Abstract
Hypertensive pregnancy (HTNP) is a risk factor for future cardiovascular disease. Exaggerated cardiovascular responses to physical stress are also considered an independent marker of cardiovascular disease risk. However, there are limited data regarding the blood pressure (BP) responses to acute stress in women, who have a history of HTNP. Hence, the aim of the study is to compare BP responses to a physical stress in postmenopausal women with a history of HTNP to age- and parity-matched women with a history of normotensive pregnancy (NP). Beat-to-beat BP and heart rate was recorded in 64 postmenopausal women with [age = 58.5 (55.2, 62.2) yr, where values are the median, 25th percentile, and 75th percentile] and without [age = 59.4 (55.9, 62.4) yr] a history of HTNP before and during isometric handgrip (IHG) exercise (30% of maximal voluntary contraction) to fatigue. Muscle metaboreflex was measured during postexercise ischemia following IHG exercise. BP variables increased similarly in response to IHG exercise [systolic: NP = 11.5 (8.9, 17.6) %, HTNP = 11.3 (9.5, 15.9) %; diastolic NP = 11.2 (7.9, 13.3) %, HTNP = 9.5 (7.1, 14.3) %; mean blood pressure: NP = 9.8 (5.0, 13.6) %, and HTNP = 7.2 (4.4, 10.4) %] and postexercise ischemia [systolic: NP = 14.1 (10.3, 23.0) %, HTNP = 15.8 (10.6, 21.4) %; diastolic NP = 12.2 (4.8, 17.0) %, HTNP = 10.4 (5.3, 17.1) %; and mean blood pressure: NP = 11.1 (6.1, 17.9) %, HTNP = 9.4 (2.9, 14.8) %] in both groups. Although having a history of HTNP is associated with future cardiovascular disease risk, results from this study suggest that the risk may not be manifested through altered cardiovascular metaboreflex response to physical stressors.
Keywords: pressor response, preeclampsia, isometric handgrip exercise
a history of hypertensive pregnancy (HTNP), which includes preeclampsia, gestational hypertension, and chronic hypertension, is a risk factor for cardiovascular disease, including development of hypertension (3, 4, 6, 10, 14). However, the mechanisms leading to later-life development of cardiovascular disease in women with a history of HTNP are unknown.
Dysregulation of sympathetic control of blood pressure (BP) is one possible mechanism contributing to cardiovascular disease and the development of hypertension in men and women (22). An exaggerated BP response to laboratory physical stress is considered an independent marker of cardiovascular risk (16, 17, 21, 28). During acute isometric handgrip (IHG) exercise and postexercise ischemia, there is occlusion of the microvasculature, accompanied by an increase in metabolites that triggers chemosensitive afferents, causing a pressor response from the reflex sympathetic excitation (1, 15). The activation of the chemosensitive afferents in skeletal muscle during IHG and especially with postexercise ischemia raises BP, in large part, by increasing muscle sympathetic nerve activity (MSNA) (15). Thus, sympathetic hyperresponsiveness of the metaboreflex can indicate a potential dysregulation in the sympathetic control of BP.
In addition to sympathetic hyperresponsiveness in women with current HTNP (22), the sympathetic hyper-responsiveness that occurs between 28 and 32 wk of gestation can predict pregnancy-induced hypertension (22). In some women with a history of HTNP, hypertension reoccurs later in life (23, 27). However, it is currently unknown whether sympathetic hyper-responsiveness persists beyond pregnancy and through the menopausal transition in women, who have had a history of HTNP. Hence, the primary aim of this study was to compare the BP responses in women with a history of HTNP 30 yr postdiagnosis to age- and parity-matched women with a history of normotensive pregnancy (NP). We hypothesized that women with a history of HTNP 30 yr postpartum would have exaggerated BP responses to IHG exercise and postexercise ischemic occlusion compared with age- and parity-matched women with a history of NP.
METHODS
Eighty postmenopausal women with and without confirmed histories of preeclampsia (HTNP) and NP between 1976 and 1982 were recruited from the Rochester Epidemiology Project (18, 24–26) to investigate the association between preeclamptic pregnancy and subclinical cardiovascular disease. Women with a confirmed diagnosis of preeclampsia (HTNP) and women without a diagnosis of preeclampsia (NP) were age- and parity-matched. All births were singletons. Sixty-four women met the inclusion criteria for this study and agreed to participate. All participants were free of acute and chronic cardiovascular disease (with the exception of controlled hypertension) and respiratory disease, they had a body mass index (BMI) of <30 kg/m2, and were nonsmokers. All participants were asked to refrain from exercise, alcohol, and caffeine for at least 24 h and were asked to fast for 4 h before the start of the study. All study procedures were approved by the Institutional Review Board of the Mayo Clinic and were performed according to the Declaration of Helsinki. All participants gave written informed consent.
Experimental procedures.
The study was performed during one experimental session in the Clinical Research Unit of the Mayo Clinic with a controlled ambient temperature between 22°C and 24°C. After obtaining the demographic and clinical measurements, the participants were asked to rest quietly in the supine position for 10 min. After the 10 min of rest, three measurements of right arm cuff BP were recorded.
In all participants, beat-to-beat BP was continuously recorded using a noninvasive finger plethysmography (Nexfin, Edwards Lifesciences, Irvine, CA) on the right hand. Heart rate was continuously recorded via a continuous 3-lead electrocardiogram (Cardiocap/5; Datex-Ohmeda, Louisville, CO).
While in the supine position, participants performed maximal voluntary contraction (MVC) on a handgrip dynamometer three times with their left arm, separated by a minute. An average of the three measurements was recorded. Baseline measurements of BP and HR were recorded during quiet rest for a period of 2 min in a supine position. Participants performed an IHG exercise at 30% MVC. Verbal feedback was given to maintain the 30% intensity until fatigue. As the participants reached fatigue during the IHG exercise, a BP cuff, placed on the upper left arm of the participant, was inflated to supra-systolic pressure (220 mmHg). All of the participants were asked to perform the IHG exercise until cuff inflation. Ischemia was maintained for 90 s followed by 2 min of quiet rest.
Data analysis.
Data were collected at 250 Hz, stored on a computer (WinDaq; DATAq Instruments, Akron, OH), and were analyzed off-line with signal processing software (PowerLab; ADInstruments, Sydney, Australia). Heart rate and systolic, diastolic, and mean arterial pressure (MAP) were continuously monitored throughout the protocol from the electrocardiogram and the BP waveform, respectively.
As the time to fatigue during the IHG exercise varied among participants, the BP and heart rate recordings were divided into three parts based on the total time to exhaustion (1st third, 2nd third, and final third). The BP response was calculated for each part as the mean pressure over each one-third interval minus baseline BP. An example of individual BP response to IHG exercise and postexercise ischemia is presented in Fig. 1.
Fig. 1.
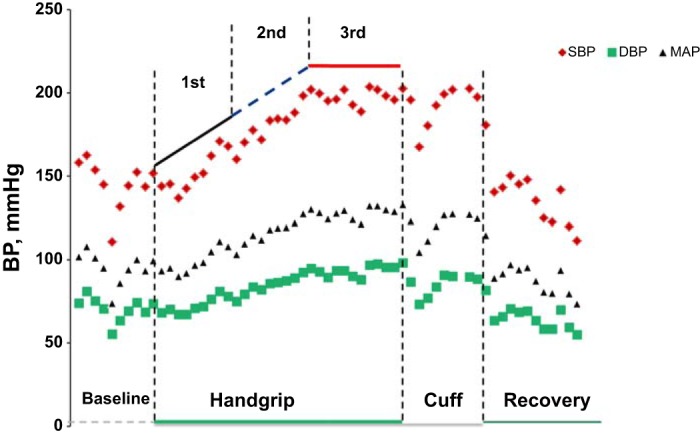
An example of individual blood pressure response to isometric handgrip (IHG) exercise and postexercise ischemia. Individual recording from a representative participant showing blood pressure (BP) response of an individual participant during baseline, IHG exercise, postexercise ischemia, and recovery. DBP, diastolic blood pressure; MAP, mean arterial pressure; SBP, systolic blood pressure.
Statistical analyses.
Serially collected data for each of five BP parameters included a single measure at baseline, three measures in response to respective forms of IHG exercise, and postexercise ischemia with the selection of the three responses, corresponding to equally spaced intervals over the participant’s total testing time. Given the multiplicity of data and the problems that arise from multiple comparisons, the repeated measurements were reduced to single measures of trend for each individual, which were then used to compare groups with a history of NP versus HTNP. Summary statistics to quantify the trend included the mean BP response (i.e., the average of each subject’s response measurement subtracted by their baseline value), the peak BP response (maximum response value, normalized for the baseline measurement), and time-response slopes based on regressions performed separately on each subject. For this, a simple linear regression model was fitted to the four total measurements made on each subject from which the least squares slope was obtained and used to summarize the response pattern. Given the consistency in results across these different types of summary measures, we reported only those pertaining to the mean BP response over the duration of IHG exercise to fatigue and postexercise ischemia, which had been computed both as a simple difference and as a percent change (rather than a simple difference) relative to the baseline measurement. The final summary measures of BP response were described with quartiles (medians, 25th, and 75th percentiles) and were assessed for group differences using the Wilcoxon rank sum test. Data analyses were carried out with the statistical software package SAS, version 9.4 (SAS Institute, Cary, NC).
RESULTS
Sixty-four women (33 with a history of NP and 31 with a history of HTNP) completed the visit for IHG exercise and postexercise cuff occlusion. The participant characteristics and baseline measurements of these women are presented in Table 1. In comparison to age- and parity-matched women with a history of NP, women with a history of HTNP had significantly higher body mass index at the time of study and were more likely to be diagnosed and treated for hypertension. There were no significant differences in any of the baseline hemodynamic variables between women with and without a history of HTNP.
Table 1.
Baseline clinical characteristics
| Variable | History of NP | History of HTNP | |
|---|---|---|---|
| (n = 33) | (n = 31) | P Value | |
| Age at study consent, yr* | 59.4 (55.9, 62.4) | 58.5 (55.2, 62.2) | 0.757 |
| Body mass index, kg/m2* | 25.0 (23.2, 29.5) | 27.8 (25.0, 32.6) | 0.040 |
| Hypertension, n | 7 (21%) | 16 (52%) | 0.011 |
| Antihypertensive medications, n | 4 (12%) | 16 (52%) | <0.001 |
| Total cholesterol, mg/dl* | 204.0 (186.0, 222.0) | 194.0 (174.0, 216.0) | 0.251 |
| LDL cholesterol, mg/dl* | 119.2 (100.6, 133.6) | 109.0 (88.2, 126.8) | 0.282 |
| HDL cholesterol, mg/dl* | 65.0 (52.0, 77.0) | 55.0 (45.0, 71.0) | 0.081 |
| Triglycerides, mg/dl* | 94.0 (67.0, 109.0) | 104.0 (81.0, 163.0) | 0.083 |
| Fasting glucose, mg/dl* | 95.0 (91.0, 98.0) | 97.0 (91.0, 108.0) | 0.172 |
| Baseline measurements | |||
| Systolic blood pressure, mmHg* | 138.2 (127.1, 148.6) | 142.6 (129.6, 151.2) | 0.498 |
| Diastolic blood pressure, mmHg* | 76.3 (72.2, 83.5) | 79.1 (74.0, 83.9) | 0.568 |
| Mean arterial pressure, mmHg* | 101.1 (92.6, 108.7) | 103.8 (94.3, 110.3) | 0.337 |
| Heart rate, beats/min* | 63.4 (58.5, 69.3) | 62.0 (55.8, 69.3) | 0.537 |
bpm, beats per minute; HTNP, hypertensive pregnancy; NP, normotensive pregnancy.
Values are the median (25th, 75th percentiles) using Wilcoxon rank sum test.
There were no significant differences between women with a history of NP and history of HTNP in the average response (change between each participant’s baseline measurement and the average of their three IHG exercise measurements) in systolic, diastolic, and mean BP during the IHG exercise (Table 2). There were also no significant differences between groups when the systolic, diastolic, and mean BP responses during IHG exercise were expressed as percent change (Table 2). Additionally, there was no significant difference between groups when the systolic, diastolic, and mean BP responses during IHG exercise were analyzed after dividing them into three time frames of the duration of hand grip to exhaustion (data not shown). The absolute systolic, diastolic, mean BP, and heart rate during IHG and postexercise ischemia are presented in Fig. 2.
Table 2.
Magnitude of change in hemodynamic variables during handgrip exercise
| History of NP | History of HTNP | ||
|---|---|---|---|
| (n = 33) | (n = 31) | P Value | |
| Absolute difference | |||
| Systolic blood pressure, mmHg | 15.9 (12.5, 24.5) | 16.0 (13.2, 22.7) | 0.934 |
| Diastolic blood pressure, mmHg | 8.3 (6.2, 11.1) | 7.7 (5.8, 10.9) | 0.650 |
| Mean arterial pressure, mmHg | 9.4 (5.2, 12.7) | 7.9 (4.7, 10.6) | 0.441 |
| Heart rate, beats per min | 8.9 (6.8, 11.4) | 7.2 (4.3, 13.1) | 0.259 |
| Percent change | |||
| Systolic blood pressure, mmHg | 11.5 (8.9, 17.6) | 11.3 (9.5, 15.9) | 1.000 |
| Diastolic blood pressure, mmHg | 11.2 (7.9, 13.3) | 9.5 (7.1, 14.3) | 0.536 |
| Mean arterial pressure, mmHg | 9.8 (5.0, 13.6) | 7.2 (4.4, 10.4) | 0.364 |
| Heart rate, beats/min | 13.8 (9.4, 19.2) | 10.4 (7.4, 20.1) | 0.349 |
Values are the median (25th and 75th percentiles).
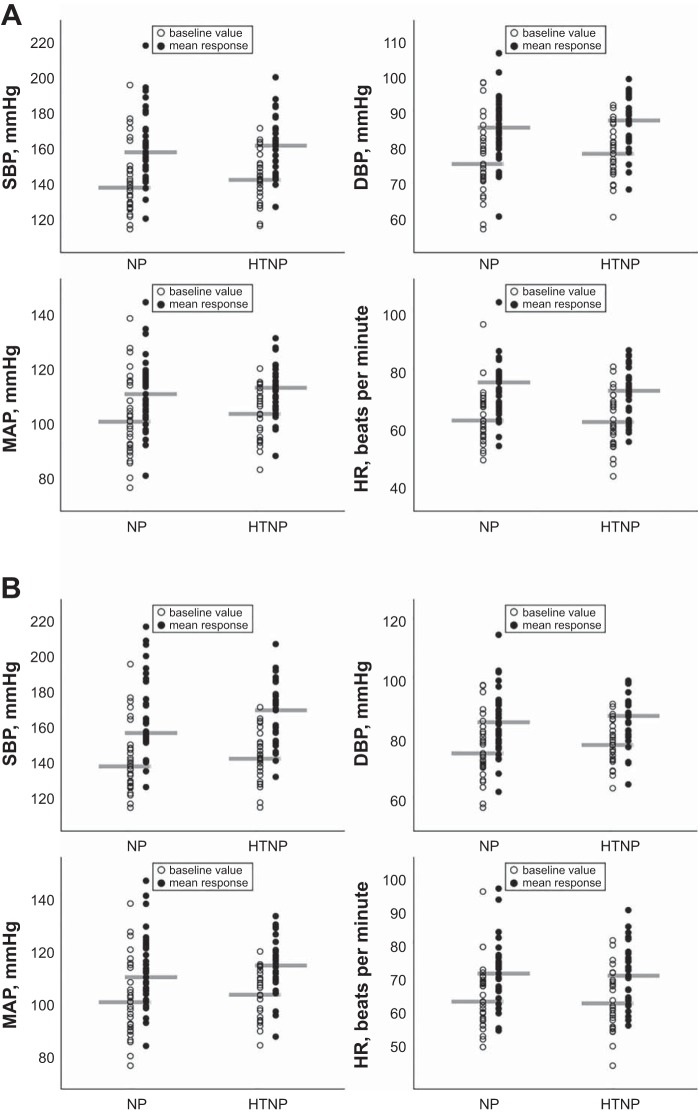
Fig. 2.
Blood pressure and heart rate responses in women with and without a history of hypertensive pregnancy. Individual data are shown in open circles (baseline) and mean response (solid circles) over the entire duration of handgrip exercise (A) and postexercise ischemia (B). Gray bars show the median value. HR, heart rate; HTNP, hypertensive pregnancy; NP, normotensive pregnancy; SBP, systolic blood pressure. Women with a history of HTNP, n = 31. Women without a history of HTNP, n = 33.
There were no significant differences in the average responses in systolic, diastolic, and mean BP (averaged over three measurements taken at 30-s intervals and normalized for baseline measurement) of postexercise cuff occlusion between women with and without a history of HTNP (Table 3). Likewise, there were no significant differences in the systolic, diastolic, and mean BP percent change during postexercise cuff occlusion between the two groups (Table 3). We further analyzed the possibility that the sex of the offspring influenced the blood pressure response. There were no significant differences in the blood pressure response to IHG and/or postexercise ischemia between pregnancies with male or female infants (data not shown).
Table 3.
Magnitude of change in hemodynamic variables during postexercise ischemia
| History of NP | History of HTNP | ||
|---|---|---|---|
| (n = 33) | (n = 31) | P Value | |
| Absolute difference | |||
| Systolic blood pressure, mmHg | 21.3 (14.1, 30.2) | 23.5 (15.0, 28.7) | 0.880 |
| Diastolic blood pressure, mmHg | 9.0 (4.4, 12.6) | 8.2 (4.4, 12.7) | 0.869 |
| Mean arterial pressure, mmHg | 10.7 (6.0, 16.2) | 10.3 (2.7, 15.6) | 0.573 |
| Heart rate, beats/min | 6.5 (3.0, 8.5) | 6.0 (3.1, 9.3) | 0.956 |
| Percent change | |||
| Systolic blood pressure, mmHg | 14.1 (10.3, 23.0) | 15.8 (10.6, 21.4) | 0.847 |
| Diastolic blood pressure, mmHg | 12.2 (4.8, 17.0) | 10.4 (5.3, 17.1) | 0.804 |
| Mean arterial pressure, mmHg | 11.1 (6.1, 17.9) | 9.4 (2.9, 14.8) | 0.500 |
| Heart rate, beats/min | 10.8 (5.0, 13.3) | 10.0 (4.5, 14.2) | 0.934 |
Values are the median (25th and 75th percentile).
Because the proportion of women with current hypertension was higher in the HTNP group, a subset analysis was used to test for group differences in BP and heart rate responses among women who are currently normotensive. In these 41 women free of hypertension (n = 26 in the NP group and n = 15 in the HTNP group), there were no significant differences in SBP, diastolic blood pressure, and MAP average responses. For instance, the median (interquartile range, IQR) MAP responses for currently normotensive women with and without a history of HTNP was 8.9 (3.0 to 15.4) and 9.7 (5.0 to 14.0) mmHg during IHG exercise (P = 0.725), and 9.7 (2.4 to 16.1) and 10.6 (5.7 to 16.0) mmHg, respectively, during postexercise cuff occlusion (P > 0.999). Although the number of women who had a history of NP within the hypertension stratum was too few to enable group comparisons, currently hypertensive women with a history of HTNP (n = 16) showed similar responses during IHG exercise [e.g., median (IQR) MAP response: 7.5 (4.7 to 10.0) mmHg] and postexercise cuff occlusion [10.3 (5.3 to 13.5) mmHg] compared with currently normotensive women with a history of HTNP.
DISCUSSION
To our knowledge, this is the first study to evaluate the long-term effects of a history of hypertensive pregnancy on sympathetic responsiveness in women. Contrary to our hypothesis, results in the current study suggest that over the period of ~30 yr after the incident event, the sympathetic responsiveness to IHG exercise and postexercise ischemia in women with a history of HTNP, who were without prior cardiovascular events, are similar to those of age- and parity-matched women with a history of NP.
In women with HTNP, previous studies have shown evidence of sympathetic dysregulation within ~5 yr of a HTNP. For example, although plasma levels of norepinephrine at rest are higher in women 5–6 yr after preeclamptic pregnancy as compared with women with uncomplicated pregnancies, 24-h arterial pressure measurements did not differ between groups (12). These data are concordant with the findings of the present study that BP did not differ at baseline between the women depending on their pregnancy history ~30 yr after the index pregnancy. These observations need to be evaluated carefully as 50% of the women with a history of HTNP were diagnosed with hypertension and were taking antihypertensive medications, while only 21% of women with a history of NP were diagnosed with hypertension. However, a subanalysis of the women without current hypertension did not uncover significant differences between groups. Our results with the pressor response to IHG and postexercise ischemia are consistent with the observations made by Mangos et al. (14), who reported no difference in sympathoexcitatory responses to orthostatic stress or cold stress between women with a history of HTNP (2–12 yr postpartum) and controls (women with uncomplicated pregnancy with no history of hypertension, renal disease, preeclampsia, or gestational hypertension).
A novel aspect of the present study is the insight into the sympathetic regulation of postmenopausal women with a history of HTNP to physical stressors. Even though the BP responses during physical stressors were not significantly different between the groups, we observed a higher rate of clinical hypertension in women with history of HTNP as compared with women with NP. Previous studies evaluated BP responses to physical stressors in premenopausal women related to pregnancy history (12, 14). This difference in hormonal status is of particular importance because the autonomic support for BP regulation changes in postmenopausal women (2). Specifically, young women regulate BP differently compared with postmenopausal women based of the difference in the relationship between muscle sympathetic nerve activity and total peripheral resistance (8, 9, 20). In this regard, it is plausible that the menopause by itself could have independently contributed to the clinical hypertension in some women with a history of HTNP. However, the women in the present study were matched for menopausal status, and ~50% of the women with a history of HTNP were diagnosed with hypertension, as opposed to only 21% in the NP group. This suggests that menopause has a minimal effect on the higher prevalence of clinical hypertension diagnosis in the women with a history of HTNP.
Additionally, Schobel et al. (22) reported that the MAP and MSNA were significantly higher in women with preeclampsia at 33 wk of gestation, as compared with normotensive pregnant and nonpregnant women; although MAP and MSNA decreased significantly postpartum in women with preeclampsia. However, the sympathetic nerve activity and hemodynamic responses to a cold-pressor test and Valsalva maneuver did not differ significantly between the groups (22). These results suggest that baseline sympathetic vasoconstrictor activity is partially mediating the increase in mean arterial pressure in preeclampsia (22). However, the study conducted by Schobel et al. (22) included only premenopausal young women, as opposed to the postmenopausal women in the current study. Second, the cold-pressor test increases blood pressure by a different physiological mechanism than the IHG with postexercise ischemia (reviewed in Refs. 13 and 19). So in addition to the hormonal status, the present study differs on the methodological technique to that used in previous literature.
It is possible that in the present study, only the baseline sympathetic vasoconstrictor activity might be higher in women with a history of HTNP, causing a higher incidence of hypertension in this group as compared with women without a history of HTNP. For example, Fischer et al. (5) have reported that women with a history of preeclampsia have a significantly higher muscle sympathetic nerve activity during 22 and 33 wk of gestation in their subsequent pregnancies, as compared with their post-partum levels. The authors speculated that pregnancy-induced sympathetic hyperactivity could manifest into preeclampsia only if the vasodilating mechanisms fail. This loss of vasodilating mechanisms may be a phenomenon similar to that observed in postmenopausal women, in which the β-adrenergic vasodilation fails to offset α-adrenergic mediated vasoconstriction typically seen in young women (8, 11). On the basis of these reports, we speculate that women with a history of HTNP tend to have higher sympathetic activity every time they encounter an endogenous stressor (pregnancy); however, it may not manifest into higher BP responses during exogenous stressors (IHG exercise). On the basis of our data on the frequency of hypertension, we also speculate that when women with a history of HTNP face another endogenous stressor (menopause), they respond differently as compared with women with NP. However, we are unable to see these manifestations in BP response to external stressors. Collectively, these studies give us an insight into the early sympathetic dysregulation in women with a history of HTNP.
A limitation of the current study is that ~50% of the women with a history of HTNP were using antihypertensive medications (Table 4). However, it has been reported that the enhanced BP response during IHG exercise is not suppressed by β-blockade (propranolol) and is reduced only ~30% by α-blockade (prazosin) in individuals with essential hypertension (7). Additionally, we performed a subset analysis to compare women with a history of HTNP who are currently normotensive and women with a history of NP who are currently normotensive, which showed no significant differences in their BP response to physical stressors. Thus, we conclude that the potential impact of antihypertensive medications on our findings is likely small. However, we cannot rule out the possibility that the women who developed hypertension may represent a unique subgroup in whom differences might manifest closer to the incident pregnancy through development of clinically defined hypertension.
Table 4.
Antihypertensive medications in both groups
| AH Medication | β-Blockers | Ca Channel Blockers | Diuretics | ACE Inhibitors | ARB | Vasodilators | ||
|---|---|---|---|---|---|---|---|---|
| HCTZ | K+ Sparing | |||||||
| NP | 4 | 1 | 0 | 3 | 0 | 1 | 1 | 0 |
| HTNP | 21 | 10 | 1 | 9 | 3 | 11 | 2 | 1 |
Values are the number of individuals. ACE, angiotensin-converting enzyme; AH, antihypertensive; ARB, angiotensin receptor blockers; Ca, calcium; HCTZ, hydrochlorothiazide; K+, potassium.
Perspectives and Significance
In conclusion, these data suggest that ~30 yr postpartum, the autonomic responsiveness in women with a history of HTNP is similar to women with a history of NP. Additionally, even though ~50% of the women with a history of HTNP are currently diagnosed with hypertension, their BP responses to physical stressors were similar to those without a current diagnosis of hypertension.
GRANTS
Financial support for this article was provided by the National Institutes of Health Grant HL-118154 (to J. N. Barnes), HL-83947 (to M. J. Joyner), P50 AG-44170 (to V. M. Miller); the American Heart Association Grant 14PRE18040000 (to R. E. Harvey); and a Clinical and Translational Science Award Grant UL1 TR000135 (to M. J. Joyner).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.M.R., R.E.H., and J.N.B. performed experiments; S.M.R., R.E.H., and J.N.B. analyzed data; S.M.R., R.E.H., B.D.L., V.M.M., M.J.J., and J.N.B. interpreted results of experiments; S.M.R. and B.D.L. prepared figures; S.M.R. and B.D.L. drafted manuscript; S.M.R., R.E.H., B.D.L., V.M.M., M.J.J., and J.N.B. edited and revised manuscript; S.M.R., R.E.H., B.D.L., V.M.M., M.J.J., and J.N.B. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the women who participated in this study, as well as K. Russell, M. Johnson, C. Johnson, S. Roberts, S. Wolhart, P. Engrav, and K. Jensen, for their technical assistance throughout the project.
REFERENCES
- 1.Baker PN, Johnson IR. The use of the hand-grip test for predicting pregnancy-induced hypertension. Eur J Obstet Gynecol Reprod Biol 56: 169–172, 1994. doi: 10.1016/0028-2243(94)90165-1. [DOI] [PubMed] [Google Scholar]
- 2.Barnes JN, Hart EC, Curry TB, Nicholson WT, Eisenach JH, Wallin BG, Charkoudian N, Joyner MJ. Aging enhances autonomic support of blood pressure in women. Hypertension 63: 303–308, 2014. doi: 10.1161/HYPERTENSIONAHA.113.02393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bellamy L, Casas JP, Hingorani AD, Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ 335: 974, 2007. doi: 10.1136/bmj.39335.385301.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chesley LC. Remote prognosis after eclampsia. Perspect Nephrol Hypertens 5: 31–40, 1976. [PubMed] [Google Scholar]
- 5.Fischer T, Schobel HP, Frank H, Andreae M, Schneider KT, Heusser K. Pregnancy-induced sympathetic overactivity: a precursor of preeclampsia. Eur J Clin Invest 34: 443–448, 2004. doi: 10.1111/j.1365-2362.2004.01350.x. [DOI] [PubMed] [Google Scholar]
- 6.Fisher KA, Luger A, Spargo BH, Lindheimer MD. Hypertension in pregnancy: clinical-pathological correlations and remote prognosis. Medicine (Baltimore) 60: 267–276, 1981. doi: 10.1097/00005792-198107000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Hamada M, Kazatani Y, Shigematsu Y, Ito T, Kokubu T, Ishise S. Enhanced blood pressure response to isometric handgrip exercise in patients with essential hypertension: effects of propranolol and prazosin. J Hypertens 5: 305–309, 1987. doi: 10.1097/00004872-198706000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Hart EC, Charkoudian N, Wallin BG, Curry TB, Eisenach J, Joyner MJ. Sex and ageing differences in resting arterial pressure regulation: the role of the β-adrenergic receptors. J Physiol 589: 5285–5297, 2011. doi: 10.1113/jphysiol.2011.212753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hart EC, Charkoudian N, Wallin BG, Curry TB, Eisenach JH, Joyner MJ. Sex differences in sympathetic neural-hemodynamic balance: implications for human blood pressure regulation. Hypertension 53: 571–576, 2009. doi: 10.1161/HYPERTENSIONAHA.108.126391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Irgens HU, Reisæter L, Irgens LM, Lie RT. Long term mortality of mothers and fathers after pre-eclampsia: population based cohort study. BMJ 323: 1213–1217, 2001. doi: 10.1136/bmj.323.7323.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kneale BJ, Chowienczyk PJ, Brett SE, Coltart DJ, Ritter JM. Gender differences in sensitivity to adrenergic agonists of forearm resistance vasculature. J Am Coll Cardiol 36: 1233–1238, 2000. doi: 10.1016/S0735-1097(00)00849-4. [DOI] [PubMed] [Google Scholar]
- 12.Lampinen KH, Rönnback M, Groop PH, Nicholls MG, Yandle TG, Kaaja RJ. Increased plasma norepinephrine levels in previously pre-eclamptic women. J Hum Hypertens 28: 269–273, 2014. doi: 10.1038/jhh.2013.84. [DOI] [PubMed] [Google Scholar]
- 13.Lovallo W. The cold pressor test and autonomic function: a review and integration. Psychophysiology 12: 268–282, 1975. doi: 10.1111/j.1469-8986.1975.tb01289.x. [DOI] [PubMed] [Google Scholar]
- 14.Mangos GJ, Spaan JJ, Pirabhahar S, Brown MA. Markers of cardiovascular disease risk after hypertension in pregnancy. J Hypertens 30: 351–358, 2012. doi: 10.1097/HJH.0b013e32834e5ac7. [DOI] [PubMed] [Google Scholar]
- 15.Mark AL, Victor RG, Nerhed C, Wallin BG. Microneurographic studies of the mechanisms of sympathetic nerve responses to static exercise in humans. Circ Res 57: 461–469, 1985. doi: 10.1161/01.RES.57.3.461. [DOI] [PubMed] [Google Scholar]
- 16.Matthews KA, Woodall KL, Allen MT. Cardiovascular reactivity to stress predicts future blood pressure status. Hypertension 22: 479–485, 1993. doi: 10.1161/01.HYP.22.4.479. [DOI] [PubMed] [Google Scholar]
- 17.McAllister RG., Jr Effect of adrenergic receptor blockade on the responses to isometric handgrip: studies in normal and hypertensive subjects. J Cardiovasc Pharmacol 1: 253–263, 1979. doi: 10.1097/00005344-197903000-00008. [DOI] [PubMed] [Google Scholar]
- 18.Melton LJI, III. History of the Rochester Epidemiology Project. Mayo Clin Proc 71: 266–274, 1996. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 19.Mitchell JH, Wildenthal K. Static (isometric) exercise and the heart: physiological and clinical considerations. Annu Rev Med 25: 369–381, 1974. doi: 10.1146/annurev.me.25.020174.002101. [DOI] [PubMed] [Google Scholar]
- 20.Narkiewicz K, Phillips BG, Kato M, Hering D, Bieniaszewski L, Somers VK. Gender-selective interaction between aging, blood pressure, and sympathetic nerve activity. Hypertension 45: 522–525, 2005. doi: 10.1161/01.HYP.0000160318.46725.46. [DOI] [PubMed] [Google Scholar]
- 21.Sannerstedt R, Julius S. Systemic haemodynamics in borderline arterial hypertension: responses to static exercise before and under the influence of propranolol. Cardiovasc Res 6: 398–403, 1972. doi: 10.1093/cvr/6.4.398. [DOI] [PubMed] [Google Scholar]
- 22.Schobel HP, Fischer T, Heuszer K, Geiger H, Schmieder RE. Preeclampsia—a state of sympathetic overactivity. N Engl J Med 335: 1480–1485, 1996. doi: 10.1056/NEJM199611143352002. [DOI] [PubMed] [Google Scholar]
- 23.Smith GC, Pell JP, Walsh D. Pregnancy complications and maternal risk of ischaemic heart disease: a retrospective cohort study of 129,290 births. Lancet 357: 2002–2006, 2001. doi: 10.1016/S0140-6736(00)05112-6. [DOI] [PubMed] [Google Scholar]
- 24.St. Sauver JL, Grossardt BR, Leibson CL, Yawn BP, Melton LJ III, Rocca WA. Generalizability of epidemiological findings and public health decisions: an illustration from the Rochester Epidemiology Project. Mayo Clin Proc 87: 151–160, 2012. doi: 10.1016/j.mayocp.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.St Sauver JL, Grossardt BR, Yawn BP, Melton LJ III, Rocca WA. Use of a medical records linkage system to enumerate a dynamic population over time: the Rochester epidemiology project. Am J Epidemiol 173: 1059–1068, 2011. doi: 10.1093/aje/kwq482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.White WM, Mielke MM, Araoz PA, Lahr BD, Bailey KR, Jayachandran M, Miller VM, Garovic VD. A history of preeclampsia is associated with a risk for coronary artery calcification 3 decades later. Am J Obstet Gynecol 214: 519.e1–519.e8, 2016. doi: 10.1016/j.ajog.2016.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilson BJ, Watson MS, Prescott GJ, Sunderland S, Campbell DM, Hannaford P, Smith WC. Hypertensive diseases of pregnancy and risk of hypertension and stroke in later life: results from cohort study. BMJ 326: 845, 2003. doi: 10.1136/bmj.326.7394.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wood DL, Sheps SG, Elveback LR, Schirger A. Cold pressor test as a predictor of hypertension. Hypertension 6: 301–306, 1984. doi: 10.1161/01.HYP.6.3.301. [DOI] [PubMed] [Google Scholar]