Abstract
Autoantibodies to the ANG II type I receptor (AT1-AA) are associated with preeclampsia (PE). We found that vitamin D supplementation reduced AT1-AA and blood pressure (MAP) in the RUPP rat model of PE. However, it was undetermined whether the decrease in AT1-AA was the mechanism whereby vitamin D lowered MAP or if it were through factors downstream of AT1-AA. Uterine artery resistance index, placental ET-1, and soluble FMS-like tyrosine kinase-1 are increased with AT1-AA-induced hypertension and are considered markers of PE in pregnant women. Therefore, we hypothesized that vitamin D would reduce PE factors during AT1-AA-induced hypertension and could lower blood pressure in a model of hypertension during pregnancy without PE features. Either ANG II (50 ng·kg−1·day) or AT1-AA (1:40) was infused from gestational day (GD) 12–19. vitamin D2 (VD2, 270 IU/day) or vitamin D3 (VD3, 15 IU/day) was administered orally from GD14–GD18. MAP (mmHg) increased in AT1-AA (121 ± 4) and ANG II (113 ± 1)-infused pregnant rats compared with normal pregnant rats (NP) (101 ± 2) but was lower in AT1-AA+VD2 (105 ± 2), AT1-AA+VD3 (109 ± 2), ANG II+VD2 (104 ± 4), and ANG II+VD3 (104 ± 3). VD2 and/or VD3 improved PE features associated with AT1-AA during pregnancy, while ANG II did not induce such features, supporting the hypothesis that AT1-AA induces PE features during pregnancy, and these are improved with vitamin D. In this study, we demonstrate that vitamin D improved many factors associated with PE and reduced blood pressure in a hypertensive model without PE features, indicating that vitamin D could be beneficial for various hypertensive disorders of pregnancy.
Keywords: hypertension, inflammation, pregnancy
several hemodynamic changes occur in pregnancy to accommodate the required increase in blood flow for sustenance of the fetoplacental unit. The renin-angiotensin system (RAS) is activated in normal pregnancies to increase salt retention, which contributes to an increase in blood volume and compensatory increase in diastolic preload and cardiac output (3, 6, 16). Despite these increases in blood volume, normal pregnant women do not have an increase in blood pressure. Compensatory vasorelaxing mechanisms are activated in pregnant women, such as increased nitric oxide bioavailability and a decreased sensitivity to vasoconstrictors, which maintains vascular tension at normal levels (10, 12, 23, 31). This careful balance of proconstrictive and prorelaxation factors is essential for the maternal vasculature to adapt and ensure adequate blood flow to the growing fetoplacental unit.
Preeclampsia (PE) is a hypertensive disorder of pregnancy characterized by vascular dysfunction and abnormal RAS activation (10–12, 19). In contrast to normal pregnant women, PE women exhibit increased vasoconstrictive sensitivity to ANG II, resulting in a decrease in vascular compliance and an increase in blood pressure (10, 12). This loss of protection from ANG II-induced vasoconstriction is associated with systemic increases in reactive oxygen species (ROS), vasoconstrictive endothelin-1 (ET-1), and anti-angiogenic soluble FMS-like tyrosine kinase-1 (sFlt-1), which are markers of endothelial dysfunction and PE severity in these patients (11, 15, 32). Drugs, such as angiotensin II type 1 (AT1) receptor blockers and angiotensin-converting enzyme (ACE) inhibitors are contraindicated in pregnancy; however, a therapeutic agent that reduces ANG II sensitivity in PE patients safely would be expected to alleviate hypertension in these patients.
Autoantibodies to the ANG II type 1 receptor (AT1-AA) are increased in PE patients, compared with normal pregnant patients, and their levels correlate with the severity of PE and have been suggested as a mechanism whereby such hypersensitivity to ANG II develops in PE (7, 21, 30, 38). Infusion of AT1-AA into pregnant rats induces many of the characteristics of PE, such as hypertension, sFlt-1, ET-1, and ROS, which are implicated in the development of hypertension and intrauterine growth restriction (IUGR) in PE patients (5, 33). AT1-AAs stimulate AT1 receptor signaling in a similar manner as ANG II to induce increases in blood pressure (5). However, we are learning that AT1-AA also stimulates the AT1 receptor to induce factors differently from ANG II during pregnancy. In fact, in this current study, we compare hypertension and PE factors stimulated by the AT1-AA with hypertension and PE factors stimulated by ANG II during pregnancy, as a model of hypertension during pregnancy without PE features. We believe that vitamin D can reduce the effect of AT1-AA-induced hypertension during pregnancy and could also be beneficial for pregnant hypertensive women with or without PE. Vitamin D downregulates RAS activation and improves endothelial function in animal models (28, 44, 45), and dysregulation of the RAS is a common cause of hypertension. Therefore, we used ANG II infusion as a model of hypertension during pregnancy without PE features to compare the beneficial effects of vitamin D supplementation when PE features are induced. PE women are at risk for vitamin D deficiency (2, 4, 9, 24, 37, 43). Emerging evidence in the literature supports vitamin D supplement regimens to reduce the risk and severity of PE (4, 18). A previous study by our laboratory demonstrated that vitamin D supplementation in the reduced uterine perfusion pressure (RUPP) rat model of PE reduces production of AT1-AA, sFlt-1, ET-1, and blood pressure (8). Collectively, these data suggest that vitamin D reduces symptoms of PE. However, it is unknown whether decreased production of AT1-AA in the RUPP model was the primary mechanism via which sFlt-1, ET-1, and blood pressure were reduced. Therefore, we sought to determine whether vitamin D reduces factors associated with PE in response to AT1-AA but also hypertension without PE features during pregnancy.
MATERIALS AND METHODS
Procedures involving animals were performed in accordance with the National Institutes of Health “Guidelines for the Care and Use of Laboratory Animals” and were approved by the Institutional Animal Care and Use Committee of the University of Mississippi Medical Center. Animals used for experiment were >250 g timed-pregnant Sprague-Dawley rats (Harlan, Indianapolis, IN) that were housed under a 12:12-h light-dark cycle.
Vitamin D administration to AT1-AA- and ANG II-infused rats.
AT1-AA was purified using a previously described method (22). Briefly, total IgG from serum of RUPP rats was purified, and AT1-AA was extracted from IgG by column purification using an epitope binding site. Pregnant rats were implanted with osmotic minipumps (model 2002; Alzet Scientific, Palo Alto, CA) containing either ANG II (50 ng·kg−1·min−1 in saline) or AT1-AA (1:40 in saline) on gestational days (GD) 12–19, as previously described (7, 22, 36). Groups of ANG II- or AT1-AA-infused rats were treated with either 270 IU of vitamin D2 (VD2) (County Line Pharmaceuticals, Brookfield, WI) or 15 IU of vitamin D3 (VD3) (Enfamil, Glenview, IL) by daily oral gavage on GD14–18, as previously described (8). Numbers of animals per group are as follows: NP: 19, NP+VD2: 5, NP+VD3: 4, ANG II: 15, ANG II+VD2: 9, ANG II+VD3: 7, AT1-AA: 5, AT1-AA+VD2: 7, and AT1-AA+VD3: 6. On GD18, animals were implanted with indwelling carotid catheters that were exteriorized after being tunneled under the skin and through the back of the neck. Blood pressure was measured in a conscious state via pressure transducer with an acclimation time of 30 min and reading time of 30 min (Cobe II Transducer CDX Sema, Birmingham, AL) on GD19 followed by the weighing of pups and placentas and the collection of tissues and blood for analysis.
Assessment of uterine artery resistance index.
On GD18, uterine artery resistance index (UARI) of rats was measured by Doppler sonography. Rats were anesthetized by isoflurane anesthesia and fixed on the platform of a Vevo 770 unit (VisualSonics, Toronto, ON, Canada) with a 30-Hz transducer, model no. 710B. Doppler velocimetry measurements were taken on uterine arteries, and 1 or 2 measurements in a placenta of each uterine horn were imaged (total of 2–4 measurements for both left and right horn combined/animal). The waveforms representing the peak systolic velocity (PSV) and the end-diastolic flow velocity (EDV) were captured, and the velocities were measured. Three waveforms were measured per frame. The equation UARI = (PSV − EDV)/PSV was used, as is used in clinical settings.
Placental and renal cortical preproendothelin-1 mRNA levels.
Quantitative real-time PCR (qRT-PCR) was utilized to determine tissue preproendothelin-1 (PPET) levels. Placenta and renal cortex tissues were isolated and quickly frozen in liquid nitrogen. The tissues were then stored at −80°C. Total RNA was extracted from the tissues using the RNeasy Protect mini kit (Qiagen, Germantown, MD) and was performed according to the manufacturer's instructions. cDNA was synthesized from 1 μg total RNA using the iScript cDNA synthesis kit (Bio-Rad, Hercules, CA). qRT-PCR was performed using iQ SYBR Green Supermix (Bio-Rad) and measured for fluorescence on a CFX96 Touch Real-Time PCR Detection System (Bio-Rad). The primer sequences provided by Life Technologies (forward: 5′-ctaggtctaagcgatccttg-3′, reverse: 5′-tctttgtctgcttggc-3′) were used for PPET, as previously described (41). Levels of mRNA were calculated using the mathematical formula for 2−ΔΔCt (2avg·Ct gene of interest − avg Ct β-actin), as recommended by Applied Biosystems (Applied Biosystems User Bulletin, no. 2, 1997) and expressed as a fold change to NP rats for all experimental rat groups.
Measurement of plasma sFlt-1 levels by ELISA.
Circulating sFlt-1 levels were measured by ELISA (R&D Systems, Minneapolis, MN). Sensitivity for the ELISA was 15.2 pg/ml, and the assay range was 31.3–4,000 pg/ml.
Analysis of circulating reactive oxygen species by 8-isoprostane measurement.
Oxidative stress was assessed by measuring plasma 8-isoprostane levels using an ELISA (Cayman Chemical, Ann Arbor, MI). Assay range for this ELISA was 0.8–500 pg/ml. Where necessary, samples were diluted to ensure values were within the standard curve range.
Statistical analysis.
Data were expressed as means ± SE. The significance of difference in mean values were performed by one-way ANOVA with Bonferroni multiple-comparison post hoc test and/or by an unpaired standard t-test for two groups. P < 0.05 was considered to be significant. The commercial program GraphPad Prism 5 (GraphPad Software, La Jolla, CA) was used for data analysis.
RESULTS
Vitamin D supplementation reduces mean arterial pressure in response to ANG II or AT1-AA.
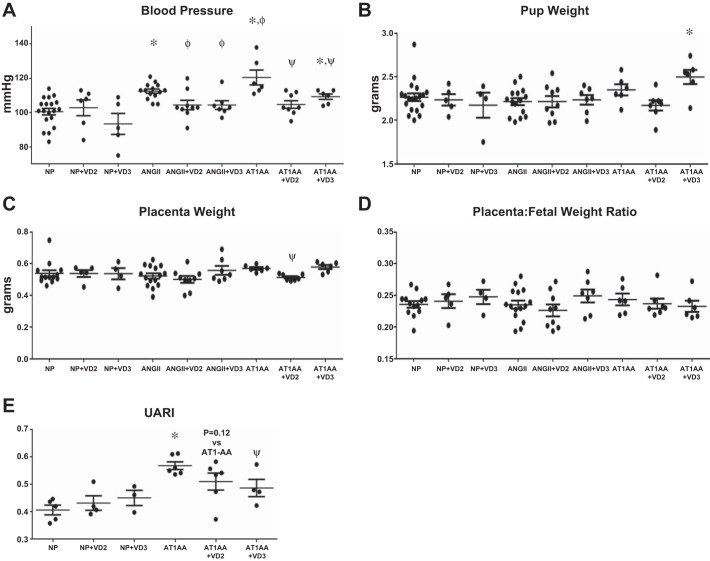
Vitamin D2 (NP+VD2) and D3 (NP+VD3) supplementation into normal pregnant rats (NP) had no significant effect on mean arterial pressure (MAP) in NP rats (Fig. 1A). AT1-AA infusion into pregnant rats significantly increased blood pressure to 121 ± 4 mmHg (P < 0.05, unpaired t-test) compared with NP (101 ± 2 mmHg). Both VD2 and VD3 treatment significantly lowered MAP to 105 ± 2 and 109 ± 2 mmHg (P < 0.05, unpaired t-test) in AT1-AA-infused rats, respectively. ANG II infusion increased blood pressure to 113 ± 1 mmHg (P < 0.05, unpaired t-test) in pregnant rats and both VD2 (104 ± 4 mmHg, P < 0.05, unpaired t-test) and VD3 (104 ± 3 mmHg, P < 0.05, unpaired t-test) reduced blood pressure relative to ANG II alone.
Fig. 1.
A: blood pressures were significantly increased in both ANG II- and AT1-AA-infused rats compared with NP. Neither VD2 nor VD3 treatment affected blood pressure in NP rats. Both VD2 and VD3 significantly reduced blood pressure in ANG II- and AT1-AA-infused rats. AT1-AA infusion increased blood pressure above levels observed in ANG II-infused rats (n: NP = 19, NP+VD2 = 6, NP+VD3 = 5, ANG II = 14, ANG II+VD2 = 9, ANG II+VD3 = 7, AT1-AA = 6, AT1-AA+VD2 = 8, and AT1-AA+VD3 = 6). Comparisons by unpaired t-test. B: there were no changes in pup weight in ANG II or AT1-AA rats compared with NP. Although pup weights were not significantly less in AT1-AA or ANGII infusion compared to NP, VD3 treatment did improve pup weight of AT1-AA infused compared to AT1-AA alone. Comparisons by unpaired t-test and one-way ANOVA. C: placental weights did not differ in ANG II- or AT1-AA-infused rats compared with NP. VD2 did reduce placental weight in AT1-AA-infused rats. No other groups treated with VD2 or VD3 had significantly altered placental weight compared with their untreated counterparts (n: NP = 13, NP+VD2 = 5, NP+VD3 = 4, ANG II = 15, ANG II+VD2 = 9, ANG II+VD3 = 7, AT1-AA = 6, AT1-AA+VD2 = 7, and AT1-AA+VD3 = 6). Comparisons by unpaired t-test. D: placental efficiency, defined as placenta:fetal weight ratio, was not changed with ANG II or AT1-AA infusion compared with NP rats. In addition, neither VD2 nor VD3 affected placenta:fetal weight ratio in either ANG II- or AT1-AA-infused pregnant rats (n: NP = 13, NP+VD2 = 5, NP+VD3 = 4, ANG II = 15, ANG II+VD2 = 9, ANG II+VD3 = 7, AT1-AA = 6, AT1-AA+VD2 = 7, and AT1-AA+VD3 = 6). Comparisons by unpaired t-test and one-way ANOVA. E: uterine artery resistance index (UARI) increased significantly in AT1-AA-infused rats compared with NP. Vitamin D treatment in NP rats did not alter UARI. Resistance was significantly decreased in AT1-AA+VD3 rats but did not dignificantly change in AT1-AA+VD2 rats (n: NP = 5, NP+VD2 = 4, NP+VD3 = 3, AT1-AA = 6, AT1-AA+VD2 = 6, and AT1-AA+VD3 = 4). Comparisons by unpaired t-test. *P < 0.05 vs. NP, ΦP < 0.05 vs. ANG II-infused, ψP < 0.05 vs. AT1-AA-infused.
Effects of vitamin D on intrauterine growth restriction in normal pregnant, AT1-AA, or ANG II-infused pregnant rats.
Although previous studies from the Xia laboratory infused AT1-AA and observed IUGR in pregnant mice, we do not commonly see this PE feature in our AT1-AA infused rats (17). This may be due to differences in concentration or route of administration between the protocols of our laboratory and those of the Xia laboratory. Nevertheless, we feel that examining pup or placental effects in response to an intervention such as vitamin D is important in evaluating the safety of vitamin D in our AT1-AA and ANG II-infused rats. Neither AT1-AA nor ANG II infusion into pregnant rats significantly affected pup weights in our study. Although pup weights were not significantly less in AT1-AA or ANGII infusion compared to NP, VD3 treatment did improve pup weight of AT1-AA infused compared to AT1-AA alone. NP rat placental weights did not differ from those of AT1-AA- or ANG II-infused pregnant rats (Fig. 1C). Vitamin D did not impact placental weights in ANG II-infused rats. Placental weight was reduced in AT1-AA+VD2 rats; however, VD3 had no effect.
Placental efficiency, as defined by placenta:fetal weight ratio, was unchanged in AT1-AA and ANG II rats compared with NP (Fig. 1D). VD2 treatment did not alter this ratio in NP, AT1-AA-infused, or ANG II-infused rats. NP+VD3, AT1-AA+VD3, or ANG II+VD3 rats did not have an altered placenta:fetal weight ratio compared with their untreated counterparts. Most importantly, these data indicate that vitamin D did not have adverse outcomes on fetal growth or survival.
Uterine artery resistance index is increased in AT1-AA-infused rats and reduced with vitamin D treatment.
Pregnant rats with AT1-AA exhibit increased uterine artery resistance index (UARI), defined by (PSV − EDV)/PSV, on GD18 compared with NP rats (0.569 ± 0.014 vs. 0.407 ± 0.018, respectively, P < 0.05, unpaired t-test) (Fig. 1E). Neither VD2 nor VD3 supplementation in NP rats (0.432 ± 0.027 and 0.451 ± 0.028, respectively) had an effect on UARI compared with NP rats. Although VD3 supplementation reduced UARI in AT1-AA-infused rats, VD2 did not have a significant effect (0.487 ± 0.031 and 0.511 ± 0.031, respectively, P < 0.05, unpaired t-test).
sFlt-1 levels are reduced in AT1-AA-infused pregnant rats treated with vitamin D.
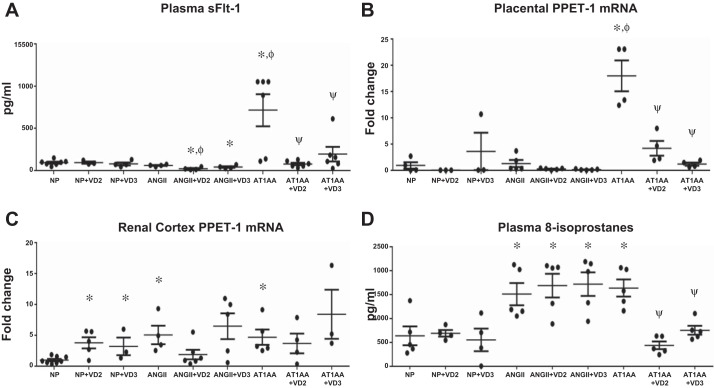
As shown in previous studies, AT1-AA infusion into pregnant rats (714.6 ± 188.5 pg/ml) increased circulating sFlt-1 levels compared with NP rats (93.9 ± 12.1 pg/ml, P < 0.05, unpaired t-test and one-way ANOVA). (Fig. 2A). Importantly, both VD2 (77.1 ± 15.5 pg/ml, P < 0.05, unpaired t-test and one-way ANOVA) and VD3 (195.1 ± 87.0 pg/ml, P < 0.05, unpaired t-test and one-way ANOVA) drastically reduced sFlt-1 in AT1-AA infused rats. ANG II infusion into pregnant rats did not alter sFlt-1 (60.4 ± 6.3 pg/ml) levels compared with NP. However, VD2 and VD3 did significantly reduce sFlt-1 in ANG II-infused rats (22.0 ± 8.2 and 41.6 ± 10.3 pg/ml, respectively, P < 0.05, unpaired t-test) to a level below that of NP rats.
Fig. 2.
A: plasma sFlt-1 levels were significantly increased in AT1-AA-infused rats. sFlt-1 was significantly reduced in AT1-AA-infused rats treated with either VD2 or VD3. ANG II infusion into pregnant rats did not alter sFlt-1 levels. VD2 did reduce sFlt-1 levels in ANG II-infused rats, but VD3 had no effect. Neither VD2 nor VD3 affected sFlt-1 levels in NP rats (n: NP = 7, NP+VD2 = 3, NP+VD3 = 4, ANG II = 4, ANG II+VD2 = 4, ANG II+VD3 = 4, AT1-AA = 6, AT1-AA+VD2 = 6, and AT1-AA+VD3 = 6). Comparisons by unpaired t-test and one-way ANOVA. B: placental preproendothelin-1 (PPET) was measured by real-time PCR and normalized to NP level. Placental PPET did not change with VD2 or VD3 treatment in NP rats. ANG II did not significantly increase placental PPET expression, and Vitamin D did not have an effect in ANG II-infused rats. AT1-AA infusion significantly increased placental PPET expression, and both VD2 and VD3 significantly reduced PPET (n: NP = 4, NP+VD2 = 3, NP+VD3 = 3, ANG II = 5, ANG II+VD2 = 5, ANG II+VD3 = 5, AT1-AA = 4, AT1-AA+VD2 = 4, and AT1-AA+VD3 = 4). Comparisons were made by unpaired t-test and one-way ANOVA. C: renal cortex expression of PPET was significantly increased in both ANG II- and AT1-AA-infused pregnant rats. VD2 modestly decreased renal cortex PPET in ANG II- and AT1-AA-infused rats; however this did not reach significance. VD3 did not alter PPET in the renal cortices of ANG II- or AT1-AA-infused pregnant rats. VD2 and VD3 administration to NP rats significantly increased PPET expression in renal cortices (n: NP = 8, NP+VD2 = 5, NP+VD3 = 3, ANG II = 4, ANG II+VD2 = 6, ANG II+VD3 = 5, AT1-AA = 5, AT1-AA+VD2 = 4, AT1-AA+VD3 = 3). Comparisons were made by unpaired t-test. D: 8-isoprostanes were measured in plasma. Isoprostanes did not change with Vitamin D treatment in NP rats. Isoprostanes were significantly increased in AT1-AA-infused rats and significantly decreased with VD2 and VD3-treated AT1-AA rats. ANG II rats had significantly increased isoprostanes compared with NP rats; however, neither VD2 nor VD3 affected isoprostane levels in ANG II rats (n: NP = 5, NP+VD2 = 4, NP+VD3 = 4, ANG II = 5, ANGII+VD2 = 5, ANG II+VD3 = 5, AT1-AA = 5, AT1-AA+VD2 = 5, and AT1-AA+VD3 = 5). Comparisons were made by unpaired t-test and one-way ANOVA. *P < 0.05 vs. NP, ΦP < 0.05 vs. ANG II-infused, ψP < 0.05 vs. AT1-AA-infused.
Placental and renal cortical ET-1 mRNA levels with vitamin D supplementation in both AT1-AA- and ANG II-infused pregnant rats.
We measured ET-1 expression by real-time PCR of its precursor, preproendothelin-1 (PPET) in placenta and renal cortex and normalized groups to NP. Placental PPET increased in AT1-AA-infused pregnant rats (18.1 ± 2.9-fold change, P < 0.05, unpaired t-test and one-way ANOVA) (Fig. 2B) but was significantly reduced with VD2 (4.3 ± 1.4-fold change, P < 0.05, unpaired t-test and one-way ANOVA) and VD3 (1.3 ± 0.3-fold change, P < 0.05, unpaired t-test and one-way ANOVA). ANG II infusion did not raise PPET expression in placental tissue (1.4 ± 0.7-fold change). Although ANG II rats treated with VD2 (0.3 ± 0.1-fold change) and VD3 (0.2 ± 0.1-fold change) had a reduction in PPET, these changes did not reach significance compared with ANG II-infused rats. Placental PPET levels in NP rats treated with VD2 (0.1 ± 0.0-fold change) and VD3 (3.7 ± 3.5-fold change) were not significantly changed compared with NP rats. Renal cortex expression of PPET was increased in both ANG II- (5.1 ± 1.5-fold change, P < 0.05, unpaired t-test) and AT1-AA-infused rats (4.7 ± 1.2-fold change, P < 0.05, unpaired t-test) compared with NP (Fig. 2C). In addition, VD2 supplementation decreased PPET in ANG II- (1.9 ± 0.8-fold change) and AT1-AA-infused (3.7 ± 1.6-fold change), although these changes did not reach significance. PPET was not altered with VD3 supplementation in ANG II (6.5 ± 2.1-fold change) or AT1-AA infused pregnant rats (8.5 ± 4.0-fold change).
Effects of vitamin D on plasma isoprostane levels in AT1-AA-infused rats treated with vitamin D.
We assessed levels of an indicator of reactive oxygen species, 8-isoprostanes, in plasma. AT1-AA-infused pregnant rats had increased isoprostane (1633 ± 179 pg/ml, P < 0.05, unpaired t-test and one-way ANOVA) compared with NP rats (634 ± 197 pg/ml) (Fig. 2D). VD2 and VD3 supplementation in AT1-AA-infused rats reduced the isoprostane levels (436 ± 81 and 752 ± 94 pg/ml, respectively, P < 0.05, unpaired t-test and one-way ANOVA). ANG II rats had significantly higher isoprostane levels compared with NP (1505 ± 233 pg/ml, P < 0.05, unpaired t-test). However, they did not change in ANG II+VD2 (1682 ± 248 pg/ml) or ANG II+VD3 (1714 ± 247 pg/ml). These data indicate that although the extent to which isoprostanes were increased was similar in AT1-AA and ANG II-infused rats, the mechanism by which vitamin D reduced isoprostanes was specific to AT1-AA-induced mechanisms.
DISCUSSION
PE is associated with elevated ET-1, ROS, sFlt-1, AT1-AA, as well as increased UARI, IUGR, and hypertension during pregnancy. Over the years, we and others have shown that infusion of AT1-AA induces all of these factors, when infused into pregnant rodents (5, 17, 33). We have recently published that VD2 or VD3 supplementation to the RUPP rat model of PE significantly lowered blood pressure and circulating factors thought to play an important role in the disease, such as CD4+ T cells, IL-6, sFlt-1, ET-1, and AT1-AA (8). However, whether reduction of AT1-AA levels by vitamin D in RUPP rats was the central mechanism via which blood pressure and such factors were reduced was not investigated. AT1-AA has been shown to induce PE characteristics independent of placental ischemia. Although ANG II infusion increased blood pressure, renal ET-1, and ROS during pregnancy, it does not stimulate sFlt-1 and other PE features (5). Therefore, ANG II infusion into pregnant rats was used as a model to study hypertension during pregnancy without PE features, which would be comparable to gestational or chronic hypertension in pregnancy. Therefore, the purpose of the present study was to determine whether vitamin D reduced blood pressure and factors associated with PE induced by AT1-AA and improved blood pressure in ANG II-treated rats as an example of hypertension without complicating factors observed in PE. As gestational hypertension is a significant risk factor for PE, reductions in blood pressure in this model could implicate vitamin D for prevention and/or treatment of PE and other disease states of pregnancy not complicated by PE. Ultimately, the outcome of these studies sheds light on the efficacy of vitamin D to improve pregnancy outcomes as part of a prenatal regimen.
Emerging studies in both the clinical population and in rodent models of PE indicate that vitamin D may reduce the immune pathogenesis of PE and improve blood pressure (4, 7, 13, 14, 18). Vitamin D has been associated with improvement of endothelial function in rodent models and in the vasculature ex vivo (28, 44). In this study, we show that vitamin D supplementation to hypertensive pregnant rats with AT1 receptor activation by either ANG II or AT1-AA, safely improves blood pressure without harming fetal weight. Furthermore, we found that markers of PE, ROS, ET-1, and sFlt-1 were reduced in AT1-AA-infused rats with vitamin D supplementation. In addition, we observed in our AT1-AA-infused rats that vitamin D2 and vitamin D3 are not identical with regard to effects on blood pressure, which may be attributable to differences in efficacy of the two isoforms of the compound in the rat (8). In comparison to AT1-AA-infused rats, ANG II-infused rats did not have an increase in sFlt-1 or placental ET-1. In addition, ROS levels were not altered with vitamin D supplementation, indicating that although both AT1-AA and ANG II activate the AT1 receptor to increase blood pressure, the downstream pathways activated during pregnancy differ. Furthermore, it is possible that during hypertension without PE features, i.e., elevated AT1-AA levels, ANG II does not have as profound of an effect to activate downstream PE mechanisms during pregnancy. Nevertheless, in either ANG II- or AT1-AA-induced hypertensive pregnancy, vitamin D safely lowered the maternal hypertensive response.
Vitamin D has a provascular effect in models of endothelial dysfunction, which may be attributed to reductions in ROS (44). In the present study, we observed a reduction in a marker of systemic ROS, 8-isoprostanes, in our AT1-AA-infused rats treated with vitamin D. In addition, our findings in the present study show that sFlt-1 induced by AT1-AA infusion was attenuated by either VD2 or VD3 supplementation. sFlt-1 has an important role in the pathogenesis of PE and is directly correlated with PE severity in human patients (25, 40). Studies have demonstrated that sFlt-1 infusion into pregnant rats and mice induces many of the characteristics of PE, including increases in blood pressure and ET-1 (26, 27). Although the exact mechanism via which AT1-AA induces increases in sFlt-1 levels remains unclear, these data demonstrate that vitamin D can alter this mechanism as a method to improve blood pressure during high levels of AT1-AA, as in the case of PE. Importantly, and indicating the specificity for AT1-AA during PE, sFlt-1 is not elevated in ANG II-induced hypertensive pregnant rats in this study.
ET-1 is a potent vasoconstrictor peptide and is increased in both human preeclamptic patients and in rodent models of PE (1, 22, 29, 35). ET-1 is induced in response to AT1 receptor activation by ANG II and by AT1-AA (5, 34). It has been proposed that AT1-AA-induced ET-1 contributes to the systemic endothelial dysfunction seen in preeclamptic patients, which is characterized by increases in the UARI (20, 22, 39). Interestingly, studies have demonstrated that vitamin D increases the production of ET-1 in some cell types (42). In fact, we observed that both VD2 and VD3 increased ET-1 expression in the renal cortices of our normal pregnant rats. In the kidney, vasodilatory endothelin receptor type B (ETB) receptors are prevalent and promote sodium excretion and a lowering of blood pressure. It is possible that vitamin D did not increase blood pressure in our normal pregnant rats despite this increase in renal cortical ET-1 expression due to ETB receptor activation; however, investigation into this mechanism was beyond the scope of the current study. Clinical studies indicate that vitamin D supplementation reduces plasma ET-1 levels and that this is associated with an improvement of factors associated with endothelial dysfunction in pregnancy such as sFlt-1 and ROS, implying that inhibition of ET-1 by vitamin D was upstream of direct transcriptional regulation of ET-1 (36, 44). In our current study, we found that both ANG II and AT1-AA-induced ET-1 in the renal cortex, most likely a mechanism contributing to the hypertension in each model. VD2 decreased ET-1 in the renal cortex in each model while VD3 seemed to have no effect. These data indicate that the AT1-dependent mechanism via which AT1-AA and ANG II activate ET-1 transcription in the renal cortex is similar and, furthermore, that the mechanism via which VD2 and VD3 interact with that mechanism is also similar. In contrast, only AT1-AA stimulated placental ET-1, and profoundly so, indicating an important difference between AT1-AA and ANG II-stimulated pathways during hypertensive pregnancy. Both VD2 and VD3 reduced placental ET-1, while only VD2 was able to significantly reduce UARI, which indicates that reductions in both placental and renal cortex ET-1 production contributed to reduce UARI in our AT1-AA-infused rats.
The stimuli by which placental ischemia leads to production of AT1-AA have been only partially elucidated. What has been well established is that AT1-AA activates the AT1 receptor and stimulates many downstream effects similar to ANG II, such as vasoconstriction and hypertension (5). However, there are many downstream mechanisms of AT1 receptor activation by AT1-AA that differ from those of ANG II. AT1-AA infusion into pregnant rats increased sFlt-1 and ET-1, whereas ANG II had no effect on sFlt-1 or placental ET-1 levels. Both ANG II and AT1-AA induce increases in systemic ROS; however, this was decreased with vitamin D treatment during AT1-AA-induced hypertension only in the placenta (5). VD2 and VD3 were able to effectively reduce sFlt-1 and placental ET-1 when AT1-AA was elevated. Importantly, these reductions also occurred with reduced blood pressure without affecting fetal outcomes.
Collectively, these data indicate that vitamin D supplementation may be beneficial not only in women with established PE and the presence of AT1-AA, but those with hypertension not complicated by PE or AT1-AA as depicted in our ANG II infusion model. Therefore, we conclude that VD2 or VD3 could be an important, inexpensive, and widely available supplement that could be added to the prevention and treatment strategies for pregnant hypertensive women, whether or not they develop PE features.
Perspectives and Significance
In this study, we have shown that vitamin D reduces some factors that are associated with pathophysiology of PE in AT1-AA-infused rats. Furthermore, we show that it is safe for blood pressure management in hypertensive pregnancies in the absence of PE. Moreover, we also demonstrated that vitamin D supplementation into NP rats did not adversely affect fetal growth, survival, or uterine artery resistance and, therefore, this study indicates that vitamin D may be a potential therapeutic to prevent increases in sFlt-1, ET-1, ROS, and blood pressure in PE, and hypertensive pregnancies at risk of PE.
GRANTS
This work was supported by National Institutes of Health Grants HL-105324, HL-124715, HL-78147, HL-51971, and HD-067541. R. Dechend is supported by the German Research Foundation (DFG Grant DE 631/9-1).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.L.F., L.M.A., D.C.C., T.I., A.H., N.C., N.U., and B.D.L. performed experiments; J.L.F., L.M.A., D.C.C., M.W.C., T.I., N.C., N.U., K.W., and B.D.L. analyzed data; J.L.F., L.M.A., D.C.C., M.W.C., N.C., K.W., F.H., and B.D.L. interpreted results of experiments; J.L.F., D.C.C., T.I., K.W., and B.D.L. prepared figures; J.L.F., L.M.A., D.C.C., and B.D.L. drafted manuscript; J.L.F., L.M.A., D.C.C., M.W.C., K.W., F.H., R.D., and B.D.L. edited and revised manuscript; J.L.F., L.M.A., D.C.C., M.W.C., T.I., A.H., N.C., N.U., K.W., F.H., R.D., and B.D.L. approved final version of manuscript.
REFERENCES
- 1.Alexander BT, Rinewalt AN, Cockrell KL, Massey MB, Bennett WA, Granger JP. Endothelin type a receptor blockade attenuates the hypertension in response to chronic reductions in uterine perfusion pressure. Hypertension 37: 485–489, 2001. doi: 10.1161/01.HYP.37.2.485. [DOI] [PubMed] [Google Scholar]
- 2.Baker AM, Haeri S, Camargo CA Jr, Espinola JA, Stuebe AM. A nested case-control study of midgestation vitamin D deficiency and risk of severe preeclampsia. J Clin Endocrinol Metab 95: 5105–5109, 2010. doi: 10.1210/jc.2010-0996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baker PN, Broughton Pipkin F, Symonds EM. Platelet angiotensin II binding and plasma renin concentration, plasma renin substrate and plasma angiotensin II in human pregnancy. Clin Sci (Lond) 79: 403–408, 1990. doi: 10.1042/cs0790403. [DOI] [PubMed] [Google Scholar]
- 4.Bodnar LM, Catov JM, Simhan HN, Holick MF, Powers RW, Roberts JM. Maternal vitamin D deficiency increases the risk of preeclampsia. J Clin Endocrinol Metab 92: 3517–3522, 2007. doi: 10.1210/jc.2007-0718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brewer J, Liu R, Lu Y, Scott J, Wallace K, Wallukat G, Moseley J, Herse F, Dechend R, Martin JN Jr, Lamarca B. Endothelin-1, oxidative stress, and endogenous angiotensin II: mechanisms of angiotensin II type I receptor autoantibody-enhanced renal and blood pressure response during pregnancy. Hypertension 62: 886–892, 2013. doi: 10.1161/HYPERTENSIONAHA.113.01648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown MA, Gallery ED, Ross MR, Esber RP. Sodium excretion in normal and hypertensive pregnancy: a prospective study. Am J Obstet Gynecol 159: 297–307, 1988. doi: 10.1016/S0002-9378(88)80071-1. [DOI] [PubMed] [Google Scholar]
- 7.Darby MM, Wallace K, Cornelius D, Chatman KT, Mosely JN, Martin JN, Purser CA, Baker RC, Owens MT, Lamarca BB. Vitamin D supplementation suppresses hypoxia-stimulated placental cytokine secretion, hypertension and CD4+ T cell stimulation in response to placental ischemia. Med J Obstet Gynecol 1: 1012, 2013. [PMC free article] [PubMed] [Google Scholar]
- 8.Faulkner JL, Cornelius DC, Amaral LM, Harmon AC, Cunningham MW Jr, Darby MM, Ibrahim T, Thomas DS, Herse F, Wallukat G, Dechend R, LaMarca BD. Vitamin D supplementation improves pathophysiology in a rat model of preeclampsia. Am J Physiol Regul Integr Comp Physiol 310: R346––R354., 2016. doi: 10.1152/ajpregu.00388.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Forman JP, Giovannucci E, Holmes MD, Bischoff-Ferrari HA, Tworoger SS, Willett WC, Curhan GC. Plasma 25-hydroxyvitamin D levels and risk of incident hypertension. Hypertension 49: 1063–1069, 2007. doi: 10.1161/HYPERTENSIONAHA.107.087288. [DOI] [PubMed] [Google Scholar]
- 10.Gant NF, Daley GL, Chand S, Whalley PJ, MacDonald PC. A study of angiotensin II pressor response throughout primigravid pregnancy. J Clin Invest 52: 2682–2689, 1973. doi: 10.1172/JCI107462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gilbert JS, Ryan MJ, LaMarca BB, Sedeek M, Murphy SR, Granger JP. Pathophysiology of hypertension during preeclampsia: linking placental ischemia with endothelial dysfunction. Am J Physiol Heart Circ Physiol 294: H541–H550, 2008. doi: 10.1152/ajpheart.01113.2007. [DOI] [PubMed] [Google Scholar]
- 12.Hanssens M, Keirse MJ, Spitz B, van Assche FA. Angiotensin II levels in hypertensive and normotensive pregnancies. Br J Obstet Gynaecol 98: 155–161, 1991. doi: 10.1111/j.1471-0528.1991.tb13361.x. [DOI] [PubMed] [Google Scholar]
- 13.Hashemipour S, Ziaee A, Javadi A, Movahed F, Elmizadeh K, Javadi EH, Lalooha F. Effect of treatment of vitamin D deficiency and insufficiency during pregnancy on fetal growth indices and maternal weight gain: a randomized clinical trial. Eur J Obstet Gynecol Reprod Biol 172: 15–19, 2014. doi: 10.1016/j.ejogrb.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 14.Haugen M, Brantsaeter AL, Trogstad L, Alexander J, Roth C, Magnus P, Meltzer HM. Vitamin D supplementation and reduced risk of preeclampsia in nulliparous women. Epidemiology 20: 720–726, 2009. doi: 10.1097/EDE.0b013e3181a70f08. [DOI] [PubMed] [Google Scholar]
- 15.Herse F, LaMarca B. Angiotensin II type 1 receptor autoantibody (AT1-AA)-mediated pregnancy hypertension. Am J Reprod Immunol 69: 413–418, 2013. doi: 10.1111/aji.12072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Immonen I, Siimes A, Stenman UH, Kärkkäinen J, Fyhrquist F. Plasma renin substrate and oestrogens in normal pregnancy. Scand J Clin Lab Invest 43: 61–65, 1983. doi: 10.3109/00365518309168223. [DOI] [PubMed] [Google Scholar]
- 17.Irani RA, Zhang Y, Blackwell SC, Zhou CC, Ramin SM, Kellems RE, Xia Y. The detrimental role of angiotensin receptor agonistic autoantibodies in intrauterine growth restriction seen in preeclampsia. J Exp Med 206: 2809–2822, 2009. doi: 10.1084/jem.20090872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ito M, Koyama H, Ohshige A, Maeda T, Yoshimura T, Okamura H. Prevention of preeclampsia with calcium supplementation and vitamin D3 in an antenatal protocol. Int J Gynaecol Obstet 47: 115–120, 1994. doi: 10.1016/0020-7292(94)90350-6. [DOI] [PubMed] [Google Scholar]
- 19.Lamarca B. Endothelial dysfunction. An important mediator in the pathophysiology of hypertension during pre-eclampsia. Minerva Ginecol 64: 309–320, 2012. [PMC free article] [PubMed] [Google Scholar]
- 20.LaMarca B, Parrish M, Ray LF, Murphy SR, Roberts L, Glover P, Wallukat G, Wenzel K, Cockrell K, Martin JN Jr, Ryan MJ, Dechend R. Hypertension in response to autoantibodies to the angiotensin II type I receptor (AT1-AA) in pregnant rats: role of endothelin-1. Hypertension 54: 905–909, 2009. doi: 10.1161/HYPERTENSIONAHA.109.137935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.LaMarca B, Wallace K, Herse F, Wallukat G, Martin JN Jr, Weimer A, Dechend R. Hypertension in response to placental ischemia during pregnancy: role of B lymphocytes. Hypertension 57: 865–871, 2011. doi: 10.1161/HYPERTENSIONAHA.110.167569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.LaMarca BD, Alexander BT, Gilbert JS, Ryan MJ, Sedeek M, Murphy SR, Granger JP. Pathophysiology of hypertension in response to placental ischemia during pregnancy: a central role for endothelin? Gend Med 5, Suppl A: S133–S138, 2008. doi: 10.1016/j.genm.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mabie WC, DiSessa TG, Crocker LG, Sibai BM, Arheart KL. A longitudinal study of cardiac output in normal human pregnancy. Am J Obstet Gynecol 170: 849–856, 1994. doi: 10.1016/S0002-9378(94)70297-7. [DOI] [PubMed] [Google Scholar]
- 24.Martins D, Wolf M, Pan D, Zadshir A, Tareen N, Thadhani R, Felsenfeld A, Levine B, Mehrotra R, Norris K. Prevalence of cardiovascular risk factors and the serum levels of 25-hydroxyvitamin D in the United States: data from the Third National Health and Nutrition Examination Survey. Arch Intern Med 167: 1159–1165, 2007. doi: 10.1001/archinte.167.11.1159. [DOI] [PubMed] [Google Scholar]
- 25.Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, Epstein FH, Sukhatme VP, Karumanchi SA. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest 111: 649–658, 2003. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murphy SR, LaMarca BB, Cockrell K, Granger JP. Role of endothelin in mediating soluble fms-like tyrosine kinase 1-induced hypertension in pregnant rats. Hypertension 55: 394–398, 2010. doi: 10.1161/HYPERTENSIONAHA.109.141473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murphy SR, LaMarca BB, Parrish M, Cockrell K, Granger JP. Control of soluble fms-like tyrosine-1 (sFlt-1) production response to placental ischemia/hypoxia: role of tumor necrosis factor-α. Am J Physiol Regul Integr Comp Physiol 304: R130–R135, 2013. doi: 10.1152/ajpregu.00069.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ni W, Watts SW, Ng M, Chen S, Glenn DJ, Gardner DG. Elimination of vitamin D receptor in vascular endothelial cells alters vascular function. Hypertension 64: 1290–1298, 2014. doi: 10.1161/HYPERTENSIONAHA.114.03971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nova A, Sibai BM, Barton JR, Mercer BM, Mitchell MD. Maternal plasma level of endothelin is increased in preeclampsia. Am J Obstet Gynecol 165: 724–727, 1991. doi: 10.1016/0002-9378(91)90317-K. [DOI] [PubMed] [Google Scholar]
- 30.Novotny SR, Wallace K, Heath J, Moseley J, Dhillon P, Weimer A, Wallukat G, Herse F, Wenzel K, Martin JN Jr, Dechend R, Lamarca B. Activating autoantibodies to the angiotensin II type I receptor play an important role in mediating hypertension in response to adoptive transfer of CD4+ T lymphocytes from placental ischemic rats. Am J Physiol Regul Integr Comp Physiol 302: R1197–R1201, 2012. doi: 10.1152/ajpregu.00623.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ouzounian JG, Elkayam U. Physiologic changes during normal pregnancy and delivery. Cardiol Clin 30: 317–329, 2012. doi: 10.1016/j.ccl.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 32.Palei AC, Spradley FT, Warrington JP, George EM, Granger JP. Pathophysiology of hypertension in pre-eclampsia: a lesson in integrative physiology. Acta Physiol (Oxf) 208: 224–233, 2013. doi: 10.1111/apha.12106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parrish MR, Murphy SR, Rutland S, Wallace K, Wenzel K, Wallukat G, Keiser S, Ray LF, Dechend R, Martin JN, Granger JP, LaMarca B. The effect of immune factors, tumor necrosis factor-α, and agonistic autoantibodies to the angiotensin II type I receptor on soluble fms-like tyrosine-1 and soluble endoglin production in response to hypertension during pregnancy. Am J Hypertens 23: 911–916, 2010. doi: 10.1038/ajh.2010.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pollock DM. Endothelin, angiotensin, and oxidative stress in hypertension. Hypertension 45: 477–480, 2005. doi: 10.1161/01.HYP.0000158262.11935.d0. [DOI] [PubMed] [Google Scholar]
- 35.Roberts JM, Taylor RN, Goldfien A. Clinical and biochemical evidence of endothelial cell dysfunction in the pregnancy syndrome preeclampsia. Am J Hypertens 4: 700–708, 1991. [DOI] [PubMed] [Google Scholar]
- 36.Shab-Bidar S, Neyestani TR, Djazayery A, Eshraghian MR, Houshiarrad A, Gharavi A, Kalayi A, Shariatzadeh N, Zahedirad M, Khalaji N, Haidari H. Regular consumption of vitamin D-fortified yogurt drink (Doogh) improved endothelial biomarkers in subjects with type 2 diabetes: a randomized double-blind clinical trial. BMC Med 9: 125, 2011. doi: 10.1186/1741-7015-9-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shand AW, Nassar N, Von Dadelszen P, Innis SM, Green TJ. Maternal vitamin D status in pregnancy and adverse pregnancy outcomes in a group at high risk for pre-eclampsia. BJOG 117: 1593–1598, 2010. doi: 10.1111/j.1471-0528.2010.02742.x. [DOI] [PubMed] [Google Scholar]
- 38.Siddiqui AH, Irani RA, Blackwell SC, Ramin SM, Kellems RE, Xia Y. Angiotensin receptor agonistic autoantibody is highly prevalent in preeclampsia: correlation with disease severity. Hypertension 55: 386–393, 2010. doi: 10.1161/HYPERTENSIONAHA.109.140061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tam Tam KB, George E, Cockrell K, Arany M, Speed J, Martin JN Jr, Lamarca B, Granger JP. Endothelin type A receptor antagonist attenuates placental ischemia-induced hypertension and uterine vascular resistance. Am J Obstet Gynecol 204: 330.e1–330.e4, 2011. doi: 10.1016/j.ajog.2011.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thadhani R, Mutter WP, Wolf M, Levine RJ, Taylor RN, Sukhatme VP, Ecker J, Karumanchi SA. First trimester placental growth factor and soluble fms-like tyrosine kinase 1 and risk for preeclampsia. J Clin Endocrinol Metab 89: 770–775, 2004. doi: 10.1210/jc.2003-031244. [DOI] [PubMed] [Google Scholar]
- 41.Wallace K, Novotny S, Heath J, Moseley J, Martin JN Jr, Owens MY, LaMarca B. Hypertension in response to CD4+ T cells from reduced uterine perfusion pregnant rats is associated with activation of the endothelin-1 system. Am J Physiol Regul Integr Comp Physiol 303: R144–R149, 2012. doi: 10.1152/ajpregu.00049.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang DS, Miura M, Demura H, Sato K. Anabolic effects of 1,25-dihydroxyvitamin D3 on osteoblasts are enhanced by vascular endothelial growth factor produced by osteoblasts and by growth factors produced by endothelial cells. Endocrinology 138: 2953–2962, 1997. [DOI] [PubMed] [Google Scholar]
- 43.Wei SQ, Audibert F, Hidiroglou N, Sarafin K, Julien P, Wu Y, Luo ZC, Fraser WD. Longitudinal vitamin D status in pregnancy and the risk of pre-eclampsia. BJOG 119: 832–839, 2012. doi: 10.1111/j.1471-0528.2012.03307.x. [DOI] [PubMed] [Google Scholar]
- 44.Wu-Wong JR, Li X, Chen YW. Different vitamin D receptor agonists exhibit differential effects on endothelial function and aortic gene expression in 5/6 nephrectomized rats. J Steroid Biochem Mol Biol 148: 202–209, 2015. doi: 10.1016/j.jsbmb.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 45.Yuan W, Pan W, Kong J, Zheng W, Szeto FL, Wong KE, Cohen R, Klopot A, Zhang Z, Li YC. 1,25-dihydroxyvitamin D3 suppresses renin gene transcription by blocking the activity of the cyclic AMP response element in the renin gene promoter. J Biol Chem 282: 29821–29830, 2007. doi: 10.1074/jbc.M705495200. [DOI] [PubMed] [Google Scholar]