Abstract
Hypertension is a global epidemic affecting over one billion people worldwide. Despite this, the etiology of most cases of human hypertension remains obscure, and treatment remains suboptimal. Excessive dietary salt and inflammation are known contributors to the pathogenesis of this disease. Recently, it has been recognized that salt can accumulate in the skin and skeletal muscle, producing concentrations of sodium greater than the plasma in hypertensive animals and humans. Such elevated levels of sodium have been shown to alter immune cell function. Here, we propose a model in which tissue salt accumulation causes an immune response leading to renal and vascular inflammation and hypertension.
Keywords: hypertension, inflammation, salt
high blood pressure (hypertension) affects over one billion people, and because of its association with stroke, heart failure, renal failure, and other terminal conditions, it is the leading modifiable risk factor for death and disability worldwide (15, 22). Moreover, an estimated 10% of hypertensive patients have elevated blood pressure despite extensive drug therapy (14). The poor efficacy of current treatments suggests that there are unknown or underappreciated culprits in the pathogenesis of this disease.
It has long been known that dietary salt (NaCl) intake is a major risk factor for hypertension; however, the underlying mechanisms remain elusive. More recently, it has become clear that hypertension is, in part, an inflammatory disease (21). Less well appreciated are compelling data demonstrating that salt often accumulates in the skin and skeletal muscle of experimental animals and humans with hypertension (18, 33). Moreover, recent studies have shown that sodium modulates immune cell function, making the immune system a possible link between salt and hypertension (2, 12, 17). In this review, we will discuss the role of salt and inflammation in the pathogenesis of hypertension and propose a model of how these factors may interact to increase blood pressure.
Link Between Salt and Hypertension
Reports on the relationship between dietary salt intake and blood pressure originate from the early 20th century when Ambard and Beaujard found that decreasing salt intake reduced blood pressure, and conversely, increasing salt intake increased blood pressure in a small group of patients (6). Since then, numerous large clinical trials have shown that reductions in salt intake are associated with decreases in blood pressure (8, 34). In the 1940s, Walter Kempner showed that a low-sodium rice diet effectively lowered blood pressure, reversed cardiomegaly, and normalized papilledema and retinal vascular changes in hypertensive patients (16). Despite the well-known relationship between salt intake and blood pressure, the mechanisms by which salt contributes to hypertension are poorly understood.
Because renal sodium retention is generally thought to be accompanied by water retention, it is widely believed that an impairment in the ability of the kidneys to excrete excess salt leads to a shift in the pressure-natriuresis curve and a resetting of the steady-state blood pressure to a higher level to facilitate adequate salt and water excretion (7). In addition, inappropriate sodium retention has been shown to cause modest elevations in plasma and cerebrospinal fluid sodium concentrations, leading to increased sympathetically mediated vasoconstriction and increased peripheral resistance (32). Data demonstrating the importance of these processes in the pathogenesis of hypertension are convincing, yet may not tell the full story of the prohypertensive role of salt.
Recent work by Titze and colleagues (18, 33) has demonstrated that sodium retention is not always accompanied by commensurate water retention. Using 23NaMRI in humans and chemical analysis in rodents, they have shown that the skin and skeletal muscle of hypertensive patients and animals contain levels of salt far greater than that of plasma. Moreover, they found that in humans, blood pressure is positively correlated with both skin and muscle sodium content (18). These important observations suggest that changes in extra-renal salt handling may be an important component of the relationship between salt and blood pressure.
Hypertensive Inflammation: Role of the Immune System in Hypertension
The concept that inflammation accompanies, and indeed contributes to, hypertension dates back decades. Lymphocytes and monocytes have been observed in the kidneys and blood vessels of hypertensive humans as early as the 1970s (24). Moreover, early studies showed that immunosuppression lowered blood pressure in rats with renal infarction, transplantation of lymph node cells from hypertensive to normotensive rats caused hypertension in the recipients, and vascular inflammation resulted from ANG II infusion (23, 25, 35). Since these initial observations, studies in both humans and animals support the concept that hypertension is, in part, an inflammatory disease.
Our group has shown that recombinase-activating gene 1 knockout mice, which lack both T and B lymphocytes, are protected from multiple forms of experimental hypertension. Adoptive transfer of T but not B lymphocytes into these mice restored the hypertension, suggesting that T lymphocytes (T cells) play a crucial role in the development of hypertension (5). Subsequent studies have shown a particularly important role of a subset of T cells that produce the inflammatory cytokine IL-17, termed Th17 cells (20). As reviewed recently, many studies by our group and others have confirmed a role of T cells and elucidated a role of other immune cells, such as monocytes, macrophages, dendritic cells, and regulatory T cells in various models of hypertension (4, 21, 27, 30).
These findings in animals are further supported by our recent report that a humanized mouse model in which the murine immune system has been replaced by the human immune system exhibits hypertension-associated inflammation in much the same way as C57BL/6J mice (10). Moreover, in human hypertensive patients, many markers of inflammation, including circulating T cells and serum IL-17, are elevated (1, 10, 20), and immunosuppression has been shown to reduce blood pressure (9). Lastly, a recent genome-wide association study found a link between blood pressure and a single nucleotide polymorphism in the SH2B3 gene, which encodes the lymphocyte adaptor protein, LNK (19). We and others have found that this gene plays a role in experimental hypertension (28, 29).
Although it is not entirely clear how immune activation raises blood pressure, it is clear that hypertension is accompanied by infiltration of immune cells into the kidneys and vasculature (21). These cells then release a variety of inflammatory cytokines, which seem to alter renal excretory function and vascular relaxation—both of which can contribute to the hypertensive process. While the consequences of immune activation in hypertension are clear, the causes are less well understood.
Salt as a Proinflammatory Stimulus
One likely culprit of hypertensive immune activation is salt. In fact, salt accumulation has been observed in various inflamed tissues, and high salt concentrations have been shown to alter the function of various types of immune cells in recent years (26, 31). Interestingly, Jantsch et al. (12) showed that sodium accumulates in cutaneous wounds and drives macrophage activation to facilitate wound healing. Moreover, Zhang et al. (39) showed that mouse and human macrophages cultured in high salt produced more inflammatory and less anti-inflammatory cytokines than those cultured in normal salt. Lastly, macrophages stimulated with IL-4 and IL-13 become less anti-inflammatory in the presence of high salt (2).
Macrophages are not the only immune cells that are affected by high salt. Recently, Yi and colleagues showed that a high-salt diet in humans increased circulating monocytes compared with a low-salt diet (38). Moreover, they observed an increase in inflammatory cytokines like IL-6 and IL-23 and a decrease in the anti-inflammatory cytokine IL-10 in the blood of patients fed a high-salt diet. The salt-induced production of IL-23 is particularly interesting, given the fact that IL-23 enhances and sustains polarization of naïve T cells into inflammatory Th17 cells (11).
In addition, high salt directly affects Th17 polarization in vitro. Two recent reports elucidated a pathway by which high salt amplifies Th17 polarization and IL-17 secretion (17, 37). Moreover, they showed that high-salt feeding exacerbated inflammation of the central nervous system in a mouse model of experimental autoimmune encephalomyelitis. The authors later showed that these proinflammatory effects of high salt in this model are likely due to direct effects of salt on T cells rather than on dendritic cells (13).
Antigen-presenting cells, including dendritic cells, macrophages, and B cells, are required for T-cell activation. Interestingly, dendritic cells are affected by osmotic stimuli. Chessa et al. (3) showed that dendritic cells that infiltrate transplanted kidneys have a different profile depending on where in the kidney they are located. Those that encounter the hyperosmotic environment of the renal medulla express more CD11b and F4/80, compared with dendritic cells found in the cortex of the kidney. Altering salt concentrations in vitro had a similar effect on bone marrow-derived mouse dendritic cells. Collectively, all of these studies demonstrate that immune cell function can be profoundly altered by the salt concentrations of the microenvironments they encounter.
Possible Role of Salt-Mediated Inflammation in the Pathogenesis of Hypertension
Because both salt and inflammation are important in the pathogenesis of hypertension, and because salt modulates immune cell function, it is possible that high-salt microenvironments encountered in hypertension can induce inflammation, tissue damage, and ultimately increased blood pressure. Although this hypothesis has yet to be proven, we can draw on some of the evidence presented in this review to support this idea. First, inflammatory Th17 cells and the IL-17 they produce are vital for the full expression of ANG II-induced hypertension in mice, and naïve T cells cultured with Th17-polarizing cytokines produce significantly more IL-17 in the presence of added NaCl (20, 37). Moreover, antigen-presenting cells like monocytes and macrophages, which act to drive T-cell proliferation, have been shown to play a role in hypertension and have also been shown to be activated by high salt (2, 12, 21, 38, 39). On the basis of these pieces of evidence, a paradigm is emerging by which the high-salt microenvironments that are known to occur in hypertension activate immune cells, such as T cells, monocytes, and dendritic cells, leading to renal and vascular inflammation and increased blood pressure (Fig. 1).
Fig. 1.
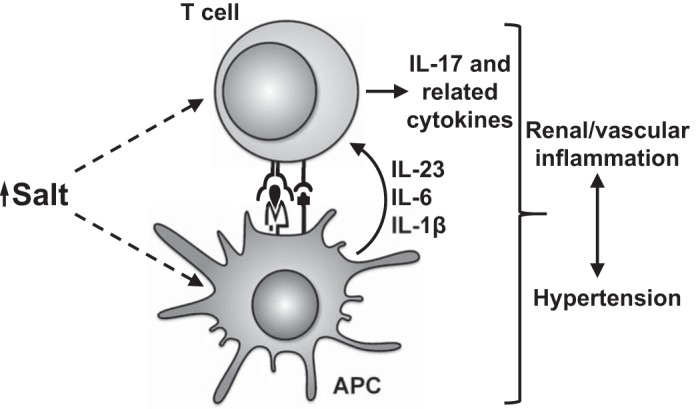
Paradigm illustrating how salt may affect immune cells to promote hypertension. High salt drives T cells toward a prohypertensive IL-17 phenotype, both directly and indirectly through activation of antigen-presenting cells (APC), such as monocytes, macrophages, and dendritic cells. This T-cell activation leads to renal and vascular inflammation, dysfunction of the kidneys and vasculature, and ultimately hypertension.
It should be noted that it is unlikely that the small changes in plasma sodium that occur upon increased salt intake would be sufficient to drive inflammation. Therefore, the proinflammatory effects of salt are likely to occur in tissues; however, it is currently unclear in which tissues immune cells may encounter high-salt concentrations in vivo. In addition to the skin and skeletal muscle, Wiig et al. (36) observed modest elevations in sodium concentration in lymph capillaries compared with the plasma of hypertensive rats. Thus, one possibility is that immune cells may encounter high salt in lymphoid tissues. Alternatively, circulating antigen-presenting cells may be activated by high salt in peripheral tissues before returning to lymphoid tissues and activating T cells. This will be a crucial point to address in future research.
Perspectives and Significance
In the past 20 yr, no new classes of agents have been widely adapted for the treatment of hypertension. As such, resistant and poorly controlled hypertension and end-organ damage caused by hypertension remain persistent problems. Because of this, the discovery of novel therapeutic targets for this disease is crucial. The immune system may be one such target. The difficulty in targeting the immune system to treat hypertension stems from the fact that the immune system serves a crucial role in host defense, and reducing immune function may put the patient at risk of infection. To circumvent this inherent risk, we have sought to determine upstream stimuli that are specific for hypertensive inflammation. If elevated tissue sodium concentration proves to be one stimulus of this process, it may be feasible to target tissue sodium as an anti-inflammatory treatment to reduce blood pressure.
GRANTS
This work was supported by the American Heart Association's Strategically Focused Research Network Grant 14SFRN20420046 and National Institutes of Health Grants R01HL-039006, P01HL-058000, P01HL-095070, P01GM-015431, R01HL108701, R01HL-105294, and K01HL-130497.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.D.F. prepared figures; J.D.F. drafted manuscript; J.D.F., A.K., and D.G.H. edited and revised manuscript; J.D.F., A.K., and D.G.H. approved final version of manuscript.
REFERENCES
- 1.Bautista LE, Vera LM, Arenas IA, Gamarra G. Independent association between inflammatory markers (C-reactive protein, interleukin-6, and TNF-α) and essential hypertension. J Hum Hypertens 19: 149–154, 2005. doi: 10.1038/sj.jhh.1001785. [DOI] [PubMed] [Google Scholar]
- 2.Binger KJ, Gebhardt M, Heinig M, Rintisch C, Schroeder A, Neuhofer W, Hilgers K, Manzel A, Schwartz C, Kleinewietfeld M, Voelkl J, Schatz V, Linker RA, Lang F, Voehringer D, Wright MD, Hubner N, Dechend R, Jantsch J, Titze J, Müller DN. High salt reduces the activation of IL-4- and IL-13-stimulated macrophages. J Clin Invest 125: 4223–4238, 2015. doi: 10.1172/JCI80919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chessa F, Mathow D, Wang S, Hielscher T, Atzberger A, Porubsky S, Gretz N, Burgdorf S, Gröne HJ, Popovic ZV. The renal microenvironment modifies dendritic cell phenotype. Kidney Int 89: 82–94, 2016. doi: 10.1038/ki.2015.292. [DOI] [PubMed] [Google Scholar]
- 4.De Miguel C, Rudemiller NP, Abais JM, Mattson DL. Inflammation and hypertension: new understandings and potential therapeutic targets. Curr Hypertens Rep 17: 507, 2015. doi: 10.1007/s11906-014-0507-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med 204: 2449–2460, 2007. doi: 10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ha SK. Dietary salt intake and hypertension. Electrolyte Blood Press 12: 7–18, 2014. doi: 10.5049/EBP.2014.12.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hall JE, Granger JP, do Carmo JM, da Silva AA, Dubinion J, George E, Hamza S, Speed J, Hall ME. Hypertension: physiology and pathophysiology. Compr Physiol 2: 2393–2442, 2012. doi: 10.1002/cphy.c110058. [DOI] [PubMed] [Google Scholar]
- 8.He FJ, Li J, Macgregor GA. Effect of longer term modest salt reduction on blood pressure: Cochrane systematic review and meta-analysis of randomised trials. BMJ 346: f1325, 2013. doi: 10.1136/bmj.f1325. [DOI] [PubMed] [Google Scholar]
- 9.Herrera J, Ferrebuz A, MacGregor EG, Rodriguez-Iturbe B. Mycophenolate mofetil treatment improves hypertension in patients with psoriasis and rheumatoid arthritis. J Am Soc Nephrol 17, Suppl 3: S218–S225, 2006. doi: 10.1681/ASN.2006080918. [DOI] [PubMed] [Google Scholar]
- 10.Itani HA, McMaster WG Jr, Saleh MA, Nazarewicz RR, Mikolajczyk TP, Kaszuba AM, Konior A, Prejbisz A, Januszewicz A, Norlander AE, Chen W, Bonami RH, Marshall AF, Poffenberger G, Weyand CM, Madhur MS, Moore DJ, Harrison DG, Guzik TJ. Activation of human T cells in hypertension: studies of humanized mice and hypertensive humans. Hypertension 68: 123–132, 2016. doi: 10.1161/HYPERTENSIONAHA.116.07237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iwakura Y, Ishigame H. The IL-23/IL-17 axis in inflammation. J Clin Invest 116: 1218–1222, 2006. doi: 10.1172/JCI28508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jantsch J, Schatz V, Friedrich D, Schröder A, Kopp C, Siegert I, Maronna A, Wendelborn D, Linz P, Binger KJ, Gebhardt M, Heinig M, Neubert P, Fischer F, Teufel S, David JP, Neufert C, Cavallaro A, Rakova N, Küper C, Beck FX, Neuhofer W, Muller DN, Schuler G, Uder M, Bogdan C, Luft FC, Titze J. Cutaneous Na+ storage strengthens the antimicrobial barrier function of the skin and boosts macrophage-driven host defense. Cell Metab 21: 493–501, 2015. doi: 10.1016/j.cmet.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jörg S, Kissel J, Manzel A, Kleinewietfeld M, Haghikia A, Gold R, Müller DN, Linker RA. High salt drives Th17 responses in experimental autoimmune encephalomyelitis without impacting myeloid dendritic cells. Exp Neurol 279: 212–222, 2016. doi: 10.1016/j.expneurol.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 14.Judd E, Calhoun DA. Apparent and true resistant hypertension: definition, prevalence and outcomes. J Hum Hypertens 28: 463–468, 2014. doi: 10.1038/jhh.2013.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet 365: 217–223, 2005. doi: 10.1016/S0140-6736(05)70151-3. [DOI] [PubMed] [Google Scholar]
- 16.Kempner W. Some effects of the rice diet treatment of kidney disease and hypertension. Bull N Y Acad Med 22: 358–370, 1946. [PubMed] [Google Scholar]
- 17.Kleinewietfeld M, Manzel A, Titze J, Kvakan H, Yosef N, Linker RA, Muller DN, Hafler DA. Sodium chloride drives autoimmune disease by the induction of pathogenic TH17 cells. Nature 496: 518–522, 2013. doi: 10.1038/nature11868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kopp C, Linz P, Dahlmann A, Hammon M, Jantsch J, Müller DN, Schmieder RE, Cavallaro A, Eckardt KU, Uder M, Luft FC, Titze J. 23Na magnetic resonance imaging-determined tissue sodium in healthy subjects and hypertensive patients. Hypertension 61: 635–640, 2013. doi: 10.1161/HYPERTENSIONAHA.111.00566. [DOI] [PubMed] [Google Scholar]
- 19.Levy D, Ehret GB, Rice K, Verwoert GC, Launer LJ, Dehghan A, Glazer NL, Morrison AC, Johnson AD, Aspelund T, Aulchenko Y, Lumley T, Köttgen A, Vasan RS, Rivadeneira F, Eiriksdottir G, Guo X, Arking DE, Mitchell GF, Mattace-Raso FU, Smith AV, Taylor K, Scharpf RB, Hwang SJ, Sijbrands EJ, Bis J, Harris TB, Ganesh SK, O’Donnell CJ, Hofman A, Rotter JI, Coresh J, Benjamin EJ, Uitterlinden AG, Heiss G, Fox CS, Witteman JC, Boerwinkle E, Wang TJ, Gudnason V, Larson MG, Chakravarti A, Psaty BM, van Duijn CM. Genome-wide association study of blood pressure and hypertension. Nat Genet 41: 677–687, 2009. doi: 10.1038/ng.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Madhur MS, Lob HE, McCann LA, Iwakura Y, Blinder Y, Guzik TJ, Harrison DG. Interleukin 17 promotes angiotensin II-induced hypertension and vascular dysfunction. Hypertension 55: 500–507, 2010. doi: 10.1161/HYPERTENSIONAHA.109.145094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McMaster WG, Kirabo A, Madhur MS, Harrison DG. Inflammation, immunity, and hypertensive end-organ damage. Circ Res 116: 1022–1033, 2015. doi: 10.1161/CIRCRESAHA.116.303697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murray CJ, Lopez AD. Measuring the global burden of disease. N Engl J Med 369: 448–457, 2013. doi: 10.1056/NEJMra1201534. [DOI] [PubMed] [Google Scholar]
- 23.Okuda T, Grollman A. Passive transfer of autoimmune induced hypertension in the rat by lymph node cells. Tex Rep Biol Med 25: 257–264, 1967. [PubMed] [Google Scholar]
- 24.Olsen F. Inflammatory cellular reaction in hypertensive vascular disease in man. Acta Pathol Microbiol Scand A 80: 253–256, 1972. [PubMed] [Google Scholar]
- 25.Olsen F. Type and course of the inflammatory cellular reaction in acute angiotensin-hypertensive vascular disease in rats. Acta Pathol Microbiol Scand A 78: 143–150, 1970. [DOI] [PubMed] [Google Scholar]
- 26.Paling D, Solanky BS, Riemer F, Tozer DJ, Wheeler-Kingshott CAM, Kapoor R, Golay X, Miller DH. Sodium accumulation is associated with disability and a progressive course in multiple sclerosis. Brain 136: 2305–2317, 2013. doi: 10.1093/brain/awt149. [DOI] [PubMed] [Google Scholar]
- 27.Rodriguez-Iturbe B. Autoimmunity in the pathogenesis of hypertension. Hypertension 67: 477–483, 2016. doi: 10.1161/HYPERTENSIONAHA.115.06418. [DOI] [PubMed] [Google Scholar]
- 28.Rudemiller NP, Lund H, Priestley JR, Endres BT, Prokop JW, Jacob HJ, Geurts AM, Cohen EP, Mattson DL. Mutation of SH2B3 (LNK), a genome-wide association study candidate for hypertension, attenuates Dahl salt-sensitive hypertension via inflammatory modulation. Hypertension 65: 1111–1117, 2015. doi: 10.1161/HYPERTENSIONAHA.114.04736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saleh MA, McMaster WG, Wu J, Norlander AE, Funt SA, Thabet SR, Kirabo A, Xiao L, Chen W, Itani HA, Michell D, Huan T, Zhang Y, Takaki S, Titze J, Levy D, Harrison DG, Madhur MS. Lymphocyte adaptor protein LNK deficiency exacerbates hypertension and end-organ inflammation. J Clin Invest 125: 1189–1202, 2015. doi: 10.1172/JCI76327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schiffrin EL. Immune mechanisms in hypertension and vascular injury. Clin Sci (Lond) 126: 267–274, 2014. doi: 10.1042/CS20130407. [DOI] [PubMed] [Google Scholar]
- 31.Schwartz L, Guais A, Pooya M, Abolhassani M. Is inflammation a consequence of extracellular hyperosmolarity? J Inflamm (Lond) 6: 21, 2009. doi: 10.1186/1476-9255-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stocker SD, Monahan KD, Browning KN. Neurogenic and sympathoexcitatory actions of NaCl in hypertension. Curr Hypertens Rep 15: 538–546, 2013. doi: 10.1007/s11906-013-0385-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Titze J, Bauer K, Schafflhuber M, Dietsch P, Lang R, Schwind KH, Luft FC, Eckardt KU, Hilgers KF. Internal sodium balance in DOCA-salt rats: a body composition study. Am J Physiol Renal Physiol 289: F793–F802, 2005. doi: 10.1152/ajprenal.00096.2005. [DOI] [PubMed] [Google Scholar]
- 34.Wang M, Moran AE, Liu J, Qi Y, Xie W, Tzong K, Zhao D. A meta-analysis of effect of dietary salt restriction on blood pressure in Chinese adults. Glob Heart 10: 291–299.e6, 2015. doi: 10.1016/j.gheart.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.White FN, Grollman A. Autoimmune factors associated with infarction of the kidney. Nephron 1: 93–102, 1964. doi: 10.1159/000179322. [DOI] [PubMed] [Google Scholar]
- 36.Wiig H, Schröder A, Neuhofer W, Jantsch J, Kopp C, Karlsen TV, Boschmann M, Goss J, Bry M, Rakova N, Dahlmann A, Brenner S, Tenstad O, Nurmi H, Mervaala E, Wagner H, Beck FX, Müller DN, Kerjaschki D, Luft FC, Harrison DG, Alitalo K, Titze J. Immune cells control skin lymphatic electrolyte homeostasis and blood pressure. J Clin Invest 123: 2803–2815, 2013. doi: 10.1172/JCI60113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu C, Yosef N, Thalhamer T, Zhu C, Xiao S, Kishi Y, Regev A, Kuchroo VK. Induction of pathogenic TH17 cells by inducible salt-sensing kinase SGK1. Nature 496: 513–517, 2013. doi: 10.1038/nature11984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yi B, Titze J, Rykova M, Feuerecker M, Vassilieva G, Nichiporuk I, Schelling G, Morukov B, Choukèr A. Effects of dietary salt levels on monocytic cells and immune responses in healthy human subjects: a longitudinal study. Transl Res 166: 103–110, 2015. doi: 10.1016/j.trsl.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang WC, Zheng XJ, Du LJ, Sun JY, Shen ZX, Shi C, Sun S, Zhang Z, Chen XQ, Qin M, Liu X, Tao J, Jia L, Fan HY, Zhou B, Yu Y, Ying H, Hui L, Liu X, Yi X, Liu X, Zhang L, Duan SZ. High salt primes a specific activation state of macrophages, M(Na). Cell Res 25: 893–910, 2015. doi: 10.1038/cr.2015.87. [DOI] [PMC free article] [PubMed] [Google Scholar]


