Abstract
Purpose of review
Whole lung tissue engineering is a relatively new area of investigation. In a short time, however, the field has advanced quickly beyond proof of concept studies in rodents and now stands on the cusp of wide-spread scale up to large animal studies. Therefore, this technology is ever closer to being directly clinically relevant.
Recent findings
The main themes in the literature include refinement of the fundamental components of whole lung engineering and increasing effort to direct induced pluripotent stem cells and lung progenitor cells toward use in lung regeneration. There is also increasing need for and emphasis on functional evaluation in the lab and in vivo, and the use of all of these tools to construct and evaluate forthcoming clinically-scaled engineered lung.
Summary
Ultimately, the goal of the research described herein is to create a useful clinical product. In the intermediate time, however, the tools described here may be employed to advance our knowledge of lung biology and the organ-specific regenerative capacity of lung stem and progenitor cells.
Keywords: organ engineering, lung engineering, whole organ engineering, regenerative medicine, lung regeneration
Introduction
Currently, the only curative option for patients with end-stage lung disease is lung transplantation. However, 20% of patients waiting for a transplant die on the waiting list [1], while transplant recipients face lifelong immunosuppression and high rates of graft failure. Despite 50 years of experience [2], the 10-year mortality in lung transplant recipients remains above 60% [3]. To address this need, several teams including our own are working to engineer whole lungs [4–7]; the goal is to create lungs on-demand from a patient’s own cells, thereby increasing organ availability and minimizing the need for post-transplantation immunosuppression. Such an achievement would have a deep impact on the care of terminally ill pulmonary patients.
In this article we describe recent progress in whole lung regeneration and highlight key scientific and clinical challenges to translating this work from the laboratory to the operating room. We discuss ongoing efforts to optimize three-dimensional scaffold production, identify key lung stem and progenitor cells for organ regeneration, and engineer bioreactor culture chambers to grow whole organs ex vivo. We review techniques to evaluate engineered lung function and highlight the need for standard practices and non-destructive sampling methods. Finally, we briefly discuss considerations for evaluating engineered human lungs in large animals and, eventually, in the clinic.
Scientific Foundations of Lung Engineering
Three components are required to engineer a tissue in vitro: 1) a scaffold to support cell attachment and growth; 2) cells with which to populate the scaffold; and 3) a bioreactor that provides the developing tissue with the necessary nutrients, waste removal, and other physical and biochemical stimuli to generate organized and functional tissue (Figure 1). Here we address each in turn.
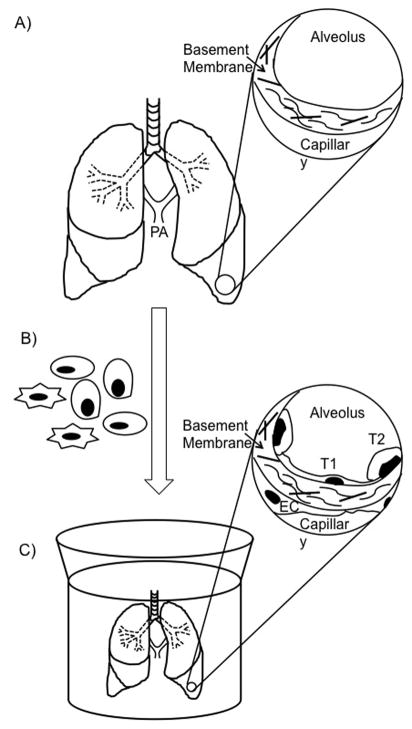
Figure 1. Production of a tissue-engineered lung.
A) A decellularized lung. Key architectural components of native lung are retained, including the trachea, bronchi, and pulmonary arteries; inset: an alveolus and an adjacent capillary that have been stripped of cells. The preserved basement membrane and retained constituent parts such as proteins and carbohydrates are indicated (arrow). B) The scaffold is mounted in a bioreactor and cells are seeded into the airway and vascular compartments of the decellularized lung scaffold via the trachea and pulmonary arteries, respectively, indicated in panel (a). C) The reseeded scaffold is cultured in a bioreactor and subject to physiological stimuli (see Figure 2); inset: an alveolus and an adjacent capillary of a decellularized scaffold that have been reseeded with epithelial and endothelial cells. After seeding, the alveolus is lined with a thin layer of alveolar type I cells as well as surfactant-producing type II cells. Endothelial cells populate the luminal surface of the capillary, completing the alveolar-capillary barrier. PA = pulmonary arteries, T1 = alveolar type I cell, T2 = alveolar type II cell, and EC = endothelial cell.
Scaffolds for Lung Regeneration
Decellularization is a process by which physical, chemical, or biological means are used to remove the cellular components of a tissue or organ, leaving the overall structure of the organ intact [8]. Using freeze-thaw cycles, enzymatic digestion [9], or detergents such as Triton X-100 or sodium deoxycholate [10], researchers obtain a 3-dimensional (3D) biological scaffold that retains architectural, biochemical, and mechanical properties of native tissue. The microscopic extracellular matrix (ECM) proteins and associated carbohydrates that remain after decellularization are biologically active and serve as both a surface on which cells can grow and as a signaling platform for these cells. Adhesion sites inherent in matrix proteins form molecular “zip codes” that are important for regulating cell attachment, migration, proliferation, and even differentiation [11]. Therefore, decellularization must balance removal of potentially antigenic cellular constituents, with the maintenance of these underlying ECM proteins. Protocols initially devised for whole rat lungs have been successfully scaled up for use with porcine [12, 13], nonhuman primate [14, 15], and human lungs [12, 15, 16]. However, a consensus on which decellularization treatment will be most efficacious and tractable for eventual clinical use has yet to be reached.
Cells for Scaffold Repopulation
The appropriate choice of cell types for seeding the decellularized scaffold remains the biggest uncertainty in the path toward regenerating a lung in vitro. The designated stem or progenitor cells must proliferate sufficiently to repopulate the surface area of a full set of human lungs (~100 m2), must confer the key functions of the lung, and must renew over time and in response to injury. Ciliated and goblet cells in the proximal airways, alveolar type I (ATI) and type II (ATII) cells in the distal alveoli, and vascular endothelial cells (ECs) in arterial, venous, and capillary networks are indispensible. In addition, alveolar lipofibroblasts and other supporting cells of mesenchymal lineage will likely be necessary. Together, these cells confer: 1) a thin alveolar epithelial layer that enables gas exchange, 2) an alveolar-capillary barrier function that prevents translocation of blood or blood components into the air spaces, 3) surfactant production to prevent alveolar collapse, 4) host defense in the form of ciliated and mucus-producing cells, and 5) a patent vasculature that produces vasoactive and antithrombotic substances such as endothelial nitric oxide synthase (eNOS) and prostacyclin.
To balance effective outcome with the practical considerations of working with a large number of cell types, investigators must place a high premium on selecting a minimum number of essential progenitors. In the proximal lung, for example, p63+/Krt5+ basal cells give rise to both ciliated and secretory epithelial cells [17]. Distally, integrin α6β4+ alveolar epithelial cells, club cells, and type II alveolar epithelial cells are candidate progenitors [18–21]. Further work is needed to define these cell populations in the human lung, since many were first identified in murine models. Selected endogenous progenitors may ultimately serve as targets for induced pluripotent stem (iPS) cell differentiation, to enable immunologic compatiblity with the intended recipient. Thus far, directed differentiation protocols have produced proximal airway cells from human ES cells [22] and distal lung cells from human iPS cells [23].
With regard to endothelial cell (EC) function, microvascular pulmonary EC may be sufficient for whole lung repopulation. However, evidence of functional heterogeneity among pulmonary ECs suggests that effective function may necessitate both microvascular and macrovascular ECs [24]. Similarly, one or more types of mesenchymal/stromal cells should be included to support scaffold maintenance and epithelial health through secretion of matrix components and growth factors [21]. The addition of stromal cells to whole lung cultures, for example, enhances the engraftment of transplanted embryonic epithelial lung progenitor cells in mice [25] and improves the barrier function of an endothelial-reseeded decellularized lung [26].
Bioreactor Designs
Construction and use of bioreactors that provide sterile, sealed environments for organ culture and that incorporate pumps, fluid reservoirs, and electronics to apply and monitor organ perfusion and ventilation (Figure 2) are in the early stages [27–29]. Work to evaluate parameters such as vascular perfusion rate, tidal volume, respiratory rate, or peak “intrathoracic” (i.e. intra-bioreactor) and intra-alveolar pressures during culture are required, as these stimuli are critical for proper lung development. Vascular perfusion provides shear stress that enhances the expression of endothelial cell-cell junctions [30] and increases expression of eNOS mRNA [31]; insufficient or inappropriately applied shear stress results in disorganized endothelial cell-cell junctions and impaired barrier permeability [32–34]. Likewise, the amount of stretch applied to alveoli during development influences both the thickness of the alveolar-capillary barrier and the relative numbers of ATI and ATII [35]; complete lack of fetal breathing movements results in pulmonary hypoplasia [36]. Therefore, special attention needs to be paid to these aspects of whole organ engineering.
Figure 2. Bioreactor for the culture of engineered lung tissue.
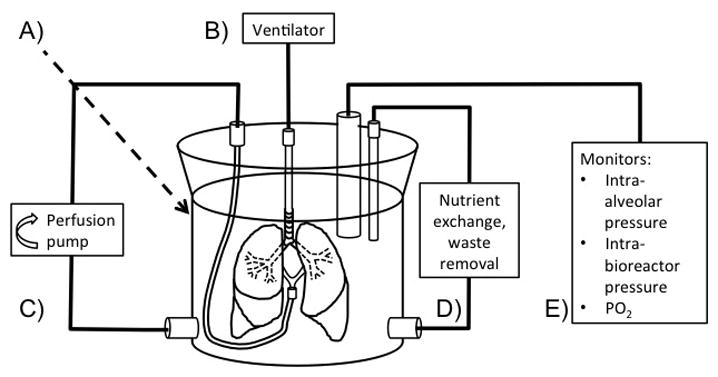
A) The bioreactor is a sterile, sealed container that houses the lung during culture. B) A ventilator is incorporated to provide positive or negative pressure ventilation with carefully chosen tidal volume, respiratory rate, and airway pressures. C) A perfusion pump circulates culture media through the lung vasculature. D) Access sites allow for provision of fresh media and other nutrients and removal of waste. E) Probes enable monitoring of intra-alveolar and intra-bioreactor pressures, the partial pressure of oxygen within the bioreactor, and other parameters. PO2 = partial pressure of oxygen.
Form vs. Function: Evaluating the Results of Engineering Efforts
To date, engineered constructs have been shown to exchange gas in vivo for a matter of hours before failing due to vascular thrombus formation and incomplete barrier function. This failure mode suggests that more research is required to identify the particular cell populations required for long-term organ function. Additional functional evaluation will be especially important as cells derived from pluripotent precursors – embryonic stem (ES) and iPS cells – are increasingly incorporated into the engineered tissues.
Cellular Phenotype in Engineered Lungs
Early studies demonstrated that implantable lung constructs could be engineered using either a combination of fetal rat lung cells and human umbilical vein endothelial cells, or mixed rat lung cells with rat lung microvascular endothelial cells [4, 5]. When these cell populations were seeded into the airways and vasculature of decellularized scaffolds and cultured in a bioreactor for approximately 1 week, the resulting construct resembled native lung histologically. Both studies reported that the cells in the constructs displayed markers of type I and II alveolar epithelial cells, airway epithelial cells, endothelial cells, and interstitial cells. Other groups have demonstrated that less mature cell populations can also begin expressing markers of type II alveolar cells when cultured on decellularized matrix [6, 37].
However, characterization of these cells relies largely on visual assessment of morphology, immunofluorescent staining of surface and intracellular markers, and assessment of gene expression by RT-PCR. Other than the use of fluorescently-tagged surfactant to demonstrate surfactant recycling [38], little functional evaluation has been applied to the cells derived by these protocols, despite the fact that functional evaluation at the cellular level is an integral part of research, regulatory practice and use, and of establishing eventual manufacturing standards.
Engineered Organ Function Ex Vivo
The function of engineered tissues must be evaluated during culture and prior to implantation. However, current criteria for donor lungs for transplant are not sufficient to evaluate tissue-engineered organs [39]. To ensure that engineered lungs satisfy minimal structural and functional parameters prior to implantation, we must develop reliable and non-destructive methods to evaluate tissue integrity and function. These parameters must include assessment of alveolar-capillary barrier function, gas exchange, respiratory mechanics, and vascular patency.
Assessment of lung scaffold structural integrity by bronchoscopy and micro-CT [5, 40] demonstrate grossly intact architecture. Barrier function of engineered lungs to polysaccharides is comparable to native lungs stored on ice, but significantly lower than that of native lungs [41]. Ex vivo perfusion of engineered lungs with red blood cells during ventilation with 98% FiO2 O2 showed that engineered lungs performed similarly to freshly isolated native lung [4]. Lung mechanics have been evaluated by measuring compliance [4–6, 37], vital capacity [4], resistance [6, 37], and elastance [6, 37]. All studies showed significant differences between native and decellularized lung; most [5, 6, 37] showed persistent differences between engineered and native lung. However, each of these studies employed slightly different methods, including volume-controlled continuous ventilation to obtain dynamic compliance values [4], direct measurements of pressure and volume to obtain quasi-static compliance [35], and pressure-controlled ventilation [6, 37]. Standardizing these assessments would facilitate direct comparison of engineering techniques and outcomes. Long-term, such assessments will be critical for decisions regarding implantation.
Techniques to measure the ability of engineered lung vasculature to prevent thrombosis have yet to be devised. Assays that evaluate the ability of the engineered vasculature to inactivate factor Xa and thrombin [42] or report of a minimum time to thrombosis for heparinized or un-heparinized whole blood could be useful in determining how the construct would fare in vivo.
Engineered Organ Function In Vivo
To date, the sum of in vivo evaluation of implanted engineered organs includes pulmonary artery blood gas measurement [4, 5, 43] and imaging by chest x-ray [5] or fluoroscopy [4, 43]. These data showed effective O2 uptake and CO2 release by engineered lung and 100% SaO2 in both engineered and native lung [5]. Engineered lung outperformed a pneumonectomized control intraoperatively and post-op on room air [4].
Long-term, more comprehensive non-invasive means must be included to evaluate barrier function and monitor pulmonary arterial pressure. Barrier function can be assessed with imaging techniques such as fluoroscopy and chest x-ray [4, 5], in combination with bronchial-alveolar lavage (BAL). BAL can help determine whether airway fluid is hydrostatic in origin or due to compromised barrier integrity [44–46]. Additional metrics such as right heart, pulmonary capillary wedge, and central venous pressures, as well as ventilation/perfusion (V/Q) scans will be crucial to evaluating engineered lung function in vivo. A standard protocol for post-operative follow-up incorporating many of these techniques should be devised well in advance of any large-mammal trial.
Practical Considerations for Clinical Use: Large Animal Experiments and Clinical Perspective
Ultimately, “performing a clinical trial using an agent about which all properties are known is, in fact, rarely possible” [47], however, large-animal models are better at predicting therapeutic responses than rodent models and so must be pursued. Pigs and non-human primates in particular would support eventual regulatory approval to test in man, and pig models have been used to investigate and validate ex-vivo lung perfusion techniques [48]. Pig scaffolds may also serve as a substrate for human cells, possibly providing an avenue from laboratory to clinical work [7, 15, 49–51].
As a specific animal model of a relevant human disease, CFTR-null and CFTR-ΔF508 piglets have been produce and may be of use in evaluating the effectiveness of engineered lungs for transplant into pulmonary patients [52, 53]. Alternatively, investigation of the ability of an engineered lung transplant to reduce mortality following acute or chronic lung injury may be more tractable [54, 55].
In either instance, we must assemble a team experienced in performing complicated thoracic surgeries and follow-up care in the setting of compromised lung function. As the first human patients are likely to have end-stage respiratory failure, protective ventilator strategies and extracorporeal circulation may prove useful for managing the patient perioperatively [56]. These tools may also be helpful post-operatively, as first-generation engineered constructs may not respond to pharmacologic intervention in the same way as native tissue, or may present unforeseen complications. As such, specialized protocols and expertise will be critical to safe clinical translation.
As cell-based therapies for lung disease move forward, it will be increasingly important that we identify the patient population most likely to benefit from this technology. Decompensating cystic fibrosis patients and end-stage pulmonary disease patients could both benefit from engineered lungs [57–59]. However, the physiology, biology, and age of each of these groups at the prospective time of transplantation are very different. Since the ideal lung construct will be made from a patient’s own cells, and the protocol for transplant will necessarily take into account age and comorbidities, we have to begin thinking about who will likely be the first candidates for transplant. Such complex decisions will require input from anesthesiologists, pulmonologists, intensivists, cardiothoracic surgeons, and ethicists in collaboration with the biological engineers currently spearheading this work.
Finally, it will be important to monitor for acute status changes necessitating additional intervention or alternate therapy - in addition to standard complications such as immune rejection and organ failure, iPS-cell derived tissues have shown some potential for teratoma formation [60, 61]. As this would be a dangerous outcome, techniques should be adopted to both minimize and allow the early detection of such complications. Both tumor surveillance and accrual of long-term outcome data will be important for safety evaluation and to provide data for continued scientific refinement.
Conclusions
Six years later after the first reports of engineered whole lung, the field has extended beyond proof of concept and must now grapple with details regarding cell source and type and refinement of culture conditions to enable proper tissue organization. Functional evaluation of the cells ultimately derived for use in scaffold repopulation and delineation of functional criteria for the overall construct are pressing issues that will be critical to advancement of this work. In tandem, careful consideration to transitioning from rodents to large animals will both improve the information that can be obtained, and will lay important groundwork for the forthcoming transition to the clinic. The inclusion of clinicians in the next stages of development is especially important as 1) they possess key areas of knowledge that can inform the design of functional assays and the selection of transplant models and patients in particular; and 2) surgeons, anesthesiologists, and intensivists will ultimately comprise the team of individuals who will handle and implement the use of whole engineered lungs in the clinic. Overall, we expect that strategic and committed collaboration between clinicians, scientists, and engineers will speed the transition of this much-needed technology from the laboratory to clinical practice.
Key points.
There is an unmet need for additional lungs available for transplant.
Functional three-dimensional lung tissue can be engineered using a decellularized extracellular matrix scaffold, key endogenous cell types, and a biomimetic bioreactor culture chamber.
In order to engineer clinically useful lung tissue on a large scale, key stem and progenitor cell advances must be made; namely, the identification and derivation of lung cells that can populate a pulmonary scaffold and confer organ function are critical milestones that have yet to be met.
Criteria for and assays to evaluate the function of derived cells, engineered tissue, and pre-implant constructs must be a focus of both engineers and clinicians.
Interdisciplinary large animal work is indispensible to the advancement of engineered organs from the laboratory to the clinic.
Acknowledgments
LEN is supported in this work by 1U01HL111016-01. KLL and MSBR are supported by NIH MSTP Training Grant T32GM007205.
Footnotes
Disclosure of funding sources: NIH 1U01HL111016-01 (LEN) and NIH MSTP Training Grant T32GM007205 (KLL, MSBR).
Disclosures
LEN has a founder and shareholder in Humacyte, Inc, which is a regenerative medicine company. Humacyte produces engineered blood vessels from allogeneic smooth muscle cells for vascular surgery. LEN’s spouse has equity in Humacyte, and LEN serves on Humacyte’s Board of Directors. LEN is an inventor on patents that are licensed to Humacyte and that produce royalties for LEN. LEN has received an unrestricted research gift to support research in her laboratory at Yale. Humacyte did not fund these studies, and Humacyte did not influence the conduct, description or interpretation of the findings in this report.
References and Recommended Reading
JUSTIFICATION FOR REFERENCES OUTSIDE OF THE “PERIOD OF REVIEW” (2016): The field of whole lung engineering is only 6 yrs old. The “outside of period” references highlighted here are the first two reports of this technology (Petersen 2010, Ott 2010) and the most recent report, which is also the most effective tissue reported to date (Ren 2015). Bonvillain is also highlighted as a first-in-field paper. Rosen 2015 is highlighted for the implications for whole lung engineering – i.e. that a mixed cell population is essential. This may serve as a “roadmap” for future lung efforts.
- 1.Flynn B, Hastie J, Sladen RN. Heart and lung transplantation. Curr Opin Anaesthesiol. 2014;27:153–60. doi: 10.1097/ACO.0000000000000058. [DOI] [PubMed] [Google Scholar]
- 2.Hardy JD, Eraslan S, Webb WR. Transplantation of the Lung. Ann Surg. 1964;160:440–8. doi: 10.1097/00000658-196409000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Valapour M, Skeans MA, Heubner BM, Smith JM, Schnitzler MA, Hertz MI, Edwards LB, Snyder JJ, Israni AK, Kasiske BL. OPTN/SRTR 2012 Annual Data Report: lung. Am J Transplant. 2014;14(Suppl 1):139–65. doi: 10.1111/ajt.12584. [DOI] [PubMed] [Google Scholar]
- **4.Ott HC, Clippinger B, Conrad C, Schuetz C, Pomerantseva I, Ikonomou L, Kotton D, Vacanti JP. Regeneration and orthotopic transplantation of a bioartificial lung. Nat Med. 2010:927–33. doi: 10.1038/nm.2193. This is one of the first two studies to demonstrate the ability of engineered lung tissue to exchange gas when implanted in a rat recipient. With the Petersen paper (following), this paper established the field of whole lung engineering in its current form. [DOI] [PubMed] [Google Scholar]
- **5.Petersen TH, Calle EA, Zhao L, Lee EJ, Gui L, Raredon MB, Gavrilov K, Yi T, Zhuang ZW, Breuer C, Herzog E, Niklason LE. Tissue-engineered lungs for in vivo implantation. Science. 2010:538–41. doi: 10.1126/science.1189345. This is the first report of whole lung engineered lung tissue that was able to exchange gas when implanted in a rat recipient. With the Ott paper (above), this paper established the field of whole lung engineering. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Price AP, England KA, Matson AM, Blazar BR, Panoskaltsis-Mortari A. Development of a Decellularized Lung Bioreactor System for Bioengineering the Lung: The Matrix Reloaded. Tissue Eng. 2010 doi: 10.1089/ten.tea.2009.0659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wagner DE, Bonenfant NR, Sokocevic D, DeSarno MJ, Borg ZD, Parsons CS, Brooks EM, Platz JJ, Khalpey ZI, Hoganson DM. Three-dimensional scaffolds of acellular human and porcine lungs for high throughput studies of lung disease and regeneration. Biomaterials. 2014;35:2664–79. doi: 10.1016/j.biomaterials.2013.11.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crapo PM, Gilbert TW, Badylak SF. An overview of tissue and whole organ decellularization processes. Biomaterials. 2011;32:3233–43. doi: 10.1016/j.biomaterials.2011.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keane TJ, Swinehart IT, Badylak SF. Methods of tissue decellularization used for preparation of biologic scaffolds and in vivo relevance. Methods. 2015;84:25–34. doi: 10.1016/j.ymeth.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 10.Tsuchiya T, Sivarapatna A, Rocco K, Nanashima A, Nagayasu T, Niklason LE. Future prospects for tissue engineered lung transplantation: decellularization and recellularization-based whole lung regeneration. Organogenesis. 2014;10:196–207. doi: 10.4161/org.27846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shojaie S, Ermini L, Ackerley C, Wang J, Chin S, Yeganeh B, Bilodeau M, Sambi M, Rogers I, Rossant J, Bear CE, Post M. Acellular lung scaffolds direct differentiation of endoderm to functional airway epithelial cells: requirement of matrix-bound HS proteoglycans. Stem Cell Reports. 2015;4:419–30. doi: 10.1016/j.stemcr.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gilpin SE, Guyette JP, Gonzalez G, Ren X, Asara JM, Mathisen DJ, Vacanti JP, Ott HC. Perfusion decellularization of human and porcine lungs: bringing the matrix to clinical scale. J Heart Lung Transplant. 2014;33:298–308. doi: 10.1016/j.healun.2013.10.030. [DOI] [PubMed] [Google Scholar]
- 13.Balestrini JL, Gard AL, Liu A, Leiby KL, Schwan J, Kunkemoeller B, Calle EA, Sivarapatna A, Lin T, Dimitrievska S, Cambpell SG, Niklason LE. Production of decellularized porcine lung scaffolds for use in tissue engineering. Integr Biol (Camb) 2015;7:1598–610. doi: 10.1039/c5ib00063g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *14.Bonvillain RW, Danchuk S, Sullivan DE, Betancourt AM, Semon JA, Eagle ME, Mayeux JP, Gregory AN, Wang G, Townley IK, Borg ZD, Weiss DJ, Bunnell BA. A nonhuman primate model of lung regeneration: detergent-mediated decellularization and initial in vitro recellularization with mesenchymal stem cells. Tissue Eng Part A. 2012;18:2437–52. doi: 10.1089/ten.tea.2011.0594. First report of non-rodent lung engineering. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *15.Balestrini JL, Gard AL, Gerhold KA, Wilcox EC, Liu A, Schwan J, Le AV, Baevova P, Dimitrievska S, Zhao L. Comparative biology of decellularized lung matrix: Implications of species mismatch in regenerative medicine. Biomaterials. 2016;102:220–30. doi: 10.1016/j.biomaterials.2016.06.025. First paper to expolore the cross-species implications of using a non-human scaffold with human cells., which has been a cornerstone of clinical-scale up of engineered lung technology. This paper suggests improved perfomance of human cells on human matrix, compared to pig matrix. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Price AP, Godin LM, Domek A, Cotter T, D’Cunha J, Taylor DA, Panoskaltsis-Mortari A. Automated decellularization of intact, human-sized lungs for tissue engineering. Tissue Eng Part C Methods. 2015;21:94–103. doi: 10.1089/ten.tec.2013.0756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hogan BL, Barkauskas CE, Chapman HA, Epstein JA, Jain R, Hsia CC, Niklason L, Calle E, Le A, Randell SH, Rock J, Snitow M, Krummel M, Stripp BR, Vu T, White ES, Whitsett JA, Morrisey EE. Repair and regeneration of the respiratory system: complexity, plasticity, and mechanisms of lung stem cell function. Cell Stem Cell. 2014;15:123–38. doi: 10.1016/j.stem.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim CF, Jackson EL, Woolfenden AE, Lawrence S, Babar I, Vogel S, Crowley D, Bronson RT, Jacks T. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell. 2005;121:823–35. doi: 10.1016/j.cell.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 19.Rawlins EL, Okubo T, Xue Y, Brass DM, Auten RL, Hasegawa H, Wang F, Hogan BL. The role of Scgb1a1+ Clara cells in the long-term maintenance and repair of lung airway, but not alveolar, epithelium. Cell Stem Cell. 2009;4:525–34. doi: 10.1016/j.stem.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chapman HA, Li X, Alexander JP, Brumwell A, Lorizio W, Tan K, Sonnenberg A, Wei Y, Vu TH. Integrin alpha6beta4 identifies an adult distal lung epithelial population with regenerative potential in mice. J Clin Invest. 2011;121:2855–62. doi: 10.1172/JCI57673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barkauskas CE, Cronce MJ, Rackley CR, Bowie EJ, Keene DR, Stripp BR, Randell SH, Noble PW, Hogan BL. Type 2 alveolar cells are stem cells in adult lung. J Clin Invest. 2013;123:3025–36. doi: 10.1172/JCI68782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wong AP, Bear CE, Chin S, Pasceri P, Thompson TO, Huan L-J, Ratjen F, Ellis J, Rossant J. Directed differentiation of human pluripotent stem cells into mature airway epithelia expressing functional CFTR protein. Nat Biotechnol. 2012:876–82. doi: 10.1038/nbt.2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ghaedi M, Calle EA, Mendez JJ, Gard AL, Balestrini J, Booth A, Bove PF, Gui L, White ES, Niklason LE. Human iPS cell-derived alveolar epithelium repopulates lung extracellular matrix. J Clin Invest. 2013:4950–62. doi: 10.1172/JCI68793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stevens T. Functional and molecular heterogeneity of pulmonary endothelial cells. Proc Am Thorac Soc. 2011;8:453–7. doi: 10.1513/pats.201101-004MW. [DOI] [PubMed] [Google Scholar]
- *25.Rosen C, Shezen E, Aronovich A, Klionsky YZ, Yaakov Y, Assayag M, Biton IE, Tal O, Shakhar G, Ben-Hur H, Shneider D, Vaknin Z, Sadan O, Evron S, Freud E, Shoseyov D, Wilschanski M, Berkman N, Fibbe WE, Hagin D, Hillel-Karniel C, Krentsis IM, Bachar-Lustig E, Reisner Y. Preconditioning allows engraftment of mouse and human embryonic lung cells, enabling lung repair in mice. Nat Med. 2015;21:869–79. doi: 10.1038/nm.3889. Highlights the importance of mixed cell population in generating effective, widespread, and functional lung tissue after injury that leaves bare extracellular matrix. Serves as an animal model to inform ex vivo laboratory efforts to generate lung tissue. [DOI] [PubMed] [Google Scholar]
- 26.Ren X, Moser PT, Gilpin SE, Okamoto T, Wu T, Tapias LF, Mercier FE, Xiong L, Ghawi R, Scadden DT, Mathisen DJ, Ott HC. Engineering pulmonary vasculature in decellularized rat and human lungs. Nat Biotechnol. 2015;33:1097–102. doi: 10.1038/nbt.3354. [DOI] [PubMed] [Google Scholar]
- 27.Bonvillain RW, Scarritt ME, Pashos NC, Mayeux JP, Meshberger CL, Betancourt AM, Sullivan DE, Bunnell BA. Nonhuman primate lung decellularization and recellularization using a specialized large-organ bioreactor. J Vis Exp. 2013:e50825. doi: 10.3791/50825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Charest JM, Okamoto T, Kitano K, Yasuda A, Gilpin SE, Mathisen DJ, Ott HC. Design and validation of a clinical-scale bioreactor for long-term isolated lung culture. Biomaterials. 2015;52:79–87. doi: 10.1016/j.biomaterials.2015.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *29.Raredon MS, Rocco KA, Gheorghe CP, Sivarapatna A, Ghaedi M, Balestrini JL, Raredon TL, Calle EA, Niklason LE. Biomimetic Culture Reactor for Whole-Lung Engineering. Biores Open Access. 2016;5:72–83. doi: 10.1089/biores.2016.0006. This paper focuses on the technical details of a large-animal or human lung bioreactor. This work highlights the requirements and challenges of large bioreactors such as those required for the production of engineered human lungs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yoshida Y, Okano M, Wang S, Kobayashi M, Kawasumi M, Hagiwara H, Mitsumata M. Hemodynamic-force-induced difference of interendothelial junctional complexes. Ann N Y Acad Sci. 1995;748:104–20. doi: 10.1111/j.1749-6632.1994.tb17311.x. discussion 20–1. [DOI] [PubMed] [Google Scholar]
- 31.Uematsu M, Ohara Y, Navas JP, Nishida K, Murphy TJ, Alexander RW, Nerem RM, Harrison DG. Regulation of endothelial cell nitric oxide synthase mRNA expression by shear stress. Am J Physiol. 1995;269:C1371–8. doi: 10.1152/ajpcell.1995.269.6.C1371. [DOI] [PubMed] [Google Scholar]
- 32.Lipowsky HH, Kovalcheck S, Zweifach BW. The distribution of blood rheological parameters in the microvasculature of cat mesentery. Circ Res. 1978;43:738–49. doi: 10.1161/01.res.43.5.738. [DOI] [PubMed] [Google Scholar]
- 33.Parker JC, Stevens T, Randall J, Weber DS, King JA. Hydraulic conductance of pulmonary microvascular and macrovascular endothelial cell monolayers. Am J Physiol Lung Cell Mol Physiol. 2006;291:L30–7. doi: 10.1152/ajplung.00317.2005. [DOI] [PubMed] [Google Scholar]
- 34.Vestweber D. VE-cadherin: the major endothelial adhesion molecule controlling cellular junctions and blood vessel formation. Arterioscler Thromb Vasc Biol. 2008;28:223–32. doi: 10.1161/ATVBAHA.107.158014. [DOI] [PubMed] [Google Scholar]
- 35.Alcorn D, Adamson TM, Lambert TF, Maloney JE, Ritchie BC, Robinson PM. Morphological effects of chronic tracheal ligation and drainage in the fetal lamb lung. J Anat. 1977;123:649–60. [PMC free article] [PubMed] [Google Scholar]
- 36.Harding R. Fetal pulmonary development: the role of respiratory movements. Equine Vet J Suppl. 1997:32–9. doi: 10.1111/j.2042-3306.1997.tb05076.x. [DOI] [PubMed] [Google Scholar]
- 37.Jensen T, Roszell B, Zang F, Girard E, Matson A, Thrall R, Jaworski DM, Hatton C, Weiss DJ, Finck C. A rapid lung de-cellularization protocol supports embryonic stem cell differentiation in vitro and following implantation. Tissue Eng Part C Methods. 2012:632–46. doi: 10.1089/ten.tec.2011.0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang SXL, Islam MN, O'Neill J, Hu Z, Yang Y-G, Chen Y-W, Mumau M, Green MD, Vunjak-Novakovic G, Bhattacharya J, Snoeck H-W. Efficient generation of lung and airway epithelial cells from human pluripotent stem cells. Nat Biotechnol. 2013 doi: 10.1038/nbt.2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chaney J, Suzuki Y, Cantu E, 3rd, van Berkel V. Lung donor selection criteria. J Thorac Dis. 2014;6:1032–8. doi: 10.3978/j.issn.2072-1439.2014.03.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wagner DE, Bonenfant NR, Sokocevic D, Desarno MJ, Borg ZD, Parsons CS, Brooks EM, Platz JJ, Khalpey ZI, Hoganson DM, Deng B, Lam YW, Oldinski RA, Ashikaga T, Weiss DJ. Three-dimensional scaffolds of acellular human and porcine lungs for high throughput studies of lung disease and regeneration. Biomaterials. 2014 doi: 10.1016/j.biomaterials.2013.11.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *41.Ren X, Moser PT, Gilpin SE, Okamoto T, Wu T, Tapias LF, Mercier FE, Xiong L, Ghawi R, Scadden DT, Mathisen DJ, Ott HC. Engineering pulmonary vasculature in decellularized rat and human lungs. Nat Biotechnol. 2015 doi: 10.1038/nbt.3354. Most successful engineered lung tissue reported to date, based on barrier function and distribution of endothelilal cells throughout the lung scaffold. Highlights the importance of cells of mesenchymal lineage in supporting endothelial cell barrier function. [DOI] [PubMed] [Google Scholar]
- 42.Dimitrievska S, Gui L, Weyers A, Lin T, Cai C, Woo W, Tuggle CT, Sundaram S, Balestrini JL, Slattery D, Tchouta L, Kyriakides TR, Tarbell JM, Linhardt RJ, Niklason LE. New Functional Tools for Antithrombogenic Activity Assessment of Live Surface Glycocalyx. Arterioscler Thromb Vasc Biol. 2016 doi: 10.1161/ATVBAHA.116.308023. This work presents a novel technique for evaluating the interaction of engineered tissue surfaces with the coagulation cascade. Work such as this and future means of evaluating this aspect of engieered tissues will be highly valuable as pre-implantation metrics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Song JJ, Kim SS, Liu Z, Madsen JC, Mathisen DJ, Vacanti JP, Ott HC. Enhanced in vivo function of bioartificial lungs in rats. Ann Thorac Surg. 2011;92:998–1005. doi: 10.1016/j.athoracsur.2011.05.018. discussion -6. [DOI] [PubMed] [Google Scholar]
- 44.Colucci G, Domenighetti G, Della Bruna R, Bonilla J, Limoni C, Matthay MA, Martin TR. Comparison of two non-bronchoscopic methods for evaluating inflammation in patients with acute hypoxaemic respiratory failure. Crit Care. 2009;13:R134. doi: 10.1186/cc7995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fein A, Grossman RF, Jones JG, Overland E, Pitts L, Murray JF, Staub NC. The value of edema fluid protein measurement in patients with pulmonary edema. Am J Med. 1979;67:32–8. doi: 10.1016/0002-9343(79)90066-4. [DOI] [PubMed] [Google Scholar]
- 46.Carlson RW, Schaeffer RC, Jr, Carpio M, Weil MH. Edema fluid and coagulation changes during fulminant pulmonary edema. Chest. 1981;79:43–9. doi: 10.1378/chest.79.1.43. [DOI] [PubMed] [Google Scholar]
- 47.Tyndall A. Mesenchymal stem cell treatments in rheumatology[mdash]a glass half full? Nature Reviews Rheumatology: Nature Research. 2014:117–24. doi: 10.1038/nrrheum.2013.166. [DOI] [PubMed] [Google Scholar]
- 48.Nelson K, Bobba C, Ghadiali S, Hayes D, Jr, Black SM, Whitson BA. Animal models of ex vivo lung perfusion as a platform for transplantation research. World journal of experimental medicine. 2014;4:7. doi: 10.5493/wjem.v4.i2.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nichols JE, Niles J, Riddle M, Vargas G, Schilagard T, Ma L, Edward K, La Francesca S, Sakamoto J, Vega S. Production and assessment of decellularized pig and human lung scaffolds. Tissue Engineering Part A. 2013;19:2045–62. doi: 10.1089/ten.tea.2012.0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.O’Neill JD, Anfang R, Anandappa A, Costa J, Javidfar J, Wobma HM, Singh G, Freytes DO, Bacchetta MD, Sonett JR. Decellularization of human and porcine lung tissues for pulmonary tissue engineering. The Annals of thoracic surgery. 2013;96:1046–56. doi: 10.1016/j.athoracsur.2013.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Price AP, Godin LM, Domek A, Cotter T, D’Cunha J, Taylor DA, Panoskaltsis-Mortari A. Automated decellularization of intact, human-sized lungs for tissue engineering. Tissue Engineering Part C: Methods. 2014;21:94–103. doi: 10.1089/ten.tec.2013.0756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rogers CS, Hao Y, Rokhlina T, Samuel M, Stoltz DA, Li Y, Petroff E, Vermeer DW, Kabel AC, Yan Z. Production of CFTR-null and CFTR-ΔF508 heterozygous pigs by adeno-associated virus–mediated gene targeting and somatic cell nuclear transfer. The Journal of clinical investigation. 2008;118:1571–7. doi: 10.1172/JCI34773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rogers CS, Stoltz DA, Meyerholz DK, Ostedgaard LS, Rokhlina T, Taft PJ, Rogan MP, Pezzulo AA, Karp PH, Itani OA. Disruption of the CFTR gene produces a model of cystic fibrosis in newborn pigs. Science. 2008;321:1837–41. doi: 10.1126/science.1163600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Matute-Bello G, Frevert CW, Martin TR. Animal models of acute lung injury. American Journal of Physiology-Lung Cellular and Molecular Physiology. 2008;295:L379–L99. doi: 10.1152/ajplung.00010.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wright JL, Cosio M, Churg A. Animal models of chronic obstructive pulmonary disease. american journal of physiology-lung cellular and molecular physiology. 2008;295:L1–L15. doi: 10.1152/ajplung.90200.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *56.Hoechter DJ, von Dossow V. Lung transplantation: from the procedure to managing patients with lung transplantation. Current Opinion in Anesthesiology. 2016;29:8–13. doi: 10.1097/ACO.0000000000000268. This work highlights the importance of expert perioperative management of transplanted lungs in order to attenuate primary graft failure and improve outcomes. Advances in clinical management and increased familiarity with “atypical” tranpslanted lungs such as the ex vivo perfused lungs (as well as engineered lungs) will be essential to the support of these technologies in the clinic. [DOI] [PubMed] [Google Scholar]
- 57.Ikpa PT, Bijvelds MJ, de Jonge HR. Cystic fibrosis: toward personalized therapies. The international journal of biochemistry & cell biology. 2014;52:192–200. doi: 10.1016/j.biocel.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 58.Weiss DJ. Concise review: current status of stem cells and regenerative medicine in lung biology and diseases. Stem Cells. 2014;32:16–25. doi: 10.1002/stem.1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hogan BL, Barkauskas CE, Chapman HA, Epstein JA, Jain R, Hsia CC, Niklason L, Calle E, Le A, Randell SH. Repair and regeneration of the respiratory system: complexity, plasticity, and mechanisms of lung stem cell function. Cell stem cell. 2014;15:123–38. doi: 10.1016/j.stem.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cunningham JJ, Ulbright TM, Pera MF, Looijenga LH. Lessons from human teratomas to guide development of safe stem cell therapies. Nature biotechnology. 2012;30:849–57. doi: 10.1038/nbt.2329. [DOI] [PubMed] [Google Scholar]
- 61.Zhang WY, de Almeida PE, Wu JC. Teratoma formation: A tool for monitoring pluripotency in stem cell research. 2012 [PubMed] [Google Scholar]