Abstract
A 60-year-old man was prescribed oral desmopressin (1-deamino-8-D-arginine vasopressin acetate trihydrate; DDAVP) for nocturnal polyuria. One week after starting to take desmopressin, he frequently felt chest pain while resting. Coronary angiography revealed no organic stenosis; however, an acetylcholine provocation test showed severe coronary spasm with ST elevation. He was diagnosed with coronary spastic angina, and we stopped the oral desmopressin and added diltiazem. While DDAVP should dilate the coronary vessels in healthy subjects, it may provoke coronary vasospasm in patients with endothelial dysfunction. We should be careful to avoid triggering coronary spasm when administering DDAVP to patients that may have potential endothelial dysfunction.
Keywords: coronary spastic angina, desmopressin, DDAVP
Introduction
Arginine vasopressin (AVP) is known to induce both systemic and coronary vasoconstriction mainly via the vasopressin V1 receptor (1-3). A previous study showed that vasopressin causes decreased coronary flow via the constriction of small coronary arteries without large-sized focal spasm (4). Desmopressin (1-deamino-8-D-arginine vasopressin acetate trihydrate; DDAVP) is a synthetic analogue of arginine vasopressin used to treat central diabetes insipidus (5) or nocturnal polyuria (6) as a selective agonist for the vasopressin V2 receptor. Although DDAVP is an agonist for the vasopressin receptor, it has been considered that DDAVP does not induce coronary spasm (vasoconstriction), given its selectivity for the vasopressin V2 receptor. We report a case of coronary spastic angina induced after oral DDAVP administration.
Case Report
A 60-year-old man was admitted to our hospital complaining of chest pain at rest. Ten days prior to this admission, he was prescribed oral desmopressin acetate hydrate (MINIRINMELT OD TabletⓇ 120 μg/day before sleeping) by a urologist at a nearby hospital for the treatment of nocturnal polyuria. After starting the administration of oral desmopressin, chest pain at rest frequently occurred, especially at night. On the day of hospital admission, he felt chest oppression at rest in succession at 2 AM, 5 AM, 6 AM, and 8 AM, and these episodes were relieved spontaneously or after sublingual administration of nitroglycerin. The patient then visited our hospital and was admitted emergently. An initial electrocardiogram (ECG) showed no significant ST-T changes (Fig. 1), the findings for troponin-T were negative (troponin-I level was 6.5 pg/mL), and transthoracic echocardiography showed no segmental asynergy at rest. His low-density cholesterol level (LDL-C) was 80.8 mg/dL.
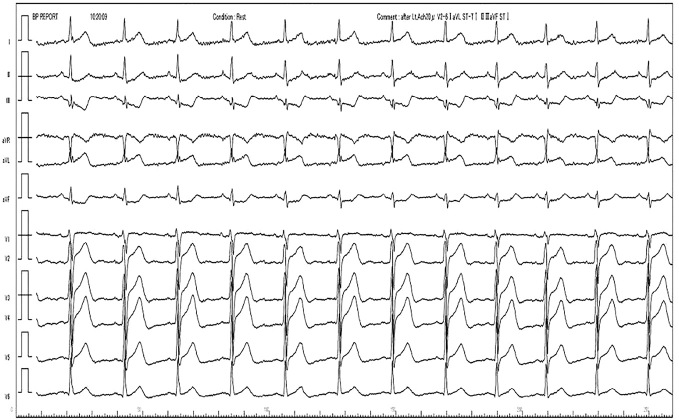
Figure 1.
ECG on admission. ECG on admission showed sinus rhythm (heart rate=76 beats per minute) without any significant ST-T changes.
The patient was an ex-smoker and had a history of pulmonary adenocarcinoma which was cured by lung lobectomy one year prior to the admission. He also had a history of unstable angina three years prior to this admission and received percutaneous coronary intervention to the left circumflex artery with a drug-eluting stent. He had hypertension, dyslipidemia, and a smoking habit as coronary risk factors. He had been prescribed aspirin, statins, and a calcium channel blocker (amlodipine besilate 2.5 mg/day) before the admission.
On Day 2, we underwent coronary angiography, which revealed no organic stenosis, including in-stent restenosis. We performed the acetylcholine provocation test to investigate whether or not his chest pain was due to coronary spasm. Despite the continuous administration of an oral calcium channel blocker during the test, the administration of only 20 μg of acetylcholine chloride into the left coronary artery induced excessive spasm (Fig. 2) and total occlusion of the left anterior descending artery, with ST segment elevation in leads I, aVL, and V2-6 accompanied by reciprocal changes in leads II, III, and aVF (Fig. 3). The intracoronary administration of nitroglycerin and nicorandil relieved the severe coronary spasm. He was diagnosed with coronary spastic angina (CSA), and we stopped the oral DDAVP and added diltiazem (200 mg/day). After that, he did not complain of any chest pain and was discharged on Day 6. He has been followed up without any recurrence of coronary artery spasm for 10 months.
Figure 2.
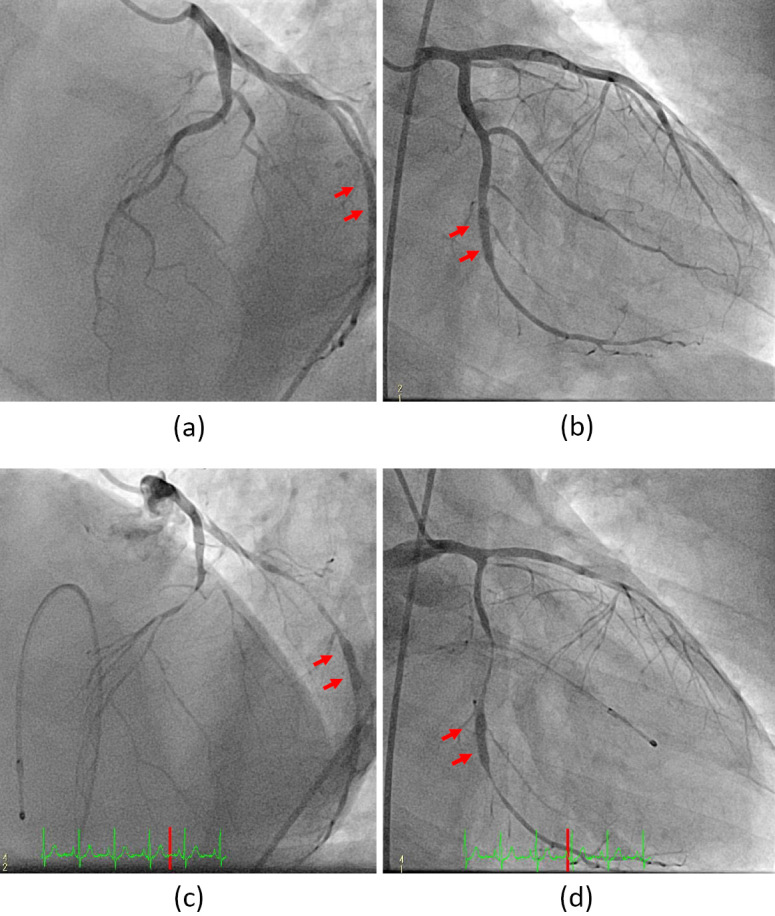
Coronary angiography and the acetylcholine provocation test. (a) The left cranial view of the left coronary artery. (b) The right caudal view of the left coronary artery. There was no organic stenosis, including in-stent restenosis. (c) The left cranial view of the left coronary artery after the administration of acetylcholine chloride. (d) The right caudal view of the left coronary artery after the administration of acetylcholine chloride. Excessive coronary spasm was induced only by 20 µg administration of acetylcholine chloride. The drug-eluting stent previously implanted in the left circumflex artery is indicated by arrows.
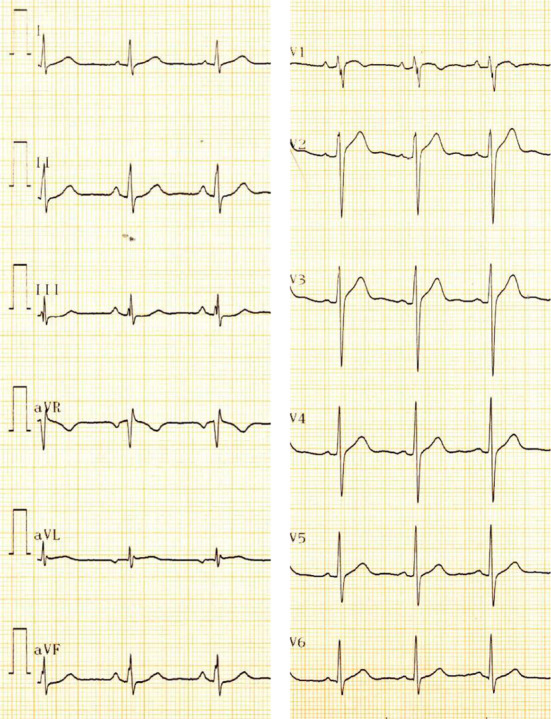
Figure 3.
ECG during acetylcholine provocation test. ST segment elevation was observed in leads I, aVL, and V2-6 accompanied by reciprocal changes in leads II, III, and aVF.
Discussion
Nocturnal polyuria, defined as waking at night to pass urine (7), can occur at any age in any population, although the prevalence of the condition increases with age (8). DDAVP, a synthetic analogue of arginine vasopressin, relieves nocturnal polyuria by decreasing night-time urine production (6). DDAVP is a selective agonist for the vasopressin V2 receptor, which is one of three subtypes of vasopressin receptors. V1 receptors are found on the vascular smooth muscle of the systemic, splanchnic, renal, and coronary circulation systems. V1 receptors activate phospholipase C, which leads to an increase in intracellular calcium and vasoconstriction (9). V2 receptors are Gs protein-linked receptors located mainly on the renal basolateral membrane of the distal tubal and collecting ducts and activate adenylyl cyclase to increase the production of cyclic adenosine monophosphate (cAMP) (9). V3 receptors are found mainly in the pituitary gland and are thought to be involved in adrenocorticotropic hormone release (9).
The intra-arterial infusion of DDAVP, a selective V2 receptor agonist, was reported to cause a dose-dependent increase in the forearm blood flow (10). Several studies have also suggested the existence of extrarenal V2 receptors that may mediate a vasodilatory effect of AVP (10-13). Another study revealed that AVP caused endothelium-dependent relaxation of the canine basilar and coronary artery, whereas the removal of the endothelium caused basilar artery contraction by AVP (14). Endothelial NO synthase (eNOS) is known to be activated by several agonists, including acetylcholine, via an increase in the intracellular free calcium level and the binding of calmodulin to eNOS (15, 16). Furthermore, cAMP-increasing agents lead to an increase in eNOS activity, and protein kinase A (PKA) induces eNOS phosphorylation in cultured endothelial cells (17-19). DDAVP and other cAMP-increasing agents can activate eNOS in human endothelial cells, which accounts for DDAVP-induced vasodilation (20).
Patients with coronary spasm are known to have endothelial dysfunction of the coronary arteries (21, 22). Acetylcholine dilates the blood vessels via eNOS when the vascular endothelium is normal and contracts the blood vessels if there is endothelial detachment or injury (23). A similar phenomenon is supposed to happen when DDAVP is used for patients who have endothelial dysfunction. DDAVP induces vasodilatory effects for normal endothelium vessels but may cause vasoconstrictive effects in vessels with an endothelial disorder, especially under the acetylcholine provocation test. Since a smoking history (24) and the previous implantation of a drug-eluting stent (25) are closely associated with the endothelial dysfunction, DDAVP might provoke coronary vasoconstriction in the patient.
A few previous case reports have suggested the association of desmopressin with coronary spasm (26, 27). Angelini et al. reported typical Prinzmetal angina in a 50-year-old man receiving desmopressin for a pituitary insufficiency (26). Alcalde et al. reported a 64-year-old woman with recurrent severe acute pulmonary edema, which was induced by coronary artery spasm while receiving desmopressin for hypopituitarism (27). However, these previous studies did not mention the date when desmopressin was started and the date when symptoms were first noted. Therefore, no direct relationship between desmopressin and coronary spasm has yet been clearly demonstrated.
Our report suggests that DDAVP may provoke vasospasm of the coronary arteries via V2 receptors, which can be visualized by coronary angiography.
We reported a case of CSA induced by oral DDAVP administration. While DDAVP should dilate coronary vessels in healthy subjects, DDAVP may provoke coronary vasospasm in patients with endothelial dysfunction. This case suggests that the mechanism of DDAVP in endothelial dysfunction is at least partially responsible for the myocardial ischemia caused by vasopressin. Further, in clinical settings, we should be alert for the emergence of coronary spasm when administering DDAVP to patients that may have endothelial dysfunction.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Nakano J. Studies on the cardiovascular effects of synthetic vasopressin. J Pharmacol Exp Ther 157: 19-31, 1967. [PubMed] [Google Scholar]
- 2. Fisher CH, Sheff RN, Novak G, White RI Jr. The effect of superior mesenteric artery vasopressin infusions on cardiac output and coronary blood flow in dogs. Invest Radiol 9: 456-461, 1974. [DOI] [PubMed] [Google Scholar]
- 3. Heyndrickx GR, Boettcher DH, Vatner SF. Effects of angiotensin, vasopressin, and methoxamine on cardiac function and blood flow distribution in conscious dogs. Am J Physiol 231(5 Pt. 1): 1579-1587, 1976. [DOI] [PubMed] [Google Scholar]
- 4. Maturi MF, Martin SE, Markle D, et al. Coronary vasoconstriction induced by vasopressin. Production of myocardial ischemia in dogs by constriction of nondiseased small vessels. Circulation 83: 2111-2121, 1991. [DOI] [PubMed] [Google Scholar]
- 5. Robinson AG. DDAVP in the treatment of central diabetes insipidus. N Engl J Med 294: 507-511, 1976. [DOI] [PubMed] [Google Scholar]
- 6. Weiss JP, Zinner NR, Klein BM, Norgaard JP. Desmopressin orally disintegrating tablet effectively reduces nocturia: results of a randomized, double-blind, placebo-controlled trial. Neurourol Urodyn 31: 441-447, 2012. [DOI] [PubMed] [Google Scholar]
- 7. van Kerrebroeck P, Weiss J. Standardization and terminology of nocturia. BJU Int 84 (Suppl 1): 1-4, 1999. [DOI] [PubMed] [Google Scholar]
- 8. Jackson S. Lower urinary tract symptoms and nocturia in men and women: prevalence, aetiology and diagnosis. BJU Int 84 (Suppl 1): 5-8, 1999. [DOI] [PubMed] [Google Scholar]
- 9. Kam PC, Williams S, Yoong FF. Vasopressin and terlipressin: pharmacology and its clinical relevance. Anaesthesia 59: 993-1001, 2004. [DOI] [PubMed] [Google Scholar]
- 10. Hirsch AT, Dzau VJ, Majzoub JA, Creager MA. Vasopressin-mediated forearm vasodilation in normal humans. Evidence for a vascular vasopressin V2 receptor. J Clin Invest 84: 418-426, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liard JF. Cardiovascular effects associated with antidiuretic activity of vasopressin after blockade of its vasoconstrictor action in dehydrated dogs. Circ Res 58: 631-640, 1986. [DOI] [PubMed] [Google Scholar]
- 12. Walker BR. Evidence for a vasodilatory effect of vasopressin in the conscious rat. Am J Physiol 251(1 Pt 2): H34-H39, 1986. [DOI] [PubMed] [Google Scholar]
- 13. Bichet DG, Razi M, Lonergan M, et al. Hemodynamic and coagulation responses to 1-desamino[8-D-arginine] vasopressin in patients with congenital nephrogenic diabetes insipidus. N Engl J Med 318: 881-887, 1988. [DOI] [PubMed] [Google Scholar]
- 14. Katusic ZS, Shepherd JT, Vanhoutte PM. Vasopressin causes endothelium-dependent relaxations of the canine basilar artery. Circ Res 55: 575-579, 1984. [DOI] [PubMed] [Google Scholar]
- 15. Pollock JS, Forstermann U, Mitchell JA, et al. Purification and characterization of particulate endothelium-derived relaxing factor synthase from cultured and native bovine aortic endothelial cells. Proc Natl Acad Sci U S A 88: 10480-10484, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Busse R, Mulsch A. Calcium-dependent nitric oxide synthesis in endothelial cytosol is mediated by calmodulin. FEBS Lett 265: 133-136, 1990. [DOI] [PubMed] [Google Scholar]
- 17. Butt E, Bernhardt M, Smolenski A, et al. Endothelial nitric-oxide synthase (type III) is activated and becomes calcium independent upon phosphorylation by cyclic nucleotide-dependent protein kinases. J Biol Chem 275: 5179-5187, 2000. [DOI] [PubMed] [Google Scholar]
- 18. Ferro A, Queen LR, Priest RM, et al. Activation of nitric oxide synthase by beta 2-adrenoceptors in human umbilical vein endothelium in vitro. Br J Pharmacol 126: 1872-1880, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Boo YC, Sorescu G, Boyd N, et al. Shear stress stimulates phosphorylation of endothelial nitric-oxide synthase at Ser1179 by Akt-independent mechanisms: role of protein kinase A. J Biol Chem 277: 3388-3396, 2002. [DOI] [PubMed] [Google Scholar]
- 20. Kaufmann JE, Iezzi M, Vischer UM. Desmopressin (DDAVP) induces NO production in human endothelial cells via V2 receptor- and cAMP-mediated signaling. J Thromb Haemost 1: 821-828, 2003. [DOI] [PubMed] [Google Scholar]
- 21. Kugiyama K, Murohara T, Yasue H, et al. Increased constrictor response to acetylcholine of the isolated coronary arteries from patients with variant angina. Int J Cardiol 52: 223-233, 1995. [DOI] [PubMed] [Google Scholar]
- 22. Kawano H, Ogawa H. Endothelial function and coronary spastic angina. Intern Med 44: 91-99, 2005. [DOI] [PubMed] [Google Scholar]
- 23. JCS Joint Working Group Guidelines for diagnosis and treatment of patients with vasospastic angina (Coronary Spastic Angina) (JCS 2013). Circ J 78: 2779-2801, 2014. [DOI] [PubMed] [Google Scholar]
- 24. Celermajer DS, Adams MR, Clarkson P, et al. Passive smoking and impaired endothelium-dependent arterial dilatation in healthy young adults. N Engl J Med 334: 150-154, 1996. [DOI] [PubMed] [Google Scholar]
- 25. Luscher TF, Steffel J, Eberli FR, et al. Drug-eluting stent and coronary thrombosis: biological mechanisms and clinical implications. Circulation 115: 1051-1058, 2007. [DOI] [PubMed] [Google Scholar]
- 26. Angelini P, Uribe C, Lozano P. Differential local spasticity in myocardial bridges. Tex Heart Inst J 39: 384-388, 2012. [PMC free article] [PubMed] [Google Scholar]
- 27. Alcalde O, Domingo E, Figueras J. Recurrent severe acute pulmonary edema caused by transient left ventricular insufficiency with mitral regurgitation related to severe coronary artery spasm. Circ Heart Fail 3: 332-335, 2010. [DOI] [PubMed] [Google Scholar]