The United States is experiencing an epidemic in obesity with an increase in childhood obesity and type 2 diabetes1. Understanding and promoting healthy lifestyles is a first step towards combating this epidemic, however, lifestyle changes are inherently difficult to achieve; highlighting the significance of understanding the neurochemistry of energy homeostasis; the balance between energy intake and expenditure. Several peptide neuromodulators modulate energy intake that in turn influence energy expenditure via downstream anabolic and catabolic responses.2 Energy expended on thermogenesis through well-defined neural circuits also contributes to overall energy balance.3 Despite basic understanding of pathways and modulators limited means exist to effect therapeutic changes in adiposity or metabolic rate other than life style changes. Here we review evidence that neural signaling metabolites may modulate the gain in thermoregulatory pathways during hibernation and torpor and offer novel insights into means to fine tune energy use. We extend prior reviews on mechanisms of metabolic suppression in hibernation4–10 by focusing primarily on neural signaling metabolites.
Hibernation is an extreme example of energy conservation that offers a system in which to explore modulatory processes fundamental to the regulation of energy balance. Here we give a general overview of hibernation and discuss evidence that in hibernation the gain in thermoregulatory and possibly adiposity signaling is modulated, in part, by neural signaling metabolites. Specifically we discuss a role for metabolite signaling molecules, adenosine, 5′-AMP and glutamate in the onset and emergence from hibernation.
Introduction to hibernation and torpor – A means of energy conservation
Hibernation is a means of energy conservation that is driven by a change in thermoregulation.11, 12 Field and laboratory studies suggest that pre-hibernation energy conservation begins with a gradual decrease in body temperature (Tb) 13–16 presumably via a decrease in thermogenesis. In the arctic ground squirrel the pre-hibernation decrease in Tb begins prior to a decline in food intake14 suggesting that decreased energy expenditure leads to a decrease in food intake. Hibernation conserves energy to such an extent that animals survive without food or water for 5–8 months despite ambient temperatures that can fall to −20°C.17, 18 Over the course of the hibernation season, the majority of energy is conserved during prolonged bouts of torpor where metabolic rate (MR) decreases to as low as 1–2% of basal metabolic rate (BMR),19, 20 and body temperature falls to within 1–2°C above ambient temperature (Ta).20 Studies in arctic ground squirrels suggest that pre-hibernation remodeling involves a gain in the sensitivity of A1 adenosine receptors (A1AR) that eventually leads to the onset of prolonged torpor.14, 21 Torpor onset is identified by an initial decrease in the rate of oxygen consumption (VO2) and a subsequent decrease in core body temperature (Tb). A single bout of torpor consists of three phases; onset, maintenance and arousal.
Several diverse species of mammals hibernate and many other species show short (less than 24h) bouts of daily torpor. Daily torpor can be classified as shallow torpor in contrast to deep torpor or hibernation.23 Daily torpor is not hibernation, but aspects of energy conservation are similar in daily torpor and hibernation and prolonged periods of lowered Tb and metabolic rate during hibernation may be called torpor, as is done in this review. Hibernation is distinguished from daily torpor by the prolonged length of the torpor bout which can last up to 2–3 weeks in some species of ground squirrels, or just a few days in hamsters. Brief (10–20h) interbout arousals interrupt prolonged torpor with predictable regularity and consume significant amounts of energy.22 Hibernation can be described as obligate or facultative. Most typically, ground squirrels are obligate hibernators while hamsters are facultative hibernators. Obligate hibernators, hibernate according to an endogenous circannual rhythm. Facultative hibernators hibernate in response to shortening day length and decreases in food or water availability. Daily torpor occurs during acute periods of food or water deprivation. Daily torpor differs from hibernation in limited duration, by less extreme decreases in MR and Tb, and by more immediate expression in response to environmental challenge23. Both hibernation and daily torpor represent regulated shut down of energy consumption and are not pathological consequence of starvation24.
Several classes of molecules have been investigated as modulators or mediators of hibernation and torpor. These include neuropeptides, nutrients or molecules that block nutrient use such as 2-deoxyglucose, neurotransmitters such as 5HT and histamine and metabolite/neuromodulators such as 5′-AMP and adenosine (reviewed in Drew et al25). The orexigenic neuropeptide NPY and the metabolites AMP and adenosine have been studied more extensively than other signaling molecules. We review briefly evidence supporting a role for these molecules. We also acknowledge that comprehensive confirmation that these putative endogenous mediators are necessary, sufficient and present in the extracellular compartment in brain during onset of torpor is lacking.
Putative endogenous mediators of torpor
Neuropeptides
Although not a neural signaling metabolite amongst neuropeptides NPY shows the greatest promise as an endogenous regulator of torpor. Injection of NPY into the third ventricle induces torpor-like hypothermia which is reversed by a NPY Y1 receptor antagonist and, like torpor, depends on photoperiod.26 Ablation of the arcuate nucleus abolishes torpor in Siberian hamsters and fasting-induced torpor in mice.27 Ghrelin, a gut appetite hormone and neuropeptide activates NPY neurons in the arcuate nucleus and deepens torpor in mice.28 These studies support the hypothesis that NPY Y1 receptor sites activated by NPY within the arcuate nucleus induce a torpor-like state through modulation of thermoregulation.26, 27, 29 The efficacious orexigenic effect of NPY, however, is difficult to reconcile within the context of hibernation because onset of hibernation is preceded by a hypophagic period.30 Indeed, TRH is the only member of the group of neuropeptides, well-known to regulate food intake that is upregulated in the hypothalamus during pre-hibernation satiety30. TRH expression during the hypophagic period fits previous findings that TRH is anorexigenic31. Nonetheless, TRH reverses hibernation in Syrian hamsters32 decreases sleep33, 34 and increases body temperature.35 Reversal of spontaneous torpor with a NPY Y1 receptor antagonist has, to our knowledge, not been tested or reported.
Adenosine
Adenosine is unique amongst the putative mediators of torpor because it affects sleep, appetite and body temperature in a manner consistent with hibernation and torpor. Indeed, adenosine promotes sleep,36 lowers body temperature37, and reduces food intake.38 Moreover, stimulation of A1AR induces torpor in hibernating species most likely through an inhibition of thermogenesis and modulation of parasympathetic tone37, 39. Spontaneous torpor is reversed by A1AR antagonists in Syrian hamsters, arctic ground squirrels and fasting-induced torpor in mice.32, 40–42
In facultative hibernators, such as the Syrian hamster (Mesocricetus auratus), A1AR dependent signaling within the CNS is necessary for torpor onset. This claim is based on evidence that the A1AR antagonist cyclopentyltheophylline (CPT) reverses spontaneous torpor when administered into the lateral ventricle in Syrian hamsters during torpor onset.42 Interestingly, CPT has no effect when delivered later in the torpor bout demonstrating that mechanisms controlling onset, maintenance and interbout arousal are mediated by distinct mechanisms. While blocking A1AR reverses torpor, CHA, an A1AR agonist, administered into the lateral ventricle of Syrian hamsters, induces a torpor-like decline in Tb at a rate similar to the rate of fall in Tb during spontaneous torpor.32, 42
In the hibernating arctic ground squirrel (AGS; Urocitellus parryii), like in hamsters, evidence suggests that torpor onset is regulated in part within the CNS by A1AR signaling. In these obligate hibernators the efficacy of the A1AR agonist 6N cyclohexyladenosine (CHA) depends on a seasonal change in sensitivity (Jinka et al., 2011). We observed that CHA injected ip or delivered via intracerebroventricular (icv) administration into the lateral ventricle induces torpor in AGS in winter, but not in summer. Moreover, we observed that CHA-induced torpor is similar to spontaneous torpor with regard to the magnitude and temporal profile of both the decrease in oxygen consumption and Tb, and that the A1AR antagonist cyclopentyl theophylline (CPT) reverses naturally occurring torpor. We then found that A1, but not A2 or A3 adenosine receptor stimulation is necessary to support spontaneous torpor.40 Circannual changes in sleep43 and euthermic Tb14 and sensitivity to CHA suggests that an increase in the gain in A1AR mediated signaling within sleep and thermoregulatory pathways underlies pre-hibernation remodeling of sleep and thermogenesis.14 Tb decreases by 1 to 2°C before animals hibernate and this gradual modification of thermogenesis and metabolism predicts onset of hibernation.14–16 Remodeling of thermoregulation and metabolism is postulated to result from changes within the hypothalamus44 as well as from altered sensitivity of A1AR.14 Increased sleep drive and lower Tb in winter ground squirrels that are not hibernating when compared to summer ground squirrels is consistent with increased sensitivity of A1AR.43
Although the house mouse (Mus Musculus) does not hibernate many strains show daily torpor in response to fasting. C57BL mice display daily torpor within 6–7h when food is withheld during the dark period (when mice eat). During onset of this fasting-induced daily torpor MR falls prior to a fall in Tb in a manner similar to what is observed in hibernation. The slow rate of cooling during onset of hibernation and daily torpor compared to a faster rate of cooling during hypothermia suggests that the rate of cooling distinguishes torpor from hypothermia. Daily torpor in C57BL mice also depends on central A1AR activation since, like hibernation in ground squirrels and hamsters, the onset of daily torpor is reversed by CNS administration of an A1AR antagonist.41
The requirement of CNS A1AR in daily torpor emphasizes a common mechanism despite several differences between daily (shallow) torpor and hibernation (deep torpor). During daily torpor Tb falls from around 37°C to between 15–25°C. By contrast, during hibernation Tb may decrease to as cold as −3°C 23 depending on the species and the ambient temperature.18 In one study, metabolic rate (MR) during daily torpor in Mus musculus, weighing 45g, decreased from a basal MR (BMR) of about 1.5mLg−1h−1 to a torpid MR (TMR) of 0.3mLg−1h−1. In these same animals Tb decreased from 37 to 19°C. By contrast MR during hibernation in a Western Jumping Mouse (Zapus princeps) weighing 33g decreased from a BMR of 1.5 mLg−1h−1 to a TMR of 0.04 mLg−1h−1 and Tb decreased from 37°C to 5°C23 (Table 1). Some of the differences in torpid metabolic rates observed during daily (shallow) torpor and hibernation (deep torpor) may be due to colder ambient temperatures and colder torpid Tb in hibernating species. Mus musculus does not tolerate ambient temperatures below about 15°C. Consequently study of daily torpor in this species is usually at ambient temperatures of 20–21°C while study of hibernation is usually at ambient temperatures of 2–5°C.
Table 1.
| Species | Metabolic Phenotype | Body Weight (g) | BMR (mLg−1h−1) | Torpid MR (mLg−1h−1) | Euthermic Tb (°C) | Minimum Tb (°C) |
|---|---|---|---|---|---|---|
| House Mouse (Mus musculus) | Daily torpor | 45 | 1.5 | 0.3 | 37 | 19 |
| Western Jumping Mouse (Zapus princeps) | Hibernation | 33 | 1.5 | 0.04 | 37 | 5 |
The laboratory rat (Rattus norvegicus), a non-hibernating species, does not show spontaneous torpor and for this reason is an ideal rodent species to investigate if mechanisms involved in daily torpor or hibernation have potential to translate to humans. Adenosine agonists promote sleep in rats and other nonhibernating species, including humans. 36, 45. The A1AR agonist CHA inhibits shivering and nonshivering thermogenesis in rats and increases parasympathetic tone to the heart, both physiological characteristics of hibernation.37 A number of studies of the effects of A1AR agonists and purine nucleotides in rats suggest that this mechanism will translate to non-hibernating species. Moreover, small seasonal fluctuations in metabolic rate have been observed in humans and it is tempting to speculate that A1AR mechanisms are involved.
As discussed above, the onset of hibernation is anticipated by a decrease in basal metabolic rate and food intake.14, 46 Gradual changes in metabolism are seasonally dependent and proposed to be controlled by the CNS via molecular mechanisms not fully understood14. These mechanisms appear to be present and operative in non-hibernating mammals. It has been shown that during the winter season humans acclimate to colder temperature, even in mild climates.47 The acclimation is independent from body mass and body composition, suggesting autonomic and endocrine changes as potential mechanisms (Ooijen et al. 2004).47 Season is also known to affect weight and appetite as well as social and physical activity, which are associated with higher risk of obesity leading to metabolic syndrome.48 Even if the effects of season are less dramatic in humans than in hibernating mammals, seasonality plays a role in physiological changes and in gene expression of non-hibernating mammals.49. In obligate hibernators season drives remodeling of mechanisms that preserve energy balance. Similar pre-hibernation mechanisms are conserved in facultative hibernators16. Less is known about fasting-induced torpor. Understanding mechanisms of remodeling, which are proposed to underlie onset of hibernation, and potentially daily torpor, is likely to unveil novel mechanisms that affect energy expenditure via modulation of known thermoregulatory neural pathways. The ability to modulate these mechanisms may enable means to regulate metabolism in a manner that reduces morbidity associated with metabolic syndrome and type 2 diabetes.
Adenosine A1AR mediate onset of hibernation through an inhibition of thermogenesis
Onset of torpor involves a suite of coordinated physiological processes; one of which is a decrease in Tb setpoint50 that can be viewed mechanistically as an inhibition of thermogenesis. Because metabolic rate is influenced by an individual’s thermoneutral zone, and thus energy expended for thermogenesis, study of hibernation presents an opportunity to reveal novel means to modulate the gain in thermoregulatory pathways. Understanding how thermogenesis is altered in hibernation may provide novel insights into treatments for obesity. By reversing mechanisms that downregulate thermogenesis and energy expenditure in hibernation we may be able to modulate the gain in thermogenesis to promote weight loss. Although large increases in thermogenesis would require large changes in heat dissipation, moderate increases in thermogenesis could be offset in cooler climates by lighter weight clothing and lower ambient temperatures.
A1AR modulates thermogenesis, one of the most energy demanding requirements of homeothermy, in part through effects on the nucleus of the solitary tract (nTS). Stimulating A1AR within the nTS37, 51 inhibits shivering and nonshivering thermogenesis. Moreover evidence suggests that sensory processing is modified within the nTS in Syrian hamster as brain temperature declines52. Inhibition of thermogenesis during onset of hibernation decreases oxygen consumption and this immediate energy savings is followed by a subsequent decline in Tb. The preoptic area of the hypothalamus (POAH), the thermosensitive region of the CNS, is another potential site where A1AR may modulate thermogenesis.50, 53 The POAH has been proposed as the main integrator of sleep and thermoregulatory information which is modulated by A1AR.53–56 Alternative means of inhibiting thermogenesis is also sufficient to induce deep hypothermia, or suspended animation in rats57, 58, but these mechanisms have not been implicated as endogenous drivers of hibernation.
Is adenosine the endogenous signaling molecule?
Adenosine is a likely candidate as an endogenous A1AR agonist that is capable of driving the onset of torpor based on the purine’s role in sleep45, thermoregulation39, 53 and energy homeostasis59. Adenosine is a neuroprotective neuromodulator and by-product of cellular metabolism that is positioned within neurons, astrocytes and peripheral tissues to modulate brain and systemic energy homeostasis through integrated biochemical and receptor mediated processes. Adenosine is an end-product and precursor of adenyl nucleotides (AMP, ADP and ATP) and has high affinity for a family of G-protein coupled receptors named; A1, A2A, A2B, A3. Through biochemical and receptor mediated processes adenosine functions as a bioenergetic network regulator.60
Extracellular adenosine mediates homeostatic sleep drive.61 Sleep deprivation leads to an increase in adenosine in basal forebrain and other brain regions and declines once sleep ensues.62 Adenosine contributes to homeostatic sleep by suppressing cortical arousal through inhibition of cell groups that promote arousal or disinhbition of cell groups that promote sleep. In this way, adenosine restores homeostasis through biochemical and hyperpolarizing influences; illustrating cross-talk between biochemical and neuromodulatory pools of adenosine. Adenosine also plays a role in thermoregulation63 and A1AR agonists are potent inhibitors of shivering and nonshivering thermogenesis.37, 51
Is AMP the endogenous signaling molecule?
AMP is well known as a biochemical regulator capable of modulating cellular energy homeostasis through biochemical mechanisms. Moreover, AMP induces a torpor-like state64, 65, identified as a decrease in the rate of oxygen consumption and Tb. Like adenosine, AMP, inhibits thermogenesis via stimulation of A1AR even when conversion to adenosine is blocked.53 This evidence as well as evidence that AMP inhibits firing of thermosensitive neurons within the hypothalamus53 argues for AMP as a purinergic thermoregulatory neuromodulator with sufficient potency to orchestrate the onset of torpor through an inhibition of thermogenesis. AMP was also shown to induce, in mice, a torpor-like decrease in Tb.65 The same study showed that AMP increased during constant darkness, and suggested that AMP functioned as a circadian metabolic signal.65 Circulating levels of AMP increased in mice when 24h darkness was used to mimic conditions of hibernation and fasting-induced daily torpor was associated with an increase in blood levels of AMP.65 Moreover, AMP induced expression of murine procolipase, a gene upregulated in peripheral tissues during the 24h dark conditions.65 The effects on Tb originally attributed to activation of AMPK65 was later found to depend on an A1AR dependent mechanism.66, 67 Additional study spurred debate as to whether the AMP-induced hypothermia was torpor. Ablation of the arcuate nucleus eliminated fasting-induced torpor, but did not eliminate the torpor-like response to AMP.67
One interpretation is that activation of A1AR within the CNS by AMP and/or adenosine is sufficient to induce a torpor-like state through an inhibition of thermogenesis and that A1AR stimulation is the mechanism responsible for lowering the thermoregulatory set point during onset of hibernation. Fasting induced torpor in mice and hibernation in ground squirrels is modulated by circadian and circannual signals. Importantly, as discussed above, the torpor-like effects of AMP in mice are enhanced under 24h darkness65 and the torpor-like effects of the A1AR agonist CHA in ground squirrels are seen only in the hibernation season.40 Indeed, the quality of response to CHA with regard to time course and magnitude of CHA-induced VO2 and Tb decline differs substantially between summer and winter ground squirrels.40 The circadian and circannual characteristics of A1AR agonists and circulating levels of AMP argue for a role for adenosine and/or AMP as neural signaling metabolites in hibernation. Nonetheless, it remains to be determined if adenosine or AMP increase in brain according to a seasonal or circadian rhythm that predicts onset of torpor.
Given the poorly understood circadian and circannual modulation of neural control of torpor, or the extreme phenotype of deep and shallow torpor it may not be feasible to translate these mechanisms to humans for therapeutic applications. However, studies in rats suggest that the thermolytic and parasympathomemetic effects of CHA in rats mimics neuronal mechanisms governing hibernation.37 Interestingly, the thermolytic potency of CHA in rats is increased by dietary restriction, although dietary restriction does not precede seasonal sensitivity to the thermolytic effects of CHA in ground squirrels.14, 39 Nonetheless, the profound effect of CHA on thermogenesis suggests the presence of the same neuronal substrate involved in the regulation of energy balance irrespective of whether the animals actually hibernate. Further study of circadian and circannual influence on the response to CNS A1AR stimulation has potential to unveil mechanisms to fine-tune metabolism.
Adenosine and 5′AMP in cellular energy homeostasis
Adenosine and 5′AMP (AMP) are reciprocal precursors of each other making them part of the same biochemical pathways involved in cellular energy homeostasis. Adenosine conversion to AMP is catalyzed by adenosine kinase. AMP conversion to adenosine is catalyzed by 5′-nucleotidases, termed ecto-5′nucleotidase (NT5E, a.k.a.CD73) to indicate that the enzyme is located outside the cell. Elevated adenosine levels are associated with local energy deficits reflected by increased levels of pyruvate and lactate and increased phosphorylation (activation) of AMPK.68
Cellular AMP is derived from ADP by the reaction catalyzed by adenylate kinase (2ADP ↔ ATP + AMP). Under normal circumstances, catabolism maintains the ATP:ADP ratio between 10:169 driving the adenylate kinase reaction towards ADP and maintaining AMP at low levels. However, under conditions of metabolic challenge the ATP:ADP ratio falls, and at steady state AMP is elevated relative to less metabolically stressful conditions. An increase in AMP: ATP activates AMPKK which phosphorylates and activates AMPK. AMPK stimulates ATP producing processes, and inhibits ATP consuming pathways.70 Adenosine and AMP have integrated roles as biochemical modulators and share roles in activating A1AR in energy conservation and homeostasis. Such redundancy would make sense given the interdependency of these metabolites. Inosine and IMP are also products of adenosine and AMP deamination.71 Although inosine’s role in energy homeostasis in mammals is not well understood, inosine serves as a sink for adenine nucleotides under fermentative conditions and thus plays an essential role in energy homeostasis in yeast.72
If adenosine or AMP are endogenous mediators of torpor, where extracellular pools of these metabolites originate is another compelling question73. Two proposed mechanisms for increasing extracellular adenosine are 1. Release of ATP with subsequent extracellular conversion of ATP to adenosine via NT5E, and 2. Transport of intracellular adenosine to the extracellular space via equilibrative nucleoside transporters. Both equilibrative and concentrative nucleoside transporters (ENTs and CNTs) transport adenosine across the plasma membrane;73–75 ENTs transport adenosine down its concentration gradient so when intracellular adenosine accumulates it is released and when extracellular adenosine accumulates it is taken up. Release via ENTs thus depends on intracellular adenosine concentrations which depend largely on expression of adenosine kinase (AdK), an enzyme compartmentalized within astrocytes in adult brain. AdK catalyzes phosphorylation of adenosine to make AMP and is regulated by cellular energy charge; AdK is stimulated by ADP/ATP as well as by AMP/adenosine76,76. Seizure after brain injury, and homeostatic sleep drive provide two examples that illustrate how adenosine kinase in astrocytes regulate intracellular and subsequently extracellular adenosine levels by release via ENTs. Astrocyte proliferation following brain injury increases AdK which then decreases intracellular and subsequently extracellular adenosine as the nucleoside flows down its concentration gradient. The decrease in extracellular adenosine promotes seizure activity77. Homeostatic sleep drive also depends on AdK and thus cellular metabolism. Mice deficient in glial AdK are unable to convert adenosine to AMP and consequently have higher intra and extracellular levels of adenosine and greater homeostatic sleep drive.36 In both of these examples adenosine originates from astrocytes via ENTs and extracellular levels of adenosine are influenced by astrocyte density (eg. in seizure) or energy status (eg. in sleep).
The functional link proposed between cellular metabolism and sleep homeostasis36 may also underlie onset of torpor. In arctic ground squirrel hypothalamus inhibition of adenosine kinase increases extracellular levels of adenosine while blockade of equilibrative nucleoside transporter has no effect (Drew et al., unpublished) suggesting that AdK regulates extracellular adenosine in these animals. Extracellular ATP is another potential source for extracellular adenosine and AMP.78 Tanycytes are glial like cells that line the 3rd and 4th ventricle 79 and are emerging as key players in energy homeostasis.80–82 Tanycytes release ATP44 that is then converted to adenosine and AMP by extracellular enzymes.73 Moreover, tanycytes are active during hibernation.83
Limitations to Energy Conservation
Prolonged torpor is interrupted by regular, albeit brief, periods of euthermy when Tb returns to pre-torpid levels. Interbout arousals occur with precise regularity and depending on species and ambient temperature occur as frequently as every few days to as infrequently as every 2 to 3 weeks. During these interrupted periods of energy conservation animals remain at pre-torpid Tb for 10–20h before re-entering torpor. Hibernation in the fat tailed lemur, under protected conditions, and in hibernating bears are the only exceptions where prolonged torpor is not interrupted. In both of these cases hibernating Tb remains above 30°C.84, 85 However, when hibernating Tb falls below 30°C, illustrated in the fat tailed lemur under unprotected conditions, torpor is interrupted by brief interbout arousals.85
Interbout arousals consume significant amounts of energy and thus represent a limit to energy conservation. 22 The energy expended for warming and maintaining high body temperature during these arousal episodes range from 23 to 86% of the total energy spent during the entire hibernation season depending on ambient temperature22. This high degree of seemingly uneconomical energy expenditure clearly indicates an essential function for these brief returns to euthermia. Many theories have been proposed for the benefit of these periodic arousals, some of which suggest that interbout arousals occur to eliminate metabolic waste products86, to replenish blood glucose levels87, 88 or to restore renal function and electrolyte balance.89 One of the most debated hypotheses is that interbout arousals allow for restorative sleep.90–92 Elucidating the mechanisms that stimulate interbout arousal may reveal novel means to modulate thermogenesis and thus metabolism. These mechanisms could provide pathways to drug discovery to modulate metabolic rate for the treatment of obesity.
Proposed mechanism for cross-talk between neural signaling and metabolism in interbout arousal
While the neurochemical mechanisms regulating interbout arousals are poorly understood,25, 93 they are expected to be related to metabolic and/or circadian cycles94, 95 and triggered by metabolic needs.87,25, 96 Pioneering work of Mary McKenna and others illustrate the importance of astrocytes and glutamate oxidation in brain energy metabolism97–99. Cross-talk between metabolic and neural signaling pools is a principle that may be fundamental to the energy economy of hibernation and torpor. Interestingly NMDA receptors play a role in arousal and a decrease in glutamate availability is being investigated as a metabolic signal to arouse. Jinka et al., 2012 showed that inhibition of NMDA type glutamate receptors (NMDAR) induces arousal from torpor in AGS and that the receptor pool involved lies outside of the CNS or within a circumventricular organ (Fig. 2). Similar results have been found in other ground squirrel species.93
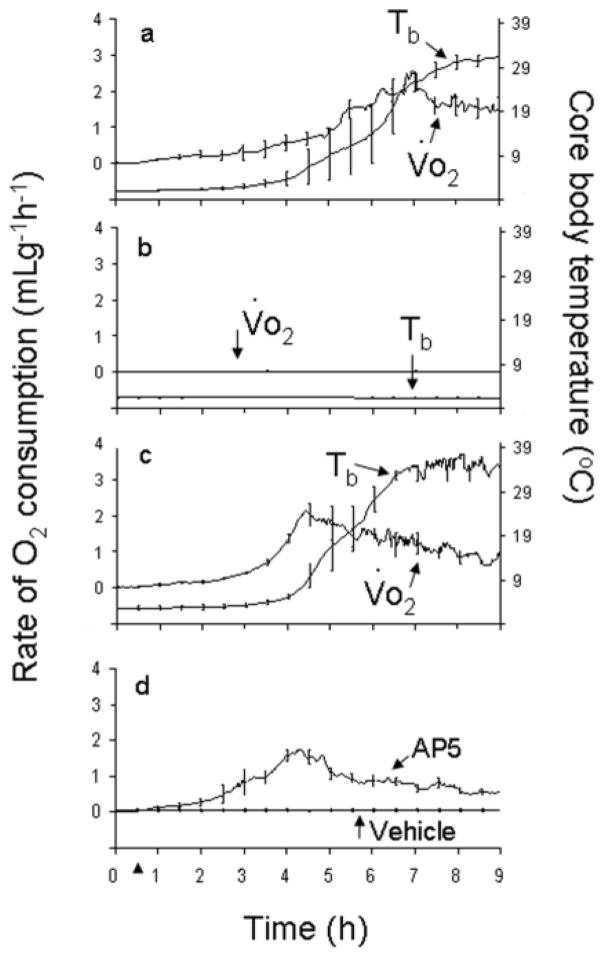
Figure 2.
Inhibiting NMDAR outside of the CNS is sufficient to induce arousal from hibernation: MK-801 (5 mg/kg; ip) induces arousal, indicated by an increase in the rate of O2 consumption (Vo2) and core body temperature (Tb) in a hibernating arctic ground squirrel (AGS) (a) while vehicle has no effect (b). Handling-induced arousal is shown for comparison (c). AP5 (5 mg/kg; ip), an NMDAR antagonist that does not cross the blood brain barrier, also induces arousal from hibernation (d). Arrowhead at 0.5h represents when drug (a, d) or vehicle (b, d) was delivered or arousal was initiated by handling (c). (From Jinka et al., 2012)
A metabolite and neurosignaling molecule such as glutamate illustrates how metabolic flux could influence neurally mediated interbout arousal. Metabolic flux influences glutamatergic signaling through effects on substrate availability and modulatory influence of metabolic end products. Glutamate is derived from glucose via the pyruvate carboxylase pathway. Recycling of glutamate from glutamine via the glu-gln cycle also maintains supplies of glutamate unless excess nitrogen drives equilibrium towards glutamine.100, 101
Both sources of glutamate, i.e., glucose and the glu-gln cycle, may become limiting during prolonged torpor. In AGS, blood glucose levels decrease from 10 to 5mM during torpor 102 until gluconeogenesis replenishes blood glucose levels during arousal.87, 103 During prolonged torpor protein catabolism produces nitrogenous waste that would normally be eliminated as urea. However, urea blood levels and urea cycle activity decrease during torpor. 87, 103,104 The subsequent buildup of nitrogen may shift the glu-gln cycle towards glutamine. Low levels of glucose in torpor would be expected to decrease glutamate supply and limit the capacity of the glu-gln cycle. An increase in glutamine and decrease in glucose are two of the most robust biomarkers of torpor.86, 103 Another biomarker of torpor is an increase in circulating ketone bodies such as β-hydroxybutyrate and acetoacetate.105 Ketones influence glutamatergic transmission by inhibiting vesicular glutamate transporters.106 These metabolic consequences of prolonged torpor offer intriguing possibilities for modulating neuronal signaling to induce interbout arousal. More research is needed to test these and alternative hypothesis regarding endogenous neural signaling in a process that is clearly essential to successful hibernation.22
In this review we have described the energy conserving phenomenon of hibernation and focused on three ubiquitous, metabolite signaling molecules, adenosine, AMP and glutamate. Much remains to be learned about cellular signaling in the energy conserving phenomenon of hibernation and readers are referred to other reviews for a more comprehensive discussion of the signaling molecules and processes known to influence hibernation and torpor, 4–10 Discovery of the signaling molecules that modulate thermogenesis during torpor onset and interbout arousal may suggest novel approaches to modulate metabolism and this knowledge may spur new ideas for treating obesity. Faced with an epidemic of obesity in the US with growing prevalence world-wide, the cross-talk between metabolites and neuronal signaling in energy expenditure is an important area for study.
Supplementary Material
Figure 1.
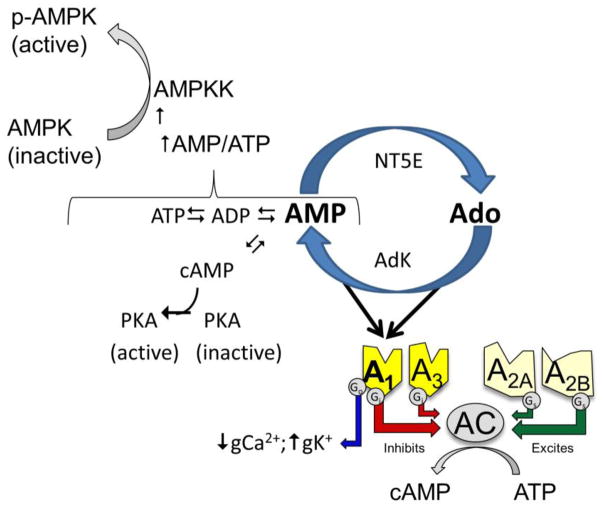
Adenosine is central to energy homeostasis where as a precursor to 5′AMP adenosine contributes to energy charge and activation of AMP kinase. Adenosine and AMP are reciprocal precursors of each other. Adenosine is converted to 5′AMP, a reaction catalyzed by adenosine kinase (AdK). 5′AMP is converted to adenosine, a reaction catalyzed by ecto-5′nucleotidase (NT5E), also known as CD73. At the receptor level both adenosine and 5′AMP stimulate the A 1 adenosine receptor.
Acknowledgments
This material is based upon work supported by the National Science Foundation under Grant Number (NSF IOS-1258179) and by the National Institute Of Neurological Disorders And Stroke of the National Institutes of Health under Award Number R03NS081637. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References Cited
- 1.Santoro N. Childhood obesity and type 2 diabetes: The frightening epidemic. World J Pediatr. 2013;9:101–102. doi: 10.1007/s12519-013-0410-8. [DOI] [PubMed] [Google Scholar]
- 2.Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404:661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- 3.Morrison SF, Madden CJ, Tupone D. Central neural regulation of brown adipose tissue thermogenesis and energy expenditure. Cell Metab. 2014;19:741–756. doi: 10.1016/j.cmet.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khan M, He L, Zhuang X. The emerging role of gpr50 receptor in brain. Biomed Pharmacother. 2016;78:121–128. doi: 10.1016/j.biopha.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 5.Cubuk C, Bank JH, Herwig A. The chemistry of cold: Mechanisms of torpor regulation in the siberian hamster. Physiology (Bethesda) 2016;31:51–59. doi: 10.1152/physiol.00028.2015. [DOI] [PubMed] [Google Scholar]
- 6.van Breukelen F, Martin SL. The hibernation continuum: Physiological and molecular aspects of metabolic plasticity in mammals. Physiology (Bethesda) 2015;30:273–281. doi: 10.1152/physiol.00010.2015. [DOI] [PubMed] [Google Scholar]
- 7.Grabek KR, Martin SL, Hindle AG. Proteomics approaches shed new light on hibernation physiology. J Comp Physiol B. 2015;185:607–627. doi: 10.1007/s00360-015-0905-9. [DOI] [PubMed] [Google Scholar]
- 8.Staples JF. Metabolic suppression in mammalian hibernation: The role of mitochondria. J Exp Biol. 2014;217:2032–2036. doi: 10.1242/jeb.092973. [DOI] [PubMed] [Google Scholar]
- 9.Swoap SJ. Thermoregulation: An orphan receptor finds its way in the cold. Curr Biol. 2012;22:R17–18. doi: 10.1016/j.cub.2011.11.046. [DOI] [PubMed] [Google Scholar]
- 10.Swoap SJ, Weinshenker D. Norepinephrine controls both torpor initiation and emergence via distinct mechanisms in the mouse. PLoS One. 2008;3:e4038. doi: 10.1371/journal.pone.0004038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Florant GL, Turner BM, Heller HC. Temperature regulation during wakefulness, sleep, and hibernation in marmots. Am J Physiol. 1978;235:R82–88. doi: 10.1152/ajpregu.1978.235.1.R82. [DOI] [PubMed] [Google Scholar]
- 12.Snapp BD, Heller HC. Suppression of metabolism during hibernation in ground squirrels (citellus lateralis) Physiol Zool. 1981;54:2970307. [Google Scholar]
- 13.Levesque DL, Tattersall GJ. Seasonal torpor and normothermic energy metabolism in the eastern chipmunk (tamias striatus) J Comp Physiol B. 2010;180:279–292. doi: 10.1007/s00360-009-0405-x. [DOI] [PubMed] [Google Scholar]
- 14.Olson JM, Jinka TR, Larson LK, Danielson JJ, Moore JT, Carpluck J, et al. Circannual rhythm in body temperature, torpor, and sensitivity to a(1) adenosine receptor agonist in arctic ground squirrels. J Biol Rhythms. 2013;28:201–207. doi: 10.1177/0748730413490667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sheriff MJ, Williams CT, Kenagy GJ, Buck CL, Barnes BM. Thermoregulatory changes anticipate hibernation onset by 45 days: Data from free-living arctic ground squirrels. J Comp Physiol B. 2012:841–847. doi: 10.1007/s00360-012-0661-z. [DOI] [PubMed] [Google Scholar]
- 16.Chayama Y, Ando L, Tamura Y, Miura M, Yamaguchi Y. Decreases in body temperature and body mass constitute pre-hibernation remodelling in the syrian golden hamster, a facultative mammalian hibernator. R Soc Open Sci. 2016;3:160002. doi: 10.1098/rsos.160002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sheriff MJ, Kenagy GJ, Richter M, Lee T, Toien O, Kohl F, et al. Phenological variation in annual timing of hibernation and breeding in nearby populations of arctic ground squirrels. Proc Biol Sci. 2010;278:2369–2375. doi: 10.1098/rspb.2010.2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barnes BM. Freeze avoidance in a mammal: Body temperatures below 0 degree c in an arctic hibernator. Science. 1989;244:1593–1595. doi: 10.1126/science.2740905. [DOI] [PubMed] [Google Scholar]
- 19.Geiser F. Reduction of metabolism during hibernation and daily torpor in mammals and birds: Temperature effect or physiological inhibition? J Comp Physiol B. 1988;158:25–37. doi: 10.1007/BF00692726. [DOI] [PubMed] [Google Scholar]
- 20.Buck CL, Barnes BM. Effects of ambient temperature on metabolic rate, respiratory quotient, and torpor in an arctic hibernator. Am J Physiol Regul Integr Comp Physiol. 2000;279:R255–262. doi: 10.1152/ajpregu.2000.279.1.R255. [DOI] [PubMed] [Google Scholar]
- 21.Drew KL, Jinka TR. Season primes the brain in an arctic hibernator to facilitate entrance into torpor mediated by adenosine a(1) receptors. 2011;31:10752–10758. doi: 10.1523/JNEUROSCI.1240-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karpovich SA, Toien O, Buck CL, Barnes BM. Energetics of arousal episodes in hibernating arctic ground squirrels. J Comp Physiol B. 2009;179:691–700. doi: 10.1007/s00360-009-0350-8. [DOI] [PubMed] [Google Scholar]
- 23.Geiser F. Metabolic rate and body temperature reduction during hibernation and daily torpor. Annu Rev Physiol. 2004;66:239–274. doi: 10.1146/annurev.physiol.66.032102.115105. [DOI] [PubMed] [Google Scholar]
- 24.Schubert KA, Boerema AS, Vaanholt LM, de Boer SF, Strijkstra AM, Daan S. Daily torpor in mice: High foraging costs trigger energy-saving hypothermia. Biol Lett. 2010;6:132–135. doi: 10.1098/rsbl.2009.0569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Drew KL, Buck CL, Barnes BM, Christian SL, Rasley BT, Harris MB. Central nervous system regulation of mammalian hibernation: Implications for metabolic suppression and ischemia tolerance. J Neurochem. 2007;102:1713–1726. doi: 10.1111/j.1471-4159.2007.04675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dark J, Pelz KM. Npy y1 receptor antagonist prevents npy-induced torpor-like hypothermia in cold-acclimated siberian hamsters. Am J Physiol Regul Integr Comp Physiol. 2008;294:R236–245. doi: 10.1152/ajpregu.00587.2007. [DOI] [PubMed] [Google Scholar]
- 27.Pelz KM, Routman D, Driscoll JR, Kriegsfeld LJ, Dark J. Monosodium glutamate-induced arcuate nucleus damage affects both natural torpor and 2dg-induced torpor-like hypothermia in siberian hamsters. Am J Physiol Regul Integr Comp Physiol. 2008;294:R255–265. doi: 10.1152/ajpregu.00387.2007. [DOI] [PubMed] [Google Scholar]
- 28.Gluck EF, Stephens N, Swoap SJ. Peripheral ghrelin deepens torpor bouts in mice through the arcuate nucleus neuropeptide y signaling pathway. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1303–1309. doi: 10.1152/ajpregu.00232.2006. [DOI] [PubMed] [Google Scholar]
- 29.Pelz KM, Dark J. Icv npy y1 receptor agonist but not y5 agonist induces torpor-like hypothermia in cold-acclimated siberian hamsters. Am J Physiol Regul Integr Comp Physiol. 2007;292:R2299–2311. doi: 10.1152/ajpregu.00790.2006. [DOI] [PubMed] [Google Scholar]
- 30.Schwartz C, Hampton M, Andrews MT. Hypothalamic gene expression underlying pre-hibernation satiety. Genes Brain Behav. 2015;14:310–318. doi: 10.1111/gbb.12199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Steward CA, Horan TL, Schuhler S, Bennett GW, Ebling FJ. Central administration of thyrotropin releasing hormone (trh) and related peptides inhibits feeding behavior in the siberian hamster. Neuroreport. 2003;14:687–691. doi: 10.1097/00001756-200304150-00006. [DOI] [PubMed] [Google Scholar]
- 32.Tamura Y, Shintani M, Nakamura A, Monden M, Shiomi H. Phase-specific central regulatory systems of hibernation in syrian hamsters. Brain Res. 2005;1045:88–96. doi: 10.1016/j.brainres.2005.03.029. [DOI] [PubMed] [Google Scholar]
- 33.Nishino S, Arrigoni J, Shelton J, Kanbayashi T, Dement WC, Mignot E. Effects of thyrotropin-releasing hormone and its analogs on daytime sleepiness and cataplexy in canine narcolepsy. J Neurosci. 1997;17:6401–6408. doi: 10.1523/JNEUROSCI.17-16-06401.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hara J, Gerashchenko D, Wisor JP, Sakurai T, Xie X, Kilduff TS. Thyrotropin-releasing hormone increases behavioral arousal through modulation of hypocretin/orexin neurons. J Neurosci. 2009;29:3705–3714. doi: 10.1523/JNEUROSCI.0431-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mullur R, Liu YY, Brent GA. Thyroid hormone regulation of metabolism. Physiol Rev. 2014;94:355–382. doi: 10.1152/physrev.00030.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bjorness TE, Dale N, Mettlach G, Sonneborn A, Sahin B, Fienberg AA, et al. An adenosine-mediated glial-neuronal circuit for homeostatic sleep. J Neurosci. 2016;36:3709–3721. doi: 10.1523/JNEUROSCI.3906-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tupone D, Madden CJ, Morrison SF. Central activation of the a1 adenosine receptor (a1ar) induces a hypothermic, torpor-like state in the rat. J Neurosci. 2013;33:14512–14525. doi: 10.1523/JNEUROSCI.1980-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang L, Qi Y, Yang Y. Astrocytes control food intake by inhibiting agrp neuron activity via adenosine a1 receptors. Cell Rep. 2015;11:798–807. doi: 10.1016/j.celrep.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 39.Jinka TR, Carlson ZA, Moore JT, Drew KL. Altered thermoregulation via sensitization of a1 adenosine receptors in dietary-restricted rats. Psychopharmacology (Berl) 2010;209:217–224. doi: 10.1007/s00213-010-1778-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jinka TR, Toien O, Drew KL. Season primes the brain in an arctic hibernator to facilitate entrance into torpor mediated by adenosine a(1) receptors. J Neurosci. 2011;31:10752–10758. doi: 10.1523/JNEUROSCI.1240-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Iliff BW, Swoap SJ. Central adenosine receptor signaling is necessary for daily torpor in mice. Am J Physiol Regul Integr Comp Physiol. 2012;303:R477–484. doi: 10.1152/ajpregu.00081.2012. [DOI] [PubMed] [Google Scholar]
- 42.Shiomi H, Tamura Y. pharmacological aspects of mammalian hibernation: Central thermoregulation factors in hibernation cycle. Nippon Yakurigaku Zasshi (Folia Pharmacol Jpn ) 2000;116:304–312. doi: 10.1254/fpj.116.304. [DOI] [PubMed] [Google Scholar]
- 43.Walker JM, Haskell EH, Berger RJ, Heller HC. Hibernation and circannual rhythms of sleep. Physiological Zoology. 1980;53:8–11. [Google Scholar]
- 44.Bolborea M, Dale N. Hypothalamic tanycytes: Potential roles in the control of feeding and energy balance. Trends Neurosci. 2013;36:91–100. doi: 10.1016/j.tins.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bjorness TE, Greene RW. Adenosine and sleep. Curr Neuropharmacol. 2009;7:238–245. doi: 10.2174/157015909789152182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sheriff MJ, Fridinger RW, Toien O, Barnes BM, Buck CL. Metabolic rate and prehibernation fattening in free-living arctic ground squirrels. Physiol Biochem Zool. 2013;86:515–527. doi: 10.1086/673092. [DOI] [PubMed] [Google Scholar]
- 47.van Ooijen AM, van Marken Lichtenbelt WD, van Steenhoven AA, Westerterp KR. Seasonal changes in metabolic and temperature responses to cold air in humans. Physiol Behav. 2004;82:545–553. doi: 10.1016/j.physbeh.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 48.Rintamaki R, Grimaldi S, Englund A, Haukka J, Partonen T, Reunanen A, et al. Seasonal changes in mood and behavior are linked to metabolic syndrome. PLoS One. 2008;3:e1482. doi: 10.1371/journal.pone.0001482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dopico XC, Evangelou M, Ferreira RC, Guo H, Pekalski ML, Smyth DJ, et al. Widespread seasonal gene expression reveals annual differences in human immunity and physiology. Nat Commun. 2015;6:7000. doi: 10.1038/ncomms8000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Heller HC, Colliver GW, Beard J. Thermoregulation during entrance into hibernation. Pflügers Arch. 1977;369:55–59. doi: 10.1007/BF00580810. [DOI] [PubMed] [Google Scholar]
- 51.Tupone D, Madden C, Algwaiz H, Morrison S. Adenosine a1-receptor agonist (cha) produces a hypothermic state by reducing bat thermogenesis. The FASEB Journal. 2012;26:1081. [Google Scholar]
- 52.Sekizawa S, Horowitz JM, Horwitz BA, Chen CY. Realignment of signal processing within a sensory brainstem nucleus as brain temperature declines in the syrian hamster, a hibernating species. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2012;198:267–282. doi: 10.1007/s00359-011-0706-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Muzzi M, Blasi F, Masi A, Coppi E, Traini C, Felici R, et al. Neurological basis of amp-dependent thermoregulation and its relevance to central and peripheral hyperthermia. J Cereb Blood Flow Metab. 2013;33:183–190. doi: 10.1038/jcbfm.2012.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Krauchi K, Deboer T. The interrelationship between sleep regulation and thermoregulation. Front Biosci (Landmark Ed) 2010;15:604–625. doi: 10.2741/3636. [DOI] [PubMed] [Google Scholar]
- 55.Barros RC, Branco LG, Carnio EC. Respiratory and body temperature modulation by adenosine a1 receptors in the anteroventral preoptic region during normoxia and hypoxia. Respir Physiol Neurobiol. 2006;153:115–125. doi: 10.1016/j.resp.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 56.Alam MN, McGinty D, Szymusiak R. Preoptic/anterior hypothalamic neurons: Thermosensitivity in wakefulness and non rapid eye movement sleep. Brain Res. 1996;718:76–82. doi: 10.1016/0006-8993(96)00035-2. [DOI] [PubMed] [Google Scholar]
- 57.Cerri M, Mastrotto M, Tupone D, Martelli D, Luppi M, Perez E, et al. The inhibition of neurons in the central nervous pathways for thermoregulatory cold defense induces a suspended animation state in the rat. J Neurosci. 2013;33:2984–2993. doi: 10.1523/JNEUROSCI.3596-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Almeida MC, Hew-Butler T, Soriano RN, Rao S, Wang W, Wang J, et al. Pharmacological blockade of the cold receptor trpm8 attenuates autonomic and behavioral cold defenses and decreases deep body temperature. J Neurosci. 2012;32:2086–2099. doi: 10.1523/JNEUROSCI.5606-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Drew K, Jinka T. The bioenergetic network of adenosine in hibernation, sleep and thermoregulation. In: Masino S, Boison D, editors. Adenosine a key link between metabolism and brain activity. New York: Springer; 2013. pp. 253–272. [Google Scholar]
- 60.Boison D, Masino SA, Geiger JD. Homeostatic bioenergetic network regulation: A novel concept to avoid pharmacoresistance in epilepsy. Expert Opin Drug Discov. 2011;6:1–12. doi: 10.1517/17460441.2011.575777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Oishi Y, Huang ZL, Fredholm BB, Urade Y, Hayaishi O. Adenosine in the tuberomammillary nucleus inhibits the histaminergic system via a1 receptors and promotes non-rapid eye movement sleep. Proc Natl Acad Sci U S A. 2008;105:19992–19997. doi: 10.1073/pnas.0810926105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Porkka-Heiskanen T, Strecker RE, McCarley RW. Brain site-specificity of extracellular adenosine concentration changes during sleep deprivation and spontaneous sleep: An in vivo microdialysis study. Neuroscience. 2000;99:507–517. doi: 10.1016/s0306-4522(00)00220-7. [DOI] [PubMed] [Google Scholar]
- 63.Barros RC, Branco LG. Role of central adenosine in the respiratory and thermoregulatory responses to hypoxia. Neuroreport. 2000;11:193–197. doi: 10.1097/00001756-200001170-00038. [DOI] [PubMed] [Google Scholar]
- 64.Dugbartey GJ, Bouma HR, Strijkstra AM, Boerema AS, Henning RH. Induction of a torpor-like state by 5′-amp does not depend on h2s production. PLoS One. 2015;10:e0136113. doi: 10.1371/journal.pone.0136113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang J, Kaasik K, Blackburn MR, Lee CC. Constant darkness is a circadian metabolic signal in mammals. Nature. 2006;439:340–343. doi: 10.1038/nature04368. [DOI] [PubMed] [Google Scholar]
- 66.Muzzi M, Blasi F, Chiarugi A. Amp-dependent hypothermia affords protection from ischemic brain injury. J Cereb Blood Flow Metab. 2013;33:171–174. doi: 10.1038/jcbfm.2012.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Swoap SJ, Rathvon M, Gutilla M. Amp does not induce torpor. Am J Physiol Regul Integr Comp Physiol. 2007;293:R468–473. doi: 10.1152/ajpregu.00888.2006. [DOI] [PubMed] [Google Scholar]
- 68.Porkka-Heiskanen T, Kalinchuk AV. Adenosine, energy metabolism and sleep homeostasis. Sleep Med Rev. 2011;15:123–135. doi: 10.1016/j.smrv.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 69.Erecinska M, Silver IA. Ions and energy in mammalian brain. Prog Neurobiol. 1994;43:37–71. doi: 10.1016/0301-0082(94)90015-9. [DOI] [PubMed] [Google Scholar]
- 70.Grahame Hardie D. Amp-activated protein kinase: A key regulator of energy balance with many roles in human disease. J Intern Med. 2014;276:543–559. doi: 10.1111/joim.12268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ipata PL, Camici M, Micheli V, Tozz MG. Metabolic network of nucleosides in the brain. Curr Top Med Chem. 2011;11:909–922. doi: 10.2174/156802611795347555. [DOI] [PubMed] [Google Scholar]
- 72.Walther T, Novo M, Rossger K, Letisse F, Loret MO, Portais JC, et al. Control of atp homeostasis during the respiro-fermentative transition in yeast. Mol Syst Biol. 2010;6:344. doi: 10.1038/msb.2009.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Latini S, Pedata F. Adenosine in the central nervous system: Release mechanisms and extracellular concentrations. J Neurochem. 2001;79:463–484. doi: 10.1046/j.1471-4159.2001.00607.x. [DOI] [PubMed] [Google Scholar]
- 74.Wall M, Dale N. Activity-dependent release of adenosine: A critical re-evaluation of mechanism. Curr Neuropharmacol. 2008;6:329–337. doi: 10.2174/157015908787386087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Young JD. The slc28 (cnt) and slc29 (ent) nucleoside transporter families: A 30-year collaborative odyssey. Biochem Soc Trans. 2016;44:869–876. doi: 10.1042/BST20160038. [DOI] [PubMed] [Google Scholar]
- 76.Mimouni M, Bontemps F, Van den Berghe G. Kinetic studies of rat liver adenosine kinase. Explanation of exchange reaction between adenosine and amp. J Biol Chem. 1994;269:17820–17825. [PubMed] [Google Scholar]
- 77.Boison D. Adenosinergic signaling in epilepsy. Neuropharmacology. 2015 doi: 10.1016/j.neuropharm.2015.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zimmermann H, Zebisch M, Strater N. Cellular function and molecular structure of ecto-nucleotidases. Purinergic Signal. 2012;8:437–502. doi: 10.1007/s11302-012-9309-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Langlet F, Mullier A, Bouret SG, Prevot V, Dehouck B. Tanycyte-like cells form a blood-cerebrospinal fluid barrier in the circumventricular organs of the mouse brain. J Comp Neurol. 2013;521:3389–3405. doi: 10.1002/cne.23355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Langlet F. Tanycytes: A gateway to the metabolic hypothalamus. J Neuroendocrinol. 2014;26:753–760. doi: 10.1111/jne.12191. [DOI] [PubMed] [Google Scholar]
- 81.Ebling FJ. On the value of seasonal mammals for identifying mechanisms underlying the control of food intake and body weight. Horm Behav. 2014;66:56–65. doi: 10.1016/j.yhbeh.2014.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Frayling C, Britton R, Dale N. Atp-mediated glucosensing by hypothalamic tanycytes. J Physiol. 2011;589:2275–2286. doi: 10.1113/jphysiol.2010.202051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bratincsak A, McMullen D, Miyake S, Toth ZE, Hallenbeck JM, Palkovits M. Spatial and temporal activation of brain regions in hibernation: C-fos expression during the hibernation bout in thirteen-lined ground squirrel. J Comp Neurol. 2007;505:443–458. doi: 10.1002/cne.21507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Toien O, Blake J, Edgar DM, Grahn DA, Heller HC, Barnes BM. Hibernation in black bears: Independence of metabolic suppression from body temperature. Science. 2011;331:906–909. doi: 10.1126/science.1199435. [DOI] [PubMed] [Google Scholar]
- 85.Dausmann KH, Glos J, Ganzhorn JU, Heldmaier G. Physiology: Hibernation in a tropical primate. Nature. 2004;429:825–826. doi: 10.1038/429825a. [DOI] [PubMed] [Google Scholar]
- 86.Serkova NJ, Rose JC, Epperson LE, Carey HV, Martin SL. Quantitative analysis of liver metabolites in three stages of the circannual hibernation cycle in 13-lined ground squirrels by nmr. Physiol Genomics. 2007;31:15–24. doi: 10.1152/physiolgenomics.00028.2007. [DOI] [PubMed] [Google Scholar]
- 87.Galster W, Morrison PR. Gluconeogenesis in arctic ground squirrels between periods of hibernation. Am J Physiol. 1975;228:325–330. doi: 10.1152/ajplegacy.1975.228.1.325. [DOI] [PubMed] [Google Scholar]
- 88.Galster WA, Morrison P. Cyclic changes in carbohydrate concentrations during hibernation in the arctic ground squirrel. Am J Physiol. 1970;218:1228–1232. doi: 10.1152/ajplegacy.1970.218.4.1228. [DOI] [PubMed] [Google Scholar]
- 89.Jani A, Martin SL, Jain S, Keys D, Edelstein CL. Renal adaptation during hibernation. Am J Physiol Renal Physiol. 2013;305:F1521–1532. doi: 10.1152/ajprenal.00675.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Strijkstra AM, Daan S. Dissimilarity of slow-wave activity enhancement by torpor and sleep deprivation in a hibernator. Am J Physiol. 1998;275:R1110–1117. doi: 10.1152/ajpregu.1998.275.4.R1110. [DOI] [PubMed] [Google Scholar]
- 91.Larkin JE, Heller HC. Sleep after arousal from hibernation is not homeostatically regulated. Am J Physiol. 1999;276:R522–529. doi: 10.1152/ajpregu.1999.276.2.R522. [DOI] [PubMed] [Google Scholar]
- 92.Daan S, Barnes BM, Strijkstra AM. Warming up for sleep? Ground squirrels sleep during arousals from hibernation. Neurosci Lett. 1991;128:265–268. doi: 10.1016/0304-3940(91)90276-y. [DOI] [PubMed] [Google Scholar]
- 93.Harris MB, Milsom WK. Is hibernation facilitated by an inhibition of arousal? In: Heldmaier G, Klingenspor M, editors. Life in the cold. Berlin: Springer-Verlag; 2000. pp. 241–250. [Google Scholar]
- 94.Tu BP, McKnight SL. Metabolic cycles as an underlying basis of biological oscillations. Nat Rev Mol Cell Biol. 2006;7:696–701. doi: 10.1038/nrm1980. [DOI] [PubMed] [Google Scholar]
- 95.Malan A. Is the torpor-arousal cycle of hibernation controlled by a non-temperature-compensated circadian clock? J Biol Rhythms. 2010;25:166–175. doi: 10.1177/0748730410368621. [DOI] [PubMed] [Google Scholar]
- 96.Carey HV, Andrews MT, Martin SL. Mammalian hibernation: Cellular and molecular responses to depressed metabolism and low temperature. Physiol Rev. 2003;83:1153–1181. doi: 10.1152/physrev.00008.2003. [DOI] [PubMed] [Google Scholar]
- 97.McKenna MC, Stridh MH, McNair LF, Sonnewald U, Waagepetersen HS, Schousboe A. Glutamate oxidation in astrocytes: Roles of glutamate dehydrogenase and aminotransferases. J Neurosci Res. 2016 doi: 10.1002/jnr.23908. [DOI] [PubMed] [Google Scholar]
- 98.Schousboe A, Scafidi S, Bak LK, Waagepetersen HS, McKenna MC. Glutamate metabolism in the brain focusing on astrocytes. Adv Neurobiol. 2014;11:13–30. doi: 10.1007/978-3-319-08894-5_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dienel GA, McKenna MC. A dogma-breaking concept: Glutamate oxidation in astrocytes is the source of lactate during aerobic glycolysis in resting subjects. J Neurochem. 2014;131:395–398. doi: 10.1111/jnc.12835. [DOI] [PubMed] [Google Scholar]
- 100.Hamberger AC, Chiang GH, Nylen ES, Scheff SW, Cotman CW. Glutamate as a cns transmitter. I. Evaluation of glucose and glutamine as precursors for the synthesis of preferentially released glutamate. Brain Res. 1979;168:513–530. doi: 10.1016/0006-8993(79)90306-8. [DOI] [PubMed] [Google Scholar]
- 101.Oz G, Berkich DA, Henry PG, Xu Y, LaNoue K, Hutson SM, et al. Neuroglial metabolism in the awake rat brain: Co2 fixation increases with brain activity. J Neurosci. 2004;24:11273–11279. doi: 10.1523/JNEUROSCI.3564-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Osborne PG, Hu Y, Covey DN, Barnes BN, Katz Z, Drew KL. Determination of striatal extracellular gamma-aminobutyric acid in non-hibernating and hibernating arctic ground squirrels using quantitative microdialysis. Brain Res. 1999;839:1–6. doi: 10.1016/s0006-8993(99)01627-3. [DOI] [PubMed] [Google Scholar]
- 103.Epperson LE, Karimpour-Fard A, Hunter LE, Martin SL. Metabolic cycles in a circannual hibernator. Physiol Genomics. 2011 doi: 10.1152/physiolgenomics.00028.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rose JC, Epperson LE, Carey HV, Martin SL. Seasonal liver protein differences in a hibernator revealed by quantitative proteomics using whole animal isotopic labeling. Comp Biochem Physiol Part D Genomics Proteomics. 2011;6:163–170. doi: 10.1016/j.cbd.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Andrews MT, Russeth KP, Drewes LR, Henry PG. Adaptive mechanisms regulate preferred utilization of ketones in the heart and brain of a hibernating mammal during arousal from torpor. Am J Physiol Regul Integr Comp Physiol. 2009;296:R383–393. doi: 10.1152/ajpregu.90795.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Juge N, Gray JA, Omote H, Miyaji T, Inoue T, Hara C, et al. Metabolic control of vesicular glutamate transport and release. Neuron. 2010;68:99–112. doi: 10.1016/j.neuron.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tang F, Lane S, Korsak A, Paton JF, Gourine AV, Kasparov S, et al. Lactate-mediated glia-neuronal signalling in the mammalian brain. Nat Commun. 2014;5:3284. doi: 10.1038/ncomms4284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gundersen V, Storm-Mathisen J, Bergersen LH. Neuroglial transmission. Physiol Rev. 2015;95:695–726. doi: 10.1152/physrev.00024.2014. [DOI] [PubMed] [Google Scholar]
- 109.Li AJ, Wiater MF, Wang Q, Wank S, Ritter S. Deletion of gpr40 fatty acid receptor gene in mice blocks mercaptoacetate-induced feeding. Am J Physiol Regul Integr Comp Physiol. 2016;310:R968–974. doi: 10.1152/ajpregu.00548.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.