 3A). There were no difference in distance to the nearest capillary from SC that were Pax7+/MyoD− or Pax7+/MyoD+ (P > 0.05, Fig. 3A) at 72 h postexercise. Before resistance training, there was no difference in the distance of Pax7+/MyoD+ or Pax7+/MyoD− cells to the nearest capillary 24 h or 72 h following a single bout of exercise in comparison to the Pre distance.
Fig. 3.
Distance between activated (MyoD+/Pax7+) and quiescent (MyoD−/Pax7+) SC to nearest capillary following a single bout of exercise before compared with after 16 wk of RT. Response to resistance exercise before 16 wk RT exercise (UTSR; A) and after (TSR; B). *Significantly different compared with active SC within time point (P < 0.05). Values are means ± SE.
TSR.
Pax7+/MyoD+ cells were located closer to the nearest capillary compared with Pax7+/MyoD− cells before the single bout of exercise (P < 0.05, Fig. 3B). However, at 24 h postexercise recovery, the difference in distance between SC and its nearest capillary was abolished, such that there was no difference between the two SC populations (Fig. 3B). At 72 h, there was a reestablishment of the relationship observed at the Pre time point, such that Pax7+/MyoD+ cells were again located closer to their nearest capillary compared with Pax7+/MyoD− cells (P < 0.05, Fig. 3B). After 16 wk resistance training, there was no difference in the distance of Pax7+/MyoD+ or Pax7+/MyoD− cells to the nearest capillary 24 or 72 h after a single bout of exercise compared with baseline measurements.
MRF Genes in Response to an Acute Bout of Resistance Exercise
UTSR.
In response to a single bout of exercise, MyoD mRNA expression did not increase from basal levels at 24 h (1.1-fold change) or 72 h postexercise recovery (1.8-fold change), compared with Pre (Fig. 4A). MRF4 mRNA expression did not significantly increase from basal expression at 24 h (1.2-fold change) or at 72 h postexercise recovery (1.3-fold change) (Fig. 4B). Myf5 mRNA expression did not significantly increase from basal expression at 24 h (1.4-fold change) or at 72 h postexercise recovery (1.1-fold change) (Fig. 4C).
Fig. 4.
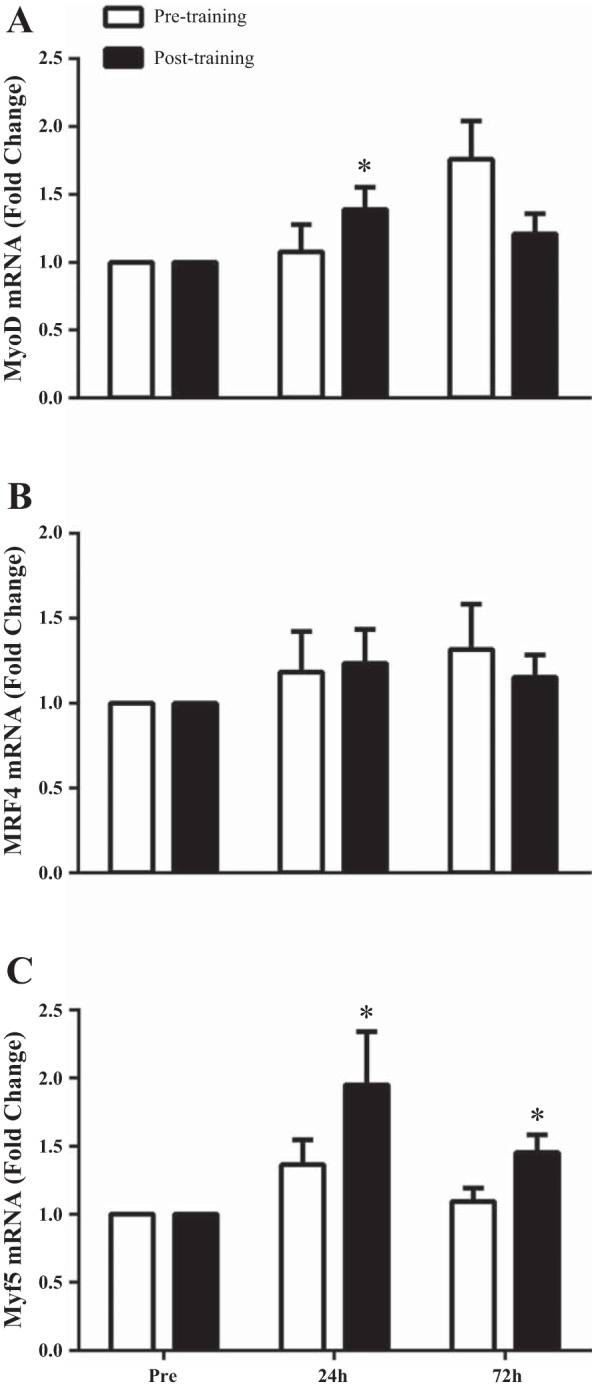
Relative expression of MyoD mRNA (A), MRF4 mRNA (B), and Myf5 mRNA (C) expression in response to a single bout of exercise before (UTSR; open bars) compared with after 16 wk of RT (TSR; filled bars) expressed as fold change from Pre. Data are normalized to β-2-microglobulin. *Significantly different compared with Pre (P < 0.05). Values are means ± SE.
TSR.
After 6 wk of RT, a single bout of exercise resulted in MyoD mRNA expression increased 1.4-fold from basal levels at 24 h postexercise recovery (P < 0.05, Fig. 4A). However, MyoD mRNA expression was no longer increased 72 h postexercise recovery compared with Pre (1.2-fold change) (P > 0.05, Fig. 4A). Myf5 mRNA expression was increased at both 24 h (2.0-fold) and 72 h (1.5-fold) postexercise compared with Pre (P < 0.05, Fig. 4C). MRF4 mRNA expression did not significantly increase from basal levels at 24 h (1.2-fold change) or at 72 h postexercise (1.2-fold change).
DISCUSSION
In the present study we observed an altered activation of the SC pool in response to a single bout of exercise after 16 wk of RT. We speculate that increased capillarization as a result of 16 wk of exercise training may be an important factor for enhancing SC activation in the postexercise period.
Activation, proliferation, and/or differentiation of SC are important events in the postexercise recovery period to support muscle fiber adaptation. Accordingly, SC number is increased substantially in the days following a single bout of resistance exercise (36). More importantly, a greater proportion of SC is in the active state following exercise, as defined by the colocalization of MyoD with Pax7 (23, 37). In the present study, before exercise training, there was an ~35% increase in active SC (MyoD+/Pax7+) 24 h after a single bout of resistance exercise. However, there was a significantly greater increase in active SC (~55%) at the same time point after 16 wk of RT. Consistent with this observation, we observed an increase in MyoD gene expression (~1.4-fold from Pre) 24 h postexercise after RT compared with no change in the untrained status response. These findings suggest an enhanced SC activation following 16 wk of RT. We suggest that this is an adaptive response to chronic exercise training that allows for an augmented postexercise response to acute exercise. To better understand the nature of this observation to an acute bout of exercise after training, we examined whether enhanced SC activation following RT in young men was accompanied by changes in muscle fiber capillarization.
Skeletal muscle fiber perfusion is essential for the delivery of oxygen, growth factors, and macronutrients to skeletal muscle fibers. Inadequate muscle fiber perfusion has been suggested to play a role in “anabolic resistance” and impaired nutritive flow in various populations (13, 32, 40). To meet increased metabolic demand and to support continuous muscle hypertrophy during resistance exercise, an increase in muscle capillarization may be required. Consistent with this notion, muscle fiber capillarization has been reported to increase significantly in response to RT in healthy young men (12, 14, 19). In agreement, we report a ~13% increase in C/Fi in type I and a ~26% increase in type II muscle fibers. Furthermore, we observed an increase in type I (~10%) and type II (~17%) CFPE index. As CFPE is regarded as a proxy measure of microvascular perfusion (16), an increase in CFPE suggests improved delivery of circulating nutrients and/or growth factors. Therefore, increases in muscle fiber vascularization and/or the reorganization of the microvascular bed after RT may result in enhanced supply of circulating growth factors during the postexercise period that could influence the SC response.
There are many growth factors that may play a role in regulating SC function (e.g., IL-6, IGF-1, myostatin, HGF) (17). Therefore, an increase in muscle fiber perfusion may result in enhanced exposure of SC to regulatory growth factors in circulation (4, 5). We and others have reported an anatomical relationship between muscle SC and capillaries (5) and have also noted that activated SC are closer to capillaries than quiescent SC (5, 25) suggesting that proximity of a SC to a capillary could be an important factor for SC function. Accordingly, it has been hypothesized that SC content (5, 10) and/or activation status (4, 5, 25) may be related to muscle fiber capillarization. In the present study, activated SC cells were located in closer proximity to capillaries compared with quiescent SC at baseline (Pre; before the single bout of resistance exercise) in both the UTSR and the TSR condition. We were unable to observe any direct or significant correlation between the increase in muscle capillarization and the altered acute SC response in the TSR. However, we observed that the temporal-spatial relationship between both quiescent and active SC and the nearest capillary had been changed in response to a single bout of exercise at 24 h after 16 wk RT. These small changes may be indicative of an adaptive response of the spatial relationship between SC and capillaries after chronic training. Whether the small changes in the relationship between active and/or quiescent SC and the distance to the nearest capillary can explain the enhanced activation of SC in response to a single bout of exercise following 16 wk of RT remains unknown and requires further study. Furthermore, SC activation status was not determined in a fiber type-specific manner, and future studies should address this issue.
Although we observed an increase in capillarization following RT that accompanied an altered SC response to resistance exercise, there remains an incomplete understanding of how the SC response to a stimulus is initiated. Indeed, there is evidence to suggest that numerous cytokines and growth factors produced by skeletal muscle and/or the microvasculature may stimulate SC in an autocrine/paracrine fashion rather than through circulation. IL-6, previously reported to have a role in SC regulation (34, 41), is produced locally by contracting muscles (39). Interestingly, cell types such as endothelial cells within the muscle have also produce IL-6 under certain conditions (35, 45), as well as IGF-1 and HGF (5). Given the established spatial relationship between capillaries and SC, it would stand to reason that cellular cross-talk between endothelial cells and SC may influence angiogenesis (5, 33). Indeed, Chazaud et al. (4) reported that human muscle progenitor cells undergoing differentiation produce VEGF, a key factor for angiogenesis (4). Taken together, these findings indicate that the relationship between microvascular capillaries and SC may be predicated not only on the exposure to systemic factors, but also the immediate paracrine cross-talk between endothelial cells and SC. Future studies should address whether cytokines released from skeletal muscle or the microvasculature stimulate the SC response through autocrine/paracrine pathways, or exposure to endocrine-derived signals delivered through the microvasculature, or some combination of both.
Given the increased muscle perfusion after 16 wk of RT, we speculate that SC may have received enhanced input from circulating growth factors and more rapidly initiated the myogenic program and migratory function of SC leading to a loss in the observed anatomical relationship between SC and capillaries in the rested state and early activated state after exercise. Although we do not find a significant correlation between the altered (post-RT) response and the increase in capillarization, recent work might lead us to speculate that capillarization may play a role in resistance training adaptation. Indeed, Snijders et al. (38) recently observed that capillarization was linked to changes in muscle cross-sectional area following resistance training in older men. The study observed that individuals who started with a higher muscle fiber capillarization at baseline had a greater muscle hypertrophy following resistance training in older men. Taken together, the changes in SC activation that accompany the increases in muscle capillarization following long-term RT warrant further study into the relationship between capillaries and the SC pool. In compromised populations, such as older adults, who can have a relatively reduced muscle capillarization (8, 31) and reduced muscle mass (43), an impaired SC activation in response to exercise has been observed (23, 37). Furthermore, it would be interesting to investigate whether increasing muscle fiber capillarization would result in an augmented SC response during the postexercise period in older adults. In conclusion, we observed that an altered activation of the SC pool in response to a single bout of resistance exercise is accompanied by increased capillarization following 16 wk RT.
GRANTS
G. Parise was supported by Natural Sciences and Engineering Research Council of Canada (NSERC) Grant (1455843), and J. P. Nederveen by a NSERC Canadian Graduate Scholarship (CGS-D).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
J.P.N., T.S., S.J., C.G.W., C.J.M., L.M.J., S.K.B., and S.M.P. performed experiments; J.P.N., T.S., S.J., and C.G.W. analyzed data; J.P.N., T.S., S.J., and G.P. interpreted results of experiments; J.P.N. prepared figures; J.P.N. drafted manuscript; J.P.N., T.S., S.J., C.G.W., C.J.M., L.M.J., S.K.B., S.M.P., and G.P. edited and revised manuscript; J.P.N., T.S., S.J., C.G.W., C.J.M., L.M.J., S.K.B., S.M.P., and G.P. approved final version of manuscript.
ACKNOWLEDGMENTS
The Pax7 hybridoma cells developed by Dr. A. Kawakami and the A4.951 developed by Dr. H. Blau were obtained from the Developmental Studies Hybridoma Bank, created by the National Institute of Child Health and Human Development and maintained at The Univ. of Iowa, Dept. of Biology, Iowa City, IA.
REFERENCES
- 1.Bellamy LM, Joanisse S, Grubb A, Mitchell CJ, McKay BR, Phillips SM, Baker S, Parise G. The acute satellite cell response and skeletal muscle hypertrophy following resistance training. PLoS One 9: e109739, 2014. doi: 10.1371/journal.pone.0109739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bergstrom J. Percutaneous needle biopsy of skeletal muscle in physiological and clinical research. Scand J Clin Lab Invest 35: 609–616, 1975. doi: 10.3109/00365517509095787. [DOI] [PubMed] [Google Scholar]
- 3.Brack AS, Rando TA. Intrinsic changes and extrinsic influences of myogenic stem cell function during aging. Stem Cell Rev 3: 226–237, 2007. doi: 10.1007/s12015-007-9000-2. [DOI] [PubMed] [Google Scholar]
- 4.Chazaud B, Sonnet C, Lafuste P, Bassez G, Rimaniol AC, Poron F, Authier FJ, Dreyfus PA, Gherardi RK. Satellite cells attract monocytes and use macrophages as a support to escape apoptosis and enhance muscle growth. J Cell Biol 163: 1133–1143, 2003. doi: 10.1083/jcb.200212046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Christov C, Chrétien F, Abou-Khalil R, Bassez G, Vallet G, Authier FJ, Bassaglia Y, Shinin V, Tajbakhsh S, Chazaud B, Gherardi RK. Muscle satellite cells and endothelial cells: close neighbors and privileged partners. Mol Biol Cell 18: 1397–1409, 2007. doi: 10.1091/mbc.E06-08-0693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clark MG. Impaired microvascular perfusion: a consequence of vascular dysfunction and a potential cause of insulin resistance in muscle. Am J Physiol Endocrinol Metab 295: E732–E750, 2008. doi: 10.1152/ajpendo.90477.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clark MG, Wallis MG, Barrett EJ, Vincent MA, Richards SM, Clerk LH, Rattigan S. Blood flow and muscle metabolism: a focus on insulin action. Am J Physiol Endocrinol Metab 284: E241–E258, 2003. doi: 10.1152/ajpendo.00408.2002. [DOI] [PubMed] [Google Scholar]
- 8.Coggan AR, Spina RJ, King DS, Rogers MA, Brown M, Nemeth PM, Holloszy JO. Skeletal muscle adaptations to endurance training in 60- to 70-yr-old men and women. J Appl Physiol (1985) 72: 1780–1786, 1992. [DOI] [PubMed] [Google Scholar]
- 9.Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature 433: 760–764, 2005. doi: 10.1038/nature03260. [DOI] [PubMed] [Google Scholar]
- 10.Emslie-Smith AM, Engel AG. Microvascular changes in early and advanced dermatomyositis: a quantitative study. Ann Neurol 27: 343–356, 1990. doi: 10.1002/ana.410270402. [DOI] [PubMed] [Google Scholar]
- 11.Gavin TP, Drew JL, Kubik CJ, Pofahl WE, Hickner RC. Acute resistance exercise increases skeletal muscle angiogenic growth factor expression. Acta Physiol (Oxf) 191: 139–146, 2007. doi: 10.1111/j.1748-1716.2007.01723.x. [DOI] [PubMed] [Google Scholar]
- 12.Green H, Goreham C, Ouyang J, Ball-Burnett M, Ranney D. Regulation of fiber size, oxidative potential, and capillarization in human muscle by resistance exercise. Am J Physiol Regul Integr Comp Physiol 276: R591–R596, 1999. [DOI] [PubMed] [Google Scholar]
- 13.Groen BB, Hamer HM, Snijders T, van Kranenburg J, Frijns D, Vink H, van Loon LJ. Skeletal muscle capillary density and microvascular function are compromised with aging and type 2 diabetes. J Appl Physiol (1985) 116: 998–1005, 2014. doi: 10.1152/japplphysiol.00919.2013. [DOI] [PubMed] [Google Scholar]
- 14.Hather BM, Tesch PA, Buchanan P, Dudley GA. Influence of eccentric actions on skeletal muscle adaptations to resistance training. Acta Physiol Scand 143: 177–185, 1991. doi: 10.1111/j.1748-1716.1991.tb09219.x. [DOI] [PubMed] [Google Scholar]
- 15.Hepple RT, Mackinnon SL, Goodman JM, Thomas SG, Plyley MJ. Resistance and aerobic training in older men: effects on V̇o2peak and the capillary supply to skeletal muscle. J Appl Physiol (1985) 82: 1305–1310, 1997. [DOI] [PubMed] [Google Scholar]
- 16.Hepple RT, Mathieu-Costello O. Estimating the size of the capillary-to-fiber interface in skeletal muscle: a comparison of methods. J Appl Physiol (1985) 91: 2150–2156, 2001. [DOI] [PubMed] [Google Scholar]
- 17.Kadi F, Charifi N, Denis C, Lexell J, Andersen JL, Schjerling P, Olsen S, Kjaer M. The behaviour of satellite cells in response to exercise: what have we learned from human studies? Pflugers Arch 451: 319–327, 2005. doi: 10.1007/s00424-005-1406-6. [DOI] [PubMed] [Google Scholar]
- 18.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔC(T) Method. Methods 25: 402–408, 2001. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 19.McCall GE, Byrnes WC, Dickinson A, Pattany PM, Fleck SJ. Muscle fiber hypertrophy, hyperplasia, and capillary density in college men after resistance training. J Appl Physiol (1985) 81: 2004–2012, 1996. [DOI] [PubMed] [Google Scholar]
- 20.McHugh MP. Recent advances in the understanding of the repeated bout effect: the protective effect against muscle damage from a single bout of eccentric exercise. Scand J Med Sci Sports 13: 88–97, 2003. doi: 10.1034/j.1600-0838.2003.02477.x. [DOI] [PubMed] [Google Scholar]
- 21.McKay BR, De Lisio M, Johnston AP, O’Reilly CE, Phillips SM, Tarnopolsky MA, Parise G. Association of interleukin-6 signalling with the muscle stem cell response following muscle-lengthening contractions in humans. PLoS One 4: e6027, 2009. doi: 10.1371/journal.pone.0006027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McKay BR, O’Reilly CE, Phillips SM, Tarnopolsky MA, Parise G. Co-expression of IGF-1 family members with myogenic regulatory factors following acute damaging muscle-lengthening contractions in humans. J Physiol 586: 5549–5560, 2008. doi: 10.1113/jphysiol.2008.160176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McKay BR, Ogborn DI, Bellamy LM, Tarnopolsky MA, Parise G. Myostatin is associated with age-related human muscle stem cell dysfunction. FASEB J 26: 2509–2521, 2012. doi: 10.1096/fj.11-198663. [DOI] [PubMed] [Google Scholar]
- 24.Mitchell CJ, Churchward-Venne TA, Bellamy L, Parise G, Baker SK, Phillips SM. Muscular and systemic correlates of resistance training-induced muscle hypertrophy. PLoS One 8: e78636, 2013. doi: 10.1371/journal.pone.0078636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nederveen JP, Joanisse S, Snijders T, Ivankovic V, Baker SK, Phillips SM, Parise G. Skeletal muscle satellite cells are located at a closer proximity to capillaries in healthy young compared with older men. J Cachexia Sarcopenia Muscle 7: 547–554, 2016. doi: 10.1002/jcsm.12105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O’Reilly C, McKay B, Phillips S, Tarnopolsky M, Parise G. Hepatocyte growth factor (HGF) and the satellite cell response following muscle lengthening contractions in humans. Muscle Nerve 38: 1434–1442, 2008. doi: 10.1002/mus.21146. [DOI] [PubMed] [Google Scholar]
- 27.Pedersen BK, Febbraio MA. Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiol Rev 88: 1379–1406, 2008. doi: 10.1152/physrev.90100.2007. [DOI] [PubMed] [Google Scholar]
- 28.Pizza FX, Baylies H, Mitchell JB. Adaptation to eccentric exercise: neutrophils and E-selectin during early recovery. Can J Appl Physiol 26: 245–253, 2001. doi: 10.1139/h01-015. [DOI] [PubMed] [Google Scholar]
- 29.Pizza FX, Davis BH, Henrickson SD, Mitchell JB, Pace JF, Bigelow N, DiLauro P, Naglieri T. Adaptation to eccentric exercise: effect on CD64 and CD11b/CD18 expression. J Appl Physiol (1985) 80: 47–55, 1996. [DOI] [PubMed] [Google Scholar]
- 30.Porter MM, Koolage CW, Lexell J. Biopsy sampling requirements for the estimation of muscle capillarization. Muscle Nerve 26: 546–548, 2002. doi: 10.1002/mus.10221. [DOI] [PubMed] [Google Scholar]
- 31.Proctor DN, Sinning WE, Walro JM, Sieck GC, Lemon PW. Oxidative capacity of human muscle fiber types: effects of age and training status. J Appl Physiol (1985) 78: 2033–2038, 1995. [DOI] [PubMed] [Google Scholar]
- 32.Rasmussen BB, Fujita S, Wolfe RR, Mittendorfer B, Roy M, Rowe VL, Volpi E. Insulin resistance of muscle protein metabolism in aging. FASEB J 20: 768–769, 2006. doi: 10.1096/fj.05-4607fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rhoads RP, Johnson RM, Rathbone CR, Liu X, Temm-Grove C, Sheehan SM, Hoying JB, Allen RE. Satellite cell-mediated angiogenesis in vitro coincides with a functional hypoxia-inducible factor pathway. Am J Physiol Cell Physiol 296: C1321–C1328, 2009. doi: 10.1152/ajpcell.00391.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Serrano AL, Baeza-Raja B, Perdiguero E, Jardí M, Muñoz-Cánoves P. Interleukin-6 is an essential regulator of satellite cell-mediated skeletal muscle hypertrophy. Cell Metab 7: 33–44, 2008. doi: 10.1016/j.cmet.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 35.Sironi M, Breviario F, Proserpio P, Biondi A, Vecchi A, Van Damme J, Dejana E, Mantovani A. IL-1 stimulates IL-6 production in endothelial cells. J Immunol 142: 549–553, 1989. [PubMed] [Google Scholar]
- 36.Snijders T, Nederveen JP, McKay BR, Joanisse S, Verdijk LB, van Loon LJ, Parise G. Satellite cells in human skeletal muscle plasticity. Front Physiol 6: 283, 2015. doi: 10.3389/fphys.2015.00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Snijders T, Verdijk LB, Smeets JS, McKay BR, Senden JM, Hartgens F, Parise G, Greenhaff P, van Loon LJ. The skeletal muscle satellite cell response to a single bout of resistance-type exercise is delayed with aging in men. Age (Dordr) 36: 9699, 2014. doi: 10.1007/s11357-014-9699-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Snijders T, Nederveen JP, Joanisse S, Leenders M, Verdijk LB, van Loon LJ, Parise G. Muscle fibre capillarization is a critical factor in muscle fibre hypertrophy during resistance exercise training in older men. J Cachexia Sarcopenia Muscle 2016. August 4. [Epub]. doi: 10.1002/jcsm.12137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Steensberg A, van Hall G, Osada T, Sacchetti M, Saltin B, Klarlund Pedersen B. Production of interleukin-6 in contracting human skeletal muscles can account for the exercise-induced increase in plasma interleukin-6. J Physiol 529: 237–242, 2000. doi: 10.1111/j.1469-7793.2000.00237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Timmerman KL, Lee JL, Dreyer HC, Dhanani S, Glynn EL, Fry CS, Drummond MJ, Sheffield-Moore M, Rasmussen BB, Volpi E. Insulin stimulates human skeletal muscle protein synthesis via an indirect mechanism involving endothelial-dependent vasodilation and mammalian target of rapamycin complex 1 signaling. J Clin Endocrinol Metab 95: 3848–3857, 2010. doi: 10.1210/jc.2009-2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Toth KG, McKay BR, De Lisio M, Little JP, Tarnopolsky MA, Parise G. IL-6 induced STAT3 signalling is associated with the proliferation of human muscle satellite cells following acute muscle damage. PLoS One 6: e17392, 2011. doi: 10.1371/journal.pone.0017392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Velloso CP. Regulation of muscle mass by growth hormone and IGF-I. Br J Pharmacol 154: 557–568, 2008. doi: 10.1038/bjp.2008.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Verdijk LB, Koopman R, Schaart G, Meijer K, Savelberg HH, van Loon LJ. Satellite cell content is specifically reduced in type II skeletal muscle fibers in the elderly. Am J Physiol Endocrinol Metab 292: E151–E157, 2007. doi: 10.1152/ajpendo.00278.2006. [DOI] [PubMed] [Google Scholar]
- 44.Vincent HK, Vincent KR. The effect of training status on the serum creatine kinase response, soreness and muscle function following resistance exercise. Int J Sports Med 18: 431–437, 1997. doi: 10.1055/s-2007-972660. [DOI] [PubMed] [Google Scholar]
- 45.Yan SF, Tritto I, Pinsky D, Liao H, Huang J, Fuller G, Brett J, May L, Stern D. Induction of interleukin 6 (IL-6) by hypoxia in vascular cells. Central role of the binding site for nuclear factor-IL-6. J Biol Chem 270: 11463–11471, 1995. doi: 10.1074/jbc.270.19.11463. [DOI] [PubMed] [Google Scholar]